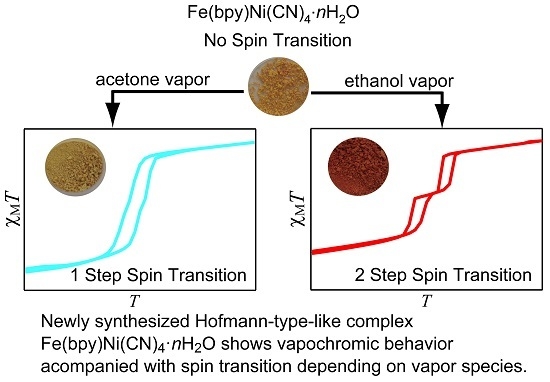
Spin-Crossover Behavior of Hofmann-Type-Like Complex Fe(4,4’-bipyridine)Ni(CN)4·nH2O Depending on Guest Species
Abstract
:1. Introduction
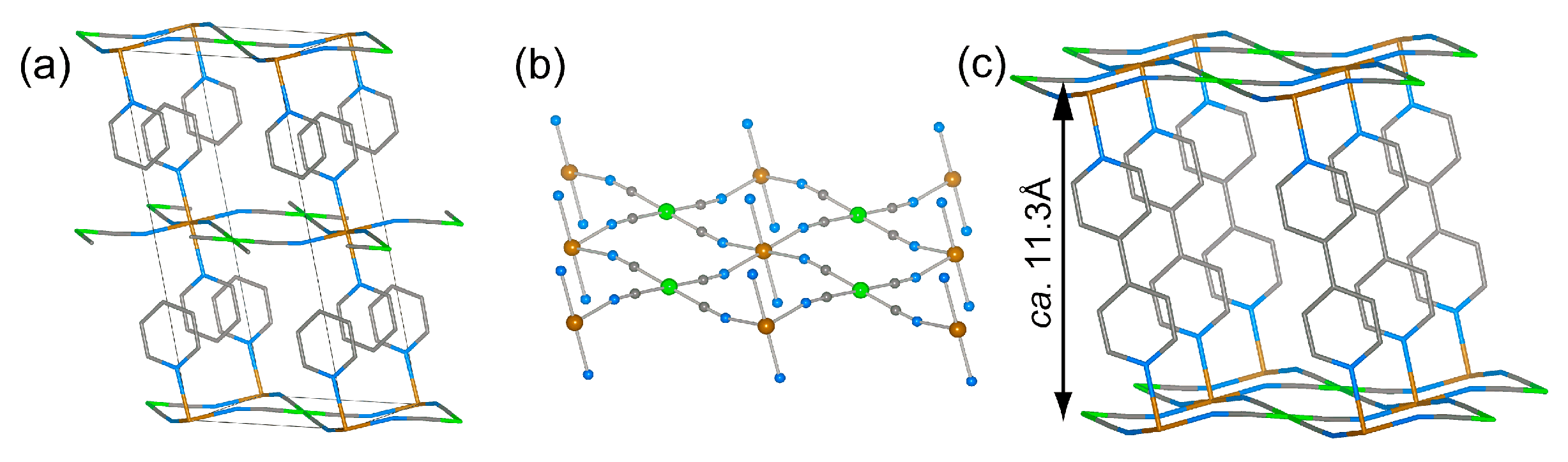
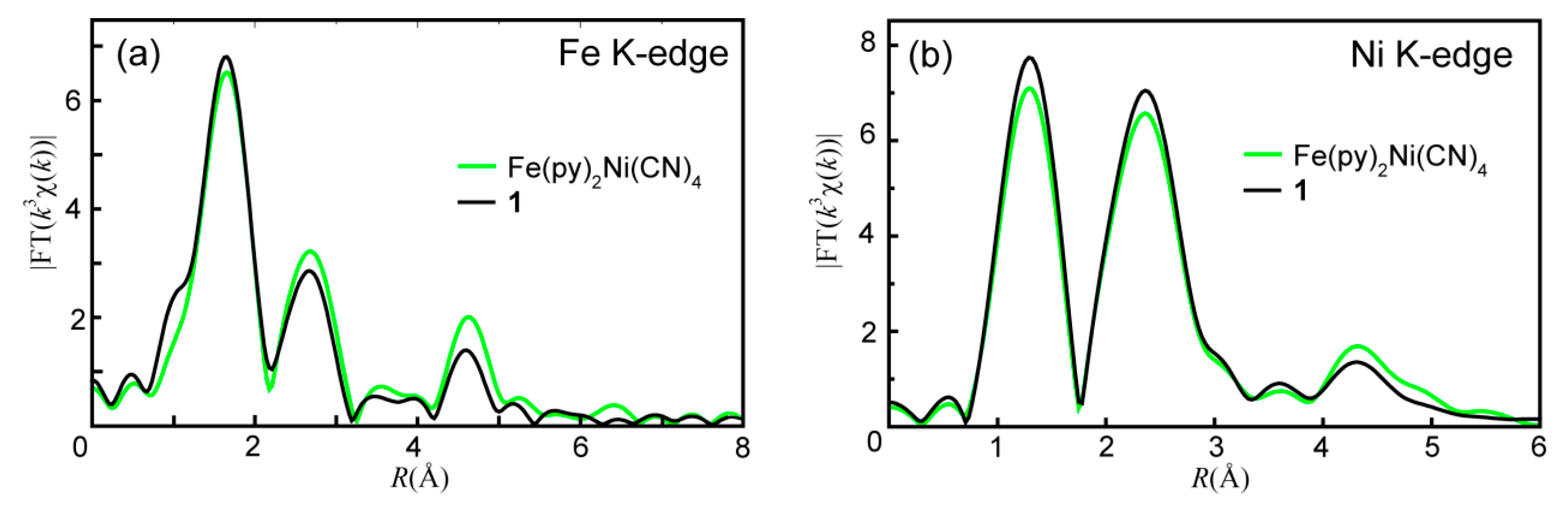
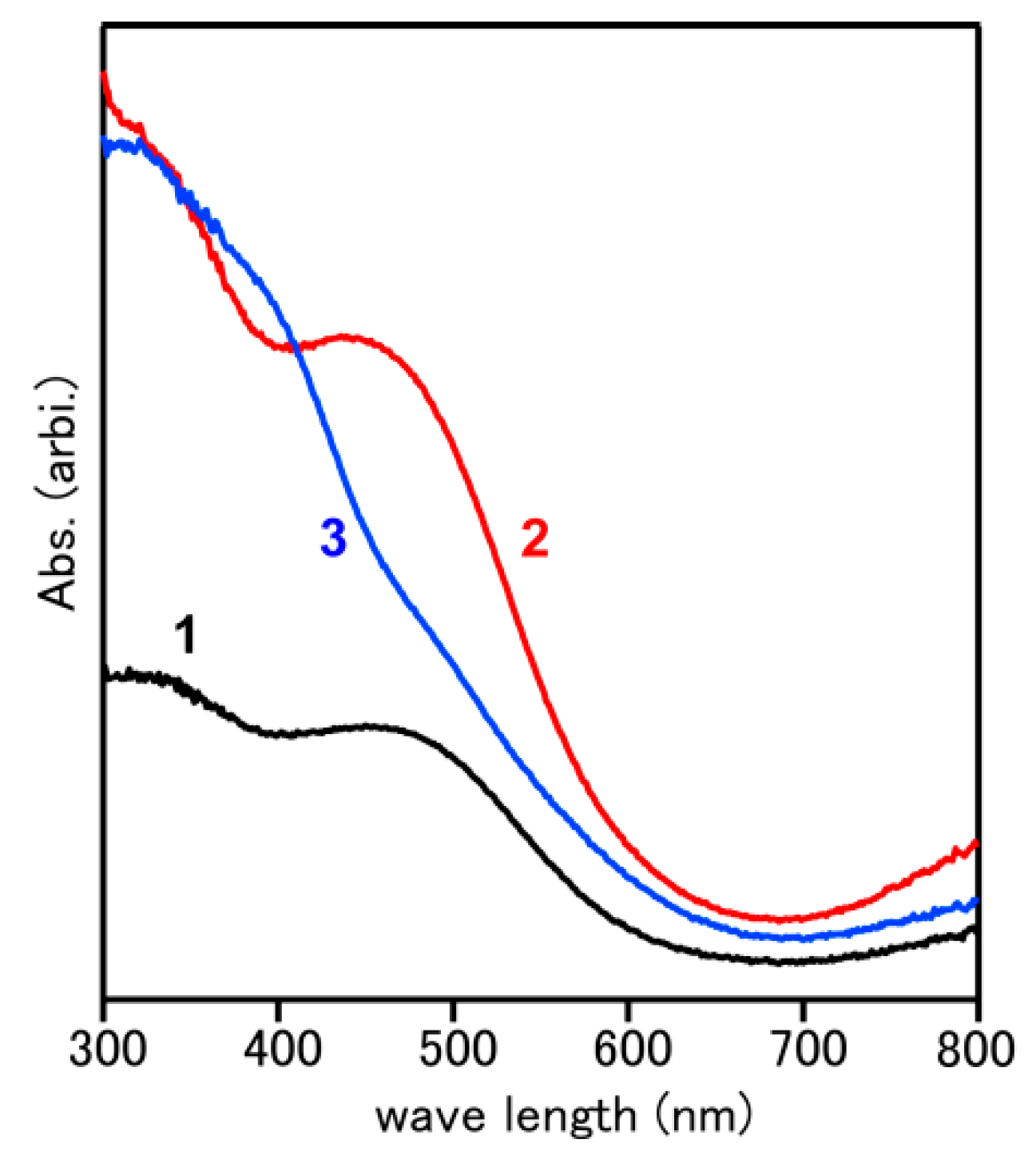
2. Results and Discussion
3. Experimental Section
4. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gütlich, P.; Hauser, A.; Spiering, H. Thermal and Optical Switching of Iron(II) Complexes. Angew. Chem. Int. Ed. Engl. 1994, 33, 2024–2054. [Google Scholar] [CrossRef]
- Gütlich, P.; Goodwin, H.A. (Eds.) Spin Crossover in Transition Metal Compounds I-III. Topics in Current Chemistry Vols. 233–235; Springer: Berlin, Germany, 2004.
- Muñoz, M.C.; Real, J.A. Thermo-, piezo-, photo- and chemo-switchable spin crossover iron(II)-metallocyanate based coordination polymers. Coord. Chem. Rev. 2011, 255, 2068–2093. [Google Scholar] [CrossRef]
- Bousseksou, A.; Molnár, G.; Salmon, L.; Nicolazzi, W. Molucular spin crossover phenomenon: Recent achievements and prospects. Chem. Soc. Rev. 2011, 40, 3313–3335. [Google Scholar] [CrossRef] [PubMed]
- Gütlich, P.; Gaspar, A.B.; Garcia, Y. Spin state switching in iron coordination compounds. Beilstein J. Org. Chem. 2013, 9, 342–391. [Google Scholar] [CrossRef] [PubMed]
- Halcrow, M.A. (Ed.) Spin-Crossover Materials—Properties and Applications; John Wiley & Sons: Chichester, UK, 2013.
- Spepherd, H.J.; Bartual-Murgui, C.; Molnár, G.; Real, J.A.; Munoz, M.C.; Salmon, L.; Bousseksou, A. Thermal and pressure-induced spin crossover in a novel three-diemnsional Hoffmann-like clathrate complex. New J. Chem. 2011, 35, 1205–1210. [Google Scholar] [CrossRef]
- Sciortino, N.F.; Scherl-Gruenwald, K.R.; Chastanet, G.; Halder, G.J.; Chapman, K.W.; Létard, J.-F.; Kepert, C.J. Hysteretic Three-Step Spin Crossover in a Thermo- and Photochromic 3D Pillared Hofmann-type Metal-Organic Framework. Angew. Chem. Int. Ed. 2012, 51, 10154–10158. [Google Scholar] [CrossRef] [PubMed]
- Arcís-Castillo, Z.; Muñoz, M.C.; Molnár, G.; Bousseksou, A.; Real, J.A. [Fe(TPT)2/3{MI (CN)2}]·nSolv (MI = Ag, Au): New Bimetallic Porous Coordination Polymers with Spin-Crossover Properties. Chem. Eur. J. 2013, 19, 6851–6861. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.J.; Tao, J.Q.; Yu, Z.; Dun, C.Y.; Liu, Y.J.; You, X.Z. A stacking spin-crossover iron(II) compound with a large hysteresis. J. Chem. Soc. Dalton Trans. 1998. [Google Scholar] [CrossRef]
- Létard, J.F.; Guionneau, P.; Rabardel, L.; Howard, J.A.K.; Goeta, A.E.; Chasseau, D.; Kahn, O. Structural, Magnetic, and Photomagnetic Studies of a Mononuclear Iron(II) Derivative Exhibiting an Exceptionally Abrupt Spin Transition. Light-Induced Thermal Hysteresis Phenomenon. Inorg. Chem. 1998, 37, 4432–4441. [Google Scholar] [CrossRef] [PubMed]
- Gütlich, P.; Garcia, Y.; Goodwin, H.A. Spin crossover phenomena in Fe(II) complexes. Chem. Soc. Rev. 2000, 29, 419–427. [Google Scholar] [CrossRef]
- Hayami, S.; Gu, Z.Z.; Shiro, M.; Einaga, Y.; Fujishima, A.; Sato, O. First Observation of Light-Induced Excited Spin State Trapping for an Iron(III) Complex. J. Am. Chem. Soc. 2000, 122, 7126–7127. [Google Scholar] [CrossRef]
- Real, J.A.; Gaspar, A.B.; Niel, V.; Muñoz, M.C. Communication between iron(II) building blocks in cooperative spin transition phenomena. Coord. Chem. Rev. 2003, 236, 121–141. [Google Scholar] [CrossRef]
- Real, J.A.; Gaspar, A.B.; Muñoz, M.C. Thermal, pressure and light switchable spin-crossover materials. Dalton Trans. 2005. [Google Scholar] [CrossRef] [PubMed]
- Scitortio, N.F.; Neville, S.M.; Létard, J.-F.; Moubaraki, B.; Murray, K.S.; Kepert, C.J. Thermal- and Light-Induced Spin-Crossover Bistability in a Disrupted Hofmann-Type 3D Framework. Inorg. Chem. 2014, 53, 7886–7893. [Google Scholar]
- Klein, Y.M.; Sciortino, N.F.; Ragon, F.; Housecroft, C.E.; Kepert, C.J.; Neville, S.M. Spin crossover intermediate plateau stabilization in a flexible 2-D Hofmann-type coordination polymer. Chem. Commun. 2014, 50, 3838–3840. [Google Scholar] [CrossRef] [PubMed]
- Powell, H.M.; Rayner, J.H. Clathrate Compound Formed by Benzene with an Ammonia-Nickel Cyanide Complex. Nature 1949, 163, 566–567. [Google Scholar] [CrossRef]
- Rayner, J.H.; Powell, H.M. Structure of Molecular Compounds. Part X. Crystal Structure of the Compound of Benzene with an Ammonia-Nickel Cyanide. J. Chem. Soc. 1952. [Google Scholar] [CrossRef]
- Iwamoto, T.; Nakano, T.; Morita, M.; Miyoshi, T.; Miyamoto, T.; Sasaki, Y. The Hofmann-type Clathrate: M(NH3)2M'(CN)4.2G. Inorg. Chem. Acta 1968, 2, 313–316. [Google Scholar] [CrossRef]
- Iwamoto, T. The Hofmann-type and related inclusion compounds. In Inclusion Compounds; Atwood, J.L., Davies, J.E.D., MacNicol, D.D., Eds.; Academic Press: London, UK, 1984; Volume 1, pp. 29–57. [Google Scholar]
- Akyüz, S.; Dempster, A.B.; Morehouse, R.L. An Infrared and Raman Spectroscopic Study of Some Metal Pyridine Tetracyanonickelate Complexes. J. Mol. Struct. 1973, 17, 105–125. [Google Scholar] [CrossRef]
- Ülkü, D. Die Kristallstruktur des Dipyridin-cadmium tetracyanonickelats Cd(C5H5N)2Ni(CN)4. Z. Kristallogr. 1975, 142, 271–280. [Google Scholar]
- Morehouse, R.L.; Aytaç, K.; Ülkü, D. Unit-cell dimension of Hofmann pyridine complexes. Z. Kristallogr. 1977, 145, 157–160. [Google Scholar] [CrossRef]
- Kitazawa, T.; Gomi, Y.; Takahashi, M.; Enomoto, M.; Miyazaki, A.; Enoki, T. Spin-crossover behaviour of the coordination polymer Fe(C5H5N)2Ni(CN)4. J. Mater. Chem. 1996, 6, 119–121. [Google Scholar] [CrossRef]
- Niel, V.; Martinez-Agudo, J.M.; Munoz, M.C.; Gaspar, A.B.; Réal, J.A. Cooperative Spin Crossover Behavior in Cyanide-Bridged Fe(II)-M(II) Bimetallic 3D Hofmann-like Networks (M = Ni, Pd, and Pt). Inorg. Chem. 2001, 40, 3838–3839. [Google Scholar] [CrossRef] [PubMed]
- Tayagaki, T.; Galet, A.; Molnar, G.; Muñoz, M.C.; Zwick, A.; Tanaka, K.; Réal, J.A.; Bousseksou, A. Metal Dilution Effects on the Spin-Crossover Properties of the Three-Dimensional Coordination Polymer Fe(pyrazine)[Pt(CN)4]. J. Phys. Chem. B 2005, 109, 14859–14867. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Tricard, S.; Félix, G.; Molnár, G.; Nicolazzi, W.; Salmon, L.; Bousseksou, A. Re-Appearance of Cooperativity in Ultra-Small Spin-Crossover [Fe(pz){Ni(CN)4}] Nanoparticles. Angew. Chem. Int. Ed. 2014, 53, 10894–10898. [Google Scholar] [CrossRef] [PubMed]
- Okabayashi, J.; Ueno, S.; Wakisaka, Y.; Kitazawa, T. Temperature-depedence EXAFS study for spin crossover complex: Fe(pyridine)2Ni(CN)4. Inorg. Chim. Acta. 2015, 426, 142–145. [Google Scholar] [CrossRef]
- Muňoz-Páez, A.; Díaz-Moreno, S.; Marcos, E.S.; Rehr, J.J. Importance of Multiple-Scattering Phenomena in XAS Structural Determinations of [Ni(CN)4]2− in Condensed Phases. Inorg. Chem. 2000, 39, 3784–3790. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, T.; Iwamoto, T.; Sasaki, Y. The Infrared Spectra of the Diamminemetal(II) Tetracyanoniccolate(II) Benzene and Aniline Clathrates. Inorg. Chem. Acta 1967, 1, 120–126. [Google Scholar] [CrossRef]
- Akyüz, S.; Dempster, A.B.; Morehouse, R.L. Host-guest interactions and stability of Hofmann-type benzene and aniline clathrates studied by i.r. spectroscopy. Spectrochim. Acta 1974, 30A, 1989–2004. [Google Scholar] [CrossRef]
- Iwamoto, T.; Miyoshi, T.; Miyamoto, T.; Sasaki, Y.; Fujiwara, S. The Metal Ammine Cyanide Aromatic Clathrates. I. The Preparation and Stoichiometry of the Diamminemetal(II) Tetracyanoniccolate(II) Dibenzene and Dianiline. Bull. Chem. Soc. Jpn. 1967, 40, 1174–1178. [Google Scholar] [CrossRef]
- Matouzenko, G.S.; Molnar, G.; Bréfuel, N.; Perrin, M.; Bousseksou, A.; Borshch, S.A. Spin-Crossover Iron(II) Coordination Polymer with Zigzag Chain Structure. Chem. Mater. 2003, 15, 550–556. [Google Scholar] [CrossRef]
- Southon, P.D.; Liu, L.; Fellows, E.A.; Price, D.J.; Halder, G.J.; Chapman, K.W.; Moubaraki, B.; Murray, K.S.; Létard, J.-F.; Kepert, C.J. Dynamic Interplay between Spin-Crossover and Host-Guest Function in a Nanoporous Metal-Organic Framework Material. J. Am. Chem. Soc. 2009, 131, 10998–11009. [Google Scholar] [CrossRef] [PubMed]
- Ohba, M.; Yoneda, K.; Agustí, G.; Muñoz, M.C.; Gaspar, A.B.; Real, J.A.; Yamasaki, M.; Ando, H.; Nakao, Y.; Sakaki, S.; et al. Bidirectional Chemo-Switching of Spin State in a Microporous Framework. Angew. Chem. Int. Ed. 2009, 48, 4767–4771. [Google Scholar] [CrossRef] [PubMed]
- Funasako, Y.; Mochida, T.; Takahashi, K.; Sakurai, T.; Ohta, H. Vapochromic Ionic Liquids from Metal-Chelate Complexes Exhibiting Reversible Changes in Color, Thermal, and Magnetic Properties. Chem.-A Eur. J. 2012, 18, 11929–11936. [Google Scholar] [CrossRef] [PubMed]
- Wenger, O.S. Vapochromism in Organometallic and Coordination Complexes: Chemical Sensors for Volatile Organic Compounds. Chem. Rev. 2013, 113, 3686–3733. [Google Scholar] [CrossRef] [PubMed]
- Jobbagy, C.; Deak, A. Stimuli-Responsive Dynamic Gold Complexes. Eur. J. Inorg. Chem. 2014, 27, 4434–4449. [Google Scholar] [CrossRef]
- Kobayashi, A.; Kato, M. Vapochromic Platinum(II) Complexes: Crystal Engineering toward Intelligent Sensing Devices. Eur. J. Inorg. Chem. 2014, 27, 4469–4483. [Google Scholar] [CrossRef]
- Naik, A.D.; Robeyns, K.; Meunier, C.F.; Léonard, A.F.; Rotaru, A.; Tinant, B.; Filinchuk, Y.; Su, B.L. Garcia Selective and Reusable Iron(II)-Based Molecular Sensor for the Vapor-Phase Detection of Alcohol. Inorg. Chem. 2014, 53, 1263–1265. [Google Scholar] [CrossRef] [PubMed]
- Hosoya, K.; Kitazawa, T.; Takahashi, M.; Takeda, M.; Meunier, J.-F.; Molnár, G.; Bousseksou, A. Unexpected isotope effect on the spin transition of the coordination polymer Fe(C5H5N)2[Ni(CN)4]. Phys. Chem. Chem. Phys. 2003, 5, 1682–1688. [Google Scholar] [CrossRef]
- Weber, B.; Bauer, W.; Pfaffeneder, T.; Dîrute, M.M.; Naik, A.D.; Rotaru, A.; Garcia, Y. Influence of Hydrogen Bonding on the Hysteresis Width in Iron(II) Spin-Crossover Complexes. Eur. J. Inorg. Chem. 2011, 2011, 3193–3206. [Google Scholar] [CrossRef]
- Kitazawa, T.; Gomi, Y.; Takahashi, M.; Takeda, M. 57Fe Mossbauer Spectra and Crystal Structure of Hofmann-type Thiophne Clathrate, Fe(NH3)2Ni(CN)4∙C4H4S. Mol. Cryst. Liq. Cryst. 1998, 311, 167–172. [Google Scholar] [CrossRef]
- Kitazawa, T.; Takahashi, Mi.; Takahashi, Ma.; Enomoto, M.; Miyazaki, A.; Enoki, T.; Takeda, M. 57Fe Mossbauer Spectroscopic and Magnetic Study of the Spin-crossover Polymer Complex, Fe(3-Chloropyridine)2Ni(CN)4. J. Radioanal. Nucl. Chem. 1999, 239, 285–290. [Google Scholar] [CrossRef]
- Molnár, G.; Guillon, T.; Moussa, N.O.; Rechignat, L.; Kitazawa, T.; Nardone, M.; Bousseksou, A. Two-step spin-crossover phenomenon under high pressure in the coordination polymer Fe(3-methylpyridine)2[Ni(CN)4]. Chem. Phys. Lett. 2006, 423, 152–156. [Google Scholar] [CrossRef]
- Rodríguez-Velamazán, J.A.; Castro, M.; Palacios, E.; Burriel, R.; Kitazawa, T.; Kawasaki, T. A Two-Step Spin Transition with a Disordered Intermediate State in a New Two-Dimensional Coordination Polymer. J. Phys. Chem. B 2007, 111, 1256–1261. [Google Scholar] [CrossRef] [PubMed]
- Kitazawa, T.; Takahashi, M.; Kawasaki, T. 2D iron(II) spin crossover complex with 3,5-lutidine. Hyperfine Interact. 2013, 218, 133–138. [Google Scholar] [CrossRef]
- Kitazawa, T.; Takahashi, M. New Hofmann-like spin crossover compound with 3,5-lutidine. Hyperfine Interact. 2014, 226, 27–34. [Google Scholar] [CrossRef]
- Sugaya, A.; Ueno, S.; Okabayashi, J.; Kitazawa, T. Crystal structure and magnetic properties of the spin crossover complex FeII(ethyl nicotinate)2[AuI(CN)2]2. New J. Chem. 2014, 38, 1955–1958. [Google Scholar] [CrossRef]
- Ueno, S.; Kawasaki, T.; Okabayashi, J.; Kitazawa, T. Structural, Electronic, and Magnetic Properties of Novel Spin-Crossover Complex: Fe(butyl nicotinate)2[Au(CN)2]2. Bull. Chem. Soc. Jpn. 2015, 88, 551–553. [Google Scholar] [CrossRef]
- Ravel, B.; Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: Data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Rad. 2005, 12, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Ankudinov, A.L.; Ravel, B.; Rehr, J.J.; Conradson, S.D. Real-space multiple-scattering calculation and interpretation of X-ray-absorption near-edge structure. Phys. Rev. B 1998, 58, 7565–7566. [Google Scholar] [CrossRef]
- Real, J.A.; Andrés, E.; Muñoz, M.C.; Julve, M.; Granier, T.; Bousseksou, A.; Varret, F. Spin Crossover in a Catenane Supramolecular System. Science 1995, 268, 265–267. [Google Scholar] [CrossRef] [PubMed]
- Halder, G.J.; Kepert, C.J.; Moubaraki, B.; Murray, K.S.; Cashion, J.D. Guest-Dependent Spin Crossover in a Nanoporous Molecular Framework Material. Science 2002, 298, 1762–1765. [Google Scholar] [CrossRef] [PubMed]
- Neville, S.M.; Moubaraki, B.; Murray, K.S.; Kepert, C.J. A thermal spin transition in a nanoporous iron(II) coordination framework material. Angew. Chem. Int. Ed. 2007, 46, 2059–2062. [Google Scholar] [CrossRef] [PubMed]
- Seredyuk, M.; Gaspar, A.B.; Ksenofontov, V.; Reiman, S.; Galyametdinov, Y.; Haase, W.; Rentschler, E.; Gütlich, P. Multifunctional materials exhibiting spin crossover and liquid-crystalline properties. Hyperfine Interact. 2005, 166, 385–390. [Google Scholar] [CrossRef]
- Niel, V.; Thompson, A.L.; Muñoz, M.C.; Galet, A.; Goeta, A.E.; Réal, J.A. Crystalline-state reaction with allosteric effect in spin-crossover, interpenetrated networks with magnetic and optical bistability. Angew. Chem. Int. Ed. 2003, 42, 3760–3763. [Google Scholar] [CrossRef] [PubMed]
- Real, J.A.; Muñoz, M.C.; Andrés, E.; Granier, T.; Gallois, B. Spin-Crossover Behavior in the Fe(tap)2(NCS)2∙nCH3CN System (tap = 1,4,5,8-Tetraazaphenanthrene; n = 1, 1/2). Crystal Structures and Magnetic Properties of Both Solvates. Inorg. Chem. 1994, 33, 3587–3594. [Google Scholar] [CrossRef]
- Hostettler, M.; Törnroos, K.W.; Chernyshov, D.; Vangdal, B.; Bürgi, H.-B. Challenges in engineering spin crossover: Structures and magnetic properties of six alcohol solvates of iron(II) tris(2-picolylamine) dichloride. Angew. Chem. Int. Ed. 2004, 43, 4589–4594. [Google Scholar] [CrossRef] [PubMed]
- Bartel, M.; Absmeier, A.; Jameson, G.N.L.; Werner, F.; Kato, K.; Takata, M.; Boča, R.; Hasegawa, M.; Mereiter, K.; Caneschi, A.; et al. Modification of Spin Crossover Behavior through Solvent Assisted Formation and Solvent Inclusion in a Triply Interpenetrating Three-Dimensional Network. Inorg. Chem. 2007, 46, 4220–4229. [Google Scholar] [CrossRef] [PubMed]
- Bartual-Murgui, C.; Salmon, L.; Akou, A.; Ortega-Villar, N.A.; Shepherd, H.J.; Muñoz, M.C.; Molnár, G.; Real, J.A.; Bousseksou, A. Synergetic Effect of Host-Guest Chemistry and Spin Crossover 3D Hofmann-like Metal-organic Frameworks [Fe(bpac)M(CN)4] (M = Pt, Pd, Ni). Chem. Eur. J. 2012, 18, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-Y.; He, C.-T.; Chen, Y.-C.; Zhang, Z.-M.; Liu, W.; Ni, Z.-P.; Tong, M.-L. Tunable cooperativity in a spin-crossover Hoffman-like metal-organic framework material by aromatic guests. J. Mater. Chem. C 2015, 3, 7830–7835. [Google Scholar] [CrossRef]
- Kosone, T.; Kitazawa, T. Guest-dependent spin transition with long range intermediate state for 2-dimensional Hofmann-like coordination polymer. Inorg. Chim. Acta. 2016, 439, 159–163. [Google Scholar] [CrossRef]
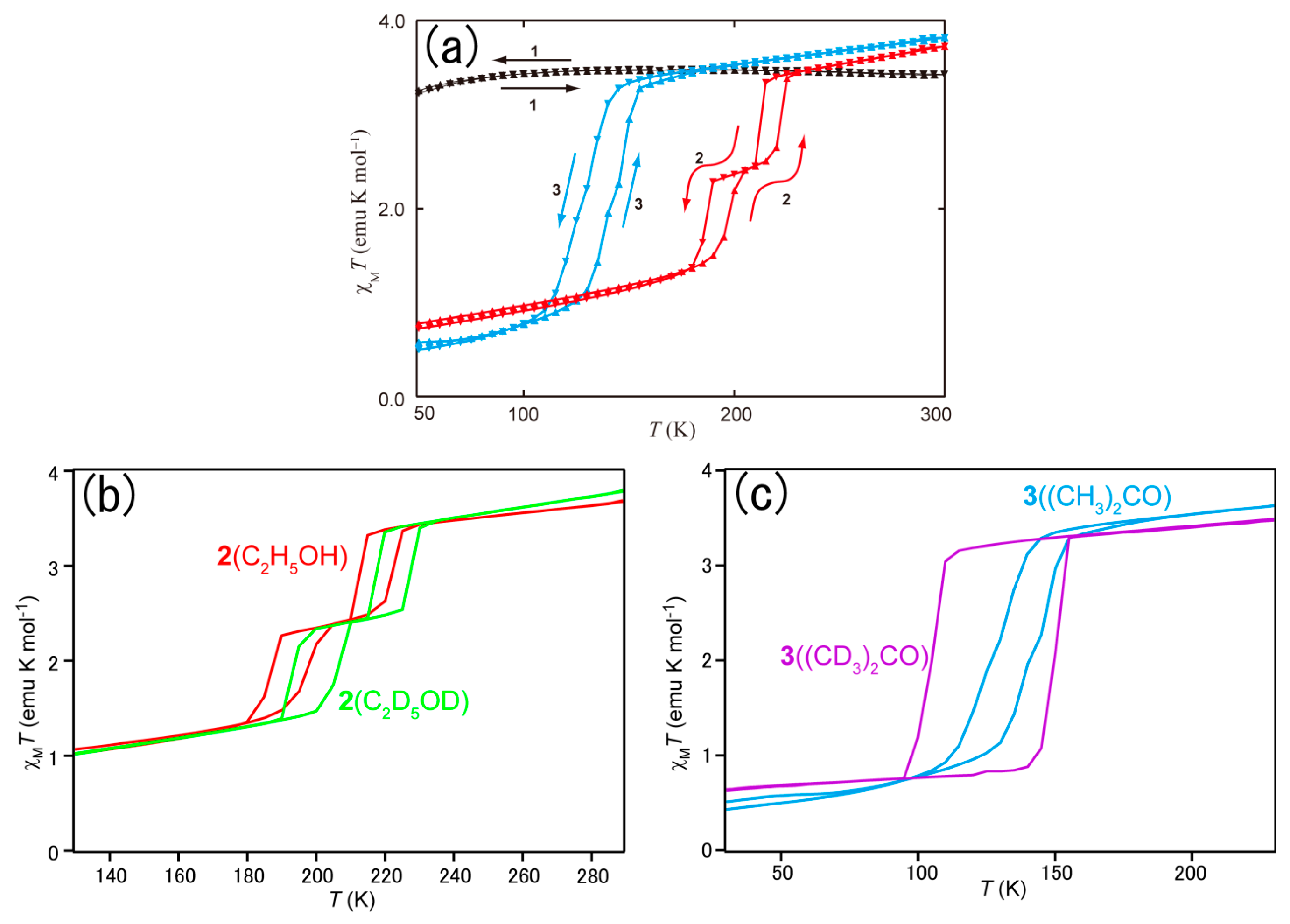
| Sample | Tc↓ a (K) | Tc↑ b (K) | Δ c (K) | Tc↓ a (K) | Tc↑ b (K) | Δ c (K) |
|---|---|---|---|---|---|---|
| 2(C2H5OH) | 187 | 197 | 10 | 213 | 223 | 10 |
| 2(C2D5OD) | 190 | 205 | 10 | 215 | 230 | 10 |
| 3((CH3)2CO) | 125 | 145 | 20 | - | - | - |
| 3((CD3)2CO) | 103 | 148 | 45 | - | - | - |
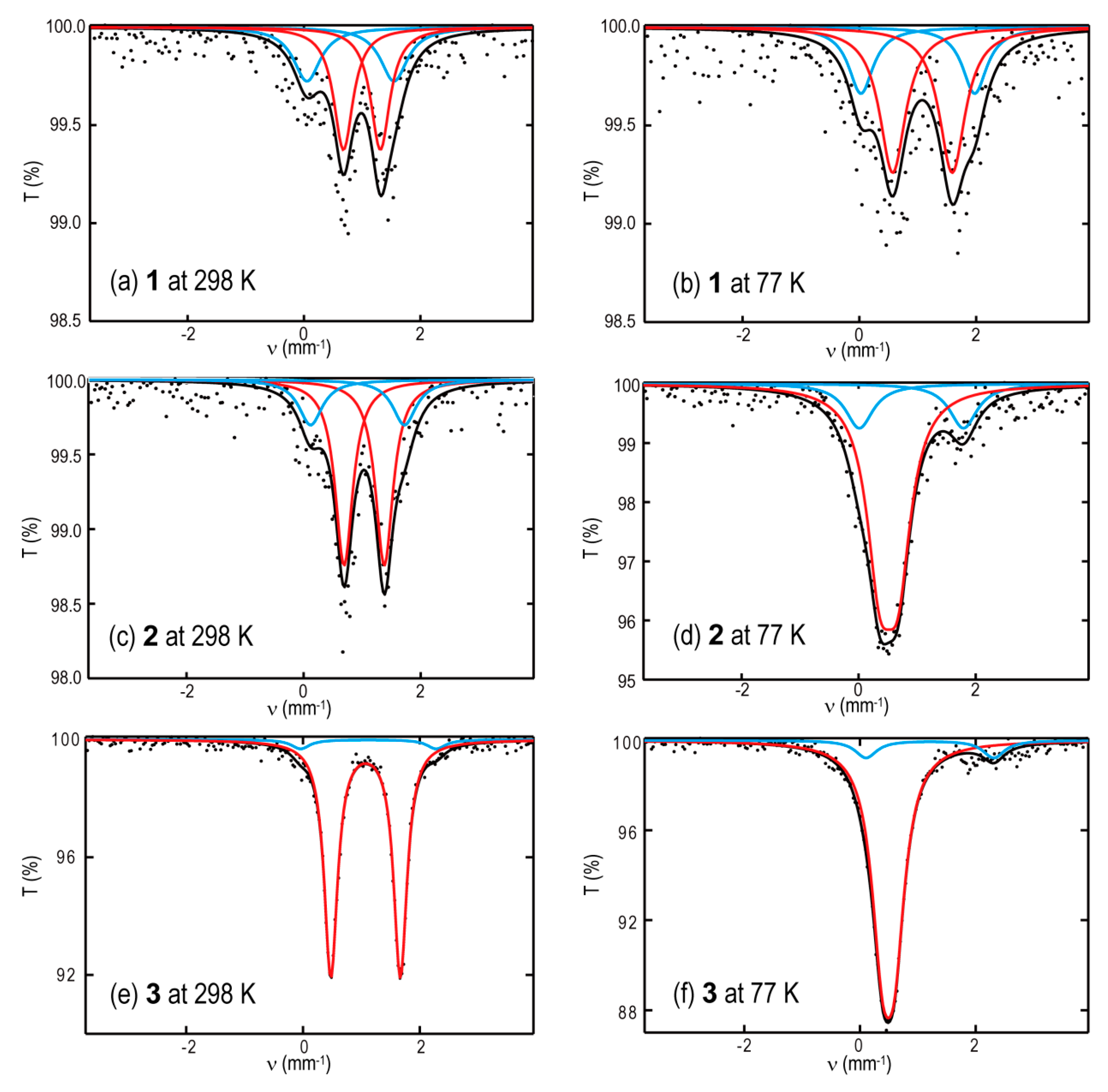
| Compound | Temp (K) | Signal | State | δ (mm s−1) | ΔEQ (mm s−1) | Area (%) |
|---|---|---|---|---|---|---|
| 1 | 298 | major | HS | 1.00(3) | 0.63(3) | 62 |
| minor | HS | 0.80(3) | 1.50(3) | 38 | ||
| 77 | major | HS | 1.13(3) | 1.10(3) | 71 | |
| minor | HS | 1.02(3) | 1.96(3) | 29 | ||
| 2 | 298 | major | HS | 1.04(2) | 0.69(2) | 75 |
| minor | HS | 0.92(2) | 1.60(2) | 25 | ||
| 77 | major | LS | 0.52(2) | 0.31(2) | 78 | |
| minor | HS | 0.90(2) | 1.79(2) | 22 | ||
| 3 | 298 | major | HS | 1.07(1) | 1.19(1) | 95 |
| minor | HS | 1.12(3) | 2.31(7) | 5 | ||
| 77 | major | LS | 0.49(1) | 0.22(1) | 90 | |
| minor | HS | 1.07(3) | 1.97(5) | 10 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hosoya, K.; Nishikiori, S.-i.; Takahashi, M.; Kitazawa, T. Spin-Crossover Behavior of Hofmann-Type-Like Complex Fe(4,4’-bipyridine)Ni(CN)4·nH2O Depending on Guest Species. Magnetochemistry 2016, 2, 8. https://doi.org/10.3390/magnetochemistry2010008
Hosoya K, Nishikiori S-i, Takahashi M, Kitazawa T. Spin-Crossover Behavior of Hofmann-Type-Like Complex Fe(4,4’-bipyridine)Ni(CN)4·nH2O Depending on Guest Species. Magnetochemistry. 2016; 2(1):8. https://doi.org/10.3390/magnetochemistry2010008
Chicago/Turabian StyleHosoya, Kazumasa, Shin-ichi Nishikiori, Masashi Takahashi, and Takafumi Kitazawa. 2016. "Spin-Crossover Behavior of Hofmann-Type-Like Complex Fe(4,4’-bipyridine)Ni(CN)4·nH2O Depending on Guest Species" Magnetochemistry 2, no. 1: 8. https://doi.org/10.3390/magnetochemistry2010008
APA StyleHosoya, K., Nishikiori, S.-i., Takahashi, M., & Kitazawa, T. (2016). Spin-Crossover Behavior of Hofmann-Type-Like Complex Fe(4,4’-bipyridine)Ni(CN)4·nH2O Depending on Guest Species. Magnetochemistry, 2(1), 8. https://doi.org/10.3390/magnetochemistry2010008