A High Pressure Investigation of the Order-Disorder Phase Transition and Accompanying Spin Crossover in [FeL12](ClO4)2 (L1 = 2,6-bis{3-methylpyrazol-1-yl}-pyrazine)
Abstract
:1. Introduction
2. Results and Discussion
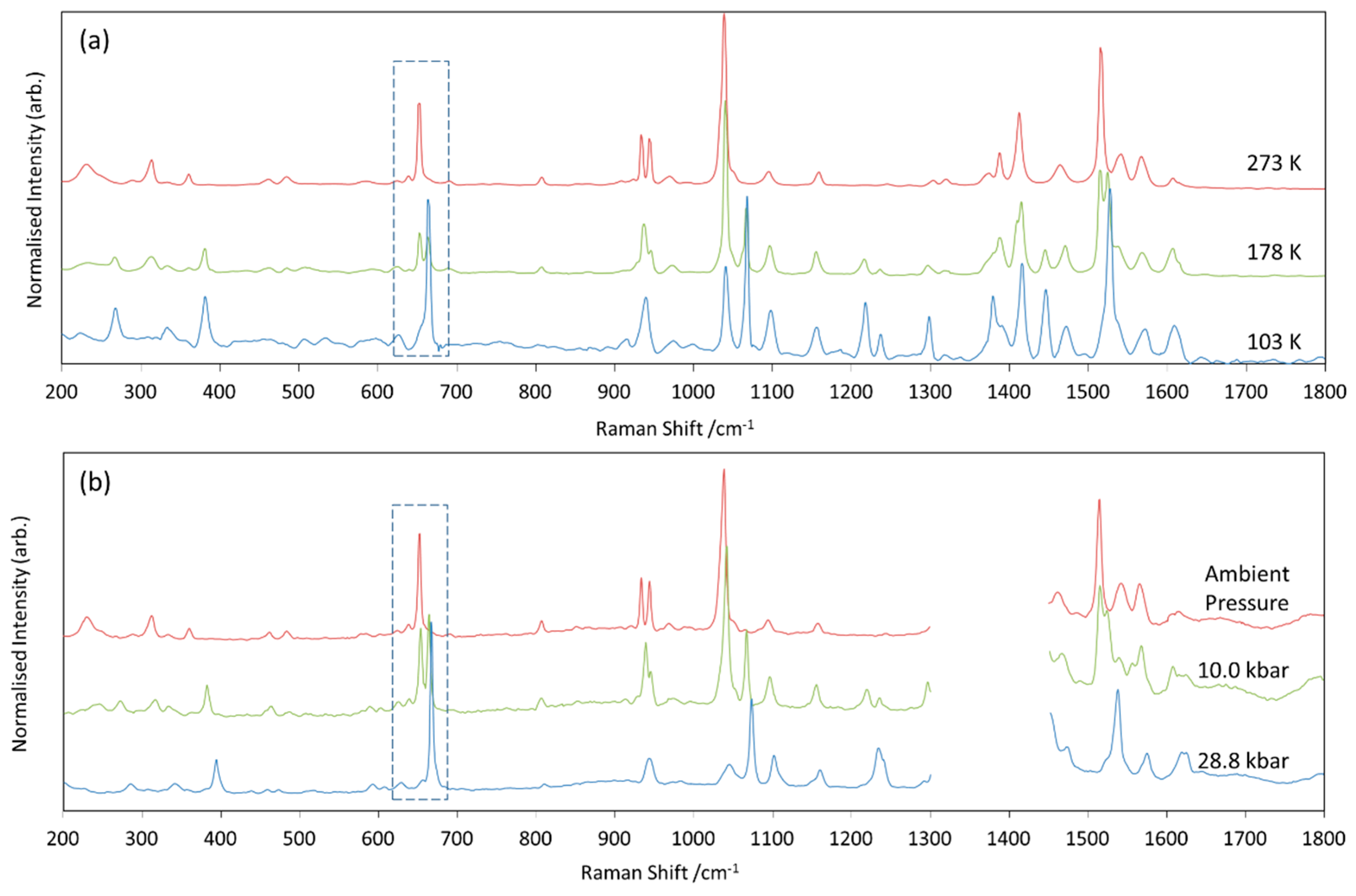
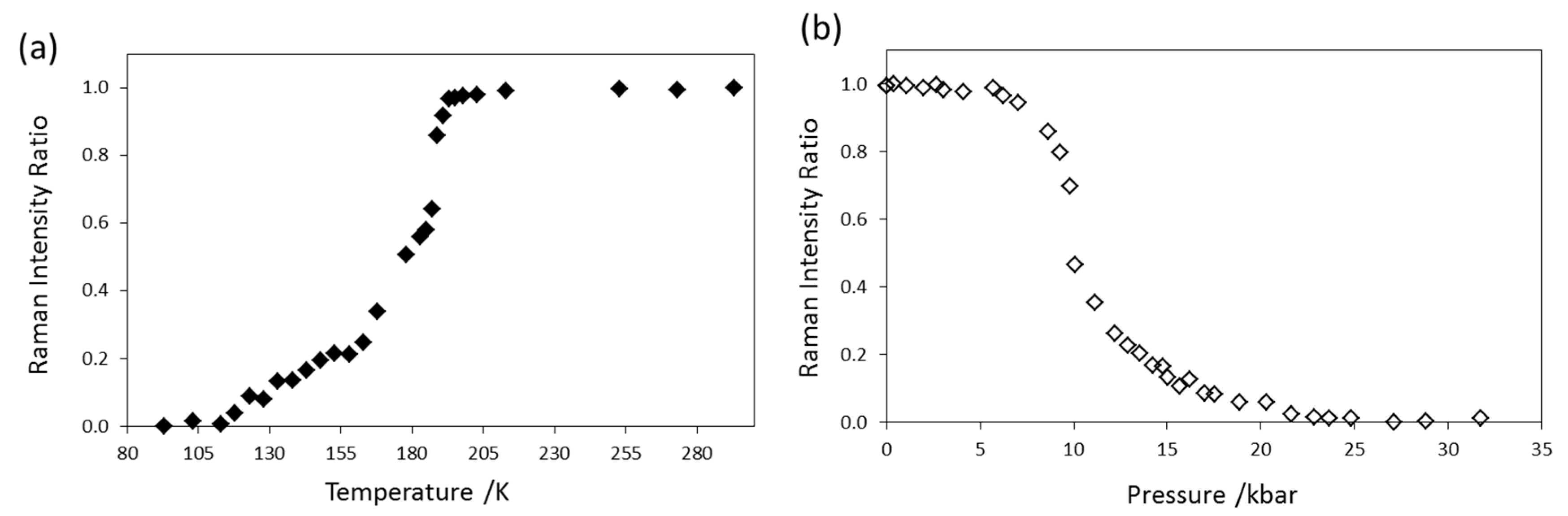
2.1. Variable Temperature and Pressure Raman Spectroscopy
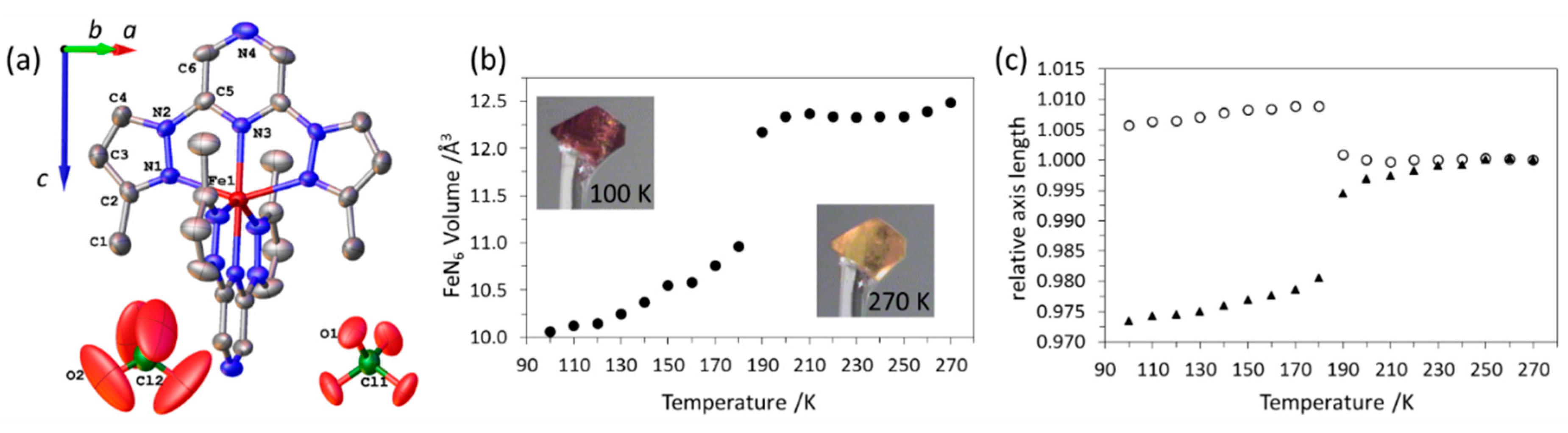
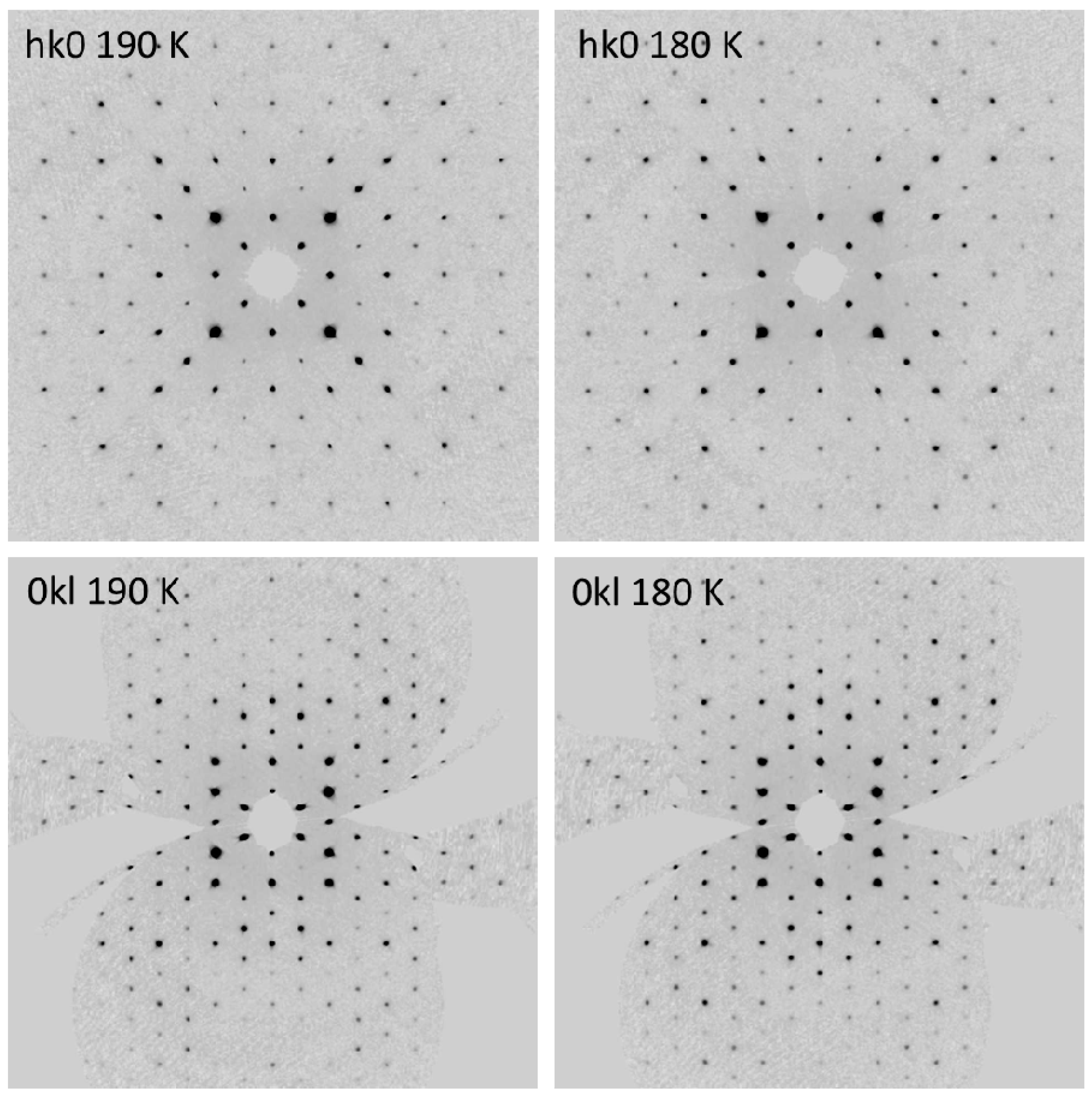
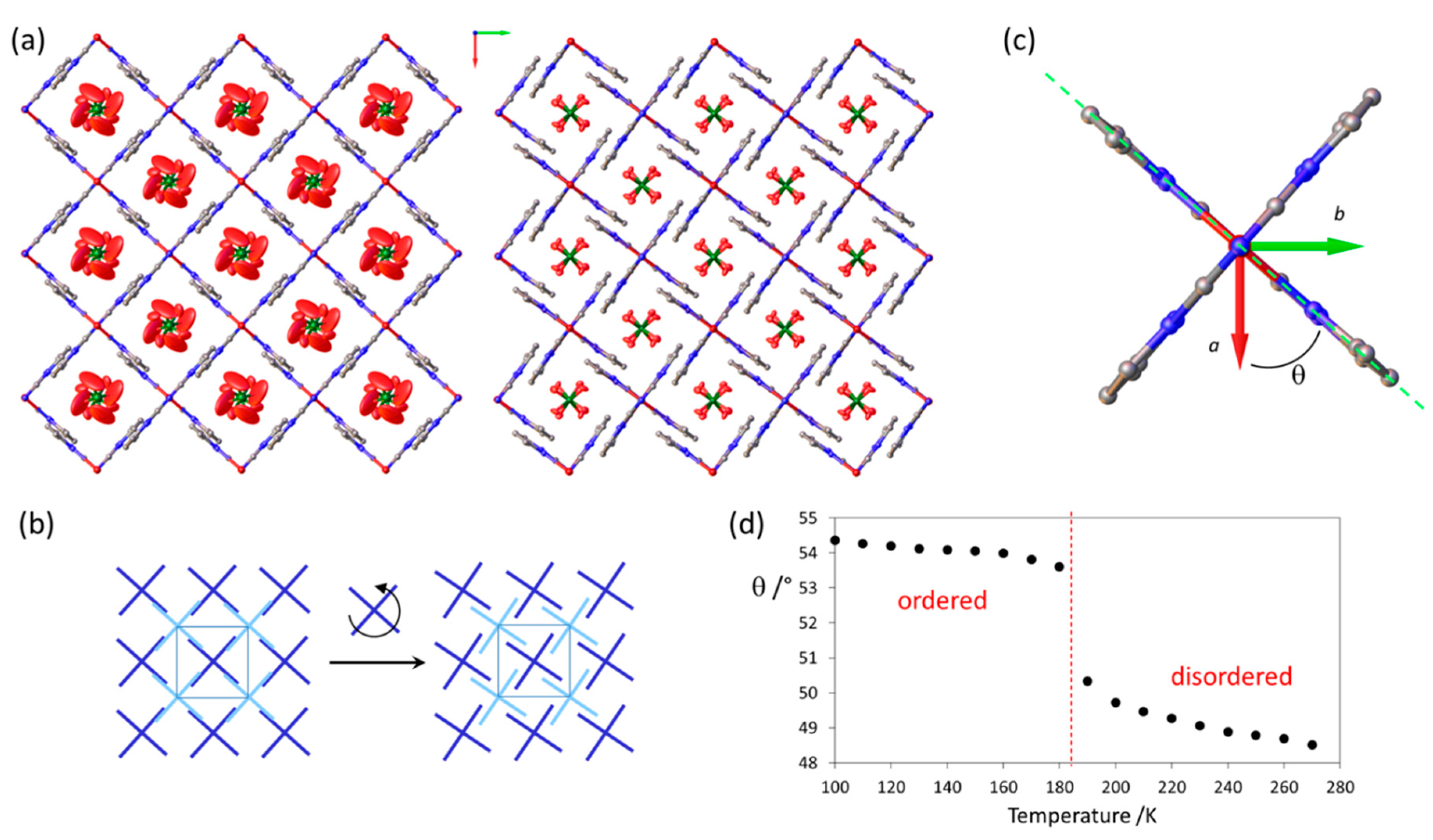
2.2. Variable Temperature X-ray Diffraction
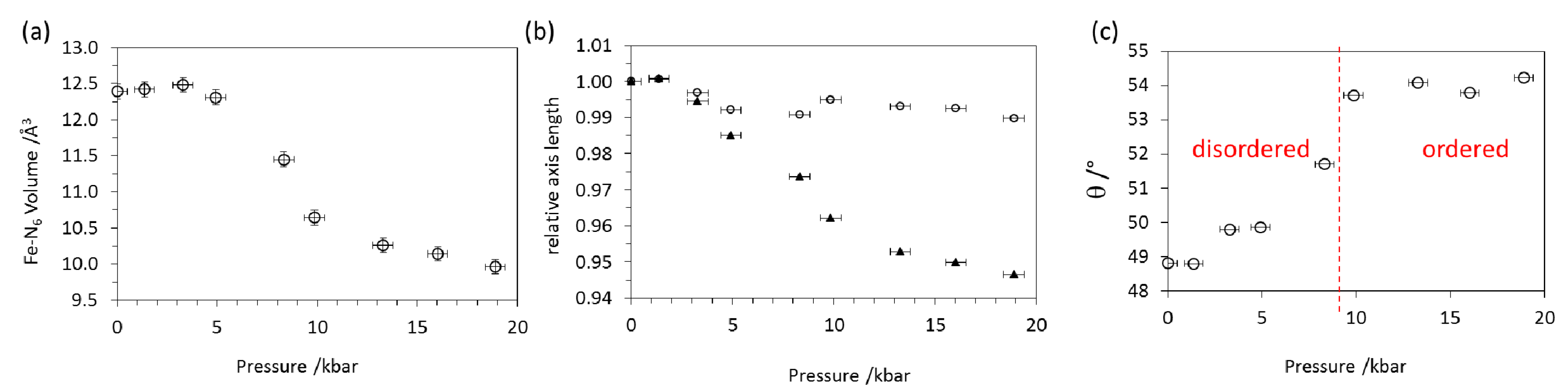
2.3. High Pressure X-ray Diffraction
3. Experimental Section
4. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Halcrow, M.A. Structure:function relationships in molecular spin-crossover complexes. Chem. Soc. Rev. 2011, 40, 4119–4142. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, H.J.; Palamarciuc, T.; Rosa, P.; Guionneau, P.; Molnár, G.; Letard, J.-F.; Bousseksou, A. Antagonism between Extreme Negative Linear Compression and Spin Crossover in [Fe(dpp)2(NCS)2]·py. Angew. Chem. Int. Ed. 2012, 51, 3910–3914. [Google Scholar] [CrossRef] [PubMed]
- Nishino, M.; Boukheddaden, K.; Konishi, Y.; Miyashita, S. Simple Two-Dimensional Model for the Elastic Origin of Cooperativity among Spin States of Spin-Crossover Complexes. Phys. Rev. Lett. 2007, 98, 247203. [Google Scholar] [CrossRef] [PubMed]
- Murray, K.S.; Kepert, C.J. Cooperativity in Spin Crossover Systems: Memory, Magnetism and Microporosity. In Spin Crossover in Transition Metal Compounds I; Gütlich, P., Goodwin, H.A., Eds.; Springer: Heidelberg, Germany, 2004; Volume 233, pp. 195–228. [Google Scholar]
- Koenig, E.; Ritter, G.; Kulshreshtha, S.K.; Nelson, S.M. The high-spin (5T2) → low-spin (1A1) transition in solid tris(2,2′-bi-2-imidazoline)iron(II) diperchlorate. Hysteresis effects, simultaneous change of spin and lattice characteristics, and order-disorder phenomena of the perchlorate anion. Inorg. Chem. 1982, 21, 3022–3029. [Google Scholar] [CrossRef]
- Matouzenko, G.S.; Bousseksou, A.; Borshch, S.A.; Perrin, M.; Zein, S.; Salmon, L.; Molnár, G.; Lecocq, S. Cooperative Spin Crossover and Order-Disorder Phenomena in a Mononuclear Compound [Fe(DAPP)(abpt)](ClO4)2 [DAPP = [Bis(3-aminopropyl)(2-pyridylmethyl)amine], abpt = 4-Amino-3,5-bis(pyridin-2-yl)-1,2,4-triazole]. Inorg. Chem. 2004, 43, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Sheu, C.F.; Pillet, S.; Lin, Y.-C.; Chen, Y.-C.; Chen, S.M.; Hsu, I.-J.; Lecomte, C.; Wang, Y. Magnetostructural Relationship in the Spin-Crossover Complex t-{Fe(abpt)2[N(CN)2]2}: Polymorphism and Disorder Phenomenon. Inorg. Chem. 2008, 47, 10866–10874. [Google Scholar] [CrossRef] [PubMed]
- Craig, G.A.; Sánchez-Costa, J.; Roubeau, O.; Teat, S.J.; Aromí, G. Coupled Crystallographic Order-Disorder and Spin State in a Bistable Molecule: Multiple Transition Dynamics. Chem. Eur. J. 2011, 17, 3120–3127. [Google Scholar] [CrossRef] [PubMed]
- Chernyshov, D.; Klinduhov, N.; Törnroos, K.W.; Hostettler, M.; Vangdal, B.; Bürgi, H.B. Coupling between spin conversion and solvent disorder in spin crossover solids. Phys. Rev. B 2007, 76, 014406. [Google Scholar] [CrossRef]
- Kershaw Cook, L.J.; Halcrow, M.A. Synthesis of 4-Hydroxy-2,6-di(pyrazol-1-yl)pyridine, and the Spin State Behaviour of Its Iron(II) Complex Salts. Magnetochemistry 2015, 1, 3–16. [Google Scholar] [CrossRef]
- Murray, K.S. Spin-Crossover Materials: Properties and Applications; Wiley: Chichester, UK, 2013; pp. 1–54. [Google Scholar]
- Elhaïk, J.; Money, V.A.; Barrett, S.A.; Kilner, C.A.; Radosavljevic Evans, I.; Halcrow, M.A. The spin-states and spin-crossover behaviour of iron(II) complexes of 2,6-dipyrazol-1-ylpyrazine derivatives. Dalton Trans. 2003. [Google Scholar] [CrossRef]
- Money, V.A.; Elhaïk, J.E.; Radosavljevic Evans, I.; Halcrow, M.A.; Howard, J.A.K. A study of the thermal and light induced spin transition in [FeL2](BF4)2 and [FeL2](ClO4)2 L = 2,6-di(3-methylpyrazol-1-yl)pyrazine. Dalton Trans. 2004. [Google Scholar] [CrossRef] [PubMed]
- Ksenofontov, V.; Gaspar, A.B.; Gutlich, P. Spin Crossover in Transition Metal Compounds III; Gütlich, P., Goodwin, H.A., Eds.; Springer: Heidelberg, Germany, 2004; Volume 235, pp. 23–64. [Google Scholar]
- Tao, J.; Wei, R.-J.; Huang, R.-B.; Zheng, L.-S. Polymorphism in spin-crossover systems. Chem. Soc. Rev. 2012, 41, 703–737. [Google Scholar] [PubMed]
- Guionneau, P.; Collet, E. Spin-Crossover Materials: Properties and Applications; Wiley: Chichester, UK, 2013; pp. 507–526. [Google Scholar]
- Tuchagues, J.-P.; Bousseksou, A.; Molnár, G.; McGarvey, J.J.; Varret, F. Spin Crossover in Transition Metal Compounds III; Gütlich, P., Goodwin, H.A., Eds.; Springer: Heidelberg, Germany, 2004; Volume 235, pp. 85–103. [Google Scholar]
- Halcrow, M.A. Spin-Crossover Materials: Properties and Applications; Wiley: Chichester, UK, 2013; pp. 147–169. [Google Scholar]
- Woodall, C.H.; Fuertes, S.; Beavers, C.M.; Hatcher, L.E.; Parlett, A.; Shepherd, H.J.; Christensen, J.; Teat, S.J.; Intissar, M.; Rodrigue-Witchel, A.; et al. Tunable Trimers: Using Temperature and Pressure to Control Luminescent Emission in Gold(I) Pyrazolate-Based Trimers. Chem. Eur. J. 2014, 20, 16933–16942. [Google Scholar] [CrossRef] [PubMed]
- Piermarini, G.J.; Block, S.; Barnett, J.D.; Forman, R.A. Calibration of the pressure dependence of the R1 ruby fluorescence line to 195 kbar. J. Appl. Phys. 1975, 46, 2774–2780. [Google Scholar] [CrossRef]
- CrysAlisPro, version 171.37.33; Oxford Diffraction Ltd.: Abingdon, UK, 2015.
- Sheldrick, G.M. A Short History of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- APEX2, v2014.9-0; Bruker AXS Inc.: Madison, WI, USA, 2014.
- Dawson, A.; Allan, D.R.; Parsons, S.; Ruf, M. Use of a CCD diffractometer in crystal structure determinations at high pressure. J. Appl. Crystallogr. 2004, 37, 410–416. [Google Scholar] [CrossRef]
- SADABS Program for Empirical Absorption Correction and Scaling of X-ray Data; Bruker AXS Inc.: Madison, WI, USA, 2005.
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shepherd, H.J.; Tonge, G.; Hatcher, L.E.; Bryant, M.J.; Knichal, J.V.; Raithby, P.R.; Halcrow, M.A.; Kulmaczewski, R.; Gagnon, K.J.; Teat, S.J. A High Pressure Investigation of the Order-Disorder Phase Transition and Accompanying Spin Crossover in [FeL12](ClO4)2 (L1 = 2,6-bis{3-methylpyrazol-1-yl}-pyrazine). Magnetochemistry 2016, 2, 9. https://doi.org/10.3390/magnetochemistry2010009
Shepherd HJ, Tonge G, Hatcher LE, Bryant MJ, Knichal JV, Raithby PR, Halcrow MA, Kulmaczewski R, Gagnon KJ, Teat SJ. A High Pressure Investigation of the Order-Disorder Phase Transition and Accompanying Spin Crossover in [FeL12](ClO4)2 (L1 = 2,6-bis{3-methylpyrazol-1-yl}-pyrazine). Magnetochemistry. 2016; 2(1):9. https://doi.org/10.3390/magnetochemistry2010009
Chicago/Turabian StyleShepherd, Helena J., George Tonge, Lauren E. Hatcher, Mathew J. Bryant, Jane V. Knichal, Paul R. Raithby, Malcolm A. Halcrow, Rafal Kulmaczewski, Kevin J. Gagnon, and Simon J. Teat. 2016. "A High Pressure Investigation of the Order-Disorder Phase Transition and Accompanying Spin Crossover in [FeL12](ClO4)2 (L1 = 2,6-bis{3-methylpyrazol-1-yl}-pyrazine)" Magnetochemistry 2, no. 1: 9. https://doi.org/10.3390/magnetochemistry2010009
APA StyleShepherd, H. J., Tonge, G., Hatcher, L. E., Bryant, M. J., Knichal, J. V., Raithby, P. R., Halcrow, M. A., Kulmaczewski, R., Gagnon, K. J., & Teat, S. J. (2016). A High Pressure Investigation of the Order-Disorder Phase Transition and Accompanying Spin Crossover in [FeL12](ClO4)2 (L1 = 2,6-bis{3-methylpyrazol-1-yl}-pyrazine). Magnetochemistry, 2(1), 9. https://doi.org/10.3390/magnetochemistry2010009







