Abstract
Anthracnose, which is caused by the fungus Colletotrichum gloeosporioides (Penz.) Penz. and Sacc. (C. gloeosporioides), is the main disease that affects soursop fruits and causes accelerated deterioration due to the rotting process. The objective of this study was to evaluate the effect of chitosan-based coatings with essential oils on the physiological, antifungal, and shelf-life properties of soursop. Chitosan-based coatings were combined with essential oils of cinnamon and thyme and applied to soursop. The parameters evaluated were respiration and ethylene by gas chromatography, shelf life, weight loss, total soluble solids, color, maturity index, and titratable acidity. The chitosan obtained had a molecular weight of 169 kDa and an 83% degree of deacetylation; respiration and ethylene values showed significant reductions of 47 and 50% with coatings. Weight loss was reduced by up to 50%, even on inoculated fruits, and shelf life increased by two days. Chitosan-based coatings with essential oils are an appropriate alternative to improve the quality of soursop and decrease the effect of C. gloeosporioides.
1. Introduction
The Annona genus includes tropical and subtropical crops of commercial importance in Mexico and around the world [1]. Among the important crops of the genus Annona in Mexico is the soursop (Annona muricata L.), which currently has commercial plantations in different states in Mexico [2]. Nayarit is the main producer—Vidal-Hernández et al. [2] reported a growing area of 2300 ha with a production of 16,100 t per year in this region. In 2019, the government of Mexico reported a national production of 30,790 tons of soursop, with a commercial value of MXN 248.170 million [3].
Soursop pulp is a source of fiber and also contains several compounds that may have health advantages if consumed in moderation. Soursop extract has demonstrated important pharmaceutical properties [4]. The main uses of soursop are consumption as fresh fruit and production of pulp, which is used as an ingredient in foods and beverages [5]. However, fruit losses are common due to diseases and pests, which occur preharvest and postharvest, causing a great economic impact [2]. Notably, the most economically important disease is anthracnose [2]. The causative agent of this disease is the fungus C. gloeosporioides and the inoculation can occur in seedlings and adult plants. It affects stems, branches, leaves, flowers, and fruits, causing low yields and deteriorating the quality of the final product [6]. In addition, soursop fruits have a short postharvest shelf life (2–7 days) due to their intense metabolic activity, high respiration rate, and ethylene production combined with their sensitivity to low temperatures [7].
A viable alternative to preserving fresh fruits and vegetables is the use of edible coatings as a protective wrap. In this sense, the use of edible coatings in highly perishable products such as fruit and vegetables provides a solution to increase their shelf life [8], due to their protection against mechanical damage and infections caused by fungi, as well as the effects on breathing rate by modifying the permeability to gases and water vapor [9,10].
Recently, chitosan (poly-D-glucosamine and N-acetyl-D-glucosamine) has been among the most-used biopolymers for coating fruits and vegetables, due to its biocompatibility, null toxicity, high malleability, and antimicrobial and antifungal properties [10,11]. Furthermore, chitosan has an excellent capacity to form biofilms which are semipermeable to CO2 and O2 gases. In addition, it has an antifungal effect, inhibiting the growth of mycelium and germination, and causing damage to hyphae and spores [12,13]. The use of chitosan-based coatings mixed with essential oils is one of the most recently tested technologies in the field of fruit coatings. The addition of essential oils of oregano, rosemary, cinnamon, cloves, garlic, eucalyptus, and chili has been shown to be efficient for the control of pathogens postharvest due to the active compounds present in these, such as carvacrol, cinnamic acid, citral, thymol, linalool, and menthol, among others, inhibiting the growth of microorganisms, enhancing the shelf life of agricultural products, and maintaining their sensory properties [8]. In this context, the aim of this research was to evaluate the effect of chitosan-based coatings with essential oils on the physiological, antifungal, and shelf-life properties of soursop fruits.
2. Materials and Methods
2.1. Harvesting, Isolation, and Identification of the Fungus C. gloeosporioides of Soursop
Soursop fruits were harvested at physiological maturity from Venustiano Carranza, Nayarit (21°32′22.77″ north latitude and 104°58′39.73″ west longitude), which has a warm and sub-humid climate. The fruits were washed twice, once with only water to remove impurities and once with a 2% chlorine solution. Finally, they were allowed to drain to dry.
Soursop fruits were stimulated to anthracnose development, and 12 cuts of the affected surfaces were inoculated in Petri dishes with PDA culture medium (potato dextrose agar, BD DifcoTM, Hercules, CA, USA) at 25 °C for 15 days (until the fungus covered the entire Petri dish). From these cultures, the mycelium and spores were obtained. DNA was extracted from the mycelium and quantified with a Nanodrop 8000 spectrophotometer (Thermo Scientific, Wilmington, NC, USA) at 260 and 280 nm. The molecular identification was performed using the ITS1-5.8S-ITS2 primer for the Internal Transcribed Spacer (ITS). The reaction was carried out on a thermocycler T100 (Bio-Rad, Hercules, CA, USA). The products were purified with the Freeze Kit N Squeeze DNA Gel Extraction Spin Columns (Bio-Rad). Finally, the sequence obtained was compared and aligned with those of the NCBI GenBank database, USA, through Nucleotide BLAST (National Center for Biotechnology Information, 2015) [14,15].
2.2. Obtaining Chitosan from Shrimp Waste
A local seafood restaurant from Celaya, Guanajuato, México donated the waste shrimp shells (Litopenaeus vannamei), which were sun-dried for 24 h and then ground in an industrial blender (TAPISA, T12 L, Cd. México, México). The chitosan conversion was performed according to the procedure reported by Acosta-Ferreira et al. [16], with slight modifications. First, the chitin was demineralized by mixing the crushed residue with a 3.6% HCl solution (w/v) for 4 h at room temperature, after which the demineralized material was mixed with a 4.5% NaOH solution (w/v) at 60 °C for 4 h to remove proteins. Finally, the chitin recuperated was subjected to alkaline deacetylation by mixing it with a 45% NaOH solution (w/v) at 120 °C for 2 h. Subsequently, the material was washed with common water to neutralize it, then dried at 45 °C for 12 h. The chitosan obtained was ground to reduce the particle size prior to use.
Determination of Degree of Deacetylation (DDA) and Molecular Weight (Mw)
The DDA of chitosan was determined using a UV/VIS Multiskan GO Microplate spectrophotometer (Thermo Scientific, Wilmington, USA), according to the methodology reported by Liu et al. [17]. A calibration curve (r2 = 0.999) was obtained with N-acetylglucosamine and (+)-glucosamine as monomers at 201 nm. The molecular weight (Mw) was determined using an Ubbelohde viscometer at 25 ± 1 °C. To obtain the standard curve (r2 = 0.9613), five chitosan concentrations (0.0014, 0.0012, 0.0010, 0.008, and 0.006 g mL−1) were dissolved in a 0.3 M CH3COOH/0.2 M CH3COONa solution and filtered through membranes with a pore size of 0.45 mm. The viscosity Mw was calculated based on the Mark–Houwink equation:
where [η] is the intrinsic viscosity, K and a are constant values that depend on the nature of the polymer and solvent as well as the temperature, and M is the relative molecular weight. In this work, K = 0.074 mL g−1 and a = 0.76 are the empirical constants that depend on the polymer nature, the solvent, and the temperature [16].
[η] = KMa
2.3. Preparation and Application of Coatings
The 1% chitosan solution (w/v) was prepared by dissolving chitosan in acetic acid 1% (v/v). The coatings were prepared by mixing a 1% chitosan solution and adding cinnamon essential oils and thyme. Four treatments were established for non-inoculated and inoculated fruits with C. gloeosporioides spores. Treatments for fruits were (1) without coating; (2) coatings with 1% chitosan; (3) 1% chitosan + 0.1% cinnamon; and (4) 1% chitosan + 0.1% thyme. To form the film on the soursop fruits, a thin layer of chitosan solution was applied with a food-grade brush and dried with a hair dryer (model 1875, CONAIR® LLC, Stamford, CT, USA) using air at room temperature.
2.4. Shelf Life and Physicochemical Analysis
For the evaluation of the shelf life of the soursop fruits, the coatings were applied to the fruits in relation to each treatment. Subsequently, they were placed in plastic trays and kept at room temperature, and the physicochemical properties (pH, weight loss, instrumental color, total soluble solids (TSS), titratable acidity (TA), and flavor index) were evaluated from day zero to day ten. The respiration (CO2) and ethylene production rates were evaluated in soursop fruits that were not inoculated.
2.4.1. Weight Loss
The weight loss was determined by calculating the difference between the initial weight of the soursop fruits in each experiment and the weight obtained at the end of each storage time, evaluated using a semi-analytical balance (Schuler Scientific, SSH-6001, Englewood, CO, USA); the results were expressed as a percentage of weight loss [18].
2.4.2. Instrumental Color
The color measurements were made using a Konica Minolta colorimeter (Model CR-410, Germany), using the CIEL L*C*h* (Commission Internationale de L’Eclairage) system. The results were expressed as L* (which represents the percentage of brightness, 0 = dark and 100 = light), C* (represents chroma or saturation, 0 = center of lightness axis), and h* (which is the hue angle, 0° = red, 90° = yellow, 180° = green, and 270° = blue) [18].
2.4.3. pH, Soluble Solid Content, Titratable Acidity, and Flavor Index
The pH was determined from soursop juice with a digital potentiometer (Hanna® Instrument, HI98130, Bedfordshire, UK), previously calibrated with saturated solution [18]. The SSC was done using an ATAGO optical refractometer (RX-50000i-Plus, Tokyo, Japan) at 20 °C, and the results were expressed as TSS [18]. The TA was carried out by titrating 20 mL of the sample (previously mixed with 25 g of fruit and 20 mL of distilled water) with 0.1 N NaOH solution and phenolphthalein as a pH indicator. The results were expressed as a percentage of citric acid [19]. The flavor index was determined as the ratio of TSS/TA [18].
2.5. Respiration and Ethylene Production Rates
The respiration and ethylene production rates were determined by placing the heavy fruits inside an airtight container of known volume; subsequently, after 2 h of keeping the fruit hermetically sealed, 10 mL of the gas was extracted from the headspace with the aid of a syringe and injected into an Agilent Gas Chromatograph (GC) (Agilent Technologies, Santa Clara, CA, USA).The GC was equipped with an open column with silica porous layer packaging connected simultaneously to a flame ionization detector (FID) at 170 °C and a thermal conductivity detector (TCD) at 170 °C; as carrier gas, N2 was used, with a flow rate of 2 mL/min. The injector and furnace of the chromatograph were maintained at 150 and 80 °C, respectively, and CO2 (460 ppm) and ethylene (100 ppm) standards (Quark INFRA) were used for quantification [20].
2.6. Statistical Analysis
The experiments were conducted in triplicate (n = 3), and the data represent the mean value. Regression analyses were applied with different models for each variable. A linear model and polynomial models were used, according to the data tendency; furthermore, an ANOVA using a completely randomized design and an LSD post hoc tests were carried out (α = 0.05) for each day and for each parameter evaluated.
3. Results and Discussion
3.1. Degree of Deacetylation and Molecular Weight of Chitosan
Chitosan obtained presented a low molecular weight (169 kDa) and 83% degree of deacetylation (DDA). The low molecular weight was due to the chemical treatment applied, which caused the depolymerization of chitosan chains (mainly during the deproteinization process under alkaline conditions) [10]. Muley et al. [21] reported the production of chitosan from shrimp shells by chemical treatment (deactivation with 50% NaOH), reaching 78.04% of DDA and 173 kDa of Mw. In addition, the chitosan obtained was similar in molecular weight and DDA properties to those supplied by Sigma-Aldrich (50–190 kDa and 75–85% DDA, CAS Number 9012-76-4). The methodology used in this work proved to be efficient in producing quality chitosan compared to commercial products. The chitosan obtained by the chemical method presented good physicochemical properties (molecular weight and degree of deacetylation) to produce coatings.
3.2. Shelf Life of Soursop Fruits
Figure 1 shows the changes in the superficial appearance of the soursop fruits with chitosan-based coatings, in the cases of both non-inoculated and inoculated with C. gloeosporioides. The results show that the coatings with cinnamon and thyme increased the shelf life because they actively controlled anthracnose disease and helped to maintain the quality of soursop fruits in inoculated and non-inoculated fruits. This relates to the effect of coatings and essential oils on the metabolic processes that occur at the cell wall level in the maturation process because, by modifying the atmosphere, they alter ethylene production and the maturation process, which translate into an increase in the shelf life of the fruit [22].
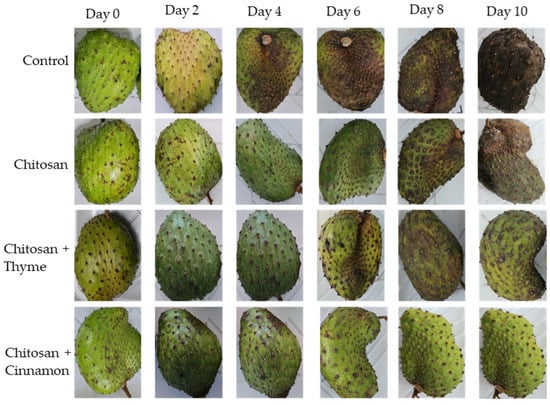
Figure 1.
Timeline over 10 days of the evaluation of soursop in its different treatments (control, chitosan coating, chitosan + thyme oil coating, and chitosan + cinnamon oil coating).
In this sense, Bill et al. [23] proved that the use of thyme in chitosan coatings decreases the severity of anthracnose in avocados, while López-Mata et al. [13] showed that the use of cinnamon in chitosan coatings prolongs the shelf life of strawberries. In addition, a study by Montalvo-González et al. [24] in soursop reported that wax-based coatings with 1-methyl cyclopropane at 25 °C reported an acceptable consumption quality up to 8 days, demonstrating an increase in shelf life, with soursop having a postharvest life of 5 to 6 days. These results are similar to those found in the present study for soursop, decreasing the severity of anthracnose compared to the control and increasing the effect of coatings when the essential oils of thyme and cinnamon were used, extending the shelf life until day 10 and maintaining acceptable quality, even in the inoculated fruits, since the pulp did not present anthracnose. Even Figure 2 confirms the beneficial effect of the chitosan coatings with thyme and cinnamon oils because it shows that C. gloeosporioides failed to enter the internal part of the fruit on day 10. Then, the chitosan-based coatings with essential oils of thyme and cinnamon proved to be a good alternative to decrease the growth of C. gloeosporioides in soursop and maintain the quality of the fruits.
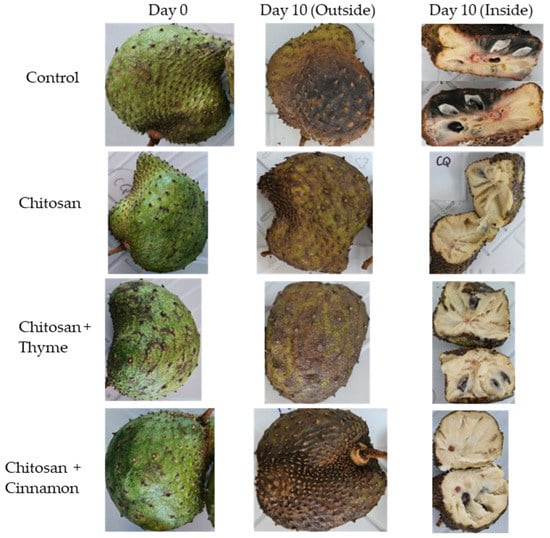
Figure 2.
Comparison of soursops inoculated with C. gloeosporioides and growth of the fungus inside the fruit (day 0 and day 10).
It is now known that the main protection barriers for fruit and vegetables are structural polysaccharides and proteins, which form networks through cohesion forces, conferring mechanical and barrier properties to gases (O2 and CO2). Coatings with essential oils in fruits have been shown to help maintain these barriers and physicochemical properties, while also offering antioxidant characteristics and antifungal and antimicrobial properties [25].
3.3. Physical and Chemical Analyses of Soursop Fruits
3.3.1. Weight Loss Determination
Figure 3 shows the effect of chitosan-based coatings on the weight loss of soursop, both non-inoculated (a) and inoculated with C. gloeosporioides (b). Results for cinnamon and thyme essential oil treatments showed a reduction of up to 20% and 19% in weight loss for the non-inoculated and inoculated soursops, respectively, after 10 days of treatment. The non-inoculated treatments showed a significant difference from the control, and the coatings with essential oils presented a significant difference compared to treatments with only chitosan. These results demonstrate that coatings with cinnamon and thyme essential oils in combination with chitosan provide an effective barrier against water loss.
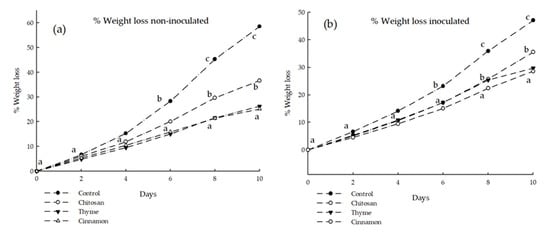
Figure 3.
Effect of the different coatings on the values of (%) WL without inoculated spores (a) and inoculated with spores (b). Different letters indicate significant difference (LSD post hoc tests were performed (α = 0.05)).
The behavior was related to the semi-permeable barrier formed by the chitosan coatings with added essential oils, which has been associated with reduced weight loss, browning, and improved fruit quality [26]. In this sense, [22] reported a weight loss of 12% in uncoated soursop at 6 days, while fruits coated with 1% chitosan lost the same weight after 9 days. On the other hand, Liu et al. [27] showed 30% weight loss in cherimoya (control), while cherimoyas coated with 1% chitosan showed 26% weight loss at 10 days of evaluation. The results obtained in this research show weight loss values in the ranges reported by these authors.
3.3.2. Instrumental Color
The effects of chitosan-based coatings on the color of soursop fruits inoculated and non-inoculated with C. gloeosporioides are shown in Figure 4 and Figure 5. For both treatments, the green color of soursop peels changed with time, as part of the physiological processes that occurred by ripening generating changes in the color saturation, causing the colors of the fruit to oscillate between yellow (carotenoids) and red-purple (anthocyanins) [28]. The non-inoculated treatment using chitosan coating with cinnamon oil preserved the green color (saturation, C*) of the fruits significantly. Likewise, the luminosity (L*) of the fruits decreased in all the evaluated treatments. The inoculated treatment showed that saturation color (C*) did not present significant differences in any of the evaluated treatments. In addition, the tonality of the fruits decreased over time towards a dark brown color; this is related to the rotting process caused by C. gloesporioides, as observed in significant differences between the control and the fruits with coatings with essential oils.
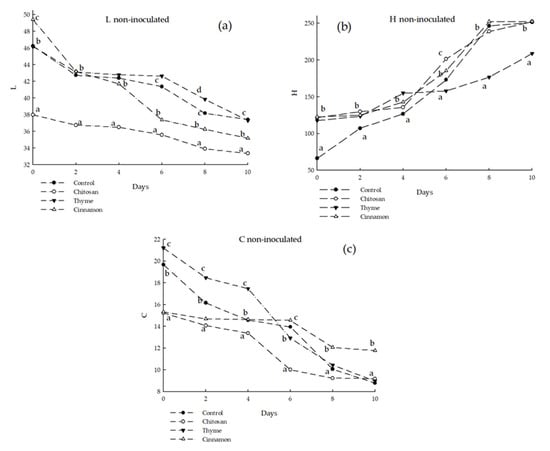
Figure 4.
Color of soursop non-inoculated with C. gloeosporioides: (a) L = luminosity; (b) H = Hue; (c) C = Chroma. Different letters indicate significant difference (LSD post hoc tests were performed (α = 0.05)).
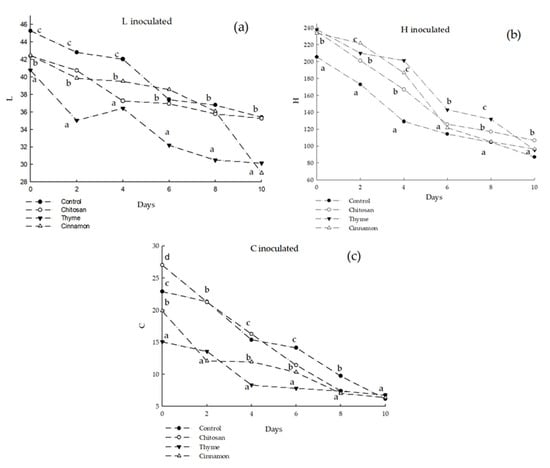
Figure 5.
Color of soursop inoculated with C. Gloeosporioides. (a) L = luminosity; (b) H = Hue; (c) C = Chroma. Different letters indicate significant difference (LSD post hoc tests were performed (α = 0.05)).
In this sense, Evangelista-Lozano et al. [28] reported brightness values similar to those obtained in the present study (L* = 44.5), while Hue was different, with lower values (h* = 96) obtaining yellow hues. Jiménez-Zurita et al. [29] evaluated the color of the soursop epidermis, concluding that the fruits had an opaque and poorly luminous green color (h* = 151.7–164.9; C* = 9.4–21.4; L* = 30.2–45.8). The color results reported are comparable to those found in the present work.
3.3.3. pH and Titratable Acidity
Regarding the pH parameter, there were no significant differences from day 2 to day 10 in all the treatments evaluated, reaching an average value of pH = 4.5 at 10 days of evaluation (Figure 6a). Ojeda et al. [30] reported pH levels ranging from 3.9 to 4.3 to 22 °C in soursop fruits, which were similar to the final values reported in the present research. Torres et al. [31] reported that the pH decrease and the TSS increase in climacteric fruits are related to the degradation of starch into reducing sugars or its conversion into pyruvic acid.
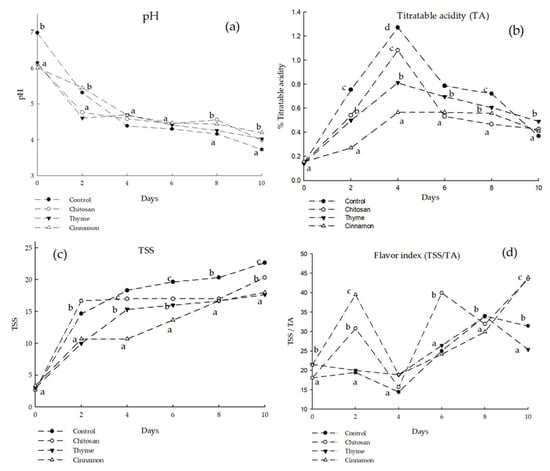
Figure 6.
Results of (a) pH; (b) TA; (c) TSS; and (d) TSS/TA without inoculation with C. gloeosporioides. Different letters indicate significant difference (LSD post hoc tests were performed (α = 0.05)) without inoculation with C. gloeosporioides.
Titratable acidity values showed a peak on day 4; however, from day 6 the acidity values decreased and remained similar until day 10 (Figure 6b). This increase in the acidity values at the beginning of the maturation process was due to the hydrolysis of starch to generate organic acids and volatile compounds; later, at the end of the ripening stage, these values decreased because organic acids were used as a substrate for conversion into sugars and in the breathing process [32]. It can be observed that the fruits coated with chitosan with cinnamon were those that presented the lowest acidity values, which agrees with what was reported by Sotelo-Alcántara et al. [7]. The authors reported that chitosan and cinnamon coatings promoted a low accumulation of organic acids in soursop (A. muricata L.). Likewise, Hernández-Ibáñez et al. [33] reported a decrease in acidity in banana fruits treated with chitosan as storage days progressed, demonstrating that the maturation process decreases the concentration of organic acids in the fruits and that chitosan-based coatings can help maintain this natural process without affecting the process (or even helping the process).
3.3.4. Total Soluble Solids and Flavor Index
The results of total soluble solids (TSS) are shown in Figure 6c. The uncoated soursop fruits showed the highest values of TSS on day 10 (22%), while the fruits coated with essential oils presented 18% less TSS after the same time. This behavior with coatings has been observed by several authors.
Jiménez-Zurita et al. [29] pointed out that soursop must have at least 9% TSS for consumption and that this value can reach up to 16%. Evangelista-Lozano et al. [28] reported values between 11 and 12% for TSS, and Arrazola-Paternina et al. [34] indicated that, at the stage of commercial maturity, it can reach 20.5% TSS. In the present study, the values of TSS were between 16 and 18 on day 10, being within the limits of consumption, while the controls reached 22% TSS. Then, the chitosan coatings did not affect the TSS values in the consumption stage. The behavior of the value of TSS is related to the degree of ripeness of the fruit since, during this process, sugar synthesis is promoted by the hydrolysis of starch into simpler carbohydrates of the disaccharide and monosaccharide types (glucose, sucrose, and fructose), which is promoted by the action of enzymes such as amylases [35].
The results of the flavor index are shown in Figure 6d, where it is possible to observe that the coatings with chitosan and cinnamon presented the highest values at 10 days. This behavior is related to the increase in sugar concentration or to the decrease in acidity values in the fruits as part of the maturation process. Sotelo-Alcántara et al. [7] reported a flavor index of 40 in soursop fruits coated with cinnamon, a result similar to that found in the present work at day 10.
3.4. Respiration and Ethylene Production Rates
Figure 7 shows the results for respiration and ethylene of coated soursop fruits in non-inoculated treatments. The results showed similar behavior in the first 4 days between the treatments; however, after 8 days, significant differences were observed between the coated and uncoated fruits, and thyme treatments were the best at reducing the respiration process (reduced to 47%). Furthermore, by day 10, fruits without essential oils were completely degraded. Due to their high rate of respiration, the fruits of soursop are classified as climacteric; this type of fruit is often harvested at physiological maturity and matures after harvest because the respiratory intensity and ethylene production are high [29]. Respiration is a process that allows the synthesis of metabolites and causes the degradation of the fruits; in this sense, the coatings based on chitosan with cinnamon and thyme increase the shelf life by at least two days due to the formation of a barrier that decreases the level of respiration and ethylene [18].
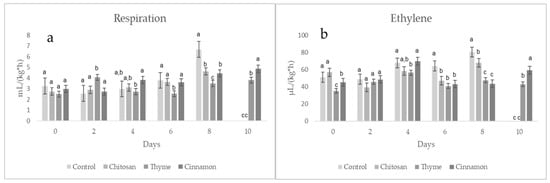
Figure 7.
Respiration (a) and ethylene (b) of coated soursop fruits (without inoculation). Different letters indicate significant difference (LSD post hoc tests were performed (α = 0.05)).
4. Conclusions
The chitosan obtained by the chemical method presented good physicochemical properties (low molecular weight and high degree of deacetylation), with values similar to those distributed by Sigma-Aldrich and with the appropriate characteristics for its application in edible coatings. The chitosan-based coatings with essential oils of cinnamon and thyme showed a visible protective effect on the soursop fruits, reducing the anthracnose disease caused by the fungus C. gloeosporioides. Moreover, after 8 days, the fruits coated with essential oils of thyme and cinnamon showed significantly lower rates of respiration and ethylene, increasing shelf life by two days, even with respect to the fruits coated without essential oils. Regarding the physicochemical parameters evaluated, only weight loss showed significant differences in fruits coated with chitosan and essential oils. Chitosan-based coatings with essential oils of thyme and cinnamon proved to be a good option to decrease the growth of C. gloeosporioides in soursop and maintain the quality of the fruits.
Author Contributions
F.M.-C.: formal analysis, methodology, writing—original draft preparation. C.N.-C.: data curation, validation, supervision; L.M.-A.: methodology, investigation, supervision; A.M.-V.: writing—review and editing, visualization; I.A.-T.: methodology, funding acquisition; E.R.R.-M.: data curation, validation, writing—review and editing; C.G.-V.: writing—original draft preparation, writing—review and editing; R.V.-G.: funding acquisition, supervision, validation; J.R.R.-N.: conceptualization, project administration, funding acquisition, writing—original draft preparation. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by SAGARPA-CONACYT (México) grant number 266891, and the APC was funded by Universidad de Guanajuato
Data Availability Statement
Not applicable.
Acknowledgments
Department of Genomic Services LANGEBIO, CINVESTAV-Campus Guanajuato, for the sequencing services.
Conflicts of Interest
The authors declare that there are no conflict of interest.
References
- Rodríguez-Núñez, J.R.; Campos-Rojas, E.; Andrés-Agustín, J.; Alia-Tejacal, I.; Peña-Caballero, V.; Madera-Santana, T.M.; Núñez-Colín, C.A. Distribution, eco-climatic characterization, and potential growing regions of Annona cherimola Mill. (Annonaceae) in Mexico. Ethnobiol. Conserv. 2021, 10. [Google Scholar] [CrossRef]
- Vidal-Hernández, L.; López-Moctezuma, H.; Vidal-Martínez, A.; Ruiz-Bello, R.; Castillo, R.D.; Chiquito-Contreras, G. La situación de las annonaceae en México: Principales plagas, enfermedades y su control. Rev. Bras. Frutic. 2014, 36, 44–54. [Google Scholar] [CrossRef][Green Version]
- SIAP. Anuario Estadístico de la Producción Agrícola y Pesquera. In Cierre agrícola SEMARNAT; Secretaria de Medio Ambiente y Recursos Naturales: Canatlán, México, 2021; Available online: https://nube.siap.gob.mx/cierreagricola/ (accessed on 15 January 2023).
- Afzaal, M.; Saeed, F.; Asghar, A.; Shah, Y.A.; Ikram, A.; Ateeq, H.; Hussain, M.; Ofoedu, C.E.; Chacha, J.S. Nutritional and Therapeutic Potential of Soursop. J. Food Qual. 2022, 1, 1–9. [Google Scholar] [CrossRef]
- Tiencheu, B.; Egbe, A.C.; Achidi, A.U.; Tenyang, N.; Ngongang, E.F.T.; Djikeng, F.T.; Foss, B.T. Nutritional, organoleptic, and phytochemical properties of soursop (Annona muricata) pulp and juice after postharvest ripening. Eur. J. Nutr. Food Saf. 2021, 13, 15–28. [Google Scholar]
- Huang, H.; Tian, C.; Huang, Y.; Huang, H. Biological control of poplar anthracnose caused by Colletotrichum gloeosporioides (Penz.) Penz. & Sacc. Egypt. J. Biol. Pest Control. 2020, 30, 104. [Google Scholar] [CrossRef]
- Sotelo-Alcántara, A.; Alia-Tejacal, I.; Rodríguez-Núñez, R.; Campos-Rojas, E.; Juárez-López, P.; Pérez-Arias, A. Postharvest effects of a chitosan-cinnamon essential oil coating on soursop fruits (Annona muricata L.). Acta Hortic 2022, 1340, 35–40. [Google Scholar] [CrossRef]
- Quintero, C.; Falguera, V.; Muñoz, A. Películas y recubrimientos comestibles: Importancia y tendencias recientes en la cadena hortofrutícola. Revista Tumbaga 2010, 5, 93–118. [Google Scholar]
- Ramos-García, L.; Bautista-Baños, S.; Barrera-Necha, L.; Bosquez-Molina, E.; Alia-Tejacal, I.; Estrada-Carrillo, M. Compuestos antimicrobianos adicionados en recubrimientos comestibles para uso en productos hortofrutícolas. Rev. Mex. Fitopatol. 2010, 28, 44–57. [Google Scholar]
- Khalid, M.A.; Niaz, B.; Saeed, F.; Afzaal, M.; Islam, F.; Hussain, M.; Mahwish Khalid, H.M.S.; Siddeeg, A.; Al-Farga, A. Edible coatings for enhancing safety and quality attributes of fresh produce: A comprehensive review. Int. J. Food Prop. 2022, 25, 1817–1847. [Google Scholar] [CrossRef]
- Rodríguez-Núñez, J.R.; Madera-Santana, T.; Sánchez-Machado, D.; López-Cervantes, J.; Soto, H. Chitosan/hydrophilic plasticizer-based films: Preparation, physicochemical and antimicrobial properties. J. Polym. Environ. 2014, 22, 41–51. [Google Scholar] [CrossRef]
- Kocira, A.; Kozłowicz, K.; Panasiewicz, K.; Staniak, M.; Szpunar-Krok, E.; Hortynska, P. Polysaccharides as edible films and coatings: Characteristics and influence on fruit and vegetable quality—A review. Agronomy 2021, 11, 813. [Google Scholar] [CrossRef]
- López-Mata, M.; Ruiz-Cruz, S.; Navarro-Preciado, C.; Ornelas-Paz, J.; Estrada-Alvarado, I.; Gassos-Ortega, L.; Rodrigo-García, J. Efecto de recubrimientos comestibles de quitosano en la reducción microbiana y conservación de la calidad de las fresas. Biotecnia 2012, 14, 33–43. [Google Scholar] [CrossRef]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for basidiomycetes application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef]
- White, T.J.; Burns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocol: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: Cambridge, MA, USA, 1990; pp. 315–322. [Google Scholar]
- Acosta-Ferreira, S.; Castillo, O.; Madera-Santana, T.; Mendoza-García, A.; Núñez-Colín, C.; Grijalva-Verdugo, C.; Villa-Lerma, G.; Morales-Vargas, A.; Rodríguez-Núñez, R. Production and physicochemical characterization of chitosan for the harvesting of wild microalgae consortia. Biotechnol. Rep. 2020, 28, e00554. [Google Scholar] [CrossRef]
- Liu, N.; Chen, X.G.; Park, H.J.; Liu, C.G.; Liu, C.G.S.; Meng, X.H.; Yu, L.J. Effect of MW and concentration of chitosan on antibacterial activity of Escherichia coli. Carbohydr. Polym. 2006, 64, 60–65. [Google Scholar] [CrossRef]
- Madera-Santana, T.; De Dios-Aguilar, A.; Colín-Chávez, C.; Mariscal-Amaro, A.; Núñez-Colín, C.; Veloz-García, R.; Guzmán-Maldonado, H.; Peña-Caballero, V.; Grijalva-Verdugo, C.; Rodríguez-Núñez, R. Recubrimiento a base de quitosano y extracto acuoso de hoja de Moringa oleífera obtenido por UMAE y su efecto en las propiedades fisicoquímicas de fresa (Fragaria x ananassa). Biotecnia 2019, 21, 155–163. [Google Scholar]
- AOAC. Official Methods of Analysis, 18th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2007. [Google Scholar]
- Saltveit, M.E. Respiratory metabolism. In Agricultural Handbook 66—The Commercial Storage of Fruits, Vegetables, and Florist and Nursery Stocks; Gross, K.C., Wang, C.Y., Saltveit, M., Eds.; Available from the National Technical Information Service, Springfield, VA orat 2016; USDA Agricultural Research Service: Washington, DC, USA, 2016. Available online: http://www.ba.ars.usda.gov/hb66/contents.html (accessed on 3 February 2022).
- Muley, A.; Chaudhari, A.; Mulchandani, H.; Singhal, R. Extraction and characterization of chitosan from prawn shell waste and its conjugation with cutinase for enhanced thermo-stability. Int. J. Biol. Macromol. 2018, 111, 1047–1058. [Google Scholar] [CrossRef]
- Ramos-Guerrero, A.; González-Estrada, R.; Romanazzi, G.; Landi, L.; Gutiérrez-Martínez, P. Effects of chitosan in the control of postharvest anthracnose of soursop (Annona muricata) fruit. Rev. Mex. Ing. Química. 2020, 19, 99–108. [Google Scholar] [CrossRef]
- Bill, M.; Sivakumar, D.; Korsten, L.; Thompson, K. The efficacy of combined application of edible coatings and thyme oil in inducing resistance components in avocado (Persea americana Mill.) against anthracnose during post-harvest storage. Crop Prot. 2014, 64, 159–167. [Google Scholar] [CrossRef]
- Montalvo-González, E.; León-Fernández, E.; Rea-Paez, H.; Mata-Montes De Oca, M.; Tovar-Gómez, B. Uso combinado de 1-Meticiclopropeno y emulsiones de cera en la conservación de guanábana (Annona muricata). Rev. Bras. Frutic. 2014, 36, 206–306. [Google Scholar] [CrossRef][Green Version]
- Aider, M. Chitosan application for active bio-based films production and potential in the food industry. LWT-Food Sci. Technol. 2010, 43, 837–842. [Google Scholar] [CrossRef]
- Salvador, A.; Cuquerella, J.; Monterde, A. Efecto del quitosano aplicado como recubrimiento en mandarinas ‘fortune’. Rev. Iberoam Tecnol. Postcos. 2003, 5, 122–127. [Google Scholar]
- Liu, K.; Liu, J.; Li, H.; Yuan, C.; Zhong, J.; Chen, Y. Influence of postharvest citric acid and chitosan coating treatment on ripening attributes and expression of cell wall related genes in cherimoya (Annona cherimola Mill.) fruit. Sci. Hortic. 2016, 198, 1–11. [Google Scholar] [CrossRef]
- Evangelista-Lozano, S.; Cruz-Castillo, G.; Pérez-González, S.; Mercado-Silva, E.; Dávila-Ortiz, G. Producción y calidad frutícola de guanábanos (Annona muricata L.) provenientes de semilla de jiutepec, Morelos, México. Rev. Chapingo Ser. Hortic. 2003, 9, 69–79. [Google Scholar] [CrossRef]
- Jiménez-Zurita, J.; Balois-Morales, R.; Alia-Tejacal, I.; Juárez-López, P.; Sumaya-Martínez, T.; Bello-Lara, E. Caracterización de frutos de guanábana (Annona muricata L.) en Tepic, Nayarit, México. Revista Mexicana de Ciencias Agrícolas 2016, 7, 1261–1270. [Google Scholar] [CrossRef]
- Ojeda, G.; Coronado, J.; Nava, R.; Sulbarán, B.; Araujo, D.; Cabrera, L. Caracterización fisicoquímica de la pulpa de la guanábana (Annona muricata) cultivada en el occidente de Venezuela. Boletín del Centro de Investigaciones Biológicas 2007, 41, 151–160. [Google Scholar]
- Torres, R.; Montes, E.J.; Pérez, O.A.; Andrade, R.D. Relation of color and maturity stage with physicochemical, properties of tropical fruits. Inf. Tecnol. 2013, 24, 51–56. [Google Scholar] [CrossRef]
- Dussán-Sarria, S.; Torres-León, C.; Reyes-Calvache, M. Efecto del recubrimiento comestible sobre los atributos fisicoquímicos de mango ‘Tommy Atkins’ mínimamente procesado y refrigerado. Acta Agron. 2014, 63, 212–221. [Google Scholar] [CrossRef]
- Hernández-Ibáñez, A.; Bautista-Baños, S.; Gutiérrez-Martínez, P. Potential of chitosan for controlling crown rot disease of banana (Musa acuminate) fruit cv. ‘Enano gigante’. In Food Science and Food Biotechnology Essentials: A Contemporary Perspective; Nevárez, M.G., Ortega-Rivas, E., Eds.; Asociación Mexicana de Ciencias de los Alimentos, A.C.: Puerto Vallarta, México, 2012; pp. 129–136. [Google Scholar]
- Arrazola-Paternina, S.; Barrera-Violeth, L.; Villalba-Cadavid, I. Determinación física y bromatológica de la guanábana cimarrona (Annona glabra L.) del departamento de Córdoba. Orinoquia 2013, 17, 159–166. [Google Scholar]
- Romanazzi, G.; Sanzani, S.; Bi, Y.; Tian, S.; Gutiérrez-Martínez, P.; Alkan, N. Induced resistance to control postharvest decay of fruit and vegetables. Postharvest Biol. Technol. 2016, 122, 82–94. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).