Abstract
The human intake of selenium (Se), which is an essential element in animals and humans, can be increased through the consumption of vegetables that have been biofortified during cultivation. There is increasing interest in wild edible plants (WEPs) due to their positive effects on health. In fact, many WEPs are rich in microelements, vitamins, dietary fibers, and several antioxidant compounds. Among WEPs, sea beet (Beta vulgaris ssp. maritima) is the wild ancestor of Swiss chard (Beta vulgaris var. cicla). The present study investigated the potential of fortifying Swiss chard and sea beet with Se. The two subspecies were cultivated in a floating system with a nutrient solution enriched with four concentrations of Se (0, 1, 3, and 5 mg L−1), and the production and quality of the baby leaves were evaluated. The addition of Se to the nutrient solution resulted in a higher leaf concentration of this microelement in both subspecies, with a positive effect on the yield (+20%) and leaf chlorophyll concentration (+25%) at the Se concentration of 1 mg L−1. The leaf concentration of nitrates was reduced by the Se treatment in sea beet regardless of the Se concentration (−24%, on average). Selenium biofortification was more effective in sea beet plants than in Swiss chard due to the higher ability of the wild species to acquire readily available minerals from the hydroponic nutrient solution. In conclusion, both subspecies accumulated a significant amount of Se without negative effects on yield or leaf quality, thus proving them to be suitable for the production of Se-enriched baby leaves.
1. Introduction
Approximately 15% of the global population is affected by selenium (Se) deficiency []. Selenium is an essential element for animals and humans. It is present in seleno-amino acids, selenoproteins, and glutathione peroxidase (GSH-Px; EC 1.11.1.9). It is also involved in the metabolism of thyroid hormones, the antioxidant defense system, and immunological processes []. The adequate daily intake (AI) of Se in adults is 70 µg [,].
Dietary supplements can be used to treat Se deficiency; however, the majority of these supplements are synthetic, and it is unclear whether they are absorbed in the body as is normally the case with natural foods such as fruits and vegetables. A viable option to prevent Se deficiency is the biofortification of vegetables with the application of Se salts []. The consumption of vegetables that are biofortified during cultivation has been proven to be effective in improving the human intake of trace elements []. Several studies have been conducted on the Se biofortification of hydroponically grown plants []. Among the hydroponic techniques, the floating system has been shown to be suitable for cultivating leafy vegetables, including Swiss chard and sea beet []. Floating systems can facilitate crop biofortification, as found in a previous work [].
Selenium can positively or negatively affect plant growth, depending on the plant species and the amount of Se applied [,]. For instance, Se can reduce the leaf nitrate concentration by increasing nitrate reductase and glutamate synthase activities []; however, evidence has also shown that the leaf nitrate concentration is not affected by Se application [,]. Selenium can also protect plants from oxidative stress induced by biotic or abiotic factors []; however, the leaf phenol concentration and the antioxidant capacity can be both increased [,] or not affected [] by Se treatment.
There is increasing interest in growing wild edible plants (WEPs) as new leafy vegetables. These species can be included in a diet due to their positive effects on health []. Indeed, many WEPs are rich in microelements, vitamins, dietary fibers, and several antioxidant compounds that have shown anticancer activities and can help to prevent chronic neurodegenerative diseases and inflammatory processes. The high antioxidant capacity is mainly due to the high concentration of phenols []. Among WEPs, sea beet (Beta vulgaris ssp. maritima, SB), the wild ancestor of Swiss chard (Beta vulgaris ssp. vulgaris var. cicla, SC), grows naturally in coastal and saline areas of the Mediterranean area, as well as in northern Europe []. Its leaves are rich in phytochemicals and antioxidants []. Swiss chard is frequently grown for the production of ready-to-eat baby leaves, which are tasty and have positive effects on health []. However, both Swiss chard and sea beet have the potential to accumulate toxic amounts of nitrate and oxalate, which could be harmful to humans [,].
The goal of the present study was to investigate the potential of fortifying Swiss chard and sea beet with Se. The effects of a nutrient solution enriched with four different concentrations of Se (0, 1, 3, and 5 mg L−1) were evaluated on the leaf Se concentration and the production and quality of baby leaves of Swiss chard and sea beet cultivated hydroponically. Since, in the literature, biofortification with Se has shown conflicting effects on leaf quality parameters, we also investigated the effect of Se enrichment on the leaf concentrations of nitrates, oxalates, pigments, and antioxidant compounds.
2. Materials and Methods
2.1. Plant Material, Growth Conditions, Treatments, and Experimental Design
Two experiments were conducted in a glasshouse at the University of Pisa, Italy (lat. 42′42″48 N, long. 10°24′52″92 E), in autumn 2021. A weather station inside the glasshouse allowed for continuous monitoring of the climatic conditions, which are reported in Table 1 (second experiment) and Table S1 (first experiment).

Table 1.
Basic information on the first experiment with plants of Swiss chard (Beta vulgaris subsp. vulgaris var. cicla) and sea beet (Beta vulgaris subsp. maritima) grown hydroponically (floating raft system) under greenhouse conditions.
Seeds of sea beet (Beta vulgaris ssp. maritima, SB) and Swiss chard (Beta vulgaris ssp. vulgaris var. cicla, SC) were provided by Gargini Sementi (Lucca, Italy) and Pennard Plants (Shepton Mallet, UK), respectively, and sown in 180-cell trays with rockwool plugs. The trays were placed in a growth chamber at 25 °C, and after five days they were moved to the greenhouse. Twenty-one days after sowing, seedlings were placed in 50 L plastic tanks containing a stagnant nutrient solution (the water depth was approximately 25 cm). The composition of the nutrient solution was as follows: N-NO3 (10 mM), NH4+ (0.4 mM), P (1.5 mM), K (9 mM), Ca (4.5 mM), Mg (2 mM), SO42− (2.3 mM), Fe (40 µM), B (40 µM), Cu (3 µM), Zn (10 µM), Mn (10 µM), and Mo (1 µM). Each tank hosted 180 plants, and the crop density was approximately 720 plants m−2 of ground area.
A full factorial experiment was conducted with two factors: plant subspecies and Se concentration. Selenium was added to the nutrient solutions three days after transplanting. Each treatment had three replicates, each consisting of a hydroponic tank. The experiment was repeated twice.
Selenium was supplied as sodium selenate (Na2SeO4) at concentrations of 0 (control), 1, 3, and 5 mg Se L−1. These concentrations were selected in accordance with the findings of earlier studies on lettuce and basil plants cultivated in floating systems [,], in order to achieve an adequate biofortification of leaves without negative impacts on crop yield.
2.2. Determinations
Plants were harvested 14 and 15 days after transplanting in the 1st and 2nd experiments, respectively. The plants were cut 1 cm above the collar. For each replicate, the fresh weight (FW) of 20 plants was measured. The dry weight (DW) was measured after drying the plant samples to a constant weight in a ventilated oven at 50 °C. The fresh and dry biomass production values were expressed as kg FW m−2 and g DW m−2, respectively, and the concentration of dry matter was expressed as the percent DW/FW ratio. The leaf area (LA) was measured using a digital planimeter (DT Area Meter MK2, Delta T-Devices). The leaf area index (LAI) was calculated as the leaf area of individual plants multiplied by the crop density, and the leaf succulence (LS) was calculated as the FW/LAI ratio.
Dried leaf samples were analyzed for the contents of Se, nitrate, and oxalate as follows.
After microwave-assisted digestion of the samples with nitric acid and hydrogen peroxide following EPA Method 3051A [], the total Se concentration was determined via inductively coupled plasma spectrometry (ICP OES 5900 Agilent, Santa Clara, CA, USA) (limit of quantification = 0.0125 mg kg−1 DW). The bioaccumulation factor (BAF) was calculated as the ratio of the Se concentration in the leaves (mg kg−1 FW) to the Se concentration in the nutrient solution (mg L−1).
Dried leaf samples were also extracted with distilled water (100 mg DW in 20 mL) at room temperature for 2 h, and the nitrate concentration was measured spectrophotometrically using the salicylic–sulfuric acid method [].
The content of total oxalate in the leaves was extracted with 0.25 M HCl (50 mg in 6 mL) at 100 °C for 15 min. The mixture was allowed to cool down, and the total extract volume was brought to 10 mL with 0.25 M HCl and then filtered through filter paper. The extraction of soluble oxalate was performed according to the same protocol, except for the use of water instead of 0.25 M HCl. The total and soluble oxalate contents were determined in the liquid phase by adding 200 mL of the sample to 1 mL of 1 M H2SO4 and 400 mL of 0.003 M K2MnO4. After 10 min at room temperature, the absorbance of the solution was read at 528 nm using a calibration curve obtained with oxalate solutions [].
The leaf concentrations of total chlorophylls, carotenoids, flavonoids, and phenols and the antioxidant capacity were determined in fresh samples, which were ultrasonically extracted with 99% v/v MeOH (100 mg in 5 mL) for 60 min and then stored for 24 h at −18 °C in the dark. The methanol extracts were used to measure the chlorophyll and carotenoid concentrations using the equation reported by Welburn and Lichtenthaler []. The total chlorophyll content was calculated as the sum of chlorophyll a and chlorophyll b. To 0.1 mL of the methanol extract, 0.06 mL of 5% NaNO2 and 0.04 mL of 10% AlCl3 were added. After five minutes, 0.4 mL of 1 M NaOH and 0.2 mL of H2O were added. Then, the content of flavonoids was measured spectrophotometrically (510 nm) using a calibration curve obtained with catechin standard solutions [].
The methanol extracts were also used for the determination of the total phenolic content, using a calibration curve obtained with gallic acid standard solutions [], and the total antioxidant capacity, which was measured via the ferric reducing ability of plasma (FRAP) [].
2.3. Estimated Dietary Intake and Health Risk Assessment
The estimated dietary intake (EDI100, µg day−1) of Se due to the daily consumption of 100 g of Se-enriched leaves was calculated and expressed as the percentage (EDI%) of the adequate intake (AI) of Se (70 µg day−1) for an adult [].
To evaluate the possible health risk due to an excessive intake of Se associated with the consumption of enriched SC or SB leaves, the health risk index (HRI) of Se was calculated as the ratio of the EDI and the tolerable upper intake level (UL) of Se (300 µg day−1) [].
The health risk index (HRI) due to an excessive intake of NO3− or oxalate was calculated, for a 60 kg adult, as the ratio between the dietary intake of nitrate or oxalate due to the daily consumption of 100 g of leaves and an acceptable daily intake of 222 mg of NO3− [] or a lethal dose of 2 g day−1 of oxalate [], respectively.
2.4. Statistical Analysis
The two experiments were conducted under almost identical environmental conditions, and the results of the two experiments were very similar. Thus, for the sake of simplicity, only the results of the second experiment are shown here, while those of the first experiment are reported in the Supplementary Material (Tables S2 and S3). Data were tested for the normality of the distribution using the Shapiro–Wilk test and for the homogeneity of variances using Levene’s test and then subjected to a two−way ANOVA, and mean values were separated using Tukey’s test (p < 0.05). To investigate whether there were clusters of related qualitative characteristics and to identify clusters across subspecies and Se concentrations, a PCA of SC and SB plants together was carried out after standardization of the data. The statistical analysis was performed using JMP Statistical Software.
3. Results
Sea beet (SB) and Swiss chard (SC) accumulated Se in leaf tissues to an extent that increased with the Se concentration in the root zone (Figure 1). The Se concentration detected in control plants was always under the quantification limit (<0.0125 mg kg−1 DW). Sea beet plants showed, on average, a higher leaf Se concentration (+31%; Figure 1) and a higher bioaccumulation factor (+14%) than SC (Figure 1).
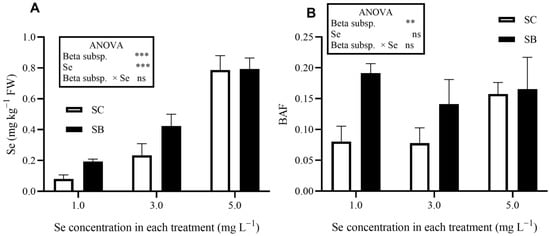
Figure 1.
Selenium concentration (A) and bioaccumulation factor (BAF, B) in leaves of Swiss chard (Beta vulgaris subsp. vulgaris var. cicla; SC) and sea beet (Beta vulgaris subsp. maritima; SB) plants grown hydroponically (floating system) with different selenium (Se) concentrations in the nutrient solutions. The Se concentration in leaves of control plants was below the limit of quantification (0.0125 mg Se kg−1 DW). Significance levels: *** p ≤ 0.001; ** p ≤ 0.01; ns = not significant.
On average, plants treated with 1 mg Se L−1 (+20%) showed a higher yield compared to controls, whereas no differences were detected at 3 and 5 mg Se L−1 compared to controls and the 1 mg L−1 treatment (Table 2). Regardless of the concentration, the addition of Se to the nutrient solution resulted in an increased LAI (+50%, on average) and reduced LS (−28%, on average) compared to controls. The average dry matter content of leaves was reduced by treatments with 1 (−23%) and 3 mg L−1 of Se (−24%) compared to controls (Table 2). On average, SB leaves showed a lower yield (−23%), a lower LS (−25%), and a higher dry matter content (+29%) compared to SC leaves (Table 2).

Table 2.
Crop yield (total leaf fresh biomass), leaf dry biomass (DM), dry matter content (percent DM/FM ratio), leaf area index (LAI), and succulence (LS) in plants of Swiss chard (Beta vulgaris subsp. vulgaris var. cicla) and sea beet (Beta vulgaris subsp. maritima) grown hydroponically (floating system) with different selenium (Se) concentrations in the nutrient solutions.
Treatments with Se reduced the nitrate concentration in SB leaves (−24%, on average), whereas exposure to 3 (+51%) and 5 mg Se L−1 (+62%) increased the nitrate concentration in SC leaves (Table 3).

Table 3.
Leaf concentrations of nitrate (NO3−); total and soluble oxalate; and total chlorophylls, carotenoids, phenols, and flavonoids and antioxidant capacity (FRAP index), expressed on a fresh weight basis, in plants of Swiss chard (Beta vulgaris subsp. vulgaris var. cicla) and sea beet (Beta vulgaris subsp. maritima) grown hydroponically (floating system) with different selenium (Se) concentrations in the nutrient solutions.
The total oxalate concentration was not affected by the Se treatments, which slightly affected the leaf concentration of soluble oxalate (Table 3).
On average, treatment with 1 mg Se L−1 increased the leaf chlorophyl concentration by +25% and treatment with 5 mg Se L−1 increased the leaf carotenoid concentration by +74% compared to controls (Table 3).
Se-biofortified leaves of SB exhibited a higher antioxidant capacity than controls (Table 3).
On average, SB showed a higher antioxidant capacity (+56%) and higher concentrations of nitrate (+92%), total oxalate (+25%), chlorophyll (+21%), and total phenols (+14%) but a lower carotenoid (−56%) concentration compared to SC (Table 3). A positive linear correlation was detected between the leaf flavonoid content and the antioxidant capacity (Figure 2).
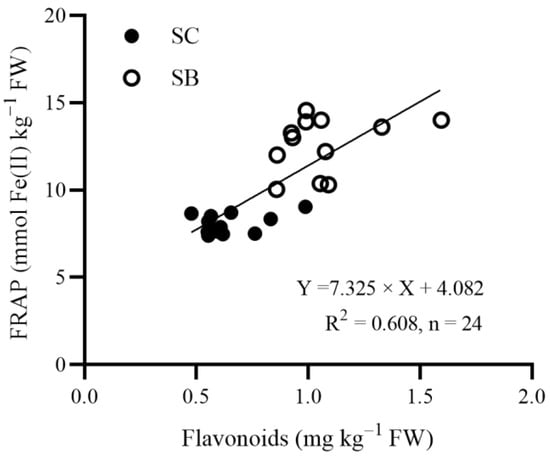
Figure 2.
Linear regression between the antioxidant capacity (measured via FRAP assay) and the total flavonoid concentration, expressed on a fresh weight (FW) basis, in the leaves of Swiss chard (Beta vulgaris subsp. vulgaris var. cicla; SC) and sea beet (Beta vulgaris subsp. maritima; SB) plants grown hydroponically (floating system) with different concentrations of selenium (Se) in the nutrient solutions.
Principal Component Analysis (PCA)
The first two principal components (PCs), explaining a cumulative variance of 78.9%, were identified on the basis of a screen plot of eigenvalues. A total of 52.9% of the total variance was explained by PC1, which correlated positively with the dry matter content and the concentrations of nitrate and total chlorophylls, flavonoids, and oxalate and the antioxidant capacity and correlated negatively with the concentration of carotenoids and LS. PC2 explained 26.0% of the total variance. It correlated negatively with the dry matter content and LS and correlated positively with the concentration of total chlorophylls and the flavonoid content’s antioxidant capacity. The relationship between the parameters measured in this study is illustrated by the loadings reported in Figure 3A. Parameters located close to each other showed a strong co-variance. A greater contribution of the parameters to the PCs is indicated by the position furthest away from the origin. On the right half of the loading plot, a cluster with the flavonoid concentration and FRAP index and a second cluster with oxalate and the dry matter content suggested a strong co-variance between these variables as well as a strong contribution of these quantities, together with the total chlorophyl concentration, to PC1. The carotenoid concentration and LS also contributed to PC1; however, they were located on its negative side. The most important variables contributing to PC2 were LS, the concentrations of soluble and total oxalate, and the dry matter content. The relationship between the analyzed samples is shown by the scores reported in Figure 3B. Two main groups were distinguished by PC1 and PC2 according to the beta species. In fact, SB plants, which showed the highest concentrations of flavonoids, chlorophylls, nitrate, oxalate, and dry matter content and the highest antioxidant capacity, were positioned on the right half of the plot (positive side of PC1). Conversely, SC plants, which were characterized by the highest leaf concentration of carotenoids and LS, were located in the left half of the plot (negative side of PC1).
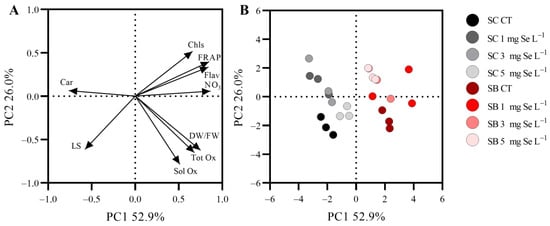
Figure 3.
Loading (A) and score plots (B) for PC1 and 2, describing variation in some qualitative characteristics (concentration of chlorophyll, Chls; carotenoid, Car; flavonoid, Flav; nitrate, NO3; dry matter, DW/FW; total oxalate, Tot Ox; soluble oxalate, Sol Ox; antioxidant capacity, FRAP; leaf succulence, LS) of the leaves of Swiss chard (Beta vulgaris subsp. vulgaris var. cicla; SC) and sea beet (Beta vulgaris subsp. maritima; SB) plants grown hydroponically (floating system) with different selenium (Se) concentrations in the nutrient solutions.
4. Discussion
Selenium supplementation has been proven to significantly increase the leaf Se concentration in many leafy vegetables such as chard [], basil [,,], lettuce [,], lamb’s lettuce, wild rocket, and spinach []. In our experiment, the addition of 1, 3, and 5 mg Se L−1, as Na2SeO4, produced Se-biofortified baby leaves of both subspecies, especially in sea beet. As a wild plant, SB usually grows in low-nutrient habitats and has a high capacity to acquire readily available minerals [] such as those dissolved in a hydroponic nutrient solution. This could explain the higher amount of Se accumulated in SB leaves compared to SC. The Se accumulation rate depends on the plant species and variety and the dose of Se used for treatments, as reported by Francini et al. []. In any case, the leaf Se concentration was lower than the values reported by Castro et al. [] in chard plants treated with 12–20 mg Se L−1 via four foliar applications (about 3.06–4.64 mg kg−1 FW). These differences were probably due to the higher concentration of Se and the different method of Se application adopted by the authors.
The leaf Se concentration ranged between 0.080 and 0.827 mg Se kg−1 FW. Thus, 100 g of fresh Se-biofortified leaves of SC or SB would satisfy from 12% to 112% (SC) and from 27% to 118% (SB) of the adequate intake (AI) of Se (Table S4). Considering the EDI of Se due to the consumption of 100 g of fresh SC or SB leaves, the health risk index (HRI) of Se was always below 1, meaning that the daily consumption of Se-biofortified leaves would not have a long-term negative effect on human health [] (Table S4). To avoid exceeding the Se UL (300 ug day−1), the maximum amounts of fresh SC and SB leaves biofortified with 5 mg L−1 of Se that can be consumed per day are 385 and 395 g, respectively.
Crop variety and species can affect the influence of Se on plant growth. Studies conducted on spinach [], lettuce [], and basil [] treated with similar concentrations of Se showed reductions in plant biomass. In contrast, Brassica chinensis plants showed a higher yield when exposed to 0.5 mg kg−1 Se, but no differences were detected in plants treated with 1 mg Se kg−1 compared to the controls []. The beneficial or toxic effects of Se on plant growth, depending on the Se dose, have also been observed in lettuce [], cabbage, and alfalfa []. In our experiment, plant growth was not negatively affected by the addition of Se to the nutrient solution. The increase in fresh biomass that we observed in plants treated with 1 mg L−1 of Se is consistent with the results by Hawrylak-Nowak et al. [] and could indicate that a small amount of Se (from 0.5 to 1 mg L−1) could increase antioxidant metabolism and stimulate plant growth []. The higher leaf concentration of chlorophylls induced by treatment with 1 mg L−1 of Se could have increased biomass production by enhancing photosynthesis, as suggested by Wang et al. [].
The increased biomass produced by SC may be a result of breeding; in fact, this crop species has undergone genetic improvements aimed at increasing the yield []. However, in a previous study carried out in spring on older plants of SC and SB cultivated hydroponically and subjected to repeated cuts, SB exhibited higher leaf biomass production than SC []. Since the present study was conducted in the fall/autumn and plants were cut only once, the different behaviors of the two subspecies could be ascribed to both the different environmental conditions and the single cut.
The greater leaf area induced in our experiment by the Se treatment could be explained by the growth-promoting effect of Se [] and is in agreement with previous experiments with cauliflower [] and cucumber []. The potential effect of the Se treatment on the leaf dry matter content is not clear, and the contrasting effects of the Se treatment observed in this work are consistent with the results reported in curly endive plants by Sabatino et al. []. These latter authors observed an increased dry matter content in curly endive plants treated with 0.16 and 0.32 mg Se L−1 via fertigation, while no differences were detected in plants treated with 0.08 and 0.63 mg Se L−1. The higher dry matter content we found in SB leaves compared to SC could lead to an improvement in the shelf life of the wild subspecies []. The lower LS induced by the increased leaf area may negatively influence the leaf texture, which is an important organoleptic parameter.
The decrease in the leaf nitrate concentration observed in Se-enriched SB plants was also detected in lettuce [,] and could be ascribed to the ability of Se to increase nitrate reductase and glutamate synthase activities. In contrast, Hernández-Castro et al. [] and Puccinelli et al. [] observed no impact of selenium treatment on the leaf nitrate concentration, which was considerably lower than the maximum values established for spinach (3500 mg kg−1 FW) grown in the fall and winter []. As previously discovered by Puccinelli et al. [], besides the higher dry matter content, the characteristics of the plant species affect nitrate build-up [] and may partially explain the increased nitrate concentration in SB leaves.
Evidence on the effect of Se on oxalate accumulation in plant tissues is scarce, except for a study conducted by Golob et al. [] on Tartary buckwheat plants. In this species, the foliar application of Se significantly decreased the formation of calcium oxalate druses in the leaves.
In our study, the exposure of plants of both subspecies to Se did not affect the leaf concentration of total oxalate and decreased the concentration of soluble oxalate only in plants treated with 3 mg L−1 of Se. In addition, the PCA highlighted a positive correlation between the concentration of total and soluble oxalate and the leaf dry matter content (Figure 3). Thus, the changes in the oxalate concentration could be ascribed to the variation in the dry matter content. The Amaranthaceae family includes species, such as SC and SB, which typically have high leaf oxalate concentrations (although even within this family there is considerable variation in this), and this may explain the differences between the two subspecies tested in our experiment. However, in SC and SB plants the concentration and ratio (from 72.7% to 89.2%) of total and soluble oxalate were comparable to those reported by Freidig et al. [] in Beta vulgaris. The higher leaf total oxalate level detected in SB was also linked to the higher dry matter content.
The higher concentrations of total chlorophylls and carotenoids observed in Se-enriched plants in our study are consistent with results obtained by Golob et al. [] in Tartary buckwheat plants and Chang et al. [] in Lycium chinense L. plants. As highlighted by the PCA results, the leaf concentrations of chlorophyll and carotenoids showed an opposite trend in the two subspecies of Beta: SB leaves showed a higher chlorophyll concentration but a lower carotenoid concentration than SC leaves. These differences may be due to the unique traits of the two subspecies. In fact, the concentrations of pigments in plant tissues depend on their genotypes [].
In our experiment, an increment in the antioxidant capacity, compared with the control, was only found in Se-enriched SB plants. The positive linear correlation between the leaf flavonoid content and the antioxidant capacity, reported in Figure 2 and highlighted by the PCA results (Figure 3), is in agreement with previous findings [,,,]. In addition, Se has been found to be effective in increasing the leaf phenolic concentration in tomato [] and basil [] grown hydroponically with the Se concentrations in the nutrient solutions ranging from 2 to 44 mg Se L−1 and from 3 to 20 mg Se L−1, respectively. Conversely, studies conducted on basil [] and carrot [] showed that Se biofortification affected neither the leaf phenol concentration nor the antioxidant capacity. These contrasting results suggest that the effects of Se on these parameters may depend on the plant species and variety, the application dose, and the growing conditions.
5. Conclusions
Our study clearly indicates that, in both Swiss chard and sea beet, the addition of sodium selenate to the nutrient solution increases the leaf concentration of selenium to levels that are effective for the biofortification of the two subspecies. Selenium biofortification was more effective in sea beet than in Swiss chard. A serving dose of 100 g of Se-biofortified leaves of Swiss chard and sea beet would satisfy from 12% to 112% and from 27% to 118% of the adequate intake of Se, respectively. Treatment with 1 mg Se L−1 also induced positive effects on leaf production and quality.
In general, sea beet showed the highest concentrations of flavonoids, chlorophylls, nitrate, oxalate, and dry content and the highest antioxidant capacity, whereas Swiss chard plants were characterized by the highest leaf concentration of carotenoids and leaf succulence.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/horticulturae9080909/s1, Table S1, Basic information on the experiment with plants of Beta vulgaris subsp. vulgaris var. cicla and Beta vulgaris subsp. maritima grown hydroponically (floating raft system) under greenhouse conditions; Table S2, Crop yield (total leaf fresh biomass), leaf dry biomass (DM), dry matter content (percent DM/FM ratio), leaf area index (LAI), leaf succulence (LS), and Se content, expressed on a dry matter basis, in plants of Swiss chard (Beta vulgaris subsp. vulgaris var. cicla) and sea beet (Beta vulgaris subsp. maritima) grown hydroponically (floating system) with different selenium (Se) concentrations in the nutrient solutions (first experiment); Table S3, Leaf contents of Se; nitrate (NO3−); total and soluble oxalate (OX); and total chlorophylls, carotenoids, phenols, and flavonoids and antioxidant capacity (FRAP index), expressed on a fresh weight basis, in plants of Swiss chard (Beta vulgaris subsp. vulgaris var. cicla; SC) and sea beet (Beta vulgaris subsp. maritima; SB) grown hydroponically (floating system) with different selenium (Se) concentrations in the nutrient solutions (first experiment); Table S4, Amount of Se provided (Se EDI100), expressed as % of adequate intake (AI), and health risk index (HRI) for Se, NO3−, and oxalates due to the consumption of 100 g of fresh leaves of Swiss chard (Beta vulgaris subsp. vulgaris var. cicla) and sea beet (Beta vulgaris subsp. maritima) plants grown hydroponically (floating system) with different selenium (Se) concentrations in the nutrient solutions.
Author Contributions
Conceptualization, M.P., A.P. and B.P.; methodology, M.P., I.R., A.P. and B.P.; formal analysis, M.P. and I.R.; investigation, M.P.; resources, A.P. and B.P.; data curation, M.P.; writing—original draft preparation, M.P.; writing—review and editing, M.P., F.M., A.P. and B.P.; supervision, M.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are contained within the article or the Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- White, P.J.; Broadley, M.R. Biofortification of Crops with Seven Mineral Elements Often Lacking in Human Diets—Iron, Zinc, Copper, Calcium, Magnesium, Selenium and Iodine. New Phytol. 2009, 182, 49–84. [Google Scholar] [CrossRef]
- Rayman, M.P. Selenium and Human Health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority. Scientific Opinion on Dietary Reference Values for Selenium. EFSA J. 2014, 12, 3846. [Google Scholar] [CrossRef]
- European Food Safety Authority. Scientific Opinion on Dietary Reference Values for Iodine. EFSA J. 2014, 12, 3660. [Google Scholar] [CrossRef]
- Gonzali, S.; Kiferle, C.; Perata, P. Iodine Biofortification of Crops: Agronomic Biofortification, Metabolic Engineering and Iodine Bioavailability. Curr. Opin. Biotechnol. 2017, 44, 16–26. [Google Scholar] [CrossRef]
- Consentino, B.B.; Ciriello, M.; Sabatino, L.; Vultaggio, L.; Baldassano, S.; Vasto, S.; Rouphael, Y.; La Bella, S.; De Pascale, S. Current Acquaintance on Agronomic Biofortification to Modulate the Yield and Functional Value of Vegetable Crops: A Review. Horticulturae 2023, 9, 219. [Google Scholar] [CrossRef]
- Izydorczyk, G.; Ligas, B.; Mikula, K.; Witek-Krowiak, A.; Moustakas, K.; Chojnacka, K. Biofortification of Edible Plants with Selenium and Iodine—A Systematic Literature Review. Sci. Total Environ. 2021, 754, 141983. [Google Scholar] [CrossRef]
- Puccinelli, M.; Carmassi, G.; Botrini, L.; Bindi, A.; Rossi, L.; Fierro-sañudo, J.F.; Pardossi, A.; Incrocci, L. Growth and Mineral Relations of Beta vulgaris var. cicla and Beta vulgaris ssp. maritima Cultivated Hydroponically with Diluted Seawater and Low Nitrogen Level in the Nutrient Solution. Horticulturae 2022, 8, 638. [Google Scholar] [CrossRef]
- Puccinelli, M.; Pezzarossa, B.; Rosellini, I.; Malorgio, F. Selenium Enrichment Enhances the Quality and Shelf Life of Basil Leaves. Plants 2020, 9, 801. [Google Scholar] [CrossRef]
- Hajiboland, R.; Amjad, L. Does Antioxidant Capacity of Leaves Play a Role in Growth Response to Selenium at Different Sulfur Nutritional Status? Plant Soil Environ. 2007, 53, 207–215. [Google Scholar] [CrossRef]
- Ramos, S.J.; Faquin, V.; Guilherme, L.R.G.; Castro, E.M.; Ávila, F.W.; Carvalho, G.S.; Bastos, C.E.A.; Oliveira, C. Selenium Biofortification and Antioxidant Activity in Lettuce Plants Fed with Selenate and Selenite. Plant Soil Environ. 2010, 56, 584–588. [Google Scholar] [CrossRef]
- Bian, Z.H.; Lei, B.; Cheng, R.F.; Wang, Y.; Li, T.; Yang, Q.C. Selenium Distribution and Nitrate Metabolism in Hydroponic Lettuce (Lactuca sativa L.): Effects of Selenium Forms and Light Spectra. J. Integr. Agric. 2020, 19, 133–144. [Google Scholar] [CrossRef]
- Hernández-Castro, E.; Trejo-Téllez, L.I.; Gómez-Merino, F.C.; Rodríguez-Mendoza, M.N.; Sánchez-García, P.; Robledo-Paz, A. Bioaccumulation of Iron, Selenium, Nitrate, and Proteins in Chard Shoots. J. Soil Sci. Plant Nutr. 2015, 15, 694–710. [Google Scholar] [CrossRef]
- Puccinelli, M.; Malorgio, F.; Maggini, R.; Rosellini, I.; Pezzarossa, B. Biofortification of Ocimum basilicum L. Plants with Selenium. Acta Hortic. 2019, 1242, 663–670. [Google Scholar] [CrossRef]
- Djanaguiraman, M.; Devi, D.D.; Shanker, A.K.; Sheeba, J.A.; Bangarusamy, U. Selenium—An Antioxidative Protectant in Soybean during Senescence. Plant Soil. 2005, 272, 77–86. [Google Scholar] [CrossRef]
- Hawrylak-Nowak, B. Enhanced Selenium Content in Sweet Basil (Ocimum basilicum L.) by Foliar Fertilization. Veg. Crops Res. Bull. 2008, 69, 63–72. [Google Scholar] [CrossRef]
- Schiavon, M.; Dall’Acqua, S.; Mietto, A.; Pilon-Smits, E.A.H.; Sambo, P.; Masi, A.; Malagoli, M. Selenium Fertilization Alters the Chemical Composition and Antioxidant Constituents of Tomato (Solanum lycopersicon L.). J. Agric. Food Chem. 2013, 61, 10542–10554. [Google Scholar] [CrossRef]
- Smoleń, S.; Baranski, R.; Ledwożyw-Smoleń, I.; Skoczylas, Ł.; Sady, W. Combined Biofortification of Carrot with Iodine and Selenium. Food Chem. 2019, 300, 125202. [Google Scholar] [CrossRef]
- Motti, R.; Bonanomi, G.; Lanzotti, V.; Sacchi, R. The Contribution of Wild Edible Plants to the Mediterranean Diet: An Ethnobotanical Case Study Along the Coast of Campania (Southern Italy). Econ. Bot. 2020, 74, 249–272. [Google Scholar] [CrossRef]
- Ceccanti, C.; Landi, M.; Guidi, L.; Pardossi, A.; Incrocci, L. Seasonal Fluctuations of Crop Yield, Total Phenolic Content and Antioxidant Activity in Fresh or Cooked Borage (Borago officinalis L.), Mallow (Malva sylvestris L.) and Buck’s-Horn Plantain (Plantago coronopus L.) Leaves. Horticulturae 2022, 8, 253. [Google Scholar] [CrossRef]
- Lombardi, T.; Bertacchi, A.; Pistelli, L.; Pardossi, A.; Pecchia, S.; Toffanin, A.; Sanmartin, C. Biological and Agronomic Traits of the Main Halophytes Widespread in the Mediterranean Region as Potential New Vegetable Crops. Horticulturae 2022, 8, 195. [Google Scholar] [CrossRef]
- Bouchmaa, N.; Ben Mrid, R.; Kabach, I.; Zouaoui, Z.; Karrouchi, K.; Chtibi, H.; Zyad, A.; Cacciola, F.; Nhiri, M. Beta Vulgaris Subsp. Maritima: A Valuable Food with High Added Health Benefits. Appl. Sci. 2022, 12, 1866. [Google Scholar] [CrossRef]
- Saini, R.K.; Ko, E.Y.; Keum, Y.-S. Minimally Processed Ready-to-Eat Baby-Leaf Vegetables: Production, Processing, Storage, Microbial Safety, and Nutritional Potential. Food Rev. Int. 2017, 33, 644–663. [Google Scholar] [CrossRef]
- Petroski, W.; Minich, D.M. Is There Such a Thing as “Anti-Nutrients”? A Narrative Review of Perceived Problematic Plant Compounds. Nutrients 2020, 12, 2929. [Google Scholar] [CrossRef]
- Kiani, A.; Sharafi, K.; Omer, A.K.; Matin, B.K.; Davoodi, R.; Mansouri, B.; Sharafi, H.; Soleimani, H.; Massahi, T.; Ahmadi, E. Accumulation and Human Health Risk Assessment of Nitrate in Vegetables Irrigated with Different Irrigation Water Sources- Transfer Evaluation of Nitrate from Soil to Vegetables. Environ. Res. 2022, 205, 112527. [Google Scholar] [CrossRef]
- Puccinelli, M.; Malorgio, F.; Incrocci, L.; Rosellini, I.; Pezzarossa, B. Effects of Individual and Simultaneous Selenium and Iodine Biofortification of Baby-Leaf Lettuce Plants Grown in Two Different Hydroponic Systems. Horticulturae 2021, 7, 590. [Google Scholar] [CrossRef]
- U.S. EPA. Method 3051A (SW-846): Microwave Assisted Acid Digestion of Sediments, Sludges, and Oils; U.S. EPA: San Francisco, CA, USA, 2007. [Google Scholar]
- Cataldo, D.A.; Maroon, M.; Schrader, L.E.; Youngs, V.L. Rapid Colorimetric Determination of Nitrate in Plant Tissue by Nitration of Salicylic Acid. Commun. Soil. Sci. Plant Anal. 1975, 6, 71–80. [Google Scholar] [CrossRef]
- Naik, V.V.; Mahavidyalaya, S.P.K.; Sindhudurg, D. Methodology in Determination of Oxalic Acid in Plnat Tissue: A Comparative Approach. J. Glob. Trends Pharm. Sci. 2014, 5, 1662–1672. [Google Scholar]
- Wellburn, A.R.; Lichtenthaler, H. Formulae and Program to Determine Total Carotenoids and Chlorophylls A and B of Leaf Extracts in Different Solvents. In Advances in Photosynthesis Research; Springer: Berlin/Heidelberg, Germany, 1984; pp. 9–12. [Google Scholar]
- Kim, D.O.; Chun, O.K.; Kim, Y.J.; Moon, H.Y.; Lee, C.Y. Quantification of Polyphenolics and Their Antioxidant Capacity in Fresh Plums. J. Agric. Food Chem. 2003, 51, 6509–6515. [Google Scholar] [CrossRef]
- Kang, H.M.; Saltveit, M.E. Antioxidant Capacity of Lettuce Leaf Tissue Increases after Wounding. J. Agric. Food Chem. 2002, 50, 7536–7541. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority. Tolerable Upper Intake Level on Vitamins and Minerals. Sci. Comm. Food 2006, 33, 480. [Google Scholar]
- European Food Safety Authority. Nitrate in Vegetables—Scientific Opinion of the Panel on Contaminants in the Food Chain. EFSA J. 2008, 6, 689. [Google Scholar] [CrossRef]
- Libert, B.; Franceschi, V.R. Oxalate in Crop Plants. J. Agric. Food Chem. 1987, 35, 926–938. [Google Scholar] [CrossRef]
- Puccinelli, M.; Malorgio, F.; Rosellini, I.; Pezzarossa, B. Uptake and Partitioning of Selenium in Basil (Ocimum basilicum L.) Plants Grown in Hydroponics. Sci. Hortic. 2017, 225, 271–276. [Google Scholar] [CrossRef]
- Francini, A.; Quattrini, E.; Giuffrida, F.; Ferrante, A. Biofortification of Baby Leafy Vegetables Using Nutrient Solution Containing Selenium. J. Sci. Food Agric. 2023, 103, 5472–5480. [Google Scholar] [CrossRef]
- Duarte, B.; Feijão, E.; Pinto, M.V.; Matos, A.R.; Silva, A.; Figueiredo, A.; Fonseca, V.F.; Reis-Santos, P.; Caçador, I. Nutritional Valuation and Food Safety of Endemic Mediterranean Halophytes Species Cultivated in Abandoned Salt Pans under a Natural Irrigation Scheme. Estuar. Coast. Shelf Sci. 2022, 265, 107733. [Google Scholar] [CrossRef]
- Ferrarese, M.; Mahmoodi Sourestani, M.; Quattrini, E.; Schiavi, M.; Ferrante, A. Biofortification of Spinach Plants Applying Selenium in the Nutrient Solution of Floating System. Veg. Crops Res. Bull. 2012, 76, 127–136. [Google Scholar] [CrossRef]
- Wang, C.; Yue, L.; Cheng, B.; Chen, F.; Zhao, X.; Wang, Z.; Xing, B. Mechanisms of Growth-Promotion and Se-Enrichment in Brassica chinensis L. by Selenium Nanomaterials: Beneficial Rhizosphere Microorganisms, Nutrient Availability, and Photosynthesis. Environ. Sci. Nano 2022, 9, 302–312. [Google Scholar] [CrossRef]
- Hawrylak-Nowak, B.; Matraszek, R.; Pogorzelec, M. The Dual Effects of Two Inorganic Selenium Forms on the Growth, Selected Physiological Parameters and Macronutrients Accumulation in Cucumber Plants. Acta Physiol. Plant. 2015, 37, 41. [Google Scholar] [CrossRef]
- Wang, P.; Grimm, B. Connecting Chlorophyll Metabolism with Accumulation of the Photosynthetic Apparatus. Trends Plant Sci. 2021, 26, 484–495. [Google Scholar] [CrossRef]
- Skorupa, M.; Gołȩbiewski, M.; Kurnik, K.; Niedojadło, J.; Kȩsy, J.; Klamkowski, K.; Wójcik, K.; Treder, W.; Tretyn, A.; Tyburski, J. Salt Stress vs. Salt Shock—The Case of Sugar Beet and Its Halophytic Ancestor. BMC Plant Biol. 2019, 19, 57. [Google Scholar] [CrossRef]
- Saeedi, M.; Soltani, F.; Babalar, M.; Izadpanah, F.; Wiesner-Reinhold, M.; Baldermann, S. Selenium Fortification Alters the Growth, Antioxidant Characteristics and Secondary Metabolite Profiles of Cauliflower (Brassica oleracea var. botrytis) Cultivars in Hydroponic Culture. Plants 2021, 10, 1537. [Google Scholar] [CrossRef]
- Shalaby, T.A.; Abd-Alkarim, E.; El-Aidy, F.; Hamed, E.S.; Sharaf-Eldin, M.; Taha, N.; El-Ramady, H.; Bayoumi, Y.; dos Reis, A.R. Nano-Selenium, Silicon and H2O2 Boost Growth and Productivity of Cucumber under Combined Salinity and Heat Stress. Ecotoxicol. Environ. Saf. 2021, 212, 111962. [Google Scholar] [CrossRef]
- Sabatino, L.; Ntatsi, G.; Iapichino, G.; D’anna, F.; De Pasqual, C. Effect of Selenium Enrichment and Type of Application on Yield, Functional Quality and Mineral Composition of Curly Endive Grown in a Hydroponic System. Agronomy 2019, 9, 207. [Google Scholar] [CrossRef]
- Mishra, V.K.; Gamage, T.V. Postharvest Physiology of Fruits and Vegetables. In Handbook of Food Preservation; CRC Press: Boca Raton, FL, USA, 2020; p. 20. ISBN 9780128132784. [Google Scholar]
- European Parliament and Council of the European Union. Commission Regulation (EU) No 1258/2011 of 2 December 2011 Amending Regulation (EC) No 1881/2006 as Regards Maximum Levels for Nitrates in Foodstuffs. Off. J. Eur. Union 2011, 320, 15–17. [Google Scholar]
- Colla, G.; Kim, H.J.; Kyriacou, M.C.; Rouphael, Y. Nitrate in Fruits and Vegetables. Sci. Hortic. 2018, 237, 221–238. [Google Scholar] [CrossRef]
- Golob, A.; Stibilj, V.; Nečemer, M.; Kump, P.; Kreft, I.; Hočevar, A.; Gaberščik, A.; Germ, M. Calcium Oxalate Druses Affect Leaf Optical Properties in Selenium-Treated Fagopyrum Tataricum. J. Photochem. Photobiol. B 2018, 180, 51–55. [Google Scholar] [CrossRef]
- Freidig, A.K.; Goldman, I.L. Variation in Oxalic Acid Content among Commercial Table Beet Cultivars and Related Crops. J. Am. Soc. Hortic. Sci. 2011, 136, 54–60. [Google Scholar] [CrossRef]
- Chang, D.C.; Park, C.S.; Kim, S.Y.; Lee, Y.B. Growth and Tuberization of Hydroponically Grown Potatoes. Potato Res. 2012, 55, 69–81. [Google Scholar] [CrossRef]
- Kopsell, D.A.; Kopsell, D.E.; Curran-Celentano, J. Carotenoid Pigments in Kale Are Influenced by Nitrogen Concentration and Form. J. Sci. Food Agric. 2007, 87, 900–907. [Google Scholar] [CrossRef]
- Lebedev, V.G.; Lebedeva, T.N.; Vidyagina, E.O.; Sorokopudov, V.N.; Popova, A.A.; Shestibratov, K.A. Relationship between Phenolic Compounds and Antioxidant Activity in Berries and Leaves of Raspberry Genotypes and Their Genotyping by SSR Markers. Antioxidants 2022, 11, 1961. [Google Scholar] [CrossRef] [PubMed]
- Muflihah, Y.M.; Gollavelli, G.; Ling, Y.C. Correlation Study of Antioxidant Activity with Phenolic and Flavonoid Compounds in 12 Indonesian Indigenous Herbs. Antioxidants 2021, 10, 1530. [Google Scholar] [CrossRef]
- Aryal, S.; Baniya, M.K.; Danekhu, K.; Kunwar, P.; Gurung, R.; Koirala, N. Total Phenolic Content, Flavonoid Content and Antioxidant Potential of Wild Vegetables from Western Nepal. Plants 2019, 8, 96. [Google Scholar] [CrossRef]
- Li, M.; Pare, P.W.; Zhang, J.; Kang, T.; Zhang, Z.; Yang, D.; Wang, K.; Xing, H. Antioxidant Capacity Connection with Phenolic and Flavonoid Content in Chinese Medicinal Herbs. Rec. Nat. Prod. 2018, 12, 239–250. [Google Scholar] [CrossRef]
- Mezeyova, I.; Hegedusova, A.; Andrejiová, A.; Mezeyová, I.; Hegedűsová, A.; Hegedűs, O.; Golian, M. Phytomass and Content of Essential Oils in Ocimum basilicum after Foliar Treatment with Selenium. Agric. Food 2016, 4, 19–27. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).