Abstract
In this study, we propagated two old Galician plum varieties in liquid medium using a temporary immersion system with RITA© bioreactors. Environmental variables including culture system, light intensity, CO2 enrichment, immersion frequency and sucrose supplementation were evaluated in relation to in vitro proliferation, physiological status and ex vitro performance. Bioreactors were superior to jars for culturing shoots in photomixotrophic conditions, producing up to 2 times more shoot numbers and up to 1.7 times more shoot length (depending on the genotype) using shoot clusters. The number and quality of shoots were positively influenced by the sucrose concentration in the medium, plus by the light and gaseous environment. For individual apical sections the best response occurred with 3% sucrose, 150 µmol m−2 s−1 photosynthetic photon flux density and 2000 ppm CO2, averaging 2.5 shoots per explant, 26 mm shoot length and 240 mm2 leaf area, while with 50 µmol m−2 s−1 light and ambient CO2 (400 ppm) values decreased to 1.2 shoots per explant, 14 mm of shoot length and 160 mm2 of leaf area. Shoots cultured photoautotrophically (without sucrose) were successfully rooted and acclimated despite of showing limited growth, low photosynthetic pigments, carbohydrate, phenolic and antioxidant contents during the multiplication phase.
1. Introduction
Plums include a large and diverse group of closely related Prunus species of the Rosaceae family. European plum (P. domestica L.) and Japanese plum (P. salicina Lindl) are currently the most globally cultivated plum species. China is the leading producing country, followed by Romania, Serbia, Chile, Iran, the USA, Turkey, Italy, France, Ukraine and Spain [1]. As many other cultivated Prunus spp. fruit trees, plums show limited intra-specific genetic variability [2]. The biodiversity loss started with the process of domestication and was exacerbated by clonal propagation, a narrow parentage range for breeding and selection of similar fruit attributes for agronomic, processing and commercial reasons [3]. Reduced genetic variability diminishes the breeding potential and increases the vulnerability to pests, diseases and environmental change [4]. Genotyping projects carried out in several European countries revealed that local cultivars often differ considerably from widespread international cultivars [5], probably because old traditional cultivars and wild relatives have been subjected to less artificial selection pressures [6]. Local varieties may show low yield and not have outstanding agronomic characteristics, but their allelic diversity provides a gene-pool reservoir for breeding for organoleptic characteristics and for adaptation in a changing environment [7,8,9,10]. The potential value of most local genotypes for current market needs is largely unknown, since they have not been sufficiently characterized from an genetic, agronomic and consumer point of view [11].
In the present study, we focus on two local plum varieties from Galicia, located in the northwest of Spain. Galicia is one of the regions where the coexistence of forest areas with small but numerous traditional farms favored during the past centuries has contributed to the maintenance of an extensive forest and agricultural and horticultural biodiversity [12,13,14]. In the last century, large-scale commercialization of food in general and of fruits in particular led to the introduction of new varieties. Plants were selected for larger size and higher yields, which displaced the traditional crops and their associated cultural methodologies. As a result, in many regions local varieties of fruits have a narrow range of distribution and are linked to self-supply practices. Together with the continuous decrease in the population of rural areas, this has promoted a rapid global genetic erosion of agrobiodiversity. There is an urgent need to preserve as much of the current fruit tree diversity as possible to prevent the irretrievable loss of this heritage [13,15]. There are breeding programs in some countries that could use this old genetic material [1,16].
Ex situ conservation of the existing genetic resources is one strategy recommended to alleviate this situation [17]. Within ex situ conservation, micropropagation is the methodology of choice in the case of species or genotypes such as plums, which are propagated vegetatively by budding, grafting or cuttings [18,19]. The first micropropagation protocols in semisolid medium were reviewed by Druart and Gruselle as early as 1986 [20]. In 1991, Druart pointed out the importance of reliable micropropagation protocols for plums to enhance the commercial propagation of virus-free plants [21]. This author also highlighted the need to develop new micropropagation protocols that could be automated. These protocols would make the whole process of in vitro plant production more economically viable and competitive with traditional vegetative propagation. However, the protocols developed afterwards were still based in semisolid medium [22,23,24,25,26]. More recently, liquid medium has been used in bioreactors as an alternative to agar-based protocols, this has the potential to enhance the quality of micropropagated plants and facilitates automation [27]. This technology has been successfully applied to a range of woody species [28], however, to the best of our knowledge it has not been used with European plum (P. domestica). In this study, we proliferated plum in a liquid medium by a temporary immersion system using commercial RITA© bioreactors and explored the feasibility of culturing plum shoots without sucrose supplementation. Variables such as culture system, growth conditions (light intensity and CO2 enrichment), frequency of immersion and sucrose supplementation were evaluated in relation to in vitro proliferation, physiological status and ex vitro performance.
The main goal was to provide information that could be developed into a commercially applicable protocol for rapid propagation of plum trees and may also be useful in rescuing rare or endangered plum germplasm. The application of bioreactors for this work represents a novel approach to plum propagation.
2. Materials and Methods
2.1. Establishment of Shoot Multiplication Cultures
Shoots of P. domestica, old Galician landrace varieties “Claudia Blanca País” (CBP) and “Collón de Frade Negro” (CFN) were collected from trees growing in the research orchard of the “Asociacion Galega da Froita Autóctona do Eume”, San Sadurniño, A Coruña, Spain (Figure 1). CBP is a productive small to medium-sized plum tree. The fruits ripen during August, having a green-yellowish skin occasionally with a pink tinge and some red spots, the flesh is light yellow, very sweet and tasty. CFN is a vigorous and productive tree with fruit maturing at the end of August. Oval fruits are normally black skinned with greenish flesh that is tasty and a bit sour close to the skin.
Figure 1.
Plant material for establishment of plum cultures. (a) Orchard of the “Asociación Galega da Froita Autóctona do Eume”, (b) Collón de Frade Negro (CFN) genotype and (c) Claudia Blanca País (CBP) genotype.
Cultures were initiated from branches with dormant buds collected in early spring from 4-year-old trees following the protocols described in Sánchez and Vieitez [29] with minor nutrient modifications. After fungicide treatment (copper oxychloride), branch segments 25- to 30-cm-long were set upright in moistened perlite and forced axillary buds to flush in a growth cabinet at 25 °C and 80–90% relative humidity with a 16 h photoperiod (90–100 µmol m−2 s−1 provided by cool-white fluorescent lamps). After 2–3 weeks, recently sprouted shoots (3–5 cm in length) were used as the source of explants. The shoots were stripped of their leaves and surface sterilized by immersion for 30 s in 70% ethanol and for 10 min in a 6 g L−1 solution of sodium hypochlorite containing 2–3 drops of Tween 80®. The shoots were then rinsed three times in sterile distilled water. Nodal segments (10 mm) were cut from the shoots and inoculated in tubes with semisolid medium (SSM). Culture medium consisted in Murashige and Skoog medium (MS) [30] supplemented with 0.5 mg L−1 of N6-benzyladenine (BA), 0.5 mg L−1 of indole-3-butyric acid (IBA), 3% sucrose and 0.65% (w/v) agar Vitroagar (Pronadisa, Spain). The medium was adjusted to pH 5.7 before being autoclaved at 120 °C for 20 min. Cultures were incubated under a 16 h photoperiod provided by cool-white fluorescent lamps (photosynthetic photon flux density (PPF) from 50 to 60 µmol m−2 s−1) at 25 °C light/20 °C dark (Standard conditions; ST). The explants were transferred every 2 weeks during the first 6 weeks after establishment. Thereafter, the explants were maintained in 50 mL of the medium described above in 300 mL glass jars (8 explants per jar) and were subcultured every 5 weeks. Preliminary work with the two plum clones indicated that they were indistinguishable and could be treated as one and the same.
2.2. Culture System, Hormonal Supplementation and Frequency of Immersion
To study the proposed variables, we performed two experiments. In the first one, clusters (15–20 mm high) with 3–4 shoots (Figure 2a) of CFN genotype were excised from shoots growing in jars in SSM. These initial explants (8 per container) were cultured in jars or RITA© vessels (Vitropic, Saint Mathieu de Tréviers, France) under ST conditions and were subcultured every 5 weeks. In jars, the explants were cultured with 50 mL of SSM, and in RITA© with 150 mL of liquid medium (LM) of the same composition as SSM without agar and immersed for 1 min 3 times per day. The proliferation medium consisted of MS supplemented with 3% sucrose, 0.65% agar and (i) 0.2 mg L−1 BA + 0.2 mg L−1 IBA, (ii) 0.2 mg L−1 BA + 0.5 mg L−1 IBA and (iii) 0.5 mg L−1 BA + 0.5 mg L−1 IBA.
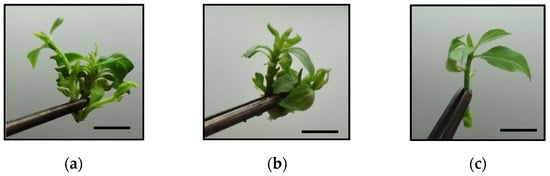
Figure 2.
(a) Cluster of CFN shoots used as initial explant for experiments regarding the culture system and the hormonal concentration of culture media, (b) cluster of CBP shoots used as initial explant for experiments regarding the culture system and the immersion frequency and (c) apical section of CBP used as initial experiment regarding growth conditions and sucrose supplementation. Bar: 10 mm.
In the second experiment, clusters of similar size as described above were excised from shoots of the CBP genotype growing in jars and were cultured in jars or RITA© vessels with MS supplemented with 0.5 mg L−1 BA + 0.5 mg L−1 IBA and 3% sucrose (Figure 2b). Agar (0.65%) was added to the jars. In RITA©, shoots were immersed for 1 min 3 or 6 times per day. After 6 weeks of culture under ST conditions, the morphological data were recorded and shoots were used for rooting experiments.
2.3. Growth Conditions and Sucrose Supplementation
Apical sections (20 mm) of CBP genotype (Figure 2c) were cultured in RITA© and were immersed in LM supplemented with 0, 1 or 3% sucrose for 1 min 6 times per day. The explants were cultured under ST conditions and with ambient CO2 (~400 ppm) or in an experimental unit previously designed for photoautotrophic micropropagation (PAM) of chestnut [31]. In the PAM experimental unit, the cultures grew under high PPF (150 µmol m−2 s−1), and CO2-enriched air (~2000 ppm) was injected to the bioreactors during immersion. The photoperiod and temperature regime were the same as under ST conditions. After 6 weeks of subculture morphological data were recorded and shoots were used for rooting experiments or biochemical analysis (monosaccharides, photosynthetic pigments, soluble phenolic compounds and antioxidant activity).
2.3.1. Biochemical Quantifications
Soluble Monosaccharides
We used the dinitrosalicylic acid (DNS) method [32,33]. Briefly, 100–150 mg of apical leaves was homogenized with 2 mL of distilled water and centrifuged for 5 min at 10,000× g. The supernatant was collected, mixed with DNS, heated in a thermoblock at 100 °C for 5 min and placed on ice for 5 min before quantitation in a spectrophotometer at 540 nm. The results were expressed as glucose equivalents on a fresh weight basis.
Total Soluble Sugars
Total soluble carbohydrates were determined by the anthrone method [34]. Fresh leaves (100 mg) were homogenized in 2 mL water and centrifuged at 10,000× g for 5 min. Samples (250 µL) were treated with 750 µL of ice-cold anthrone reagent (1 g/L in 96% H2SO4). The reaction mixture was heated in a water bath (40 °C) for 40 min and rapidly cooled to 0 °C. Absorbance was measured at 620 nm. The results were expressed as sucrose equivalents on a fresh weight basis.
Photosynthetic Pigments
The two uppermost fully expanded leaves per explant were collected, weighed and extracted with dimethylformamide. Chlorophyll a, b and total carotenoids were quantified using the method described by Wellburn [35].
Total Soluble Phenolic Compounds
The extraction was performed according to [36]. Individual shoots were collected, and leaves were homogenized with methanol 80%. The mixture was centrifuged at 10,000× g for 5 min and the supernatant used for analysis of soluble phenolic compounds and antioxidants.
Total soluble phenolic compounds were assayed using the Folin–Ciocalteau method [37]. The soluble phenols content was calculated from a standard curve based on gallic acid different concentrations and results were expressed as gallic acid equivalents on a fresh weight basis.
Antioxidant Activity
The extraction was performed as described for soluble phenols. Antioxidant activity of plant extracts was determined through spectrophotometry using a 1,1-diphenyl-2-picrylhydrazyl (DPPH) scavenging radical assay [38], and results were expressed as TROLOX mM equivalents per g on a fresh weight basis.
2.4. Rooting and Acclimatization
Shoots of CBP and CFN genotypes (longer than 15 mm) cultured in jars or bioreactors with 3% sucrose were treated with MS with the macronutrients reduced to half strength (½MS) with 25 mg L−1 IBA, 0.7% agar and 3% sucrose. After 24 h, shoots were transferred to jars with SSM of the same composition but without IBA. Alternatively, shoots were inserted in rockwool cubes (2 cm of side) soaked in liquid medium (Figure 3a) and introduced in Plantform™ bioreactors (Plantform, Hjärup, Sweden) without the inner baskets (Figure 3b,c). Plantforms™ were aerated for 1 min at a frequency of 6 times every 24 h for 6 weeks.
Figure 3.
(a,b) Shoots of CBP inserted in rockwool cubes for rooting expression and (c) shoots inserted in a Plantform™ bioreactor without the inner basket.
Shoots of CBP cultured in bioreactors with different sucrose were treated with ½MS with 25 mg L−1 IBA, 0.7% agar and the same sucrose concentration that was present in the multiplication medium. After 24 h, shoots were transferred to IBA-free medium for rooting expression and distributed between the three sucrose treatments. Shoots were inserted in rockwool cubes and placed in Plantform™ bioreactors as described above.
Rooted shoots were transferred to plastic plug trays (size of the plugs 52 × 52 mm by 60 mm height) filled with a peat:perlite (3:1) mixture and placed in a controlled environmental chamber (Fitotron SGC066, Sanyo Gallencamp PLC, Leicestershire, UK) with a photoperiod of 16 h light:8 h dark, a photosynthetic photon flux from 240–250 µmol m−2·s−1 and a temperature of 25 °C (day) and 20 °C (night), with a relative humidity of 85%. After 3 weeks in the phytotron, plantlets were transferred to 1.3 L pots and placed into a greenhouse between April and June. The percentage of survival and plantlet growth were recorded when transferred to the greenhouse and 4 weeks later.
2.5. Data Recording and Statistical Analysis
The parameters analyzed were: (a) the number of normal shoots longer than 15 mm produced by each explant (NS); (b) the length of the longest shoot per explant (SL); (c) the length (LL) and width (LW) of the largest leaf per explant; (d) leaf biomass; (e) monosaccharides; (f) total soluble sugars; (g) total soluble phenolic compounds; (h) total antioxidant activity; (i) photosynthetic pigments; (j) rooting and acclimatization percentage. Leaf area was calculated as ½ LL*LW.
For proliferation experiments, three jars or RITA© vessels (24 explants) were used for each treatment and the experiments were repeated twice. For rooting experiments, 18 shoots per treatment were used.
The data were analyzed by Levene’s test (to verify the homogeneity of variance) and were then subjected to analysis of variance (ANOVA), followed by comparison of group means (Tukey-b test), or to the Welch ANOVA, followed by Games–Howell post-hoc comparison (when heteroscedasticity was detected). When an interaction between two factors was indicated by the two-way ANOVA, Bonferroni’s adjustment was applied to detect the simple main effects in multiple post-hoc comparisons. Statistical analyses were performed using SPSS 26.0 (IBM).
3. Results
3.1. Culture System and Hormonal Composition of the Media
Plum shoots grew successfully in jars and bioreactors (Figure 4), but the culture system and the hormonal composition of the media significantly influenced the performance of shoots. The best results for number and length of shoots and leaf size were obtained in bioreactors (Figure 5). MS medium supplemented with 0.5 mg/L BA and IBA produced more and longer shoots (Figure 5a,b), whereas the size of leaves decreased when more BA and IBA were added to the medium (Figure 5c,d).

Figure 4.
CFN shoots cultured in jars (a) and in bioreactors (b) in MS supplemented with 0.5 mg L−1 BA, 0.5 mg L−1 IBA and 3% sucrose.
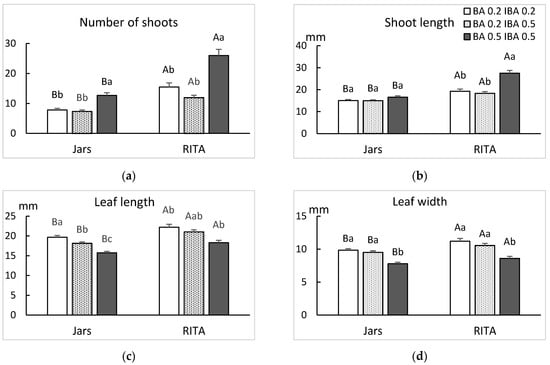
Figure 5.
Effect of the hormonal composition of the media and the culture system on the growth of the plum genotype CFN. (a) Number of shoots, (b) shoot length, (c) length and (d) width of the largest leaf. Different uppercase letters indicate significant differences between culture system and different lowercase letters indicate significant differences between the hormonal treatments (p < 0.05).
3.2. Culture System and Number of Immersions
Table 1 shows the effect of culturing the CBP genotype in jars or in RITA© bioreactors with 3 or 6 immersions per day. The use of bioreactors significantly increased all the growth parameters. The highest values were obtained with six immersions, although significant differences between the two immersion frequencies were only detected in leaf length.

Table 1.
Effect of the number of immersions and the culture system on the growth of the plum genotype CBP cultured with 3% sucrose. (a) Shoot length (SL), length (LL) and width (LW) of the largest leaf; (b) Number of shoots (NS). Data are mean values and standard error of 48 explants. Different letters indicate significant differences (p < 0.05).
3.3. Growth Conditions and Sucrose Supplementation
The number and quality of shoots were significantly affected by the sucrose concentration in the medium and by the light and gaseous environment (Figure 6).
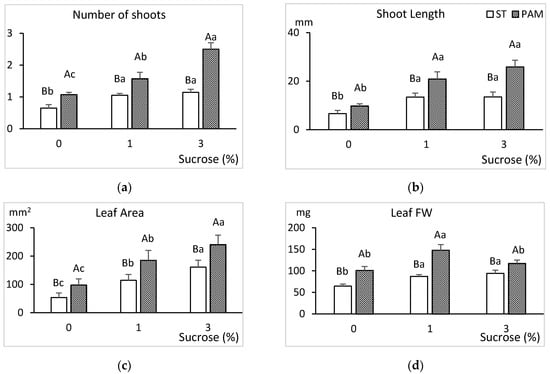
Figure 6.
Effect of growth conditions and sucrose supplementation on the growth of the genotype CBP cultured in RITA© with 6 immersions/day. (a) Number of shoots, (b) shoot length (mm), (c) leaf area and (d) leaf biomass. Different uppercase letters indicate significant differences in relation to the growth conditions, and different lowercase letters indicate significant differences in relation to the sucrose supplementation (p < 0.05). ST: standard conditions, PAM: photoautotrophic conditions and FW: fresh weight.
The best response was obtained in PAM conditions for all the sucrose treatments (Figure 6). Sucrose significantly enhanced the growth of shoots and leaves, and the best response growth responses were obtained with 1–3% sucrose in PAM conditions. Shoots cultured in PAM with 1% sucrose produced more and longer shoots, as well as larger leaves than shoots cultured in ST conditions with 3% sucrose. Explants cultured without sucrose showed limited growth, especially in ST conditions. Frequently, only 20–30% of the explants produced vigorous shoots longer than 15 mm, meaning that shoots suitable for the rooting experiments were barely obtained. For this treatment, the average number of new shoots (0.7) and their short height (7 mm average) were too small for sub-culturing. Leaves of shoots grown without sucrose had the lowest concentration of photosynthetic pigments. Pigments increased which sucrose supplementation but were not significantly influenced by growth conditions (Figure 7).
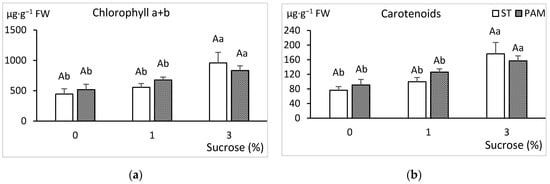
Figure 7.
Effect of growth conditions and sucrose supplementation on the photosynthetic pigments of leaves of the genotype CBP cultured in RITA© with 6 immersions/day. (a) Chlorophyll a+ chlorophyll b and (b) total carotenoids. Different uppercase letters indicate significant differences in relation to the growth conditions, and different lowercase letters indicate significant differences in relation to the sucrose supplementation (p < 0.05). ST: standard conditions, PAM: photoautotrophic conditions and FW: fresh weight.
Carbohydrate content in leaves was influenced by the sucrose treatment and its interaction with the growth conditions (Figure 8).
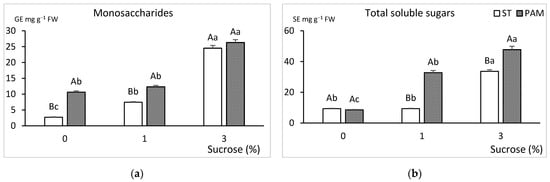
Figure 8.
Effect of growth conditions and sucrose supplementation on the carbohydrate concentration of leaves of genotype CBP cultured in RITA© with 6 immersions/day. (a) Monosaccharides and (b) total soluble sugars. Different uppercase letters indicate significant differences in relation to the growth conditions, and different lowercase letters indicate significant differences in relation to the sucrose supplementation (p < 0.05). ST: standard conditions, PAM: photoautotrophic conditions and FW: fresh weight. SE: sucrose equivalents and GE: glucose equivalents.
Generally, carbohydrate content (Figure 8a,b) increased with sucrose supplementation and with PAM conditions. There were marked differences between the highest and the lowest concentrations of total soluble sugars and monosaccharides. In monosaccharides, the lowest content was observed in shoots cultured without sucrose in ST conditions. The highest values were for 3% sucrose, with no significant differences between growth conditions (Figure 8a). Total sugars followed a similar trend to the monosaccharides (Figure 8b).
Soluble phenolic compounds and antioxidant activity were quantified to estimate the shoot stress levels in different growth conditions and with different concentrations of sucrose (Figure 9). In ST conditions sugar enhanced phenolic production, but this was not reflected consistently in PAM conditions (Figure 9a). Regarding antioxidants, in the PAM treatment the observed levels were elevated with increased sugar content and were also higher than the corresponding ST treatments (Figure 9b).
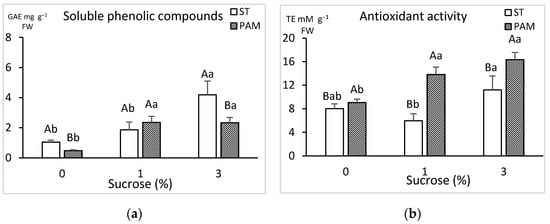
Figure 9.
Effect of growth conditions and sucrose supplementation on the phenolic and antioxidant concentration of leaves of genotype CBP cultured in RITA© with 6 immersions/day. (a) Total soluble phenolics and (b) total antioxidant activity. Different uppercase letters indicate significant differences in relation to the growth conditions, and different lowercase letters indicate significant differences in relation to the sucrose supplementation (p < 0.05). ST: standard conditions, PAM: photoautotrophic conditions and FW: fresh weight. GAE: gallic acid equivalents and TE: Trolox equivalents.
3.4. Effect of the Culture System on Rooting and Acclimation
Shoots of CFN and CBP genotypes cultured in jars or RITA© with 3% sucrose were successfully rooted and acclimated irrespective of the culture system. Six weeks after auxin treatment, the length of the rooted shoots ranged from 30 to 40 mm, and 6 weeks later their height was 10-fold longer, averaging 37 cm (Figure 10 and Figure 11).

Figure 10.
Shoots of CBP cultured in jars and RITA© with 3% sucrose and rooted in jars. (a) Shoots proliferated in jars 6 weeks after auxin treatment and (b,c) shoots proliferated in RITA© (6 immersions/day) 12 weeks after auxin treatment.

Figure 11.
Shoots of CFN cultured in jars and RITA© with 3% sucrose and rooted in rockwool cubes. (a) Shoots proliferated in jars 6 weeks after auxin treatment and (b,c) plantlets from shoots proliferated in RITA© after being transferred to the phytotron (b) and 3 months later in the greenhouse (c).
Rooting and acclimation responses averaged 90%, and we did not observe differences in the percentages of shoots rooted in SSM or in rockwool cubes. Shoots rooted in cubes were easier to handle without compromising the integrity of the roots, whereas some roots were broken when the shoots were extracted from the jars, and cubes were used thereafter.
3.5. Effect of Sucrose Supplementation on Rooting and Acclimation
Figure 12 and Figure 13 show the rooting response of CBP shoots cultured in ST or PAM with 0, 1 or 3% sucrose and rooted in rockwool cubes soaked with medium containing 0, 1 or 3% sucrose. Figure 12 shows quantitative data and Figure 13 the appearance of the shoots after rooting and during acclimation. None of the three investigated factors (growth conditions, sucrose during multiplication and rooting) had a significant effect on rooting (p values were 0.238, 0.536 and 0.128 for growth conditions, sucrose during multiplication and sucrose during rooting, respectively), and shoots from all the treatments rooted and acclimated successfully (Figure 12 and Figure 13). However, when shoots were multiplied without sucrose the proportion of rootable shoots was lower than in the rest of treatments, meaning more bioreactors had to be used to obtain enough shoots for the rooting experiments.
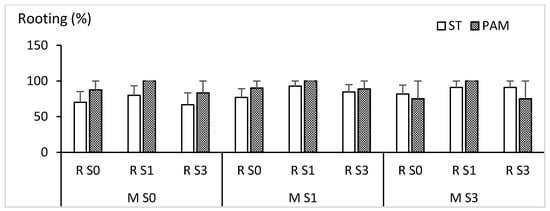
Figure 12.
Shoots of CBP cultured in RITA© with 0–3% sucrose in ST or PAM conditions and rooted in rockwool cubes soaked with 0–3% sucrose. ST: standard conditions and PAM: photoautotrophic conditions. M S0, M S1 and M S3: sucrose percentage (0, 1 or 3%) of the multiplication medium in RITA© bioreactors. R S0, R S1 and R S3: sucrose percentage (0, 1 or 3%) of the rooting expression medium.

Figure 13.
(a,b) Shoots of CBP cultured in RITA© without sucrose in PAM conditions and rooted in rockwool cubes soaked with 1% (a) and 3% sucrose (b); (c–e) shoots cultured in ST with 1% sucrose and rooted with 0, 1 and 3% sucrose 6 weeks after root induction (c), after 2 weeks in the phytotron (d) and after 2 months in the greenhouse (e). ST: standard conditions and PAM: photoautotrophic conditions.
4. Discussion
In the present study, we demonstrated the feasibility of micropropagating two local plum varieties from the northwest of Spain. We obtained high proliferation and rooting rates and the plantlets were successfully acclimated. The multiplication of both clones was more efficient in bioreactors than in jars with semisolid medium. Similar results have been reported for other woody plants such as calabash tree [39], eucalyptus [40], apple [41], teak [42,43], pistachio [44], chestnut [45], hazelnut [46], yerba mate [47], willow [48], olive [49], alder [50] and pear [51].
Most bioreactors include forced ventilation systems, which increase gas exchange [28,52]. It has been reported that this feature promotes the photosynthetic ability of tissues, enabling the decrease or even the elimination of the conventional sugar supplementation [53,54]. For this reason, bioreactors have been used for the photoautotrophic propagation of several plants including eucalyptus [55,56,57,58], apple [59], poplar [60], bamboo [61] and willow [62], but to the best of our knowledge there are no reports of photoautotrophic protocols for plum or any other Prunus species. In the current study, we explored the feasibility of using commercial RITA© bioreactors for the propagation of plum photoautotrophically (without exogenous sugars) in an experimental unit which provided good results with willow [62]. Previously, we carried out some experiments photomixotrophically (using 3% sucrose) to find the best growing conditions for the plum genotypes under study.
In the photomixotrophic using shoot clusters as initial explants, the shoot number and shoot length were the highest in treatments with BA 0.5 and IBA 0.5, whereas leaf size was negatively correlated. Typically, leaf size decreases with increasing cytokinin supplementation [42,63,64]. Increasing exposure of the plant material to more immersions of up to six per day increased plant growth, whereas using more frequent immersion produced hyperhydric shoots (data not shown). Immersion every 3–6 h has been successfully used for other tree species such as calabash tree [39], Fraxinus mandshurica [65], teak, chestnut [45], yerba mate [47], willow [48,62] and apple [66].
For photoautotrophic propagation, we used the best conditions ascertained above, but we selected apical sections as initial explants. This material does not produce as many shoots as clusters, but it is more uniform and maybe less affected by the carry-over as it is more distant from the previous nutritional conditions. In these experiments, we found that sucrose supplementation affected beneficially the number and quality of shoots. Using high light intensity and CO2 enrichment provided further improvement in growth to explants cultured with 1 and 3% sucrose, but it did not compensate for the absence of sugar supplementation. The explants could be grown photoautotrophically (without sucrose), but the number and size of shoots limited the practical and future value of this option. Increased gaseous exchange enabled shoot growth of Paulownia fortunei [67], Samanea saman [68], Eucalyptus spp. [55,56,57,58], Macadamia tetraphylla [69], Bambusa vulgaris [61] and Salix viminalis [62] in media with low sucrose or without any supplementary carbohydrate, whereas the exposure of Vernonia condensata [70], Fraxinus mandshurica [65], Juglans regia [71], Populus deltoides [72] and Pfaffia glomerata [73] to these environmental conditions resulted in a limited growth in sucrose-free medium and a beneficial effect of sucrose supplementation, as observed in plum.
It has been claimed that culturing plants in low sugar or no sugar media can easily enhance photosynthetic competence [74], but the results of this study do not corroborate this hypothesis. Carbohydrates are the direct products of photosynthesis, and in our study the highest carbohydrate accumulation occurred in leaves of shoots grown with 3% sucrose. Shoots cultured without sucrose showed limited growth and low sugar content, possibly indicating a low photosynthetic competence. In V. condensata, [70] Solanum tuberosum [74,75] and Nicotiana tabacum shoots [74] accumulated more carbohydrates when they were cultured with sucrose. Interestingly, in tobacco and potato, the low content of sugars observed in plants cultured without sucrose was associated with high photosynthetic capacity and successful growth [74], whereas in plum the shoots cultured without sucrose showed the lowest sugar content and limited growth, possibly indicating a low photosynthetic competence. In our experimental conditions, photosynthetic pigments increased with sugar but not with additional light or CO2 provided by the PAM system and did not correlate exactly with the growth of the explants. Similarly, in V. condensata, total chlorophyll content was higher in shoots cultured with sugar but did not follow the same trend of shoot length or biomass accumulation, although in that study photosynthetic pigments did increase with CO2 exchange [70]. A poor correlation between photosynthetic pigments and growth in addition to this study has also been reported for other plants such as myrtle [76], chestnut [77], apple [66], tobacco, potato, strawberry and rapeseed [74].
Plants produce reactive oxygen species throughout their life cycle. The environmental conditions of micropropagated plants differ from those found in nature and can increase this stress, potentially causing cell damage [78]. Plants counteract reactive oxygen species through enzymatic and non-enzymatic mechanisms [78,79,80]. Within non-enzymatic antioxidants, phenolics play a major role in defense against reactive oxygen species. Their concentration in explants subjected to different culture conditions may provide useful information about the plant’s physiological state [81]. In the current study, we quantified phenolic compounds and antioxidant activity to estimate the influence of sucrose and the growth conditions on the stress levels of plum cultured in bioreactors. Within each growth conditions (ST or PAM), we observed higher phenol content and antioxidant activity with increased sucrose in the culture medium. The addition of sucrose to V. condensata leads to an increase in phenolic and flavonoid compounds [70], and in bamboo [82], rose [83], teak [42] and olive [84], the phenol content was associated with treatments producing more and longer shoots.
In plum, growth and biochemical parameters suggest that shoots cultured without sucrose undergo more stress than shoots cultured with this sugar. However, the limited proliferation and the low content in pigments, carbohydrates, phenols and total antioxidants did not hinder rooting and acclimation of shoots propagated without sucrose. We did not observe significant differences between rooting performance of shoots multiplied or rooted with sucrose 0, 1 or 3%, the only inconvenience of the use of sucrose-free medium being the low proportion of vigorous shoots from these treatments. Rooting percentages were high, averaging 81, 89 and 86% for shoots multiplied without sucrose, with 1% and 3% sucrose, respectively. Shoots multiplied with high light intensity and CO2 enrichment rooted slightly better than those multiplied in ST conditions (89 versus 82%), but these differences were not significant. Likewise, the use of semisolid medium or rockwool cubes soaked in liquid medium did not affect the rooting and acclimation percentages; the best advantage of using rockwool cubes was to facilitate the handling of shoots during transfer to substrate without causing root damage. The use of fibrous or porous support materials for rooting has been recommended as a simple and cost effective means of micropropagation [85,86] and was beneficial for other plants such as sweet potato [87], American and European chestnut [88,89], cannabis [90] and peach [91], and our results demonstrate their applicability to plum as a way to facilitate large-scale propagation.
Reports on P. domestica micropropagation include commercial [20,21,24] and local varieties faced with varying degrees of vulnerability [22,23,26,92], and to the best of our knowledge, all these methods have been developed using semisolid medium. Even with a fully prescribed/defined protocol, in these conditions production is limited by the size of containers and physical manipulation restrictions [21] and is not easily scaled up and commercialized. To date, the use of liquid medium for the micropropagation of Prunus species has been limited to P. avium [93], P. armeniaca [94], P. cerasifera [95] and a hybrid rootstock of P. cerasifera and P. dulcis [96]. Published results are consistent with our higher proliferation rates found in temporary immersion bioreactors compared with semisolid medium. In our study, we investigated the effect of high light intensity and CO2 enrichment, which provided further improvement in growth and enabled us to reduce sucrose concentration. This protocol can be applied to shoot clusters instead of individual apical sections of plum, which increases proliferation, and be extended to other fruit trees and to larger bioreactors used for commercial applications, making the micropropagation process more economically viable. The medium-sized RITA© bioreactors (1L) of this study allowed us to test several treatments with a limited amount of plant material. This approach can be useful for fine-tuning protocols to cope with genotypic differences in micropropagation requirements, which are an ongoing challenge for fruit trees.
5. Conclusions
The use of RITA© bioreactors for plum shoot micropropagation was highly beneficial. The best multiplication rates were obtained with clusters of shoots, which were immersed for 1 min six times per day in MS BA 0.5 mg L−1 IBA 0.5 mg L−1 with 3% sucrose and cultured with PPF 50 µmol m−2 s−1 and ambient air. When individual apical sections were used as initial explants, growth was enhanced by using media with 1% or 3% sucrose, PPF 150 µmol m−2 s−1 and CO2-enriched air. The absence of sucrose decreased growth and increased the levels of stress indicators, especially when shoots were cultured under 50 µmol m−2 s−1 PPF and ambient air. However, these shoots reacted positively to rooting and were successfully acclimated.
This is the first report in the photoautotrophic micropropagation of Prunus domestica. By using bioreactors, we obtained successful results for rooting and acclimation, whereas the multiplication phase in medium without sugar has to be improved before being more widely applicable.
Author Contributions
Conceptualization, N.V., M.Á.B. and C.S.; formal analysis, D.G. and N.V.; investigation, D.G., N.V. and A.A.; writing—original draft, D.G., C.B.C. and N.V.; writing—review and editing, D.G., C.B.C. and N.V.; funding acquisition, N.V., M.Á.B. and C.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Xunta de Galicia (Spain) (project IN607A 2021), by CYTED (P117RT0522), and by CSIC (PIE 202140E015, COOPB20584).
Data Availability Statement
Not applicable.
Acknowledgments
We thank Beatriz Cuenca (Maceda Nursery, TRAGSA) for her advice on photoautotrophic micropropagation, Mª José Cernadas for technical assistance and the Asociación Galega de Froita Autóctona do Eume for providing the plant material. We dedicate this article to the memory of our dear colleague and friend, Brais Bogo Graña, who started the experiments in bioreactors but died prematurely before this research was completed.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sottile, F.; Caltagirone, C.; Giacalone, G.; Peano, C. Unlocking Plum Genetic Potential: Where Are We At ? Horticulturae 2022, 8, 128. [Google Scholar] [CrossRef]
- Zhebentyayeva, T.; Shankar, V.; Scorza, R.; Callahan, A.; Ravelonandro, M.; Castro, S.; DeJong, T.; Saski, C.A.; Dardick, C. Genetic characterization of worldwide Prunus domestica (plum) germplasm using sequence-based genotyping. Hortic. Res. 2019, 6, 12. [Google Scholar] [CrossRef]
- Miller, A.J.; Gross, B.L. From forest to field: Perennial fruit crop domestication. Am. J. Bot. 2011, 98, 1389–1414. [Google Scholar] [CrossRef]
- Goldschmidt, E.E. The Evolution of Fruit Tree Productivity: A Review. Econ. Bot. 2013, 67, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Gaši, F.; Sehic, J.; Grahic, J.; Hjeltnes, S.H.; Ordidge, M.; Benedikova, D.; Blouin-Delmas, M.; Drogoudi, P.; Giovannini, D.; Höfer, M.; et al. Genetic assessment of the pomological classification of plum Prunus domestica L. accessions sampled across Europe. Genet. Resour. Crop Evol. 2020, 67, 1137–1161. [Google Scholar] [CrossRef]
- Lansari, A.; Kester, D.E.; Iezzoni, A.F. Inbreeding, coancestry, anf founding clones of almonds of California, Mediterranean shores, and Russia. J. Am. Soc. Hortic. Sci. 1994, 119, 1279–1285. [Google Scholar] [CrossRef]
- Maxted, N.; Kell, S.; Ford-Lloyd, B.; Dulloo, E.; Toledo, Á. Toward the systematic conservation of global crop wild relative diversity. Crop Sci. 2012, 52, 774–785. [Google Scholar] [CrossRef]
- Caballero, A.; García-Dorado, A. Allelic diversity and its implications for the rate of adaptation. Genetics 2013, 195, 1373–1384. [Google Scholar] [CrossRef]
- Marconi, G.; Ferradini, N.; Russi, L.; Concezzi, L.; Veronesi, F.; Albertini, E. Genetic characterization of the apple germplasm collection in central Italy: The value of local varieties. Front. Plant Sci. 2018, 9, 1460. [Google Scholar] [CrossRef] [PubMed]
- Aranzana, M.J.; Decroocq, V.; Dirlewanger, E.; Eduardo, I.; Gao, Z.S.; Gasic, K.; Iezzoni, A.; Jung, S.; Peace, C.; Prieto, H.; et al. Prunus genetics and applications after de novo genome sequencing: Achievements and prospects. Hortic. Res. 2019, 6, 58. [Google Scholar] [CrossRef]
- Urrestarazu, J.; Errea, P.; Miranda, C.; Santesteban, L.G.; Pina, A. Genetic diversity of Spanish Prunus domestica L. germplasm reveals a complex genetic structure underlying. PLoS ONE 2018, 13, e0195591. [Google Scholar] [CrossRef] [PubMed]
- Ferrás-Sexto, C.; O’Flanagan, P. Small-holdings and sustainable family farming in Galicia and Ireland. A comparative case study. Norois 2012, 224, 61–76. [Google Scholar] [CrossRef]
- Pereira-Lorenzo, S.; Urrestarazu, J.; Ramos-Cabrer, A.M.; Miranda, C.; Pina, A.; Dapena, E.; Moreno, M.A.; Errea, P.; Llamero, N.; Díaz-Hernández, M.B.; et al. Analysis of the genetic diversity and structure of the Spanish apple genetic resources suggests the existence of an Iberian genepool. Ann. Appl. Biol. 2017, 171, 424–440. [Google Scholar] [CrossRef]
- Goded, S.; Ekroos, J.; Domínguez, J.; Guitián, J.A.; Smith, H.G. Effects of organic farming on bird diversity in North-West Spain. Agric. Ecosyst. Environ. 2018, 257, 60–67. [Google Scholar] [CrossRef]
- FAO. Contributing to Food Security and Sustainability in a Changing World; FAO: Rome, Italy, 2011; ISBN 9789251067482. [Google Scholar]
- Miloševic, T.; Miloševic, N. Plum (Prunus spp.) Breeding. In Advances in Plant Breeding Strategies: Fruits; Al-Khayri, J.M., Jain, S.M., Johnson, D.V., Eds.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 165–215. [Google Scholar]
- Dulloo, M.E.; Hunter, D.; Borelli, T. Ex situ and in situ conservation of agricultural biodiversity: Major advances and research needs. Not. Bot. Horti Agrobot. Cluj-Napoca 2010, 38, 123–135. [Google Scholar] [CrossRef]
- Postman, J.; Hummer, K.; Stover, E.; Krueger, R.; Forsline, P.; Grauke, L.J.; Zee, F.; Ayala-Silva, T.; Irish, B. Fruit and Nut Genebanks in the U.S. National Plant Germplasm System. HortScience 2006, 41, 1188–1194. [Google Scholar] [CrossRef]
- Pence, V.C. In Vitro Methods and the Challenge of Exceptional Species for Target 8 of the Global Strategy for Plant Conservation 1. Ann. Mo. Bot. Gard. 2013, 99, 214–220. [Google Scholar] [CrossRef]
- Druart, P.; Gruselle, R. Plum (Prunus domestica). In Biotechnology in Agriculture and Forestry, Vol 1: Trees I; Bajaj, Y., Ed.; Springer: Berlin/Heidelberg, Germany, 1986; pp. 130–154. ISBN 3-540-15581-3. [Google Scholar]
- Druart, P. In Vitro Culture and Micropropagation of Plum (Prunus spp.). In High-Tech and Micropropagation II Biotechnology in Agriculture and Forestry, Vol 18; Bajaj, Y.P.S., Ed.; Springer: Berlin/Heidelberg, Germany, 1992; pp. 279–303. [Google Scholar]
- Andreu, P.; Marín, J.A. In vitro culture establishment and multiplication of the Prunus rootstock ‘Adesoto 101′ (P. insititia L.) as affected by the type of propagation of the donor plant and by the culture medium composition. Sci. Hortic. 2005, 106, 258–267. [Google Scholar] [CrossRef]
- Ruzic, D.; Vujovic, T.; Cerovic, R. In vitro preservation of autochthonous plum genotypes. Bulg. J. Agric. Sci. 2012, 18, 55–62. [Google Scholar]
- Wolella, E.K. Surface sterilization and in vitro propagation of Prunus domestica L. cv. Stanley using axillary buds as explants. J. Biotech Res. 2017, 8, 18–26. [Google Scholar]
- Alburquerque, N.; Faize, L.; Burgos, L. Silencing of Agrobacterium tumefaciens oncogenes ipt and iaaM induces resistance to crown gall disease in plum but not in apricot. Pest Manag. Sci. 2017, 73, 2163–2173. [Google Scholar] [CrossRef] [PubMed]
- Vujović, T.; Jevremović, D.; Marjanović, T.; Glišić, I. In vitro propagation and medium-term conservation of autochthonous plum cultivar “Crvena Ranka”. Acta Agric. Serbica 2020, 25, 141–147. [Google Scholar] [CrossRef]
- Etienne, H.; Berthouly, M. Temporary immersion systems in plant micropropagation. Plant Cell Tissue Organ Cult. 2002, 69, 215–231. [Google Scholar] [CrossRef]
- Vidal, N.; Sánchez, C. Use of bioreactor systems in the propagation of forest trees. Eng. Life Sci. 2019, 19, 896–915. [Google Scholar] [CrossRef]
- Sanchez, M.C.; Vieitez, A.M. In vitro morphogenetic competence of basal sprouts and crown branches of mature chestnut. Tree Physiol. 1991, 8, 59–70. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Cuenca Valera, B.; Aldrey Villar, A.; Blanco Beiro, B.; Vidal González, N. Use of a Continuous Immersion System (CIS) for micropropagation of chestnut in photoautotrophic and photomixotrophic conditions. In Woody Plant Production Integrating Genetic and Vegetative Propagation Technologies, Proceedings of the 3rd International Conference of the IUFRO Unit 2.09.02, Vitoria-Gasteiz, Spain, 8–12 September 2014; Park, Y.S., Bonga, J.M., Eds.; IUFRO: Vienna, Austria, 2015; pp. 112–116. [Google Scholar]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Lindsay, H. A colorimetric estimation of reducing sugars in potatoes with 3,5-dinitrosalicylic acid. Potato Res. 1973, 16, 176–179. [Google Scholar] [CrossRef]
- Yemm, E.W.; Willis, A.J. The Estimation of Carbohydrates in Plant Extracts by Anthrone. Biochem. J. 1954, 57, 508–514. [Google Scholar] [CrossRef]
- Wellburn, A.R. The Spectral Determination of Chlorophylls a and b, as well as Total Carotenoids, Using Various Solvents with Spectrophotometers of Different Resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Díaz, J.; Bernal, A.; Pomar, F.; Merino, F. Induction of shikimate dehydrogenase and peroxidase in pepper (Capsicum annuum L.) seedlings in response to copper stress and its relation to lignification. Plant Sci. 2001, 161, 179–188. [Google Scholar] [CrossRef]
- Singleton, V.; Rossi, J. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–168. [Google Scholar]
- Blois, M.S. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Murch, S.J.; Liu, C.; Romero, R.M.; Saxena, P.K. In vitro Culture and Temporary Immersion Bioreactor Production of Crescentia cujete. Plant Cell Tissue Organ Cult. 2004, 78, 63–68. [Google Scholar] [CrossRef]
- McAlister, B.; Finnie, J.; Watt, M.; Blakeway, F. Use of the temporary immersion bioreactor system (RITA®) for production of commercial Eucalyptus clones in Mondi Forests (SA). Plant Cell Tissue Organ Cult. 2005, 81, 347–358. [Google Scholar] [CrossRef]
- Zhu, L.-H.; Li, X.-Y.; Welander, M. Optimisation of growing conditions for the apple rootstock M26 grown in RITA containers using temporary immersion principle. Plant Cell Tissue Organ Cult. 2005, 81, 313–318. [Google Scholar] [CrossRef]
- Quiala, E.; Cañal, M.J.; Meijón, M.; Rodríguez, R.; Chávez, M.; Valledor, L.; de Feria, M.; Barbón, R. Morphological and physiological responses of proliferating shoots of teak to temporary immersion and BA treatments. Plant Cell Tissue Organ Cult. 2012, 109, 223–234. [Google Scholar] [CrossRef]
- Aguilar, M.E.; Garita, K.; Kim, Y.W.; Kim, J.-A.; Moon, H.K. Simple Protocol for the Micropropagation of Teak (Tectona grandis Linn.) in Semi-Solid and Liquid Media in RITA Bioreactors and ex Vitro Rooting. Am. J. Plant Sci. 2019, 10, 1121–1141. [Google Scholar] [CrossRef]
- Akdemir, H.; Süzerer, V.; Onay, A.; Tilkat, E.; Ersali, Y.; Çiftçi, Y.O. Micropropagation of the pistachio and its rootstocks by temporary immersion system. Plant Cell Tissue Organ Cult. 2014, 117, 65–76. [Google Scholar] [CrossRef]
- Vidal, N.; Blanco, B.; Cuenca, B. A temporary immersion system for micropropagation of axillary shoots of hybrid chestnut. Plant Cell Tissue Organ Cult. 2015, 123, 229–243. [Google Scholar] [CrossRef]
- Latawa, J.; Shukla, M.R.; Saxena, P.K. An efficient temporary immersion system for micropropagation of hybrid hazelnut. Botany 2016, 94, 1–8. [Google Scholar] [CrossRef]
- Luna, C.V.; Gonzalez, A.M.; Mroginski, L.A.; Sansberro, P.A. Anatomical and histological features of Ilex paraguariensis leaves under different in vitro shoot culture systems. Plant Cell Tissue Organ Cult. 2017, 129, 457–467. [Google Scholar] [CrossRef]
- Regueira, M.; Rial, E.; Blanco, B.; Bogo, B.; Aldrey, A.; Correa, B.; Varas, E.; Sánchez, C.; Vidal, N. Micropropagation of axillary shoots of Salix viminalis using a temporary immersion system. Trees 2018, 32, 61–71. [Google Scholar] [CrossRef]
- Benelli, C.; De Carlo, A. In vitro multiplication and growth improvement of Olea europaea L. cv Canino with temporary immersion system (Plantform™). 3 Biotech 2018, 8, 317. [Google Scholar] [CrossRef]
- San José, M.C.; Blázquez, N.; Cernadas, M.J.; Janeiro, L.V.; Cuenca, B.; Sánchez, C.; Vidal, N. Temporary immersion systems to improve alder micropropagation. Plant Cell Tissue Organ Cult. 2020, 143, 265–275. [Google Scholar] [CrossRef]
- Lotfi, M.; Bayoudh, C.; Werbrouck, S.; Mars, M. Effects of meta–topolin derivatives and temporary immersion on hyperhydricity and in vitro shoot proliferation in Pyrus communis. Plant Cell Tissue Organ Cult. 2020, 143, 499–505. [Google Scholar] [CrossRef]
- Georgiev, V.; Schumann, A.; Pavlov, A.; Bley, T. Temporary immersion systems in plant biotechnology. Eng. Life Sci. 2014, 14, 607–621. [Google Scholar] [CrossRef]
- Xiao, Y.; Niu, G.; Kozai, T. Development and application of photoautotrophic micropropagation plant system. Plant Cell Tissue Organ Cult. 2011, 105, 149–158. [Google Scholar] [CrossRef]
- Watt, M.P. The status of temporary immersion system (TIS) technology for plant micropropagation. Afr. J. Biotechnol. 2012, 11, 14036–14043. [Google Scholar] [CrossRef]
- Kirdmanee, C.; Kitaya, Y.; Kozai, T. Effects of CO2 enrichment and supporting materialin vitro on photoautotrophic growth ofEucalyptus plantletsin vitro andex vitro. Vitr. Cell. Dev. Biol.-Plant 1995, 31, 144–149. [Google Scholar] [CrossRef]
- Zobayed, S.M.A.; Afreen, F.; Kozai, T. Physiology of Eucalyptus plantlets grown photoautotrophically in a scaled-up vessel. Vitr. Cell. Dev. Biol.-Plant 2001, 37, 807–813. [Google Scholar] [CrossRef]
- Zobayed, S. Mass Propagation of Eucalyptus camaldulensis in a Scaled-up Vessel Under In Vitro Photoautotrophic Condition. Ann. Bot. 2000, 85, 587–592. [Google Scholar] [CrossRef]
- Tanaka, M.; Giang, D.T.T.; Murakami, A. Application of a novel disposable film culture system to photoautotrophic micropropagation of Eucalyptus uro-grandis (Urophylia × grandis). Vitr. Cell. Dev. Biol.-Plant 2005, 41, 173–180. [Google Scholar] [CrossRef]
- Fuljahn, S.; Tantau, H.-J. Process engineering as a means of regulating the microclimate in a photoautotrophic in vitro culture. Acta Hortic. 2009, 817, 143–150. [Google Scholar] [CrossRef]
- Arencibia, A.D.; Gómez, A.; Poblete, M.; Vergara, C. High-performance micropropagation of dendroenergetic poplar hybrids in photomixotrophic Temporary Immersion Bioreactors (TIBs). Ind. Crops Prod. 2017, 96, 102–109. [Google Scholar] [CrossRef]
- García-Ramírez, Y.; Barrera, G.P.; Freire-Seijo, M.; Barbón, R.; Concepción-Hernández, M.; Mendoza-Rodríguez, M.F.; Torres-García, S. Effect of sucrose on physiological and biochemical changes of proliferated shoots of Bambusa vulgaris Schrad. Ex Wendl in temporary immersion. Plant Cell Tissue Organ Cult. 2019, 137, 239–247. [Google Scholar] [CrossRef]
- Gago, D.; Vilavert, S.; Bernal, M.Á.; Sánchez, C.; Aldrey, A.; Vidal, N. The Effect of Sucrose Supplementation on the Micropropagation of Salix viminalis L. Shoots in Semisolid Medium and Temporary Immersion Bioreactors. Forests 2021, 12, 1408. [Google Scholar] [CrossRef]
- Greenboim-Wainberg, Y.; Maymon, I.; Borochov, R.; Alvarez, J.; Olszewski, N.; Ori, N.; Eshed, Y.; Weiss, D. Cross Talk between Gibberellin and Cytokinin: The Arabidopsis GA Response Inhibitor SPINDLY Plays a Positive Role in Cytokinin Signaling. Plant Cell 2005, 17, 92–102. [Google Scholar] [CrossRef]
- Silva, L.A.S.; Costa, A.D.O.; Batista, D.S.; Silva, M.L.D.; Costa Netto, A.P.D.; Rocha, D.I. Exogenous gibberellin and cytokinin in a novel system for in vitro germination and development of African iris (Dietes bicolor). Rev. Ceres 2020, 67, 402–409. [Google Scholar] [CrossRef]
- Deng, Z.; Chu, J.; Wang, Q.; Wang, L. Effect of different carbon sources on the accumulation of carbohydrate, nutrient absorption and the survival rate of Chinese Ash (Fraxinus mandshurica) explants in vitro. Afr. J. Agric. Res. 2012, 7, 3111–3119. [Google Scholar] [CrossRef]
- Kim, N.-Y.; Hwang, H.-D.; Kim, J.-H.; Kwon, B.-M.; Kim, D.; Park, S.-Y. Efficient production of virus-free apple plantlets using the temporary immersion bioreactor system. Hortic. Environ. Biotechnol. 2020, 61, 779–785. [Google Scholar] [CrossRef]
- Sha Valli Khan, P.S.; Kozai, T.; Nguyen, Q.T.; Kubota, C.; Dhawan, V. Growth and Water Relations of Paulownia fortunei Under Photomixotrophic and Photoautotrophic Conditions. Biol. Plant. 2003, 46, 161–166. [Google Scholar] [CrossRef]
- Mosaleeyanon, K.; Cha-um, S.; Kirdmanee, C. Enhanced growth and photosynthesis of rain tree (Samanea saman Merr.) plantlets in vitro under a CO2-enriched condition with decreased sucrose concentrations in the medium. Sci. Hortic. 2004, 103, 51–63. [Google Scholar] [CrossRef]
- Cha-um, S.; Chanseetis, C.; Chintakovid, W.; Pichakum, A.; Supaibulwatana, K. Promoting root induction and growth of in vitro macadamia (Macadamia tetraphylla L. ‘Keaau’) plantlets using CO2-enriched photoautotrophic conditions. Plant Cell Tissue Organ Cult. 2011, 106, 435–444. [Google Scholar] [CrossRef]
- Fortini, E.A.; Batista, D.S.; Mamedes-Rodrigues, T.C.; Felipe, S.H.S.; Correia, L.N.F.; Chagas, K.; Silva, P.O.; Rocha, D.I.; Otoni, W.C. Gas exchange rates and sucrose concentrations affect plant growth and production of flavonoids in Vernonia condensata grown in vitro. Plant Cell Tissue Organ Cult. 2021, 144, 593–605. [Google Scholar] [CrossRef]
- Hassankhah, A.; Vahdati, K.; Lotfi, M.; Mirmasoumi, M.; Preece, J.; Assareh, M.-H. Effects of Ventilation and Sucrose Concentrations on the Growth and Plantlet Anatomy of Micropropagated Persian Walnut Plants. Int. J. Hort. Sci. Technol. 2014, 1, 111–120. [Google Scholar]
- Mingozzi, M.; Montello, P.; Merkle, S. Adventitious shoot regeneration from leaf explants of eastern cottonwood (Populus deltoides) cultured under photoautotrophic conditions. Tree Physiol. 2009, 29, 333–343. [Google Scholar] [CrossRef]
- Saldanha, C.W.; Otoni, C.G.; Notini, M.M.; Kuki, K.N.; da Cruz, A.C.F.; Neto, A.R.; Dias, L.L.C.; Otoni, W.C. A CO2-enriched atmosphere improves in vitro growth of Brazilian ginseng [Pfaffia glomerata (Spreng.) Pedersen]. Vitr. Cell. Dev. Biol.-Plant 2013, 49, 433–444. [Google Scholar] [CrossRef]
- Ševčíková, H.; Lhotáková, Z.; Hamet, J.; Lipavská, H. Mixotrophic in vitro cultivations: The way to go astray in plant physiology. Physiol. Plant. 2019, 167, 365–377. [Google Scholar] [CrossRef]
- Badr, A.; Angers, P.; Desjardins, Y. Metabolic profiling of photoautotrophic and photomixotrophic potato plantlets (Solanum tuberosum) provides new insights into acclimatization. Plant Cell Tissue Organ Cult. 2011, 107, 13–24. [Google Scholar] [CrossRef]
- Lucchesini, M.; Monteforti, G.; Mensuali-Sodi, A.; Serra, G. Leaf ultrastructure, photosynthetic rate and growth of myrtle plantlets under different in vitro culture conditions. Biol. Plant. 2006, 50, 161–168. [Google Scholar] [CrossRef]
- Sáez, P.L.; Bravo, L.A.; Latsague, M.I.; Sánchez, M.E.; Ríos, D.G. Increased light intensity during in vitro culture improves water loss control and photosynthetic performance of Castanea sativa grown in ventilated vessels. Sci. Hortic. 2012, 138, 7–16. [Google Scholar] [CrossRef]
- Gaspar, T.; Franck, T.; Bisbis, B.; Kevers, C.; Jouve, L.; Hausman, J.F.; Dommes, J. Concepts in plant stress physiology. Application to plant tissue cultures. Plant Growth Regul. 2002, 37, 263–285. [Google Scholar] [CrossRef]
- Ahmad, P.; Jaleel, C.A.; Salem, M.A.; Nabi, G.; Sharma, S. Roles of enzymatic and nonenzymatic antioxidants in plants during abiotic stress. Crit. Rev. Biotechnol. 2010, 30, 161–175. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Gharibi, S.; Tabatabaei, B.E.S.; Saeidi, G.; Goli, S.A.H. Effect of Drought Stress on Total Phenolic, Lipid Peroxidation, and Antioxidant Activity of Achillea Species. Appl. Biochem. Biotechnol. 2016, 178, 796–809. [Google Scholar] [CrossRef]
- García-Ramírez, Y.; Gonzáles, M.G.; Mendoza, E.Q.; Seijo, M.F.; Cárdenas, M.L.O.; Moreno-Bermúdez, L.J.; Ribalta, O.H. Effect of BA Treatments on Morphology and Physiology of Proliferated Shoots of Bambusa vulgaris Schrad. Ex Wendl in Temporary Immersion. Am. J. Plant Sci. 2014, 05, 205–211. [Google Scholar] [CrossRef][Green Version]
- Malik, M.; Warchoł, M.; Pawłowska, B. Liquid culture systems affect morphological and biochemical parameters during Rosa canina plantlets in vitro production. Not. Bot. Horti Agrobot. Cluj-Napoca 2018, 46, 58–64. [Google Scholar] [CrossRef]
- Regni, L.; Del Buono, D.; Micheli, M.; Facchin, S.L.; Tolisano, C.; Proietti, P. Effects of Biogenic ZnO Nanoparticles on Growth, Physiological, Biochemical Traits and Antioxidants on Olive Tree In Vitro. Horticulturae 2022, 8, 161. [Google Scholar] [CrossRef]
- Newel, C.; Growns, D.; McComb, J. The influence of medium aeration on in vitro rooting of Australian plant microcuttings. Plant Cell Tissue Organ Cult. 2003, 75, 131–142. [Google Scholar] [CrossRef]
- Dutta Gupta, S.; Prasad, V.S.S. Matrix-Supported Liquid Culture Systems for Efficient Micropropagation of Floricultural Plants. In Floriculture, Ornamental and Plant Biotechnology; Teixeira da Silva, J., Ed.; Global Science Books: Kagawa, Japan, 2006; pp. 487–495. [Google Scholar]
- Afreen-Zobayed, F.; Zobayed, S.M.A.; Kubota, C.; Kozai, T.; Hasegawa, O. Supporting material affects the growth and development of in vitro sweet potato plantlets cultured photoautotrophically. Vitr. Cell. Dev. Biol.-Plant 1999, 35, 470–474. [Google Scholar] [CrossRef]
- Maner, L.; Merkle, S. Polymerized peat plugs improve American chestnut somatic embryo germination in vitro. J. Am. Chestnut Found. 2010, 24, 16. [Google Scholar]
- Cuenca, B.; Sánchez, C.; Aldrey, A.; Bogo, B.; Blanco, B.; Correa, B.; Vidal, N. Micropropagation of axillary shoots of hybrid chestnut (Castanea sativa × C. crenata) in liquid medium in a continuous immersion system. Plant Cell Tissue Organ Cult. 2017, 131, 307–320. [Google Scholar] [CrossRef]
- Kodym, A.; Leeb, C.J. Back to the roots: Protocol for the photoautotrophic micropropagation of medicinal Cannabis. Plant Cell Tissue Organ Cult. 2019, 138, 399–402. [Google Scholar] [CrossRef] [PubMed]
- Adelberg, J.; Naylor-Adelberg, J.; Miller, S.; Gasic, K.; Schnabel, G.; Bryson, P.; Saski, C.; Parris, S.; Reighard, G. In vitro co-culture system for Prunus spp. and Armillaria mellea in phenolic foam rooting matric. Vitr. Cell. Dev. Biol.-Plant 2021, 57, 387–397. [Google Scholar] [CrossRef]
- Gianní, S.; Sottile, F. In vitro storage of plum germplasm by slow growth. Hortic. Sci. 2016, 42, 61–69. [Google Scholar] [CrossRef]
- Godoy, S.; Tapia, E.; Seit, P.; Andrade, D.; Sánchez, E.; Andrade, P.; Almeida, A.M.; Prieto, H. Temporary immersion systems for the mass propagation of sweet cherry cultivars and cherry rootstocks: Development of a micropropagation procedure and effect of culture conditions on plant quality. Vitr. Cell. Dev. Biol.-Plant 2017, 53, 494–504. [Google Scholar] [CrossRef]
- Zare Khafri, A.; Solouki, M.; Zarghami, R.; Fakheri, B.; Mahdinezhad, N.; Naderpour, M. In vitro propagation of three Iranian apricot cultivars. Vitr. Cell. Dev. Biol.-Plant 2021, 57, 102–117. [Google Scholar] [CrossRef]
- Damiano, C.; Monticelli, S.; La Starza, S.R.; Gentile, A.; Frattarelli, A. Temperate fruit plant propagation through temporary immersion. Acta Hortic. 2003, 625, 193–200. [Google Scholar] [CrossRef]
- Cantabella, D.; Mendoza, C.R.; Teixidó, N.; Vilaró, F.; Torres, R.; Dolcet-Sanjuan, R. GreenTray® TIS bioreactor as an effective in vitro culture system for the micropropagation of Prunus spp. rootstocks and analysis of the plant-PGPMs interactions. Sci. Hortic. 2022, 291, 110622. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).