Abstract
The gray mold caused by Botrytis cinerea is the main concern for the postharvest conservation of table grapes worldwide, and the use of sulfur dioxide (SO2) is the most common practice for its control. The aim of this work was to assess the postharvest conservation of the hybrid table grape ‘BRS Nubia’ by using a new technology known as a field ultra-fast SO2-generating pad before packaging the grapes. The fruits were harvested in the 2021 season from a commercial vineyard located at Marialva, Parana, Brazil. The experiment was conducted in a completely randomized design, with 4 treatments and 4 replications, and each plot consisted of 10 bunches. The treatments included: (a) control; (b) field ultra-fast SO2-generating pad (FUFR) during the 4 h before packaging; (c) dual-release SO2-generating pad (DR) during cold storage; and (d) FUFR pad during the 4 h before packaging in combination with the DR pad during cold storage. The bunches were packaged in 0.5 kg plastic clamshells and placed in carton boxes with a capacity of 10 units each. The treatments were evaluated after 30 and 45 days in cold storage (1 ± 1 °C) by means of the incidence of gray mold, shattered berries, stem browning, bunch mass loss and bleaching. After 45 days, the boxes were removed from cold storage and kept without the pads and liners for 3 days at room temperature (22 ± 1 °C), and the incidence of gray mold, shattered berries and stem browning were assessed. The use of the FUFR pad before packaging and the DR pad during cold storage, combined or not, were efficient at controlling gray mold, keeping the incidence of the disease very low. Both pads were also efficient at preventing the mass loss of grapes, but the percentage of shattered berries was lower when the FUFR pad was used. This allowed grapes to arrive already sanitized to the packing house, and no additional SO2-generating pads were needed, representing savings in economic terms for packaging operations. The stem browning, the chemical properties, and the color attributes of berries were not influenced by any treatment.
1. Introduction
The demand of the national and international markets for a high-quality standard of table grapes has been increasing progressively; therefore, it is of fundamental importance that the quality of the bunches is maintained until they reach their final destination [1]. In addition to the nutritional value, the visual aspect of the grapes is a parameter of significant relevance for the consumer market. Freshness, color, firmness, flavor, aroma and fabric integrity are part of the set of attributes that define its quality and, consequently, are determinant purchase criteria for the consumer. To meet this demand for quality, several hybrid table grapes were released in the last decade by the Table Grape Breeding Program of Embrapa Grape and Wine, Brazil, in particular, ‘BRS Nubia’, which presents black and crunchy berries, neutral flavor and tolerance for downy mildew [2]. BRS is the Embrapa acronym for protected cultivars developed in breeding programs.
After harvesting, the grape is exposed to injuries caused by handling. In the meantime, some factors such as injuries caused by handling, mass loss and injuries caused by pathogens can compromise its quality. The main factor responsible for postharvest losses of table grapes is the fungus Botrytis cinerea, the causal agent of the gray mold disease [3,4]. This pathogen occurs in all producing regions of the world, causing losses of economic importance in the pre- and postharvest phases, and may remain latent in the field and only express itself during the transport and the cold storage of grapes [5,6]. Thus, even if the phytosanitary treatment carried out in the field is effective, the disease can cause losses during the postharvest period [7].
The association between cold storage and the use of adequate packaging is the postharvest conservation strategy that most contributes to increasing the shelf-life of the produce, favoring its commercialization [8]. Aiming to control gray mold, fumigation of the cold chamber by using sulfur dioxide gas (SO2) was initially developed for table grapes. Later, SO2-generating pads were developed [9], and they have been used all over the world. Their functioning is based on the reaction of the active ingredient, which can be sodium metabisulfite (Na2S2O5) or potassium (K2S2O5), with the humidity of the air, leading to the continuous emission of gas inside the packages. In addition to controlling fungi, the SO2 influences some physiological processes of the fruit itself by presenting an antioxidant action [10], keeping the stems fresh and green. However, a high-intensity dose of SO2 can damage grapes by causing bleaching or affecting the sunken regions of the berries. Therefore, for export purposes, levels of SO2 tolerance (10 ppm) have been proposed by EU countries with the objective of protecting the consumer and environment.
Dual release SO2-generating pads, containing fast and slow-release phases combined in just one pad [11,12], are commonly used to control gray mold during cold storage. The use of the newly developed field ultra-fast SO2-generating pad may further reduce gray mold incidence. This pad was developed to be used inside the field harvest box for a period of 4–6 h before grapes are handled and packaged into carton boxes in the packing house by killing the fungus spores before the grapes are packaged. To our knowledge, no other works have focused on field ultra-fast pads so far, and in addition, no information is known about the association of this new pad with the commonly used dual-release pad, especially for the postharvest conservation of ‘BRS Nubia’ grape.
We evaluated in this trial the use of the field ultra-fast SO2-generating pad before packaging to control gray mold in ‘BRS Nubia’ hybrid table grapes in combination with dual-release SO2-generating pads.
2. Materials and Methods
2.1. Location
The grapes ‘BRS Nubia’ (Vitis spp.) were harvested from a commercial vineyard located in Marialva, PR (23°29′06″ S, 51°47′31″ W, elevation 602 m a.s.l.) in May during the 2021 season when bunches reached full ripeness. The 9-year-old vines were grafted onto ‘IAC 766 Campinas’ rootstock and trained in an overhead trellis system. The vineyard has a history of occurrence of B. cinerea. According to Köppen, the region is classified as subtropical (Cfa). The temperature varies from 14 °C to 30 °C, being rarely lower than 9 °C or higher than 34 °C, and annual precipitation is approximately 1596 mm [13].
2.2. Treatments and Experimental Design
The treatments included: (a) control (without any type of SO2-generating pad); (b) field ultra-fast SO2-generating pad before packaging; (c) dual-release SO2-generating pad during cold storage; and (d) field ultra-fast SO2-generating pad before packaging in combination with a dual-release SO2-generating pad during cold storage.
A completely randomized design was used as a statistical model, consisting of 4 treatments and 4 replications, and each plot was composed of a corrugated cardboard box containing 10 bunches.
SO2-generating pads containing 98% of the active ingredient (a.i.), sodium metabisulfite (Na2S2O5), were supplied by Suragra S. A. (San Bernardo, Chile). The field ultra-fast SO2-generating pads had 1.4 g of a.i. and were designed to release the SO2 gas very quickly in a short period of time, with a maximum of 6 h. The dual-release SO2-generating pads had 5 g of the a.i. (1 g and 4 g for the fast- and the slow-release phases, respectively). The field ultra-fast and the dual-release SO2-generating pads had dimensions of 46 × 26 cm.
Right after harvesting in the field, around 20 kg of grapes were placed in plastic harvest boxes of 20 kg capacity lined with perforated plastic liners with 0.3% ventilation area (VA). For treatments b and d, one field ultra-fast SO2-generating pad was placed above the grapes, and the liners were immediately sealed (Figure 1). An SO2 dosimeter (Gastec Co., Kanagawa, Japan) was placed inside the harvest box to confirm the continuous release of the gas. After the sealing, the boxes were transferred to the packing house. After 4 h at a temperature of 25 °C, the liners of these treatments were unsealed, and the grapes were removed, pre-cooled, and handled in a sorting table.

Figure 1.
Field ultra-fast SO2-generating pad treatment right after harvesting. (A): Plastic harvest box lined with a perforated plastic liner. (B): Freshly harvested ‘BRS Nubia’ grapes. (C): Laying of a field ultra-fast SO2—generating pad above grapes. (D): Sealing of the liner.
For treatments a and c, grapes were not subjected to the field ultra-fast SO2-generating pad, and the box liner remained open (not sealed) during this period.
The grapes of all treatments were individually packaged in vented plastic clamshells with a capacity of 0.5 kg and packaged into corrugated cardboard boxes measuring 60 × 40 × 10 cm with a storage capacity of 10 plastic clamshells each, previously lined with perforated plastic liners of 0.3% VA (Figure 2).
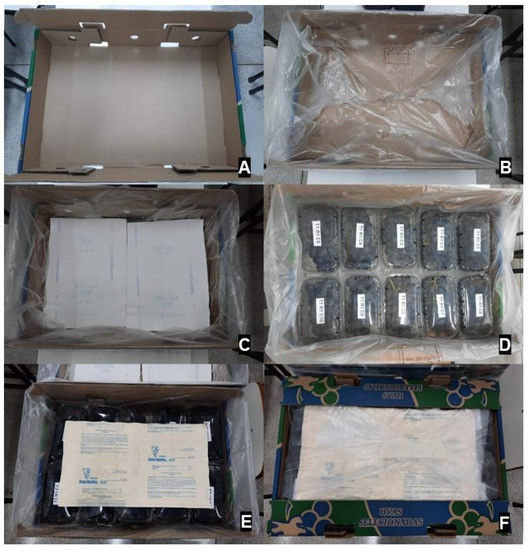
Figure 2.
Packaging steps of ‘BRS Nubia’ table grape. (A): Carton box and perforated plastic liners. (B): Sheet of moisture-absorbing paper on the bottom of the box. (C): Placement of vented plastic clamshells with grapes. (D): Dual release SO2-generating pad on top of clamshells. (E): Sealing of the liners with adhesive tape. (F): Carton box ready to be placed under cold storage.
For all treatments, below the plastic clamshells, at the bottom of the box, a sheet of moisture-absorbent paper was placed, and above the clamshells, according to the treatment, the dual-release SO2-generating pads were placed. After the grape packaging process, the boxes were stored for a period of 45 days in a cold chamber at 1 ± 1 °C and high relative humidity. After 45 days of cold storage, SO2-generating pads and liners were removed, and boxes were kept for 3 days at room temperature (22 ± 1 °C).
2.3. Assessments
The treatments were assessed after 30 and 45 days of the beginning of cold storage. At 30 days, the following variables were analyzed: incidence of gray mold (% of berries with symptoms), shattered berries (% of loose berries), stem browning, loss of mass and bleaching (% of berries with bleaching). In addition to these variables, at 45 days, the soluble solids content (SSC), titratable acidity (TA), pH and berry color attributes were also assessed. After 3 days at room temperature, the following variables were evaluated: incidence of gray mold, shattered berries and stem browning.
The incidence of gray mold was calculated using the following formula: incidence (%) = (number of affected berries/total of berries) × 100 [14]. The bunch mass loss was obtained by weighing the bunches at the initial time of storage and at the time of each evaluation [15]:
The stem browning was evaluated through visual assessment according to the methodology described by Ngcobo et al. [16], assigning notes in accordance with the level of darkness: (1) fresh and green; (2) some light browning; (3) significant browning; and (4) severe browning.
Shattered berries and bleaching were assessed by counting the loose and discolored berries from the bunch inside the clamshells, respectively, and both were expressed as a percentage [17].
The SSC was obtained using a portable digital refractometer (Model PAL-1, Atago, Tokyo, Japan), using the juice extracted from 10 berries from each plot, and the results were expressed in °Brix. To determine the AT, the juice was titrated with a standardized 0.1 N NaOH solution in a semi-automatic titrator, using 10 mL of the juice extracted from the berries previously, with pH = 8.2 and the endpoint of the titration. Results were expressed in % of tartaric acid [18].
The assessment of the berry color attributes (CIELab) was performed using a colorimeter (CR10 Plus, Konica-Minolta, Ramsey, NJ, USA) on 10 berries of each plot, in which L* represents lightness (L* = 100 = white; L* = 0 = black); a* represents the color intensity from green to red (a* more negative = greener; a* more positive = redder), and b* represents the color intensity from yellow to blue (b* more positive = more yellow; b* more negative = bluer). The values of a* and b* were used to calculate the chroma (C*) and hue angle (h°).
2.4. Statistical Analysis
The dataset was subjected to analysis of variance (ANOVA), and means were compared by Fisher’s LSD test at 5% probability. The non-parametric Kruskal–Wallis test was performed as an alternative to ANOVA for the variable incidence of gray mold, provided that the assumption of normality of residuals and equality of variances was not accepted from the Shapiro–Wilk test, Barlett test and via test graphical analysis. The Dunn’s test was used as a post-hoc test to find the datasets that differ after the Kruskal–Wallis statistical test rejects the null hypothesis that the performance of comparisons across datasets is similar, and this test performs tests performance in pairs. Statistical analyses were performed using the R software.
3. Results and Discussion
At 30 and 45 days of cold storage, grapes subjected to treatments with SO2-generating pads had a lower incidence, or even absence of, gray mold when compared with the control (Table 1). There was no significant difference between the SO2-generating pads, all of which reduced the incidence of gray mold during the cold storage period when compared with the control. After 3 days at room temperature, even with all treatments stored under similar conditions, the highest incidence of the disease remained in the control bunches. In treatments in which the SO2-generating pads were used, after 3 days at room temperature, there was an increase in the incidence of the disease (Table 1).

Table 1.
Gray mold incidence (means ± standard deviations) of ‘BRS Nubia’ table grape after 30 and 45 days at cold storage (CS) (1 ± 1 °C) and after 3 days at room temperature (RT) (22 ± 1 °C), individually packaged in ventilated plastic clamshells with different SO2-generating pads and perforated plastic liners.
The combination of the field ultra-fast SO2-generating pad combined with the dual-release SO2-generating pad resulted in a total absence of symptoms of gray mold in grapes throughout the storage period (Table 1). The field ultra-fast SO2-generating pad released a continuous dose of gas over a period of 6 h, as confirmed by the dosimeter placed inside the harvest box. As described by Mühlbeier et al. [12], the dual-release SO2-generating pad (with the combination of fast- and slow-release phases in just one pad) initially releases a high dose of gas, which peaks over a period of 24 to 48 h after the pad comes into contact with the moisture in the package, then decreases.
As previously described, the SO2 treatments were efficient in controlling gray mold. Thus, considering the economic aspect, it would be feasible to choose the use of only one SO2-generating pad instead of the combination of two. The use of field ultra-fast SO2-generating pads before packaging allows bunches to arrive sanitized at the packing house, ready to be packaged, and no additional treatment is needed. In addition, the residue levels of SO2 tolerance were lower than the limit allowed for consumption, and no off-flavors were detected in berries.
The use of SO2-generating pads during cold storage is one of the main decay control methods in table grapes, especially regarding gray mold [1,11,19], and its use is necessary when the objective is to store the grape bunches for long periods.
Regarding the mass loss, at 30 and 45 days of cold storage, the highest mean was observed in the control and in the treatment in which both SO2-generating pads were used (Table 2), indicating a possible sensitivity of this grape cultivar to higher doses of SO2. On the other hand, when the pads were used separately (only the field ultra-fast or the dual-release SO2-generating pad), the mass loss was lower. In general, mass loss remained low in all treatments, with the highest means observed at the end of storage, being 2.5 and 2.7%, respectively. The observed effects of the mass loss in the grape bunches can also be related to the characteristics of each cultivar, and for the ‘BRS Nubia’ grape, under the conditions of this study, the mass loss was low and not visible. It should be considered that a wilting appearance of the berries, as a consequence of the loss of mass, occurs when the loss reaches about 4 to 5%, thus impairing the ideal appearance and firmness for consumption [20].

Table 2.
Mass loss (means ± standard deviations) of ‘BRS Nubia’ table grape after 30 and 45 days in cold storage (CS) (1 ± 1 °C) individually packaged in ventilated plastic clamshells with different SO2-generating pads and perforated plastic liners.
It is possible that in the case of the control, where the highest percentage of diseased berries was detected, the mass loss was caused by the respiratory rate of the bunches, which supposedly was higher due to the parasitism process of the pathogen. Regarding the association of SO2-generating pads, it is likely that the greater dose of gas released during the storage period also promoted an increase in the respiratory rate of the bunches, causing higher mass loss.
The packaging used also influenced the transmission of water vapor. The lower the transmission rate, the higher the relative humidity inside the package and, consequently, the lower the mass loss [21,22]. Therefore, the associated use of a coating or perforated plastic liner combined or not with a SO2-generating pad is indicated to reduce water loss during the postharvest handling of grapes [23]. In this work, the low values recorded for bunch mass loss were probably due to the associated effects of cold storage and the proper use of packaging. However, even after controlling for these factors, the bunches continue to lose water, mainly due to the respiratory process of the fruits [24].
In terms of stem browning, no significant differences were detected among treatments (Table 3). During the cold storage of grapes, water loss and stem oxidation are expected, which are the factors responsible for stem browning and loss of grape quality. In general, it was observed that ‘BRS Nubia’ grapes had considerable stem browning scores upon assessment, and most treatments reached a score of almost 3 (significant browning) at the end of the storage.

Table 3.
Stem browning scores (means ± standard deviations) of ‘BRS Nubia’ table grape after 30 and 45 days at cold storage (CS) (1 ± 1 °C) and after 3 days at room temperature (RT) (22 ± 1 °C), individually packaged in ventilated plastic clamshells with different SO2-generating pads and perforated plastic liners.
Regarding shattered berries (Table 4), the highest means were observed in the control and in the treatment with the dual-release SO2-generating pad. After 45 days in cold storage and 3 days at room temperature, this treatment remained superior in relation to shattered berries, followed by the control and the other treatments. It is assumed that the high incidence of gray mold observed in the bunches of the control treatment may have been one of the factors that influenced the shattered berries, as the presence of the fungus in grapes causes the berries to soften and, consequently, favors a greater occurrence of loose berries [25]. Treatments with the dual-release SO2-generating pad resulted in the highest means of shattered berries. During the initial packaging of the grapes, it was observed that the cultivar has a high occurrence of natural shattered berries. In addition, plastic clamshells are used to provide a combination of practicality, protection and advertising of table grapes [26], and in a way, they also prevent shattered berries from being a problem because even if the berries are loose, they will remain inside the package.

Table 4.
Shattered berries (means ± standard deviations) of ‘BRS Nubia’ table grape after 30 and 45 days in cold storage (CS) (1 ± 1 °C) and after 3 days at room temperature (RT) (22 ± 1 °C), individually packaged in ventilated plastic clamshells with different SO2-generating pads and perforated plastic liners.
There were no differences in the chemical properties of the grapes throughout the assessments (Table 5), such as SSC, TA and pH. Besides, there were no differences regarding the berry color attributes (Table 6), implying that the use of techniques, appropriate packaging, and postharvest conservation enabled the main physicochemical properties of table grapes to be maintained under suitable conditions for prolonged periods [27]. Finally, as previously reported by Youssef et al. [28], ‘BRS Nubia’ table grape seems to not be sensitive to bleaching, provided that no symptoms of this disorder were detected in any of the treated bunches.

Table 5.
Soluble solids content (SSC), titratable acidity (TA), SSC/TA and pH (means ± standard deviations) of ‘BRS Nubia’ table grape after 45 days of cold storage (CS) (1 ± 1 °C) individually packaged in ventilated plastic clamshells with different SO20generating pads and perforated plastic liners.

Table 6.
Lightness (L*); color intensity towards green to red (a*); color intensity towards yellow to blue (b*); chroma (C*) and hue angle (h°) (means ± standard deviations) of ‘BRS Nubia’ table grape after 45 days in cold storage (CS) (1 ± 1 °C), individually packaged in ventilated plastic clamshells with different SO2-generating pads and perforated plastic liners.
4. Conclusions
The use of the field ultra-fast SO2-generating pad before packaging or the dual-release SO2-generating pad during cold storage, combined or not, are efficient at controlling gray mold in ‘BRS Nubia’ grapes, keeping the incidence of the disease very low. Both pads are also efficient at preventing the mass loss of grapes, but the percentage of shattered berries is lower when the field ultra-fast SO2-generating pad is used. This allows grapes to arrive already sanitized to the packing house, and no additional SO2 pad is needed, representing savings in economic terms for packaging operations. The stem browning, the chemical properties, and the color attributes of berries are not influenced by any treatment.
Author Contributions
B.C.D., M.T.H., A.C.d.A. and S.R.R. conceived and designed the experiments. B.C.D., M.T.H., B.E.B. and A.C.d.A. performed the experiments. B.C.D. analyzed the data. B.C.D. and S.R.R. wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Champa, H. Pre and postharvest practices for quality improvement of table grapes (Vitis vinifera L.). J. Nation. Sci. Found. Sri Lanka 2015, 43, 3–9. [Google Scholar] [CrossRef]
- Maia, J.D.G.; Ritschel, P.; Lazzarotto, J.J. A Viticultura de Mesa no Brasil. Produção para o Mercado Nacional e Internacional. Territoires du vin. 2018. Available online: https://preo.u-bourgogne.fr/territoiresduvin/index.php?id=1546 (accessed on 23 March 2021).
- Martínez-Romero, D.; Guillén, F.; Valverde, J.M.; Bailén, G.; Zapata, P.; Serrano, M.; Castillo, S.; Valero, D. Influence of carvacrol on survival of Botrytis cinerea inoculated in table grapes. Int. J. Food Microbiol. 2007, 115, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Michailides, T.J.; Xiao, C.L. Fungicide-resistant phenotypes in Botrytis cinerea populations and their impact on control of gray mold on stored table grapes in California. Eur. J. Plant Pathol. 2019, 154, 203–213. [Google Scholar] [CrossRef]
- Melgarejo-Flores, B.G.; Ortega-Ramírez, L.A.; Silva-Espinoza, B.A.; González-Aguilar, G.A.; Miranda, M.R.A.; Ayala-Zavala, J.F. Antifungal protection and antioxidant enhancement of table grapes treated with emulsions, vapors, and coatings of cinnamon leaf oil. Postharvest Biol. Technol. 2013, 86, 321–328. [Google Scholar] [CrossRef]
- Parisi, M.C.M.; Henrique, C.M.; Prati, P. Doenças pós-colheita: Um entrave na comercialização. Informações Tecnológicas. Apta Reg. Pesqui. Tecnol. 2015, 12, 5. [Google Scholar]
- Zoffoli, J.P.; Latorre, B.A.; Rodríguez, E.J.; Aldunce, P. Modified atmosphere packaging using chlorine gas generators to prevent Botrytis cinerea on table grapes. Postharvest Biol. Technol. 1999, 15, 135–142. [Google Scholar] [CrossRef]
- Yamashita, F.; Tonzar, A.C.; Fernandes, J.G.; Moriya, S.; Benassi, M.T. Influência de diferentes embalagens de atmosfera modificada sobre a aceitação de uvas finas de mesa var. Itália mantidas sob refrigeração. Food Sci. Technol. 2000, 20, 1–9. [Google Scholar] [CrossRef]
- Gentry, J.P.; Nelson, K.E. Further studies on control of decay of table by 2-stage generation of sulfur dioxide within unvented containers. Am. J. Enol. Vitic. 1968, 19, 70–81. [Google Scholar]
- Neves, L.C.; Silva, V.X.; Benedette, R.M.; Prill, M.A.S.; Vieites, R.L.; Roberto, S.R. Conservação de uvas ‘Crimson Seedless’ e ‘Itália’, submetidas a diferentes tipos de embalagens e dióxido de enxofre (SO2). Rev. Bras. Frutic. 2008, 30, 65–73. [Google Scholar] [CrossRef]
- Lichter, A.; Zutahy, Y.; Kaplunov, T.; Lurie, S. Evaluation of table grapes storage in boxes with sulfur dioxide-releasing pads with either an internal plastic liner or external wrap. HortTechnology 2008, 18, 206–214. [Google Scholar] [CrossRef]
- Mühlbeier, D.T.; Ribeiro, L.T.M.; Higuchi, M.T.; Khamis, Y.; Chaves Júnior, O.J.; Koyama, R.; Roberto, S.R. SO2-generating pads reduce gray mold in clamshell-packaged ‘Rubi’ table grapes grown under a two-cropping per year system. Semin. Ciências Agrárias 2021, 42, 1069–1086. [Google Scholar] [CrossRef]
- Nitsche, P.R.; Caramori, P.H.; Ricce, W.D.S.; Pinto, L.F.D. Atlas Climático do Estado do Paraná; IAPAR: Londrina, Brazil, 2019. Available online: http://www.idrparana.pr.gov.br/Pagina/Atlas-Climatico (accessed on 23 March 2021).
- Youssef, K.; Roberto, S.R. Applications of salt solutions before and after harvest affect the quality and incidence of postharvest gray mold of ‘Italia’ table grapes. Postharvest Biol. Technol. 2014, 87, 95–102. [Google Scholar] [CrossRef]
- Mattiuz, B.; Miguel, A.C.A.; Galati, V.C.; Nachtigal, J.C. Efeito da temperatura no armazenamento de uvas apirênicas minimamente processadas. Rev. Bras. Frutic. 2009, 31, 44–52. [Google Scholar] [CrossRef]
- Ngcobo, M.E.K.; Opara, U.L.; Thiart, G.D. Effects of packaging liners on cooling rate and quality attributes of table grape (cv. Regal Seedless). Packag. Technol. Sci. 2011, 25, 73–84. [Google Scholar] [CrossRef]
- Henríquez, J.L.; Pinochet, S. Impact of ventilation area of the liner bag, in the performance of SO2 generator pads in boxed table grapes. Acta Hortic. 2016, 1144, 267–272. [Google Scholar] [CrossRef]
- Zenebon, O.; Pascuet, N.S.; Tiglea, P. Procedimentos e determinações gerais. In Métodos Físico-Químicos para Análise de Alimentos, 4th ed.; Instituto Adolfo Lutz: São Paulo, Brasil, 2008; pp. 85–104. Available online: http://www.ial.sp.gov.br/resources/editorinplace/ial/2016_3_19/analisedealimentosial_2008.pdf (accessed on 23 March 2021).
- Palou, L.; Serrano, M.; Martínez-Romero, D.; Valero, D. New approaches for postharvest quality retention of table grapes. Fresh Prod. 2010, 4, 103–110. [Google Scholar]
- Netto, A.G.; Gayet, J.P.; Bleinroth, E.W.; Mútallo, M.; Garcia, E.; Garcia, A.E.; Ardito, E.F.G.; Bordin, M. Uva para Exportação: Procedimentos de Colheita e Pós-Colheita; EMBRAPA-SPI FRUPEX: Brasília, Brazil, 1993; p. 40. [Google Scholar]
- Cia, P.; Benato, E.A.; Valentini, S.R.T.; Sanches, J.; Ponzo, F.S.; Flores, D.; Terra, M.M. Atmosfera modificada e refrigeração para conservação pós colheita de uva ‘Niagara Rosada’. Pesqui. Agropecu. Bras. 2010, 45, 1058–1065. [Google Scholar] [CrossRef][Green Version]
- Zagory, D.; Kader, A.A. Modified atmosphere packaging of fresh produce. Food Technol. 1988, 42, 70–77. [Google Scholar]
- Crisosto, C.H.; Garner, D.; Crisosto, G.M.; Sibbett, S.; Day, K.R. Late harvest and delayed cooling induce internal browning of ‘Ya Li’ and ‘Seuri’ chinese pears. HortScience 1994, 29, 667–670. [Google Scholar] [CrossRef]
- Kluge, R.A.; Nachtigal, J.C.; Bilhalva, A.B. Fatores que afetam a qualidade e a deterioração das frutas em pós-colheita. In Fisiologia e Manejo Pós-Colheita de Frutas de Clima Temperado, 2nd ed.; Editora Rural: São Paulo, Brasil, 2002; pp. 21–26. [Google Scholar]
- Bulit, J.; Dubos, B. Botrytis bunch rot and blight. In Compendium of Grape Diseases; Pearson, R.C., Goheen, A.C., Eds.; APS Press: St. Paul, MN, USA, 1990; pp. 13–15. [Google Scholar]
- Saito, S.; Xiao, C.L. Evaluation of sulfur dioxide-generating pads and modified atmosphere packaging for control of postharvest diseases in blueberries. Acta Hortic. 2017, 1180, 123–128. [Google Scholar] [CrossRef]
- Kader, A.A. Postharvest Technology of Horticultural Crops—An Overview from Farm to Fork. Ethiop. J. Appl. Sci. Technol. 2013, 1, 1–8. [Google Scholar]
- Youssef, K.; Chaves Junior, O.J.; Mühlbeier, D.T.; Roberto, S.R. Sulphur Dioxide Pads Can Reduce Gray Mold While Maintaining the Quality of Clamshell-Packaged ‘BRS Nubia’ Seeded Table Grapes Grown under Protected Cultivation. Horticulturae 2020, 6, 20. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).