Abstract
Oleander is very frequently planted as an ornamental shrub in urban areas of the Mediterranean. Its most common pest is the aphid Aphis nerii, and heavy infestations produce aesthetic damage and disturbances to the citizens, and they are frequently sprayed with insecticides in order to reduce the population density. One alternative method is conservation biological control which is enhanced by the provisioning of alternative food, refuges, and reproduction sites. In this study, the usefulness of four flowering service plants (Lobularia maritima, Calendula officinalis, Coreopsis grandiflora, and Achillea millefolium) is evaluated for aphid infestation levels and aphid natural enemy abundances. Aphid levels were consistently lower in oleander plots surrounded by service plants. Natural enemy abundances tended to be higher in plots surrounded by service plants, but significant differences could not be found until the fourth year of the study, when more lacewings, total predators, and mummies were higher in plots surrounded by service plants. On these plots, hoverflies, ladybeetles, and lacewings were the most common predators. Paragus sp. and Hippodamia variegata were the prevalent hoverfly and ladybeetle species, respectively. The potential toxicity effect on the prevalence of natural enemy species is discussed. On service plants, significantly more hoverflies were recorded on L. maritima than on C. officinalis and C. grandiflora, and more true bugs were recorded on C. officinalis and A. millefolium than on L. maritima or C. grandiflora. Our results suggest that planting service plants (such as sweet alyssum, marigold, or yarrow) surrounding oleanders can improve conservation biological control by enhancing the abundance of aphid natural enemies and thus a reduction of the abundance of A. nerii.
1. Introduction
Oleander (Nerium oleander L., Gentianales: Apocynaceae) is a Eurasian plant species that is cultivated and distributed worldwide. It is very frequently planted as an ornamental shrub in urban areas of the Mediterranean as fences in streets or parks, but also in roundabouts and in central hedges on highways [1,2].
Several arthropod groups live on oleander (aphids, scales, mites, etc.), but the most common one in the Mediterranean region is the milkweed-oleander aphid Aphis nerii Boyer de Fonscolombe (Hemiptera: Aphididae). It is a native of the Mediterranean region and an invasive species throughout much of the world, living mainly on Apocynaceae and forming big colonies on growing shoots of oleanders [3,4].
In the Mediterranean region, infestations of A. nerii on oleanders produce aesthetic damage and disturbances to citizens. In order to reduce the aphids’ population density, they are frequently sprayed with insecticides. However, concerns about the use of pesticides in urban areas are increasing. According to the European Union Directive 129/2009, integrated pest management strategies should be developed to control pest of agricultural crops but also of non-agricultural areas (such as green spaces), and the use of pesticides should be regarded as the last tool for managing pests. To implement this directive, alternatives to chemical control need to be developed, an option being biological control.
Methods that enhance biodiversity have been proposed as the main strategy for biological pest control to be used in urban areas [5,6,7,8]. This strategy includes the management of the habitat in a way that attracts predators and parasitoids already living in urban ecosystems to increase their pest control efficiency. The provision of alternative prey or hosts, food for adult parasitoids, and refuges and places for egg laying have been proclaimed as effective resources for the establishment of natural enemies and the development of their ecological functions. The provision of resources must be integrated in a way that is spatially and temporally favorable to natural enemies and practical for pest control advisors to implement [9]. Shrewsbury and Raupp [10,11] reported the azalea lace bug, Stephanitis pyroides (Scott) (Hemiptera: Tingidae), to be extremely abundant in simple landscapes and rare in complex ones. Planting herbaceous species with flowering periods that overlap with the pest attack period is one method to increase diversity and provide resources necessary for the natural enemy’s establishment and improve its fitness and function [12,13,14,15,16]. In urban green areas, service plants have been employed to enhance the biological control of different ornamental plant pests [12,13], but no specific studies on aphids have been found.
The aim of the present study was to determine how increasing biodiversity by planting flowering herbaceous species around oleanders can contribute to the reduction of aphid population densities. For this, (1) we compared aphid and natural enemy abundances on oleanders growing with or without flowering plants in their close surroundings, and (2) we determined the abundance of natural enemies on the flowering plants.
2. Materials and Methods
The experiment was conducted during four growing seasons (2012–2015) in the Arboretum Pius Font i Quer in Lleida, Catalonia, Spain, NE of the Iberian Peninsula (coordinates, 41°37′33″ N and 0°35′55″ E, altitude 183 m).
The experimental setup consisted of eight plots of 9 square meters. In each plot, nine plants of ornamental oleander (1 m in height), obtained from a nursery in Lleida, were planted during the first of the four study years.
Plots were randomly assigned to be surrounded by flowering plants or not. These plants will be termed “service plants” in the following text. Four of the plots were surrounded by a strip (50 cm wide) of service plants (F) and four plots were not (NF). After planting the service plant, the distance between the plots was 2 m. The service plant species used in the experiment were: sweet alyssum (Lobularia maritima (L.) Desv.), marigold (Calendula officinalis L.), large-flowered tickseed (Coreopsis glandiflora Hogg ex Sweet), and yarrow (Achillea millefolium L.). Sweet alyssum is a Brassicaceae with white flowers and a long flowering period from spring to early winter. The three other plant species belong to the Asteraceae family. Marigold has orange flowers and a flowering period from March to September. Large-flowered tickseed has yellow-golden flowers, flowering from May to September. Finally, yarrow has a variety of flower colours (white to purple) and flowers from May to October. We selected a variety of pink flowers in order to increase the range of flower colours in the experiment. All the flowering periods correspond to the growing season of the Mediterranean region. Sweet alyssum was selected because it has already been used as a natural enemy attraction plant in several horticultural studies [17,18,19,20,21]. The flowers of Asteraceae have been reported to be attractive to several predators in horticultural and other crops [20,21,22,23,24,25]. In the F plots, each side of the plot was randomly planted with one of these four species. Plants were obtained from the same nursery as oleanders. The oleanders and service plants were drip irrigated and maintained during the four years by the staff of the arboretum.
The beginning of the sampling period varied between years. In 2012, the sampling started in mid-June when the plantation of oleanders was finished in 2013 in mid-July due to a late aphid infestation that season, and in 2014 and 2015, the second half of May. After the onset of sampling, the plots were monitored every 7–15 days, according to the development of aphid populations.
Plant sampling was performed on days without rain between 8:00 and 14:00 h. Nine oleander apical shoots (25 cm long) per plot were monitored, and the occurrence of A. nerii was recorded according to the aphid scale of abundance developed by [26] (Table 1). The levels of the scale correlated well with the number of aphids in the sampling unit, and the honeydew damage of the aphids, measured as the number of drops fallen per hour [26,27]. It has previously been used to determine aphid abundances in several tree and shrub species of urban green areas in Spain [27]. The number of predators on these nine shoots per plot was also recorded, as well. If possible, predators were identified in the field at the genus or species level. If not, they were identified to the lowest taxon level that we were able to recognize. The number of not yet emerged aphid mummies was also recorded, but they were not identified during field samplings. However, some mummies were regularly brought to the laboratory and maintained in rearing cages for three weeks at 22 °C, 16:8 day/night photoperiod and 70% relative humidity until parasitoid adult emergence. The parasitoid species that emerged were identified.

Table 1.
Scale of infestation severity of aphids in oleanders [26,27].
Additionally, we recorded the presence of aphids and the number of predators occurring on the service plants. For that, we observed each service plant strip for one minute. The identification criteria were the same as those described for oleanders.
Data Analysis
- (1)
- Because the aphid abundance was estimated as levels of infestation, this variable was categorical and ordinal, and the differences between treatments (NF vs. F) were analysed by a Chi-squared test, taking into account the total absolute frequency of each level along the yearly samplings;
- (2)
- Data of the abundance of predators and mummies on oleander were checked for normality by a Shapiro–Wilk test and analysed by an ANOVA of repeated measures where the treatment was the main effect and the sampling date the repeated factor;
- (3)
- The number of predators recorded on service plants was low on most of the sampling dates. For this reason, the cumulated number across the sampling period was analysed. Predator abundances were compared between service plants. Data were checked by a Shapiro–Wilk test and because the conditions of normality were not achieved, even when the data were transformed to log (x + 1), the comparison between F and NF plots was made by a Kruskall–Wallis test. When significant differences occurred, multiple comparisons were performed with the pairwise Dunn’s test, applying the Bonferroni correction. Mummies on service plants were not analysed because of the low numbers that could be recorded.
Statistical analyses were performed using R software version 3.5.3, Vienna, Austria [28].
3. Results
3.1. Aphid Infestation
The occurrence of aphid species other than A. nerii, particularly Aphis fabae, was very low, and only winged individuals and small colonies were found, especially in 2012 and 2013. They have, thus, not been included in the results. Therefore, the aphid key species on oleander was A. nerii, and all the results presented refer to it.
Aphis nerii occurred on oleanders from May to the end of October. However, its population dynamics and infestation severity varied from year to year. The periods of peak aphid abundance occurred at the end of July in 2012, the end of August in 2013, at the end of May in 2014, and from mid-July to mid-August in 2015. After the peaks, the aphid populations decreased, but, different from what is usual for many aphid species on different plants, the populations of A. nerii did not collapse, and the decrease of the population was rather slow. In 2015, the aphid abundance was even quite stable during most of the sampling period. Plots surrounded by service plants (F) showed a lower proportion of high infestation levels than plots not surrounded (NF) (Figure 1) quite regularly through the season, and this difference was consistently significant during the four-year study (Table 2).
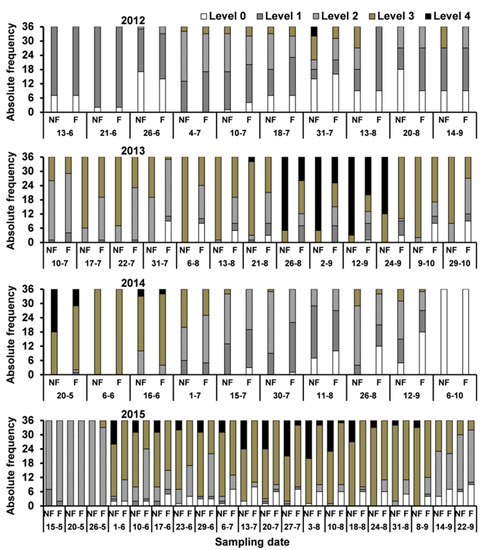
Figure 1.
Absolute frequency of infestation levels of A. nerii on oleanders surrounded with (F) and without (NF) service plants in the study period (2012–2015). Levels correspond to the scale of infestation severity of aphids in oleanders cited in Table 1 [26,27].

Table 2.
Chi-squared test and p-values for comparing absolute frequencies of infestation levels in plots surrounded with (F) and without (NF) service plants (sweet alyssum, marigold, large-flowered tickseed, and yarrow) in each of the study years (2012–2015).
3.2. Predator Abundance on Oleander
The abundance of predators varied between predator groups and years. Hoverflies (Diptera: Syrphidae) were, as a group, the most abundant predators in all four study years. Ladybeetles (Coleoptera: Coccinellidae) and lacewings (Neuroptera: Chrysopidae) were the other two most common predators. These three groups comprised more than 90% of the specimens recorded. The comparison of the predator abundances between the F and NF plots are, thus, only presented for these three groups and for the total of the predators. Other predators occurring through the sampling periods were pirate bugs of the genus Orius (Hemiptera: Anthocoridae), damsel bugs (Hemiptera: Nabidae), true bugs (Hemiptera: Miridae), big eye bugs (Hemiptera, Lygaeidae), earwigs from the species Forficula auricularia (Dermaptera: Forficulidae), larvae of Aphidoletes aphidimyza (Diptera: Cecidomyiidae), larvae of chamaemiids (Diptera: Chamaemiidae) from the genus Leucopis sp., rove beetles (Coleoptera: Staphylidae), mites (Acari: Trombididae), and spiders (not identified).
Most of the hoverflies were recorded as larvae and, therefore, cannot be identified to a genus or species level. Among the adults, we identified the following species and genera: Eupeodes corollae (F.), Episyrphus balteatus (De Geer), Scaeva pyrastri (L.), Sphaerophoria scripta (L.), Sphaerophoria rueppellii (Wiedemann), Paragus sp., and Melanostoma sp. Paragus sp. was the most abundant hoverfly in each year, especially in 2015, followed by S. scripta and E. balteatus. No significant differences in the hoverfly abundance between the F and NF plots were found (Table 3), although a trend towards a higher abundance on the F than the NF plots was frequently observed (Figure 2).

Table 3.
F and p values from the ANOVA of repeated measures for the main aphid predators and aphid mummy abundance in each study year.
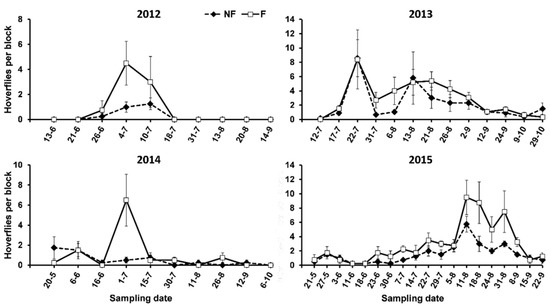
Figure 2.
Abundance of hoverflies on oleanders surrounded (F) and not surrounded (NF) by service plants in the study period.
Hippodamia variegata (Goeze) was the most abundant ladybeetle on the oleanders every year. It represented 92% of all ladybeetles found in the study, and all of the development stages of this species were recorded. Adults of other species were observed: Propylea quatuordecimpunctata (L.), Oenopia conglobata (L.), Adalia decempunctata (L.), Coccinella septempunctata L., and the genus Scymnus sp. Except for Scymnus sp., which was recorded in three out of the four years, the occurrence of the other species was casual. No significant differences in ladybeetle abundance were found between the F and NF plots (Table 3), although we regularly recorded more individuals on the F than the NF plots (Figure 3).
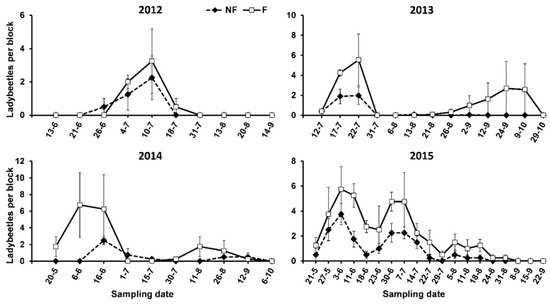
Figure 3.
Abundance of ladybeetles in oleanders surrounded (F) and not surrounded (NF) by service plants in the study period.
Eggs, larvae, pupae, and adults of Chrysopidae were recorded. All of the adults and nearly all of the egg clusters belonged to the complex Chrysoperla carnea (Stephens). Egg clusters of other species were observed sporadically. The abundance of lacewings varied very much from one year to the other, with the abundance being very low in 2012 and 2014, with higher abundances in 2013 and, especially, in 2015. Significantly higher abundances on the F than on the NF plots were found in 2015 (Table 3, Figure 4).
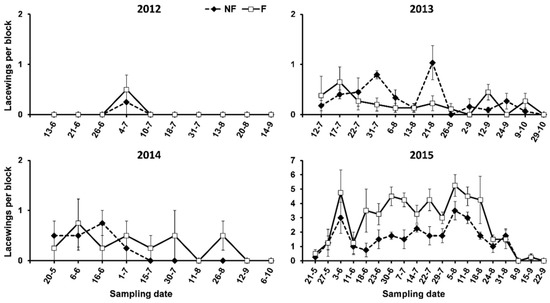
Figure 4.
Abundance of lacewings on oleanders in plots surrounded (F) and not surrounded (NF) by service plants in the study period.
When the cumulated number of predators was analysed, their abundances were higher on the F than the NF plots in most of the sampling dates, but significant differences were only found in 2015 (Table 3, Figure 5).
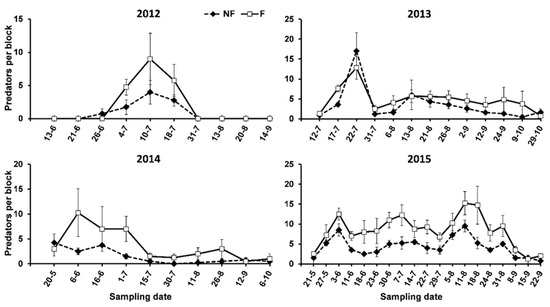
Figure 5.
Abundance of total predators on oleanders surrounded (F) and not surrounded (NF) by service plants in the study period.
3.3. Mummies on Oleanders
Most of the mummies were recorded during the first half of the seasons (Figure 6) and followed a similar pattern of abundance, firstly increasing exponentially and decreasing more or less quickly after peaking. In 2012 and 2013, mummies were recorded mainly in July, and the peak number occurred in the middle of the month. In 2014 and 2015, the mummies were already recorded in May, and the number of them peaked at the beginning or at the end of June in 2014 and 2015, respectively. Significant differences between the F and NF plots in mummy abundance only occurred in 2015 (Table 3), although we recorded more on the F plots in some of the other years (Figure 6).
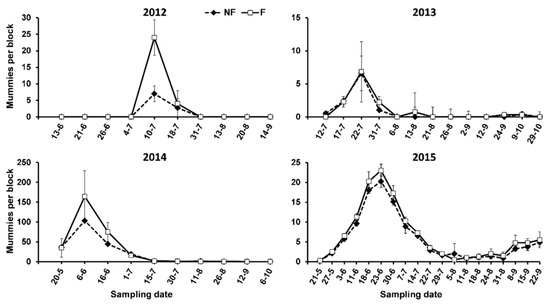
Figure 6.
Abundance of aphid mummies on oleanders surrounded (F) and not surrounded (NF) by service plants in the study period.
The mummies reared in the laboratory until adult emergence always resulted in Lysiphlebus testaceipes (Cresson).
3.4. Aphid Occurrence on Service Plants
Only small colonies of aphids were observed on the service plants. The aphid abundance was not recorded. Aphids on sweet alyssum were identified as Aphis gossypii (Glover), and aphids on the other service plants (Asteraceae) belonged to the Aphis fabae group.
3.5. Predator Abundance on Service Plants
A total of 691 individuals were recorded during the study period. Hoverflies, true bugs, spiders, lacewings, and ladybeetles were the most common predators that could be recorded. Hoverflies and true bugs accounted for 35.0% and 32.1% of the total predators, respectively. On the contrary, ladybeetles only accounted for 5.5%. Lacewings accounted for 11.2% and spiders 15.7%. Sporadically, specimens of other predators such as pirate bugs, damsel bugs, big eye bugs, earwigs, praying mantis (Dyctioptera: Mantidae), rove beetles, and ground beetles (Coleoptera: Carabidae) were observed.
Paragus sp., S. scripta, and Melanostoma sp. were the most abundant hoverflies, especially Paragus sp., which accounted for 67.5% of the total hoverflies. The presence of E. corollae, E. balteatus, and S. rueppellii was also recorded. Among the ladybeetles, H. variegata accounted for 52.6% and Scymnus sp. for 28.9%. Other ladybeetles recorded were C. septempunctata, O. conglobata, and A. decempunctata. True bugs and spiders were not identified in detail and were considered as a group. The lacewings were identified as a C. carnea group.
The service plants harboured the predator groups differently (Figure 7). Significantly more hoverflies were recorded on sweet alyssum than on calendula, and coreopsis and more true bugs were recorded on calendula and yarrow than on sweet alyssum or large-flowered tickseed (Table 4).
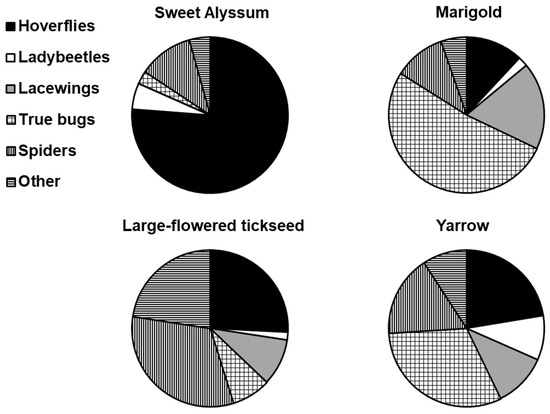
Figure 7.
Proportion (%) of predatory groups recorded on service plants: sweet alyssum (L. maritima), marigold (C. officinalis), large-flowered tickseed (C. grandiflora), and yarrow (A. millefolium).

Table 4.
Mean rank, χ² and p of the Kruskall–Wallis test from the abundance comparison of predator groups between service plant species. For each predator, values followed by different letters show statistical differences between service plants.
4. Discussion
The inclusion of flowering service plants can increase the structural complexity and floristic diversity of urban landscapes [10,11], and this is expected to be important for biodiversity conservation and to ensure the provision of ecosystem services as a biological control [6,7,8,12,13,29,30]. Our results indicate that the presence of flowering service plants such as sweet alyssum, marigold, large-flowered tickseed, and yarrow can contribute to the conservation and augmentation of the most common predators that prey on the main pest of oleanders, A. nerii.
4.1. Aphid Infestation
Outbreaks of A. nerii only occur in places where the plants’ growing conditions result in an increased proportion of actively growing shoot terminals [31], as it occurs in urban landscapes. This was what happened in the studied experimental plots, where aphid populations were very abundant, especially in 2013, 2014, and 2015. In 2012, the plants were probably too young to allow the aphids to develop populations that were as large as in the following three years.
The consistently lower infestation in the F compared to the NF plots in every year of the four-year study suggests that the presence of service plants efficiently reduces A. nerii infestations. One pathway could be via the open soil through which the plant canopy temperature is elevated, which may increase the development rate and fecundity of A. nerii. However, it was shown that the crape myrtle aphid, Tinocallis kahawaluokalani (Kirkaldy), decreased its abundance with hotter canopy temperatures [8]. The effect of service plants in reducing aphid populations by promoting biological control by predators or parasitoids has been reported for several crops, in part by using the same service plant species as we have used [18,32,33,34,35,36,37,38,39,40,41,42]. In urban landscapes, service plants have also been employed to enhance the biological control of different pests [12,13,43], but no previous studies reporting aphid reduction have been found.
4.2. Natural Enemies of A. nerii on Oleanders
Although the abundance of the predator groups between oleander plots surrounded by service plants and plots not surrounded by service plants were not significantly different, except in 2015, the abundances of hoverflies, ladybeetles, lacewings, total predators, and mummies tended to be higher on oleanders surrounded by service plants.
Explanations for the lack of significant differences could be the consequence of the limitations of the sampling methods: (1) The movements of the adult predators between the plots or between the plots and nearby plants were not recorded [44]. No data on the time that a predator remained in each type of plot (NF and F) was recorded, but this could be a factor to consider in future studies in order to find the differences between the plots. (2) The sampling was only performed during the day, when some of the most abundant predators may have foraged in the night [12], e.g., hoverfly larvae. (3) Not all components of the natural enemy community were sampled; for example, ground-dwelling predators (such as ground beetles, rove beetles, or spiders), which can also prey on aphid populations in urban areas [45]. (4) Plant age may be an additional reason. In fact, statistically significant differences were only found three years after the oleanders had been planted. In spite of this, our results suggest that natural enemies have the potential to contribute to the reduction of A. nerii abundance. This is in line with an increase in aphid parasitism due to the higher plant richness in urban agroecosystems shown in [30].
Oleanders are toxic due to high levels of cardiac glycoside cardenolides, particularly olenadrin and neriin [46,47]. These toxins are ingested by aphids, sequestered, and excreted in the honeydew [48] and could have determined the species composition of the predatory groups.
Aphis nerii on oleanders is poisonous to many coccinellid species, except for H. variegata [47] and Cydonia vicinia (Mulsant) [49] (El-Shazly 2002). Accordingly, the main species in our experiment was H. variegata, and it was the only one for which the occurrence of larvae was recorded. Only the adults of the other species were recorded, and they were probably there searching for aphids but were unable to reproduce on the oleander. No records of larvae of other coccinellid species have been reported from field samplings of oleander in Lleida and the other cities of Catalonia [50,51].
The lower fitness of the hoverfly, S. rueppellii, predating on A. nerii compared to the individuals predating on Rhoplaosiphum padi L. and Myzus persicae Sulzer was attributed to the plant’s toxicity [52]. No data on Paragus sp. could be found, but a higher tolerance to aphid toxicity could be a reason for the dominance of this genus in our study.
Aphis nerii may negatively affect the fitness of C. carnea [53]. In spite of this, we recorded larvae and adults on our plots, as well as in other oleanders of the city and the region [51].
Detrimental effects on parasitoids may also occur. Aphis nerii has been reported as an unsuitable host species for Binodoxys communis (Gahan), Aphidius ervi (Haliday), Diaretiella rapae (M’Intosh), and Aphelinus abdominals (Dalman) [54,55]. Like in most areas of Catalonia, the collected mummies reared in our lab always resulted in L. testaceipes [56]. This suggests an adaptation of this parasitoid to the oleander aphid. No defensive endosymbionts have been reported in A. nerii [57], and factors that allow this adaptation may be focused more on the aphids’ toxin tolerance [55] than on aphid defence symbionts.
4.3. Aphids and Predators on Service Plants
Aphid-specific and polyphagous predators were recorded on all four of the service plants but in different proportions. These differences suggest that although all of these plants harbor natural enemies, the addition of plants with different functional traits may improve their attractiveness, as they play different roles for distinct natural enemies.
In our experiment, hoverflies were the most abundant predators recorded on sweet alyssum. This plant has been reported to be attractive to several aphid and polyphagous predators, such as hoverflies, ladybeetles, spiders, pirate bugs, and others [34,35,38,41,58]. In the Mediterranean region, sweet alyssum has been reported to enhance native hoverfly populations in greenhouses providing pollen food resources for adults [59]. Yarrow has been reported as attractive to hoverflies as well [22], and, in our study, hoverflies were the second relatively abundant predator group on this service plant. Paragus sp. was present on both the service plants and the oleanders, which suggests the ability of these species to move between them.
Besides this, marigold and yarrow harbored more true bugs than the other service plants. The role of marigold as a floral resource for true bugs and pirate bugs has previously been reported [23,37]. However, true or pirate bugs were not prevalent in the oleanders. On the other side, marigold was a poor resource for lacewings, which agrees with the findings of field surveys carried out in woody Mediterranean agroecosystems [24].
None of the service plants were especially attractive for H. variegata, and it seems that the occurrence of this ladybeetle on oleander is not enhanced by service plants. Probably, H. variegata, being an aphidophagous species able to avoid the toxic effects of A. nerii, is attracted directly by oleander/aphid cues and not by alternative food resources provided by the service plant species included in this study.
Lacewings and spiders were prevalent on the large-flowering tickseed. This plant species could, thus, contribute to the occurrence of the lacewings on the oleanders. In the case of spiders, the lack of correspondence between their relative abundance on the oleanders and on the large-flowering tickseed may be due to the different spider species living on herbaceous plants and shrubs [60], but this is speculative because a spider species or groups were not identified.
The abundance of natural enemies in the service plants was different between plants; this may be related to the different functional traits of each plant and may contribute to enhancing the attraction of different guilds of natural enemies [23].
5. Conclusions
Aphis nerii is the prevalent aphid species on oleanders, and Paragus sp. and H. variegata are the most abundant predators. Oleanders surrounded by service plants tended to harbor more natural enemies (hoverflies, ladybeetles, lacewings, and mummies), although significant differences could only be found in the fourth year of the study. The service plants used in the trials (L. maritima, C. officinalis, C. grandiflora, and A. millefolium) harbored predator groups differently. Our results show that the presence of flowering service plants can help to reduce A. nerii infestation levels and to enhance the presence of its main natural enemies. Therefore, the inclusion of service plants with flowering periods that overlap with oleander aphid infestation can contribute to the conservation biological control of A. nerii in Mediterranean regions where oleanders are planted as fences, in roundabouts, or in small islands in public gardens.
Author Contributions
B.L. and X.P. conceived and designed the experiments; B.L. performed the experiments; X.P. analysed the data, and F.M. contributed to the data analysis; X.P. wrote the manuscript; all authors read, discussed, and approved the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. F.M. thanks the national funding by the FCT—the Foundation for Science and Technology—through the institutional scientific employment program contract. The authors are grateful to the Foundation for Science and Technology (FCT, Portugal) for their financial support through national funds, FCT/MCTES (PIDDAC) to CIMO (UIDB/00690/2020 and UIDP/00690/2020) and SusTEC (LA/P/0007/2020). B.L. was funded by the project UdL-Impuls (the University of Lleida and Bank of Santander) X10020.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available from the authors upon reasonable request.
Acknowledgments
We thank César del Arco and Antoni Conesa for allowing us to set up this study in the arboretum Pius Font i Quer in Lleida. We also want to thank Miguel Velo and Pierluigi Forlano for helping us with the sampling. We thank Verena Rösch for reviewing the manuscript and the English language.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Starý, P.; Lumbierres, B.; Pons, X. Opportunistic changes in the host range of Lysiphlebus testaceipes (Cr.), an exotic aphid parasitoid expanding in the Iberian Peninsula. J. Pest. Sci. 2004, 77, 139–144. [Google Scholar] [CrossRef]
- Farooqui, S.; Tagi, T. Nerium oleander: Its application in basic and applied science. A review. Int. J. Pharm. Pharm. Sci. 2018, 10, 1–4. [Google Scholar] [CrossRef]
- Blackman, R.L.; Eastop, V.F. Aphids on the World’s Plants. An Online Identification and Information Guide. 2021. Available online: http://www.aphidsonworldsplants.info (accessed on 1 July 2022).
- Martín, A.; Planas, S.; Pons, X.; Taberner, A.; Camp, F.; Giné, J. Guía de Gestión Integrada de Plagas en Parques y Jardines; Ministerio de Agricultura, Pesca y Alimentación, Secretaria General Técnica, Centro de Publicaciones: Madrid, Spain, 2020. [Google Scholar]
- Shrewsbury, P.; Leather, S.R. Using biodiversity for pest suppression in urban landscapes. In Biodiversity and Insect Pests: Key Issues for Sustainable Management, 1st ed.; Gurr, G.M., Wratten, S.D., Snyder, W.E., Read, D.M.Y., Eds.; John Wiley and Sons Ltd.: Chichester, UK, 2012; pp. 293–308. [Google Scholar]
- Philpott, S.M.; Bichier, P. Local and landscape drivers of predation services in urban gardens. Ecol. Appl. 2017, 27, 966–976. [Google Scholar] [CrossRef] [PubMed]
- Rocha, E.A.; Souza, E.N.F.; Beakley, L.A.D.; Burley, C.; Mott, J.L.; Rue-Glutting, G.; Fellowes, M.D.E. Influence of urbanization and plants on the diversity and abundance of aphids and their ladybird and hoverfly predators in domestic gardens. Eur. J. Entomol. 2018, 115, 140–149. [Google Scholar] [CrossRef]
- Parsons, S.E.; Sozanski, K.S.; Wilson, A.A.; Frank, S.D. Effect of temperature and habitat complexity on an urban tree pest (Tinocallis kahawaluokalani), natural enemies, and predation services in city. Urban Ecosyst. 2020, 23, 13–26. [Google Scholar] [CrossRef]
- Landis, D.A.; Wratten, S.D.; Gurr, G.M. Habitat management to conserve natural enemies of arthropod pests in agriculture. Annu. Rev. Entomol. 2000, 45, 175–201. [Google Scholar] [CrossRef]
- Shrewsbury, P.M.; Raupp, M.J. Evaluation of components of vegetational texture for predicting azalea lace bug, Stephanitis pyroides (Heteroptera: Tingidae), abundance in managed landscapes. Environ. Entomol. 2000, 20, 919–926. [Google Scholar] [CrossRef]
- Shrewsbury, P.M.; Raupp, M.J. Do top-down or bottom-up forces determine Stephanitis pyroides abundance in urban landscapes? Ecol. Appl. 2006, 16, 262–272. [Google Scholar] [CrossRef]
- Shrewsbury, P.M.; Lashomb, J.H.; Hamilton, G.C.; Zhang, J.; Patts, J.; Casagrande, R.A. The influence of flowering plants on herbivore and natural enemy abundance in ornamental landscapes. Int. J. Ecol. Environ. Sci. 2004, 30, 23–33. [Google Scholar]
- Ellis, J.A.; Walter, A.D.; Tooker, J.F.; Ginzel, M.D.; Reagel, P.F.; Lacey, E.S.; Bennet, A.B.; Grossman, E.M.; Hanks, L.M. Conservation biological control in urban landscapes: Manipulating parasitoids of bagworm (Lepidoptera: Psychidae) with flowering forbs. Biol. Control 2005, 34, 99–107. [Google Scholar] [CrossRef]
- Mody, K.; Lerch, D.; Müller, A.-K.; Simons, N.K.; Blüthgen, N.; Harnish, M. Flower power in the city: Replacing roadside shrubs by wildflower meadows increases insect numbers and reduces maintenance costs. PLoS ONE 2020, 15, e0234327. [Google Scholar] [CrossRef] [PubMed]
- Heimpel, G.E.; Jervis, M.A. Does nectar improve biological control by parasitoids? In Plant-Provided Food for Carnivorous Insects: A Protective Mutualism and Its Applications; Wäckers, F.L., Rijn, P.C.J., Bruin, J., Eds.; Cambridge University Press: Cambridge, UK, 2005; pp. 269–304. [Google Scholar]
- Wäckers, F.L.; Romeis, J.; van Rijn, P. Nectar and pollen feeding by insect herbivores and implications for multitrophic interactions. Annu. Rev. Entomol. 2007, 52, 301–323. [Google Scholar] [CrossRef] [PubMed]
- Fiedler, A.K.; Landis, D.A.; Wratten, S.D. Maximizing ecosystem services from conservation biological control: The role of habitat management. Biol. Control 2008, 45, 254–271. [Google Scholar] [CrossRef]
- Araj, S.-E.; Wratten, S.D. Comparing existing weeds and commonly used insectary plants as floral resources for a parasitoid. Biol. Control 2015, 81, 15–20. [Google Scholar] [CrossRef]
- Arnó, J.; Oveja, M.F.; Gabarra, R. Selection of flowering plants to enhance the biological control of Tuta absoluta using parasitoids. Biol. Control 2018, 122, 41–50. [Google Scholar] [CrossRef]
- Chen, Y.; Mao, J.; Reynolds, O.L.; Chen, W.; He, W.; You, M.; Gurr, G.M. Alyssum (Lobularia maritima) selectively attracts and enhances the performance of Cotesia vestalis, a parasitoid of Plutella xylostella. Sci. Rep. 2020, 10, 6447. [Google Scholar] [CrossRef]
- Zhang, L.; Quin, Z.; Liu, P.; Yin, Y.; Felton, G.W.; Shi, W. Influence of plant physical and anatomical characteristics on the ovipositional preference of Orius sauteri (Hemiptera: Anthocoridae). Insects 2021, 12, 326. [Google Scholar] [CrossRef]
- Colley, M.R.; Luna, J.M. Relative attractiveness of potential insectary plants to aphidophagous hoverflies (Diptera: Syrphidae). Environ. Entomol. 2000, 29, 1054–1059. [Google Scholar] [CrossRef]
- Balzan, M.V. Flowering banker plants for the delivery of multiple agroecosystems services. Arthropod Plant Interact. 2017, 11, 743–754. [Google Scholar] [CrossRef]
- Alcalá Herrera, R.; Ruano, F.; Gálvez Ramírez, C.; Frischie, S.; Campos, M. Attraction of Green lacewings (Neuroptera: Chrysopidae) to native plants used as ground cover in woody Mediterranean ecosystems. Biol. Control 2019, 139, 104066. [Google Scholar] [CrossRef]
- Ardanuy, A.; Figueras, M.; Matas, M.; Arnó, J.; Agustí, A.; Alomar, O.; Albajes, R.; Gabarra, R. Banker plants and landscape composition influence colonisation precocity of tomato greenhouses by mirid predators. J. Pest Sci. 2021, 95, 447–459. [Google Scholar] [CrossRef]
- Lumbierres, B.; Fornells, E.; Pons, X. Fenología, dinámica poblacional y daños de Eucallipterus tiliae Linnaeus (Horn., Aphididae) en tilos ornamentales de la ciudad de Lleida. Bol. Sanid. Veg. Plagas 2004, 30, 533–561. [Google Scholar]
- Pons, X.; Lumbierres, B. New aphid species recently affecting trees in urban green areas of Catalonia. In Proceedings of the AFPP—2ème Conférence sut l’entretien des espaces verts, jardins, gazons, forêts, zones aquatiques et autres zones non agricoles, Angers, France, 28–29 October 2009; pp. 36–45. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Haaland, C.; Naisbit, R.E.; Bersier, L.-F. Sown wildflower strips for insect conservation: A review. Insect Conserv. Divers 2011, 4, 60–80. [Google Scholar] [CrossRef]
- Arnold, J.E. Biological control services from parasitic Hymenoptera in urban agriculture. Insects 2022, 13, 467. [Google Scholar] [CrossRef] [PubMed]
- Hall, R.W.; Ehler, L.E. Population ecology of Aphis nerii on oleander. Environ. Entomol. 1980, 9, 338–344. [Google Scholar] [CrossRef]
- Hickman, J.M.; Wratten, S.D. Use of Phacelia tanacetifolia strips to enhance biological control of aphids by hoverfly larvae in cereal fields. J. Econ. Entomol. 1996, 89, 832–840. [Google Scholar] [CrossRef]
- Nicholls, C.I.; Parella, M.P.; Altieri, M.A. Reducing the abundance of leafhoppers and thrips in a northern California organic vineyard through maintenance of full seasonal floral diversity with summer cover crops. Agric. For. Entomol. 2000, 2, 107–113. [Google Scholar] [CrossRef]
- Hogg, B.N.; Nelson, E.H.; Mills, N.J.; Daane, K. Floral resources enhance aphid suppression by a hoverfly. Entomol. Exp. Appl. 2011, 141, 138–144. [Google Scholar] [CrossRef]
- Hogg, B.N.; Bugg, R.L.; Daane, K.M. Attractiveness of common insectary and harvestable floral resources to beneficial insects. Biol. Control 2011, 56, 76–84. [Google Scholar] [CrossRef]
- Tschumi, M.; Albrecht, M.; Collatz, J.; Dubsky, V.; Entling, M.H.; Najar-Rodriguez, A.J.; Jacot, K. Tailored flower strips promote natural enemy biodiversity and pest control in potato crops. J. Appl. Ecol. 2016, 53, 1169–1176. [Google Scholar] [CrossRef]
- Zhao, J.; Guo, X.; Tan, X.; Desneux, N.; Zappala, L.; Zhang, F.; Wang, S. Using Calendula officinalis as a floral resource to enhance aphid and thrips suppression by the flower bug Orius sautieri (Hemiptera: Anthocoridae). Pest Manag. Sci. 2017, 73, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.L.; Gontijo, L.M. Alyssum flowers promote biological control of collar pests. BioControl 2017, 62, 195–196. [Google Scholar] [CrossRef]
- Damien, M.; Le Land, C.; Desneux, N.; Alford, L.; Al Hasan, D.; Geoeges, R.; van Baaren, J. Flowering cover crops in winter increase pest control but not trophic link diversity. Agric. Ecosyst. Environ. 2017, 247, 418–425. [Google Scholar] [CrossRef]
- Jado, R.H.; Araj, S.E.; Abu-Irmaileh, B.; Shields, M.W.; Wratten, S.D. Floral resources to enhance the potential of the parasitoid Aphidius colemani for biological control of the aphid Myzus persicaex. J. Appl. Entomol. 2019, 143, 23–42. [Google Scholar] [CrossRef]
- Tiwari, S.; Sharma, S.; Wratten, S.D. Flowering alyssum (Lobularia maritima) promote arthropod diversity and biological control of Myzus persicae. J. Asia-Pac. Entomol. 2020, 23, 634–640. [Google Scholar] [CrossRef]
- Aparicio, Y.; Riudavets, J.; Gabarra, R.; Agustí, N.; Rodriguez-Gasol, N.; Alins, G.; Blasco-Moreno, A.; Arnó, J. Can insectary plants enhance the presence of natural enemies of the Green peach aphid (Hemiptera: Aphididae) in Mediterranean peach orchards? J. Econ. Entomol. 2021, 114, 784–793. [Google Scholar] [CrossRef]
- Nighswander, G.P.; Sinclair, J.S.; Dale, A.G.; Qiu, J.; Iannone III, B.V. Importance of plant diversity and structure for urban garden pest resistance. Landsc. Urban Plan. 2021, 215, 104211. [Google Scholar] [CrossRef]
- Tooker, J.F.; Hanks, L.M. Influence of plant community structure on natural enemies of pine needle scale (Homoptera: Diaspididae) in urban landscapes. Environ. Entomol. 2000, 29, 1305–1311. [Google Scholar] [CrossRef]
- Rocha, E.A.; Fellowes, M.D.E. Urbanisation alters ecological interactions: Ant mutualists increase and specialist insect predators decrease on an urban gradient. Sci. Rep. 2020, 10, 6406. [Google Scholar] [CrossRef]
- Rotschild, M.J.; Von Euw, J.; Reichtein, T. Cardiac glycosides in the oleander aphid, Aphis nerii. J. Insect Physiol. 1970, 16, 1141–1145. [Google Scholar] [CrossRef]
- Hodek, I.; Evans, E.W. Food relationships. In Ecology and Behavior of the Ladybird Beetles (Coccinellidae); Hodek, I., Honek, A., van Emden, H.F., Eds.; Blackwell Publishing Ltd.: Chichester, UK, 2012; pp. 141–274. [Google Scholar]
- Malcom, S.B. Chemical defence in chewing and sucking insect herbivore: Plan derived carnedolides in the monarch butterfly and the oleander aphid. Chemoecology 1990, 1, 12–21. [Google Scholar] [CrossRef]
- El-Shazly, M.M. Observations on oleander (Nerium oleander L., Apocynaceae) ecosystem in Giza, Egypt. In Proceedings of the 4th International Conference on Urban Pests; Pocahontas Press: Dublin, Ireland, 2002; pp. 225–234. [Google Scholar]
- Pons, X.; Lumbierres, B. Aphids on ornamental shrubs and trees in an urban area of the Catalan coast: Bases for an IPM. In Aphids in a New Millennium; Simon, J.C., Dedryver, C.A., Rispe, C., Hullé, N., Eds.; INRA Editions: Versailles, France, 2004; pp. 359–364. [Google Scholar]
- Lumbierres, B.; Starý, P.; Pons, X. Parasitoids and predators of aphids associated with public green areas of Lleida (NE Iberian Peninsula). Adv. Hortic. Sci. 2005, 19, 69–75. [Google Scholar]
- Amorós-Jiménez, R.; Marcos-García, M.A. Fitness-related parameters of the aphid predator Sphaerophoria rueppellii (Diptera, Syrphidae) feeding on three different aphid pests. Bol. Asoc. Esp. Entomol. 2020, 44, 299–315. [Google Scholar]
- Sohail, M.; Muhammad, R.; Somomro, Q.A. Effect of eleander aphid (Aphis nerii Boyer de Fonscolombe) on the mortality and biological parameters of green lacewing Chrysoperla carnea Stephens. Pak. J. Zool. 2020, 52, 231–237. [Google Scholar]
- Desneux, N.; Barta, R.J.; Hoelmer, K.A.; Hopper, K.R.; Heimpel, G.E. Multifaceted determinants of host specificity in an aphid parasitoid. Oecologia 2009, 160, 387–398. [Google Scholar] [CrossRef]
- Monticelli, L.S.; Outreman, Y.; Frago, E.; Desneux, N. Impact of host endosymbionts on parasitoid host range—From mechanisms to communities. Curr. Opin. Insect Sci. 2019, 32, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Pons, X.; Lumbierres, B.; Madeira, F.; Starý, P. Aphid-parasitoid diversity in urban green areas: A background for conservative control strategies. Biodiversity 2018, 19, 172–178. [Google Scholar] [CrossRef]
- Desneux, N.; Asplen, M.K.; Brady, C.M.; Heimpel, G.E.; Hopper, K.R.; Luo, C.; Monticelli, L.; Oliver, K.M.; White, J.A. Interspecific variation in facultative symbiont infection among native and exotic pest population: Potential implication for biological control. Biol. Control 2018, 116, 27–35. [Google Scholar] [CrossRef]
- Irvin, N.A.; Pierce, C.; Hoddle, M.S. Evaluating the potential of flowering plants for enhancing predatory hoverflies (Syrphidae) for biological control of Diaphorina citri (Liviidae) in California. Biol. Control 2021, 157, 104574. [Google Scholar] [CrossRef]
- Pineda, A.; Marcos-García, M.A. Use of selected plants in greenhouses to enhance aphidophagous hoverfly populations (Diptera: Syrphidae). Ann. Soc. Entomol. Fr. 2008, 44, 487–492. [Google Scholar] [CrossRef]
- Otoshi, M.D.; Bichier, P.; Philpott, S.M. Local and landscape correlates of spider activity density and species richness in urban gardens. Environ. Entomol. 2015, 44, 1043–1051. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).