Abstract
Citrus is one of the most important fruit crops in the world. Citrus junos Siebold ex Tanaka cv. Pujiang Xiangcheng is a new rootstock with a good grafting affinity and multiple abiotic stress tolerance. In this study, the effects of ‘Pujiang Xiangcheng’ and two commonly used rootstocks for Citrus maxima (Burm.) Merrill cv. Guanxi Miyou, considering their growth, photosynthetic performance, phytohormones and fruit quality, were evaluated. There was little difference between three graft combinations in the height and diameter of the scions. However, the rootstock ‘Pujiang Xiangcheng’ can cause an up-regulated photosynthetic capacity. In addition, ‘Guanxi Miyou’ on ‘Pujiang Xiangcheng’ had the highest total sugar (TS) content (129.28 mg·g−1) and the lowest titratable acid (TA) content (0.78 g·100 mL−1) in 2020 and the highest TSS (11.84%) in 2021. There was no significant difference in the TSS-to-acid ratio between the fruit of ‘Guanxi Miyou’ on trifoliate orange (13.41) and ‘Pujiang Xiangcheng’ (15.68), but it was significantly higher than that of ‘Guanxi Miyou’ on sour pummelo (11.61). Moreover, the comprehensive fruit quality of ‘Guanxi Miyou’ on ‘Pujiang Xiangcheng’ was better than on sour pummelo and trifoliate orange based on two-year statistics. We suggest that ‘Pujiang Xiangcheng’ might be the appropriate rootstock of ‘Guanxi Miyou’ in production compared with sour pummelo and trifoliate orange.
1. Introduction
Citrus is one of the most important fruit crops in the world in terms of production and commercial propagation by grafting [1]. In addition to propagation, grafting can avoid a juvenile state and affect citrus tree growth, productivity, and fruit quality, and improve the tolerance to biotic/abiotic stresses [2,3,4]. Many studies have demonstrated that the rootstock–scion interaction can affect the absorption of water and nutrients, and modulate phytohormones to affect plant growth [5,6,7,8]. Studies on lemon, sweet orange, mandarin, and grapefruit have found that different rootstocks have an effect on the soluble solids, acids, and ascorbic acid contents of fruit [4,9,10,11]. Therefore, the selection of an appropriate scion–rootstock combination is pivotal for successful citrus production.
In general, the selection and use of rootstock are mainly based on its compatibility, the soil conditions of the orchard, and tradition of the local citriculture [3]. Pummelo (Citrus maxima (Burm.) Merrill) possess abundant primary metabolites, such as organic acids, sugars, and amino acids [12].There are several types of rootstocks used in pummelo production in China [13]. Trifoliate orange (Poncirus trifoliata) is the most common rootstock and has been widely used in citriculture [14,15], while it is incompatible with some varieties of lemon (Citrus limon) and pummelo (C. maxima) [16,17,18]. Sour pummelo is another important traditional pummelo rootstock because of its good compatibility and growth vigor, but the tall canopy makes harvesting difficult. Citrus junos originated from southwest China, which has been widely used as an iron-deficiency-, alkaline-, cold-, and acid-tolerant citrus rootstock [13,19,20]. In our previous citrus rootstock breeding program, we reported a new rootstock cultivar, C. junos Siebold ex Tanaka cv. Pujiang Xiangcheng, with vigorous growth, a spherical crown, upright and dense hare branches, as well as tolerance to cold weather and robust adaptation to basic soil conditions [21].
Over a long history of grafting, citrus has resulted in a wealth of information characterizing graft-transmissible traits [22,23]. However, many aspects of rootstock and scion interactions are still poorly understood. In this study, the performance of C. maxima (Burm.) Merrill cv. Guanxi Miyou grafted onto three different citrus rootstocks, trifoliate orange, sour pummelo and new rootstock ‘Pujiang Xiangcheng’, were investigated to (1) study the growth, photosynthetic performance, phytohormone of seedlings, and to (2) determine the fruit quality of the three graft combinations. The results will provide information regarding the promotion and utilization of this new citrus rootstock ‘Pujiang Xiangcheng’.
2. Material and Methods
2.1. Experiment Site and Plant Material
The experiment was carried out in the orchard of Sichuan Agricultural University, Chengdu (30°56′ N, 103°65′ E and 518 m above mean sea level), Sichuan, China. The climate is a subtropical humid monsoon climate with 1012.4 mm annual mean rainfall and the annual mean temperature is 15.9 °C. The soil type was purple soil with a pH range from 6.8 to 7.4. Two-year-old seedlings of Citrus junos Siebold ex Tanaka cv Pujiang Xiangcheng (Cj), trifoliate orange (Pt), and sour pummelo (Cg) were used as the rootstocks, and Citrus maxima (Burm.) Merrill cv. Guanxi Miyou (Gx) was used as the scion. The graft combinations were grafted by a splice grafting method (defined as Gx/Cj, Gx/Pt, and Gx/Cg) on 7 March 2016. Each scion–rootstock combination had 50 plants.
2.2. Growth Measurement
The survival rate, defined as the percentage of survived grafting plants was recorded 4 months after grafting. The vegetative growth parameters were determined on 139 DAG (days after grafting). The growth index included the scion length, scion diameter, rootstock diameter, and summer shoot length. These indexes were measured with a vernier caliper and ruler. The scion length was taken from the tip of the main stem to the graft union, including the length of spring shoot and summer shoot. The summer shoot length was taken from the terminal bud scar to the tip. The scion diameter and rootstock diameter were taken 5 cm above and below the graft union, respectively. The number of new branches was also calculated. The experimental design was composed of three replicates for each of the graft combination, with every replicate containing ten healthy plants with the same growth potential.
2.3. Chlorophyll Contents Determination
For the measurement of the chlorophyll contents, five fully mature scion leaves were taken from each combination. Here, 0.5 g of leaf disks were cut up and suspended in 10 mL of 80% acetone and kept overnight in darkness. The absorbance of the extract was determined at 470, 663, and 645 nm with a UV2550 spectrophotometer (Shimadzu, Kyoto, Japan). The chlorophyll contents were calculated according to Lichtenthaler and Wellburn (1983): chlorophyll a (Chl a) = 12.7A663 − 2.69A645, chlorophyll b (Chl b) = 22.9A645 − 4.68A663, total chlorophyll (T-Chl) = Chl a + Chl b, Carotenoid (Car) = (1000A470 − 2.05 Chl a − 114.8 Chl b)/245.
2.4. Photosynthetic Characteristics Determination
The photosynthetic characteristics of each graft combination were determined on 70, 98, 139, 404 and 447 DAG. Three trees were selected to determine the photosynthetic rate. Three mature function leaves fully exposed to light in the upper branches were selected from each tree. The measurement was taken from the middle of the leaf surface and avoided the veins. Photosynthetic rate (Pn), transpiration rate (Tr), stomatal conductance (Gs), and intercellular CO2 concentration (Ci) of the leaves were measured and recorded with a LI-6400 portable photosynthesis system (Li-Cor, Inc., Lincoln, NE, USA). The following parameters were set: light intensity of 1200 μmol m−2 s−1, CO2 concentration of 400 μmol m−2 s−1, and air temperature of 25 °C.
2.5. Endogenous Phytohormone Determination
The samples were collected on 70, 98, 139, 404 and 447 DAG for the phytohormone determination. The leaves from ten grafted plants were taken together as one sample, and three samples were taken for each graft combination. The healthy leaves were wiped clean, quickly frozen in liquid nitrogen and brought back to the lab and stored at −80 °C. The phytohormone in the leaves, including GA3, IAA and ABA, was determined through the High-Performance Liquid Chromatography (HPLC, 1260 Infinity, Agileng Technilogies, Santa Clara, CA, USA) method. The extraction, purification, and quantitative determination of GA3, IAA and ABA were carried out following the methods described by He et al. [17]. After grinding the leaves with liquid nitrogen, the phytohormones were extracted with 80% methanol (v/v). The crude extract was condensed by vacuum evaporating. Ethyl acetate at pH 3.0 was used to re-extract the phytohormone. Extracts were purified by Sep-Pak C18 columns and then dissolved in 50% aqueous MeCN.
2.6. Fruit Harvested and Quality Determination
In the 2020 and 2021 harvest season, three fruits per tree from three trees were collected to evaluate the fruit quality according to the standards of GB/T 8210-2011. First the juice was squeezed with a juicer and strained through gauze three times. The total soluble solid (TSS) content of the juice was measured by a temperature-compensated digital refractometer (ATAGO, Tokyo, Japan) and expressed as percentage. The titratable acid (TA) was determined by titrating 10 mL of juice dilution (10 × dilution) with 0.1 N NaOH (pH = 8.1). The amount of ascorbic acid was measured with a 2,6-dichlorophenolindophenol sodium salt titration. A total of 0.5 mL juice and 4.5 mL 1% oxalic acid were titrated with 0.08 g·L−1 2,6-dichlorophenolindophenol sodium salt. The total sugar (TS) was determined by using a Fehling reagent [24]. To make an invert sugar solution, 10 mL of juice, 40 mL of ddH2O, and 5 mL of 5% HCl were added into 100 mL volumetric flask and kept in thermostatic water bath at 80–90 °C for 30 min, and then one drop of the phenolphthalein indicator was added. Then, 40% sodium hydroxide was added to neutralize the HCl, and then ddH2O was used to make up the volume to 100 mL. The TSS-to-acid ratio was calculated as the ratio between TSS and TA [25].
2.7. Data Analysis
The data were organized with Microsoft Excel, and statistical analysis was done following the methods of analysis of variance using SPSS 18.0 (SPSS, Inc., Chicago, IL, USA). Significant differences between the means of the treatments were determined with a 95% confidence (p < 0.05) limit by Tukey’s honestly significant difference (HSD) test.
3. Results
3.1. The Growth of ‘Guanxi Miyou’ Grafted onto Three Citrus Rootstocks
Graft compatibility depends on the rootstock and scion for graft union formation [26]. The selection and use of rootstock are mainly based on its compatibility [27]. The data show that there were no significant differences in the survival rate ranging from 87.88% to 93.55% of ‘Guanxi Miyou’ grafted onto different rootstocks (Table 1). The graft unions all grew well with three citrus rootstocks after 13 months (Figure 1). This suggests that the new rootstock cultivar ‘Pujiang Xiangcheng’ revealed a good compatibility with ‘Guanxi Miyou’. Tree growth was significantly affected by the rootstock. Until the measurement, the average scion height (cm) and diameter (cm) were 52.33 cm and 1.69 cm for Gx/Cj, 55.40 cm and 1.62 cm for Gx/Pt, and 72.53 cm and 2.11 cm for Gx/Cg, respectively. Compared with other rootstocks, ‘Guanxi Miyou’ grafted onto sour pummelo showed the most vigorous growth status, followed by trifoliate orange and ‘Pujiang Xiangcheng’.

Table 1.
The growth indexes of ‘Guanxi Miyou’ grafted onto three citrus rootstocks on 139 DAG.

Figure 1.
Field performance of ‘Guanxi Miyou’ scion grafted onto three citrus rootstocks at different stages.
3.2. Chlorophyll Contents
At the early grafting stage (70 DAG), the Gx/Pt combination had much higher chlorophyll contents than the Gx/Cj and Gx/Cg combinations (Figure 2). From 98 to 404 DAG, the chlorophyll contents maintained the same level among the three combinations. Significant differences in chlorophyll contents were detected among the three grafting combinations after 14 months. Gx/Cg had the highest chlorophyll contents, with 10.28 mg·g−1 for chlorophyll a and 28.35 mg·g−1 for chlorophyll b, followed by Gx/Pt and Gx/Cj. The carotenoid content revealed a fluctuating change after grafting among the three combinations. At 447 DAG, ‘Guanxi Miyou’ grafted onto sour pummelo showed the maximum value for the carotenoid content, with a value of (2.22 mg·g−1), which was significantly higher than the Gx/Cj and Gx/Pt unions.
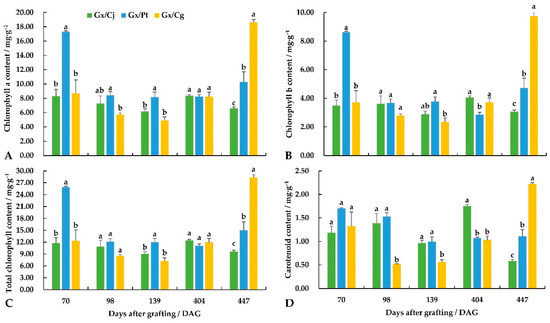
Figure 2.
The changes in the chlorophyll and carotenoid content in the leaves among the different stages of ‘Guanxi Miyou’grafted onto three citrus rootstocks. (A) Chlorophyll a content. (B) Chlorophyll b content. (C) Total chlorophyll content. (D) Carotenoid content. Different lowercase letters indicate a significant difference at the 0.05 level at the same sampling stage. The same as below.
3.3. Photosynthesis Characteristics
The net photosynthetic rate, transpiration rate, and stomatal conductance almost revealed an increase–decrease–increase trend among the three combinations (Figure 3). After nearly 15 months, Gx/Cj showed the highest Pn (13.70 μmol·m−2·s−1), Tr (4.59 mmol·m−2·s−1), and Gs (0.40 mol·m−2·s−1), which were significantly higher than Gx/Pt and Gx/Cg. The intercellular CO2 concentration maintained a similar level at the same stage among the three combinations, except for the significantly lower value of 404 DAG.
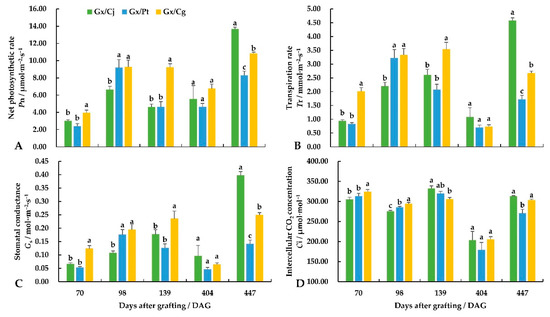
Figure 3.
The changes in the net photosynthetic rate (Pn) (A), transpiration rate (Tr) (B), stomatal conductance (Gs) (C) and intercellular CO2 concentration (Ci) (D) in the leaves for different stages of ‘Guanxi Miyou’ grafted onto three citrus rootstocks. Different lowercase letters indicate a significant difference at the 0.05 level at the same sampling stage.
3.4. Phytohormone Content
The rootstocks significantly affected the phytohormone of scion. The GA3 contents showed obvious significant differences at the same grafting stage among three combinations (Figure 4). The GA3 content in Gx/Cj and Gx/Cg decreased rapidly, then increased and reached the maximum of 5.05 μg·g−1 by 447 DAG. On the contrary, GA3 in Gx/Pt increased sharply and reached the maximum (3.99 μg·g−1), and then decreased to the minimum (1.02 μg·g−1) at the late grafting stage. At the early grafting stage, Gx/Pt revealed the highest IAA content (9.75 μg·g−1), which was 3.02 and 2.36 times that of Gx/Cj and Gx/Cg, respectively. The IAA content in both Gx/Cj and Gx/Cg showed a sharp decrease and then a gradual increase trend with the maximum of 5.01 μg·g−1 at nearly 15 months, much higher than Gx/Pt. Overall, the ABA contents among the three combinations showed a gradual decrease with fluctuations, and fell into a range from 1.23 to 2.39 μg·g−1 at the late grafting stage.
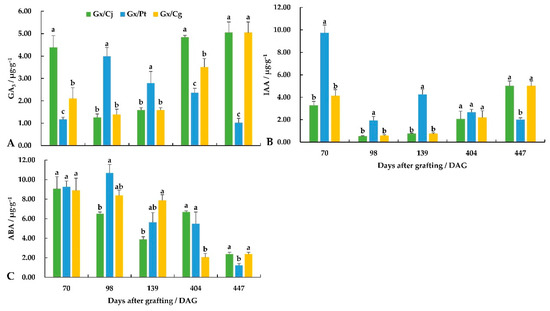
Figure 4.
The changes in GA3 (A), IAA (B) and ABA (C) content in the leaves for different stages of ‘Guanxi Miyou’ grafted onto three citrus rootstocks. Different lowercase letters indicate a significant difference at the 0.05 level at the same sampling stage.
3.5. Fruit Quality
The fruit quality parameters of ‘Guanxi miyou’ were assessed from 2020 to 2021. Different rootstock–scion combinations have a significant effect on fruit quality (Table 2). The TSS content in the fruit of Gx/Cj was significantly higher than Gx/Cg in 2020 and Gx/Cg and Gx/Pt in 2021, and there was no significant difference between Gx/Cj and Gx/Pt in 2020. Gx/Pt and Gx/Cj had the highest TS content in 2021 and 2020, respectively, and both were significantly higher than Gx/Cg. In 2020, the TA content in the fruit of Gx/Cj was the lowest, whereas there was no significant difference between the three grafting combinations in 2021. The content of Vc was not affected by the rootstocks and had no difference in both of the years considered. The TSS-to-acid ratio is a direct index to measure fruit flavor and can comprehensively judge fruit quality. In our results, the TSS-to-acid ratio of Gx/Cj was significantly higher than Gx/Cg in 2020 and 2021. Moreover, Gx/Cj also had the highest value in both years, although there was no significant difference with Gx/Pt. These results indicate that the comprehensive quality of the Gx/Cj fruit was better.

Table 2.
Effect of different rootstocks on the fruit quality of ‘Guanxi Miyou’.
4. Discussion
Compatibility is the prerequisite for successful grafting and is influenced by the genotypes of rootstocks [28,29]. ‘Guanxi miyou’ grafted onto ‘Pujiang Xiangcheng’ and sour pummelo showed a high survival rate (Table 1) and smooth graft union (Figure 5), representing a good graft compatibility. The rootstock also has an impact on scion growth and development [30]. Studies have shown that two species that are closely related in taxonomy have a high affinity for grafting [3,31,32]. The scion of the sour pummelo rootstock showed more growth potential, maybe because of their closer genetic relationship. Trifoliate orange is known as a dwarfing rootstock for citrus, which have a low to moderate vigor [33]. Compared with the trifoliate orange and sour pummelo, ‘Pujiang Xiangcheng’ showed an effectively limited scion size and influence on the growth of the rootstock (Table 1). This may be a better choice for high-density cultivated orchards to in order to decrease harvest costs and facilitate crop management.

Figure 5.
Fruit character of ‘Guanxi Miyou’ grafted onto three citrus rootstocks. (A) ‘Guanxi Miyou’ grafted onto ‘Pujiang xiangcheng’. (B) ‘Guanxi Miyou’ grafted onto sour pummelo. (C) ‘Guanxi Miyou’ grafted onto trifoliate orange. (D) Graft union of Gx/Pt.
For fruit crops, as the fundamental driving force for plant development, fruit yield and quality are influenced by the photosynthetic rate [34,35]. In previous studies, photosynthetic activity has been widely used as a critical physiological characteristic [36]. Photosynthesis draws the most attention in research, which could result in an improvement in the productivity of crops [37]. Citrus tree photosynthetic characteristics are significantly affected by rootstocks throughout the growing season [38]. In our study, at the early grafting stage, photosynthetic products cannot be transferred or the transfer speed is slow, because the communication between the rootstock and scion is blocked. Then, the measured increased in Pn, Tr, and gs was induced by ‘Pujiang Xiangcheng’, even though it had the lowest chlorophyll content (Figure 2 and Figure 3). Size-controlling rootstocks trifoliate orange significantly decreased the leaf photosynthetic capacities of ‘Guanxi miyou’ scion, and the same results were found in apple trees [39].
The phytohormones play pivotal roles in various aspects of plant growth and developmental processes [40]. Grafting can affect the alteration of the hormonal balance between the rootstock–scion [41]. Many studies have indicated the important role of auxin pathway genes in the vascular reconnection establishment during the grafting process [42,43]. Here, we further demonstrated that IAA played a pivotal role in plant grafting because of the high content in the beginning stage (Figure 4). GA3 and ABA exhibited fluctuant changes during the grafting process. This might be related to the different developmental stages.
The rootstock has a significant effect on most of the biochemical characteristics of fruit. The ripening index (TSS-to-acid ratio) is the parameter included in most quality standards for citrus fruit [9]. In our results, the TSS-to-acid ratio of Gx/Cj was significantly higher than Gx/Cg and as good as Gx/Pt in both of the years considered. It is worth noting that rootstocks have more of an effect on TSS than that of TA, which was consistent with the results in ‘Clementine’ mandarin [44], ‘Arrayana’ mandarin [45], and ‘Ray Ruby’ grapefruit [46]. There are two main explanations for the effect of stock on scion fruit quality. One is that different responses of rootstocks to the same temperature led to differences in the photosynthetic rate and assimilate accumulation [10]. Sucrose is the main sugar transported through the phloem in citrus [47]. A higher net photosynthetic flux leads to higher photo assimilate compounds transported from the leaves to the fruits. Another explanation is that the water transport efficiency of the rootstocks is different, resulting in differences in the water balance within the plant [48]. In the specific case of Gx/Cj, the highest TSS and TS values could be explained by their higher photosynthetic rate compared with Gx/Pt and Gx/Cg (Figure 3).
The grafting technique has been widely used in citrus breeding. Rootstocks have an impact on citrus growth, improve the tolerance of scions, and expand the cultivation area, which have gained much attention during recent years [22]. The selection of rootstock is very important for citrus production. Our results show that rootstock ‘Pujiang Xiangcheng’ can reduce ‘Guanxi Miyou’ growth vigor and the up-regulated photosynthetic capacity, which can lead to a good fruit quality.
Author Contributions
X.W. conceived the idea; X.W. and W.H. designed the experiments; W.H. and R.X. performed the experiments, W.H. and R.X. performed the data analysis, J.C., L.L., Q.C. and Y.W. provided the experiment materials; W.H. and R.X. wrote the original manuscript; H.T. and X.W. critically revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Sichuan Provincial Postdoctoral Science Foundation, Sichuan Science and Technology Program (2020ZHCG0027) and by the Shuangzhi Project Innovation Team of Sichuan Agricultural University (grant no. P202107).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Huang, Y.; Xu, Y.; Jiang, X.; Yu, H.; Jia, H.; Tan, C.; Hu, G.; Hu, Y.; Rao, M.J.; Deng, X. Genome of a citrus rootstock and global DNA demethylation caused by heterografting. Hortic. Res.-Engl. 2021, 8, 69. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Ahsan, M.U.; Frank, M.H. Getting to the root of grafting-induced traits. Curr. Opin. Plant Biol. 2021, 59, 101988. [Google Scholar] [CrossRef] [PubMed]
- Warschefsky, E.J.; Klein, L.L.; Frank, M.H.; Chitwood, D.H.; Londo, J.P.; von Wettberg, E.J.B.; Miller, A.J. Rootstocks: Diversity, domestication, and impacts on shoot phenotypes. Trends Plant Sci. 2016, 21, 418–437. [Google Scholar] [CrossRef] [PubMed]
- Morales, J.; Bermejo, A.; Navarro, P.; Forner-Giner, M.Á.; Salvador, A. Rootstock effect on fruit quality, anthocyanins, sugars, hydroxycinnamic acids and flavanones content during the harvest of blood oranges ‘Moro’ and ‘Tarocco Rosso’ grown in Spain. Food Chem. 2021, 342, 128305. [Google Scholar] [CrossRef]
- Dubey, A.K.; Sharma, R.M. Effect of rootstocks on tree growth, yield, quality and leaf mineral composition of lemon (Citrus limon (L.) Burm.). Sci. Hortic. 2016, 200, 131–136. [Google Scholar] [CrossRef]
- Du, W.; Hussain, S.B.; Jin, L.; Liu, X.; Li, R.; Han, Z.; Liu, Y.; Pan, Z.; Peng, S. Characteristics of boron distribution in the ‘Newhall’ navel orange plant with two root systems. Plant Physiol. Biochem. 2021, 167, 42–48. [Google Scholar] [CrossRef]
- Fan, Y.; Li, Z.; Xie, B.; Liang, X.; Huang, X. A study of shoot growth, leaf photosynthesis, and nutrients in ‘lingfengjing’litchi grafted onto seedlings of different cultivars. Horticulturae 2022, 8, 282. [Google Scholar] [CrossRef]
- Balfagón, D.; Rambla, J.L.; Granell, A.; Arbona, V.; Gómez-Cadenas, A. Grafting improves tolerance to combined drought and heat stresses by modifying metabolism in citrus scion. Environ. Exp. Bot. 2022, 195, 104793. [Google Scholar] [CrossRef]
- Continella, A.; Pannitteri, C.; La Malfa, S.; Legua, P.; Distefano, G.; Nicolosi, E.; Gentile, A. Influence of different rootstocks on yield precocity and fruit quality of ‘Tarocco Scirè’ pigmented sweet orange. Sci. Hortic. 2018, 230, 62–67. [Google Scholar] [CrossRef]
- Sau, S.; Ghosh, S.N.; Sarkar, S.; Gantait, S. Effect of rootstocks on growth, yield, quality, and leaf mineral composition of Nagpur mandarin (Citrus reticulata Blanco.), grown in red lateritic soil of West Bengal, India. Sci. Hortic. 2018, 237, 142–147. [Google Scholar] [CrossRef]
- Emmanouilidou, M.G.; Kyriacou, M.C. Rootstock-modulated yield performance, fruit maturation and phytochemical quality of ‘Lane Late’ and ‘Delta’ sweet orange. Sci. Hortic. 2017, 225, 112–121. [Google Scholar] [CrossRef]
- Deng, M.; Lin, Y.; Dong, L.; Jia, X.; Shen, Y.; Liu, L.; Chi, J.; Huang, F.; Zhang, M.; Zhang, R. Physicochemical and functional properties of dietary fiber from pummelo (Citrus grandis L. Osbeck) and grapefruit (Citrus paradisi Mcfad.) cultivars. Food Biosci. 2021, 40, 100890. [Google Scholar] [CrossRef]
- Zhu, S.; Nong, J.; Luo, G.; Li, Q.; Wang, F.; Jiang, D.; Zhao, X. Varied tolerance and different responses of five citrus rootstocks to acid stress by principle component analysis and orthogonal analysis. Sci. Hortic. 2021, 278, 109853. [Google Scholar] [CrossRef]
- Meng, L.; Zhang, Q.; Yang, J.; Xie, G.; Liu, J. PtrCDPK10 of Poncirus trifoliata functions in dehydration and drought tolerance by reducing ROS accumulation via phosphorylating PtrAPX. Plant Sci. 2020, 291, 110320. [Google Scholar] [CrossRef] [PubMed]
- Ming, R.; Zhang, Y.; Wang, Y.; Khan, M.; Dahro, B.; Liu, J.H. The JA-responsive MYC2-BADH-like transcriptional regulatory module in Poncirus trifoliata contributes to cold tolerance by modulation of glycine betaine biosynthesis. New Phytol. 2021, 229, 2730–2750. [Google Scholar] [CrossRef]
- He, W.; Xie, R.; Wang, Y.; Chen, Q.; Wang, H.; Yang, S.; Luo, Y.; Zhang, Y.; Tang, H.; Gmitter, F.G.; et al. Comparative transcriptomic analysis on compatible/incompatible grafts in Citrus. Hortic. Res.-Engl. 2022, 9, uhab072. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Wang, Y.; Chen, Q.; Sun, B.; Tang, H.; Pan, D.; Wang, X. Dissection of the mechanism for compatible and incompatible graft combinations of Citrus grandis (L.) Osbeck (‘Hongmian Miyou’). Int. J. Mol. Sci. 2018, 19, 505. [Google Scholar] [CrossRef] [PubMed]
- Bevington, K.B. Development of union abnormalities in grafts between lemon (Citrus limon) and Poncirus trifoliata. Aust. J. Agr. Res. 1976, 27, 661. [Google Scholar] [CrossRef]
- Dong, T.; Xi, L.; Xiong, B.; Qiu, X.; Huang, S.; Xu, W.; Wang, J.; Wang, B.; Yao, Y.; Duan, C. Drought resistance in Harumi tangor seedlings grafted onto different rootstocks. Funct. Plant Biol. 2021, 48, 529–541. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Cao, J.; Su, M.; Feng, G.; Xu, Y.; Yi, H. Genome-wide comprehensive analysis of transcriptomes and small RNAs offers insights into the molecular mechanism of alkaline stress tolerance in a citrus rootstock. Hortic. Res.-Engl. 2019, 6, 33. [Google Scholar] [CrossRef]
- Fu, X.; Huang, X.; Chen, T.; Zhang, J.; Wang, Y.; Chen, Q.; Lei, Q.; Tang, H.; Wang, X. A new citrus rootstock ‘Pujiang Xiangcheng’ (Citrus junos). J. Fruit Sci. 2017, 37, 917–920. [Google Scholar] [CrossRef]
- Zhu, S.; Huang, T.; Yu, X.; Hong, Q.; Xiang, J.; Zeng, A.; Gong, G.; Zhao, X. The effects of rootstocks on performances of three late-ripening navel orange varieties. J. Integr. Agric. 2020, 19, 1802–1812. [Google Scholar] [CrossRef]
- Liao, L.; Li, Y.; Bi, X.; Xiong, B.; Wang, X.; Deng, H.; Zhang, M.; Sun, G.; Jin, Z.; Huang, Z. Transcriptome analysis of Harumi tangor fruits: Insights into interstock-mediated fruit quality. Front. Plant Sci. 2022, 13, 995913. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.; Wang, G.; Li, Y.; Shen, X.; Chen, X.; Song, F.; Wu, S.; Chen, Q.; Mao, Z. Replanting affects the tree growth and fruit quality of gala apple. J. Integr. Agric. 2014, 13, 1699–1706. [Google Scholar] [CrossRef]
- López-Ortega, G.; García-Montiel, F.; Bayo-Canha, A.; Frutos-Ruiz, C.; Frutos-Tomás, D. Rootstock effects on the growth, yield and fruit quality of sweet cherry cv. ‘Newstar’ in the growing conditions of the region of Murcia. Sci. Hortic. 2016, 198, 326–335. [Google Scholar] [CrossRef]
- Habibi, F.; Liu, T.; Folta, K.; Sarkhosh, A. Physiological, biochemical, and molecular aspects of grafting in fruit trees. Hortic. Res.-Engl. 2022, 9, uhac032. [Google Scholar] [CrossRef]
- Pérez-Grajales, M.; Pérez-Reyes, T.Q.; Cruz-Álvarez, Ó.; Castro-Brindis, R.; Martínez-Damián, M.T. Compatibility of the rootstock CM-334 and its response on the yield, physicochemical quality and content of capsaicinoids in Capsicum pubescens. Itea-Inf. Tec. Econ. Ag. 2021, 117, 332–346. [Google Scholar] [CrossRef]
- Thomas, H.R.; Frank, M.H. Connecting the pieces: Uncovering the molecular basis for long-distance communication through plant grafting. New Phytol. 2019, 223, 582–589. [Google Scholar] [CrossRef]
- Alfaro, J.M.; Bermejo, A.; Navarro, P.; Quiñones, A.; Salvador, A. Effect of rootstock on citrus fruit quality: A review. Food Rev. Int. 2021; ahead-of-print. 1–19. [Google Scholar] [CrossRef]
- Zheng, X.; Zhao, Y.; Shan, D.; Shi, K.; Wang, L.; Li, Q.; Wang, N.; Zhou, J.; Yao, J.; Xue, Y. MdWRKY9 overexpression confers intensive dwarfing in the M26 rootstock of apple by directly inhibiting brassinosteroid synthetase MdDWF4 expression. New Phytol. 2018, 217, 1086–1098. [Google Scholar] [CrossRef]
- Raiol-Junior, L.L.; de Carvalho, E.V.; Moreira, A.S.; Marques, J.P.R.; Stuchi, E.S.; Peña, L.; Girardi, E.A. Graft compatibility classification within aurantioideae based on biometric traits and the anatomy of graft union. Agriculture 2022, 12, 76. [Google Scholar] [CrossRef]
- Goldschmidt, E.E. Plant grafting: New mechanisms, evolutionary implications. Front. Plant Sci. 2014, 5, 727. [Google Scholar] [CrossRef] [PubMed]
- Mademba-Sy, F.; Lemerre-Desprez, Z.; Lebegin, S. Use of flying dragon trifoliate orange as dwarfing rootstock for citrus under tropical climatic conditions. Hortscience 2012, 47, 11–17. [Google Scholar] [CrossRef]
- Opazo, I.; Toro, G.; Salvatierra, A.; Pastenes, C.; Pimentel, P. Rootstocks modulate the physiology and growth responses to water deficit and long-term recovery in grafted stone fruit trees. Agric. Water Manag. 2020, 228, 105897. [Google Scholar] [CrossRef]
- Zhou, Y.; Tian, X.; Yao, J.; Zhang, Z.; Wang, Y.; Zhang, X.; Li, W.; Wu, T.; Han, Z.; Xu, X. Morphological and photosynthetic responses differ among eight apple scion-rootstock combinations. Sci. Hortic. 2020, 261, 108981. [Google Scholar] [CrossRef]
- Bhusal, N.; Han, S.; Yoon, T. Impact of drought stress on photosynthetic response, leaf water potential, and stem sap flow in two cultivars of bi-leader apple trees (Malus × domestica Borkh.). Sci. Hortic. 2019, 246, 535–543. [Google Scholar] [CrossRef]
- Nowicka, B.; Ciura, J.; Szymańska, R.; Kruk, J. Improving photosynthesis, plant productivity and abiotic stress tolerance–current trends and future perspectives. J. Plant Physiol. 2018, 231, 415–433. [Google Scholar] [CrossRef]
- Martinez-Cuenca, M.R.; Primo-Capella, A.; Quinones, A.; Bermejo, A.; Forner-Giner, M.A. Rootstock influence on iron uptake responses in Citrus leaves and their regulation under the Fe paradox effect. PeerJ 2017, 5, e3553. [Google Scholar] [CrossRef]
- Xu, H.; Ediger, D. Rootstocks with different vigor influenced scion–water relations and stress responses in ambro-siatm apple trees (Malus Domestica var. Ambrosia). Plants 2021, 10, 614. [Google Scholar] [CrossRef]
- Aloni, B.; Cohen, R.; Karni, L.; Aktas, H.; Edelstein, M. Hormonal signaling in rootstock–scion interactions. Sci. Hortic. 2010, 127, 119–126. [Google Scholar] [CrossRef]
- Albacete, A.; Martinez-Andujar, C.; Martinez-Perez, A.; Thompson, A.J.; Dodd, I.C.; Perez-Alfocea, F. Unravelling rootstockxscion interactions to improve food security. J. Exp. Bot. 2015, 66, 2211–2226. [Google Scholar] [CrossRef]
- Xu, D.; Yang, Y.; Tao, S.; Wang, Y.; Yuan, H.; Sharma, A.; Wang, X.; Shen, C.; Yan, D.; Zheng, B. Identification and expression analysis of auxin-responsive GH3 family genes in Chinese hickory (Carya cathayensis) during grafting. Mol. Biol. Rep. 2020, 47, 4495–4506. [Google Scholar] [CrossRef] [PubMed]
- Saravana, K.R.; Gao, L.X.; Yuan, H.W.; Xu, D.B.; Liang, Z.; Tao, S.C.; Guo, W.B.; Yan, D.L.; Zheng, B.S.; Edqvist, J. Auxin enhances grafting success in Carya cathayensis (Chinese hickory). Planta 2018, 247, 761–772. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Curk, F.; Anjum, M.A.; Pailly, O.; Tison, G. Performance evaluation of common clementine on various citrus rootstocks. Sci. Hortic. 2013, 150, 278–282. [Google Scholar] [CrossRef]
- Chaparro-Zambrano, H.N.; Velásquez-Ramírez, H.A.; Ordúz-Rodríguez, J.O. Evaluation of ‘Arrayana’ tangerine (Citrus reticulata Blanco) grafted onto different rootstocks in tropical lowlands of Colombian Orinoquia, 2005-2011 (second cycle). Agronomía 2017, 35, 29–34. [Google Scholar] [CrossRef]
- McCollum, G.; Bowman, K.D. Rootstock effects on fruit quality among ‘Ray Ruby’ grapefruit trees grown in the Indian River District of Florida. Hortscience 2017, 52, 541–546. [Google Scholar] [CrossRef]
- Huang, L.; Grosser, J.W.; Gmitter, F.G., Jr.; Charles, A.S.; Wang, Y. Effects of scion/rootstock combination on flavor quality of orange juice from Huanglongbing (HLB)-affected trees: A two-year study of the targeted metabolomics. J. Agric. Food Chem. 2020, 68, 3286–3296. [Google Scholar] [CrossRef]
- Barry, G.H.; Castle, W.S.; Davies, F.S. Rootstocks and plant water relations affect sugar accumulation of citrus fruit via osmotic adjustment. J. Am. Soc. Hortic. Sci. 2004, 129, 881–889. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).