Abstract
BRs (Brassinosteroids) regulate many essential pathways related to growth, cell elongation, cell expansion, plant architecture, and fruit development. The potential exogenous application of BR-derivatives has been proven to stimulate plant growth and development, including quality attributes of fruits, whereas its biosynthesis inhibition has shown the opposite effect. In this study, BR-insensitive tomato mutants were used to reveal the potential function of BR signaling in the regulation of fruit development to elaborate the regulatory mechanism of BR signaling in tomato fruits. The BR-signaling mutant exhibited a typical dwarf phenotype and reduced vegetative growth, fruit size, and weight. Microscopic and transcriptional evaluation of the abs1 mutant fruits implies that reduced cell size and number are responsible for the phenotypic variations. Additionally, we also found that the altered content of phytohormones, such as auxin, gibberellin, cytokinin, and ethylene levels, contributed to altered fruit development. Moreover, fruit growth and cell development-specific gene expression levels were downregulated in BR-insensitive plants; culminating in reduced cell size, cell number, and cell layers. These findings provide insight into physio-chemical changes during fruit development in response to BR-insensitivity.
1. Introduction
Tomato is a model plant for molecular biotechnology research in horticulture. Multiple phases are involved in tomato fruit growth. Immediately after fertilization in the first stage, there is fast cell division, which causes the number of pericarp cells to gradually rise. Two weeks after from pollination, the rate of cell division abruptly decreases, signaling the end of this stage. Fruit weight increases during the second stage as a result of cell expansion. The third phase is when the fruit reaches the mature green (MG) stage. The tomato fruit undergoes complex metabolic changes as it reaches the MG stage, which initiates the ripening process. The two stages breaking (BK) and red ripening (RR) are tomato fruit ripening stages [1].
In plants, several elements, including temperature, light, and hormones, drive the fruit developmental process [2,3]. Hormones are substances that work at low concentrations and are crucial components of the signaling system that underlies the growth and development of plants [4]. BRs were recognized as the new (sixth) class of phytohormones due to their potential biological activity [5]. Based on its potential use in crop development, a thorough functional model of BR action in plants has subsequently been developed [5]. BRs have also been implicated in the transcription of several genes, especially within relatively short periods after BR treatment [6,7]. Studies to date have demonstrated that BRs bind to the Brassinosteroid Insensitive 1 (bri1) gene, activate it, start a signal transduction cascade through the transcription factor BZR/BES1, and control the expression of genes implicated in different pathways [7,8]. Brassinosteroid (BR) signaling is negatively regulated by the GSK3-like enzyme known as BIN2.
Accordingly, several bio-functional analyses of bri1 orthologs have been reported. Loss-of-function of bri1 mutants (d61) in rice exhibited dwarfed plant height and rigid leaves, with less effect on fertility under dense planting conditions. This was ascribed to better photosynthetic capacity and leaf area index (LAI), thus further demonstrating bri1 as a potential factor for crop improvement [8]. Overexpression of wheat bri1 in Arabidopsis improved seed germination, flowering, and yield [9]. In a similar manner, lower expression levels of strawberry bri1 mRNA reduced the development of red coloration [10]. Additionally, increased bri1 expression improved tomato fruit quality and yield, as well as the potency of BR signaling [11]. On the other hand, there is still no information on how BR signaling disruption affects fruit development in tomato plants. At the curl3 gene, the abs1 mutant is a weak (intermediate) recessive allele that results in dwarfed plants phenotype. The genetically and phenotypically stable abs1 mutant is the consequence of a missense mutation in the kinase domain (H1012Y) of the tomato curl3 gene. In this study, we employed an abs1 tomato dwarf mutant to investigate the impact of BR signaling strength on tomato fruit development.
Our findings showed that the abs1 mutant exhibited weaker BR signal strength, less vigorous vegetative development, and smaller fruit size. These findings demonstrate a distinctive regulatory role of BR signaling in tomato fruit development and its potential for enhancing crop performance.
2. Materials and Methods
2.1. Plant Material
Tomato abs1 mutant (Accession: LA4481) and its corresponding wild-type (Micro-Tom) were attained from TGRC (Tomato Genetics Resource Center, UC Davis, Davis, CA, USA) and grown at 23 °C, 16:8 h (day:night) with an irradiance of 280 ± 30 μmol m−2 s−1 in a growth chamber.
2.2. Quantitative Gene Expression Profiling
Trizol reagent (Invitrogen, Florida, USA) was used to extract total RNA, and cDNA was synthesized using Hi-Script II QRT kit (Vazyme, Wuhan, China). In order to perform a quantitative PCR, a 10 μL reaction mixture was used, which was comprised of 5 μL of SYBR Green I Master, 4 μL of the template, 0.5 μL of forward primer (10 μM), and 0.5 μL of reverse primer (10 μM). The tomato β-actin gene (SGN-U580422) was used as the internal control. Primers used for each gene are given in the Table 1.

Table 1.
Forward and reverse primers used in this study.
2.3. Morphological Characterization of Fruits
To evaluate morphological diversity among the BR-insensitive fruit and wild-type tomato fruits, fruit morphological traits were measured. All parameters were measured using three biological replicates.
2.4. Anatomical Observations of Fruit Pericarp
For microscopic observations, fruit pericarp tissues of abs1 mutants and wild-type tomatoes at different developmental stages were embedded and stained as previously described by Feng et al. [12]. Using a Nikon Eclipse 80i (Nikon, Tokyo, Japan) equipped with a digital camera, stained sections were observed and recorded.
2.5. Extraction and Quantification of Phytohormones
Fruit tissues were collected and homogenized for the measurement of endogenous hormones from wild-type and abs1 mutant fruits. Enzyme-linked immunosorbent assay (ELISA, China Agricultural University, Beijing, China) was used to measure gibberellic acid (GA3), cytokinins (ZR), and indole acetic acid (IAA). The amount of ethylene (ET) produced by same maturity stage fruits was measured using a gas chromatographic FID detector (DB-130 m, 0.32 m, 0.25 m chromatographic column; Agilent 7890B, Agilent Technologies, Santa Clara, CA, USA) after one milliliter of gas was extracted from each plastic bag using a syringe.
2.6. Statistical Analyses
All data were subjected to Student’s t-test using SPSS (version 10.0; IBM, Chicago, IL, USA) and a statistically significant difference was determined if the p-value ≤ 0.05.
3. Results
3.1. BR Signaling Components Expression during Fruit Development
The expression pattern was investigated using a tissue-specific expression analysis of curl3 (Slbri1) gene during several developmental stages in fruit tissues. All tissues expressed curl3 constitutively, and mature fruits accumulated considerably less abundance, whereas the immature green fruit tissues recorded the highest expression (Figure 1).
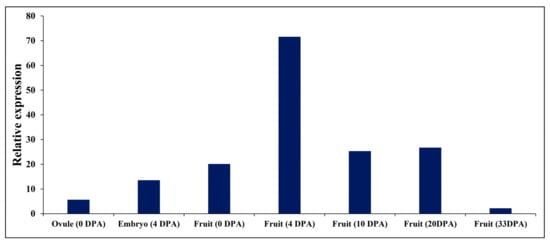
Figure 1.
Temporal expression of curl3 gene in WT (wild-type) fruits. The expression profile for curl3 at the different fruit development stages in the WT plants was attained from TFGD (http://ted.bti.cornell.edu/) on 17 April 2019. DPA, Days post anthesis.
3.2. The Altered Transcript Level of BR Signaling Components in abs1 Mutant
The transcription factor (BZR1) which regulates BR-regulated genes was significantly downregulated in abs1 mutants (Figure 2). In divergence, the mRNA abundance level of bin2 was observed to be higher in abs1 mutant plants than wild-type plants (Figure 2).
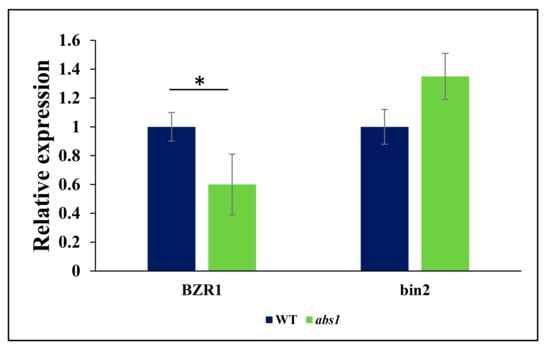
Figure 2.
Relative expression of BR-signaling components. The relative mRNA expression of BR-signaling components in WT (wild-type) and abs1 fruits by qRT-PCR. The experiment was carried out using three biological and three technical replicates, and the values are presented as relative expression. The error bar represents the standard error. Asterisk indicates significant differences (*: p ≤ 0.05, Student’s t-test).
3.3. BR-Signaling Disruption Causes a Decrease in Phytohormone Production
Hormonal crosstalk is crucial for the optimal growth and development of tomato fruit. In order to determine whether BR signaling disruption impacted the endogenous phytohormone contents to inhibit fruit development, we measured the endogenous level of GA3, IAA, ZR, and ET in the abs1 mutants for the immature green and fully red ripe fruits. Under the control condition, both genotypes recorded different endogenous concentrations of the measured phytohormones. The concentration of ZR was significantly lower in abs1 mutants in immature green fruits (Figure 3a). IAA and GA3 were slightly decreased in immature green fruits with a less observable change in red ripe fruits (Figure 3b,c). Interestingly, ethylene production in fully ripe abs1 fruits was 40% lower than in wild-type tomatoes (Figure 3d), suggesting a synergistic relation between BR signaling and ethylene production during tomato fruit ripening.
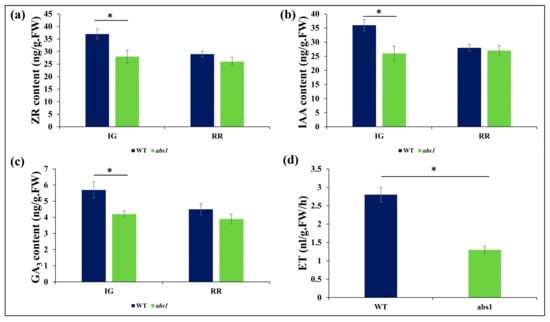
Figure 3.
Endogenous quantification of phytohormones. (a) ZR, (b) IAA, (c) GA3, and (d) ET (RR stage) in WT and abs1 mutant fruits at immature green (IG) and red ripe (RR) stages. The error bar represents standard error. Asterisks indicate significant differences (*: p ≤ 0.05, Student’s t-test).
3.4. BR-Signaling Disruption Decreased Fruit Size in abs1 Mutant
When the abs1 mutant and wild-type plants were grown in a controlled environment, abs1 mutants exhibited decreased fruit size during all developmental stages (Figure 4a,b). The highest decrease in size was observed in immature and mature green fruit. Similarly, the weight of abs1 mutant fruits was decreased during all development stages in comparison with wild-type fruits (Figure 4c).
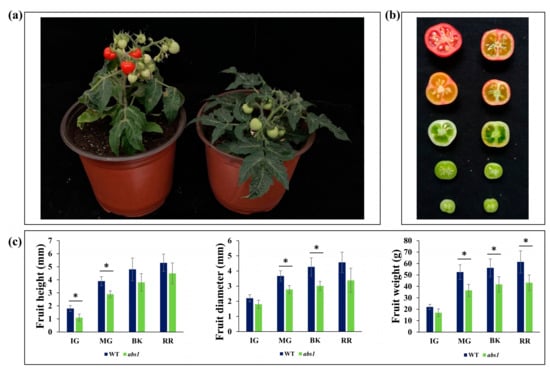
Figure 4.
Characterization of morphological traits of abs1 mutant and WT fruits. (a) The visual appearance of WT (left) and abs1 plants (right). (b) The visual appearance of WT (left) and abs1 fruits (right) at different maturity stages. (c) Average fruit height, fruit diameter, and fruit weight for WT fruit and abs1 fruit. The error bar represents standard error. Asterisks indicate significant differences (*: p ≤ 0.05, Student’s t-test).
3.5. BR-Signaling Disruption Decreases Cell Division and Expansion during Early Developmental Stages
It has been well reported that cell division persists in tomatoes for 2 weeks after anthesis. As cell division slows, cell size increases to facilitate the formation of fruitlets in the next 6–7 weeks [13]. Our results demonstrated that the abs1 mutants recorded reduced BR signaling intensity resulting in relatively smaller fruit sizes than wild-type fruits. Anatomical cross-sections of BR-insensitive mutant and pericarps of wild-type fruits were analyzed using developing fruits at different developmental stages (Figure 5a). The three different layers of tomato fruits (endocarp, exocarp, and mesocarp) were anatomically visualized under a microscope during different development stages. Pericarps of abs1 mutant and wild-type fruits revealed an apparent difference in cell size, especially in the exocarp (Figure 5b). In the immature green fruits, the mesocarpic and endocarpic cells showed cell expansion, whereas the exocarpic cells were still at the cell division stage. Cell size in the mesocarp and endocarp of abs1 mutant and wild-type fruits differed significantly. The mean cell number in abs1 mutants restrainedly increased during all development stages of fruit compared to that of wild-type fruits (Figure 5b). Alterations in the exocarps of wild-type fruits and abs1 mutants were more evident in fully mature fruits. Comparatively, microscopic observation of fully-ripe wild-type fruits and abs1 mutant fruits revealed irregular cell shapes, evidenced by a 57% decreased cell size in abs1 mutants compared to the wild-types fruits. Similarly, abs1 mutants developed comparatively fewer cell layers (approximately 12) than wild-type fruits (approximately 19), a decrease of 40% as compared to wild-type fruit (Figure 5). These results demonstrate that inhibition of BR-signaling occasioned a significant decrease in the number of cell layers and cell area, as exocarp cells of tomato fruits comprise 4–5 cell layers exteriorly of fruits while endocarpic cells encompass 1–2 cell layers interiorly of fruits. Mesocarpic cell size and number may have accounted for the differences in fruit size for both abs1 mutants and wild-type plants.

Figure 5.
The microscopic observations of WT and abs1 fruits. (a) Pericarp cross-section of the fruits of WT and abs1 mutant plants at different maturity stages; anthesis, immature green (IG), mature green (MG), fully red ripe (RR). (b) The average cell size, number of cells, and number of cell layers were calculated using ImageJ. Bars = 100 µm. The error bar represents the standard error. Asterisks indicate significant differences (*: p ≤ 0.05, **: p ≤ 0.01, Student’s t-test).
3.6. The Altered Transcript Level of Fruit Size-Determining Genes in abs1 Mutant Fruit
The BR signaling downstream events that initiate and regulate the cell expansion process are yet to be fully deciphered. We proposed that the expression of specific genes and many factors might affect fruit size. We investigated the transcription of fruit size-specific genes in abs1 mutants and wild-type fruits using quantitative-PCR. Genes analyzed included tomato sucrose transporter (SlSUT), tomato protein kinase (SlWEE1), tomato auxin transcription factor (SlIAA9), tomato cyclin-dependent kinase (SlCDKA1 and SlCDKB2), brassinolide responsive gene (GAox20), and tomato fruit weight gene (SlFW2.2). The SlSUT1 gene expression in abs1 mutants was lower compared to the fruits from wild-type plants, signifying that BR-insensitive plants are deficient in sugar transporters with potentially limited carbon supply capability. Cell mitotic process has been reported to be regulated by SlWEE1, and its expression in the abs1 mutants was substantially lower compared to the wild-type plants. Similarly, both CDKA1 and CDKB2 genes expression in abs1 mutant fruits was lower compared to the wild-type fruits. SlIAA9 was expressed slightly higher in fruits of wild-type tomatoes than in the abs1 mutants. Furthermore, GAox20 expression levels in the abs1 mutants were slightly lower than in the wild-type fruits (Figure 6). SlFW2.2 is reported to regulate cell mitotic processes. It was more highly expressed in the wild-type tomatoes than the abs1 mutants during the early fruit development stage. Thus, SlFW2.2 might be associated with the reduction of fruit size.

Figure 6.
The relative expression of genes involved in cell expansion and cell division using qRT-PCR. The experiment was carried out using three biological and three technical replicates, and the values are presented as relative expression. The error bar represents the standard error. Asterisks indicate significant differences (*: p ≤ 0.05, Student’s t-test).
4. Discussion
The control of tomato growth and development is greatly influenced by BRs [14,15,16]. Based on substantial research in Arabidopsis thaliana, the significance of BRs and BR signaling in plant growth and development has been extensively described [17]. In this study, BR-insensitive tomato mutants were used to reveal the function of BR signaling in controlling fruit growth in order to decipher the regulatory mechanism of BR signaling in tomato fruits.
The distribution of curl3 expression in various tissues showed that BR signaling is actively expressed throughout the developmental stages of fruit development. We employed a tomato mutant carrying a missense mutation in the kinase domain, H1012Y of the tomato curl3 gene suppressing the brassinosteroid receptor (curl3), and the results showed that brassinolide signaling might act as a positive regulator of fruit growth and development in the tomato plant. Compared to the wild-type plants, abs1 mutants exhibited reduced plant expansion and leaf area, delayed ripening, and lower yield (data not shown). However, BRs have been proven as plant growth hormones with direct potential for enhancing biological yield in crops [18]. Our findings showed a 3.5-fold difference in yields among abs1 mutants compared to the wild-type plants (data not shown). This decreased yield may be attributed to the lower number of fruits, and reduced fruit sizes and weight. Earlier studies revealed broad physiological and developmental processes controlled by the interactions and crosstalk between BRs and other phytohormones [19]. Recently, studies on phytohormonal molecular components have shown the involvement of hormonal activities during fruit development [20].
In our study, BR-signaling disruption influenced the phytohormone regulation of fruit growth and development, and thus, the decreased size of fruit in the abs1 mutants might be closely associated with the interaction among phytohormones. Fruit development is associated with GA, BR, and auxin signaling. Interaction between GA, BR, and auxin, as well as other phytohormones, is facilitated by ARF proteins and Aux/IAA. Auxin is a key phytohormone in tomato fruit development; as it functionally plays a crucial role in the growth period of tomato fruits [21]. Brassinosteroid is functionally similar to indole acetic acid (IAA) as a phytohormone. It regulates crop growth and development through cellular dissection and expansion [21]. In the current study, IAA concentrations recorded in mature green abs1 mutants were considerably lower than the wild-type tomato fruits, which inhibited the growth, development, and elongation of the cells. Cytokinins have been shown to regulate diverse biochemical and cellular processes in crop development [22]. Specifically, cytokinin is known as an essential plant hormone for reproductive growth; it stimulates cell division and differentiation with a direct effect on the number and magnitude of procreative organs [23]. Moreover, ZR contents recorded in the abs1 mutants decreased significantly in the developmental stages compared to that of fruits of the wild-type tomato. This might have accounted for decreased fruit size in the abs1 mutants as a result of reduced cellular activity (division). Another essential phytohormone in crop development is gibberellins (GAs). GAs are essential for ovary growth, root and stem elongation, flowering time, and seed germination [24]. In tomato, GAs stimulates cell expansion and early fruiting. We established that the endogenous concentration of GA3 in the abs1 mutant fruits was also lower than in the wild-type. This might have inhibited further cellular activities (cell expansion and division), culminating in decreased fruit size. These results indicate BR signaling modulation of phytohormones in regulating fruit growth and ripening of tomatoes. Thus, this may validate the novel BR signaling function but requires further study to fully comprehend its interaction mechanism with other phytohormones during tomato fruit development.
The developmental cycle of immature tomato fruit reveals three discrete stages (I, II, and III) characterized by conspicuous morphological and cellular changes [20,25]. Fruit set and pistillate growth occur at stage I, while cell enlargement occurs at stage II, with cellular expansion ensuing in stage III [26]. Research on ripening-related genes and fruit-ripening mutants point to a yet-to-be-defined fruit developmental regulatory cascade. Several genes have been identified to play a central role in the development of tomato fruit. BR-signal transduction possibly mediates the expressions of genes associated with cellular activities (cell division and expansion), which are essential in determining flower and fruit sizes. SlCDKA1 is a principal element in cell division and its abnormal regulation in the tomato lines may have accounted for the variable fruit sizes, as is evident in the pericarp (thinner) and lesser cell layers. Thus, the CDKA1 expressions were significantly greater in wild-type tomato fruits as compared to the abs1 mutants, indicating CDKA1 regulatory function in the fruit development. We measured the mRNA levels of CDKB2 in the abs1 mutants and wild-type tomato. The abs1 mutants recorded lower mRNA than the wild-type. We infer that alteration in CDKB2 expression may partly account for the altered phenotypes of the fruits. Moreover, WEE1 kinase impedes CDKA phosphorylation, whereas down-regulating SlWEE1 yielded fruits with reduced and thinner pericarps [27]. WEE1 expression in the abs1 mutant was also considerably reduced than that of wild-type. SlFW2.2 gene is a weight-related quantitative trait locus [28], reported to have an adverse regulatory role in cell division essential for determining fruit size. Our analysis showed that expression of FW2.2 in immature green fruits was significantly lower than in wild-type fruits, but mature fruits showed no observable change. The ability of abs1 mutants to halt a cell division phase serves as a promising mechanism to control fruit development. The downregulation of GAox20 indicates that GA may be essential to the process of cell growth to improve fruit size. This is in line with what Czerednik et al., has reported [29]. Thus, BR-signaling potentially plays a vital regulatory role in major cellular activities (cell division and expansion) in tomato fruit development by regulating associated molecular components essential for fruit development (Figure 7).

Figure 7.
Proposed BR-signaling pathway involvement in tomato fruit size regulation. BRs attach to Bri1 (Slcurl3) initiating the BR-signaling cascade. BES/BZR transcription factors binds with BR-responsive genes and activates cell expansion. Bin2 acts as BES/BZR inhibitor and subordinates the BR-signaling strength thus affecting the fruit developmental process.
5. Conclusions
The BR signaling mutant exhibited a typical dwarf phenotype along with reduced vegetative growth, fruit size, and fruit weight. Microscopic and transcriptional evaluation of the abs1 mutant fruits implies that reduced cell size and number are responsible for the phenotypic variations. Moreover, the downregulation of fruit growth and cell development-specific gene expression levels in BR-insensitive plants culminated in reduced cell size, cell number, and cell layers. These findings collectively provide a comprehensive understanding of the physio-chemical alterations that occur throughout fruit development as a result of BR-insensitivity. These insights may enhance efforts to improve the productivity of tomato crops through genetic engineering of curl3 via precise control of its expression.
Author Contributions
Conceptualization, M.A.M. and Y.Z.; methodology, M.A.M., F.L., P.G. and Y.W. (Yaru Wang); software, X.Z.; validation, J.T.; formal analysis, M.A.M.; investigation, Y.Z.; data curation, W.G. and Y.W. (Ying Wang); writing—original draft preparation, M.A.M. and H.D.; writing—review and editing, Y.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the National Key Research & Development Plan (2021YFD1200201; 2018YFD1000800); National Natural Science Foundation of China (31972426; 31991182); Wuhan Biological Breeding Major Project (2022021302024852); Key Project of Hubei Hongshan Laboratory (2021hszd007); International Cooperation Promotion Plan of Shihezi University (GJHZ202104); Fundamental Research Funds for the Central Universities (2662022YLPY001).
Acknowledgments
The authors would like to pay their gratitude to the anonymous reviewers for their precious time, and valuable suggestions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Quinet, M.; Angosto, T.; Yuste-Lisbona, F.J.; Blanchard-Gros, R.; Bigot, S.; Martinez, J.P.; Lutts, S. Tomato fruit development and metabolism. Front. Plant Sci. 2019, 29, 1554. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Wang, X.; Zhu, Z.; Sun, M.; Li, M.; Hou, L. Expression analysis of genes related to auxin metabolism at different growth stages of pak choi. Hortic. Plant J. 2020, 6, 25–33. [Google Scholar] [CrossRef]
- Zhu, Z.; Bai, Y.; Lv, M.; Tian, G.; Zhang, X.; Li, L.; Jiang, Y.; Ge, S. Soil fertility, microbial biomass, and microbial functional diversity responses to four years fertilization in an apple orchard in north China. Hortic. Plant J. 2020, 6, 223–230. [Google Scholar] [CrossRef]
- Wang, L.; Zou, Y.; Kaw, H.Y.; Wang, G.; Sun, H.; Cai, L.; Li, C.; Meng, L.Y.; Li, D. Recent developments and emerging trends of mass spectrometric methods in plant hormone analysis: A review. Plant Methods 2020, 16, 16–54. [Google Scholar] [CrossRef]
- Kim, T.W.; Guan, S.; Sun, Y.; Deng, Z.; Tang, W.; Shang, J.X.; Sun, Y.; Burlingame, A.L.; Wang, Z.Y. Brassinosteroid signal transduction from cell-surface receptor kinases to nuclear transcription factors. Nat. Cell Biol. 2009, 11, 10. [Google Scholar] [CrossRef]
- Mumtaz, M.A.; Hao, Y.; Mehmood, S.; Shu, H.; Zhou, Y.; Jin, W.; Chen, C.; Li, L.; Altaf, M.A.; Wang, Z.W. Physiological and transcriptomic analysis provide molecular insight into 24-epibrassinolide mediated cr(vi)-toxicity tolerance in pepper Plants. Environ. Pollut. 2022, 306, 119375. [Google Scholar] [CrossRef]
- Bartwal, A.; Arora, S. Brassinosteroids: Molecules with Myriad Roles. In Co-Evolution of Secondary Metabolites. Reference Series in Phytochemistry; Reference Series in Phytochemistry; Springer: Cham, Switzerland, 2020; pp. 869–895. [Google Scholar]
- Morinaka, Y.; Sakamoto, T.; Inukai, Y.; Agetsuma, M.; Kitano, H.; Ashikari, M.; Matsuoka, M. Morphological alteration caused by brassinosteroid insensitivity increases the biomass and grain production of rice. Plant Physiol. 2006, 141, 924–931. [Google Scholar] [CrossRef]
- Singh, A.; Breja, P.; Khurana, J.P.; Khurana, P. Wheat Brassinosteroid-Insensitive1 (TaBRI1) interacts with members of TaSERK gene family and cause early flowering and seed yield enhancement in Arabidopsis. PLoS ONE 2016, 11, e0153273. [Google Scholar] [CrossRef]
- Chai, Y.M.; Zhang, Q.; Tian, L.; Li, C.L.; Xing, Y.; Qin, L.; Shen, Y.Y. Brassinosteroid is involved in strawberry fruit ripening. Plant Growth Regul. 2013, 69, 63–69. [Google Scholar] [CrossRef]
- Nie, S.; Huang, S.; Wang, S.; Cheng, D.; Liu, J.; Lv, S.; Li, Q.; Wang, X. Enhancing brassinosteroid signaling via overexpression of tomato (Solanum lycopersicum) SlBRI1 improves major agronomic traits. Front. Plant Sci. 2017, 8, 1386. [Google Scholar] [CrossRef]
- Feng, Y.; Yin, Y.; Fei, S. Down-regulation of BdBRI1, a putative brassinosteroid receptor gene produces a dwarf phenotype with enhanced drought tolerance in Brachypodium distachyon. Plant Sci. 2015, 234, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Bünger-Kibler, S.; Bangerth, F. Relationship between cell number, cell size and fruit size of seeded fruits of tomato (Lycopersicon esculentum Mill.), and those induced parthenocarpically by the application of plant growth regulators. Plant Growth Regul. 1982, 1, 143–154. [Google Scholar] [CrossRef]
- Liu, L.; Jia, C.; Zhang, M.; Chen, D.; Chen, S.; Guo, R.; Guo, D.; Wang, Q. Ectopic expression of a BZR1-1D transcription factor in brassinosteroid signalling enhances carotenoid accumulation and fruit quality attributes in tomato. Plant Biotechnol. J. 2014, 12, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Mumtaz, M.A.; Munir, S.; Liu, G.; Chen, W.; Wang, Y.; Yu, H.; Mahmood, S.; Ahiakpa, J.K.; Tamim, S.A.; Zhang, Y. Altered brassinolide sensitivity1 transcriptionally inhibits chlorophyll synthesis and photosynthesis capacity in tomato. Plant Growth Regul. 2020, 92, 417–426. [Google Scholar] [CrossRef]
- Mumtaz, M.A.; Wang, Y.; Li, F.; Shang, L.; Wang, Y.; Zhang, X.; Tao, J.; Gai, W.; Dong, H.; Ahiakpa, J.K.; et al. Hindered tomato reproductive development by altered brassinosteroid sensitivity1 mutant. Plant Growth Regul. 2022, 96, 473–481. [Google Scholar] [CrossRef]
- Zheng, L.; Gao, C.; Zhao, C.; Zhang, L.; Han, M.; An, N.; Ren, X. Effects of brassinosteroid associated with auxin and gibberellin on apple tree growth and gene expression patterns. Hortic. Plant J. 2019, 5, 93–108. [Google Scholar] [CrossRef]
- Montoya, T.; Nomura, T.; Farrar, K.; Kaneta, T.; Yokota, T.; Bishop, G.J. Cloning the tomato curl3 gene highlights the putative dual role of the leucine-rich repeat receptor kinase tBRI1/SR160 in plant steroid hormone and peptide hormone signaling. Plant Cell 2002, 14, 3163–3176. [Google Scholar] [CrossRef]
- Nolan, T.; Liu, S.; Guo, H.; Li, L.; Schnable, P.; Yin, Y. Identification of brassinosteroid target genes by chromatin immunoprecipitation followed by high-throughput sequencing (Chip-seq) and RNA-sequencing. Methods Mol. Biol. 2017, 1564, 63–79. [Google Scholar]
- Srivastava, A.; Handa, A.K. Hormonal regulation of tomato fruit development: A molecular perspective. J. Plant Growth Regul. 2005, 24, 67–82. [Google Scholar] [CrossRef]
- Kumar, R.; Khurana, A.; Sharma, A.K. Role of plant hormones and their interplay in development and ripening of fleshy fruits. J. Exp. Bot. 2014, 65, 4561–4575. [Google Scholar] [CrossRef]
- Werner, T.; Schmülling, T. Cytokinin action in plant development. Curr. Opin. Plant Biol. 2009, 12, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, S.; Kikuchi, K.; Fukuda, M.; Honda, I.; Imanishi, S. Roles and regulation of cytokinins in tomato fruit development. J. Exp. Bot. 2012, 63, 5569–5579. [Google Scholar] [CrossRef] [PubMed]
- Gomez, M.D.; Barro-Trastoy, D.; Escoms, E.; Saura-Sańchez, M.; Sańchez, I.; Briones-Moreno, A.; Vera-Sirera, F.; Carrera, E.; Ripoll, J.J.; Yanofsky, M.F.; et al. Gibberellins negatively modulate ovule number in plants. Development 2018, 9, dev163865. [Google Scholar] [CrossRef]
- McAtee, P.; Karim, S.; Schaffer, R.; David, K. A dynamic interplay between phytohormones is required for fruit development, maturation, and ripening. Front. Plant Sci. 2013, 17, 4–79. [Google Scholar] [CrossRef]
- Shinozaki, Y.; Hao, S.; Kojima, M.; Sakakibara, H.; Ozeki-Iida, Y.; Zheng, Y.; Fei, Z.; Zhong, S.; Giovannoni, J.J.; Rose, J.K.C.; et al. Ethylene suppresses tomato (Solanum lycopersicum) fruit set through modification of gibberellin metabolism. Plant J. 2015, 83, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, N.; Gévaudant, F.; Hernould, M.; Chevalier, C.; Mouras, A. The cell cycle-associated protein kinase WEE1 regulates cell size in relation to endoreduplication in developing tomato fruit. Plant J. 2007, 51, 642–655. [Google Scholar] [CrossRef] [PubMed]
- Frary, A.; Nesbitt, T.C.; Frary, A.; Grandillo, S.; Van Der Knaap, E.; Cong, B.; Liu, J.; Meller, J.; Elber, R.; Alpert, K.B.; et al. fw2.2: A quantitative trait locus key to the evolution of tomato fruit size. Science 2000, 7, 85. [Google Scholar] [CrossRef]
- Czerednik, A.; Busscher, M.; Angenent, G.C.; De Maagd, R.A. The cell size distribution of tomato fruit can be changed by overexpression of CDKA1. Plant Biotechnol. J. 2015, 13, 259–268. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).