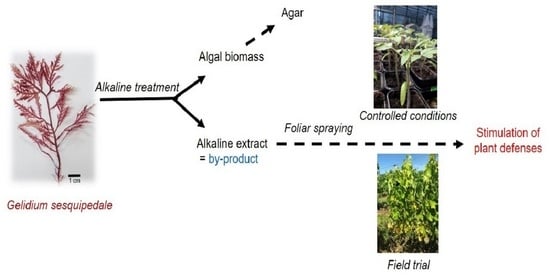
A New Promising Plant Defense Stimulator Derived from a By-Product of Agar Extraction from Gelidium sesquipedale
Abstract
1. Introduction
2. Materials and Methods
2.1. By-Product Origin, Production and Preparation
2.2. Elemental Analyses
2.3. Organic Compounds Assays
2.3.1. Carbohydrate Assays
2.3.2. Protein Assay
2.3.3. Phenolic Compounds Assay
2.4. Greenhouse Experiments
2.5. Plant Defense Activities
2.6. Quantification of Defense Gene Expression by Real Time q-PCR
2.7. Field Trial on Grapevine against P. viticola Artificially Inoculated
2.8. Statistical Analyses
3. Results
3.1. SL35 Composition
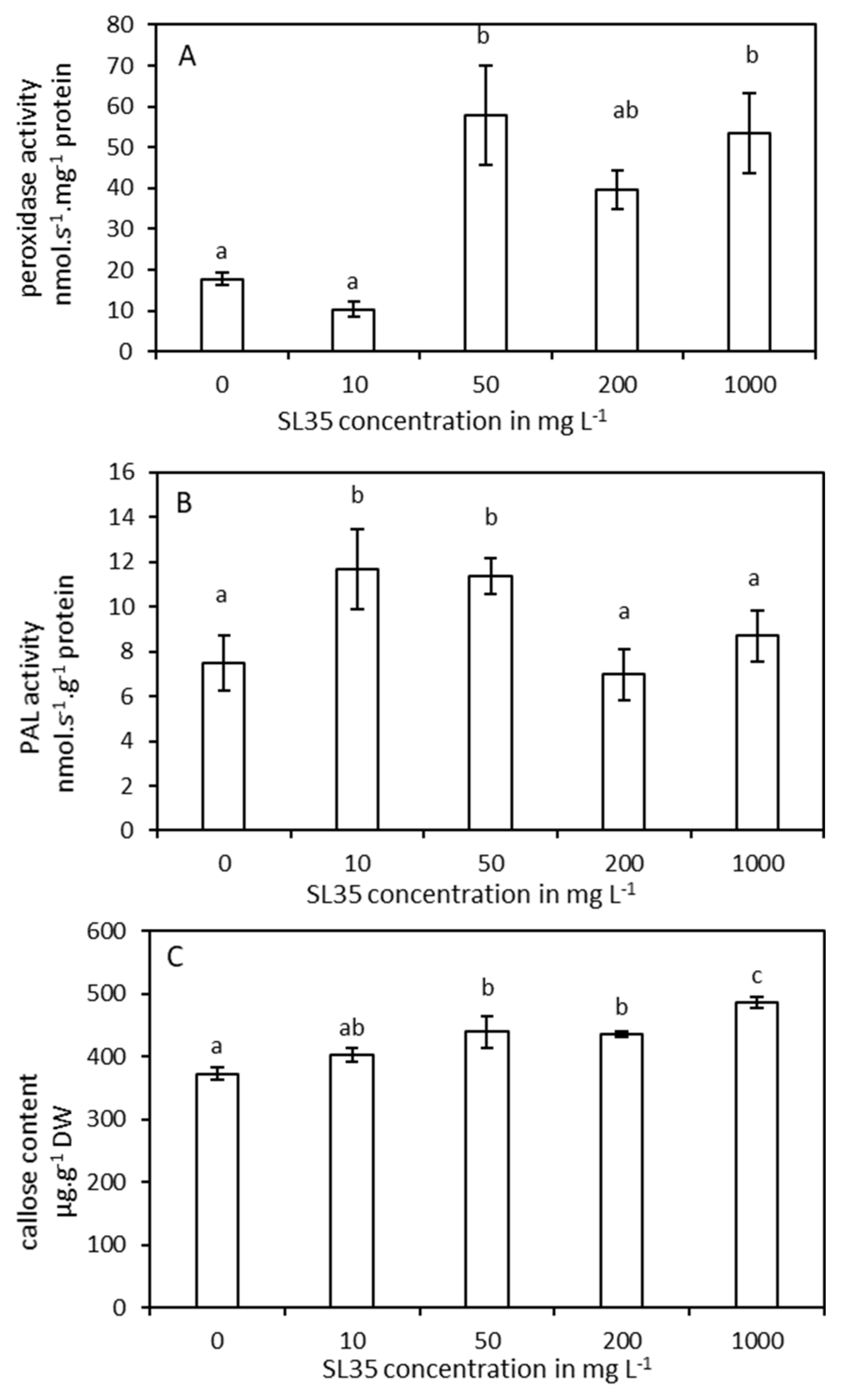
3.2. Biological Activity of SL35: Effect of the Concentration on the Stimulation of Tomato Plant Defenses
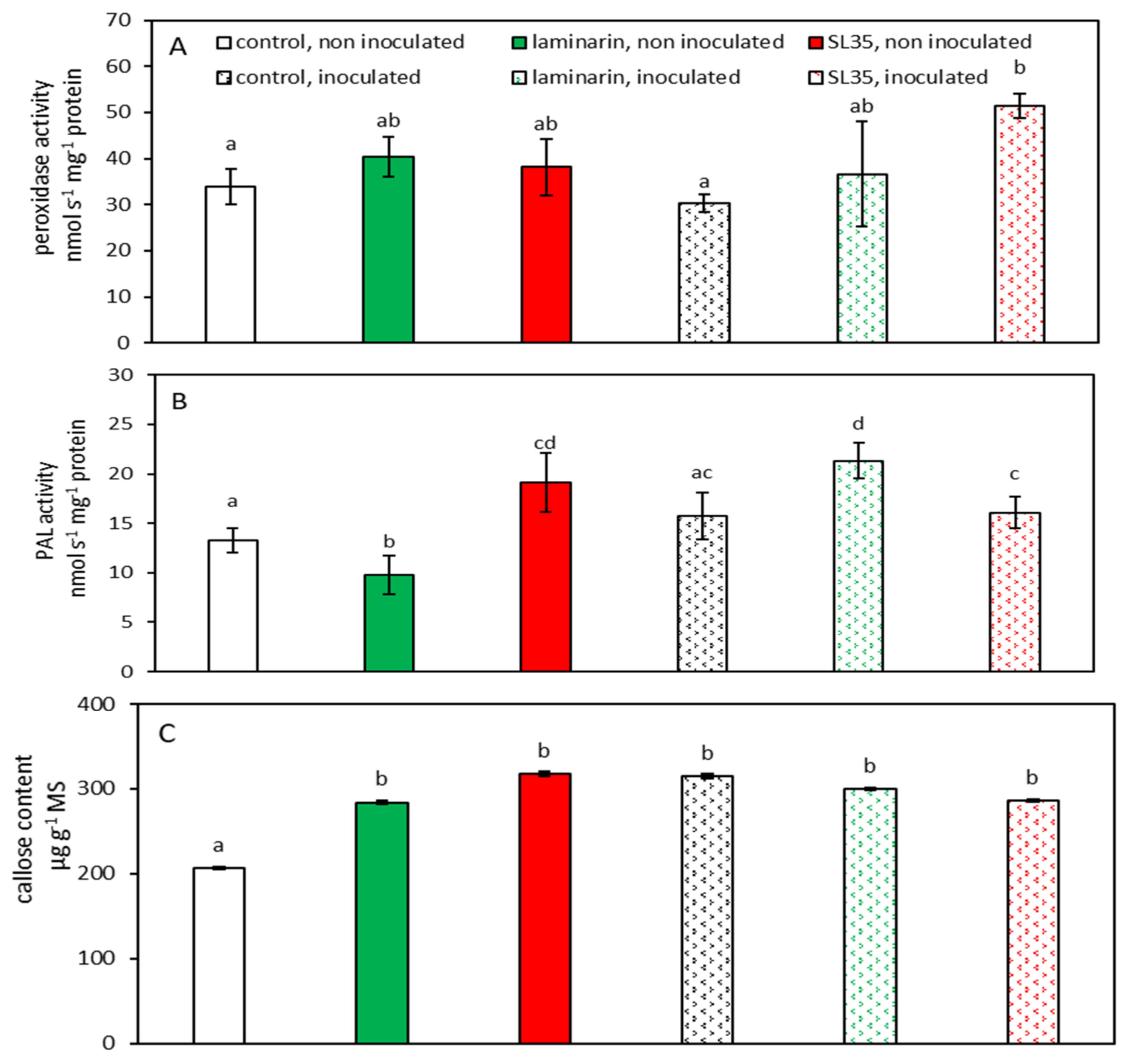
3.3. Biological Activities of SL35 in Presence or Not of Pathogen: Comparison with Commercial Standard
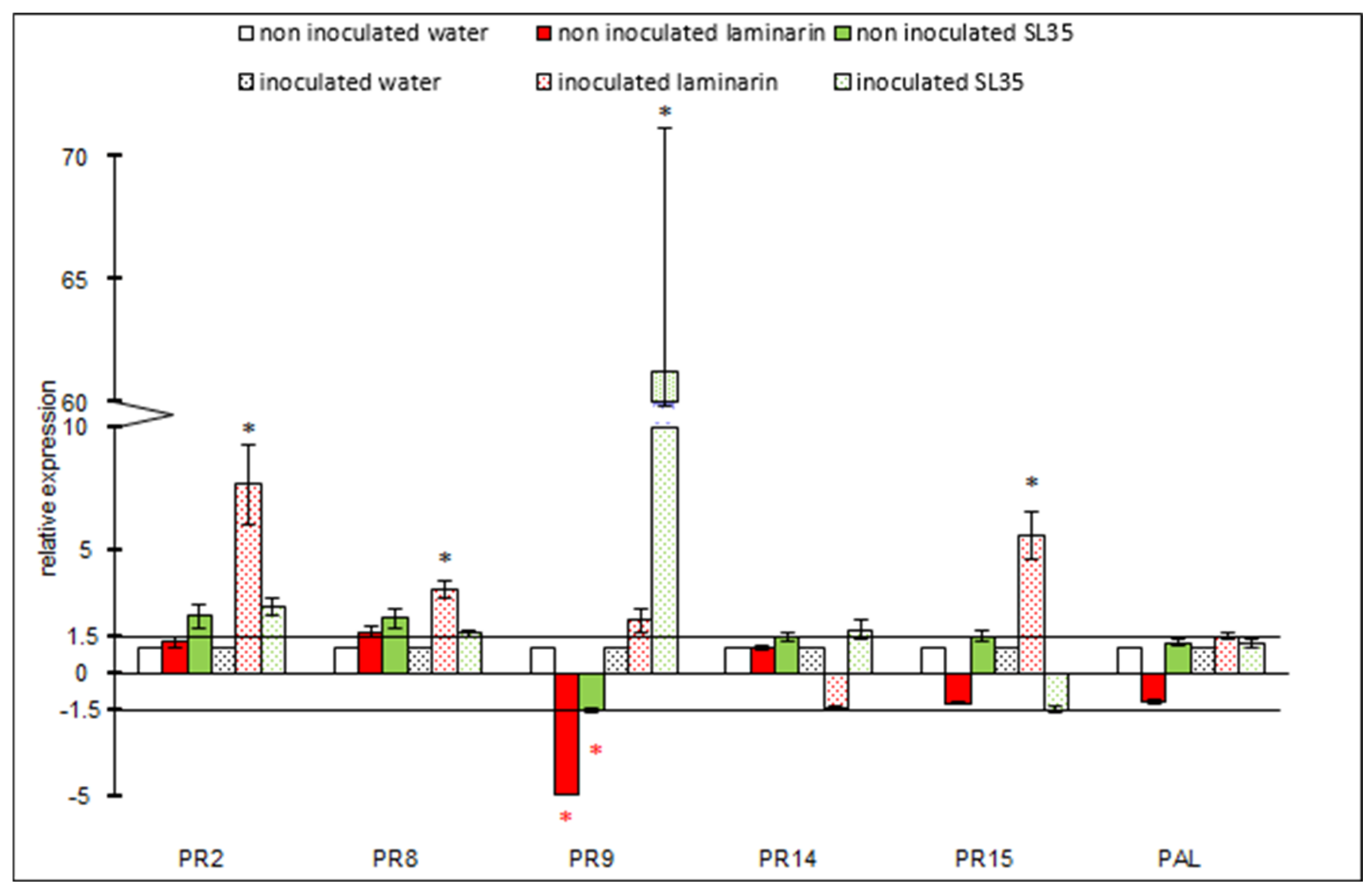
3.4. Evaluation of Defense Responses by Quantification of Gene Expression
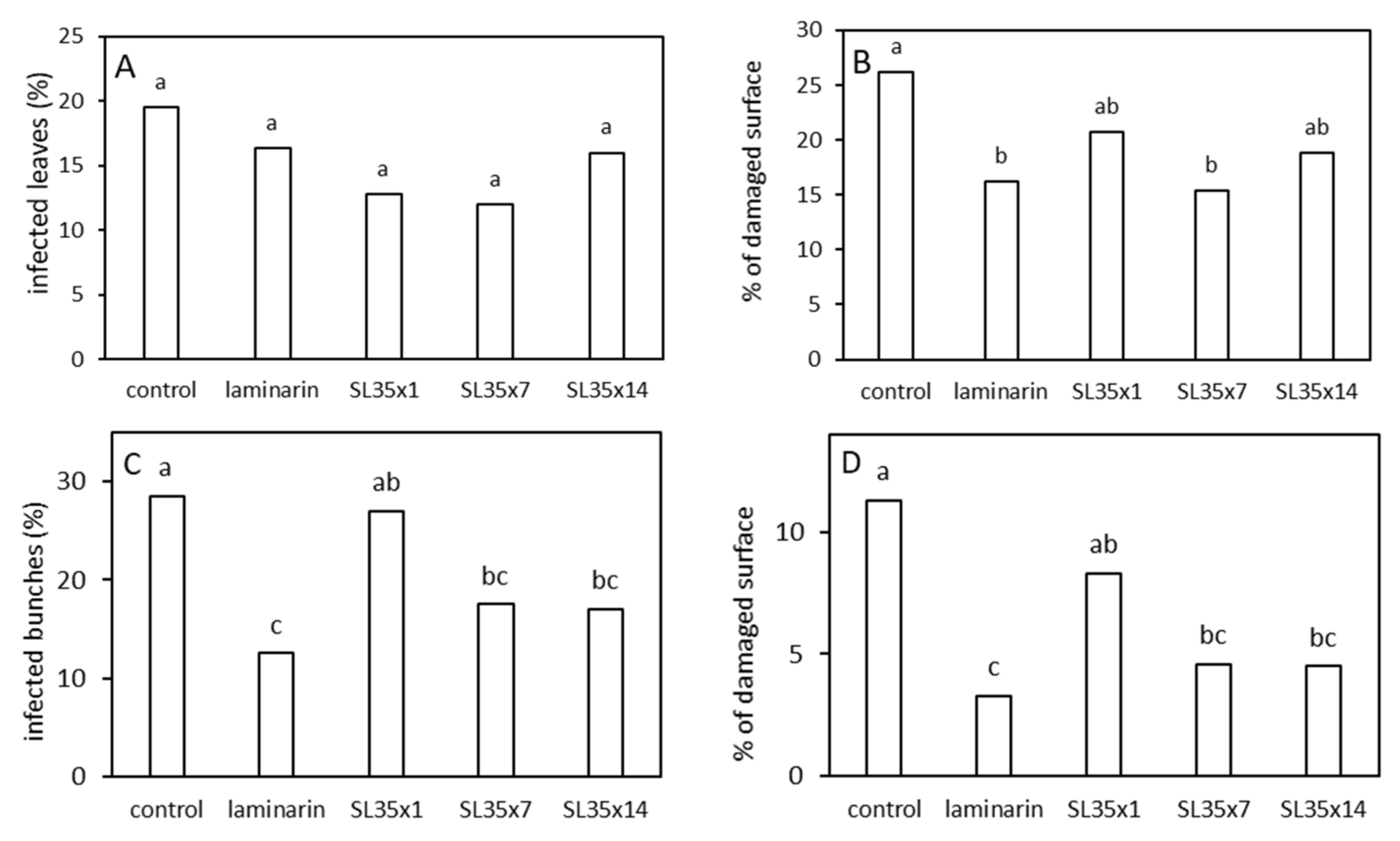
3.5. Field Trial on Grapevine against P. viticola Artificially Inoculated
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yadav, I.C.; Devi, N.L. Pesticides Classification and Its Impact on Human and Environment. In Environmental Science and Engineering; Chapter 7; Studium Press LLC: Houston, TX, USA, 2017; Volume 6, pp. 140–158. [Google Scholar]
- Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/faostat/en/#data/RP/visualize (accessed on 17 August 2022).
- Hammerschmidt, R. Introduction: Definitions and some history. In Induced Resistance for Plant Defence; Walters, D., Newton, A., Lyon, G., Eds.; Blackwell Publishing: Hoboken, NJ, USA, 2007; pp. 1–8. [Google Scholar]
- Proestos, C.; Chorianopoulos, N.; Nychas, G.J.; Komaitis, M. RP-HPLC analysis of the phenolic compounds of plant extracts. Investigation of their antioxidant capacity and antimicrobial activity. J. Agric. Food Chem. 2005, 53, 1190–1195. [Google Scholar] [CrossRef]
- Bellincampi, D.; Cervone, F.; Lionetti, V. Plant cell wall dynamics and wall-related susceptibility in plant–pathogen interactions. Front. Plant Sci. 2014, 5, 228. [Google Scholar] [CrossRef] [PubMed]
- Héloir, M.-C.; Adrian, M.; Brulé, D.; Claverie, J.; Cordelier, S.; Daire, X.; Dorey, S.; Gauthier, A.; Lemaître-Guillier, C.; Negrel, J.; et al. Recognition of Elicitors in Grapevine: From MAMP and DAMP Perception to Induced Resistance. Front. Plant Sci. 2019, 10, 1117. [Google Scholar] [CrossRef] [PubMed]
- Poornananda, M.; Jameel, K. Impact of abiotic elicitors on in vitro production of plant secondary metabolites: A review. J. Adv. Res. Biotech. 2016, 1, 7. [Google Scholar]
- Trouvelot, S.; Héloir, M.-C.; Poinssot, B.; Gauthier, A.; Paris, F.; Guillier, C.; Combier, M.; Trdá, L.; Daire, X.; Adrian, M. Carbohydrates in plant immunity and plant protection: Roles and potential application as foliar sprays. Front. Plant Sci. 2014, 5, 592. [Google Scholar] [CrossRef] [PubMed]
- Abdul Malik, N.A.; Kumar, I.S.; Nadarajah, K. Elicitor and Receptor Molecules: Orchestrators of Plant Defense and Immunity. Int. J. Mol. Sci. 2020, 21, 963. [Google Scholar] [CrossRef] [PubMed]
- Vera, J.; Castro, J.; Gonzalez, A.; Moenne, A. Seaweed polysaccharides and derived oligosaccharides stimulate defense responses and protection against pathogens in plants. Mar. Drugs 2011, 9, 2514–2525. [Google Scholar]
- Lebbar, M.S.; Faugeron-Girard, C.; Gloaguen, V. Use of An Extract or An Extract Fraction of Agarophyte Red Algae as A Plant Defense Elicitor/Stimulator and Application of Said Extract or Said Extract Fraction. French Patent FR 1870542, 7 May 2018. [Google Scholar]
- Lebbar, S.; Fanuel, M.; Le Gall, S.; Falourd, X.; Ropartz, D.; Bressollier, P.; Gloaguen, V.; Faugeron-Girard, C. Agar extraction by-products from Gelidium sesquipedale as a source of glycerol-galactosides. Molecules 2018, 23, 3364. [Google Scholar] [CrossRef]
- Nguyen, D.N.; Grybos, M.; Rabiet, M.; Deluchat, V. How do colloid separation and sediment storage methods affect water-mobilizable colloids and phosphorus? An insight into dam reservoir sediment. Colloids Surf. 2020, 606, 125505. [Google Scholar] [CrossRef]
- Bascle, S.; Bourven, I.; Baudu, M. Nature and accessibility of organic matter in lacustrine sediment. J. Soils Sediments 2021, 21, 1–19. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Blumenkrantz, N.; Asboe-Hansen, G. New method for quantitative determination of uronic acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Piló-Veloso, D.; Nascimento, E.A.; Morais, S.A.L. Isolamento e análise estrutural de ligninas. Química Nova 1993, 16, 435–448. [Google Scholar]
- Faugeron-Girard, C.; Gloaguen, V.; Koçi, R.; Célérier, J.; Raynaud, A.; Moine, C. Use of a Pleurotus ostreatus complex cell wall extract as elicitor of plant defenses: From greenhouse to field trial. Molecules 2020, 25, 1094. [Google Scholar] [CrossRef]
- Francini, A.; Nali, C.; Picchi, V.; Lorenzini, G. Metabolic changes in white clover clones exposed to ozone. Environ. Exp. Bot. 2007, 60, 11–19. [Google Scholar] [CrossRef]
- Hirano, Y.; Pannatier, E.G.; Zimmermann, S.; Brunner, I. Induction of callose in roots of Norway spruce seedlings after short-term exposure to aluminium. Tree Physiol. 2004, 24, 1279–1283. [Google Scholar] [CrossRef]
- Grassot, V.; Da Silva, A.; Saliba, J.; Maftah, A.; Dupuy, F.; Petit, J.M. Highlights of glycosylation and adhesion related genes involved in myogenesis. BMC Genom. 2014, 15, 621. [Google Scholar] [CrossRef]
- Chun, S.C.; Chandrasekaran, M. Chitosan and chitosan nanoparticles induced expression of pathogenesis-related proteins genes enhances biotic stress tolerance in tomato. Int. J. Biol. Macromol. 2019, 125, 948–954. [Google Scholar] [CrossRef]
- Babu, A.N.; Jogaiah, S.; Ito, S.I.; Nagaraj, A.K.; Tran, L.S.P. Improvement of growth, fruit weight and early blight disease protection of tomato plants by rhizosphere bacteria is correlated with their beneficial traits and induced biosynthesis of antioxidant peroxidase and polyphenol oxidase. Plant Sci. 2015, 231, 62–73. [Google Scholar] [CrossRef]
- Jogaiah, S.; Abdelrahman, M.; Tran, L.S.P.; Shin-Ichi, I. Characterization of rhizosphere fungi that mediate resistance in tomato against bacterial wilt disease. J. Exp. Bot. 2013, 64, 3829–3842. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, N.; Buonanno, M.; Ayoub, J.; Barone, A.; Monti, S.M.; Rigano, M.M. Identification of non-specific Lipid Transfer Protein gene family members in Solanum lycopersicum and insights into the features of Sola l 3 protein. Sci. Rep. 2019, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Feng, C.; Zhang, A.; Zhang, Y.; Chang, D.; Wang, Y.; Ma, Q. The dual role of oxalic acid on the resistance of tomato against Botrytis cinerea. World J. Microbiol. Biotechnol. 2019, 35, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Feng, G.; Chen, K. Defense responses of harvested tomato fruit to burdock fructooligosaccharide, a novel potential elicitor. Postharvest Biol. Technol. 2009, 52, 110–116. [Google Scholar] [CrossRef]
- Becker, S.; Scheffel, A.; Polz, M.F.; Hehemann, J.H. Accurate quantification of laminarin in marine organic matter with enzymes from marine microbes. Appl. Environ. Microbiol. 2017, 83, e03389-16. [Google Scholar] [CrossRef] [PubMed]
- Klarzynski, O.; Plesse, B.; Joubert, J.M.; Yvin, J.C.; Kopp, M.; Kloareg, B.; Fritig, B. Linear β-1, 3 glucans are elicitors of defense responses in tobacco. Plant Physiol. 2000, 124, 1027–1038. [Google Scholar] [CrossRef]
- Usov, A.I. Polysaccharides of the red algae. Adv. Carbohydr. Chem. Biochem. 2011, 65, 115–217. [Google Scholar]
- Izumi, K. Chemical heterogeneity of the agar from Gelidium amansii. Carbohydr. Res. 1971, 17, 227–230. [Google Scholar] [CrossRef]
- Cui, M.; Wu, J.; Wang, S.; Shu, H.; Zhang, M.; Liu, K.; Liu, K. Characterization and anti-inflammatory effects of sulfated polysaccharide from the red seaweed Gelidium pacificum Okamura. Int. J. Biol. Macromol. 2019, 129, 377–385. [Google Scholar] [CrossRef]
- Zhang, P.Y.; Wang, J.C.; Liu, S.H.; Chen, K.S. A novel burdock fructooligosaccharide induces changes in the production of salicylates activates defence enzymes and induces systemic acquired resistance to Colletotrichum orbiculare in cucumberseedlings. J. Phytopathol. 2009, 157, 201–207. [Google Scholar] [CrossRef]
- Abouraïcha, E.; El Alaoui-Talibi, Z.; El Boutachfaiti, R.; Petit, E.; Courtois, B.; Courtois, J.; El Modafar, C. Induction of natural defense and protection against Penicillium expansum and Botrytis cinerea in apple fruit in response to bioelicitors isolated from green algae. Sci. Hortic. 2015, 181, 121–128. [Google Scholar] [CrossRef]
- An, Q.D.; Zhang, G.L.; Wu, H.T.; Zhang, Z.C.; Zheng, G.S.; Luan, L.; Murata, Y.; Li, X. Alginate-deriving oligosaccharide production by alginase from newly isolated Flavobacterium sp. LXA and its potential application in protection against pathogens. J. Appl. Microbiol. 2009, 106, 161–170. [Google Scholar] [CrossRef]
- Feng, H.; Xia, W.; Shan, C.; Zhou, T.; Cai, W.; Zhang, W. Quaternized chitosan oligomers as novel elicitors inducing protection against B. cinerea in Arabidopsis. Int. J. Biol. Macromol. 2015, 72, 364–369. [Google Scholar] [CrossRef]
- Pogorelko, G.; Lionetti, V.; Bellincampi, D.; Zabotina, O. Cell wall integrity. Plant Signal. Behav. 2013, 8, 9. [Google Scholar] [CrossRef]
- Gauthier, A.; Trouvelot, S.; Kelloniemi, J.; Frettinger, P.; Wendehenne, D.; Daire, X.; Joubert, J.M.; Ferrarini, A.; Delledonne, M.; Flors, V.; et al. The sulfated laminarin triggers a stress transcriptome before priming the SA- and ROS-dependent defenses during grapevine’s induced resistance against Plasmopara viticola. PLoS ONE 2014, 6, e88145. [Google Scholar] [CrossRef]
- De Borba, M.C.; Velho, A.C.; de Freitas, M.B.; Holvoet, M.; Maia-Grondard, A.; Baltenweck, R.; Stadnik, M.J. A Laminarin-Based Formulation Protects Wheat Against Zymoseptoria tritici via Direct Antifungal Activity and Elicitation of Host Defense-Related Genes. Plant Dis. 2022, 106, 1408–1418. [Google Scholar] [CrossRef]
- Xin, Z.; Cai, X.; Chen, S.; Luo, Z.; Bian, L.; Li, Z.; Ge, L.; Chen, Z. A disease resistance elicitor laminarin enhances tea defense against a piercing herbivore Empoasca (Matsumurasca) onukii Matsuda. Sci. Rep. 2019, 9, 814. [Google Scholar] [CrossRef]
- Sinha, M.; Singh, R.P.; Kushwaha, G.S.; Iqbal, N.; Singh, A.; Kaushik, S.; Kaur, P.; Sharma, S.; Singh, T.P. Current overview of allergens of plant pathogenesis related protein families. Sci. World J. 2014, 2014, 543195. [Google Scholar] [CrossRef]
- Wanderley-Nogueira, A.C.; Belarmino, L.C.; Soares-Cavalcanti, N.D.M.; Bezerra-Neto, J.P.; Kido, E.A.; Pandolfi, V.; Abdelnoor, R.V.; Binneck, E.; Carazzole, M.F.; Benko-Iseppon, A.M. An overall evaluation of the resistance (R) and pathogenesis-related (PR) superfamilies in soybean, as compared with Medicago and Arabidopsis. Genet. Mol. Biol. 2012, 35, 260–271. [Google Scholar] [CrossRef]
- Aziz, A.; Poinssot, B.; Daire, X.; Adrian, M.; Bézier, A.; Lambert, B.; Pugin, A. Laminarin elicits defense responses in grapevine and induces protection against Botrytis cinerea and Plasmopara viticola. Mol. Plant Microbe Interact. 2003, 16, 1118–1128. [Google Scholar] [CrossRef]
- Ménard, R.; de Ruffray, P.; Fritig, B.; Yvin, J.C.; Kauffmann, S. Defense and resistance-inducing activities in tobacco of the sulfated β-1, 3 glucan PS3 and its synergistic activities with the unsulfated molecule. Plant Cell Physiol. 2005, 46, 1964–1972. [Google Scholar] [CrossRef]
- Bae, E.K.; Lee, H.; Lee, J.S.; Noh, E.W.; Jo, J. Molecular cloning of a peroxidase gene from poplar and its expression in response to stress. Tree Physiol. 2006, 26, 1405–1412. [Google Scholar] [CrossRef]
- Sels, J.; Mathys, J.; de Coninck, B.M.A.; Cammue, B.P.A.; de Bolle, M.F.C. Plant pathogenesis-related (PR) proteins: A focus on PR peptides. Plant Physiol. Biochem. 2008, 46, 941–950. [Google Scholar]
| Gene | References | Primers in the Corresponding Gene |
|---|---|---|
| PR2b | - | F: CCGTTGGAAACGAAGTTGAT |
| R: TCATCAGCATGGCCAAAATA | ||
| PR8 | [23] | F: TGC AGG AAC ATT CAC TGG AG |
| R: TAA CGT TGT GGC ATG ATG GT | ||
| PR9 | [24] | F: GCTTTGTCAGGGGTTGTGAT |
| [25] | R: TGCATCT CTAGCAACCAACG | |
| PR14 | [26] | F: CTCCATGCCTCCCTTATCTTC |
| R: CATGCTGTCTTTCGATCCG | ||
| PR15 | [27] | F: GGGCTAAATCCACCTCA |
| R: GGCACCACGAACATCTC | ||
| PAL | [28] | F: CTTTGATGCAGAAGCTGAGACA |
| R: TCGTCCTCGAAAGCTACAATCT | ||
| β-actin | [23] | F: AGG CAC ACA GGT GTT ATG GT |
| R: AGC AAC TCG AAG CTC ATT GT |
| Dates | Developmental Stage |
|---|---|
| 5 May | First leaf unfolded (BBCH 11) |
| 12 May | Inflorescences clearly visible (BBCH 53) |
| 20 May | Inflorescences fully developed; flowers separating (BBCH 57) |
| 27 May | Beginning of flowering: 10% of flowerhoods fallen (BBCH 61) |
| 8 June | Fruit set: young fruits beginning to swell, remains of flowers lost (BBCH71) |
| 15 June | Pea-sized berries, hanging bunches (BBCH 75) |
| 24 June | Berries beginning to touch (BBCH 77) |
| Types of Components | Analysis Method | CONTENTS |
|---|---|---|
| Mineral content | calcination | 440 ± 17 mg g−1 DW |
| Na | MP-AES | 140 ± 9 mg g−1 DW |
| K | MP-AES | 40.5 ± 0.7 mg g−1 DW |
| Si | MP-AES | 0.48 ± 0.0 mg g−1 DW |
| Ca | MP-AES | 0.43 ± 0.1 mg g−1 DW |
| Elemental analysis | ||
| C | CHNS | 170 ± 7.4 mg g−1 DW |
| H | CHNS | 27.6 ± 1.5 mg g−1 DW |
| N | CHNS | 24 ± 1 mg g−1 DW |
| S | CHNS | 86 ± 6.4 mg g−1 DW |
| P | LCK349TM and LCK350TM kits | 4.3 ± 0.1 mg g−1 DW |
| Organic compounds | ||
| Total sugars | Colorimetry | 125.4 ± 0.2 mg g−1 DW |
| Uronic acids * | Colorimetry | 9.3 ± 0.2% |
| Proteins | Colorimetry | 16.9 ± 2 mg g−1 DW |
| Phenolic compounds | Colorimetry | 7.7 ± mg g−1 DW |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koçi, R.; Dupuy, F.; Lebbar, S.; Gloaguen, V.; Faugeron Girard, C. A New Promising Plant Defense Stimulator Derived from a By-Product of Agar Extraction from Gelidium sesquipedale. Horticulturae 2022, 8, 958. https://doi.org/10.3390/horticulturae8100958
Koçi R, Dupuy F, Lebbar S, Gloaguen V, Faugeron Girard C. A New Promising Plant Defense Stimulator Derived from a By-Product of Agar Extraction from Gelidium sesquipedale. Horticulturae. 2022; 8(10):958. https://doi.org/10.3390/horticulturae8100958
Chicago/Turabian StyleKoçi, Rromir, Fabrice Dupuy, Salim Lebbar, Vincent Gloaguen, and Céline Faugeron Girard. 2022. "A New Promising Plant Defense Stimulator Derived from a By-Product of Agar Extraction from Gelidium sesquipedale" Horticulturae 8, no. 10: 958. https://doi.org/10.3390/horticulturae8100958
APA StyleKoçi, R., Dupuy, F., Lebbar, S., Gloaguen, V., & Faugeron Girard, C. (2022). A New Promising Plant Defense Stimulator Derived from a By-Product of Agar Extraction from Gelidium sesquipedale. Horticulturae, 8(10), 958. https://doi.org/10.3390/horticulturae8100958