The Effect of Harvest Date on Temporal Cannabinoid and Biomass Production in the Floral Hemp (Cannabis sativa L.) Cultivars BaOx and Cherry Wine
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Field Management
2.3. Data Collection
2.4. Statistical Analysis
3. Results and Discussion
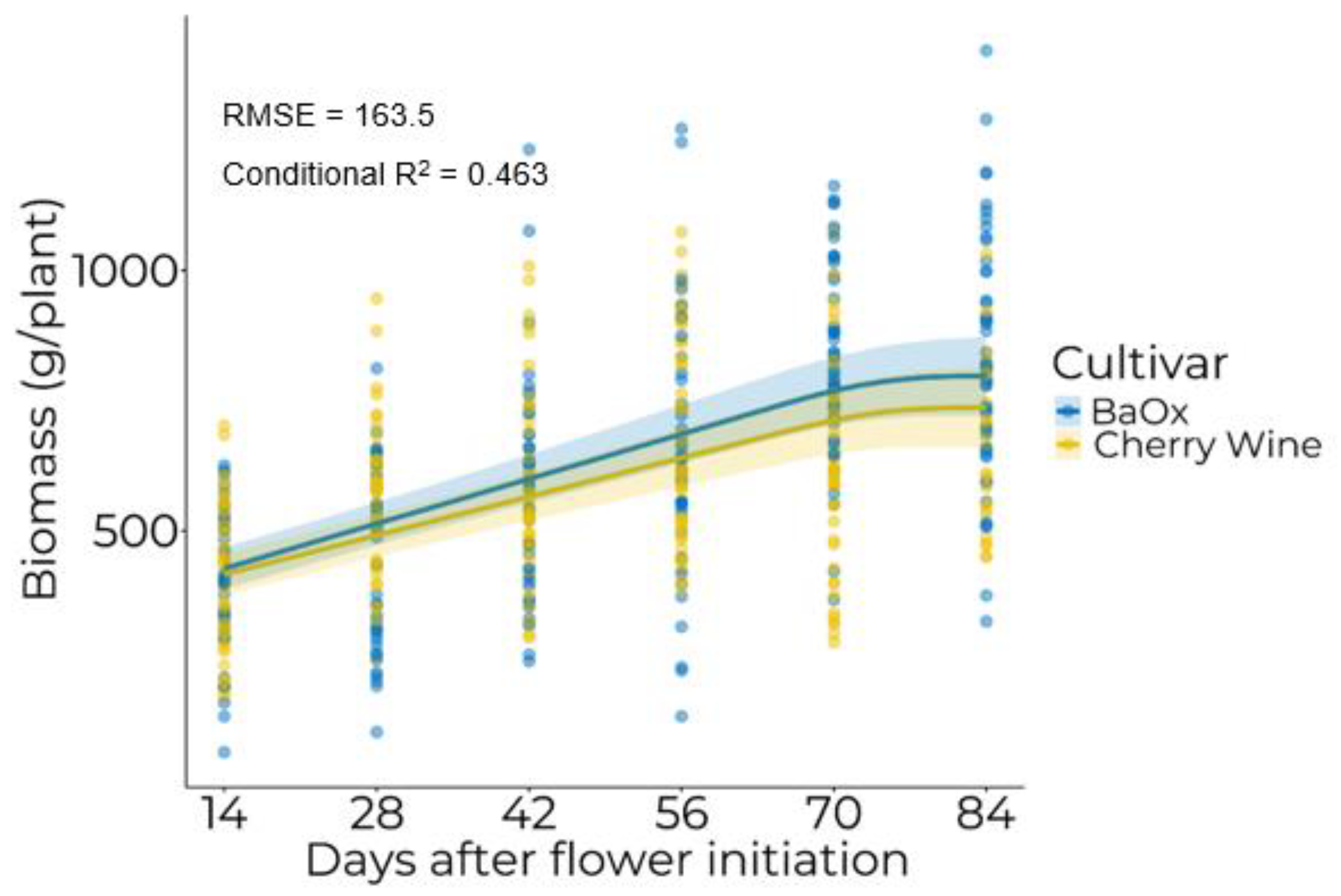
3.1. Biomass Accumulation
Ym if x ≥ XS
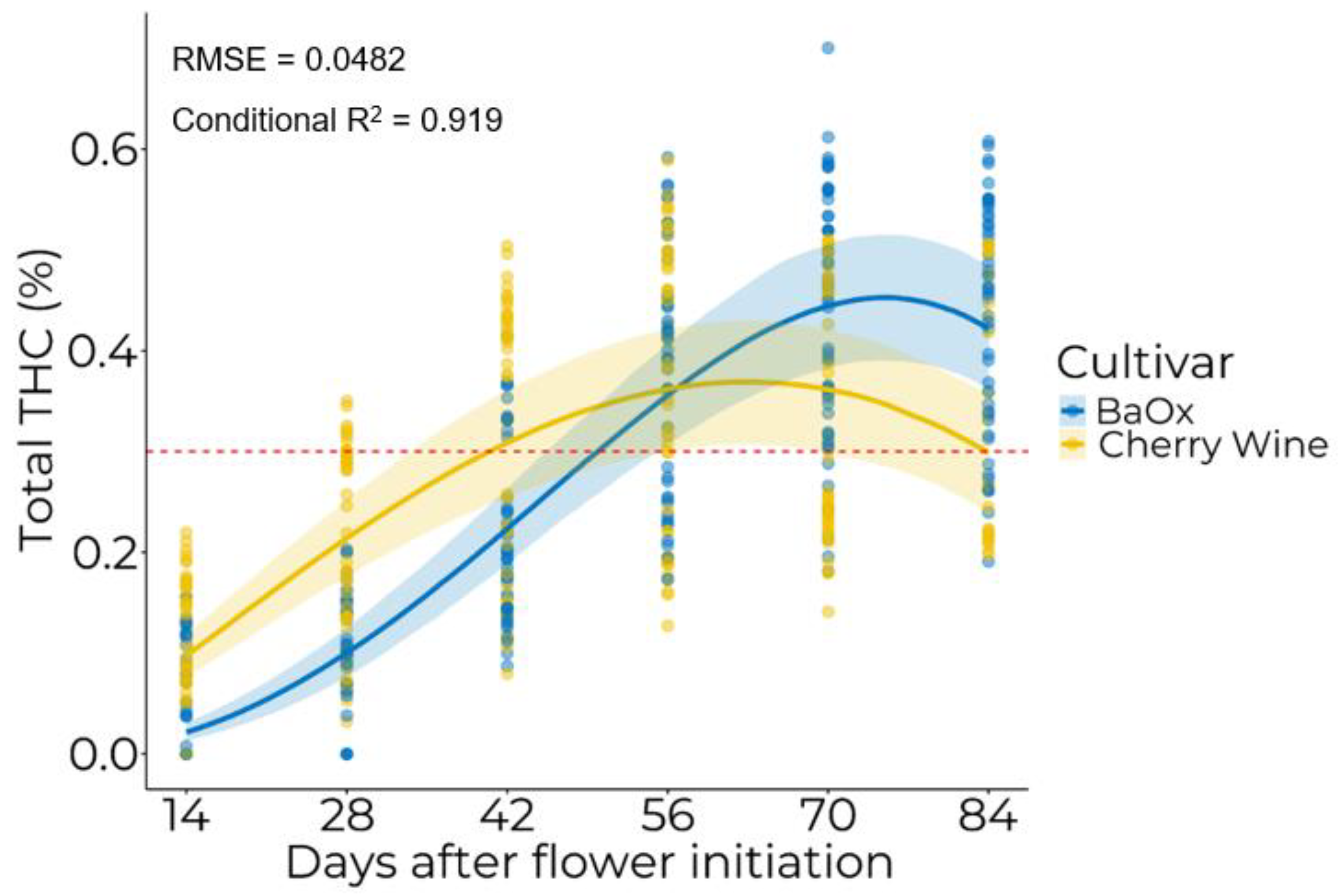
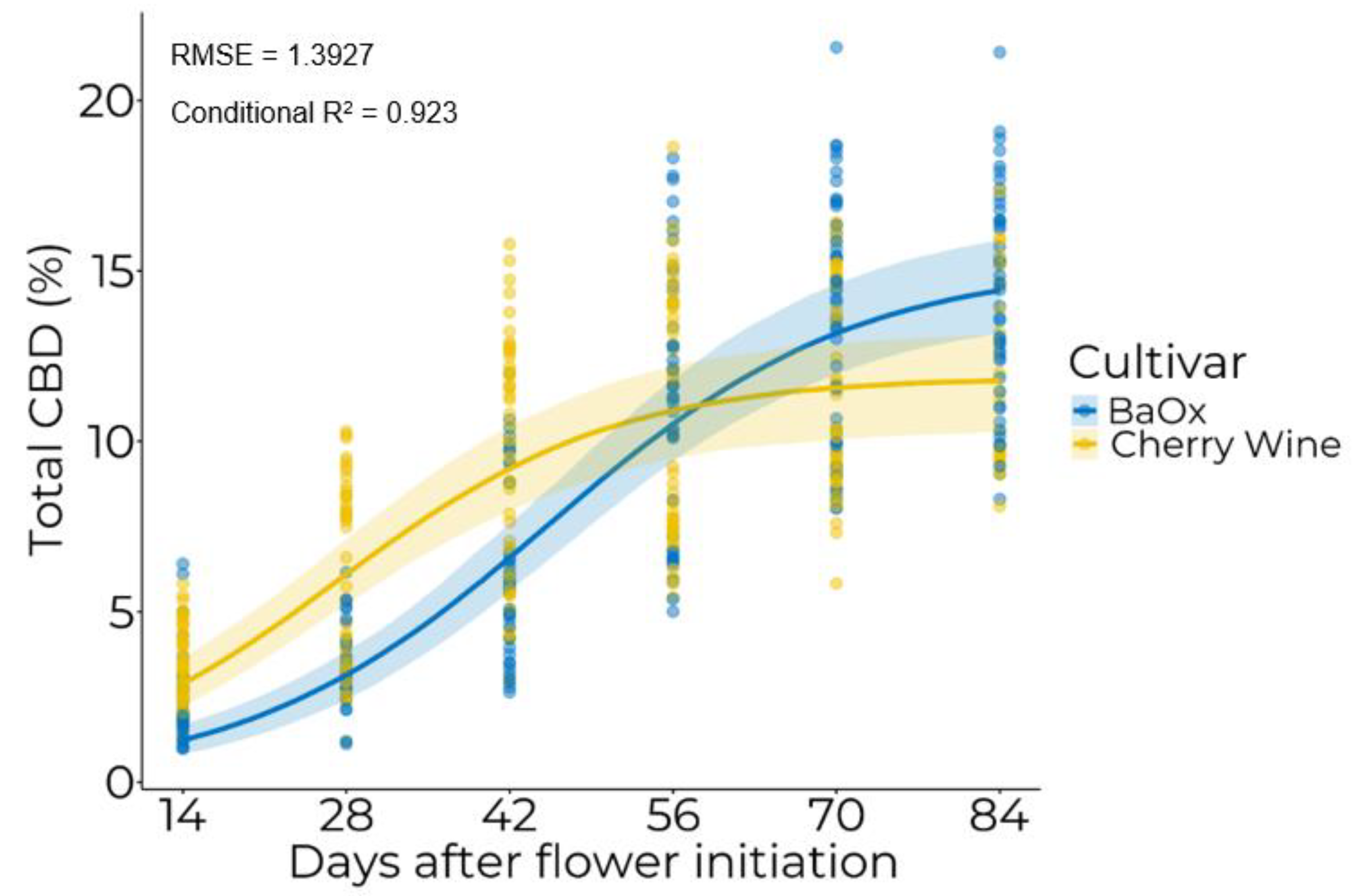
3.2. Temporal Accumulation of Cannabinoids
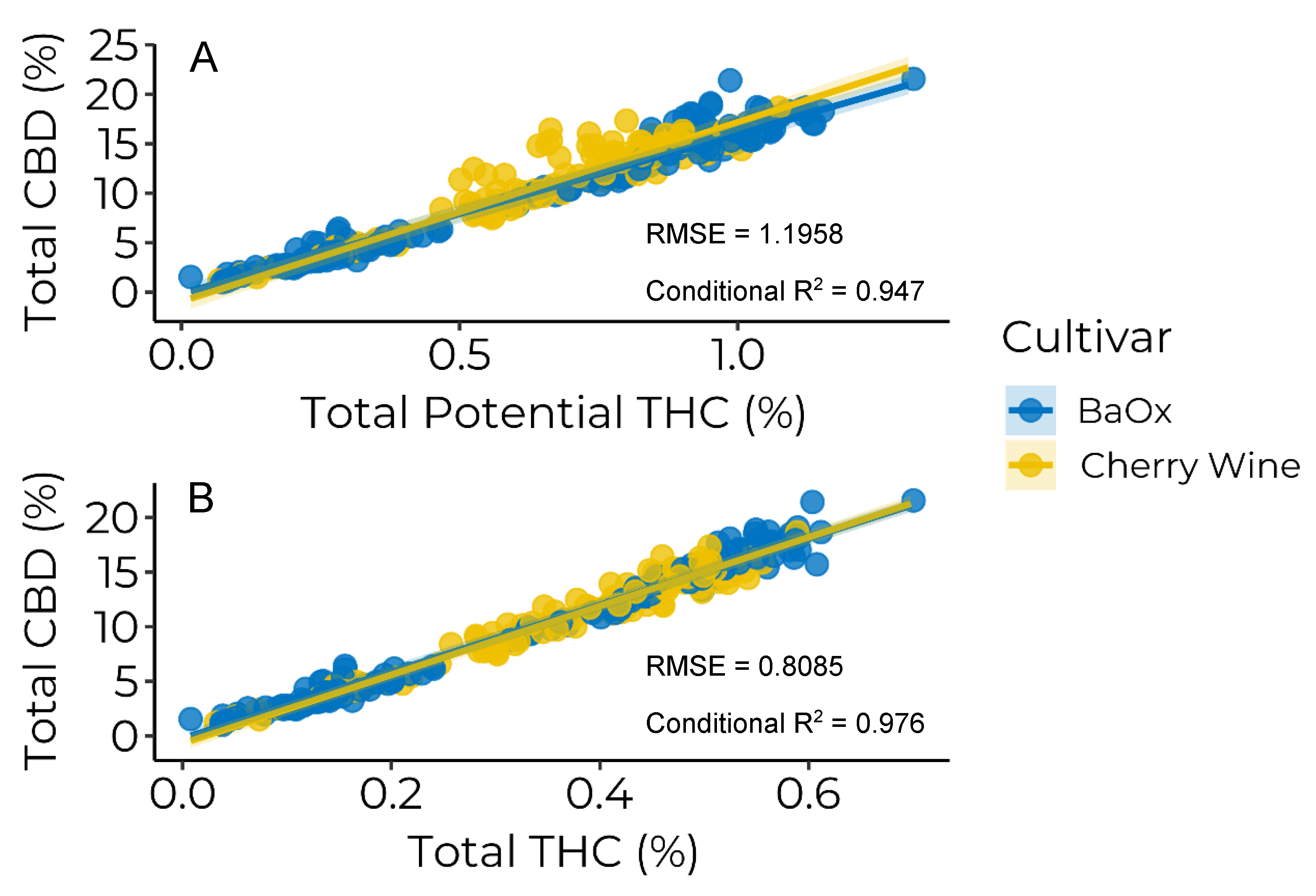
3.3. Cannabinoid Ratios
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Radwan, M.; Chandra, S.; Gul, S.; ElSohly, M. Cannabinoids, Phenolics, Terpenes and Alkaloids of Cannabis. Molecules 2021, 26, 2774. [Google Scholar] [CrossRef] [PubMed]
- Happyana, N.; Agnolet, S.; Muntendam, R.; Van Dam, A.; Schneider, B.; Kayser, O. Analysis of cannabinoids in laser-microdissected trichomes of medicinal Cannabis sativa using LCMS and cryogenic NMR. Phytochemistry 2012, 87, 51–59. [Google Scholar] [CrossRef]
- Perrotin-Brunel, H.; Buijs, W.; van Spronsen, J.; van Roosmalen, M.J.; Peters, C.J.; Verpoorte, R.; Witkamp, G.-J. Decarboxylation of Δ9-tetrahydrocannabinol: Kinetics and molecular modeling. J. Mol. Struct. 2011, 987, 67–73. [Google Scholar] [CrossRef]
- Dussy, F.E.; Hamberg, C.; Luginbühl, M.; Schwerzmann, T.; Briellmann, T.A. Isolation of Δ9-THCA-A from hemp and analytical aspects concerning the determination of Δ9-THC in cannabis products. Forensic Sci. Int. 2005, 149, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Pellati, F.; Borgonetti, V.; Brighenti, V.; Biagi, M.; Benvenuti, S.; Corsi, L. Cannabis sativa L. and Nonpsychoactive Cannabinoids: Their Chemistry and Role against Oxidative Stress, Inflammation, and Cancer. BioMed Res. Int. 2018, 2018, 1691428. [Google Scholar] [CrossRef] [PubMed]
- de Meijer, E.P.M.; Bagatta, M.; Carboni, A.; Crucitti, P.; Moliterni, V.M.C.; Ranalli, P.; Mandolino, G. The Inheritance of Chemical Phenotype in Cannabis sativa L. Genetics 2003, 163, 335–346. [Google Scholar] [CrossRef]
- Stack, G.M.; Toth, J.A.; Carlson, C.H.; Cala, A.R.; Marrero-González, M.I.; Wilk, R.L.; Gentner, D.R.; Crawford, J.L.; Philippe, G.; Rose, J.K.C.; et al. Season-long characterization of high-cannabinoid hemp (Cannabis sativa L.) reveals variation in cannabinoid accumulation, flowering time, and disease resistance. GCB Bioenergy 2021, 13, 546–561. [Google Scholar] [CrossRef]
- Zirpel, B.; Kayser, O.; Stehle, F. Elucidation of structure-function relationship of THCA and CBDA synthase from Cannabis sativa L. J. Biotechnol. 2018, 284, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Aizpurua-Olaizola, O.; Soydaner, U.; Öztürk, E.; Schibano, D.; Simsir, Y.; Navarro, P.; Etxebarria, N.; Usobiaga, A. Evolution of the Cannabinoid and Terpene Content during the Growth of Cannabis sativa Plants from Different Chemotypes. J. Nat. Prod. 2016, 79, 324–331. [Google Scholar] [CrossRef]
- De Backer, B.; Maebe, K.; Verstraete, A.; Charlier, C. Evolution of the Content of THC and Other Major Cannabinoids in Drug-Type Cannabis Cuttings and Seedlings During Growth of Plants*. J. Forensic Sci. 2012, 57, 918–922. [Google Scholar] [CrossRef]
- Massuela, D.C.; Hartung, J.; Munz, S.; Erpenbach, F.; Graeff-Hönninger, S. Impact of Harvest Time and Pruning Technique on Total CBD Concentration and Yield of Medicinal Cannabis. Plants 2022, 11, 140. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, E. Marijuana Harvest: How to Maximize Quality and Yield in Your Cannabis Garden; Rosenthal, E., Ed.; Quick American Archives: San Francisco, CA, USA, 2017. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. 2022. Available online: https://www.R-project.org/ (accessed on 29 June 2021).
- Miguez, F. Nlraa: Nonlinear Regression for Agricultural Applications. R Package Version 1.2. 2022. Available online: https://CRAN.R-project.org/package=nlraa (accessed on 14 July 2022).
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; Team, R.C. Nlme: Linear and Nonlinear Mixed Effects Models. R Package Version 3.1–120. 2015. Available online: https://CRAN.R-project.org/package=nlme (accessed on 14 July 2022).
- Moreno-Sanz, G. Can You Pass the Acid Test? Critical Review and Novel Therapeutic Perspectives of Δ9-Tetrahydrocannabinolic Acid A. Cannabis Cannabinoid Res. 2016, 1, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Berthold, E.C.; McCurdy, C.R.; Benevenute, S.D.S.; Brym, Z.T.; Freeman, J.H. Development of Cannabinoids in Flowers of Industrial Hemp (Cannabis sativa L.): A Pilot Study. J. Agric. Food Chem. 2020, 68, 6058–6064. [Google Scholar] [CrossRef] [PubMed]
- Campbell, B.J.; Berrada, A.F.; Hudalla, C.; Amaducci, S.; McKay, J.K. Genotype × Environment Interactions of Industrial Hemp Cultivars Highlight Diverse Responses to Environmental Factors. Agrosystems, Geosci. Environ. 2019, 2, 1–11. [Google Scholar] [CrossRef]
- Toth, J.A.; Smart, L.B.; Smart, C.D.; Stack, G.M.; Carlson, C.H.; Philippe, G.; Rose, J.K.C. Limited effect of environmental stress on cannabinoid profiles in high-cannabidiol hemp (Cannabis sativa L.). GCB Bioenergy 2021, 13, 1666–1674. [Google Scholar] [CrossRef]
- Yangsud, J.; Santasanasuwan, S.; Ahkkarachinoreh, P.; Maha, A.; Madaka, F.; Suksaeree, J.; Songsak, T.; Vutthipong, A.; Monton, C. Stability of cannabidiol, ∆9-tetrahydrocannabinol, and cannabinol under stress conditions. Adv. Tradit. Med. 2021, 21, 475–484. [Google Scholar] [CrossRef]
- Trofin, I.G.; Dabija, G.; Vaireanu, D.; Filipescu, L. Long-term storage and cannabis oil stability. Rev. Chim. 2012, 63, 293–297. [Google Scholar]
- Toth, J.A.; Stack, G.M.; Cala, A.R.; Carlson, C.H.; Wilk, R.L.; Crawford, J.L.; Viands, D.R.; Philippe, G.; Smart, C.D.; Rose, J.K.C.; et al. Development and validation of genetic markers for sex and cannabinoid chemotype in Cannabis sativa L. GCB Bioenergy 2020, 12, 213–222. [Google Scholar] [CrossRef]
| Model | Goodness-of-Fit Criteria z | |
|---|---|---|
| AICc | BIC | |
| Logistic | 6885.408 | 6919.173 |
| Linear-plateau | 6877.525 | 6911.289 |
| Quadratic-plateau | 6880.481 | 6922.607 |
| Asymptotic | 6917.306 | 6946.878 |
| Parameter | |||
|---|---|---|---|
| Cultivar | A (g plant−1) | B (g day−1) | XS (DAFI a) |
| BaOx | 271.2 | 7.4 | 77.3 |
| Cherry Wine | 410.5 | 4.1 | 64.1 |
| p-value | 0.5923 | <0.0001 | 0.1510 |
| Response z | Model | Goodness-of-Fit Criteria y | |
|---|---|---|---|
| AICc | BIC | ||
| Total THC % | Logistic | −1403.124 | −1365.121 |
| Four-Parameter Logistic | −1381.136 | −1351.522 | |
| Gompertz | −1372.822 | −1343.208 | |
| Beta growth function | −1432.406 | −1394.403 | |
| Four-parameter Beta growth function | −1422.664 | −1393.051 | |
| Total CBD % | Logistic | 2057.301 | 2095.305 |
| Four-Parameter Logistic | 2136.931 | 2166.54 | |
| Gompertz | NC x | NC | |
| Beta growth function | 2161.043 | 2199.047 | |
| Four-parameter Beta growth function | 2186.523 | 2216.137 | |
| Parameter | |||
|---|---|---|---|
| Cultivar | W.max (%) | TE (DAFI a) | TM (DAFI) |
| BaOx | 0.45 | 75.20 | 44.67 |
| Cherry Wine | 0.36 | 63.19 | 16.54 |
| p-value | 0.0187 | <0.0001 | <0.0001 |
| Parameter | |||
|---|---|---|---|
| Cultivar | W.max (%) | xmid (DAFI a) | Scale (% DAFI−1) |
| BaOx | 15.20 | 45.5 | 13.0 |
| Cherry Wine | 11.88 | 26.9 | 11.8 |
| p-value | 0.9958 | <0.0001 | 0.3925 |
| Cultivar | Intercept | Slope | Intercept | Slope |
|---|---|---|---|---|
| ----Total Potential THC a---- | ------Total THC b----- | |||
| %CBD | %CBD %THCP−1 | %CBD | %CBD %THC−1 | |
| BaOx | −0.196 | 16.2 | −0.253 | 30.6 |
| Cherry Wine | −0.987 | 18.2 | −0.728 | 31.5 |
| p-value | 0.0669 | <0.0001 | 0.0340 | 0.0975 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Linder, E.R.; Young, S.; Li, X.; Henriquez Inoa, S.; Suchoff, D.H. The Effect of Harvest Date on Temporal Cannabinoid and Biomass Production in the Floral Hemp (Cannabis sativa L.) Cultivars BaOx and Cherry Wine. Horticulturae 2022, 8, 959. https://doi.org/10.3390/horticulturae8100959
Linder ER, Young S, Li X, Henriquez Inoa S, Suchoff DH. The Effect of Harvest Date on Temporal Cannabinoid and Biomass Production in the Floral Hemp (Cannabis sativa L.) Cultivars BaOx and Cherry Wine. Horticulturae. 2022; 8(10):959. https://doi.org/10.3390/horticulturae8100959
Chicago/Turabian StyleLinder, Eric R., Sierra Young, Xu Li, Shannon Henriquez Inoa, and David H Suchoff. 2022. "The Effect of Harvest Date on Temporal Cannabinoid and Biomass Production in the Floral Hemp (Cannabis sativa L.) Cultivars BaOx and Cherry Wine" Horticulturae 8, no. 10: 959. https://doi.org/10.3390/horticulturae8100959
APA StyleLinder, E. R., Young, S., Li, X., Henriquez Inoa, S., & Suchoff, D. H. (2022). The Effect of Harvest Date on Temporal Cannabinoid and Biomass Production in the Floral Hemp (Cannabis sativa L.) Cultivars BaOx and Cherry Wine. Horticulturae, 8(10), 959. https://doi.org/10.3390/horticulturae8100959






