Chemical Composition and Polyphenol Compounds of Vaccinium floribundum Kunth (Ericaceae) from the Volcano Chimborazo Paramo (Ecuador)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Sample Preparation
2.3. Extraction Procedure for Total Polyphenols Content (TPC) and Antioxidant Activity (AA)
2.3.1. Quantification of Total Polyphenols Content (TPC)
2.3.2. Determination of Antioxidant Activity by Two Different Methods
ABTS Method
DPPH Method
2.4. Determination of Sugars and Organic Acids Profile
2.5. Minerals Analysis
2.6. Identification and Quantification of Phenolic Compounds by HPLC-DAD-ESI-MSn
2.6.1. Extraction and Determination of Phenolic Compounds Non-Anthocyanin
2.6.2. Identification and Quantification of Anthocyanins
2.7. Statistical Analyses
3. Results and Discussion
3.1. Antioxidant Activity (AA) and Total Polyphenol Content (TPC)
3.2. Sugars and Organic Acids Profile
3.3. Mineral Content
3.4. Identification and Quantification of Phenolic Compounds Non-Anthocyanin and Anthocyanins
3.5. Principal Component Analysis (PCA)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Llivisaca, S.; Manzano, P.; Ruales, J.; Flores, J.; Mendoza, J.; Peralta, E.; Cevallos-Cevallos, J.M. Chemical, Antimicrobial, and Molecular Characterization of Mortiño (Vaccinium floribundum Kunth) Fruits and Leaves. Food Sci. Nutr. 2018, 6, 934–942. [Google Scholar] [CrossRef]
- Chang, S.; Alasalvar, C.; Shahidi, F. Superfruits: Phytochemicals, Antioxidant Efficacies, and Health Effects—A Comprehensive Review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1580–1604. [Google Scholar] [CrossRef]
- Vasco, C.; Riihinen, K.; Ruales, J.; Kamal-Eldin, A. Chemical Composition and Phenolic Compound Profile of Mortiño (Vaccinium floribundum Kunth). J. Agric. Food Chem. 2009, 57, 8274–8281. [Google Scholar] [CrossRef]
- Garzón, G.A.; Narváez, C.E.; Riedl, K.M.; Schwartz, S.J. Chemical composition, anthocyanins, non-anthocyanin phenolics and antioxidant activity of wild bilberry (Vaccinium meridionale Swartz) from Colombia. Food Chem. 2010, 122, 980–986. [Google Scholar] [CrossRef]
- Muñoz, V.; Caviedes, M. Determinación de Métodos Para Producción de Mortiño (Vaccinium floribundum Kunth), Con Fines de Propagación y Producción Comercial; Universidad San Francisco de Quito—USFQ: Quito, Ecuador, 2011. [Google Scholar]
- Torres, M.; Trujillo, D.; Arahana, V. Cultivo In Vitro del Mortiño (Vaccinium floribundum Kunth). ACI AV. Ciencias Ing. 2010, 2, B9–B15. [Google Scholar] [CrossRef]
- Ortiz, J.; Marín-Arroyo, M.-R.; Noriega-Domínguez, M.-J.; Navarro, M.; Arozarena, I. Color, Phenolics, and Antioxidant Activity of Blackberry (Rubus Glaucus Benth.), Blueberry (Vaccinium floribundum Kunth.), and Apple Wines from Ecuador. J. Food Sci. 2013, 78, C985–C993. [Google Scholar] [CrossRef]
- Schreckinger, M.; Wang, J.; Yousef, G.; Lila, M.; Gonzalez, E. Antioxidant Capacity and in Vitro Inhibition of Adipogenesis and Inflammation by Phenolic Extracts of Vaccinium floribundum and Aristotelia Chilensis. J. Agric. Food Chem. 2010, 58, 8966–8976. [Google Scholar] [CrossRef]
- Freire, A. Botánica Sistemática Ecuatoriana; Missouri Botanical Garden: St Louis, MO, USA, 2004; pp. 1–209. [Google Scholar]
- Coba, P.; Coronel, D.; Verdugo, K.; Paredes, M.F.; Yugsi, E.; Huachi, L. Estudio etnobotánico del mortiño (Vaccinium floribundum) como alimento ancestral y potencial alimento funcional. Granja Rev. Cienc. 2012, 16, 5–13. [Google Scholar] [CrossRef]
- Prior, R.L.; Cao, G.; Martin, A.; Sofic, E.; McEwen, J.; O’Brien, C.; Lischner, N.; Ehlenfeldt, M.K.; Kalt, W.; Krewer, G.; et al. Antioxidant capacity as influenced by total phenolic and anthocyanin content, maturity, and variety of Vaccinium species. J. Agric. Food Chem. 1998, 46, 2686–2693. [Google Scholar] [CrossRef]
- Cho, M.J.; Howard, L.R.; Prior, R.L.; Clark, J.R. Flavonol glycosides and antioxidant capacity of various blackberry and blueberry genotypes determined by high-performance liquid chromato graphy/mass spectrometry. J. Sci. Food Agric. 2005, 85, 2149–2158. [Google Scholar] [CrossRef]
- Gu, C.; Howell, K.; Dunshea, F.R.; Suleria, H.A.R. LC-ESI-QTOF/MS Characterisation of Phenolic Acids and Flavonoids in Polyphenol-Rich Fruits and Vegetables and Their Potential Antioxidant Activities. Antioxidants 2019, 8, 405. [Google Scholar] [CrossRef]
- Acosta-Montoya, O.; Vaillant, F.; Cozzano, S.; Mertz, C.; Pérez, A.M.; Castro, M.V. Phenolic content and antioxidant capacity of tropical highland blackberry (Rubus adenotrichus Schltdl.) during three edible maturity stages. Food Chem. 2010, 119, 1497–1501. [Google Scholar] [CrossRef]
- Chaovanalikit, A.; Wrolstad, R.E. Total anthocyanins and total phenolics of fresh and processed cherries and their antioxidant properties. J. Food Sci. 2004, 69, FCT67–FCT72. [Google Scholar] [CrossRef]
- Baenas, N.; Ruales, J.; Moreno, D.A.; Barrio, D.A.; Stinco, C.M.; Martínez-Cifuentes, G.; Meléndez-Martínez, A.J.; García-Ruiz, A. Characterization of Andean Blueberry in bioactive compounds, evaluation of biological properties, and in vitro bioaccessibility. Foods 2020, 9, 1483. [Google Scholar] [CrossRef]
- Guijarro-Fuertes, M.; Andrade-Cuvi, M.; Bravo-Vásquez, J.; Ramos-Guerrero, L.; Vernaza, M. Andean blueberry (Vaccinium floribundum) bread: Physicochemical properties and bioaccessibility of antioxidants. Food Sci. Technol. 2019, 39, 56–62. [Google Scholar] [CrossRef]
- Vizuete, K.S.; Kumar, B.; Vaca, A.V.; Debut, A.; Cumbal, L. Mortiño (Vaccinium floribundum Kunth) Berry Assisted Green Synthesis and Photocatalytic Performance of Silver–Graphene Nanocomposite. J. Photochem. Photobiol. A Chem. 2016, 329, 273–279. [Google Scholar] [CrossRef]
- Rojas-Ocampo, E.; Torrejón-Valqui, L.; Muñóz-Astecker, L.D.; Medina-Mendoza, M.; Mori-Mestanza, D.; Castro-Alayo, E.M. Antioxidant capacity, total phenolic content and phenolic compounds of pulp and bagasse of four Peruvian berries. Heliyon 2021, 7, e07787. [Google Scholar] [CrossRef] [PubMed]
- Schreckinger, M.; Lila, M.; Yousef, G.; Gonzalez, E. Inhibition of -Glucosidase and -Amylase by Vaccinium floribundum and Aristotelia chilensis Proanthocyanidins. ACS Symp. Ser. 2012, 1109, 71–82. [Google Scholar] [CrossRef]
- Alarcón-Barrera, K.S.; Armijos-Montesinos, D.S.; García-Tenesaca, M.; Iturralde, G.; Jaramilo-Vivanco, T.; Granda-Albuja, M.G.; Giampieri, F.; Alvarez-Suarez, J.M. Wild Andean Blackberry (Rubus glaucus Benth) and Andean Blueberry (Vaccinium floribundum Kunth) from the Highlands of Ecuador: Nutritional Composition and Protective Effect on Human Dermal Fibroblasts Against Cytotoxic Oxidative Damage. J. Berry Res. 2018, 8, 223–236. [Google Scholar] [CrossRef]
- Caranqui-Aldaz, J.M.; Romero-Saltos, H.; Hernández, F.; Martínez, R. Reproductive phenology of Vaccinium floribundum Kunth (Ericaceae) and codification according to the BBCH scale based on evidence from the volcano Chimborazo paramo (Ecuador). Sci. Hortic. 2022, 303, 111207. [Google Scholar] [CrossRef]
- Wojdyło, A.; Oszmianski, J.; Laskowski, P. The polyphenolic compounds and antioxidant activity of new and old apple varieties. J. Agric. Food Chem. 2008, 56, 520–6530. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Reventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. BiolMed. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Hernández, F.; Noguera-Artiaga, L.; Burló, F.; Wojdyło, A.; Carbonell-Barrachina, Á.A.; Legua, P. Physico-chemical, nutritional, and volatile composition and sensory profile of Spanish jujube (Ziziphus jujuba Mill.) fruits. J. Sci. Food Agric. 2016, 96, 2682–2691. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.T.; Netzel, M.E.; O’Hare, T.J. Optimisation of extraction procedure and development of LC-DAD-MS methodology for anthocyanin analysis in anthocyanin-pigmented corn kernels. Food Chem. 2020, 319, 126515. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, A.; Mishra, S.P.; Pradhan, S.; Choudhary, S.; Singla, S.; Zahra, K.; Aggarwal, L.M. An Assessment of Serum Oxidative Stress and Antioxidant Parameters in Patients Undergoing Treatment for Cervical Cancer. Free Radic. Biol. Med. 2021, 167, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Sellappan, S.; Akoh, C.C.; Krewer, G. Phenolic compounds and antioxidant capacity of Georgia-grown blueberries and blackberries. J. Agric. Food Chem. 2002, 50, 2432–2438. [Google Scholar] [CrossRef]
- Esquivel-Alvarado, D.; Muñoz-Arrieta, R.; Alfaro-Viquez, E.; Madrigal-Carballo, S.; Krueger, C.; Reed, J.D. Composition of Anthocyanins and proanthocyanins in three tropical Vaccinium species from Costa Rica. J. Agric. Food Chem. 2020, 68, 2872–2879. [Google Scholar] [CrossRef] [PubMed]
- Remorini, D.; Tavarini, S.; Degl, E.; Loreti, F.; Massai, R.; Guidi, L. Effect of root stock and harvesting time on the nutritional quality of peel and flesh of nectarine fruits. Food Chem. 2008, 110, 361–367. [Google Scholar] [CrossRef]
- Nuncio-Jáuregui, N.; Munera-Picazo, S.; Calín-Sánchez, A.; Wojdyło, A.; Hernández, F.; Carbonell-Barrachina, A.A. Bioactive compound composition of pomegranate fruits removed during thinning. J. Food Compos. Anal. 2015, 37, 11–19. [Google Scholar] [CrossRef]
- Llerena, W.; Samaniego, I.; Angós, I.; Brito, B.; Ortiz, B.; Carrillo, W. Biocompounds Content Prediction in Ecuadorian Fruits Using a Mathematical Model. Foods 2019, 8, 284. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, H.; Zhong, S.; Liu, X.; Li, T.; Zong, C. Variation in sugar and organic acid content of fruit harvested from different Vaccinium uliginosum populations in the Changbai Mountains of China. J. Am. Soc. Hort. Sci. 2019, 144, 420–428. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Schmitzer, V.; Slatnar, A.; Stampar, F.; Veberic, R. Composition of sugars, organic acids, and total phenolics in 25 wild or cultivated berry species. J. Food Sci. 2012, 77, C1064–C1070. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Schmitzer, V.; Slatnar, A.; Stampar, F.; Veberic, R. A comparison of fruit quality parameters of wild bilberry (Vaccinium myrtillus L.) growing at different locations. J. Sci. Food. Agric. 2015, 95, 776–785. [Google Scholar] [CrossRef]
- Famiani, F.; Battistelli, A.; Moscatello, S.; Cruz-Castillo, J.G.; Walker, R.P. The organic acids that are accumulated in the flesh of fruits: Occurrence, metabolism and factors affecting their contents—A review. Rev. Chapingo Ser. Hortic. 2015, 21, 97–128. [Google Scholar] [CrossRef]
- Etienne, A.; Génard, M.; Lobit, P.; Mbeguié-A-Mbéguié, D.; Bugaud, C. What controls fleshy fruit acidity? A review of malate and citrate accumulation in fruit cells. J. Exp. Bot. 2013, 64, 1451–1469. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, F.A.; Kadioglu, A.; Bertoft, E.; Acar, C.; Turna, I. Effect of fruit maturation on sugar and organic acid composition in two blueberries (Vaccinium arctostaphylos and V. myrtillus) native to Turkey. N. Z. J. Crop Hortic. Sci. 2001, 29, 137–141. [Google Scholar] [CrossRef]
- Kafkas, E.; Kosar, M.; Türemis, N.; Baser, K.H.C. Analysis of sugars, organic acids and vitamin C contents of blackberry genotypes from Turkey. Food Chem. 2006, 97, 732–736. [Google Scholar] [CrossRef]
- Correira, S.; Gonçalves, B.; Aires, A.; Silva, A.; Ferreira, L.; Carvalho, R.; Fernandes, H.; Freitas, C.; Carnide, V.; Silva, A. Effect of harvest year and altitude on nutritional and biometric characteristics of blueberry cultivars. J. Chem. 2016, 2016, 8648609. [Google Scholar] [CrossRef]
- Kalt, W.; McDonald, J.E. Chemical Composition of Lowbush Blueberry Cultivars. J. Am. Soc. Hort. Sci. 1996, 121, 142–146. [Google Scholar] [CrossRef]
- Milivojevic, J.; Maksimović, V.; Nikolić, M.; Bogdanović, J.; Maletić, R.; Milatović, D. Chemical and antioxidant properties of cultivated and wild Fragaria and Rubus berries. J. Food Qual. 2011, 34, 1–9. [Google Scholar] [CrossRef]
- Cobo, M.; Gutiérrez, B.; Torres, A.; Torres, M. Preliminary Analysis of the Genetic Diversity and Population Structure of Mortiño (Vaccinium floribundum Kunth). Biochem. Syst. Ecol. 2016, 64, 14–21. [Google Scholar] [CrossRef]
- Osborne, C.A.; Lulich, J.; Thumchai, R.P.; Ulrich, L.K.; Koehler, L.A.; Bird, K.A.; Bartges, J.W. Feline Urolithiasis: Etiology and Pathophysiology. Vet. Clin. North Am. Small Anim. 1996, 26, 217–232. [Google Scholar] [CrossRef]
- Karlsons, A.; Osvalde, A.; Čekstere, G.; Pormale, J. Research on the mineral composition of cultivated and wild blueberries and cranberries. Agron. Res. 2018, 16, 454–463. [Google Scholar] [CrossRef]
- Miljković, V.M.; Nikolić, G.S.; Zvezdanović, J.; Mihajlov-Krstev, T.; Arsić, B.B.; Miljković, M.N. Phenolic profile, mineral content and antibacterial activity of the methanol extract of Vaccinium myrtillus L. Not. Bot. Horti Agrobot. 2018, 46, 122–127. [Google Scholar] [CrossRef]
- Prencipe, F.P.; Bruni, R.; Guerrini, A.; Rossi, D.; Benvenuti, S.; Pellati, F. Metabolite Profiling of Polyphenols in Vaccinium Berries and Determination of Their Chemopreventive Properties. J. Pharm. Biomed. Anal. 2014, 89, 257–267. [Google Scholar] [CrossRef]
- Wojdyło, A.; Nowicka, P.; Grimalt, M.; Legua, P.; Almansa, M.S.; Amorós, A.; Carbonell-Barrachina, A.A.; Hernández, F. Polyphenol Compounds and Biological Activity of Caper (Capparis spinosa L.) Flowers Buds. Plants 2019, 8, 539. [Google Scholar] [CrossRef]
- Wang, S.Y.; Zheng, W. Effect of plant growth temperature on antioxidant capacity in strawberry. J. Agric. Food Chem. 2001, 49, 4977–4982. [Google Scholar] [CrossRef]
- Jaakola, L.; Hohtola, A. Effect of latitude on flavonoid biosynthesis in plants. Plant Cell Environ. 2010, 33, 1239–1247. [Google Scholar] [CrossRef]
- Bueno, J.M.; Sáez-Plaza, P.; Ramos-Escudero, F.; Jiménez, A.M.; Fett, R.; Asuero, A.G. Analysis and antioxidant capacity of anthocyanin pigments. Part II: Chemical structure, color, and intake of anthocyanins. Crit. Rev. Anal. Chem. 2012, 42, 126–151. [Google Scholar] [CrossRef]
- Sunil, L.; Shetty, N.P. Biosynthesis and regulation of anthocyanin pathway genes. Appl. Microbiol. Biotechnol. 2022, 106, 1783–1798. [Google Scholar] [CrossRef] [PubMed]
- Chalker-Scott, L. Environmental Significance of Anthocyanins in Plant Stress Responses. Photochem. Photobiol. 1999, 70, 1–9. [Google Scholar] [CrossRef]
- Li, X.-J.; Hou, J.-H.; Zhang, G.-L.; Liu, R.-S.; Yang, Y.-G.; Hu, Y.-X.; Lin, J.-X. Comparison of anthocyanin accumulation and morphoanatomical features in apple skin during color formation at two habitats. Sci. Hortic. 2004, 99, 41–53. [Google Scholar] [CrossRef]
- Maier, A.; Hoecker, U. COP1/SPA ubiquitin ligase complexes repress anthocyanin accumulation under low light and high light conditions. Plant Siganl. Behav. 2015, 10, e970440. [Google Scholar] [CrossRef] [PubMed]
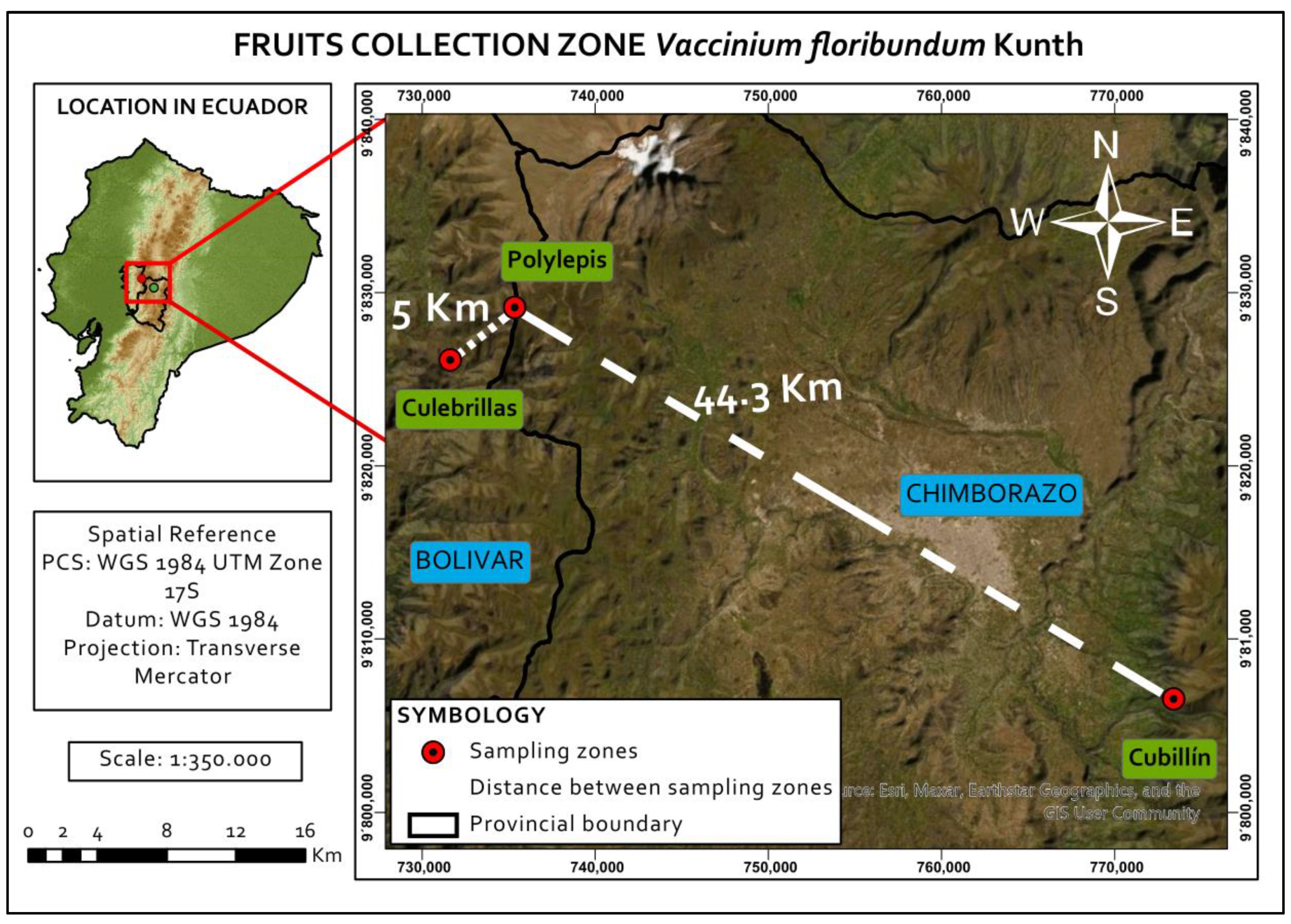

| Locality Name | Province | Coordinates | Altitude (m) | Vegetation Type | Mean Temperature(°C) | Mean Precipitation(mm) |
|---|---|---|---|---|---|---|
| Culebrillas | Bolívar | 01°34.20′ S 78.55.5′ W | 4000 | Herbaceous paramo | 3.1 | 967 |
| Polylepis | Chimborazo | 01°32.41′ S 78°53.5′ W | 4076 | Herbaceous paramo | 3.1 | 967 |
| Cubillín | Chimborazo | 01°45′ S 78°31′ W | 3500 | High mountain forest | 7.0 | 1000 |
| Factor | ABTS | DPPH | TPC |
|---|---|---|---|
| (mmol Trolox kg−1 DM) | (mg GAE 100 g−1 DM) | ||
| ANOVA Test Ϯ | |||
| Zone | *** | *** | *** |
| Stage | *** | *** | *** |
| sampling zone × stage | *** | *** | *** |
| Tukey’s multiple range test ‡ | |||
| Zone | |||
| Polylepis | 47.76 b | 77.22 a | 2231.45 b |
| Cubillín | 41.12 c | 44.85 c | 1649.69 c |
| Culebrillas | 65.74 a | 69.22 b | 2651.57 a |
| Stage ¥ | |||
| Stage 7 | 63.34 a | 72.16 a | 2559.12 a |
| Stage 8 | 39.74 b | 55.37 b | 1796.02 b |
| Study zone × Stage | |||
| Polylepis * Stage 7 | 79.53 a | 86.64 a | 3089.38 a |
| Polylepis * Stage 8 | 16.00 e | 67.80 b | 1373.52 e |
| Cubillín * Stage 7 | 41.43 d | 50.97 c | 1717.46 d |
| Cubillín * Stage 8 | 40.81 d | 38.72 d | 1581.91 d |
| Culebrillas* Stage 7 | 69.07 b | 78.86 a | 2870.53 b |
| Culebrillas* Stage 8 | 62.42 c | 59.57 bc | 2432.62 c |
| Factor | Organic Acids | Sugars | ||||||
|---|---|---|---|---|---|---|---|---|
| Citric | Malic | Quinic | Sucrose | Glucose | Fructose | Mannose | Sorbitol | |
| ANOVA Test Ϯ | ||||||||
| Zone | *** | *** | *** | *** | *** | *** | *** | *** |
| Stage | *** | *** | *** | *** | NS | *** | *** | *** |
| Zonex Stage | *** | *** | *** | *** | *** | *** | *** | *** |
| Tukey’s multiple range test ‡ | ||||||||
| Zone | ||||||||
| Polylepis | 41.98 b | 22.30 c | 108.39 c | 9.96 b | 110.05 a | 60.80 b | 31.80 b | 106.46 c |
| Cubillín | 65.05 a | 28.35 b | 199.48 a | 61.42 a | 71.75 b | 50.61 b | 35.82 b | 190.55 a |
| Culebrillas | 58.06 a | 36.71 a | 155.99 b | 13.05 b | 106.94 a | 79.19 a | 134.04 a | 157.76 b |
| Stage ¥ | ||||||||
| Stage 7 | 63.10 a | 26.22 b | 177.32 a | 41.56 a | 91.91 | 39.08 b | 79.30 a | 178.97 a |
| Stage 8 | 46.96 b | 32.02 a | 131.92 b | 14.73 b | 100.58 | 87.99 a | 55.14 b | 124.20 b |
| Zone × Stage | ||||||||
| Polylepis * Stage 7 | 50.76 b | 10.21 e | 153.20 b | 12.90 bc | 116.43 a | 64.35 b | 34.14 cd | 145.12 b |
| Polylepis * Stage 8 | 33.20 c | 34.96 b | 63.57 c | 7.02 c | 103.66 ab | 57.25 b | 29.45 cd | 67.80 c |
| Cubillín * Stage 7 | 75.98 a | 25.23 cd | 216.84 a | 104.74 a | 60.04 c | 24.19 c | 21.00 d | 217.02 a |
| Cubillín * Stage 8 | 54.11 b | 31.47 bc | 182.12 ab | 18.10 b | 83.46 bc | 77.03 b | 50.64 c | 164.07 b |
| Culebrillas * Stage 7 | 62.56 ab | 19.04 de | 161.91 b | 7.04 c | 99.27 ab | 28.69 c | 182.76 a | 174.77 ab |
| Culebrillas * Stage 8 | 53.56 b | 54.37 a | 150.07 b | 19.07 b | 114.61 a | 129.69 a | 85.32 b | 140.75 b |
| Factor | K | Na | Ca | Mg | Cu | Mn | Fe | Zn |
|---|---|---|---|---|---|---|---|---|
| Macro-Elements (g kg−1) | Micro-Elements (mg kg−1) | |||||||
| ANOVA Test Ϯ | ||||||||
| Zone | ** | *** | *** | *** | ** | *** | *** | *** |
| Stage | ** | *** | NS | *** | NS | NS | *** | NS |
| Zonex Stage | ** | *** | *** | *** | ** | *** | *** | *** |
| Tukey’s multiple range test ‡ | ||||||||
| Zone | ||||||||
| Polylepis | 8.85 a | 0.30 a | 4.88 a | 1.24 a | 5.63 a | 47.63 b | 80.95 b | 29.61 a |
| Cubillín | 6.83 b | 0.24 b | 1.80 c | 0.41 c | 4.74 b | 93.05 a | 126.89 a | 14.40 c |
| Culebrillas | 8.10 a | 0.22 b | 3.47 b | 0.93 b | 4.84 ab | 22.96 c | 70.90 b | 17.79 b |
| Stage ¥ | ||||||||
| Stage 7 | 8.52 a | 0.28 a | 3.18 | 0.92 a | 5.11 | 56.76 | 70.84 b | 21.60 |
| Stage 8 | 7.33 b | 0.23 b | 3.58 | 0.79 b | 5.03 | 52.34 | 114.99 a | 19.60 |
| Zone × Stage | ||||||||
| Polylepis * Stage 7 | 10.13 a | 0.36 a | 3.92 b | 1.28 a | 5.06 ab | 48.97 b | 69.81 bc | 32.42 a |
| Polylepis * Stage 8 | 7.57 b | 0.25 bc | 5.84 a | 1.20 a | 6.20 a | 46.30 b | 92.10 b | 26.80 a |
| Cubillín * Stage 7 | 7.15 b | 0.27 b | 1.87 c | 0.43 c | 5.02 ab | 93.04 a | 61.29 c | 15.82 bc |
| Cubillín * Stage 8 | 6.50 b | 0.21 bc | 1.72 c | 0.39 c | 4.42 b | 93.07 a | 192.49 a | 12.99 c |
| Culebrillas * Stage 7 | 8.27 ab | 0.19 a | 3.74 b | 1.06 a | 5.22 ab | 28.26 c | 81.41 bc | 16.55 bc |
| Culebrillas * Stage 8 | 7.93 b | 0.24 ab | 3.20 bc | 0.81 b | 4.46 b | 17.67 c | 60.38 c | 19.02 b |
| Peak No. | a Rt (min) | bMS/MS (m/z) | Name of Compounds c | Chemical Family |
|---|---|---|---|---|
| P1 | 7.3 | 353,191 | 3-O-Caffeoylquinic acid | Hydroxycinnamic Acid |
| P2 | 9.1 | 337,163 | 3-Coumaroylquinic acid | Hydroxycinnamic Acid |
| P3 | 9.9 | 707,353,191 | 5-O-Caffeoylquinic acid | Hydroxycinnamic Acid |
| P4 | 12.6 | 335,179 | Caffeoylshikimic acid | Hydroxycinnamic Acid |
| P5 | 15.9 | 433,323 | Caffeic acid derivate | Hydroxycinnamic Acid |
| P6 | 16 | 463,301 | Quercetin 3-hexoside | Flavonols |
| P7 | 17.8 | 463,301 | Quercetin 5-hexoside | Flavonols |
| P8 | 18 | 433,301 | Quercetin 3-pentoside | Flavonols |
| P9 | 18.1 | 447,301 | Quercetin-3-O-rhamnoside | Flavonols |
| Peak No. | a Rt (min) | Molecular Ion [M + H] (m/z) | bMS/MS (m/z) | Name of Compounds c |
|---|---|---|---|---|
| An1 | 2.3 | 627 | 303,465 | Delphinidin 3-O-glucoside |
| An2 | 4.3 | 611 | 287,449 | Cyanidin-3,5-di-O-glucoside |
| An3 | 4.8 | 449 | 287,213,137 | Cyanidin 3-O-glucoside |
| An4 | 4.9 | 419 | 287,137,213 | Cyanidin-3-O-arabinoside |
| An5 | 4.9 | 479 | 317,302,274 | Petunidin-3-O-glucoside |
| An6 | 5.1 | 463 | 301,286,201 | Peonidin-3-O-glucoside |
| An7 | 5.0 | 579 | 271,433 | Pelargonidin-3-O-rutinoside |
| Factor | Hydroxycinnamic Acid | Flavonols | Anthocyanins | ∑Total Polyphenols | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P1 | P2 | P3 | P4 | P5 | P6 | P7 | P8 | P9 | An1 | An2 | An3 | An4 | An5 | An6 | An7 | ||
| ANOVA Test Ϯ | |||||||||||||||||
| Zone | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| Stage | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| Zone xStage | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| Tukey’s multiple range test ‡ | |||||||||||||||||
| Zone | |||||||||||||||||
| Polylepis | 1.56 c | 1.56 c | 291.68 c | 140.89 c | 69.73 c | 61.40 c | 75.54 c | 35.08 b | 24.34 c | 0.07 a | 0.04 a | 6.48 b | 12.30 b | 0.00 b | 0.35 b | 0.03 a | 721.14 c |
| Cubillín | 247.37 a | 95.05 b | 595.05 b | 343.98 a | 221.44 a | 165.64 b | 224.88 b | 149.97 a | 367.79 b | 0.00 b | 0.001 c | 10.71 a | 18.69 a | 0.91 a | 0.67 a | 0.006 c | 2442.21 b |
| Culebrillas | 192.67 b | 142.15 a | 2032.71 a | 196.16 b | 138.23 b | 196.03 a | 281.66 a | 1.29 c | 509.90 a | 0.00 b | 0.02 b | 6.08 b | 11.47 b | 0.01 b | 0.12 c | 0.01 b | 3708.58 a |
| Stage ¥ | |||||||||||||||||
| Stage 7 | 179.22 a | 93.33 a | 577.24 b | 271.86 a | 183.31 a | 174.01 a | 228.07 a | 122.94 a | 339.93 a | 0.00 b | 0.001 b | 4.46 b | 9.74 b | 0.06 b | 0.42 a | 0.008 b | 2184.64 a |
| Stage 8 | 115.18 b | 65.85 b | 1369.06 a | 182.17 b | 102.96 b | 108.03 b | 159.98 b | 1.29 b | 261.43 b | 0.05 a | 0.04 a | 11.06 a | 18.57 a | 0.54 a | 0.34 b | 0.02 a | 2396.65 a |
| Zone × Stage | |||||||||||||||||
| Polylepis * Stage 7 | 1.56 d | 1.56 d | 581.80 b | 280.22 bc | 137.89 cd | 105.71 c | 124.17 c | 68.88 b | 47.40 c | 0.00 b | 0.00 c | 5.55 c | 10.44 c | 0.00 c | 0.70 b | 0.02 b | 1365.96 c |
| Polylepis * Stage 8 | 1.56 d | 1.56 d | 1.56 c | 1.56 e | 1.56 e | 17.08 d | 26.91 d | 1.29 c | 1.29 c | 0.15 a | 0.09 a | 7.42 c | 14.17 bc | 0.00 c | 0.00 e | 0.04 a | 76.32 d |
| Cubillín * Stage 7 | 302,36 a | 110.06 b | 667.27 b | 363.20 a | 235.87 a | 172.4 b | 226.14 b | 298.64 a | 370,36 b | 0,00 b | 0.00 c | 6.77 c | 12.97 bc | 0.19 b | 0.47 c | 0.00 d | 2766.40 b |
| Cubillín * Stage 8 | 192,39 bc | 80.05 c | 522.84 b | 324.75 ab | 207.01 ab | 159.23 b | 223.62 b | 1.29 c | 365.23 b | 0.00 b | 0.002 c | 14.65 a | 24.41 a | 1.63 a | 0.87 a | 0.01 c | 2118.03 b |
| Culebrillas * Stage 7 | 233,75 b | 168.36 a | 482.64 b | 172.14 d | 176.16 bc | 244.28 a | 333.90 a | 1.29 c | 602.03 a | 0.00 b | 0.004 c | 1.06 d | 5.81 d | 0.00 c | 0.08 de | 0.00 d | 2421.57 b |
| Culebrillas * Stage 8 | 151,59 c | 115.95 b | 3582.78 a | 220.19 cd | 100.31 d | 147.78 bc | 229.42 b | 1.29 c | 417.78 b | 0.00 b | 0.05 b | 11.10 b | 17.12 b | 0.01 c | 0.15 d | 0.02 b | 4995.60 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caranqui-Aldaz, J.M.; Muelas-Domingo, R.; Hernández, F.; Martínez, R. Chemical Composition and Polyphenol Compounds of Vaccinium floribundum Kunth (Ericaceae) from the Volcano Chimborazo Paramo (Ecuador). Horticulturae 2022, 8, 956. https://doi.org/10.3390/horticulturae8100956
Caranqui-Aldaz JM, Muelas-Domingo R, Hernández F, Martínez R. Chemical Composition and Polyphenol Compounds of Vaccinium floribundum Kunth (Ericaceae) from the Volcano Chimborazo Paramo (Ecuador). Horticulturae. 2022; 8(10):956. https://doi.org/10.3390/horticulturae8100956
Chicago/Turabian StyleCaranqui-Aldaz, Jorge M., Raquel Muelas-Domingo, Francisca Hernández, and Rafael Martínez. 2022. "Chemical Composition and Polyphenol Compounds of Vaccinium floribundum Kunth (Ericaceae) from the Volcano Chimborazo Paramo (Ecuador)" Horticulturae 8, no. 10: 956. https://doi.org/10.3390/horticulturae8100956
APA StyleCaranqui-Aldaz, J. M., Muelas-Domingo, R., Hernández, F., & Martínez, R. (2022). Chemical Composition and Polyphenol Compounds of Vaccinium floribundum Kunth (Ericaceae) from the Volcano Chimborazo Paramo (Ecuador). Horticulturae, 8(10), 956. https://doi.org/10.3390/horticulturae8100956







