Resistance of Tunisian Melon Landraces to Podosphaera xanthii
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Fungal Isolates
2.2. Phenotypic Evaluation by Artificial Inoculations
2.3. Phenotypic Evaluation under Natural Infection
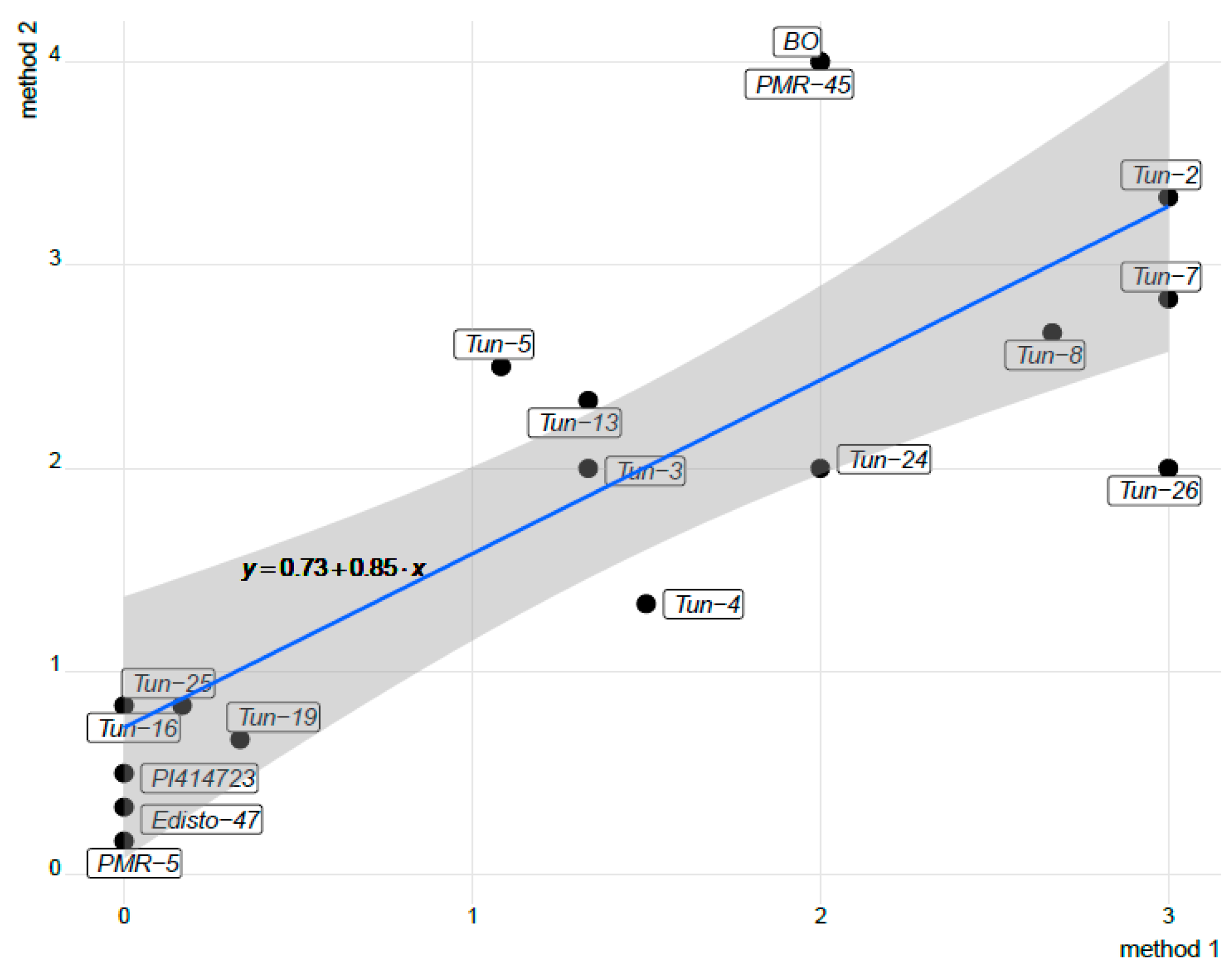
2.4. Statistical Analyses
3. Results
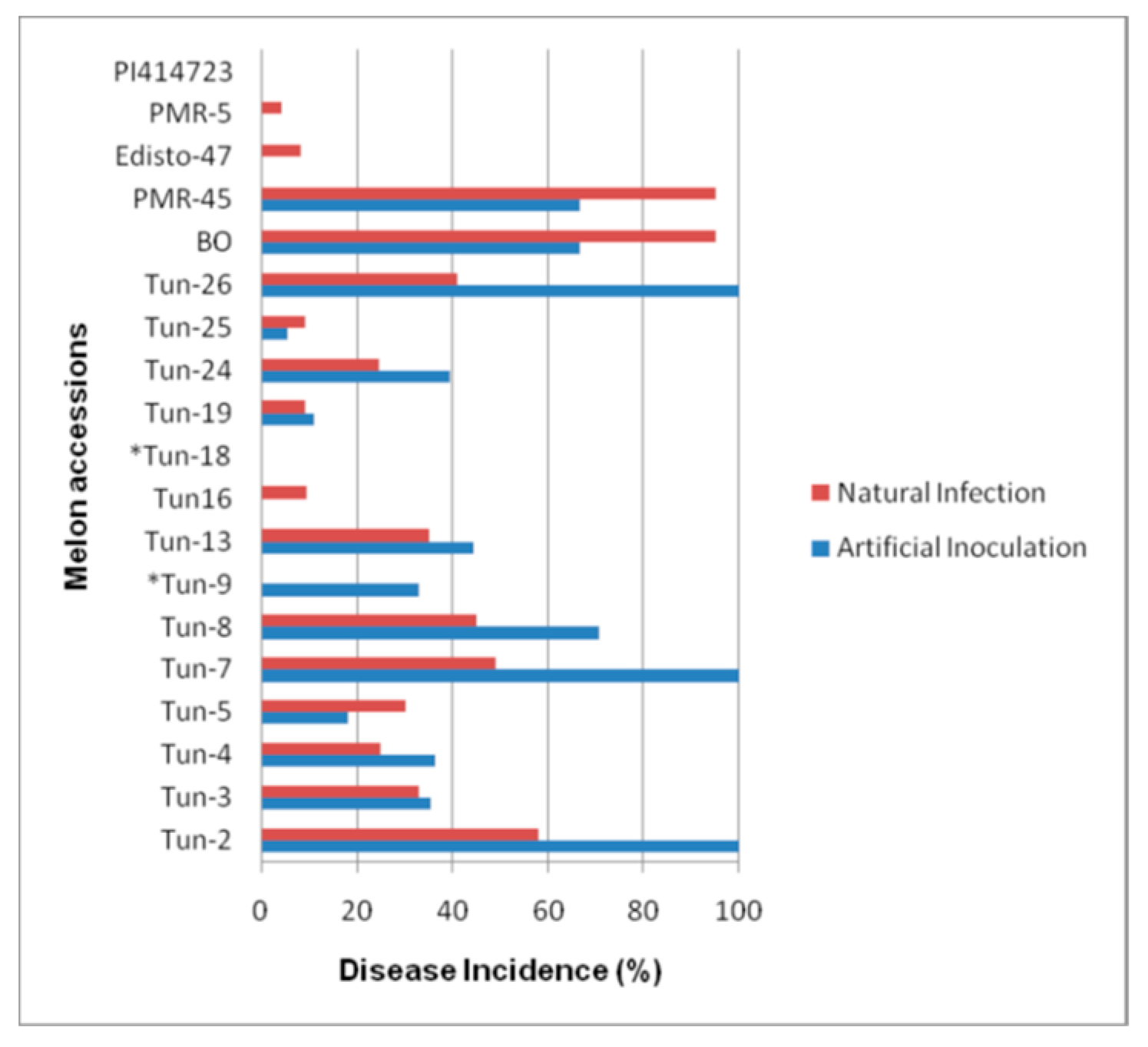
3.1. Phenotypic Evaluation by Artificial Inoculations
3.2. Phenotypic Evaluation under Natural Infection and Identification of The Prevalent Race
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mccreight, J. Melon-Powdery Mildew Interactions Reveal Variation in Melon Cultigens and Podosphaera xanthii Races 1 and 2. J. Am. Soc. Hort. Sci. 2006, 131, 59–65. [Google Scholar] [CrossRef]
- Křístková, E.; Lebeda, A.; Sedláková, B. Species Spectra, Distribution and Host Range of Cucurbit Powdery Mildews in the Czech Republic, and in Some Other European and Middle Eastern Countries. Phytoparasitica 2009, 37, 337–350. [Google Scholar] [CrossRef]
- Lebeda, A.; Křístková, E.; Sedláková, B.; McCreight, J.D.; Coffey, M.D. Cucurbit Powdery Mildews: Methodology for Objective Determination and Denomination of Races. Eur. J. Plant Pathol. 2016, 144, 399–410. [Google Scholar] [CrossRef]
- Hollomon, D.W.; Wheeler, I.E. Controlling Powdery Mildews with Chemistry. In The Powdery Mildews: A Comprehensive Treatise; American Phytopathological Society (APS Press): St. Paul, MN, USA, 2002; pp. 249–255. [Google Scholar]
- Pérez-García, A.; Romero, D.; Fernández-Ortuño, D.; López-Ruiz, F.; De Vicente, A.; Torés, J.A. The Powdery Mildew Fungus Podosphaera fusca (Synonym Podosphaera xanthii), a Constant Threat to Cucurbits. Mol. Plant Pathol. 2009, 10, 153–160. [Google Scholar] [CrossRef]
- McCreight, J.D. Genes for Resistance to Powdery Mildew Races 1 and 2U.S. in Melon PI 313970. HortScience 2003, 38, 591–594. [Google Scholar] [CrossRef]
- Hosoya, K.; Kuzuya, M.; Murakami, T.; Kato, K.; Narisawa, K.; Ezura, H. Impact of Resistant Melon Cultivars on Sphaerotheca fuliginea. Plant Breed. 2000, 119, 286–288. [Google Scholar] [CrossRef]
- Bardin, M.; Nicot, P.C.; Normand, P.; Lemaire, J.M. Virulence Variation and DNA Polymorphism in Sphaerotheca fuliginea, Causal Agent of Powdery Mildew of Cucurbits. Eur. J. Plant Pathol. 1997, 103, 545–554. [Google Scholar] [CrossRef]
- Del Pino, D.; Olalla, L.; Pérez-García, A.; Rivera, M.; García, S.; Moreno, R.; Vicente, A.; Tores, J. Occurrence of Races and Pathotypes of Cucurbit Powdery Mildew in Southeastern Spain. Phytoparasitica 2002, 30, 459–466. [Google Scholar] [CrossRef]
- Yuste-Lisbona, F.J.; López-Sesé, A.I.; Gómez-Guillamón, M.L. Inheritance of Resistance to Races 1, 2 and 5 of Powdery Mildew in the Melon TGR-1551. Plant Breed. 2010, 129, 72–75. [Google Scholar] [CrossRef]
- Perchepied, L.; Bardin, M.; Dogimont, C.; Pitrat, M. Relationship between Loci Conferring Downy Mildew and Powdery Mildew Resistance in Melon Assessed by Quantitative Trait Loci Mapping. Phytopathology 2005, 95, 556–565. [Google Scholar] [CrossRef]
- Grimault, V.; Houdault, S.; Himmel, P. Development of Differential Hosts to Identify Commercially Relevant Races of Melon Podosphaera xanthii against Which Vegetable Seed Companies Make Claims of Resistance. Cucurbit Genet. Coop. Rpt. 2020, 43, 1–3. [Google Scholar]
- Pitrat, M. Melon Genetic Resources: Phenotypic Diversity and Horticultural Taxonomy. In Genetics and Genomics of Cucurbitaceae; Grumet, R., Katzir, N., Garcia-Mas, J., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 25–60. [Google Scholar] [CrossRef]
- Yuste-Lisbona, F.J.; Capel, C.; Sarria, E.; Torreblanca, R.; Gómez-Guillamón, M.L.; Capel, J.; Lozano, R.; López-Sesé, A. Genetic Linkage Map of Melon (Cucumis melo L.) and Localization of a Major QTL for Powdery Mildew Resistance. Mol. Breed. 2011, 27, 181–192. [Google Scholar] [CrossRef]
- Howlader, J.; Hong, Y.; Natarajan, S.; Sumi, K.R.; Kim, H.-T.; Park, J.-I.; Nou, I.-S. Development of Powdery Mildew Race 5-Specific SNP Markers in Cucumis melo L. Using Whole-Genome Resequencing. Hortic. Environ. Biotechnol. 2020, 61, 347–357. [Google Scholar] [CrossRef]
- Nunes, E.W.; Esteras, C.; Ricarte, A.O.; Martínez-Perez, E.; Gómez-Guillamón, M.L.; Nunes, G.H.; Picó, M.B. Brazilian Melon Landraces Resistant to Podosphaera xanthii Are Unique Germplasm Resources. Ann. Appl. Biol. 2017, 171, 214–228. [Google Scholar] [CrossRef]
- Chikh-Rouhou, H.; Gómez-Guillamón, M.L.; González, V.; Sta-Baba, R.; Garcés-Claver, A. Cucumis melo L. Germplasm in Tunisia: Unexploited Sources of Resistance to Fusarium Wilt. Horticulturae 2021, 7, 208. [Google Scholar] [CrossRef]
- Chikh-Rouhou, H.; Mezghani, N.; Mnasri, S.; Mezghani, N.; Garcés-Claver, A. Assessing the Genetic Diversity and Population Structure of a Tunisian Melon (Cucumis melo L.) Collection Using Phenotypic Traits and SSR Molecular Markers. Agronomy 2021, 11, 1121. [Google Scholar] [CrossRef]
- Chikh-Rouhou, H.; Tlili, I.; Ilahy, R.; R’Him, T.; Sta-Baba, R. Fruit Quality Assessment and Characterization of Melon Genotypes. Int. J. Veg. Sci. 2021, 27, 3–19. [Google Scholar] [CrossRef]
- Chikh-Rouhou, H.; Ben Belgacem, A.M.; Sta-Baba, R.; Tarchoun, N.; Gómez-Guillamón, M.L. New Source of Resistance to Aphis Gossypii in Tunisian Melon Accessions Using Phenotypic and Molecular Marker Approaches. Phytoparasitica 2019, 47, 405–413. [Google Scholar] [CrossRef]
- Ballantyne, B. Powdery Mildew on Cucurbitaceae: Identity, Distribution, Host Range and Sources of Resistance. Proc. Linn. Soc. New South Wales 1975, 99, 100–120. [Google Scholar]
- Lebeda, A.; Sedláková, B. Screening for Resistance to Cucurbit Powdery Mildew (Golovinomyces cichoracearum, Podosphaera xanthii). In Mass Screening Techniques for Selecting Crops Resistant to Diseases; IAEA: Vienna, Austria, 2010; Volume 19, pp. 295–307. ISBN 978-92-0-105110-3. Available online: https://www.iaea.org/publications/7758/mass-screening-techniques-for-selecting-crops-resistant-to-disease (accessed on 1 August 2021).
- McGrath, M.T. Heterothallism in Sphaerotheca fuliginea: Mycologia: Volume 86, No 4. Available online: https://www.tandfonline.com/doi/abs/10.1080/00275514.1994.12026445 (accessed on 1 August 2021).
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2013. [Google Scholar]
- Lenth, R.V. R Package Emmeans: Estimated Marginal Means. R. 3 August 2022. Available online: https://github.com/rvlenth/emmeans (accessed on 1 August 2022).
- Wickham, H. Ggplot2 Book. Perl. 11 August 2022. Available online: https://github.com/hadley/ggplot2-book (accessed on 1 August 2022).
- Cullis, B.R.; Smith, A.B.; Coombes, N.E. On the Design of Early Generation Variety Trials with Correlated Data. J. Agric. Biol. Environ. Stat. 2006, 11, 381. [Google Scholar] [CrossRef]
- Dhillon, N.; Monforte, A.; Pitrat, M.; Pandey, S.; Singh, P.; Reitsma, K.; Garcia-Mas, J.; Sharma, A.; Mccreight, J. Melon Landraces of India: Contributions and Importance. Plant Breed Rev. 2011, 35, 85–150. [Google Scholar] [CrossRef]
- Chikh-Rouhou, H.; Gómez-Guillamón, M.L.; Garcés-Claver, A. Melon Germplasm from Tunisia with Immense Breeding Value. CGC Rep. 2021, 44, 7–11. [Google Scholar]




| Codes | Accessions | Horticultural Group | Resistance to Fusarium wilt [17] | Resistance to Aphid [20] |
|---|---|---|---|---|
| TUN-2 | Maazoun Menzel Chaker | inodorus | + | − |
| TUN-3 | Maazoun Mehdia (MM2009) | inodorus | + | − |
| TUN-4 | Maazoun Fethi | inodorus | − | − |
| TUN-5 | Fakous (FL) | flexuosus | + | − |
| TUN-7 | Trabelsi | inodorus | − | − |
| TUN-8 | Galaoui | reticulatus | − | − |
| TUN-9 | Dziri (DZ P5 2011) | inodorus | + | − |
| TUN-13 | Arbi1 | inodorus | − | − |
| TUN-16 | Sarachika | inodorus | + | − |
| TUN-18 | Rupa | cantalupensis | + | − |
| TUN-19 | Chamem (Ananas type) | reticulatus | + | + |
| TUN-24 | Maazoun (Kairouan) | inodorus | − | − |
| TUN-25 | Asli | inodorus | − | − |
| TUN-26 | Stambouli | inodorus | + | − |
| Disease Score | Reaction Classification * | |||||
|---|---|---|---|---|---|---|
| Race 2 | Race 5 | Race 3.5 | Race 2 | Race 5 | Race 3.5 | |
| TUN-2 | 3.00 ± 0.00 | 2.85 ± 0.12 | 2.79 ± 0.39 | S | S | S |
| TUN-3 | 1.30 ± 0.30 | 2.65 ± 0.18 | 3.00 ± 0.00 | S | S | S |
| TUN-4 | 1.50 ± 0.25 | 3.00 ± 0.00 | 2.75 ± 0.39 | S | S | S |
| TUN-5 | 1.10 ± 0.20 | 3.00 ± 0.00 | - | S | S | -° |
| TUN-7 | 3.00 ± 0.00 | 2.85 ± 0.14 | 2.95 ± 0.14 | S | S | S |
| TUN-8 | 2.70 ± 0.20 | 3.00 ± 0.00 | 2.83 ± 0.40 | S | S | S |
| TUN-9 | 1.00 ± 0.50 | 3.00 ± 0.00 | - | R | S | - |
| TUN-13 | 1.40 ± 0.40 | 2.60 ± 0.20 | 3.00 ± 0.00 | S | S | S |
| TUN-16 | 0.00 ± 0.00 | 2.50 ± 0.24 | 3.00 ± 0.00 | R | S | S |
| TUN-18 | 0.00 ± 0.00 | 2.65 ± 0.20 | 2.93 ± 0.17 | R | S | S |
| TUN-19 | 0.40 ± 0.25 | 2.00 ± 0.35 | - | R | S | - |
| TUN-24 | 2.00 ± 0.54 | 3.00 ± 0.00 | 3.00 ± 0.00 | S | S | S |
| TUN-25 | 0.20 ± 0.20 | 2.50 ± 0.20 | 2.95 ± 0.15 | R | S | S |
| TUN-26 | 3.00 ± 0.00 | 2.85 ± 0.14 | 2.75 ± 0.39 | S | S | S |
| Differential set | ||||||
| Bola Oro | 2.00 ± 0.10 | 2.85 ± 0.14 | 3.00 ± 0.00 | S | S | S |
| PMR45 | 2.00 ± 0.00 | 2.50 ± 0.20 | 3.00 ± 0.00 | S | S | S |
| Edisto47 | 0.00 ± 0.00 | 2.85 ± 0.20 | 3.00 ± 0.00 | R | S | S |
| PMR5 | 0.00 ± 0.00 | 0.50 ± 0.10 | 2.00 ± 0.20 | R | R | S |
| PI414723 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.37 ± 0.25 | R | R | R |
| Sources | df | Sum of Square | F Value | P (>F) |
|---|---|---|---|---|
| Block:Method | 2 | 0.831 | 0.796 | 0.395 |
| Accession (G) | 15 | 222.89 | 28.673 | <2.2 × 10−16 *** |
| Method (M) | 1 | 11.26 | 21.730 | 3.276 × 10−7 *** |
| Interaction G × M | 15 | 32.76 | 4.214 | 1.175 × 10−5 *** |
| Residuals | 158 | 81.88 |
| Accession | BLUP for Disease Scoring |
|---|---|
| PMR-5 | 0.267 |
| Edisto-47 | 0.340 |
| PI414723 | 0.414 |
| TUN-16 | 0.561 |
| TUN-19 | 0.634 |
| TUN-25 | 0.634 |
| TUN-4 | 1.443 |
| TUN-3 | 1.663 |
| TUN-5 | 1.774 |
| TUN-13 | 1.811 |
| TUN-24 | 1.958 |
| TUN-26 | 2.399 |
| TUN-8 | 2.546 |
| TUN-7 | 2.766 |
| BO | 2.840 |
| PMR-45 | 2.840 |
| TUN-2 | 2.987 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chikh-Rouhou, H.; Garcés-Claver, A.; Kienbaum, L.; Ben Belgacem, A.; Gómez-Guillamón, M.L. Resistance of Tunisian Melon Landraces to Podosphaera xanthii. Horticulturae 2022, 8, 1172. https://doi.org/10.3390/horticulturae8121172
Chikh-Rouhou H, Garcés-Claver A, Kienbaum L, Ben Belgacem A, Gómez-Guillamón ML. Resistance of Tunisian Melon Landraces to Podosphaera xanthii. Horticulturae. 2022; 8(12):1172. https://doi.org/10.3390/horticulturae8121172
Chicago/Turabian StyleChikh-Rouhou, Hela, Ana Garcés-Claver, Lydia Kienbaum, Abdelmonem Ben Belgacem, and Maria Luisa Gómez-Guillamón. 2022. "Resistance of Tunisian Melon Landraces to Podosphaera xanthii" Horticulturae 8, no. 12: 1172. https://doi.org/10.3390/horticulturae8121172
APA StyleChikh-Rouhou, H., Garcés-Claver, A., Kienbaum, L., Ben Belgacem, A., & Gómez-Guillamón, M. L. (2022). Resistance of Tunisian Melon Landraces to Podosphaera xanthii. Horticulturae, 8(12), 1172. https://doi.org/10.3390/horticulturae8121172








