Jania adhaerens Primes Tomato Seed against Soil-Borne Pathogens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Alga and Preparation of the Water-Soluble Polysaccharides
2.2. FT-IR Analyses of the Extract
2.3. Plant Material and Pathogens
2.4. Seed Treatment and Growing Substrate
2.5. Systemic-Induced Resistance Bioassays in Greenhouse Pot Experiments
2.5.1. Rhizoctonia solani (RS) and Pythium ultimum (PU)
2.5.2. Fusarium oxysporum (FO)
2.6. Expression of PR Protein and Polyphenol Pathway Genes and Enzymatic Activities
2.6.1. Expression of PR Protein and Polyphenol Pathway Genes
2.6.2. Chitinase and β-1,3-Glucanase Activities
2.7. Statistical Analysis
3. Results
3.1. FT-IR Analyses
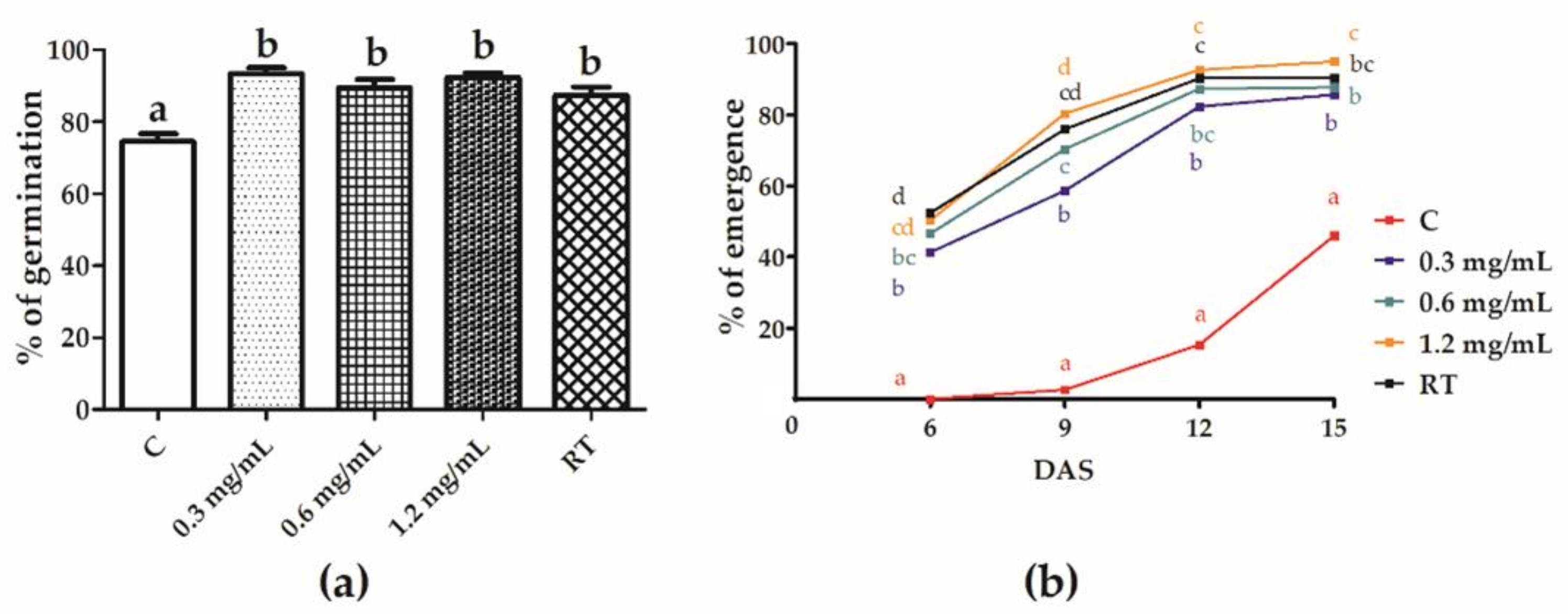
3.2. Effect of Seed Treatment on Seed Germination and Seedling Emergence
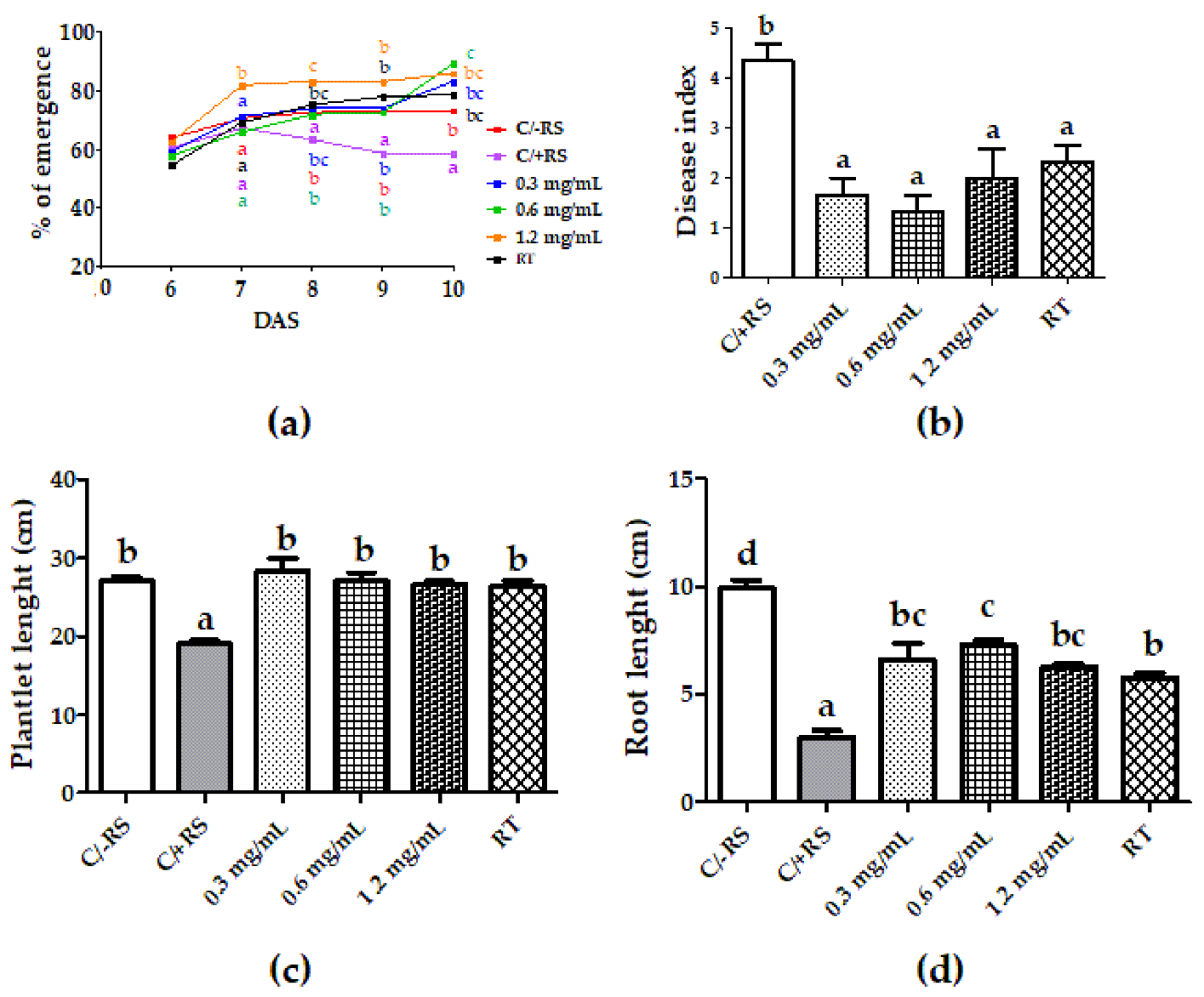
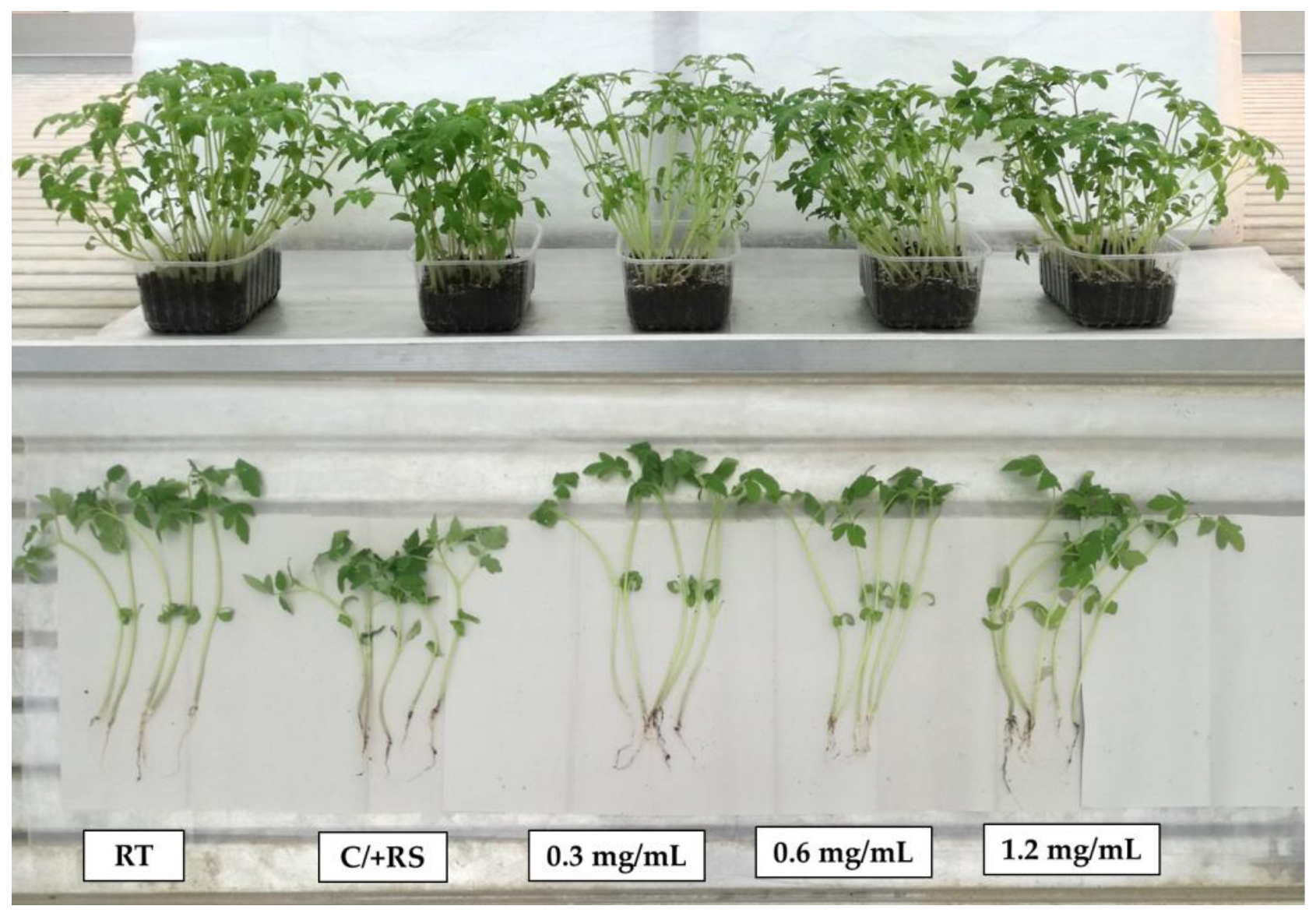
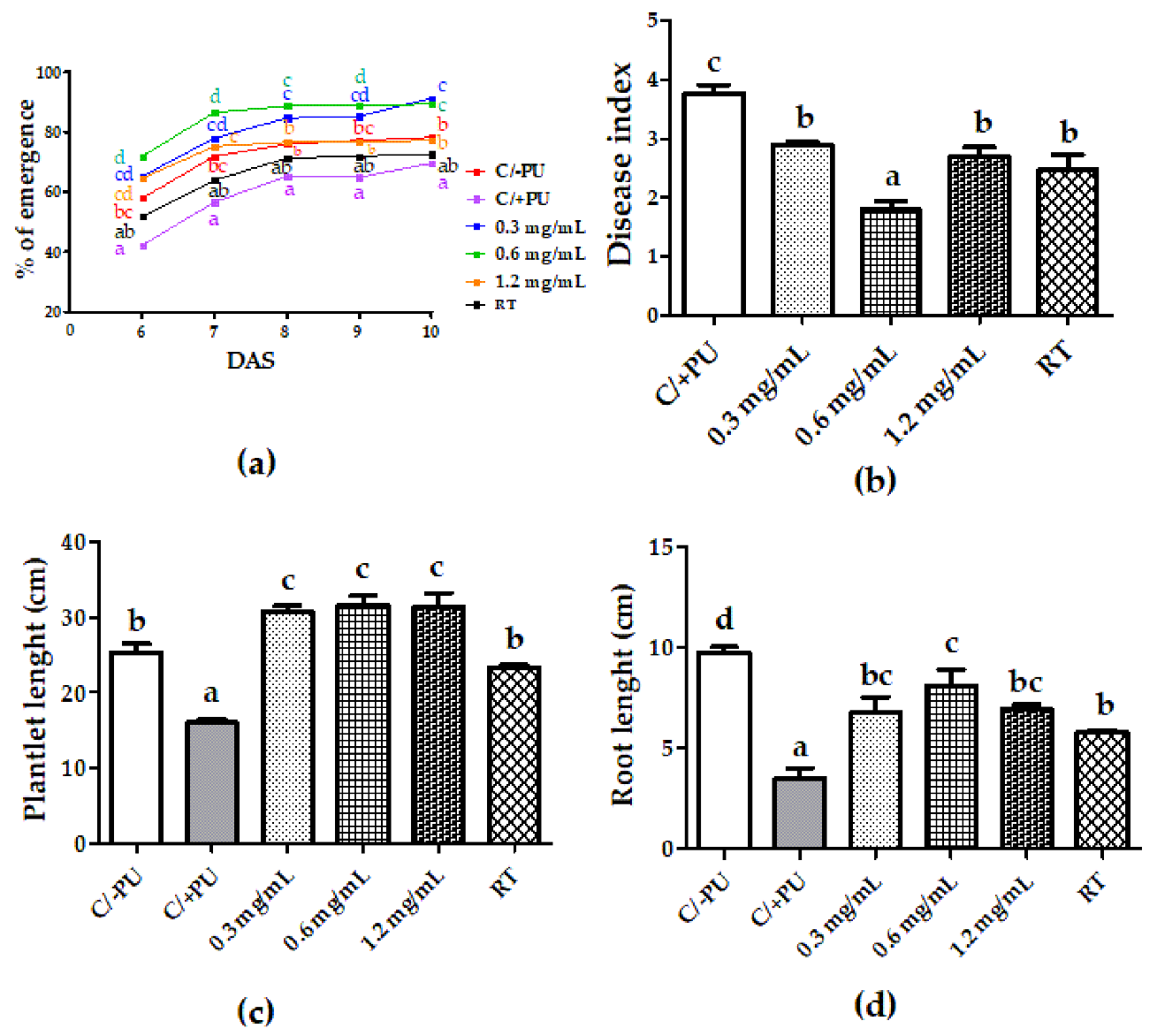
3.3. Systemic-Induced Resistance Bioassays in Greenhouse Pot Experiments
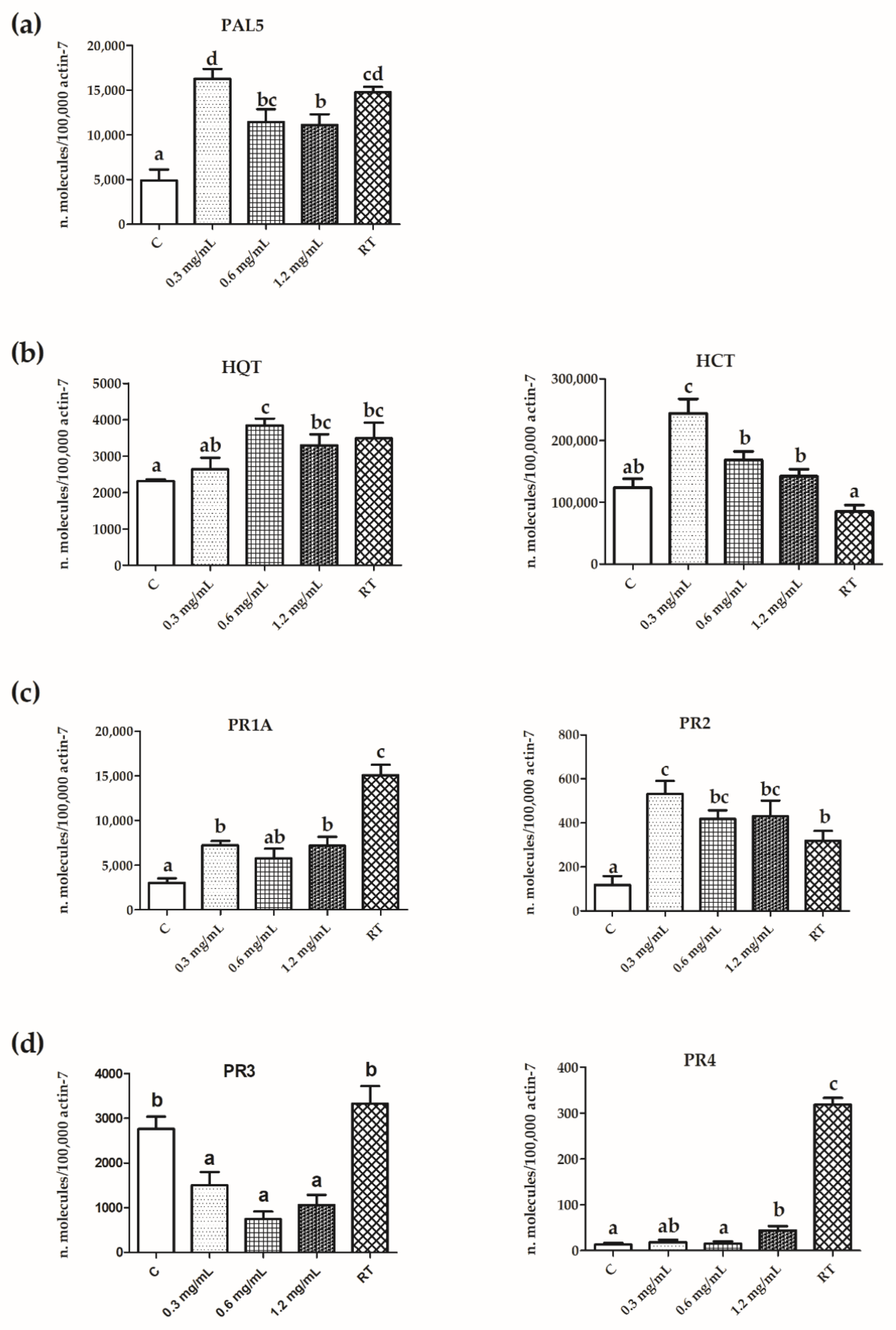
3.4. Expression of PR Protein and Polyphenol Pathway Genes
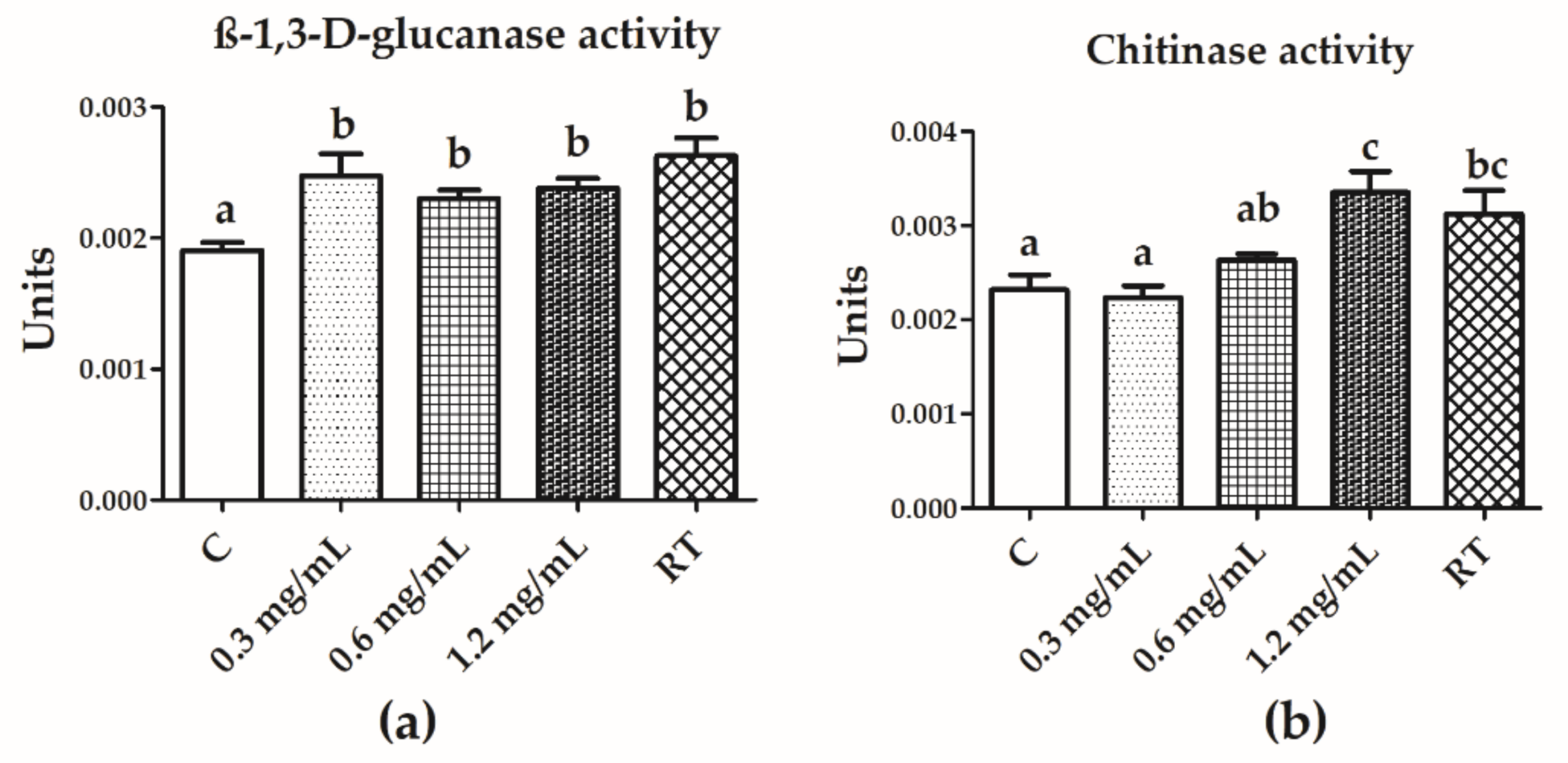
3.5. Chitinase and β-1,3-d-Glucanase Activities
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hentati, F.; Barkallah, M.; Ben Atitallah, A.; Dammak, M.; Louati, I.; Pierre, G.; Fendri, I.; Attia, H.; Michaud, P.; Abdelkafi, S. Quality characteristics and functional and antioxidant capacities of algae-fortified fish burgers prepared from common barbel (Barbus barbus). Biomed. Res. Int. 2019, 2019, 2907542. [Google Scholar] [CrossRef] [PubMed]
- Kousha, M.; Daneshvar, E.; Esmaeli, A.R.; Jokar, M.; Khataee, A.R. Optimization of Acid Blue 25 removal from aqueous solutions by raw, esterified and protonated Jania adhaerens biomass. Int. Biodeter. Biodegr. 2012, 69, 97–105. [Google Scholar] [CrossRef]
- Ramu Ganesan, A.; Kannan, M.; Karthick Rajan, D.; Pillay, A.A.; Shanmugam, M.; Sathishkumar, P.; Johansen, J.; Tiwari, B.K. Phycoerythrin: A pink pigment from red sources (rhodophyta) for a greener biorefining approach to food applications. Crit. Rev. Food Sci. Nutr. 2022, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Hamed, S.M.; Abd El-Rhman, A.A.; Abdel-Raouf, N.; Ibraheem, I.B. Role of marine macroalgae in plant protection & improvement for sustainable agriculture technology. Beni-Suef Univ. J. Basic. Appl. Sci. 2018, 7, 104–110. [Google Scholar]
- Navarro, D.A.; Stortz, C.A. The system of xylogalactans from the red seaweed Jania rubens (Corallinales, Rhodophyta). Carbohyd. Res. 2008, 343, 2613–2622. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, D.; Christiaen, D.; Arad, S.M. Chelating properties of extracellular polysaccharides from Chlorella spp. Appl. Environ. Microbial. 1987, 53, 2953–2956. [Google Scholar] [CrossRef] [PubMed]
- Mercier, L.; Lafitte, C.; Borderies, G.; Briand, X.; Esquerré-Tugayé, M.T.; Fournier, J. The algal polysaccharide carrageenans can act as an elicitor of plant defence. New Phytol. 2001, 149, 43–51. [Google Scholar] [CrossRef]
- Lefevere, H.; Bauters, L.; Gheysen, G. Salicylic acid biosynthesis in plants. Front. Plant Sci. 2020, 11, 338. [Google Scholar] [CrossRef]
- Seyfferth, C.; Tsuda, K. Salicylic acid signal transduction: The initiation of biosynthesis, perception and transcriptional reprogramming. Front. Plant Sci. 2014, 5, 697. [Google Scholar] [CrossRef]
- Bruehl, G.W. Soilborne Plant Pathogens; Macmillan Publishing Co.: New York, NY, USA, 1987; ISBN 0029491304. [Google Scholar]
- Martin, F.N.; Loper, J.E. Soilborne plant diseases caused by Pythium spp.: Ecology, epidemiology, and prospects for biological control. Crit. Rev. Plant Sci. 1999, 18, 111–181. [Google Scholar] [CrossRef]
- Kamoun, S.; Furzer, O.; Jones, J.D.; Judelson, H.S.; Ali, G.S.; Dalio, R.J.; Roy, G.S.; Schena, L.; Zambounis, A.; Panabières, F.; et al. The Top 10 oomycete pathogens in molecular plant pathology. Mol. Plant. Pathol. 2015, 16, 413–434. [Google Scholar] [CrossRef]
- Lübeck, M. Molecular characterization of Rhizoctonia solani. In Applied Mycology and Biotechnology, 1st ed.; Khachatourians, G.G., Arora, D.K., Eds.; Volume 4: Fungal genomics; Elsevier Science B.V.: Amsterdam, The Netherlands, 2004; pp. 205–224. ISBN 0444516425. [Google Scholar]
- Marcou, S.; Wikström, M.; Ragnarsson, S.; Persson, L.; Höfte, M. Occurrence and anastomosis grouping of Rhizoctonia spp. inducing black scurf and greyish-white felt-like mycelium on carrot in Sweden. J. Fungi 2021, 7, 396. [Google Scholar] [CrossRef] [PubMed]
- Gordon, T.R. Fusarium oxysporum and the Fusarium wilt syndrome. Annu. Rev. Phytopathol. 2017, 55, 23–39. [Google Scholar] [CrossRef] [PubMed]
- Fravel, D.; Olivain, C.; Alabouvette, C. Fusarium oxysporum and its biocontrol. New Phytol. 2003, 157, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Michielse, C.B.; Rep, M. Pathogen profile update: Fusarium oxysporum. Mol. Plant. Pathol. 2009, 10, 311. [Google Scholar] [CrossRef] [PubMed]
- Galletti, S.; Cianchetta, S.; Righini, H.; Roberti, R. A Lignin-rich extract of giant reed (Arundo donax L.) as a possible tool to manage soilborne pathogens in horticulture: A preliminary study on a model pathosystem. Horticulturae 2022, 8, 589. [Google Scholar] [CrossRef]
- Abo-Elyousr, K.A.; Ali, E.F.; Sallam, N.M. Alternative control of tomato wilt using the aqueous extract of Calotropis procera. Horticulturae 2022, 8, 197. [Google Scholar] [CrossRef]
- La Torre, A.; Caradonia, F.; Matere, A.; Battaglia, V. Using plant essential oils to control Fusarium wilt in tomato plants. Eur. J. Plant Pathol. 2016, 144, 487–496. [Google Scholar] [CrossRef]
- Sharma, A.; Rajendran, S.; Srivastava, A.; Sharma, S.; Kundu, B. Antifungal activities of selected essential oils against Fusarium oxysporum f. sp. lycopersici 1322, with emphasis on Syzygium aromaticum essential oil. J. Biosci. Bioeng. 2017, 123, 308–313. [Google Scholar]
- Ramírez, P.G.; Ramírez, D.G.; Mejía, E.Z.; Ocampo, S.A.; Díaz, C.N.; Martínez, R.I.R. Extracts of Stevia rebaudiana against Fusarium oxysporum associated with tomato cultivation. Sci. Hortic. 2020, 259, 108683. [Google Scholar] [CrossRef]
- Ozkaya, H.O.; Ergun, T. The effects of Allium tuncelianum extract on some important pathogens and total phenolic compounds in tomato and pepper. Pak. J. Bot. 2017, 49, 2483–2490. [Google Scholar]
- Sanaullah, N.A.R.; Atiq, M.; Rehman, A.; Khan, S.; Bashir, M.; Hameed, A.; Khan, B.; Shakir Baloch, M.; Kachelo, G.A. Antifungal potency of three plant extracts against Rhizoctonia solani damping-off disease in tomato. Int. J. Biosci. 2018, 13, 309–316. [Google Scholar]
- Hlokwe, M.T.P.; Kena, M.A.; Mamphiswana, D.N. Application of plant extracts and Trichoderma harzianum for the management of tomato seedling damping-off caused by Rhizoctonia solani. S. Afr. J. Sci. 2020, 116, 1–5. [Google Scholar] [CrossRef]
- Righini, H.; Francioso, O.; Di Foggia, M.; Prodi, A.; Quintana, A.M.; Roberti, R. Tomato seed biopriming with water extracts from Anabaena minutissima, Ecklonia maxima and Jania adhaerens as a new agro-ecological option against Rhizoctonia solani. Sci. Hortic. 2021, 281, 109921. [Google Scholar] [CrossRef]
- Akladious, S.A.; Isaac, G.S.; Abu-Tahon, M.A. Induction and resistance against Fusarium wilt disease of tomato by using sweet basil (Ocimum basilicum L.) extract. Can. J. Plant. Sci. 2015, 95, 689–701. [Google Scholar] [CrossRef]
- Righini, H.; Roberti, R.; Baraldi, E. Use of algae in strawberry management. J. Appl. Phycol. 2018, 30, 3551–3564. [Google Scholar] [CrossRef]
- Vera, J.; Castro, J.; Gonzalez, A.; Moenne, A. Seaweed polysaccharides and derived oligosaccharides stimulate defense responses and protection against pathogens in plants. Mar. Drugs 2011, 9, 2514–2525. [Google Scholar] [CrossRef]
- Vicente, T.F.L.; Lemos, M.F.L.; Félix, R.; Valentão, P.; Félix, C. Marine macroalgae, a source of natural inhibitors of fungal phytopathogens. J. Fungi 2021, 7, 1006. [Google Scholar] [CrossRef]
- Ben Salah, I.; Aghrouss, S.; Douira, A.; Aissam, S.; El Alaoui-Talibi, Z.; Filali-Maltouf, A.; El Modafar, C. Seaweed polysaccharides as bio-elicitors of natural defenses in olive trees against verticillium wilt of olive. J. Plant Interact. 2018, 13, 248–255. [Google Scholar] [CrossRef]
- Ansari, F.A.; Shriwastav, A.; Gupta, S.K.; Rawat, I.; Guldhe, A.; Bux, F. Lipid extracted algae as a source for protein and reduced sugar: A step closer to the biorefinery. Bioresour. Technol. 2015, 179, 559–564. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Xue, Y.T. Optimization of microwave assisted extraction, chemical characterization and antitumor activities of polysaccharides from Porphyra haitanensis. Carbohydr. Polym. 2019, 206, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Vaquero, M.; O’Doherty, J.V.; Tiwari, B.K.; Sweeney, T.; Rajauria, G. Enhancing the extraction of polysaccharides and antioxidants from macroalgae using sequential hydrothermal-assisted extraction followed by ultrasound and thermal technologies. Mar. Drugs 2019, 17, 457. [Google Scholar] [CrossRef] [PubMed]
- Elarrouss, H.; Elmernissi, N.; Benhima, R.; El Kadmiri, I.M.; Bendaou, N.; Smouni, A.; Wahbya, I. Microalgae polysaccharides a promising plant growth biostimulant. J. Algal Biomass Util. 2016, 7, 55–63. [Google Scholar]
- Ozawa, T.; Yamamoto, J.; Yamagishi, T.; Yamazaki, N.; Nishizawa, M. Two fucoidans in the holdfast of cultivated Laminaria japonica. J. Nat. Med. 2006, 60, 236–239. [Google Scholar] [CrossRef] [PubMed]
- Sinurat, E.; Rosmawaty, P.; Saepudin, E. Characterization of fucoidan extracted from Binuangeun’s Brown Seaweeds. Int. J. Chem. Environ. Biol. Sci. 2015, 3, 329–332. [Google Scholar]
- Shen, S.; Cheng, H.; Li, X.; Li, T.; Yuan, M.; Zhou, Y.; Ding, C. Effects of extraction methods on antioxidant activities of polysaccharides from camellia seed cake. Eur. Food Res. Technol. 2014, 238, 1015–1021. [Google Scholar] [CrossRef]
- Álvarez-Gómez, F.; Korbee, N.; Figueroa, F.L. Analysis of antioxidant capacity and bioactive compounds in marine macroalgal and lichenic extracts using different solvents and evaluation methods. Cienc. Mar. 2016, 42, 271–288. [Google Scholar] [CrossRef]
- Nawaz, A.; Amjad, M.; Khan, S.M.; Afzal, I.; Ahmed, T.; Iqbal, Q.; Iqbal, J. Tomato seed invigoration with cytokinins. J. Anim. Plant. Sci. 2013, 23, 121–128. [Google Scholar]
- Burnett, F.J.; Boor, T.; Dussart, F.; Smith, J. Developing Sustainable Management Methods for Clubroot. Project Report No. 608. Agriculture and Horticulture Development Board. 2019. Available online: https://pure.sruc.ac.uk/ws/portalfiles/potal/18991123/pr608_final_project_report.pdf (accessed on 12 July 2022).
- Li, Q.; Yu, P.; Chen, X.; Li, G.; Zhou, D.; Zheng, W. Facilitative and inhibitory effect of litter on seedling emergence and early growth of six herbaceous species in an early successional old field ecosystem. Sci. World J. 2014, 2014, 101860. [Google Scholar] [CrossRef]
- De Cal, A.; Pascual, S.; Melgarejo, P. Infectivity of chlamydospores vs microconidia of Fusarium oxysporum f. sp. lycopersici on tomato. J. Phytopathol 1997, 145, 231–233. [Google Scholar] [CrossRef]
- Roberti, R.; Galletti, S.; Burzi, P.L.; Righini, H.; Cetrullo, S.; Perez, C. Induction of defence responses in zucchini (Cucurbita pepo) by Anabaena sp. water extract. Biol. Control 2015, 82, 61–68. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Bargabus, R.L.; Zidack, N.K.; Sherwood, J.E.; Jacobsen, B.J. Screening for the identification of potential biological control agents that induce systemic acquired resistance in sugar beet. Biol. Control 2004, 30, 342–350. [Google Scholar] [CrossRef]
- Rao, C.N.R. Chemical Applications of Infrared Spectroscopy; Academic Press, Inc.: New York, NY, USA; London, UK, 1963; p. 681. [Google Scholar]
- Xu, R.B.; Yang, X.; Wang, J.; Zhao, H.T.; Lu, W.H.; Cui, J.; Cheng, C.L.; Zou, P.; Huang, W.W.; Wang, P.; et al. Chemical composition and antioxidant activities of three polysaccharide fractions from pine cones. Int. J. Mol. Sci. 2012, 13, 14262–14277. [Google Scholar] [CrossRef]
- Hong, T.; Yin, J.Y.; Nie, S.P.; Xie, M.Y. Applications of infrared spectroscopy in polysaccharide structural analysis: Progress, challenge and perspective. Food Chem. X 2021, 12, 100168. [Google Scholar] [CrossRef]
- Wiercigroch, E.; Szafraniec, E.; Czamara, K.; Pacia, M.Z.; Majzner, K.; Kochan, K.; Malek, K. Raman and infrared spectroscopy of carbohydrates: A review. Spectrochim. Acta A 2017, 185, 317–335. [Google Scholar] [CrossRef]
- Shukla, P.S.; Mantin, E.G.; Adil, M.; Bajpai, S.; Critchley, A.T.; Prithiviraj, B. Ascophyllum nodosum-based biostimulants: Sustainable applications in agriculture for the stimulation of plant growth, stress tolerance, and disease management. Front. Plant Sci. 2019, 10, 655. [Google Scholar] [CrossRef]
- Arioli, T.; Mattner, S.W.; Winberg, P.C. Applications of seaweed extracts in Australian agriculture: Past, present and future. J. Appl. Phycol. 2015, 27, 2007–2015. [Google Scholar] [CrossRef]
- Khan, W.; Rayirath, U.P.; Subramanian, S.; Jithesh, M.N.; Rayorath, P.; Hodges, D.M.; Critchley, A.T.; Craigie, J.S.; Norrie, J.; Prithiviraj, B. Seaweed extracts as biostimulants of plant growth and development. J. Plant Growth Regul. 2009, 28, 386–399. [Google Scholar] [CrossRef]
- O’ Keeffe, E.; Hughes, H.; McLoughlin, P.; Tan, S.P.; McCarthy, N. Methods of analysis for the in vitro and in vivo determination of the fungicidal activity of seaweeds: A mini review. J. Appl. Phycol. 2019, 31, 3759–3776. [Google Scholar] [CrossRef]
- Sharma, H.S.; Fleming, C.; Selby, C.; Rao, J.R.; Martin, T. Plant biostimulants: A review on the processing of macroalgae and use of extracts for crop management to reduce abiotic and biotic stresses. J. Appl. Phycol. 2014, 26, 465–490. [Google Scholar] [CrossRef]
- Abdel-Raouf, N.; Al-Homaidan, A.A.; Ibrahim, I.B.M. Agricultural importance of algae. Afr. J. Biotechnol. 2012, 1, 11648–11658. [Google Scholar] [CrossRef]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef]
- Arunkumar, K.; Sivakumar, S.R.; Rengasamy, R. Review on bioactive potential in seaweeds (marine macroalgae): A special emphasis on bioactivity of seaweeds against plant pathogens. Asian J. Plant Sci. 2010, 9, 227–240. [Google Scholar] [CrossRef]
- Righini, H.; Somma, A.; Cetrullo, S.; D’Adamo, S.; Flamigni, F.; Martel Quintana, A.; Roberti, R. Inhibitory activity of aqueous extracts from Anabaena minutissima, Ecklonia maxima and Jania adhaerens on the cucumber powdery mildew pathogen in vitro and in vivo. J. Appl. Phycol. 2020, 32, 3363–3375. [Google Scholar] [CrossRef]
- Arun, M.N.; Hebbar, S.S.; Senthivel, T.; Nair, A.K.; Padmavathi, G.; Pandey, P.; Singh, A. Seed priming: The way forward to mitigate abiotic stress in crops. In Plant Stress Physiology-Perspectives in Agriculture; Hasanuzzaman, M., Nahar, K., Eds.; IntechOpen: London, UK, 2022; Volume 11, p. 173. [Google Scholar]
- El-Mougy, N.S.; Abdel-Kader, M.M. Long term activity of bio-priming seed treatment for biological control of faba bean root rot pathogens. Aust. Plant Pathol. 2008, 37, 464–471. [Google Scholar] [CrossRef]
- Rachidi, F.; Benhima, R.; Kasmi, Y.; Sbabou, L.; Arroussi, H.E. Evaluation of microalgae polysaccharides as biostimulants of tomato plant defense using metabolomics and biochemical approaches. Sci. Rep. 2021, 11, 101860. [Google Scholar] [CrossRef]
- Chaliha, C.; Rugen, M.D.; Field, R.A.; Kalita, E. Glycans as modulators of plant defense against filamentous pathogens. Front. Plant Sci. 2018, 9, 928. [Google Scholar] [CrossRef]
- Lagogianni, C.S.; Tsitsigiannis, D.I. Effective biopesticides and biostimulants to reduce aflatoxins in maize fields. Front. Microbiol. 2019, 10, 2645. [Google Scholar] [CrossRef]
- de Borba, M.C.; Velho, A.C.; de Freitas, M.B.; Holvoet, M.; Maia-Grondard, A.; Baltenweck, R.; Magnin-Robert, M.; Randoux, B.; Hilbert, J.L.; Reignault, P.; et al. A laminarin-based formulation protects wheat against Zymoseptoria tritici via direct antifungal activity and elicitation of host defense-related genes. Plant Dis. 2022, 106, 1408–1418. [Google Scholar] [CrossRef]
- Aruna, P.; Mansuya, P.; Sridhar, S.; Kumar, J.S.; Babu, S. Pharmacognostical and antifungal activity of selected seaweeds from Gulf of Mannar region. Recent Res. Sci. Technol. 2010, 2, 115–119. [Google Scholar]
- Damonte, E.B.; Matulewicz, M.C.; Cerezo, A.S. Sulfated seaweed polysaccharides as antiviral agents. Curr. Med. Chem. 2004, 11, 2399–2419. [Google Scholar] [CrossRef] [PubMed]
- Matsuhiro, B.; Conte, A.F.; Damonte, E.B.; Kolender, A.A.; Matulewicz, M.C.; Mejías, E.G.; Pujol, C.A.; Zúñiga, E.A. Structural analysis and antiviral activity of a sulfated galactan from the red seaweed Schizymenia binderi (Gigartinales, Rhodophyta). Carbohyd. Res. 2005, 340, 2392–2402. [Google Scholar] [CrossRef] [PubMed]
- Pujol, C.A.; Scolaro, L.A.; Ciancia, M.; Matulewicz, M.C.; Cerezo, A.S.; Damonte, E.B. Antiviral activity of a carrageenan from Gigartina skottsbergii against intraperitoneal murine Herpes simplex virus infection. Planta Med. 2006, 72, 121–125. [Google Scholar] [CrossRef]
- Souza, B.W.S.; Cerqueira, M.A.; Bourbon, A.I.; Pinheiro, A.C.; Martins, J.T.; Teixeira, J.A.; Coimbra, M.A.; Vicente, A.A. Chemical characterization and antioxidant activity of sulfated polysaccharide from the red seaweed Gracilaria birdiae. Food Hydrocoll. 2012, 27, 287–292. [Google Scholar] [CrossRef]
- Abdel Latef, A.A.H.; Srivastava, A.K.; Saber, H.; Alwaleed, E.A.; Tran, L.S.P. Sargassum muticum and Jania rubens regulate amino acid metabolism to improve growth and alleviate salinity in chickpea. Sci. Rep. 2017, 7, 10537. [Google Scholar] [CrossRef]
- Safinaz, A.F.; Ragaa, A.H. Effect of some red marine algae as biofertilizers on growth of maize (Zea mays L.) plants. Int. Food Res. J. 2013, 20, 1629–1632. [Google Scholar]
- Zodape, S.T.; Mukherjee, S.; Reddy, M.P.; Chaudhary, D.R. Effect of Kappaphycus alvarezii (Doty) doty ex silva. extract on grain quality, yield and some yield components of wheat (Triticum aestivum L.). Int. J. Plant Prod. 2009, 3, 97–101. [Google Scholar]
- Zodape, S.T.; Gupta, A.; Bhandari, S.C.; Rawat, U.S.; Chaudhary, D.R.; Eswaran, K.; Chikara, J. Foliar application of seaweed sap as biostimulant for enhancement of yield and quality of tomato (Lycopersicon esculentum Mill.). J. Sci. Ind. Res. 2011, 70, 215–219. [Google Scholar]
- Jayaraman, J.; Norrie, J.; Punja, Z.K. Commercial extract from the brown seaweed Ascophyllum nodosum reduces fungal diseases in greenhouse cucumber. J. Appl. Phycol. 2011, 23, 353–361. [Google Scholar] [CrossRef]
- Witmer, X.; Nonogaki, H.; Beers, E.P.; Bradford, K.J.; Welbaum, G.E. Characterization of chitinase activity and gene expression in muskmelon seeds. Seed Sci. Res. 2003, 13, 167–178. [Google Scholar] [CrossRef]
- Morohashi, Y.; Matsushima, H. Development of β-1, 3-glucanase activity in germinated tomato seeds. J. Exp. Bot. 2000, 51, 1381–1387. [Google Scholar] [CrossRef]
- Ghule, M.R.; Ramteke, P.K.; Ramteke, S.D.; Kodre, P.S.; Langote, A.; Gaikwad, A.V.; Holkar, S.K.; Jambhekar, H. Impact of chitosan seed treatment of fenugreek for management of root rot disease caused by Fusarium solani under in vitro and in vivo conditions. 3 Biotech 2021, 11, 290. [Google Scholar] [CrossRef] [PubMed]
- Pettongkhao, S.; Bilanglod, A.; Khompatara, K.; Churngchow, N. Sulphated polysaccharide from Acanthophora spicifera induced Hevea brasiliensis defense responses against Phytophthora palmivora infection. Plants 2019, 8, 73. [Google Scholar] [CrossRef]
- Xu, X.; Chen, Y.; Li, B.; Zhang, Z.; Qin, G.; Chen, T.; Tian, S. Molecular mechanisms underlying multi-level defense responses of horticultural crops to fungal pathogens. Hortic. Res. 2022, 9, uhac066. [Google Scholar] [CrossRef] [PubMed]


| Gene Names | Forward | Reverse | Annealing T (°C) |
|---|---|---|---|
| Actin-7 | GGGATGGAGAAGTTTGGTGGTGG | CTTCGACCAAGGGATGGTGTAGC | 61 |
| PAL5 | CACTGTAAGCCAAGTAGCCAAA | CTGCAGGGGTCATCAGCATA | 59 |
| HCT | CGGACGTTACCATCACTGGA | AAGGAGGACTCAGTAGCTTTG | 59 |
| HQT | GGTGTTTTGTTTGTTGAGGCTG | GACTCCGCCACACTTGAAAC | 59 |
| FLS | GATTTGGCCTCCTCCTGCTA | TCCAAACCAAGCCCAAGTGA | 59 |
| PR1a | AGGATGCAACACTCTGGTGG | GCACAACCAAGACGTACCGA | 60 |
| PR2 | GGTGGATCCAATTCGCAAGC | ACCTGAGAACCCACCAGACT | 59 |
| PR3 | AGAGTTCCAGGGTACGGTGT | CCAATTCGACTTTCCGCTGC | 59 |
| PR4 | GATGCTGACAAGCCTCTGGA | CCCTCAAGCATCTACCGCAT | 59 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Righini, H.; Roberti, R.; Cetrullo, S.; Flamigni, F.; Quintana, A.M.; Francioso, O.; Panichi, V.; Cianchetta, S.; Galletti, S. Jania adhaerens Primes Tomato Seed against Soil-Borne Pathogens. Horticulturae 2022, 8, 746. https://doi.org/10.3390/horticulturae8080746
Righini H, Roberti R, Cetrullo S, Flamigni F, Quintana AM, Francioso O, Panichi V, Cianchetta S, Galletti S. Jania adhaerens Primes Tomato Seed against Soil-Borne Pathogens. Horticulturae. 2022; 8(8):746. https://doi.org/10.3390/horticulturae8080746
Chicago/Turabian StyleRighini, Hillary, Roberta Roberti, Silvia Cetrullo, Flavio Flamigni, Antera Martel Quintana, Ornella Francioso, Veronica Panichi, Stefano Cianchetta, and Stefania Galletti. 2022. "Jania adhaerens Primes Tomato Seed against Soil-Borne Pathogens" Horticulturae 8, no. 8: 746. https://doi.org/10.3390/horticulturae8080746
APA StyleRighini, H., Roberti, R., Cetrullo, S., Flamigni, F., Quintana, A. M., Francioso, O., Panichi, V., Cianchetta, S., & Galletti, S. (2022). Jania adhaerens Primes Tomato Seed against Soil-Borne Pathogens. Horticulturae, 8(8), 746. https://doi.org/10.3390/horticulturae8080746







