Abstract
Gypsophila paniculata is the only species in the genus Gypsophila that has been used as cut flowers, and the sequencing of its genome has just been completed, opening a new chapter in its molecular genetic breeding. The molecular marker system is the basis for genetic molecular research in the era of genomics, whereas it is still a gap for G. paniculata. In this study, we constructed a genome-wide InDel marker system of G. paniculata after genome resequencing of another wild-type accession with white flowers. Consequently, 407 InDel markers at a distance of ~2 Mb were designed for all 17 chromosomes. Later, the validation of these markers by PCR revealed that 289 markers could distinguish alleles of the two wild-type alleles clearly. The predicted polymorphisms of two wild-type alleles were then transferred to the commercial cultivars, which displayed a rich polymorphism among four commercial cultivars. Our research established the first genome-level genetic map in G. paniculata, providing a comprehensive set of marker systems for its molecular research.
1. Introduction
Gypsophila paniculata is a perennial herbaceous shrub from the genus Gypsophila, which comprises about 150 species of annual, biennial and perennial plants [1,2]. It is usually used as a filler in flower arrangements, making it an important cut flower in the global market. Differing from crops, flower type and colour as well as its inner quality and biotic or abiotic stress resistance are the main goals for ornamental breeding [3]. Although breeding efforts have been invested in the creation of new varieties and the improvement of desirable traits, conventional crosses and subsequent phenotypic selection for specific traits remain the dominant breeding methods used in the breeding of G. paniculata, which has severely hindered its breeding efficiency [4]. As a consequence, there are currently fewer commercial varieties on the world floricultural market, such as ‘Million Stars’, ‘Perfect’, ‘Dream Pink’ and ‘Huixing 1′ [5]. In contrast to its important position in the floricultural industry, the molecular genetics research of G. paniculata is limited, which hinders the improvement of the cultivars to some extent.
Molecular markers have been widely used in genetic and evolutionary research of various ornamental species including Rosa, Paeonia, Dendrobium, etc., in their germplasm characterization, genetic mapping, diversity analysis and molecular marked-assisted selection in breeding [6,7,8,9,10,11]. In the development history of molecular markers, DNA-based marker systems such as RFLP (restriction fragment length polymorphism) have been replaced progressively by PCR-based markers such as RAPD (random amplified polymorphic DNA), SSR (simple sequence repeat), SNPs (single-nucleotide polymorphisms) and InDels (insertions/deletions) [12]. A few pieces of research about the assessment of genetic diversity among wild species and commercial hybrids from Gypsophila using RAPD markers and chloroplast simple sequence repeat (cpSSR) markers have been reported [13,14], but the genetic map or marker system covering the whole genome of G. paniculata lacks. Benefiting from the recent progress in genome sequencing technology, reference genomes with high quality have been accessed in various ornamental plants, bringing floral research to a genome-wide level [15,16]. In addition, the SNP and InDel markers have become the most used molecular markers for plant research due to their abundant polymorphisms, genome-wide distribution and co-dominance [17,18,19]. For instance, high-density genetic maps based on SNPs facilitate the identification the genetic regulators of key ornamental traits such as flower type as well as the resistance gene in roses, carnation and chrysanthemum [20,21,22,23]. Although powerful, the genotyping of SNPs relies on the KASP assay, which requires a special machine and is expensive. In contrast, InDel makers are easy to use, low in cost and efficient, as the InDel marker-based mapping system relies on simple PCR and gel electrophoresis procedures [24].
This study aims to construct a genome-wide InDel marker system of G. paniculata, providing a useful tool to facilitate its breeding. Recently, the genome sequence of G. paniculata has been assembled and released to the public [25], meeting our goal to develop markers through genome resequencing. Thus, a wild-type accession of G. paniculata with white flower (WT-W) was re-sequenced using next-generation sequencing technology, and a series of molecular markers distributed genome-wide were identified, including SNP, InDel, SV (structure variation) and CNV (copy number variations by comparing with the reference genome of a wild-type accession with pink flower (WT-P)). As hypothesized, a set of InDel markers with a high level of polymorphism was developed using the information generated by genome resequencing. Moreover, the InDel markers also displayed polymorphism among four commercial cultivars. Our work provides the first genome-wide genetic map of G. paniculata, supporting the further genetic study and molecular breeding of this species.
2. Materials and Methods
2.1. Plant Materials
Two G. paniculata wild-type accessions with pink (WT-P) and white flowers (WT-W) were used in this study (Figure 1). The WT-P plant was used for the de novo genome sequenced previously, providing the reference genome data thereby. The WT-W plant was used for genome resequencing to generate InDel markers. Meanwhile, we selected four commercial cultivars of G. paniculata (‘YX1′, ‘YX2′, ‘YX3′ and ‘YX4′) to identify and validate the polymorphic InDel markers. ‘YX1′, ‘YX2′ and ‘YX4′ are three representative commercial varieties of G. paniculata with white petals, and the difference is the flower size (‘YX1′>‘YX4′>‘YX2′, from large to small’), whereas the flower colour of ‘YX3′ is pink with a similar size as ‘YX2′ (Figure 1). All of the above plant materials were provided by Yuxi Yunxing Biological Technology Co., Ltd. (Yuxi, China).
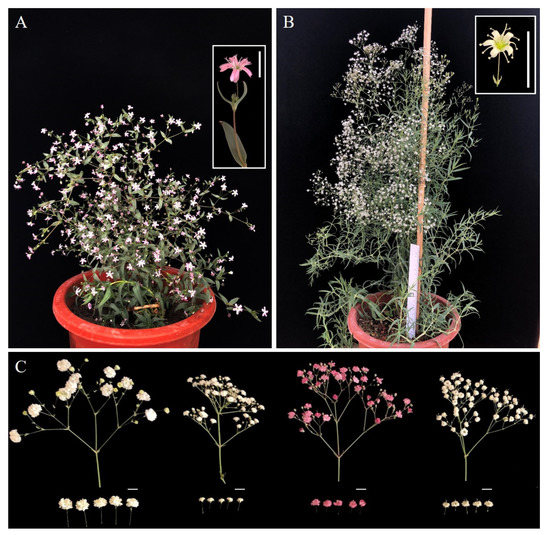
Figure 1.
The phenotype of G. paniculata wild-type accessions and commercial cultivars used in this study. (A). The pink flower wild type of G. paniculata (WT-P). (B). The white flower wild type of G. paniculata (WT-W). (C). The flower phenotype of four commercial cultivars (‘YX1′, ‘YX2′, ‘YX3′ and ‘YX4′, from left to right). Bar = 1 cm.
2.2. Variation Detection by Genome Resequencing
The fresh young leaves of G. paniculata WT-W were used for genome resequencing. The MGISEQ-2000 PE150 sequencer was applied to conduct genome sequencing, after which the original reads (8.66 Gb) were filtered to generate clean reads (8.05 Gb) for subsequent analysis. Using in-house scripts, we filtered any sequencing reads with the following: reads with adapter sequences, consecutive bases on the ends with base quality < Q20, read length < 50 bp and singletons. The clean reads were then aligned to the G. paniculata reference genome using BWA mem (v0.7.17) with default settings [26]. The alignment results were sorted using Samtools (v1.9) [27].
SNP and InDel were called using GATK HaplotypeCaller (v4.1.4.1, Broad Institute, Cambridge, MA, USA) with default settings [28]. We further filtered the calls using GATK VariantFiltration with the following parameters: SNP filtering (QD < 2.0, FS > 60.0, MQ < 40.0, MQRankSum< −12.5, ReadPosRankSum < −8.0); InDel filtering (QD < 2.0, FS > 200.0, ReadPosRankSum < −20.0). CNV were detected using CNVnator (v0.3.2) with default settings [29]. SV were identified using Manta (v1.6.0) [30]. Mutational positions, genomic regions and potential amino acid changes were assessed using ANNOVAR (v2019, Wang Kai, PA, USA) [31]. Circos (v0.69, Martin Krzywinski, Vancouver, BC, Canada) was used to plot the genome-wide distribution of variation [32].
2.3. Development of InDel Markers
We selected the InDels that were over 10 bp long and distributed ~2 Mb. The positions with excess InDels which might interfere with the PCR verification were excluded. After selecting the suitable InDels, a ~400 bp genome sequence covering each InDel was used as the template for primer design. The primers were designed on NCBI and named after the chromosome number and the physical position (N-XX.XX, Table S1).
2.4. PCR Analyses of InDel Markers
The total DNA of two wild-type plants and four commercial cultivars was extracted from fresh leaves using the CTAB method [33]. Template DNA was amplified with the designed primers in a 10 µL system (7.3 µL ddH2O; 1 µL 10× Taq buffer; 0.8 µL dNTPs; 0.2 µL primers; 0.1 µL Taq enzyme; 0.4 µL DNA template) using the following PCR program: 5 min of full denaturation at 95 °C; 29 cycles (95 °C, 30 s; 56 °C, 30 s; 72°C, 30 s); 72 °C extension for 7 min. After the standard PCR, 3 µL DNA loading buffer was added to the PCR product. Then, the mixture was separated in 3.5% agarose gel.
3. Results
3.1. Genome Resequencing and Sequence Polymorphism Identification
The successful mapping of QTLs relies on the genetic maps with high-density molecular markers between the accessions. Previously, the genome sequence data of G. paniculata, a wild-type accession with pink flowers (WT-P), was assembled onto 17 chromosomes [25]. To develop sufficient molecular markers for G. paniculata genetic research, we detected sequence polymorphisms between WT-P and another wild-type accession with white flowers (WT-W) through genome resequencing by the high-throughput sequencing platform MGISEQ-2000 PE150. After filtering, a total of 8.05 Gb of clean reads was generated, 82.23% of which were mapped to the reference genome, displaying an average sequencing depth of 5.70 (Figure 2B). Different kinds of natural genetic variations were detected between the reference and resequencing genome, including 2,377,499 SNPs, 1,366,056 InDels, 1403 SVs, and 28 CNVs, whose densities were shown on the circus map (Figure 2A). Interestingly, the InDels preferred to distribute at the end of the chromosomes rather than the centromeric region, as shown by the circus map. Meanwhile, the length of most InDels (>80%) was less than 5 bp, and the InDels between 5 and 10 bp accounted for 10% of this variation. There were about ~5% (68,302/1,366,056) InDels over 10 bp which are suitable for genome-wide marker construction (Figure 2C).
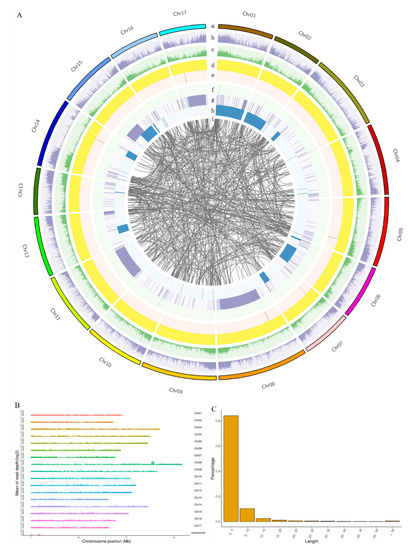
Figure 2.
Resequencing of WT-W based on the WT-P genome sequence. (A) Genomic structure variation distribution between the two G. paniculata wild-type accessions. a: reference sequence. b: SNP density distribution. c: InDel distribution density. d: CNV duplication. e: CNV deletion. f: SV insertion. g: SV deletion. h: SV inversion. i: SV translocation. Abbreviations include SNP: Single Nucleotide Polymorphism; InDel: Insertion/Deletion; CNV: Copy Number Variations; SV: Structure Variation. (B) The sequencing coverage depth distribution map of each chromosome of G. paniculata. The mean of read depth was calculated using the coverage depth (10,000 bp as the statistical window) by logarithm (log2). (C) The distribution of the InDel length between WT-P and WT-W.
3.2. Construction of InDel Markers for Polymorphism Analysis
To develop InDel markers that can discriminate alleles between WT-P and WT-W, insertions or deletions over 10 bp were chosen as candidates with the interval of the neighbouring markers set as ~2 Mb. Sequence fragments about 400 bp long that contained either the insertions or deletions were used as templates to design primers. In total, 407 pairs of primers were designed for 17 chromosomes (Figure 3). To validate the newly designed markers, PCR analysis was conducted and the products were analysed by gel electrophoresis. Of the 407 markers, 289 markers distinguished the alleles of WT-P and WT-W clearly. Another 34 markers produced close bands on the 3.5% gel, but could still discriminate the alleles of WT-P and WT-W. These markers can be used when the chromosome region has limited markers, probably separated by gel with higher concentration. The success rates of the designed primers varied across the chromosomes from 40% to 92.9%, and the average success rate was as high as 71.0% (Table 1). Our data provided the successful establishment of genome-wide InDel markers based on a genetic map for G. paniculata. Nevertheless, it has to be acknowledged that for some chromosomes, such as Chr.4, Chr.7, Chr.12 and Chr.14, there were obvious gaps between two available markers, which was probably due to the low density of InDels on the centromeric region of these chromosomes. Thus, it might be essential to develop other molecular markers such as SNPs to compensate for these empties in the future.
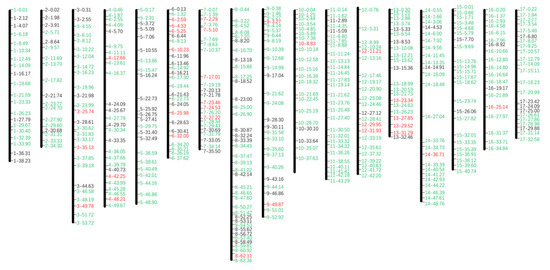
Figure 3.
The physical map of 407 InDel markers distributed across all 17 chromosomes of G. paniculata genome. The name code of the InDel marker was presented as a chromosome number with the physical distance. Green markers discriminate alleles between WT-P and WT-W. Red markers amplified close bands on gel, and black markers were unavailable.

Table 1.
The successful rates of InDel markers for all 17 chromosomes.
3.3. InDel Marker Polymorphisms among Commercial Cultivars
The wild and commercial cultivars possess excellent agronomic traits, for example, wild types are generally more resistant, while the commercial varieties display larger flowers and more petals. However, limited research focuses on the genetic regulators underlying these traits, causing the relative mechanisms to remain unknown. To explore the applicability of the InDel markers designed in distinguishing the alleles between wild-type and commercial varieties, PCR amplification was conducted using the genomic DNA of WT-P and four commercial varieties (YX1-4) as templates. Out of the 407 pairs of primers, 191 were able to discriminate alleles between WT-P and commercial cultivars. The polymorphism of the InDel markers between WT-P and commercial cultivars was then analysed by pairwise comparisons (Table 2). In total, the number of available markers for each pair of accessions ranged from 31 (YX1 vs. YX4) to 173 (YX1 vs. WT-P), with an average of 92. The InDel markers were suitable to discriminate alleles between WT-P and commercial cultivars (an average of 171 markers available) since a high degree of polymorphism was observed (Figure 4), whereas the markers available between the commercial cultivars were no more than 50. This implies that the commercial cultivars are closely related, which is consistent with the observation that all four commercial cultivars bloom white flowers but differ only in flower size.

Table 2.
Number of InDel markers that were polymorphic in pairwise comparison of five G. paniculata accessions.
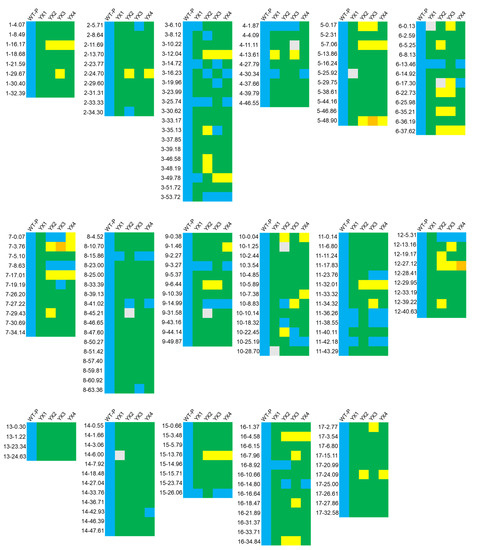
Figure 4.
Matrix of the polymorphisms using the InDel markers among the five accessions of G. paniculata. Blue squares are WT-P bands, green, yellow and orange squares represent bands different from WT-P, and grey squares mean no bands detected.
4. Discussion
The elaboration of the key regulatory mechanism underlying one or several traits as well as the fast selection of elite progenies is crucial for plant breeding. When obtaining a certain mutant, the identification of the allele(s) related to the phenotype is usually performed by the forward genetics, in which F2 rough mapping provides an approximate location of the mutation causative allele(s) on chromosomes without the requirement of a large amount of samples and high-throughput sequencing, narrowing down the targets for further fine mapping. In the last decades, the development of PCR-based markers such as RAPD, SSR and amplified fragment length polymorphisms (AFLPs) have fulfilled the shortage of map-based cloning [34]. In the field of ornamental breeding, these sequence-related amplified polymorphisms (SRAP) markers have been applied to the vase life-associated or disease-resistant QTL mapping and analyse chrysanthemum, carnation and lily, to cite a few [35].
Nevertheless, the development of such markers is labour intensive, and their application is limited in certain situations, since they are usually not genome-wide. Earlier, benefiting from the availability of an annotated reference genome and sequenced accessions, genetic markers based on InDels have been developed in Arabidopsis, accelerating the identification of the mutated allele(s) [36]. With the booming of sequencing technology and the following drop in sequencing expense, plentiful plant genomes were released for crops and horticultural plants [37,38,39,40]. The resequencing-based InDel makers were then developed in cotton [41], rice [42], Brassica [43], buckwheat [44], jute [45], melon [46], chickpea [47], cucumber [48], etc., used for research such as disease-related gene identification or accession discrimination. However, the systematic development of such markers has not been reported in ornamental species.
In this study, we constructed a genome-wide InDel marker system for G. paniculata through genome resequencing. Similar to the early report in jute [45], InDels detected in the G. paniculata genome are quite abundant, but most of them are shorter than 5 bp, which makes them hard to use as markers. Regardless, the number of the ~5% InDels that are longer than 10 bp is as large as 68302, equivalent to 91 InDels per Mb, which is more than needed. To meet the demand for mapping (1 maker/2 Mb), 409 InDels distributed on 17 chromosomes were selected, and the relative primers were then designed. Of these, 289 can discriminate alleles from 2 wild types donating the genome sequence data, coming to a success rate of 70.6%. Although we expected to obtain an available marker every 2 Mb, the outcome was barely satisfactory. There were usually missing available InDel markers in the middle (calculated by physical distance) of the chromosome, such as Chr. 4, 7 and 14 (Figure 3). The same situation happened during the development of InDel markers in rice [42] and Capsicum spp. [49]. It might be dissolved by adding other molecular markers when mapping a certain QTL, or the InDels shorter than 10 bp can also be developed as markers based on a high-resolution melting curve, as reported [50]. Since the discrimination of genetic resources and extension of the application of markers are crucial in the breeding process [44], we then detected the polymorphisms of the designed InDel markers in four best-selling cultivars. Over 170 makers were available to differentiate each commercial cultivar and WT-P, whereas only less than 50 markers worked for discrimination between the 4 commercial cultivars. It makes sense, since all the commercial cultivars bloom with white flowers and may share more common genetic information rather than WT-P.
Used not only as the filler flower but also as a preserved flower which decorates the environment after colourful staining, the status of cut flower G. paniculata is rising, leading to a massive demand for the innovation of this species. Molecular genetics play a more and more important role in floricultural breeding, in which a molecular marker system covering the whole genome is the basis for genetic molecular research in the era of genomics. However, it is still a gap for G. paniculata. Here, we provide the first genetic map of G. paniculata in this study, consisting of a comprehensive set of InDel markers for the molecular research of G. paniculata. The success in our case also implies that the development of InDel markers covering the whole genome is cost- and labour-effective with a high success rate, deserving to be applied in other ornamental species for which cross-breeding is the main method for cultivar innovation.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae8100921/s1, Table S1: Primers used in this study.
Author Contributions
C.J. and F.L. conceived and designed the research; B.L. performed the experiments and analysed the data; C.J. and F.L. wrote the manuscript and revised the manuscript; J.R. and C.Y. provided the wildtype plants and commercial cultivars. F.L. supervised the project. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Natural Science Foundation of China (31960608), Yunnan Fundamental Research Projects (202101AT070147) and High-level Talent Introduction Program of Yunnan Province—Industrial Talent Special Project (YNQR-CYRC-2020-004).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lu, D.; Nicholas, J.T. Gypsophila Linnaeus; Science Press: St. Louis, MA, USA, 2001; Volume 6. [Google Scholar]
- Li, F.; Wang, G.; Yu, R.; Wu, M.; Shan, Q.; Wu, L.; Ruan, J.; Yang, C. Effects of Seasonal Variation and Gibberellic Acid Treatment on the Growth and Development of Gypsophila paniculata. HortScience 2019, 54, 1370–1374. [Google Scholar] [CrossRef]
- Kuligowska, K.; Lütken, H.; Müller, R. Towards development of new ornamental plants: Status and progress in wide hybridization. Planta 2016, 244, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Zvi, M.M.B.; Zuker, A.; Ovadis, M.; Shklarman, E.; Ben-Meir, H.; Zenvirt, S.; Vainstein, A. Agrobacterium-mediated transformation of gypsophila (Gypsophila paniculata L.). Mol. Breed. 2008, 22, 543–553. [Google Scholar]
- Li, F.; Mo, X.; Wu, L.; Yang, C. A Novel Double-flowered Cultivar of Gypsophila paniculata Mutagenized by 60Co γ-Ray. HortScience 2020, 55, 1531–1532. [Google Scholar] [CrossRef]
- Xue, Y.; Liu, R.; Xue, J.; Wang, S.; Zhang, X. Genetic Diversity and Relatedness Analysis of Nine Wild Species of Tree Peony Based on Simple Sequence Repeats Markers. Hortic. Plant J. 2021, 7, 579–588. [Google Scholar] [CrossRef]
- Sousa, A.; Souza, M.M.; Melo, C.; Sodré, G. ISSR markers in wild species of Passiflora L. (Passifloraceae) as a tool for taxon selection in ornamental breeding. Genet. Mol. Res. Gmr 2015, 14, 18534. [Google Scholar] [CrossRef]
- Conceição, L.; Belo, G.; Souza, M.; Santos, S.; Cerqueira-Silva, C.; Corrêa, R. Confirmation of cross-fertilization using molecular markers in ornamental passion flower hybrids. Genet. Mol. Res. 2011, 10, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.-g.; Hu, Y.-d.; Zhao, R.-x.; Yan, S.; Zhang, X.-q.; Zhao, T.-m.; Chun, Z. Genome-wide researches and applications on Dendrobium. Planta 2018, 248, 769–784. [Google Scholar] [CrossRef]
- Yang, C.; Ma, Y.; Cheng, B.; Zhou, L.; Yu, C.; Luo, L.; Pan, H.; Zhang, Q. Molecular Evidence for Hybrid Origin and Phenotypic Variation of Rosa Section Chinenses. Genes 2020, 11, 996. [Google Scholar] [CrossRef]
- Fan, Y.; Wang, Q.; Dong, Z.; Yin, Y.; Teixeira da Silva, J.A.; Yu, X. Advances in molecular biology of Paeonia L. Planta 2020, 251, 23. [Google Scholar] [CrossRef]
- Semagn, K.; Babu, R.; Hearne, S.; Olsen, M. Single nucleotide polymorphism genotyping using Kompetitive Allele Specific PCR (KASP): Overview of the technology and its application in crop improvement. Mol. Breed. 2014, 33, 1–14. [Google Scholar] [CrossRef]
- Cao, T.X.; Piao, X.C.; Wu, S.Q.; Wang, S.M.; Lian, M.L. Analysis of RAPD Fingerprint of Shoots and Its Vitrification Shoots in vitro of Gypsophila paniculata L. Plant Physiol. Commun. 2007, 43, 288–290. [Google Scholar]
- Calistri, E.; Buiatti, M.; Bogani, P. Characterization of Gypsophila species and commercial hybrids with nuclear whole-genome and cytoplasmic molecular markers. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2016, 150, 11–21. [Google Scholar]
- Li, M.; Wen, Z.; Meng, J.; Cheng, T.; Zhang, Q.; Sun, L. The genomics of ornamental plants: Current status and opportunities. Ornam. Plant Res. 2022, 2, 6. [Google Scholar] [CrossRef]
- Rouet, C.; O’Neill, J.; Banks, T.; Tanino, K.; Derivry, E.; Somers, D.; Lee, E.A. Mapping Winterhardiness in Garden Roses. J. Am. Soc. Hortic. Sci. 2022, 147, 216–238. [Google Scholar] [CrossRef]
- Mieulet, D.; Aubert, G.; Bres, C.; Klein, A.; Droc, G.; Vieille, E.; Rond-Coissieux, C.; Sanchez, M.; Dalmais, M.; Mauxion, J.-P.; et al. Unleashing meiotic crossovers in crops. Nat. Plants 2018, 4, 1010–1016. [Google Scholar] [CrossRef]
- Li, F. Meiotic Recombination Suppressors of Arabidopsis Thaliana. Ph.D. Thesis, Ghent University, Ghent, Belgium, 2018. [Google Scholar]
- De Maagd, R.A.; Loonen, A.E.H.M.; Chouaref, J.; Pele, A.; Meijerdekens, F.; Fransz, P.; Bai, Y. CRISPR/Cas inactivation of RECQ4 increases homeologous crossovers in an interspecific tomato hybrid. Plant Biotechnol. J. 2020, 18, 805–813. [Google Scholar] [CrossRef]
- Song, X.; Xu, Y.; Gao, K.; Fan, G.; Zhang, F.; Deng, C.; Dai, S.; Huang, H.; Xin, H.; Li, Y. High-density genetic map construction and identification of loci controlling flower-type traits in Chrysanthemum (Chrysanthemum × morifolium Ramat.). Hortic. Res. 2020, 7, 108. [Google Scholar] [CrossRef] [PubMed]
- Rouet, C.; Lee, E.A.; Banks, T.; O’Neill, J.; LeBlanc, R.; Somers, D.J. Identification of a polymorphism within the Rosa multiflora muRdr1A gene linked to resistance to multiple races of Diplocarpon rosae W. in tetraploid garden roses (Rosa × hybrida). Theor. Appl. Genet. 2020, 133, 103–117. [Google Scholar] [CrossRef] [PubMed]
- Saint-Oyant, L.H.; Ruttink, T.; Hamama, L.; Kirov, I.; Lakhwani, D.; Zhou, N.-N.; Bourke, P.; Daccord, N.; Leus, L.; Schulz, D. A high-quality genome sequence of Rosa chinensis to elucidate ornamental traits. Nat. Plants 2018, 4, 473–484. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, X.; Lin, S.; Yang, S.; Yan, X.; Bendahmane, M.; Bao, M.; Fu, X. Mapping a double flower phenotype-associated gene DcAP2L in Dianthus chinensis. J. Exp. Bot. 2020, 71, 1915–1927. [Google Scholar] [CrossRef] [PubMed]
- Gull, S.; Haider, Z.; Gu, H.; Raza Khan, R.A.; Miao, J.; Wenchen, T.; Uddin, S.; Ahmad, I.; Liang, G. InDel marker based estimation of multi-gene allele contribution and genetic variations for grain size and weight in rice (Oryza sativa L.). Int. J. Mol. Sci. 2019, 20, 4824. [Google Scholar] [CrossRef]
- Li, F.; Gao, Y.; Jin, C.; Wen, X.; Geng, H.; Cheng, Y.; Qu, H.; Liu, X.; Feng, S.; Zhang, F.; et al. The chromosome-level genome of Gypsophila paniculata reveals the molecular mechanism of floral development and ethylene insensitivity. Hortic. Res. 2022, 9, uhac176. [Google Scholar] [CrossRef]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. The sequence alignment/map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef] [PubMed]
- McKenna, A.; Hanna, M.; Banks, E.; Sivachenko, A.; Cibulskis, K.; Kernytsky, A.; Garimella, K.; Altshuler, D.; Gabriel, S.; Daly, M. The Genome Analysis Toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010, 20, 1297–1303. [Google Scholar] [CrossRef]
- Abyzov, A.; Urban, A.E.; Snyder, M.; Gerstein, M. CNVnator: An approach to discover, genotype, and characterize typical and atypical CNVs from family and population genome sequencing. Genome Res. 2011, 21, 974–984. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Schulz-Trieglaff, O.; Shaw, R.; Barnes, B.; Schlesinger, F.; Källberg, M.; Cox, A.J.; Kruglyak, S.; Saunders, C.T. Manta: Rapid detection of structural variants and indels for germline and cancer sequencing applications. Bioinformatics 2016, 32, 1220–1222. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Li, M.; Hakonarson, H. ANNOVAR: Functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010, 38, e164. [Google Scholar] [CrossRef] [PubMed]
- Krzywinski, M.; Schein, J.; Birol, I.; Connors, J.; Gascoyne, R.; Horsman, D.; Jones, S.J.; Marra, M.A. Circos: An information aesthetic for comparative genomics. Genome Res. 2009, 19, 1639–1645. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Cheng, Y.; Zhao, X.; Yu, R.; Li, H.; Wang, L.; Li, S.; Shan, Q. Haploid induction via unpollinated ovule culture in Gerbera hybrida. Sci. Rep. 2020, 10, 1702. [Google Scholar] [CrossRef] [PubMed]
- Peters, J.L.; Cnudde, F.; Gerats, T. Forward genetics and map-based cloning approaches. Trends Plant Sci. 2003, 8, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Van Huylenbroeck, J. Ornamental Crops; Springer: Amsterdam, The Netherlands, 2018; Volume 11. [Google Scholar]
- Hou, X.; Li, L.; Peng, Z.; Wei, B.; Tang, S.; Ding, M.; Liu, J.; Zhang, F.; Zhao, Y.; Gu, H. A platform of high-density INDEL/CAPS markers for map-based cloning in Arabidopsis. Plant J. 2010, 63, 880–888. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Su, L.; Hu, S.; Xue, J.-Y.; Liu, H.; Liu, G.; Jiang, Y.; Du, J.; Qiao, Y.; Fan, Y.; et al. A chromosome-level genome assembly of rugged rose (Rosa rugosa) provides insights into its evolution, ecology, and floral characteristics. Hortic. Res. 2021, 8, 141. [Google Scholar] [CrossRef]
- Liang, Y.; Li, F.; Gao, Q.; Jin, C.; Dong, L.; Wang, Q.; Xu, M.; Sun, F.; Bi, B.; Zhao, P.; et al. The genome of Eustoma grandiflorum reveals the whole-genome triplication event contributing to ornamental traits in cultivated lisianthus. Plant Biotechnol. J. 2022, 20, 1856–1858. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Li, P.; Li, L.; Zhang, Q. Research advances in and prospects of ornamental plant genomics. Hortic. Res. 2021, 8, 65. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L. Advance of Horticultural Plant Genomes. Hortic. Plant J. 2019, 5, 229–230. [Google Scholar] [CrossRef]
- Feng, J.; Zhu, H.; Zhang, M.; Zhang, X.; Guo, L.; Qi, T.; Tang, H.; Wang, H.; Qiao, X.; Zhang, B. Development and utilization of an InDel marker linked to the fertility restorer genes of CMS-D8 and CMS-D2 in cotton. Mol. Biol. Rep. 2020, 47, 1275–1282. [Google Scholar] [CrossRef]
- Hechanova, S.L.; Bhattarai, K.; Simon, E.V.; Clave, G.; Karunarathne, P.; Ahn, E.-K.; Li, C.-P.; Lee, J.-S.; Kohli, A.; Hamilton, N. Development of a genome-wide InDel marker set for allele discrimination between rice (Oryza sativa) and the other seven AA-genome Oryza species. Sci. Rep. 2021, 11, 8962. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Wang, Y.; Zhai, W.; Deng, J.; Wang, H.; Cui, Y.; Cheng, F.; Wang, X.; Wu, J. Development of InDel markers for Brassica rapa based on whole-genome re-sequencing. Theor. Appl. Genet. 2013, 126, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Sohn, H.-B.; Kim, S.-J.; Hong, S.-Y.; Park, S.-G.; Oh, D.-H.; Lee, S.; Nam, H.Y.; Nam, J.H.; Kim, Y.-H. Development of 50 InDel-based barcode system for genetic identification of tartary buckwheat resources. PLoS ONE 2021, 16, e0250786. [Google Scholar] [CrossRef]
- Yang, Z.; Dai, Z.; Xie, D.; Chen, J.; Tang, Q.; Cheng, C.; Xu, Y.; Wang, T.; Su, J. Development of an InDel polymorphism database for jute via comparative transcriptome analysis. Genome 2018, 61, 323–327. [Google Scholar] [CrossRef]
- Islam, M.R.; Hossain, M.R.; Jesse, D.M.I.; Jung, H.-J.; Kim, H.-T.; Park, J.-I.; Nou, I.-S. Development of molecular marker linked with bacterial fruit blotch resistance in melon (Cucumis melo L.). Genes 2020, 11, 220. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Roorkiwal, M.; Kale, S.; Garg, V.; Yadala, R.; Varshney, R.K. InDel markers: An extended marker resource for molecular breeding in chickpea. PLoS ONE 2019, 14, e0213999. [Google Scholar] [CrossRef] [PubMed]
- Adedze, Y.M.N.; Lu, X.; Xia, Y.; Sun, Q.; Nchongboh, C.G.; Alam, M.; Liu, M.; Yang, X.; Zhang, W.; Deng, Z. Agarose-resolvable InDel markers based on whole genome re-sequencing in cucumber. Sci. Rep. 2021, 11, 3872. [Google Scholar] [CrossRef] [PubMed]
- Guo, G.; Zhang, G.L.; Pan, B.G.; Diao, W.P.; Liu, J.B.; Ge, W.; Gao, C.Z.; Zhang, Y.; Jiang, C.; Wang, S.B. Development and application of InDel markers for Capsicum spp. based on whole-genome re-sequencing. Scientific reports. Sci. Rep. 2019, 9, 3691. [Google Scholar]
- Chen, R.; Chang, L.C.; Cai, X.; Wu, J.; Liang, J.L.; Lin, R.M.; Song, Y.; Wang, X.W. Development of InDel markers for Brassica rapa based on a high-resolution melting curve. Hortic. Plant J. 2021, 7, 31–37. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).