Abstract
Sulphurous acid derived from sulfur dioxide (SO2) emission leads to the pollution of irrigation water and the inhibition of plant growth. The safe concentration threshold of NaHSO3 in plants should be clarified to promote agricultural production. In this study, Orychophragmus violaceus seedlings were used as experimental materials and five NaHSO3 concentrations (i.e., 0, 1, 2, 5, 10 mmol·L−1) were simultaneously sprayed on the leaf surface of different seedlings separately. Leaf physiology responses under different concentrations were analyzed. The NaHSO3 did not promote photosynthesis in O. violaceus under the 1 and 2 mmol·L−1 treatments. It was conducive to the net photosynthetic rate (PN), photorespiration rate (Rp), chlorophyll content, actual photochemical quantum yield (YII) and photochemical quenching (qP) under the 5 mmol·L−1 treatment. However, quantum yield of regulated energy dissipation (YNPQ) and nonphotochemical quenching (NPQ) were inhibited. Under the 10 mmol·L−1 treatment, PN, chlorophyll content, YII, qP, dark respiration rate (Rd) and electron transport rate (ETR) showed significant decreases, while the photorespiration portion (Sp) significantly increased. Our results demonstrated that NaHSO3 provided a sulfur source for plant growth and interfered with the redox reaction of the plant itself, and its role as a photorespiratory inhibitor might be masked.
1. Introduction
Orychophragmus violaceus is a member of the family Brassicaceae that is widely used for beautifying the city and ecological restoration [1]. O. violaceus is also a healthy seasonal vegetable that can be eaten year round and is widely distributed, especially in Yunnan, Guizhou and other southern cities [2]. The plant species has high economic and ornamental value. Sulfur dioxide (SO2) is a widely diffused air pollutant, which is easily dissolved in the water of rivers or lakes and which forms sulfite and sulfuric acid. If the water source polluted by SO2 is used for irrigation or spraying on greening plants, it may not be conducive to the plants’ growth. Studies have shown that the toxicity of SO2 to plants was mainly attributed to the highly active intermediate bisulfite [3]. Katainen et al. has also reported that the treatment of sphagnum moss with 0.1 mmol·L−1 of H2SO3 increased the net photosynthetic rate [4]. Therefore, HSO3- may have a two-way effect on the photosynthesis and growth of plants when it is used for irrigation.
NaHSO3 is one of the most commonly used sulfites, which can be used as a photosynthetic accelerator in agricultural production [5,6,7,8]. However, the effect of NaHSO3 on the photosynthetic growth of plants depends on its concentration. Studies have shown that 0.5 mmol·L−1 of NaHSO3 is the best concentration to promote the photosynthetic oxygen release of Anabaena, while 1 mmol·L−1 of NaHSO3 can increase the net photosynthetic rate of Satsuma mandarin by approximately 15% [9,10]. In general, low concentrations of NaHSO3 (<1 mmol·L−1) can significantly improve the photosynthetic oxygen release rate and dry matter accumulation of algae and other lower plants [6,10,11], while most higher plants after low concentrations of NaHSO3 (<8 mmol·L−1) spraying can significantly enhance the photosynthetic carbon assimilation ability [7,8,9,12]. Bisulfite can represent a sulfur source for plants. Botryococcus braunii reportedly stopped growing after surviving for 12 days in a sulfur-free medium, but grew well under a bisulfite treatment of 0.1 or 0.8 mmol·L−1 [6]. However, the promotion of plant growth by the addition of low concentrations of NaHSO3 is not just attributed to the supply of sulfur nutrients. At present, the effect of NaHSO3 on the photorespiration of plants is still controversial. Kang et al. [7] demonstrated that 5 mmol·L−1 of NaHSO3 inhibited the photorespiration rate of Caragana korshinskii, and the content of glyoxylic acid decreased significantly. However, Chen et al. [8] found that photosynthetic and photorespiration rates increased simultaneously after soybean leaves were treated with 5 mmol·L−1 of NaHSO3. Under normal conditions, photorespiration consumes approximately a quarter of the total output of photosynthesis and the portion of photorespiration will increase when the atmospheric carbon dioxide significantly affects the stomata [13]. In recent years, studies on the effects of foliar sprays of NaHSO3 on plants have mainly focused on the response of the photorespiration rate to NaHSO3 [7,8], whereas the proportion of photorespiration in total photosynthesis has not yet been reported. Therefore, variations in the portion of photorespiration must be determined when studying the photosynthetic physiological mechanism of NaHSO3 in plants. In addition, high concentrations of NaHSO3 (>8 mmol·L−1) can cause certain toxicity to the photosynthetic physiology of plants. Ten mmol·L−1 of NaHSO3 significantly decreased the net photosynthetic rate of strawberry leaves [9]. The photosynthetic electron transport of pea leaves was inhibited by high concentrations of sulfite [14]. It is interesting to note that NaHSO3 is a chemical compound with both oxidizing and reducing properties. Sulfite in plants can be reduced to sulfide by sulfite reductase or oxidized to sulfate by sulfite oxidase [15]. During photosynthesis, plants produce and accumulate different forms of reactive oxygen species (i.e., ROS) and reducing agents (i.e., ascorbic acid, thioredoxin and reduced glutathione), which are important regulators of photosynthesis-related gene expression [16]. Wei et al. showed that HSO3− could react with superoxide anion to form SO42− [17]. However, it has also been reported that NaHSO3 oxidation destroys the structure of algae cell membranes [18]. When NaHSO3, which has both oxidation and reduction properties, enters the plant, the normal redox reaction will be disturbed and indirectly affect photosynthesis. However, few reports have focused on the regulation of plant redox by NaHSO3.
O. violaceus was used as experimental material in this study, the mechanisms of different concentrations of NaHSO3 on photosynthesis were investigated, the safe concentration threshold of NaHSO3 in plant leaves was clarified and the theoretical basis for promoting agricultural production and reducing agricultural ecological environment pollution could be provided.
2. Materials and Methods
2.1. Plant Culture and Treatment
The experiment was carried out in the Key Laboratory of Modern Agricultural Equipment and Technology of the Ministry of Education, College of Agricultural Engineering, Jiangsu University (N 32°11′ and E 119°27′). The seeds of O. violaceus were placed on wet gauze and germinated in a light incubator with a light intensity of 40 μmol·m−2·s−1. Water was sprayed every day to keep the gauze moist. The seeds were seeded in a 12-hole seedling tray with perlite and exposed to white light. Seedlings were cultivated in the tray with a small amount of 1/4-strength Hoagland solution until the 2 leaf stage. The culture conditions were as follows: photoperiod of 12 h, CO2 concentration of 390 ± 10 μmol·mol−1, relative humidity of air of 60 ± 5%, day/night cycle temperature of 28 °C/20 °C and light intensity of 280 ± 20 μmol·m−2·s−1.
After 45 days of growth, the leaves of different seedlings were sprayed with 0 (CK), 1 (NS1), 2 (NS2), 5 (NS3) and 10 (NS4) mmol·L−1 of NaHSO3 solutions. The spraying was conducted from 9:00 to 10:00 in the morning. The 50 mL NaHSO3 solution was sprayed on plants in each pot every 5 days, and the seedlings were sprayed 5 cm from the top in all directions. During the treatment period, the leaves of the seedlings were sprayed every 5 days for a total of 5 times, and the experiment was carried out 25 days after the spray treatment.
2.2. Gas Exchange Measurements
The third fully expanded leaves from the top were chosen for the gas exchange measurement at 9:00–12:00 a.m. on a sunny day. A portable LI-6400XT photosynthesis measurement system (LI-COR Inc., Lincoln, NE, USA) was used. The flow rate was set to 500 μmol·s−1, and the leaf temperature was 30 ± 2 °C. The net photosynthetic rate (PN), stomatal conductance (gs), intercellular carbon dioxide (Ci), transpiration rate (E) and other photosynthetic parameters were selected from the two response curves under a light intensity of 800 μmol·s−1 and a CO2 concentration of 400 μmol·mol−1. The PN-PAR response curves were always fitted using the nonrectangular hyperbola equation [19], which is expressed as follows:
where PN is the net photosynthetic rate (μmol·m−2·s−1); I is the photosynthetically active radiation (μmol·m−2·s−1); (apparent quantum efficiency) is the initial slope of the PN-PAR curves (μmol·μmol−1); Amax is the net photosynthetic rate at light saturation (μmol·m−2·s−1); k is the curve representing the degree of curvature of the curve angle, the value of which is [0,1]; and Rd is the dark respiration rate (μmol·m−2·s−1). The atmospheric CO2 concentration during the measurement was 400 μmol·mol−1. For every measurement, the PAR was set at 800, 600, 400, 300, 250, 200, 150, 100, and 50 μmol·m−2·s−1. After those photosynthetic parameters were acquired, the light saturation point (LSP) and light compensation point (LCP) for the photosynthetic capacity were obtained.
The PN-Ci response curves were always fitted using the rectangular hyperbola equation [19], which is expressed as follows:
where PN is the net photosynthetic rate (μmol·m−2·s−1); CE (carboxylation efficiency) is the initial slope of the PN-PAR curves (mol·m−2·s−1); Ci is the intercellular CO2 concentration (μmol·mol−1); Bmax is the net photosynthetic rate at CO2 saturation (μmol·m−2·s−1); and Rt is the total respiratory rate (μmol·m−2·s−1). The photosynthetically active radiation during the measurement was 800 μmol·mol−1. For every measurement, the CO2 concentration was set at 1500, 1200, 1000, 800, 600, 400, 350, 300, 250, 200, 100, and 50 μmol·mol−1. After those photosynthetic parameters were acquired, the CO2 saturation point (CSP) and CO2 compensation point (CCP) for the photosynthetic capacity were obtained.
The plant photorespiration portion was calculated as follows [20]:
where the definitions of Rd and Rt are the same as those in Formulas (1) and (2); Rp was the photorespiration rate (μmol·m−2·s−1); PN and Pt are the net photosynthesis rate (μmol·m−2·s−1) and total photosynthetic rate (μmol·m−2·s−1) under specific CO2 concentrations and light intensities, respectively; and Sp is the photorespiratory portion.
2.3. Chlorophyll-A Fluorescence (ChlF) Measurement
The ChlF parameters were measured on the third fully expanded leaves from the top, which were the same leaves used for gas exchange measurements. Before the measurements, the leaves were dark-adapted for 30 min to ensure complete relaxation of all reaction centers. ChlF under dark adaptation was measured using a modulated chlorophyll fluorescence imaging system (IMAGING-PAM, Heinz Walz Gmbh) from 19:00 to 21:00. The minimum chlorophyll fluorescence (Fo) was determined using a measuring beam, whereas the maximum chlorophyll fluorescence (Fm) was recorded after a 0.8 s saturating light pulse (2800 μmol·m−2·s−1). Actinic light (340 μmol·m−2·s−1) was then applied for 3 min to drive photosynthesis. Maximum fluorescence in the light-saturated stage (F’m), basic fluorescence after induction (F’o) and fluorescence yield in the steady state (Fs) were determined. The actual photochemical quantum yield (YII) was calculated as (Fm’-F)/Fm’. The quantum yield of regulated energy dissipation (YNPQ) was calculated as 1-YII-1/(NPQ + 1 + qL(Fm/Fo − 1)). The quantum yield of nonregulated energy dissipation (YNO) was calculated as 1/(NPQ + 1 + qL(Fm/Fo1)). The photochemical quenching coefficient (qP) was calculated as (F’mFs)/(F’mF’o), while the nonphotochemical quenching coefficient (NPQ) was calculated as (FmF’m)/F’m = Fm/F’m1. Subsequently, the photosynthetic electron transport rate (ETR) was calculated as PAR × YII × 0.85 × 0.5, where 0.5 and 0.85 are the fractions of the excitation energy distributed to PSII and the fractional light absorbance, respectively, PAR is the photosynthetically active radiation, and PSII is photosystem II.
2.4. Chlorophyll and Carotene Content
The third fully expanded fresh leaves from the top were picked and immediately ground and extracted with 95% ethanol under dark conditions until the leaves turned white. The absorbance of chlorophyll a (Chl a), chlorophyll b (Chl b) and carotene was measured with a 7230 G spectrophotometer at 665 nm (OD665), 649 nm (OD649) and 470 nm (OD470), respectively. The corresponding chlorophyll concentration was calculated from the measured optical density values, and the chlorophyll content was determined by using the following formula [21].
where C is the chlorophyll concentration (mg·L−1); V is the the amount applied for the extraction (mL); A is the dilution ratio; W is the fresh weight of the sample (g).
2.5. Statistical Analysis
All measurements were based on 3 replicate plants. The statistical analysis included a 1-way analysis of variance (ANOVA), and significant differences between the means were tested using Duncan’s multiple range test at 95% confidence.
3. Results
3.1. Effects of Foliage Spraying of NaHSO3 on Gas Exchange of O. violaceus
The values of PN, gs and E in the NS3 treatment were significantly higher than those in the CK (Figure 1A,B,D). However, the values of PN, gs and E in the NS1, NS2, NS4 and CK treatments showed no significant difference. The values of PN, gs and E in the NS4 treatment were significantly lower than those in the NS3 treatment (Figure 1A,B).
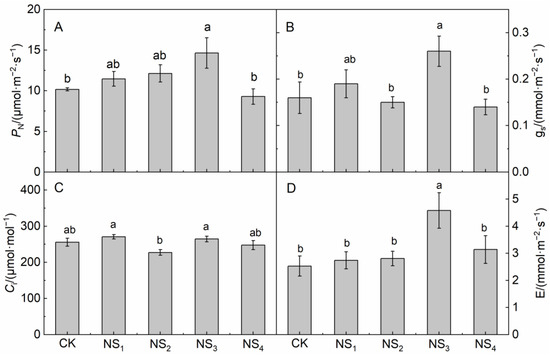
Figure 1.
Effects of foliage spraying of NaHSO3 on et photosynthetic rate (PN)(A), stomatal conductance (gs)(B), intercellular carbon dioxide concentration (Ci)(C), and transpiration rate(E)(D) of O. violaceus. Values are the means of five repetitions ± SE. Bars with different letters show significant differences at p < 0.05 (Duncan).
3.2. Responses of Net Photosynthetic Rate of O. violaceus to Photosynthetically Active Radiation (PAR) and Intracellular CO2 Concentration (Ci) under Foliage Spraying of NaHSO3
The correlation coefficients (R2) of the PN-PAR curve fitted by the nonrectangular hyperbolic model and the PN-Ci curve fitted by the rectangular hyperbolic model were all higher than 0.98, which indicated that the two models fit the curves mentioned above well.
Different concentrations of NaHSO3 affected the light response process differently. When the PAR was less than 200 μmol·m−2·s−1, the PN increased rapidly as the PAR increased, but significant differences were not observed between the values of PN in different treatments (Figure 2A). When the PAR was greater than 200 μmol·m−2·s−1, the PN value in the NS4 treatment increased more slowly than that in the CK as PAR increased. The PN value was significantly lower than that in the CK when the PAR reached the light saturation point (LSP). The foliar application of 10 mmol·L−1 of NaHSO3 decreased the LSP and inhibited the photosynthetic efficiency (Figure 2A). The PN in the NS3 treatment exhibited a clearer increase than that in the CK as the PAR increased. The foliar application of 5 mmol·L−1 of NaHSO3 promoted the photosynthetic capacity of O. violaceus (Figure 2A).
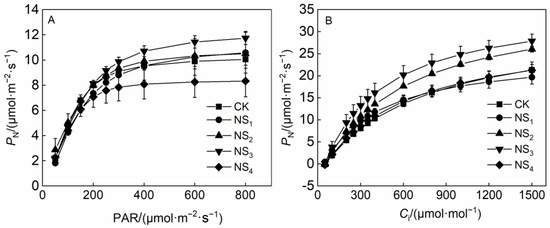
Figure 2.
Net photosynthetic rate (PN) −photosynthetically active radiation (PAR) curve (A) and net photosynthetic rate-intercellular carbon dioxide (Ci) curve (B) of O. violaceus under different concentrations of NaHSO3. Values are the means ± SE. Symbols with different letters show significant differences at p < 0.05 (Duncan).
Different concentrations of NaHSO3 affected the CO2 response process differently (Figure 2B). When the CO2 concentration was less than 400 μmol·mol−1, the values of PN clearly increased as the CO2 concentration increased but slowed down when the CO2 concentration was greater than 600 μmol·mol−1 (Figure 2B). The values of PN in the NS2 and NS3 treatments were higher than those in the CK, and the PN value in the NS3 treatment was the highest. The values of PN in the NS1 and NS4 treatments exhibited no significant difference compared to those in the CK under different CO2 concentrations (Figure 2B). The photosynthetic capacity of O. violaceus was the highest under the foliar application of 5 mmol·L−1 of NaHSO3, which was the optimal concentration.
Significant differences were not observed between the values of Amax and Bmax in all treatments (Table 1). The apparent quantum efficiency (α) is an important index that reflects the light energy utilization rate of plants [22]. The light compensation point (LCP) reflects the ability of plants to overcome their own assimilation resistance. The lower the LCP, the less the consumption of photosynthetic products and the stronger the ability to use low light intensity [23]. In this study, the values of α and LCP in the NS4 treatment decreased by 20.97% and 76.08% of those in the CK, respectively (Table 1). The O. violaceus treated with 10 mmol·L−1 of NaHSO3 showed improvement in the ability to use weak light and lower consumption of photosynthetic products to resist the stress of high concentrations of sulfite. The initial carboxylation efficiency (CE) can reflect the activity of ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) and the ability of plants to utilize CO2 [24]. In this study, the value of CE in the NS3 treatment increased by 105.77% relative to that in the CK, and the values in the other treatments exhibited no significant difference compared to those in the CK (Table 1).

Table 1.
Photosynthetic parameters under different concentrations of NaHSO3.
As the NaHSO3 concentration increased, the values of the total respiratory rate (Rt) initially increased and then decreased in the NS4 treatment and the value of Rt in the NS3 treatment increased by 80.63% compared with that in the CK (Figure 3B). The values of the photorespiration rate (Rp) and Rt in each treatment showed the same change trends as follows: NS3 > NS2 > NS4 > NS1 > CK (Figure 3A,B). The values of the photorespiration rate (Rd) gradually decreased as the NaHSO3 concentration increased, and the value in the NS4 treatment decreased by 85.56% of that in the CK (Figure 3A). The values of the photorespiratory portion (Sp) gradually increased, and the value in the NS4 treatment increased by 53.85% of that in the CK (Figure 3A).
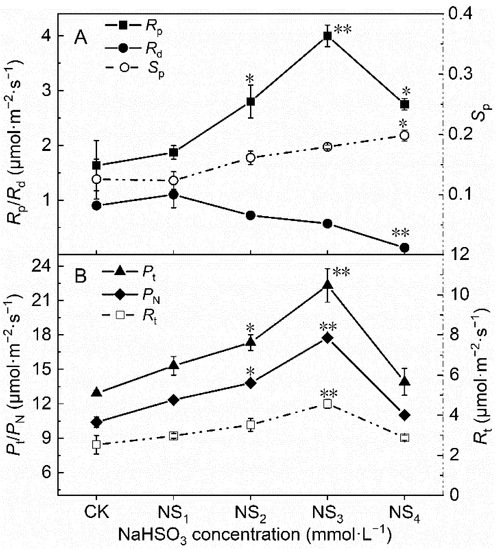
Figure 3.
Photorespiration related parameters ((A): Rp, Rd and Sp; (B):Pt, PN and Rt) under different concentrations of NaHSO3. Significant differences between the control and treatment groups are indicated by asterisks (* p < 0.05, ** p < 0.01). Rd: the dark respiration rate; Rp: the photorespiration rate; Sp: the photorespiratory portion; PN: the net photosynthetic rate; Pt: the total photosynthetic rate; Rt: the total respiratory rate.
3.3. Effects of Foliage Spraying of NaHSO3 on Chlorophyll Content in Leaves of O. violaceus
Chlorophyll is a necessary molecule for the photosynthesis of plants. The NS1, NS2 and NS3 treatments promoted the synthesis of chlorophyll a and b in O. violaceus (Table 2). The chlorophyll b contents in the NS4 treatment had no significant difference compared with those in the CK (Table 2). The chlorophyll a, chlorophyll b and total chlorophyll contents in the NS4 treatment were slightly lower than those in the NS1, NS2 and NS3 treatments (Table 2). The chlorophyll a/b in each treatment had no significant difference compared with that in the CK (Table 2). The NaHSO3 promoted the synthesis of chlorophyll in O. violaceus as a synchronous change of chlorophyll a and chlorophyll b. There was no significant difference in carotenoids between the CK and other treatments (Table 2).

Table 2.
Effects of foliage spraying of NaHSO3 on chlorophyll content in O. violaceus leaves.
3.4. Effect of Foliage Spraying of NaHSO3 on Chlorophyll a Fluorescence Parameters of O. violaceus Leaves
The light energy absorbed by the PSII reaction center is mainly distributed into three parts: photochemical pathway (YII), energy used for photoprotection mechanism (YNPQ) and other nonphotochemical energy (YNO) and YII + YNPQ + YNO = 1 [25]. With increasing NaHSO3 concentrations, the value of YII gradually increased. The value in the NS3 treatment increased by 17.95% of that in the CK, and thereafter, a decreasing trend was observed (Table 3). However, YNPQ is the opposite of YII and showed an initial decrease and then an increase. In the NS3 treatment, YNPQ decreased by 16.28% relative to that in the CK, although the value of YNO in each treatment had no significant difference compared with that in the CK (Table 3). Photochemical quenching (qP) and nonphotochemical quenching (NPQ) are two forms of energy dissipation in chloroplasts [26]. qP is the part of light energy used for photochemical electron transfer, which reflects the utilization of light energy to a certain extent, while NPQ is the part where excess light energy is dissipated in the form of heat energy [26]. The values of NPQ in the NS2 and NS3 treatments decreased by 26.09% and 17.39% of those in the CK, respectively, while the values of qP in NS2 and NS3 increased by 18.57% and 14.29% of those in the CK, respectively (Table 3). The values of NPQ and qP in the NS4 treatment exhibited no significant difference compared to those in the CK. The reduction in the photochemical reaction in the NS4 treatment might be due to the excessive NaHSO3 stress on O. violaceus, which would offset the appropriate amount of NaHSO3 to promote the photochemical pathway. The apparent photosynthetic electron transport rate (ETR) mainly reflects the electron transport in the PS II reflection center [27]. The value of ETR in the NS4 treatment decreased by 18.90% of that in the CK, while the values in other treatments showed no significant difference compared with those in the CK.

Table 3.
Effects of foliage spraying of NaHSO3 on chlorophyll a fluorescence parameters in O. violaceus leaves.
4. Discussion
Sulfur is an essential mineral element for plants, and it is fourth in the list of major plant nutrients after nitrogen, phosphorus and potassium [28]. Higher plants mainly uptake inorganic sulfate from the soil by their roots, and they can also absorb the atmospheric SO2 and exogenous HSO3−, SO3− and S2− through leaf stomata. During the process of sulfur metabolism, exogenous sulfur is first converted into the form of sulfate (SO42−), which can be absorbed by plants. After activation and reduction, sulfite (SO32−) can be produced, which has potential cytotoxicity [29]. Many metabolic pathways of SO32− are observed in plants, and their metabolites are closely related to chlorophyll synthesis. First, SO32− is reduced to sulfide (S2−) under the action of sulfite reductase and S2− reacts with acetylserine (OAS) to form cysteine (Cys) [30]. As the precursor of sulfur-containing amino acids, Cys is further synthesized into various sulfur-containing proteins, thus guaranteeing the early synthesis of chlorophyll; then, SO32− in chloroplasts could enter the thiolipid reduction pathway to synthesize sulfoquinovosyldiacylglycerol (SQDG) through two consecutive steps. SQDG is a sulfur-containing nonphosphorus glycerolipid that participates in the formation of the granum lamellae of chloroplasts, and its content is positively correlated with the chlorophyll concentration in the process of chloroplast dedifferentiation and regeneration [31]. Although sulfur is not the main component of chlorophyll, it obviously affects the synthesis of chlorophyll. It is noteworthy that the variation of chlorophyll content will directly affect the absorption, transformation and utilization of light energy by plants [32]. Ribulose-1,5-bisphosphate (RuBP) carboxylase/oxygenase (Rubisco) catalyzes the first step of the reaction of CO2 assimilation and photorespiration carbon oxidation in photosynthesis and is considered the main factor controlling the rate of photosynthesis. To ensure its catalytic ability, Rubisco must be activated by Rubisco activase (RCA). Studies have found that NaHSO3 could promote the expression of RCA genes at the transcription and translation levels, thus enhancing the initial activity of Rubisco in plants [33]. RCA activity was sensitive to the ATP/ADP ratio observed in the chloroplast matrix, and the activation of RCA depended on the hydrolysis of ATP and was inhibited by ADP [34]. Wang et al. [35] reported that 1 mmol·L−1 of NaHSO3 acted similarly as phenazine methyl sulfate (PMS), a cofactor that catalyzed cyclic photophosphorylation, by promoting photophosphorylation and increasing the ATP supply, thereby maintaining high levels of photosynthesis. Moreover, 5 mmol·L−1 of NaHSO3, as a sulfur source absorbed and utilized by plants, may play an active role in O. violaceus. On the one hand, the increase in HSO3- in the leaves accelerated the metabolism of sulfate, and its metabolites directly or indirectly promoted the increase in chlorophyll content, which was conducive to the absorption of light energy by the treated leaves, which was consistent with the results of Li et al. [36]. Meanwhile, the ratio of light energy to the photochemical pathway and light protection mechanism was adjusted. As a result, more light energy was allocated to the photochemical pathway (YII increased significantly, while YNPQ decreased significantly), the light energy utilization rate increased and the final photosynthetic rate increased. On the other hand, NaHSO3 may increase the expression of RCA genes by promoting photophosphorylation and increasing the supply of ATP, thus increasing the initial activity of Rubisco, which can catalyze the two reactions of RuBP carboxylation (photosynthesis) and oxidation (photorespiration) simultaneously, and the photosynthetic rate and photorespiration rate increase synchronously.
Photorespiration is a process in which plants fix oxygen and release CO2 under light conditions. Photorespiration can alleviate photoinhibition, eliminate toxic intermediate products and provide raw materials for other metabolic activities, and it plays an active role in photosynthesis [37]. Studies have suggested that the activities of RuBP carboxylase and RuBP oxygenase in high-yield genotype wheat were higher than those in low-yield genotype wheat. High photosynthesis and photorespiration intensities are important preconditions for ensuring high wheat yield [38]. The electron transport rate of PSII and ATP production increased when a low concentration of NaHSO3 was sprayed on citrus leaves, which decreased photoinhibition and thereby increased the net photosynthetic rate [12]. Foliage sprayed with an appropriate concentration of NaHSO3 increased the total photosynthetic and photorespiration rates, which was consistent with the results reported in the studies mentioned above. Photorespiration consumed excess light energy and protected the photosynthetic apparatus when the consumption ratio of photorespiration to photosynthate was maintained, which indirectly maintained photosynthesis. However, photorespiration consumes photosynthetic products without producing ATP, and it has also been considered a negative factor in photosynthesis. Stomata are important channels for CO2 and water exchange between plants and the environment [39]. In this study, the stomatal conductance of O. violaceus leaves in the NS4 treatment decreased, which may be due to the stress caused by higher concentrations of NaHSO3. To respond to the deficiency of water and CO2 caused by the decrease in stomatal conductance, the gene expression of carbonic anhydrase (CA) in leaves is upregulated, which catalyzes the conversion of intracellular HCO3− into H2O and CO2 [40]. The contents of chlorophyll a and b, the electron transport rate and the photosynthetic rate of soybean all reportedly increased when the leaves were sprayed with an appropriate concentration of HCO3− [41]. This demonstrated that the effect of HCO3− on plants was similar to that of HSO3− in this study. One possible hypothesis was that competition may occur between HCO3− and HSO3− in the process of photosynthesis due to their similar structure. Under high-concentration NaHSO3 treatment, excessive HSO3− accumulated in the cell sap to compete with HCO3− for the active site of CA, thereby hindering the combination of HCO3− and CA. Plants could not offset the deficiency of H2O and CO2 in their leaves by converting intracellular HCO3− when stomatal conductance decreased. The carboxylation of RuBP was inhibited, and the total photosynthetic rate decreased. In addition, O. violaceus would suffer from stress when they were sprayed with high concentrations of NaHSO3 (the reason for stress will be explained later). A high photorespiration rate and Sp in plants had a protective effect against photosynthetic apparatus damage in response to stress conditions, while a high proportion of photorespiration would also consume photosynthates and therefore decrease the net photosynthetic rate.
Among sulfites, the valence of sulfur is +4, which is both reducing and oxidizing. Sulfite dissolved in water can not only obtain electrons to form sulfur precipitates but also lose electrons to form sulfates: SO32− + 3H2O + 4e− ⇌ S + 6OH− E = −0.66; SO32− + H2O − 2e− ⇌ SO42− + 2H+ E = +0.2. Sulfite in plants has both reduction and oxidation properties, and it has dual effects on plant photosynthesis due to its concentration, which is protective or inhibitory. As a nucleophilic substance, sulfite can attack diverse substrates by splitting the disulfide bonds into peptides and cause inactivation of these compounds, which is called sulfitolysis. Sulfitolysis can lead to chlorophyll destruction, photosynthesis suppression, necrotic damage and growth retardation [42]. Therefore, if sulfite accumulates in plants and cannot be metabolized rapidly, it will cause serious damage at the cellular and even the entire plant level [30]. Sulfate oxidase (SO) plays a vital role in relieving this toxicity, and it can serve as a ‘safety valve’ to detoxify excess amounts of sulfite and protect the cells from sulfitolysis [43]. Wei et al. [44] found that an appropriate amount of NaHSO3 could react with the superoxide anion produced by the PSI receptor of Chlamydomonas reinhardtii; as a result, an anaerobic environment was established, hydrogenase (H2ase) was activated and the hydrogen production capacity was significantly improved. Golan and Whitaker [45] also proved that NaHSO3 could be used as a reducing agent to inhibit the activity of mushroom polyphenol oxidase (PPO), thereby playing a certain role in preventing browning. Therefore, NaHSO3 had a certain degree of reducibility. At appropriate concentrations, it oxidized into sulfate to enter sulfate metabolism, and it detoxified or reacted with active oxygen to reduce the damage of strong oxidizing substances to cells. However, when the concentration of NaHSO3 increased to a certain extent, its oxidation led to adverse impacts on plants. Lüttge et al. [18] indicated that a certain concentration of bisulfite compounds interfered with membrane proteins and lipids, which impaired membrane integrity and inhibited photosynthetic CO2 fixation and ion transport processes. Lin et al. [46] reported that the active oxygen content in the leaves of rice seedlings increased significantly as the NaHSO3 concentration increased. Chlorophyll a fluorescence technology is often used to study photosynthesis under adversity [47,48]. In this study, the ETR and YII in the NS4 treatment decreased significantly compared to those in the NS3 treatment, while the YNPQ increased. The results demonstrated that the leaves of O. violaceus suffered from mild stress when they were sprayed with 10 mmol·L−1 of NaHSO3. Excessive HSO3− not only had oxidative properties but also induced the production of active oxygen. These strong oxidizing substances attacked the cell biofilm system of plants, injured the photosynthetic apparatus and even a variety of organelles and affected the processes of photosynthetic CO2 absorption and ion transport, thereby inhibiting the photosynthetic carbon assimilation and reducing the efficiency of photosynthetic electron transport. To avoid further damage to plants caused by excess light energy, plants need to convert part of the captured light energy into heat energy through a heat dissipation mechanism. Physiological activities, such as protein synthesis, nutrient absorption and transport were affected under stress, which reduced the dark respiration rate.
5. Conclusions
The 5 mmol·L−1 of NaHSO3 was the appropriate concentration, which promoted the photosynthetic capacity and increased production, while a concentration of 10 mmol·L−1 inhibited the photosynthesis and caused pollution of O. violaceus. Photorespiration had a certain protective effect on plants that suffered from stress, but an excessive photorespiration portion consumed photosynthates and decreased the net photosynthetic rate. 5 mmol·L−1 of NaHSO3 absorbed by plants could be considered a sulfur source. The results helped to better understand the dose effect of HSO3− on plant photosynthetic physiology, which provided a theoretical basis for the reasonable utilization of NaHSO3 and promotion of agricultural production.
Author Contributions
Conceptualization, Z.L. and Y.W.; methodology, Z.L., D.X. and Y.W.; validation, K.Z., J.X. and R.Y.; resources, Z.L.; data curation, T.C. and R.D.; writing—original draft preparation, Z.L.; writing—review and editing, D.X. and Y.W.; project administration, Z.L.; funding acquisition, Y.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by by the project of the National Key Research and Development Program of China [2016YFC0502602], the National Natural Science Foundation of China [No. U1612441], Support Plan Projects of Science and Technology Department of Guizhou Province [No. (2021)YB453].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets during or analyzed during the current study available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study.
References
- Zhang, Y.; Ji, H.B. Physiological responses and accumulation characteristics of turfgrasses exposed to potentially toxic elements. J. Environ. Manag. 2019, 246, 796–807. [Google Scholar] [CrossRef] [PubMed]
- Xing, D.K.; Wu, Y.Y.; Fu, W.G.; Li, Q.L.; Wu, Y.S. Regulated deficit irrigation scheduling of Orychophragmus violaceus based on photosynthetic physiological response traits. Trans. ASABE 2016, 59, 1853–1860. [Google Scholar]
- Bayat, L.; Askari, M.; Amini, F.; Zahedi, M. Effects of Rhizobium inoculation on Trifolium resupinatum antioxidant system under sulfur dioxide pollution. Biol. J. Microb. 2014, 2, 37–50. [Google Scholar]
- Katainen, H.S.; Mäkinen, E.; Jokinen, J.; Kellomäki, S. Effects of SO2 on the photosynthetic and respiration rates in scots pine seedlings. Environ. Pollut. 1987, 46, 241–251. [Google Scholar] [CrossRef]
- Tombuloglu, H.; Ablazov, A.; Filiz, E. Genome-wide analysis of response to low sulfur (LSU) genes in grass species and expression profiling of model grass species Brachypodium distachyon under S deficiency. Turk. J. Biol. 2016, 40, 934–943. [Google Scholar] [CrossRef]
- Yang, S.L.; Wang, J.; Cong, W.; Cai, Z.L.; Ouyang, F. Effects of bisulfite and sulfite on the microalga Botryococcus braunii. Enzym. Microb. Technol. 2004, 35, 46–50. [Google Scholar] [CrossRef]
- Kang, T.; Wu, H.D.; Lu, B.Y.; Luo, X.J.; Gong, C.M.; Bai, J. Low concentrations of glycine inhibit photorespiration and enhance the net rate of photosynthesis in Caragana korshinskii. Photosynthetica 2018, 56, 512–519. [Google Scholar] [CrossRef]
- Chen, G.K.; Wang, X.Y.; Kang, H.J.; Sun, J. Effect of different NaHSO3 concentrations on gas exchange and fluorescence parameters in beans and maize. J. Nucl. Agr. Sci. 2017, 31, 379–385. (In Chinese) [Google Scholar]
- Guo, Y.P.; Hu, M.J.; Zhou, H.F.; Zhang, L.C.; Su, J.H.; Wang, H.W.; Shen, Y.G. Different pathways are involved in the enhancement of photosynthetic rate by sodium bisulfite and benzyladenine, a case study with strawberry (Fragaria × Ananassa Duch) plants. Plant Growth Regul. 2006, 48, 65–72. [Google Scholar] [CrossRef]
- Wang, L.; Ming, C.; Wei, L.; Gao, F.; Lv, Z.; Wang, Q.; Ma, W. Treatment with moderate concentrations of NaHSO3 enhances photobiological H production in the cyanobacterium Anabaena sp. strain PCC 7120. Int. J. Hydrogen Energy 2010, 35, 12777–12783. [Google Scholar] [CrossRef]
- Wang, H.; Mi, H.; Ye, J.; Deng, Y.; Shen, Y. Low concentrations of NaHSO3 increase cyclic photophosphorylation and photosynthesis in cyanobacterium Synechocystis PCC 6803. Photosynth. Res. 2003, 75, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.P.; Hu, M.J.; Zhou, H.F.; Zhang, L.C.; Su, J.H.; Wang, H.W.; Shen, Y.G. Low concentrations of NaHSO3 increase photosynthesis, biomass, and attenuate photoinhibition in Satsuma mandarin (Citrus unshiu Marc.) plants. Photosynthetica 2006, 44, 333–337. [Google Scholar] [CrossRef]
- Busch, F.A. Photorespiration in the context of Rubisco biochemistry, CO2 diffusion and metabolism. Plant J. 2020, 101, 919–939. [Google Scholar] [CrossRef] [PubMed]
- Veeranjaneyulu, K.; Charlebois, D.; Soukpoé-Kossi, C.N.; Leblanc, R.M. Sulfite inhibition of photochemical activity of intact pea leaves. Photosynth. Res. 1992, 34, 271–278. [Google Scholar] [CrossRef]
- Galina, B.; Dmiry, Y.; Albert, B.; Vladislav, G.; Lnna, G.K.; Aaron, F.; Rachel, A.; Robert, F.; Moshe, S. Sulfite oxidase activity is essential for normal sulfur, nitrogen and carbon metabolism in tomato leaves. Plants 2015, 4, 573–605. [Google Scholar]
- Queval, G.; Foyer, C.H. Redox regulation of photosynthetic gene expression. Philos. Trans. R. Soc. B 2012, 367, 3475–3485. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Yi, J.; Wang, L.; Huang, T.; Gao, F.; Wang, Q.; Ma, W. Light intensity is important for hydrogen production in NaHSO3 treated Chlamydomonas reinhardtii. Plant Cell Physiol. 2017, 58, 451–457. [Google Scholar]
- Lüttge, U.; Osmond, C.B.; Ball, E.; Brinckmann, E.; Kinze, G. Bisulfite compounds as metabolic inhibitors: Nonspecific effects on membranes. Plant Cell Physiol. 1972, 13, 505–514. [Google Scholar]
- Ye, Z.P. A review on modeling of responses of photosynthesis to light and CO2. Chin. J. Plant Ecol. 2010, 34, 727–740. (In Chinese) [Google Scholar]
- Wu, Y.Y.; Rao, S.; Zhang, K.Y.; Lu, Y.; Zhao, L.H.; Liang, Z. A Quantitative Method for Determining the Portion of Photorespiratory Pathway in Plants. China Patent 2016105277715, 13 February 2018. [Google Scholar]
- Wang, J.; Lu, W.; Yu, T.; Yang, Q. Leaf morphology, photosynthetic performance, chlorophyll fluorescence, stomatal development of Lettuce (Lactuca sativa L.) exposed to different ratios of red light to blue light. Front. Plant Sci. 2016, 7, 250. [Google Scholar]
- Herrmann, H.; Schwartz, J.M.; Johnson, G.N. From empirical to theoretical models of light response curves—Linking photosynthetic and metabolic acclimation. Photosynth. Res. 2020, 145, 5–14. [Google Scholar] [CrossRef]
- Duan, M.; Yang, W.C.; Mao, X.M. Effects of water deficit on photosynthetic characteristics of spring wheat under plastic mulching and comparison of light response curve models. Trans. Chin. Soc. Agri. Mach. 2018, 49, 219–227. (In Chinese) [Google Scholar]
- Ren, B.; Li, J.; Tong, X.J.; Mei, Y.M.; Meng, P.; Zhang, J.S. Simulation on photosynthetic-CO2 response of quercus variabilis and Robinia pseudoacacia in the southern foot of the Taihang Mountain, China. Chin. J. Appl. Ecol. 2018, 29, 1–10. (In Chinese) [Google Scholar]
- Kramer, D.M.; Johnson, G.; Kiirats, O.; Edwards, G.E. New fluorescence parameters for the determination of QA redox state and excitation energy fluxes. Photosynth. Res. 2004, 79, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Zai, X.M.; Zhu, S.N.; Qin, P.; Wang, X.Y.; Luo, F.X. Effect of Glomus mosseae on chlorophyll content, chlorophyll fluorescence parameters, and chloroplast ultrastructure of beach plum (Prunus maritima) under NaCl stress. Photosynthetica 2012, 50, 323–328. [Google Scholar] [CrossRef]
- Hu, H.; Wang, L.H.; Wang, Q.Q.; Jiao, L.Y.; Hua, W.Q.; Zhou, Q.; Huang, X.H. Photosynthesis, chlorophyll fluorescence characteristics and chlorophyll content of soybean seedlings under combined stress of bisphenol A and cadmium. Environ. Toxicol. Chem. 2014, 33, 2455–2462. [Google Scholar] [CrossRef] [PubMed]
- Anjum, N.A.; Gill, R.; Kaushik, M.; Hasanuzzaman, M.; Pereira, E.; Tuteja, N.; Gill, S.S. ATP-sulfurylase, sulfur-compounds and plant stress tolerance. Front. Plant Sci. 2015, 6, 210. [Google Scholar] [CrossRef]
- Stanislav, K.; Mario, M.; Hideki, T. Sulfur nutrition: Impacts on plant development, metabolism, and stress responses. J. Exp. Bot. 2019, 70, 4069–4073. [Google Scholar]
- Brychkova, G.; Grishkevich, V.; Fluhr, R.; Sagi, M. An essential role for tomato sulfite oxidase and enzymes of the sulfite network in maintaining leaf sulfite homeostasis. Plant Physiol. 2013, 161, 148–164. [Google Scholar] [CrossRef]
- Krzysztof, Z. Encyclopedia of Lipidomics, 1st ed.; Springer: Dordrecht, The Netherlands, 2017; pp. 1–4. [Google Scholar]
- Masuda, T. Recent overview of the Mg branch of the tetrapyrrole biosynthesis leading to chlorophylls. Photosynth. Res. 2008, 96, 121–143. [Google Scholar] [CrossRef]
- Chen, Y.; Jin, J.H.; Jiang, Q.S.; Yu, C.L.; Chen, J.; Xu, L.G.; Jiang, D.A. Sodium bisulfite enhances photosynthesis in rice by inducing Rubisco activase gene expression. Photosynthetica 2014, 52, 475–478. [Google Scholar] [CrossRef]
- Portis, A.R. Rubisco activase—Rubisco’s catalytic chaperone. Photosynth. Res. 2003, 75, 11–27. [Google Scholar] [CrossRef]
- Wang, H.W.; Wei, J.M.; Shen, Y.G. Spraying low concentration sodium bisulfite can promote the photosynthetic phosphorylation and photosynthesis of wheat leaves. Sci. Bull. 2000, 45, 394–398. (In Chinese) [Google Scholar] [CrossRef]
- Li, J.; Liu, X.L.; Zhang, C.L.; Guan, C.Y.; Dai, L.L.; Zhang, Y.L.; Tan, L.T.; Ma, N.; Yuan, Z.J. Effects of NaHSO3 on photosynthetic characteristics and nitrogen metabolism of rapeseed seedlings. Chin. J. Oil Crop Sci. 2014, 36, 761–769. (In Chinese) [Google Scholar]
- Sunil, B.; Saini, D.; Bapatla, R.B.; Aswani, V.; Raghavendra, A.S. Photorespiration is complemented by cyclic electron flow and the alternative oxidase pathway to optimize photosynthesis and protect against abiotic stress. Photosynth. Res. 2019, 139, 67–69. [Google Scholar] [CrossRef]
- Aliyev, J.A. Photosynthesis, photorespiration and productivity of wheat and soybean genotypes. Physiol. Plant. 2012, 145, 369–383. [Google Scholar] [CrossRef] [PubMed]
- Matthew, H.; James, H.; Mcelwain, J.C. Differences in the response sensitivity of stomatal index to atmospheric CO2 among four genera of Cupressaceae conifers. Ann. Bot. Lond. 2010, 3, 411–418. [Google Scholar]
- Hu, H.; Boisson-Dernier, A.; Israelsson-Nordström, M.; Böhmer, M.; Xue, S.; Ries, A.; Godoski, J.; Kuhn, M.J.; Schroeder, I.J. Carbonic anhydrases are upstream regulators of CO2 controlled stomatal movements in guard cells. Nat. Cell Biol. 2010, 12, 87–93. [Google Scholar] [CrossRef]
- Hao, J.J.; Huang, C.H.; Lu, H.; Yu, Y. Influence of K+, Na+ and HCO3- on photosynthesis of soybean seedlings. Soybean Sci. 2012, 31, 436–439. (In Chinese) [Google Scholar]
- Yarmolinsky, D.; Brychkova, G.; Fluhr, R.; Sagi, M. Sulfite reductase protects plants against sulfite toxicity. Plant Physiol. 2013, 161, 725–743. [Google Scholar] [CrossRef]
- Hänsch, R.; Mendel, R.R. Sulfite oxidation in plant peroxisomes. Photosynth. Res. 2005, 86, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Li, X.; Fan, B.; Ran, Z.; Ma, W. A stepwise NaHSO3 addition mode greatly improves H2 photoproduction in Chlamydomonas reinhardtii. Front. Plant Sci. 2018, 9, 1532. [Google Scholar] [CrossRef] [PubMed]
- Golan, A.; Whitaker, J.R. Effect of ascorbic acid, sodium bisulfite, and thiol compounds on mushroom polyphenol oxidase. J. Agr. Food Chem. 1984, 32, 1003–1009. [Google Scholar] [CrossRef]
- Lin, Z.F.; Liu, N.; Chen, S.W.; Lin, G.Z.; Mo, H. Bisulfite (HSO3) hydroponics induced oxidative stress and its effect on nutrient element compositions in rice seedlings. Bot. Stud. 2011, 52, 173–181. [Google Scholar]
- Liu, X.; Li, M.L.; Li, J.M.; Su, C.L.; Lian, S.; Zhang, H.B.; Li, Y.X.; Ge, K.; Li, L. AhGLK1 affects chlorophyll biosynthesis and photosynthesis in peanut leaves during recovery from drought. Sci. Rep. 2018, 8, 139–158. [Google Scholar] [CrossRef]
- Ghassemi-Golezani, K.; Hosseinzadeh-Mahootchi, A.; Farhangi-Abriz, S. Chlorophyll a fluorescence of safflower affected by salt stress and hormonal treatments. SN Appl. Sci. 2020, 2, 121–158. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).