In Vitro Propagation and Genetic Stability Assessment Using the ISSR Markers of Stachys byzantina K. Koch, a Promising Ornamental Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Explant Disinfestation
2.2. Establishment of In Vitro Cultures
2.3. Shoot Multiplication
2.3.1. Effect of Growth Regulators
2.3.2. Effect of Nutrient Medium
2.4. Rooting of Microshoots
2.4.1. In Vitro Rooting
2.4.2. Ex Vitro Rooting
2.5. Acclimatization of Young Plantlets
2.6. Genetic Stability Assessment Using ISSR Markers
2.7. Statistical Analysis
3. Results and Discussion
3.1. Establishment of In Vitro Cultures
3.2. Shoot Multiplication
3.2.1. Effect of Growth Regulators
3.2.2. Effect of Nutrient Medium
3.3. Rooting of Microshoots
3.3.1. In Vitro Rooting
3.3.2. Ex Vitro Rooting
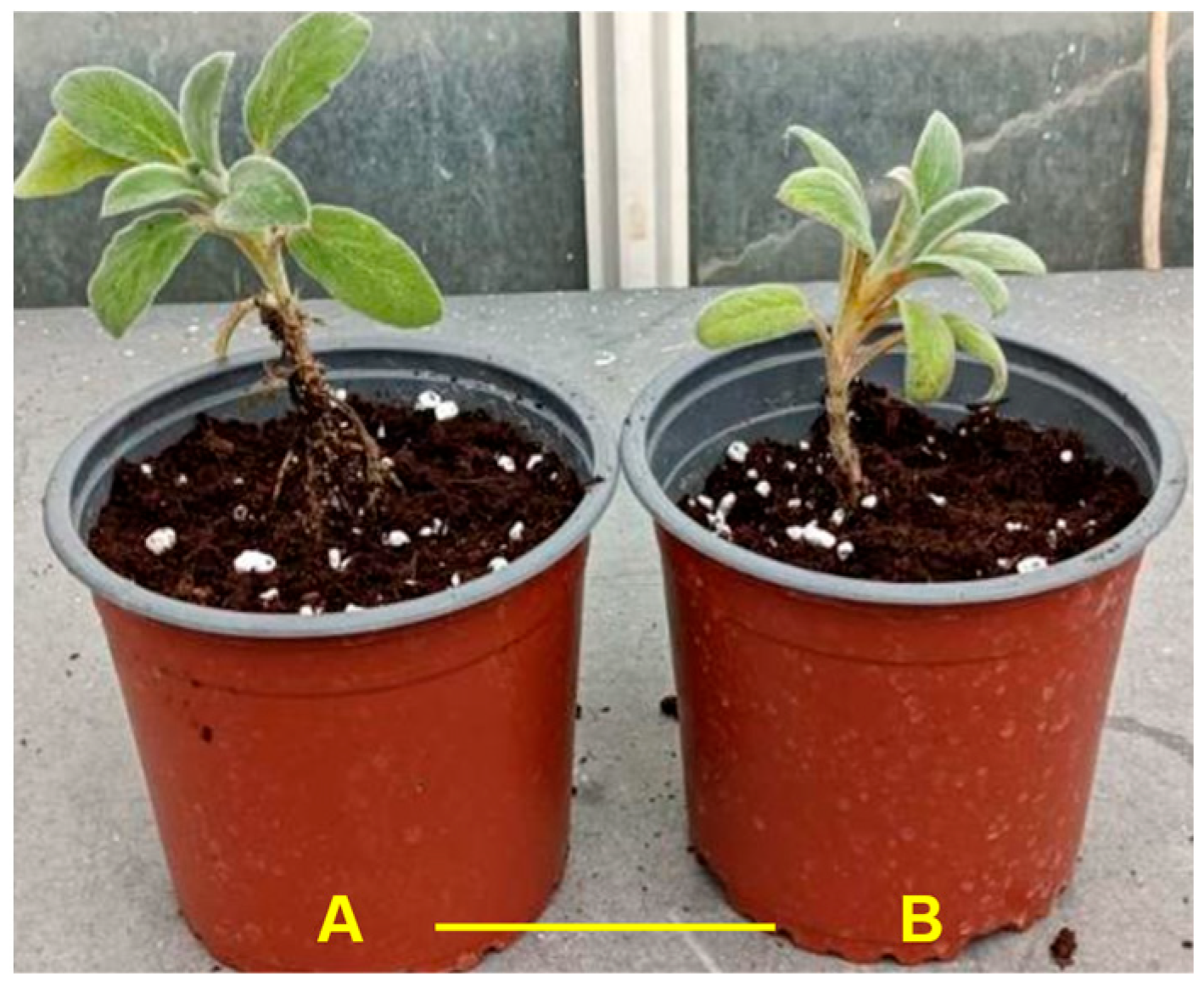
3.4. Acclimatization of Young Plantlets
3.5. Genetic Stability
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seaton, K.; Bettin, A.; Grüneberg, H. New ornamental plants for horticulture. In Horticulture: Plants for People and Places, Volume 1; Dixon, G.R., Aldous, D.E., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 435–463. ISBN 978-94-017-8577-8. [Google Scholar]
- Vascular Flora of Greece Web (Vascular Plants of Greece-An Annotated Checklist). Available online: https://portal.cybertaxonomy.org/flora-greece/intro (accessed on 1 August 2023).
- Asnaashari, S.; Delazar, A.; Alipour, S.S.; Nahar, L.; Williams, A.S.; Pasdaran, A.; Mojarab, M.; Azad, F.F.; Sarker, S. Chemical composition, free-radical-scavenging and insecticidal activities of the aerial parts of Stachys byzantina. Arch. Biol. Sci. 2010, 62, 653–662. [Google Scholar] [CrossRef]
- North Carolina Plant Toolbox, Stachys byzantina—Plant Information. Available online: https://plants.ces.ncsu.edu/plants/stachys-byzantina/?utm_source=chatgpt.com (accessed on 5 April 2025).
- Devecchi, M. The use of Labiatae of ornamental interest in the design of parks and gardens. Acta Hortic. 2006, 723, 51–58. [Google Scholar] [CrossRef]
- Kemp, S.; Hadley, P.; Blanuša, T. The Influence of plant type on green roof rainfall retention. Urban Ecosyst. 2019, 22, 355–366. [Google Scholar] [CrossRef]
- Naghibi, F.; Mosadegh, M.; Mohammadi, M.S.; Ghorbani, A. Labiatae family in folk medicine in Iran: From ethnobotany to pharmacology. Iran. J. Pharm. Res. 2005, 2, 2. [Google Scholar]
- Aminfar, P.; Abtahi, M.; Parastar, H. Gas chromatographic fingerprint analysis of secondary metabolites of Stachys lanata (Stachys byzantine C. Koch) Combined with Antioxidant Activity Modelling Using Multivariate Chemometric Methods. J. Chromat. A 2019, 1602, 432–440. [Google Scholar] [CrossRef]
- Tomou, E.-M.; Barda, C.; Skaltsa, H. Genus Stachys: A review of traditional uses, phytochemistry and bioactivity. Medicines 2020, 7, 63. [Google Scholar] [CrossRef]
- Legkobit, M.P.; Khadeeva, N.V. Variation and morphogenetic characteristics of different Stachys species during microclonal propagation. Russ. J. Genet. 2004, 40, 743–750. [Google Scholar] [CrossRef]
- Grigoriadou, K.; Krigas, N.; Sarropoulou, V.; Papanastasi, K.; Tsoktouridis, G.; Maloupa, E. In vitro propagation of medicinal and aromatic plants: The case of selected Greek species with conservation priority. Vitro Cell. Dev. Biol.-Plant 2019, 55, 635–646. [Google Scholar] [CrossRef]
- Katsanou, C.E.; Kostas, S.; Bantis, F.; Bertsouklis, K.; Hatzilazarou, S. Optimizing in vitro propagation of “Ladania” (Cistus creticus L.) through interaction of light spectra and plant growth regulators. Agronomy 2025, 15, 774. [Google Scholar] [CrossRef]
- Rai, A.C.; Kumar, A.; Modi, A.; Singh, M. Advances in Plant Tissue Culture: Current Developments and Future Trends; Academic Press: Cambridge, MA, USA, 2022; ISBN 0-323-99819-4. [Google Scholar]
- Ahmad, N.; Faisal, M.; Ahmad, A.; Alatar, A.A.; Qahtan, A.A.; Alok, A. Thidiazuron induced in vitro clonal propagation of Lagerstroemia speciosa (L.) Pers.—An important avenue Tree. Horticulturae 2022, 8, 359. [Google Scholar] [CrossRef]
- Yasemin, S.; Beruto, M. A Review on flower bulb micropropagation: Challenges and opportunities. Horticulturae 2024, 10, 284. [Google Scholar] [CrossRef]
- Bhojwani, S.S.; Dantu, P.K. Micropropagation. In Plant Tissue Culture: An Introductory Text; Springer: New Delhi, India, 2013; pp. 245–274. ISBN 978-81-322-1025-2. [Google Scholar]
- Podwyszyńska, M.; Orlikowska, T.; Trojak-Goluch, A.; Wojtania, A. Application and improvement of in vitro culture systems for commercial production of ornamental, fruit, and industrial plants in Poland. Acta Soc. Bot. Pol. 2022, 91, 914. [Google Scholar] [CrossRef]
- Bairu, M.W.; Fennell, C.W.; van Staden, J. The effect of plant growth regulators on somaclonal variation in Cavendish banana (Musa AAA Cv.‘Zelig’). Sci. Hortic. 2006, 108, 347–351. [Google Scholar] [CrossRef]
- Haisel, D.; Hofman, P.; Vágner, M.; Lipavska, H.; Tichá, I.; Schäfer, C.A.; Čapková, V. Ex vitro phenotype stability is affected by in vitro cultivation. Biol. Plant. 2001, 44, 321–324. [Google Scholar] [CrossRef]
- Zietkiewicz, E.; Rafalski, A.; Labuda, D. Genome fingerprinting by Simple Sequence Repeat (SSR)-Anchored polymerase chain reaction amplification. Genomics 1994, 20, 176–183. [Google Scholar] [CrossRef]
- Ai, X.; Wen, Y.; Wang, B. Assessment of genetic stability on in vitro propagation of Ardisia crenata var. bicolor using ISSR markers. Int. J. Plant Biol. 2023, 14, 218–227. [Google Scholar] [CrossRef]
- Kader, A.; Sinha, S.N.; Ghosh, P. A standardized protocol for genetically stable artificial seed production of Ficus religiosa L. using ISSR fingerprinting. J. Appl. Genet. 2023, 64, 275–287. [Google Scholar] [CrossRef]
- Jung, W.-S.; Chung, I.-M.; Kim, S.-H.; Chi, H.-Y.; Yu, C.Y.; Ghimire, B.K. Direct shoot organogenesis from Lycium chinense Miller leaf explants and assessment of genetic stability using ISSR markers. Agronomy 2021, 11, 503. [Google Scholar] [CrossRef]
- Zamani, M.; Sonboli, A.; Goldansaz, M.; Mirjalili, M.H. In vitro micropropagation and conservation of endangered medicinal plant Nepeta asterotricha Rech. f. (Lamiaceae): Genetic fidelity, phytochemical and biological assessment. Physiol. Mol. Biol. Plants 2024, 30, 67–80. [Google Scholar] [CrossRef]
- Thakur, M.; Sharma, V.; Luharch, R. Propagation of plum (Prunus salicina L.) cultivar frontier in vitro through control of shoot tip necrosis (stn) and validation of genetic integrity using ISSR markers. Plant Physiol. Rep. 2021, 26, 238–246. [Google Scholar] [CrossRef]
- Yıldırım, M.; Tuğ, G.N.; Yaprak, A.E. Analyses of genetic diversity and population structure of endemic and endangered species Sideritis gulendamii (Lamiaceae) and implications for its conservation. Genet. Resour. Crop Evol. 2024, 71, 4331–4345. [Google Scholar] [CrossRef]
- Chirumamilla, P.; Gopu, C.; Jogam, P.; Taduri, S. Highly efficient rapid micropropagation and assessment of genetic fidelity of regenerants by ISSR and SCoT markers of Solanum khasianum Clarke. Plant Cell Tiss. Org. Cult. 2021, 144, 397–407. [Google Scholar] [CrossRef]
- Hatzilazarou, S.; Kostas, S.; Nendou, T.; Economou, A. Conservation, regeneration and genetic stability of regenerants from alginate-encapsulated shoot explants of Gardenia jasminoides Ellis. Polymers 2021, 13, 1666. [Google Scholar] [CrossRef]
- Akin, B.; Bingöl, N.A.; Bulman, G.C. A first approach for the micropropagation of threatened endemic subspecies of Stachys cretica subsp. kutahyensis. Biologia 2024, 79, 1653–1661. [Google Scholar] [CrossRef]
- Cüce, M.; Bekircan, T.; Laghari, A.H.; Sökmen, M.; Sökmen, A.; Uçar, E.Ö.; Kılıç, A.O. Antioxidant phenolic constituents, antimicrobial and cytotoxic properties of Stachys annua L. from both natural resources and micropropagated plantlets. Indian J. Tradit. Knowl. 2017, 16, 407–416. [Google Scholar]
- Hadi, S.; Ahmadabadi, M.; Valizadeh Kamran, R. Efficient in vitro callus induction, regeneration and shoot multiplication protocols for Stachys inflata subsp. caucasica (Stschegl.); A rare medicinal plant. J. Med. Plants -Prod. 2024, 13, 570–576. [Google Scholar]
- Mantovska, D.I.; Zhiponova, M.K.; Petrova, D.; Alipieva, K.; Bonchev, G.; Boycheva, I.; Evstatieva, Y.; Nikolova, D.; Tsacheva, I.; Simova, S.; et al. Exploring the phytochemical composition and biological potential of Balkan endemic species Stachys scardica Griseb. Plants 2023, 13, 30. [Google Scholar] [CrossRef]
- Gamborg, O.; Murashige, T.; Thorpe, T.; Vasil, I. Plant tissue culture media. In Vitro 1976, 12, 473–478. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- McCown, B. Woody plant medium (WPM)-a mineral nutrient formulation for microculture for woody plant species. Hort. Sci. 1981, 16, 453. [Google Scholar]
- Tsaktsira, M.; Chavale, E.; Kostas, S.; Pipinis, E.; Tsoulpha, P.; Hatzilazarou, S.; Ziogou, F.-T.; Nianiou-Obeidat, I.; Iliev, I.; Economou, A. Vegetative propagation and ISSR-based genetic identification of genotypes of Ilex aquifolium ‘Agrifoglio commune’. Sustainability 2021, 13, 10345. [Google Scholar] [CrossRef]
- Kartsonas, E.; Papafotiou, M. Mother plant age and seasonal influence on in vitro propagation of Quercus euboica Pap., an endemic, rare and endangered oak species of Greece. Plant Cell Tiss. Org. Cult. 2007, 90, 111–116. [Google Scholar] [CrossRef]
- Wang, Y.; Yao, R. Plantlet regeneration of adult Pinus massoniana Lamb. Trees using explants collected in March and thidiazuron in culture medium. J. For. Res. 2017, 28, 1169–1175. [Google Scholar] [CrossRef]
- Bertsouklis, K.; Paraskevopoulou, A.T.; Petraki, E. In vitro regeneration from adult node explants of Juniperus oxycedrus. Not. Bot. Horti Agrobot. 2023, 51, 13062. [Google Scholar] [CrossRef]
- Loureiro, J.; Capelo, A.; Brito, G.; Rodriguez, E.; Silva, S.; Pinto, G.; Santos, C. Micropropagation of Juniperus phoenicea from adult plant explants and analysis of ploidy stability using flow cytometry. Biol. Plant. 2007, 51, 7–14. [Google Scholar] [CrossRef]
- Babu, G.A.; Mosa Christas, K.; Kowsalya, E.; Ramesh, M.; Sohn, S.-I.; Pandian, S. Improved sterilization techniques for successful in vitro micropropagation. In Commercial Scale Tissue Culture for Horticulture and Plantation Crops; Gupta, S., Chaturvedi, P., Eds.; Springer Nature: Singapore, 2022; pp. 1–21. ISBN 978-981-19-0054-9. [Google Scholar]
- Mantovska, D.I.; Kapchina, V.M.; Yordanova, Z.P. In vitro propagation of the Balkan endemic species Stachys leucoglossa Griseb. Bulg. J. Agric. Sci. 2019, 25, 1211–1215. [Google Scholar]
- Panayotova, L.G.; Ivanova, T.A.; Bogdanova, Y.Y.; Gussev, C.V.; Stanilova, M.I.; Bosseva, Y.Z.; Stoeva, T.D. In vitro cultivation of plant species from sandy dunes along the Bulgarian Black Sea coast. Phytol. Balc. 2008, 14, 119–123. [Google Scholar]
- Rezali, N.I.; Sidik, N.J.; Saleh, A.; Osman, N.I.; Adam, N.A.M. The effects of different strength of MS media in solid and liquid media on in vitro growth of Typhonium flagelliforme. Asian Pac. J. Trop. Biomed. 2017, 7, 151–156. [Google Scholar] [CrossRef]
- Ohki, S.; Sawaki, S. The effects of inorganic salts and growth regulators on in vitro shoot proliferation and leaf chlorophyll content of Delphinium cardinale. Sci. Hortic. 1999, 81, 149–158. [Google Scholar] [CrossRef]
- Pant, M.; Bisht, P.; Gusain, M.P. Effect of sucrose and media strength on in vitro multiplication in Swertia shirata Buch.-Ham ex wall: An endangered medicinal herb. Int. J. Pharm. Pharm. Sci. 2018, 10, 40–42. [Google Scholar] [CrossRef]
- Hida, W.N.; Norrizah, J.; Aminah, S.S.; Ruzaina, S.S.; Faezah, P. Effect of medium strength and hormones concentration on regeneration of Pogostemon cablin using nodes explant. Asian J. Biotechnol. 2012, 4, 46–52. [Google Scholar] [CrossRef][Green Version]
- Kevers, C.; Hausman, J.-F.; Faivre-Rampant, O.; Evers, D.; Gaspar, T. Hormonal control of adventitious rooting: Progress and questions. Angew. Bot. 1997, 71, 71–79. [Google Scholar]
- Pacurar, D.I.; Perrone, I.; Bellini, C. Auxin is a central player in the hormone cross-talks that control adventitious rooting. Physiol. Plant. 2014, 151, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Hatzilazarou, S.; Rifaki, N.; Patsou, M.; Kostas, S.; Economou, A.S. In vitro propagation of Viburnum dentatum L. ‘Lucidum Aiton’. Prop. Ornam. Plants 2009, 9, 39–42. [Google Scholar]
- Aygun, A.; Dumanoglu, H. In vitro shoot proliferation and in vitro and ex vitro root formation of Pyrus elaeagrifolia Pallas. Front. Plant Sci. 2015, 6, 225. [Google Scholar] [CrossRef]
- Gaba, V.P. Plant growth regulators in plant tissue culture and development. In Plant Development and Biotechnology; CRC Press: Boca Raton, FL, USA, 2005; pp. 87–99. [Google Scholar]
- Muraseva, D.; Kostikova, V. In vitro and ex vitro rooting of Spiraea betulifolia subsp. aemiliana (Rosaceae), an ornamental shrub. EDP Sci. 2020, 24, 00058. [Google Scholar] [CrossRef]
- Wang, F.; Xin, X.; Wei, H.; Qiu, X.; Liu, B. In vitro regeneration, ex vitro rooting and foliar stoma studies of Pseudostellaria heterophylla (Miq.) Pax. Agronomy 2020, 10, 949. [Google Scholar] [CrossRef]
- Yan, H.; Liang, C.; Yang, L.; Li, Y. In vitro and ex vitro rooting of Siratia grosvenorii, a traditional medicinal plant. Acta Physiol. Plant. 2010, 32, 115–120. [Google Scholar] [CrossRef]
- McClelland, M.; Smith, M.; Carothers, Z. The effects of in vitro and ex vitro root initiation on subsequent microcutting root quality in three woody plants. Plant Cell Tiss. Org. Cult. 1990, 23, 115–123. [Google Scholar] [CrossRef]
- Phulwaria, M.; Shekhawat, N.; Rathore, J.; Singh, R. An efficient in vitro regeneration and ex vitro rooting of Ceropegia bulbosa Roxb.—A threatened and pharmaceutical important plant of Indian Thar Desert. Ind. Crops Prod. 2013, 42, 25–29. [Google Scholar] [CrossRef]
- Kormanek, M.; Małek, S.; Banach, J.; Durło, G.; Jagiełło-Leńczuk, K.; Dudek, K. Seasonal changes of perlite–peat substrate properties in seedlings grown in different sized container trays. New For. 2021, 52, 271–283. [Google Scholar] [CrossRef]
- Cabrera, P.I.; Johnson, J.R. Fundamentals of Container Media Management: Part 1. Greenhouse and Nursery Crops Fact Sheets & Bulletins; Rutgers Fact Sheet FS 812; The State University of New Jersey: New Brunswick, NJ, USA, 2014; p. 3. [Google Scholar]
- Mateja, Š.; Dominik, V.; Franci, Š.; Gregor, O. The effects of a fogging system on the physiological status and rooting capacity of leafy cuttings of woody species. Trees 2007, 21, 491–496. [Google Scholar] [CrossRef]
- Economou, A.S. From microcutting rooting to microplant establishment: Key points to consider for maximum success in woody plants. Acta Hortic. 2013, 988, 43–56. [Google Scholar] [CrossRef]
- Hatzilazarou, S.; Kostas, S.; Economou, A.; Scaltsoyiannes, A. Efficient propagation of Nerium oleander L. through tissue culture. Prop. Ornam. Plants 2017, 17, 64–74. [Google Scholar]
- Kataoka, I. Influence of rooting substrates on the morphology of papaya root formed in vitro. Jap. J. Trop. Agric. 1994, 38, 251–257. [Google Scholar]
- Biswas, P.; Kumar, N. Application of molecular markers for the assessment of genetic fidelity of in vitro raised plants: Current status and future prospects. In Molecular Marker Techniques; Kumar, N., Ed.; Springer Nature: Singapore, 2023; pp. 233–256. ISBN 978-981-99-1611-5. [Google Scholar]
- Martínez, O. Selection of molecular markers for the estimation of somaclonal variation. In Plant Cell Culture Protocols; Loyola-Vargas, V.M., Ochoa-Alejo, N., Eds.; Methods in Molecular Biology; Springer: New York, NY, USA, 2018; Volume 1815, pp. 103–129. ISBN 978-1-4939-8593-7. [Google Scholar]
- Alatar, A.; Faisal, M. Encapsulation of Rauvolfia tetraphylla microshoots as artificial seeds and evaluation of genetic fidelity using RAPD and ISSR markers. J. Med. Plants Res. 2012, 6, 1367–1374. [Google Scholar] [CrossRef]
- Hatzilazarou, S.; Kostas, S.; Joachim, M.; Economou, A. Regeneration of Viburnum dentatum L. from alginate-encapsulated shoot explants after short-term cold storage and assessment of genetic stability using ISSR analysis. Agronomy 2020, 10, 1660. [Google Scholar] [CrossRef]
- Bramhanapalli, M.; Thogatabalija, L.; Gudipalli, P. Efficient in vitro plant regeneration from seedling-derived explants and genetic stability analysis of regenerated plants of Simarouba glauca DC. by RAPD and ISSR markers. Vitro Cell. Dev. Biol.-Plant 2017, 53, 50–63. [Google Scholar] [CrossRef]
- Ilczuk, A.; Jacygrad, E. In vitro propagation and assessment of genetic stability of acclimated plantlets of Cornus alba L. using RAPD and ISSR markers. Vitro Cell. Dev. Biol.-Plant 2016, 52, 379–390. [Google Scholar] [CrossRef]
- Soares, D.M.M.; Sattler, M.C.; Ferreira, M.F.d.S.; Praça-Fontes, M.M. Assessment of genetic stability in three generations of in vitro propagated Jatropha curcas L. plantlets using ISSR markers. Trop. Plant Biol. 2016, 9, 229–238. [Google Scholar] [CrossRef]
- Amin, S.; Wani, T.A.; Kaloo, Z.A.; Singh, S.; John, R.; Majeed, U.; Shapoo, G.A. Genetic stability using RAPD and ISSR markers in efficiently in vitro regenerated plants of Inula royleana DC. Meta Gene 2018, 18, 100–106. [Google Scholar] [CrossRef]
- Ali, H.; Musa, I.F.; Abu Bakar, N.A.; Karsani, S.A.; Yaacob, J.S. In vitro regeneration and ISSR-based genetic fidelity analysis of Orthosiphon stamineus Benth. Agronomy 2019, 9, 778. [Google Scholar] [CrossRef]





| Explant Collection Period | Survival Rate (%) |
|---|---|
| Spring | 61.0 ± 3.3 b * |
| Summer | 70.0 ± 3.3 a |
| Concentration (μΜ) | Number of Shoots | Length of Shoots (cm) | ||
|---|---|---|---|---|
| BAP | KIN | BAP | KIN | |
| 0 (control) | 3.7 ± 0.25 c * | 3.7 ± 0.25 c | 5.7 ± 0.35 a * | 5.7 ± 0.35 a |
| 5 | 2.9 ± 0.28 d | 6.0 ± 0.50 a | 2.7 ± 0.28 d | 3.6 ± 0.50 c |
| 10 | 1.9 ± 0.28 e | 4.9 ± 0.28 b | 2.9 ± 0.38 d | 4.3 ± 0.30 b |
| 20 | 1.7 ± 0.28 e | 3.0 ± 0.50 d | 2.4 ± 0.15 d | 3.7 ± 0.20 c |
| Nutrient Medium Strength | Number of Shoots | Length of Shoots (cm) | ||
|---|---|---|---|---|
| WPM | MS | WPM | MS | |
| 1:1 | 2.2 ± 0.20 cd * | 2.9 ± 0.10 a | 2.2 ± 0.11 c * | 3.2 ± 0.15 a |
| 1:2 | 2.1 ± 0.23 cd | 2.5 ± 0.10 bc | 2.5 ± 0.10 bc | 3.0 ± 0.11 a |
| 1:4 | 1.8 ± 0.20 d | 2.9 ± 0.50 a | 2.8 ± 0.47 ab | 2.9 ± 0.10 ab |
| Concentration (μΜ) | Rooting (%) | Number of Roots | Length of Roots (cm) | |||
|---|---|---|---|---|---|---|
| IBA | NAA | IBA | NAA | IBA | NAA | |
| 0 (control) | 0 | 0 | 0 | 0 | 0 | 0 |
| 1 | 0 | 10.0 ± 2.70 c * | 0 | 2.0 ± 1.0 c * | 0 | 0.7 ± 0.2 cd * |
| 5 | 13.3 ± 8.82 c | 46.7 ± 3.35 b | 1.3 ± 0.57 c | 2.6 ± 0.2 b | 0.5 ± 0.1 d | 1.1 ± 0.1 b |
| 10 | 16.7 ± 3.35 c | 73.3 ± 6.65 a | 1.7 ± 0.57 c | 2.9 ± 0.2 a | 0.8 ± 0.2 c | 1.6 ± 0.2 a |
| Substrate | Rooting (%) | Number of Roots | Length of Roots (cm) | |||
|---|---|---|---|---|---|---|
| +K-IBA | −K-IBA | +K-IBA | −K-IBA | +K-IBA | −K-IBA | |
| Peat | 73.3 ± 3.3 b * | 16.7 ± 3.35 c | 4.8 ± 0.2 b * | 1.0 ± 0.1 d | 4.2 ± 0.26 b * | 1.4 ± 0.3 c |
| Peat–Perlite (1:1) | 86.7 ± 6.65 a | 13.3 ± 3.35 c | 6.2 ± 0.26 a | 1.3 ± 0.2 c | 5.1 ± 0.26 a | 0.9 ± 0.2 d |
| Rooting | Survival (%) |
|---|---|
| In vitro | 73.3 ± 3.35 b * |
| Ex vitro | 96.7 ± 3.86 a |
| Primer | Primer Sequence (5′-3′) | Annealing Temperature (°C) | Number of Bands |
|---|---|---|---|
| ISSR UBC 807 | AGA GAG AGA GAG AGA GT | 56 | 9 |
| ISSR UBC 809 | AGA GAG AGA GAG AGA GG | 58 | 8 |
| ISSR UBC 810 | GAG AGA GAG AGA GAG AT | 52 | 7 |
| ISSR UBC 811 | GAG AGA GAG AGA GAG AC | 54 | 7 |
| ISSR UBC 812 | GAG AGA GAG AGA GAG AA | 54 | 8 |
| ISSR UBC 815 | CTC TCT CTC TCT CTC TG | 50 | 9 |
| ISSR UBC 816 | CAC ACA CAC ACA CAC AT | 54 | 7 |
| ISSR UBC 818 | CAC ACA CAC ACA CAC AG | 56 | 9 |
| ISSR UBC 821 | GTG TGT GTG TGT GTG TT | 54 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hatzilazarou, S.; Kantere, C.; Kotoula, A.-A.; Economou, A.; Bertsouklis, K.; Darras, A.; Kostas, S. In Vitro Propagation and Genetic Stability Assessment Using the ISSR Markers of Stachys byzantina K. Koch, a Promising Ornamental Species. Horticulturae 2025, 11, 530. https://doi.org/10.3390/horticulturae11050530
Hatzilazarou S, Kantere C, Kotoula A-A, Economou A, Bertsouklis K, Darras A, Kostas S. In Vitro Propagation and Genetic Stability Assessment Using the ISSR Markers of Stachys byzantina K. Koch, a Promising Ornamental Species. Horticulturae. 2025; 11(5):530. https://doi.org/10.3390/horticulturae11050530
Chicago/Turabian StyleHatzilazarou, Stefanos, Chara Kantere, Aikaterini-Angeliki Kotoula, Athanasios Economou, Konstantinos Bertsouklis, Anastasios Darras, and Stefanos Kostas. 2025. "In Vitro Propagation and Genetic Stability Assessment Using the ISSR Markers of Stachys byzantina K. Koch, a Promising Ornamental Species" Horticulturae 11, no. 5: 530. https://doi.org/10.3390/horticulturae11050530
APA StyleHatzilazarou, S., Kantere, C., Kotoula, A.-A., Economou, A., Bertsouklis, K., Darras, A., & Kostas, S. (2025). In Vitro Propagation and Genetic Stability Assessment Using the ISSR Markers of Stachys byzantina K. Koch, a Promising Ornamental Species. Horticulturae, 11(5), 530. https://doi.org/10.3390/horticulturae11050530









