Abstract
With the global population projected to reach 8.6 billion by 2050 and urbanization on the rise, sustainable food production in cities becomes imperative. Vertical farming presents a promising solution to meet this challenge by utilizing space-efficient, controlled-environment agriculture techniques. In a vertical farming system, high quality, high nutritional value products can be produced with minimum water consumption, using LEDs as energy-efficient light sources. Microgreens are a new market category of vegetables among sprouts and baby leaf greens. The most critical challenge in their cultivation is the choice of growing medium, lighting, and light spectrum, which affect photosynthesis, plant growth, and yield. This review explores various cultivation methods, including hydroponics, within the context of vertical farming. Using current research, it investigates the effect of LED lighting on the physiological properties and growth of microgreens and baby leaf lettuce, but further research is needed to determine the response of the varieties and the optimal light spectrum ratios to meet their needs.
1. Introduction
According to estimates, the world’s population will reach 8.6 billion by 2050, and about 80% of the population will be accommodated by cities [1,2,3]. To feed the growing population, sustainable food that can be produced in cities is needed. The production of such food requires the consideration of all aspects of sustainability, including environmental, social, and economic development [4,5]. Globally, the number of cultivatable agricultural areas is diminishing both in quality and quantity due to urbanization, natural disasters, global warming, irrational soil cultivation, and the potential uncontrolled use of pesticides, which affect soil fertility and thus the production of quality food [6,7,8]. According to the FAO statistical database, in 2021, there were almost 5 billion hectares of agricultural land worldwide in total. About one-third of this is used as cropland, while the remaining two-thirds are meadows and pastures for grazing animals. The size of cultivatable areas is showing a continuously decreasing trend [9], within which urban areas are those most exposed to the lack of arable cropland. 2015 was declared the International Year of Soils [10]. The same declaration stated that the sustainable management of soils is crucial for addressing the challenges posed by an increasing global population.
Soil health, along with water availability, constitutes one of the most critical resources for humanity, as human life depends on the soil’s fertility and bounty. Consequently, soil degradation presents a significant threat to food security by reducing agricultural yields, necessitating increased input use by farmers, and potentially leading to the abandonment of degraded soils [10,11]. Food security means having consistent, year-round access to the quantity and variety of safe foods needed by all household members to maintain active and healthy lives, without facing significant risks of losing that access [11,12]. Unfortunately, food safety is not provided to everyone. It is estimated that between 690 and 783 million people worldwide experienced hunger in 2022, an increase of 122 million compared to the period before the COVID-19 pandemic. Unfortunately, the lack of food security is not limited to hunger. In 2022, 2.4 billion people, including a disproportionate number of women and those in rural areas, lacked year-round access to nutritious, safe, and sufficient food. The ongoing effects of the pandemic on disposable incomes, the rising cost of a healthy diet, and overall inflation left billions unable to afford a healthy diet. As a result, millions of children under five years old continue to suffer from stunting (148 million), wasting (45 million), and overweight (37 million) [12,13,14,15]. One of the most promising new technologies to address current challenges in agriculture, such as soil degradation, and therefore food insecurity, is soilless farming. This revolutionary innovation in agriculture provides an effective strategy for consistently producing high-quality and high-yield crops [16].
Soilless agriculture can solve the increasing food scarcity issue, especially in urban areas [17]. In closed cultivation, soil-free cultivation ensures year-round production, as the adverse effects of climate are not present, water use and nutrient supply are controlled, and a healthy product can be produced without chemicals. Hydroponics as a soilless growing system is widely used as a modern urban growing system, especially in urban horticulture. There have been some successful applications of soilless growing systems in urban areas. In Tokyo, where land has become extremely valuable due to population growth, hydroponic systems are being used to grow rice in underground vaults without soil [18]. These systems allow for four crop rotations per year, compared to the traditional single crop. Hydroponics has also been successfully applied in Israel’s dry climate [19]. The Middle Eastern region is defined by a large population, limited arable land, extreme weather conditions, and numerous areas unsuitable for crop cultivation due to severe water scarcity; therefore, hydroponics provides a possible solution for safe plant-based food production.
The design and implementation of vertical farms is a proposed new method to address the issue of sustainability and meet the increasing food demand, which can offer an alternative for producing an adequate quantity and quality of plant-based food. Vertical farming can increase the yield per unit area compared to traditional cultivation [20,21,22]. Vertical farming is the practice of growing plants on vertically stacked levels. It often includes the continuous monitoring and control of environmental factors (Controlled Environment Agriculture, CEA), which aim to optimize plant growth [23,24].
A healthy diet is based on the consumption of vitamin-rich plant products with high levels of bioactive substances, which, in recent times, have become increasingly important in the human diet. A new direction in product development in the food industry has emerged, involving the production of fresh, plant-based, whole foods, for which there is increasing consumer demand. Products known as microgreens and baby leaf greens are becoming increasingly popular in indoor gardening. These are young, immature green plants harvested just a few days or weeks after germination, containing a high amount of bioactive ingredients [25]. They have a short growing cycle and shallow rooting, making them suitable for hydroponic cultivation in controlled indoor systems. However, in protected-environment growing systems, great care is needed to ensure that the light, temperature, water supply, pH, CO2 levels, and nutrient solution quality are appropriate to the needs of plants [26]. Researchers agree that lighting plays a key role in the development of lettuce in controlled indoor cultivation [27,28], but depending on the varieties and use, the mode, wavelength ratio, and combination of illumination can have different morphological, phytochemical, and physiological effects on plants [29,30,31].
Choosing the right type and composition of artificial lighting is a challenge in lettuce and microgreen production in indoor vertical farm systems. In photosynthesis, red light illumination is important for the functioning of the photosynthetic apparatus and the formation of assimilates and biomass, while blue light affects chloroplast development and chlorophyll and phytochemical formation [32,33], but can also induce stomatal closure [34]. Too much red illumination results in poor morphology of microgreens [35], so it is advisable to combine other modes of illumination during cultivation. Taking into account the ratio of light reflection, transmission, and absorption on leaves, and the composition of illumination, the spectrum of light absorbed by green leaves influences the photosynthesis of plants and the development of leaf area, so their ratio is crucial in the vertical farming system. Absorption of red and blue light occurs mainly in the upper part of the canopy, but as the leaf area increases, it may be advantageous to use, for example, green and far-red light spectral supplements, as these are better utilized in the lower leaves [24,36]. Green light not only penetrates into the deeper mesophyll layer of the leaves, stimulating chloroplasts there, but also increases photosynthesis [37,38]. The response of plants often varies depending on the proportion of blue light, but these responses can vary depending on the species.
Previous research has investigated the use of artificial lighting in indoor vertical farm systems and has shown that the types of multispectral lighting, particularly the R/B and R/FR illumination ratios, affect lettuce biology-cultivation characteristics [27,28,39,40] and increase the anthocyanin content in arugula, kale, and red cabbage microgreens, but had no effect on mustard microgreens [33,41]. The composition, amount, and duration of the illumination led to different results, depending on the reaction of the varieties used in the experiments. Knowledge is lacking on the effect of light composition on the physiological processes of species, which ultimately affect water use efficiency, yield, and nutritional quality. In this study, the aim was to understand the impact of the composition of the light spectrum and illumination, which influence the physiological processes and development of baby leaf lettuce.
In the first part of this work, we review the types of soilless systems, vertical farm systems that can be used to produce quality food in urban environments. Section 2 discusses soilless systems in indoor farming, their types, and the importance of vertical farming compared to traditional outdoor horticulture. Section 3 addresses what illumination systems, i.e., light sources and light intensities, are most commonly used in vertical farms. In the second part, special attention is given to the use of energy-efficient lighting (LEDs), the composition of the lighting that ensures sustainable microgreen and baby leaf lettuce production in a vertical farming system. Section 4 shows the importance of the growing medium and light spectrum composition in an indoor vertical farm system for the cultivation of microgreens and their phytonutrient content. Section 5 discusses the critical points of growing baby lettuce in a vertical farm system: the use of cost-effective LED sources and multispectral lighting options and their effects on plant physiology, development, water consumption, and yield. The results achieved so far can be utilized and further developed for the cultivation of different varieties in vertical farm systems in the future.
Despite the fact that it is approaching common practice in horticulture and agriculture worldwide, the technology of artificial lighting and soilless cultivation is still considered foreign and conventional farmers may find it difficult to embrace the technology. Without underestimating the importance of traditional crop production, farmers require convincing that vertical farms are not meant to replace conventional crop production, but are able to take some burden off of the soil and offer a great opportunity to complement conventional plant production, which is increasingly adapting integrated crop production and precision farming (including reasonable tillage and use of chemical plant protection), thereby preserving soil life and the service capacity of the soils.
2. Soilless Farming Systems and Vertical Farms
2.1. Soilless Farming Systems
In soilless growing systems, the controlled environment significantly contributes to the accelerated growth of plants by allowing precise management of critical factors such as light intensity and spectrum, temperature, humidity, and carbon dioxide concentration. The availability of essential nutrients is complemented by the ability to optimize these environmental variables. The soilless production system was originally developed to eliminate soil-borne pathogens. This technology is useful in areas where environmental stress is a serious problem or where space is limited. Vertical farms can use various forms of liquid-based soilless cultivation, such as hydroponics, aquaponics and aeroponics.
Soilless cultivation methods involve growing plants in nutrient-rich solutions (water containing dissolved fertilizers), where the plant roots receive mechanical support through various porous media, which can be organic (peat moss, coconut fiber, sawdust, bark), inorganic (sand, gravel, vermiculite, perlite), or synthetic (rockwool), or by omitting the support medium entirely. In liquid hydroponic systems, plants are grown without any solid medium, while in aggregate systems, a solid medium is used to support the plant roots [42]. Soilless systems can be further categorized into two types: open systems or closed systems [43].
In the technically simple construction of the initially created open systems the nutrient solution is delivered to the plant roots and is not reused, in order to limit the spread of pathogens that infect the roots [44]. However, their use has raised environmental concerns since the excess nutrient solution can pollute the environment when it escapes from the cultivation equipment [45], and valuable water and nutrients are also wasted [46]. Closed system hydroponic cultivation equipment was developed to minimize pollution. In a closed system, the nutrient solution can be recycled by recovering and replenishing nutrients and water according to the needs of the plant, and its pH can also be adjusted [47]. Different forms of soilless cultivation are used on vertical farms, such as hydroponics, aquaponics, and aeroponics, but the most common form of watering in these systems is hydroponics. It refers to the growing of crops in aggregate-free nutrient solutions. The plant roots directly contact the nutrient solution, which is strictly controlled to preserve its appropriate chemical composition and is also circulated [23,48]. Hydroponics involves different forms of watering, including but not limited to deep-water culture, nutrient film technique, ebb/flow (flood and drain), and wick system (in this case the plants are planted in an absorbent growing media). With the hydroponic system, a large number of crops and vegetables can be produced. Various experimental results have shown that leafy vegetable crops (lettuce, spinach, parsley, celery etc.) can be successfully and easily grown in hydroponic systems. Lettuce and spinach are the most promising species for integrated hydroponic and aquaculture systems, due to their higher growth and nutrient uptake capacity [49]. Although soilless cultivation is a beneficial technology in many ways, it has limitations. The main challenges of soilless growing systems are high initial investment, high use of energy, the limited number of plant species that can be cultivated, higher production costs, and the specialized skills required for operation [22,50,51].
One of its most serious disadvantages is that if some plant pathogen does get into the system, it can quickly spread throughout the entire plant population with the help of water [48], which can lead to the complete destruction of the plant population. In this specific case, the main concern is plant diseases (along with some other less significant pathogens) mainly caused by eukaryotic, fungus-like microorganisms called Oomycetes, which can cause significant losses in soilless crops by affecting both yield and crop quality. In these systems, the main source of the introduction and spread of pathogenic microorganisms is recirculated irrigation water, since the zoospores of the fungus actively swim to infect their target using two flagella [52]. The rapid spread of the inoculum is facilitated by the recycling of the nutrient solution, thereby increasing the risk of root diseases in the entire culture [53,54,55,56,57]. Limited oxygen supply can limit production and cause yield loss. Maintaining pH, electrical conductivity (EC), and the concentration of the nutrient solution is extremely important. Also, a weakened plant population is more exposed to pathogens.
Although hydroponic cultivation systems are typically considered safer than open-field agriculture, recent product recalls and foodborne outbreak investigations have identified hydroponically grown leafy greens as the source of contamination [58,59,60].
Even though aquaponics and aeroponics are more advanced soilless growing systems, hydroponics is still the most widely used. Whilst they have not been used in the experiment, acknowledging their importance, we would like to introduce them with a brief summary below.
- Aquaponics
The aquaponics system is an advanced version of the hydroponic system, combining plant cultivation and fish farming in the same ecosystem [61]. Fish are bred in indoor ponds that produce a nutrient-rich product used as a nutrient source for plants raised in vertical farms [62]. The plants filter and clean the wastewater, which is then returned to the fishponds. Aquaponics is used more often in smaller-scale vertical farming systems, as it is not suitable for growing crops of the same quality and quantity that commercial vertical systems are capable of. The latter also generally focus on cultivating a few fast-growing vegetables, which do not require aquaponics components. This simplifies economic and production issues and maximizes efficiency and crop security. However, as an emerging production system, new standardized aquaponics systems can help popularize this closed-loop system [63].
- Aeroponics
The term aeroponics was coined by NASA in the 1990s, meaning “soilless cultivation of plants in an airy/misty environment”. The aeroponic system is by far the most efficient plant cultivation system in vertical farms, as it uses up to 90% less water than the most efficient hydroponic systems. Plants grown in such aeroponic systems have been shown to absorb more minerals and vitamins, making the plants healthier and potentially more nutritious. There are two types of aeroponics: high-pressure atomization, which atomizes the nutrient solution in a very fine mist that settles on the root surface, and aero-hydro systems, which are a hybrid of hydroponics and aeroponics, where the majority of the roots are grown in air or mist, and one end of the roots (the root bed) grows into the nutrient solution [23,64].
2.2. Vertical Farms
For the production of such delicate products as microgreens and baby leaf greens, special cultivation equipment, so-called vertical farms, or (as they are often called in Asian countries) plant factories were created; however, they are not exclusively used for this purpose, as they may also be used to cultivate mature vegetable species. Plants are usually grown in a variety of soilless systems, under controlled cultivation conditions and artificial lighting. Vertical farms, using soilless farming systems, are the most dynamically developing sector of indoor plant cultivation, which was brought to life by the global demand for sustainability and high-quality food production. Vertical farming, characterized by the cultivation of crops in vertically stacked layers, represents a significant innovation in agriculture, promising to address the challenges of urban food production and sustainability. This approach, often integrated with closed hydroponic systems where plants are grown in a water-based, nutrient-rich solution without root media, has garnered attention for its potential to enhance food security, reduce water usage, and minimize agricultural land footprint [63,65,66].
Based on the most precise definition, a vertical farm is a plant-growing facility that meets six criteria. It is a thermally insulated and almost airtight, warehouse-like, opaque structure that contains a hydroponic multi-tier shelf system. Being highly energy-efficient and not radiating heat, primarily LED based light sources are used. It also contains automatic air conditioning, CO2 regulation, a nutrient solution unit with water pumps, and a central control unit for all the above.
The aim of vertical farms is to cultivate high-value crops characterized by superior yield and quality, while maximizing resource efficiency and cost-effectiveness, minimizing yield and quality vulnerabilities, and reducing environmental pollutant emissions [65]. With proper planning and management, vertical farms may offer potential advantages over traditional greenhouse systems, although they share common characteristics; for example, no soil required for cultivation. For this reason, both greenhouses and vertical farms can be built anywhere, but vertical farms can be assembled in abandoned buildings with limited or no natural light, which cannot be used for other purposes (e.g., parking garages, warehouses). In contrast, greenhouse horticulture functions within a (semi-) controlled environment, relying primarily on solar energy for photosynthesis and maintaining temperature [67]. In vertical farming using hydroponic plant cultivation and crops being harvested in an immature phenological phase plants can be sown more densely, even vertically [68], can be grown all year round as their development is not affected by climate change [69], and the quality and nutritional value of the yield are generally higher than in traditional soil-based plant cultivation [70]. The quality of the crop, such as its phytonutrient concentration, can be improved by manipulation of light and nutrients. There is no pesticide and herbicide residue, nor salt accumulation [47,71], and plants do not need to be washed before consumption. Practical experiences have shown that in conventional lettuce production from field to table one of the most expensive steps is washing and cleaning, in order to make the plant ready for consumption. Crops grown in vertical farms have a longer shelf life, because the bacterial load is generally less than 300 colony forming units (CFU) g−1, which is 1/100th to 1/1000th that of field crops [72]. It is also worth noting that microgreens and baby leaf greens grown in vertical farms do not require extensive preparation in the kitchen with little cutting involved, therefore the plants suffer less damage, which results in reduced respiration prolonged shelf life. Regarding their environmental aspect, transportation cost can be reduced by building vertical farms near urban areas, and the high efficiency of resource use (water, CO2, fertilizer, etc.) means a minimal release of pollutants into the external environment [65,73].
Plants have a shorter growing period, and their growth is faster because the roots do not encounter mechanical obstacles, so almost all nutrients are easily accessible to them [74].
3. Lighting Systems in Vertical Farms
According to Kikuchi et al. [75], vertical farm systems can be classified into two groups: in one, sunlight fulfills the photosynthesis and photoperiod requirements of plants, and in the other, artificial illumination is employed, and choosing the type of light source and duration of illumination ensures plant development. Following extensive experimentation with various lighting sources, researchers have determined that sunlight is the most efficient and cost-effective option [76]. The considerable durability and electricity consumption of these light sources [77], along with the reliance on fossil fuels for their energy supply, present critical challenges that need to be addressed [78]. Although attempts have been made to use natural sunlight on vertical farms, experience has proven that without intervention, uniform light distribution cannot be ensured, so plants grown on lower shelves cannot be provided with sufficient light. The experiment of Choubchilangroudi et al. [76] demonstrates the effectiveness of using mirrors to transmit sunlight and illuminate dark areas within a vertical farm. They stated that the correct placement of reflective panels (flat mirrors) and their ability to adjust to the sun ray’s angle will optimize sunlight utilization throughout the day. By simulating this concept with a vertical farm model, they found that the lower two-thirds of the floor equipped with reflectors received significantly more sunlight compared to a floor without a reflector system. Their study demonstrates that well-designed and strategically placed reflective panels in a vertical farm can effectively transmit sunlight to the lower floors during the daytime, supporting plant growth. In vertical farms—for the sake of yield security—only artificial lighting is used with light sources operating on various principles, which can be categorized into three types according to the principle of light emission: incandescence, discharge light emission, and electroluminescence [65]. The spectrum of light from incandescent sources is continuous and spans from infrared to visible light, often yielding a warm, yellowish hue [79].
Over the last decade, advances in lighting technology have led to the development of luminescence-based LEDs, which provide energy-efficient lighting and can be used to maintain plant growth. Electroluminescence is the direct conversion of electrical energy into light energy within a material. When an electric field is applied to certain materials, they emit light without significant heating. Organic Light-Emitting Diodes (OLEDs) and LEDs operate on this principle. LEDs in particular have gained considerable attention due to their high efficiency, long life, and potential applications in plant factories with artificial lighting [80]. LED light sources are rapidly spreading, especially in the indoor cultivation of horticultural crops, as opposed to conventional lighting, which includes fluorescent light sources used in the growth chamber or high-pressure sodium (HPS) light sources in the greenhouse [81].
However, ensuring uniform lighting in vertical farm systems depends on three major factors: the type of luminaires, the mounting height relative to the crop level, and the horizontal positioning of the luminaires [28]. The composition of the light above the plant surface and the photosynthetic photon flux density (PPFD) within the plant determine the growth of plants. In the process of photosynthesis, plants convert some of the photosynthetic photons from incident light into chemical energy, carbohydrates, while the remaining absorbed light is converted into heat [82]. A close correlation between fresh weight of lettuce and PPFD of 100–250 μmol m−2 s−1 was observed when plants were illuminated with warm white light and 660 nm red LED for a 16 h photoperiod [83].
However, as leaf area increases, PPFD decreases and the spectral distribution of light over the canopy differs from the internal distribution. For instance, green light penetrates the canopy more easily than blue and red light, due to the fact that the light transmittance, reflectance, and absorptance of green leaves are about 30%, 20%, and 50%, respectively [24], while those of blue and red light are about 0%, 100%, and 100%, respectively. The absorption of blue and red light occurs mainly in the upper part of the canopy and as the canopy grows, the leaves absorb most of this light, while only 50% of the green light is absorbed by the leaves, about 20% is reflected, and 30% transmits through the leaves of the plant [36]. Despite the transmission, there is still more green light and far-red light in lower leaves than red and blue light. This is because green or far-red supplemental lighting enhances CO2 uptake and biomass development if the lettuce is grown on a nutrient-rich substrate, allowing blue and red light to penetrate the deeper canopy layer [27]. Green light penetrates deeper into the leaf and increases photosynthesis by stimulating chloroplasts located deep in the mesophyll. Additionally, green light is able to reach the lower part of the canopy, which is extremely important for dense canopy production techniques that are common in controlled environment agriculture [37,38].
In the vertical farm system (VF), LED lighting and the fan system are significant electricity consumers [84]. In Sweden [85], a commercial vertical farm has better water consumption efficiency, lower greenhouse gas (GHG) emissions, and lower energy use efficiency compared to other traditional cultivation methods such as Spanish or Italian greenhouse cultivation. The GHG emissions for VF lettuce were approximately 0.98 kg CO2 eqv kg−1 of edible lettuce, but this can be lower if more renewable energy is used [85]. In a greenhouse vertical system, the efficiency of energy use can be increased with photovoltaic panels placed on the greenhouse roof, which provide a positive shading effect for tomatoes, peppers, and onions under strong sunlight with less than 20% coverage of the surface [86,87,88]. Plants grown on vertical shelves using LED lighting can achieve efficient photosynthetic efficiency and year-round production without the use of chemicals [89]. In vertical farms, there is the opportunity to grow leafy vegetables and medicinal and aromatic plants with short life cycles, compact sizes, and the ability to meet rapid market demands [60,90].
4. Microgreen Production in Vertical Farms
Microgreens represent a new category of vegetables among the various sprouts and baby leaf greens. They have a stronger flavor, a variety of leaf or cotyledon colors and shapes, and can be useful as a “superfood” [91]. Their yield is influenced by light, temperature, irrigation, and nutrient supply, but these are also important factors in shelf life [92]. Grown in a greenhouse under controlled conditions, or mostly in a soilless system under artificial light, improves the growth, yield, quality, and bioactive substances accumulation of the plant, without being limited by light conditions [93,94]. In the commercial cultivation of micro-vegetables, one of the most critical challenges is the choice of growing medium [93], lighting, and light spectrum composition [94] to influence photosynthesis, plant growth and phytonutrients level [95].
Harvesting usually takes place when the first true leaf appears at the desired height, which varies from about 1–3 weeks from sowing depending on plant type and growing conditions. It has been shown that the broccoli micro-vegetable, harvested 10 days after sowing, has a higher vitamin C and vitamin E content than the mature plant [96,97]. Harvesting of plants differs depending on the growing medium: radish micro-vegetables were harvested 7 days after sowing [98], broccoli after 10 days, red cabbage after 11 days [95,99], and spinach after 17–18 days [100]. The shelf life of micro-vegetables varies depending on the variety and growing medium. Spinach microgreens grown in glasshouse soil retain high vitamin C, tocopherol, and beta-carotene content in the refrigerator at 10 °C for 9 days [101], while red cabbage microgreens grown in organic soilless potting mix retain high vitamin C and beta-carotene content for 7 days at 10 °C [102]. In hydroponic cultivation, the micro-vegetable quality of broccoli is preserved for 21 days at 5 °C [101]. However, not all plants are suitable for consumption as micro vegetables. Tomatoes (Solanum lycopersicum), peppers (Capsicum spp.), aubergines (Solanum melongena), and those plants that contain anti-nutritive substances (in this case solanine) in the sprout state are not suitable. Interestingly, plants whose adult plants are not edible, such as legumes and sunflower, can be eaten as micro-vegetables [103,104].
Effect of Light Spectrum on the Growth of Microgreens
Lighting is the foremost environmental factor that affects plant growth and development, and cultivation in enclosed spaces greatly depends on the quality of light. Energy-efficient LEDs are suitable for this purpose and are successfully used in closed cultivation systems. Since the response of leafy vegetables to light greatly depends on the genotype, the developmental stage, it is necessary to determine the lighting requirements of different developmental stages to achieve maximum yield for different varieties [105]. Plants, even varieties within species, react differently to varying light spectra, but research agrees that red light is important for the photosynthetic apparatus and influences the transport of assimilates [32]. In photosynthesis, blue light affects chloroplast development, chlorophyll formation, and formation of phytochemicals, but the response of plants is highly dependent on the dose of blue light [33]. The effect of green lighting is similar to blue, participating in photosynthesis through phytochrome and cryptochrome pigments [106]. Plant water management changes under blue LED lighting, or if its ratio is high in lighting, stoma conductivity increases in cucumber seedlings [107]. Hosotani et al. [34] demonstrated that weak blue light, combined with strong red light, induces stomata opening, but blue light alone triggers stomata closure. Ying et al. [41] showed that a 30% blue to 70% red ratio of LED lighting increases the anthocyanin content in arugula, kale, and red cabbage microgreens (though not in mustard), and increases the total phenolic content in kale and mustard microgreens, compared to a 5% blue to 95% red ratio. However, too much red-light illumination leads to the so-called “red light syndrome”, which is manifested in poor morphology of microgreens, and faulty gene expression [108]. A combination of red light and other light sources, especially blue light, can effectively regulate stomatal aperture and improve carbon uptake by plants, thus preventing the formation of “red light syndrome” [35,109].
5. Lettuce Production in Vertical Farms
Lettuce (Lactuca sativa) is one of the most popular leafy greens cultivated worldwide. Its production in vertical farms is an area of growing interest due to the potential benefits in sustainability and space efficiency. It is a widely consumed vegetable recognized for its nutritional benefits and the presence of health-promoting bioactive compounds. For the consumer, color is important when choosing a product. The greenness of the leaves depends on the amount of chlorophyll content and is the determining factor for the consumer. In comparing green and red lettuce, some studies have concluded that red lettuce may have a higher level of phenols than green, making red lettuce an excellent source of antioxidants in daily meals [110]. Green lettuce, on the other hand, contains higher levels of chlorophyll than red lettuce and is considered to have antioxidant properties and benefits for the human body [111]. Nicolle et al. [112] reported that the amounts of beta-carotene and lutein were higher in red oak leaf varieties than in green-leaved Batavia and butterhead types, while others [113] have measured higher carotenoid levels in green leaf lettuce than in red leaf lettuce. However, the mode of illumination and the composition of the light spectrum can induce different responses in green and red leaf lettuce varieties in terms of growth and chlorophyll and carotenoid content [39,114]. Nitrate accumulation in green leafy vegetables can be significant in cultivation and a serious problem for consumers. In soilless cropping systems, however, it can be kept under control [26,115]. The results of Signore et al. [115] show that under low light conditions, red light can reduce nitrate concentration in leaves, especially by increasing nitrate reductase activity.
5.1. Effect of Lighting on the Physiological Processes of Baby Leaf Lettuce Varieties
The physiological response of lettuce varieties to lighting mode, intensity, and spectrum composition varies. The daily light integral (DLI) is determined by multiplying photosynthetic photon flux density (PPFD) by the photoperiod, representing the total radiation over a 24 h period, which influences biomass growth and leaf pigmentation in plants. For example, a 1% increase in light can boost the harvestable yield of many horticultural crops by 0.8% to 1% [83]. The composition and duration of illumination have different effects on the biological and production properties of plants when growing lettuce in an indoor vertical farm system (Table 1).
In their experiments, Ali et al. [39] investigated which composition and mode of LED lighting is best suited to improve the growth and phytochemical properties of baby leaf lettuce (Lactuca sativa L. var. acephala). The plants were grown in a closed, hydroponic system, where the average photosynthetic photon flux density (PPFD) at seedling level was 150 µmol s−1 m−2, applying a 16 h photoperiod over a 30-day growth cycle, with a plant density of 1150 plants m−2. The spectral quantum distribution of the lighting was red: 77.1%; green + yellow: 17.9%; blue: 5% for both continuous and pulsing (2 Hz frequency) LED lighting. They demonstrated that both LED modes increased yield, leaf length, and leaf width compared to the control that was carried out in a glasshouse where the plants were grown under monitored conditions and natural light with an average PPFD 65 µmol s−1 m−2 [39]. Chlorophyll fluorescence (Fv/Fm), which determines the photochemical efficiency of the photosynthetic system (PSII), slightly increased under pulsing LED, thus being more effective in enhancing the rate of photosynthesis than in continuous LED mode [39,116,117]. According to Miliauskiené et al. [118], lettuce grown under multispectral pulsed irradiation showed excellent growing characteristics, e.g., a 32% increase in fresh biomass, 36% increase in dry biomass, and 48% increase in leaf area. Others [40] observed different reactions of lettuce varieties under different lighting modes; under continuous red-blue LED light, the dry matter content of arugula leaves increased, and the total chlorophyll concentration decreased, while under such light conditions, the chlorophyll concentration in corn salad (Valerianella locusta L.) leaves was higher. A higher Fv/Fm value is shown under a higher proportion of blue light than under monochromatic red illumination [119], thereby increasing the plants’ photosynthetic capacity, photosynthetic rate, and stomatal density [120]. According to Viršilé et al. [121], the intensity of LED light causes variability in several growth parameters; higher LED intensity reduces leaf area, leaf number, and plant height in red lettuce, and moderate LED intensity contributed to an increase in fresh weight.
A similar conclusion was reached in our previous experiment, where green Batavia lettuce was grown under white LED (WL) illumination (Figure 1, Figure 2 and Figure 3). Cultivar Bassari (Nunhems Netherlands BV, Nunhem, The Netherlands), a green Batavia lettuce (Lactuca sativa L.), was seeded into a 264-cell plastic tray with O1 type (2.1 cm × 2.1 cm × 3.5 cm) Growfoam plugs (Foamplant, Groningen, The Netherlands). Immediately after sowing, trays were placed into a light-insulated germination chamber at a constant temperature of 20 °C. After emergence, seedlings were placed into the vertical farm where the lettuce plants were exposed to different combined spectral illumination for 12 h per day. Photosynthetic active radiation (PAR) was between 400–700 nm wavelength, and the light spectrum of the LED chips used falls close to this range, as shown in Figure 1. The broad spectrum (340–730 nm) white light (WL) was provided by BrightLife LED® (LED Lightning Ltd., Budapest, Hungary), the white light was complemented by ultraviolet A (UV) (360–400 nm) from the LED chip (WL + UV) and far-red light (FR) (640–740 nm) from the LED chip (WL + FR). The ratio of red (R) to blue (B) light intensity was 4R:1B (LED RB), which is appropriate for lettuce growth according to previous research [81]. All light sources were made by (LED Lightning Ltd., Budapest, Hungary). These illumination combinations compared with the popular red (R-620–640 nm) and blue (B 457–477 nm) LED (RB) lamps had different effects on plant growth (Figure 1 and Figure 2).
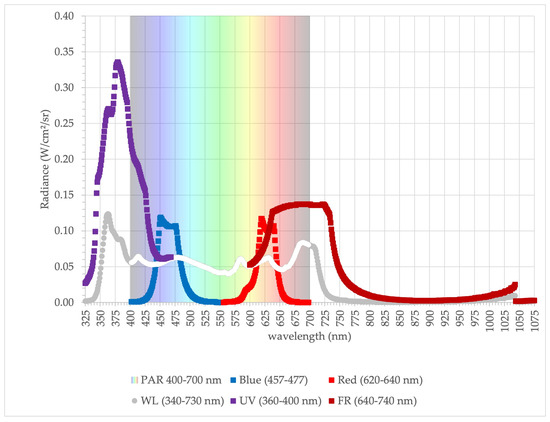
Figure 1.
Average radiance spectra of the 3-light source of LEDs compared to spectra of traditional red-blue lamp.

Figure 2.
Average size of lettuce resulting from different light spectra: red-blue (RB), white light (WL), white light with far red (WL + FR), white light with UV-A (WL + UV), respectively, in hydroponics.
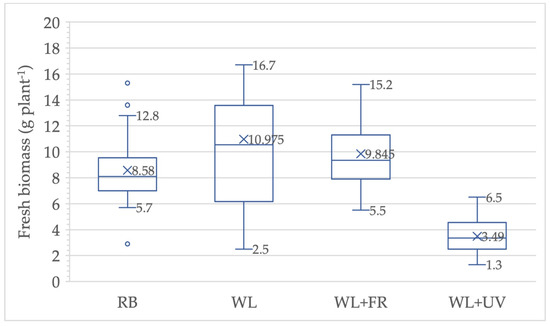
Figure 3.
Fresh biomass of lettuce grown on red/blue (RB), white (WL), white light with far-red (WL + FR) and white light with ultraviolet (WL + UV) lighting with the average, minimum, and maximum values depicted.
The use of different light sources caused a significant visible difference in size and weight of harvested plants (Figure 2). Far-red (FR) enhances CO2 uptake in lettuce leaves, biomass development [27], and photosynthesis when combined with shorter wavelengths (i.e., red and blue) [122]. When white light is supplemented with FR, the biomass weight per plant is not significantly different from that of plants under standard RB. In summary, lettuce was more sensitive to the addition of UV light compared to FR; however, it may reduce yields significantly (Figure 2 and Figure 3).
Son and Oh [119] demonstrated that growing two lettuce varieties (Sunmang and Grand Rapids TBR) under different ratios of B:R LED lighting for four weeks, the fresh mass of the plants decreased as the B (blue) ratio increased. The 10B:90R lighting ratio was favorable for the plants’ fresh and dry mass and leaf surface area. The response of the varieties also differed: the green-leaved Grand Rapids TBR produced 39% more fresh material under high red light, the red-leaved Sunmang 70% more. The extent of dry matter accumulation in plants depends on the light conditions of the developmental stages. Spalholz et al. [123] found that under low blue and strong red (20B:80R) lighting, initially (on day 17), the dry matter content of red and green oak-leaf lettuce plants was 15–39% greater than those that received 100B (blue) and sunlight-equivalent (SUN) illumination. Later, on day 42, the leaf surface area of the plants was 39–75% larger under 100B and sunlight-equivalent (SU) illumination than under various B/R treatments. A higher proportion of blue light (50B, 80B) resulted in the formation of thicker leaves with higher chlorophyll content. A different result was obtained by Cossu et al. [124] when they studied green-leaved (Falstaff, Cassandra) and red-leaved (Copacabana, Hoja Roble) varieties in a vertical farm system with a light spectrum composition of 58% white, 33% red, and 11% blue wavelengths. After 34 days, harvesting the 8–10 cm tall plant population, the green-leaved varieties produced 70% more yield than the red-leaved varieties and the study concluded that green-colored baby leaf varieties are more suitable for vertical farm cultivation and generate higher profits.
Recently, it has been established that green wavelength inhibits plant growth depending on blue wavelength, which justifies investigating the interactive effects of different wavelengths on plant growth and metabolic modifications. In a blue and red background (B16R84), 24% green light supplementation increased lettuce (Waldmann’s Green) yield [125], while others have reported that green LED illumination increased to 30% did not affect dry matter content in the same cultivar [126]. The effect of green light (GL) was also manifested at different developmental stages: at 14 and 21 days after sowing, the biomass and shoot diameter of the lettuce cultivar “Outredgeous” increased significantly, but not significantly at later, mature stages (28 days after sowing), compared to white LED illumination, indicating that GL stimulates rapid growth of lettuce at early stages [127]. An increase in leaf area was shown in the 3-week-old “Red Cos” lettuce seedlings when blue and red irradiation was supplemented with green light (GL), but this light composition did not increase the biomass in the more mature 5-week-old plants [127]. These experiments have confirmed that green LED supplementation in the early development, i.e., seedling stage, is favorable for growth.
Frutos-Totosa et al. [128] studied to what extent the spectrum composition of LED light influences the physiological processes of arugula (Eruca sativa Mill.) and corn salad (Valerianella locusta L.). They found that a higher proportion of red/blue lighting provided the greatest fresh weight and the maximum efficiency of the photosynthetic system (PSII), but with an increase in the red fraction, the chlorophyll and carotene content decreased. Chlorophyll fluorescence parameters varied depending on the species; it was higher in arugula than in corn salad, however, the PSII photochemical efficiency was greatest in a low ratio of red/far-red (Red:Far-Red) light spectrum. According to Wojciechowska et al. [40], a higher FV/Fm value can be achieved with red + blue lighting on corn salad than under ambient light surrounding the plants. Others [129] achieved the greatest fresh weight in arugula under continuous white LED lighting.


Table 1.
Summary of artificial LED lighting and its effect on the cultivation of lettuce (Lactuca sativa) in the vertical farm system.
Table 1.
Summary of artificial LED lighting and its effect on the cultivation of lettuce (Lactuca sativa) in the vertical farm system.
| LED Lighting | Lighting Conditions | Effect on Plants | Reference |
|---|---|---|---|
| Continuous 12 h | Red: 41–100%; blue: 0–59%; PPFD: 171 µmol s−1 m−2 | More R produced higher biomass, but absence of B induced abnormal leaf shape and lower phenolics and antioxidant content. | [120] |
| Continuous 16 h | Red/blue: 100, 12, 8, 4, 1, 0; PPFD: 150 µmol s−1 m−2 | R/B = 1 resulted in the best stomatal intensity and nitrate uptake, and R/B = 12 produced the highest shoot weight. | [121] |
| Continuous 16, 18, 24 h | Red: 76%; green: 14%; blue: 4%; FR: 6%; PPFD: 120, 144, 150, 180, 216, 270 µmol s−1 m−2 | Higher DLI resulted in higher biomass, but photoperiods were more efficient compared to light intensity within the same DLI. | [84] |
| Continuous and pulsing 16 h (2 Hz) | Red: 77.1%; green + yellow: 17.9%; blue: 5%; PPFD: 150 µmol s−1 m−2; natural light PPFD: 65 µmol s−1 m−2 | Pulsed LED produced better leaf performance index and energy efficiency. Significantly lower nitrate in continuous light. | [40] |
| Continuous and pulsing 16 h (30 kHz, 10 kHz, 3 kHz, 1 kHz, 0.3 kHz) | Red: 70%; white: 20%; blue: 10%; 182–187 µmol s−1 m−2; natural light PPFD: 246 µmol s−1 m−2 | Pulsed LED produced better energy efficiency with the same production quantity and Fv/Fm. | [117] |
| Continuous and pulsing 16 h (1 kHz, 0.5 kHz) | Red: 75%; green: 10%; blue: 15%; FR: 10% DLI 14.4 µmol m−2 day−1 | Pulsed LED produced better energy efficiency with the same production quantity. | [119] |
| Continuous 16 h | Red: 50%, blue: 50%; red: 67%, far-red: 33%; blue: 59, green: 14%, far-red: 7%, white: 19%; red: 59, green: 14%, far-red: 7%, white: 19%; PPFD: 150 µmol s−1 m−2 | Application of wider spectra resulted in higher organic carbon content and total dry matter. | [28] |
| Continuous 12, 16, 18, 24 h | Red: 88%; blue: 10%; FR: 2% DLI 13, 17, 19,5, 26 µmol m−2 day−1 | For optimal nitrate assimilation, lettuce required 300–400 µmol s−1 m−2 PPFD and 16–18 h photoperiod. Light intensity was more efficient than photoperiod and insufficient light quantity resulted in reduced biomass and increase in nitrate and nitrite contents. | [122] |
| Continuous 16 h | Far-red: 10–30%, white 0–70%; PPFD: 400–550 µmol s−1 m−2 | Far-red photons (701–750 nm) alone have low efficiency but adding them to white light significantly increased gross photosynthesis. | [123] |
| Continuous 18 h | Red/Blue: 100, 4, 1, 0.25, 0; UV: 5.1%, Blue: 20, Green: 26.1%, Red: 26.3%, FR: 22.6%; PPFD: 200 µmol s−1 m−2 | Application of wider spectra resulted higher fresh and dry biomass. | [124] |
5.2. Lighting Up the Cultivation of Baby Leaf Lettuce Varieties
Due to the use of modern soilless systems, the water use efficiency (WUE) of lettuce grown in vertical farms (VF) is higher than that in greenhouses and open fields. The WUE of lettuce expressed as grams of fresh weight (FW) per liter of water consumed (g FW L−1 H2O) in VF reached 80 g FW L−1 H2O, which is significantly higher than in greenhouses or open fields (60 and 20 g FW L−1 H2O, respectively) [71]. Determining the optimal plant density is important in VFs, where higher stock density is treated as an increase in yield per unit area. Higher stock density, along with precise cultivation techniques, can lead to a reduction in water consumption by the plant, greater light collection from the foliage surface, and intensive photosynthesis [130]. In lettuce cultivation, it has been shown that in VFs or growing chambers, the average plant density is 140 plants m−2 [131]; higher plant density, especially in early growth stages, can lead to significant increases in WUE [132]. In vertical farms, there was no significant difference in yield, fresh weight, dry matter content, stomatal conductivity, or leaf chlorophyll content (SPAD) between Canasta variety plants grown in ebb-and-flow substrate culture and high-pressure aeroponic systems, but WUE was more efficient in the aeroponic system than in the ebb-and-flow system [132]. Li et al. [133] found that in a nutrient film technique system, two lettuce varieties (Nenglv naiyoung and Dasusheng) achieved greater fresh shoot weight than in an aeroponic system. However, for both varieties, the root–shoot ratio increased when they were grown in an aeroponic system, indicating that more biomass was transferred to the root.
In the experiment by Carotti et al. [132], the plant density of the Canasta variety in the ebb-and-flow system was 153 plants m−2, and in the aeroponic system, it was 131 plants m−2, with both systems using the control light spectrum RB3 (red and blue spectrum 3:1), equivalent to 200 micromoles m−2 s−1. Since far-red radiation has been shown to enhance photosynthesis when combined with shorter wavelengths (i.e., red and blue) [122], in this experiment the red and blue (RB3) spectra were supplemented with long wavelengths of red (far-red) waves at different levels (30, 50, 70 µmol m−2 s−1 FR) in ebb-and-flow systems. In the same system, plant densities of 153, 270, and 733 plants m−2 under RB3 lighting were examined. The authors found that even a 30% FR supplementation increased the yield of Canasta lettuce, and the highest value was achieved at RB3-70 (70 FR addition) compared to the control (RB3), and leaf area index (LAI) also achieved a 96% increase with 30 and 70 FR supplementation. However, a 30 µmol m−2 s−1 (FR) supplementation, with the same amount of red and blue radiation, did not affect stomatal conductivity, and by increasing the FR supplementation from 0 to 30–70 µmol m−2 s−1 (FR), the SPAD decreased by 20–21%. Finally, it was found that by adding 30–50 µmol m−2 s−1 (FR) to the red and blue spectra, WUE can increase significantly (46–64%) without compromising the dry matter content of the plant. Lee et al. [29], when changing the red to far-red (R/FR) ratio from 0.7 to 4.1, found that the Sunmang variety achieved a 30–50% increase in leaf fresh weight compared to red and blue spectra, while Zou et al. [134] found a 56% increase in the Tiberious variety when adding 50 µmol m−2 s−1 far-red radiation to the red and blue spectra. The far-red (FR) light significantly increased dry weight, stem length, leaf length, and width, but decreased the levels of anthocyanins, carotenoids, and chlorophyll [30].
In summary, the indoor vertical farm system has the advantage of producing chemical-free, nutrient-rich microgreens and baby leaf lettuces regardless of environmental factors, and a consistent food supply due to the short growing period of the plants. However, the soilless growing systems used here require great care, including pH, electrical conductivity (EC), nutrient solution quality, and composition. Red light affects photosynthesis and dry matter formation, while blue light affects stomatal function; mainly a combination of these was studied. Nevertheless, the ratio of red and blue light and the additional illuminations, so-called multispectral illuminations, affect the fresh biomass and leaf area. The red/near infrared (R/FR) ratio, combined with red and blue light, affects leaf surface water use efficiency (WUE), which varies between species. White light (WL) additions to FR light change the photosynthesis and dry matter content of the varieties, but ultraviolet (UV) supplement (WL + UV) light is the most unfavourable.
6. Conclusions and Future Perspectives
Vertical farming is weather-independent, chemical-free cultivation, a major innovation in agriculture and a promising solution for urban food production. This review examines different cultivation methods, including hydroponic cultivation in vertical farming, and looks at the impact of LED lighting technology on plant growth and yield. It also presents current research findings on the production of microgreen products, in addition to a focus on baby leaf lettuce cultivation. Several studies highlight that the success of vertical farming of microgreens and baby leaf lettuce is influenced by the growing medium, the choice of illumination light spectrum. The choice of blue–red light ratio or the addition of far-red (FR) has different effects on vigor, yield, and dry matter content depending on the variety. Previous research has shown that a red/blue light ratio of 3:1 or 4:1 is favorable for dry matter formation in lettuce and that dry matter accumulation is higher in green leaf lettuce than in red leaf lettuce. However, the effect of supplementary illuminations such as far-red (FR) or green light (GL) on the development of different lettuce varieties on a blue/red background or on a white background needs further research.
Climate change is threatening outdoor vegetable production, so indoor vertical growing can play a major role in providing high nutritional value, healthy food all year round. As plants grow under artificial lighting, the choice of energy efficient light sources (LEDs), the composition of the light spectrum, and the duration and the mode of illumination (continuous or pulsed) are crucial in vertical farming systems. The water, nutrient, and light requirements of the plants differ during the development of the varieties, and further research is needed to meet these requirements in such production systems. In the future, studies should be carried out on the needs and responses of the varieties at the developmental stages to ensure cost-effective vertical lettuce production in vertical farm systems.
Author Contributions
Conceptualization, writing, and editing N.B. and Z.P.; writing—original draft preparation, E.N.; writing—review and editing, L.H. and S.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Kutatási Fejlesztési és Innovációs Hivatal (NKFIH). Grant numbers: 2020-1.1.2-PIACI-KFI-2021-00328, KA220-VET-779E2179. This work was supported by the Flagship Research Groups Programme of the Hungarian University of Agriculture and Life Sciences and the ÚNKP-23-3 New National Excellence Program of the Ministry for Culture and Innovation from the source of the National Research, Development and Innovation Fund.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Despommier, D. The Vertical Farm: Controlled Environment Agriculture Carried out in Tall Buildings Would Create Greater Food Safety and Security for Large Urban Populations. J. Verbraucherschutz Und Leb. 2011, 6, 233–236. [Google Scholar] [CrossRef]
- Islam, R.; Siwar, C. The Analysis of Urban Agriculture Development in Malaysia. Adv. Environ. Biol. 2012, 6, 1068–1078. [Google Scholar]
- Al-Chalabi, M. Vertical Farming: Skyscraper Sustainability? Sustain. Cities Soc. 2015, 18, 74–77. [Google Scholar] [CrossRef]
- Kalantari, F.; Tahir, O.M.; Joni, R.A.; Fatemi, E. Opportunities and Challenges in Sustainability of Vertical Farming: A Review. J. Landsc. Ecol. 2018, 11, 35–60. [Google Scholar] [CrossRef]
- Abou-Hussein, S.D. Climate Change and Its Impact on the Productivity and Quality of Vegetable Crops (Review Article). J. Appl. Sci. Res. 2012, 8, 4359–4383. [Google Scholar]
- Lakhiar, I.A.; Yan, H.; Zhang, J.; Wang, G.; Deng, S.; Bao, R.; Zhang, C.; Syed, T.N.; Wang, B.; Zhou, R.; et al. Plastic Pollution in Agriculture as a Threat to Food Security, the Ecosystem, and the Environment: An Overview. Agronomy 2024, 14, 548. [Google Scholar] [CrossRef]
- Lakhiar, I.A.; Yan, H.; Zhang, C.; Wang, G.; He, B.; Hao, B.; Han, Y.; Wang, B.; Bao, R.; Syed, T.N.; et al. A Review of Precision Irrigation Water-Saving Technology under Changing Climate for Enhancing Water Use Efficiency, Crop Yield, and Environmental Footprints. Agriculture 2024, 14, 1141. [Google Scholar] [CrossRef]
- Fedoroff, N.V.; Battisti, D.S.; Beachy, R.N.; Cooper, P.J.M.; Fischhoff, D.A.; Hodges, C.N.; Knauf, V.C.; Lobell, D.; Mazur, B.J.; Molden, D.; et al. Radically Rethinking Agriculture for the 21st Century. Science (1979) 2010, 327, 833–834. [Google Scholar] [CrossRef]
- FAO Land Statistics and Indicators 2000–2021. FAOSTAT Analytical Brief Series 71, 14. 2023. Available online: https://openknowledge.fao.org/server/api/core/bitstreams/5c8b2707-1bcf-4c29-90e2-3487e583f71e/content (accessed on 11 April 2024).
- The General Assembly of UN Draft Resolution Submitted by the Vice-Chair of the Committee. Ms. Farrah Brown (Jamaica), on the Basis of Informal Consultations on Draft Resolution A/C.2/68/L.21. 2013. Available online: https://www.fao.org/fileadmin/user_upload/GSP/docs/iys/World_Soil_Day_and_International_Year_of_Soils__UNGA_Resolution_Dec._2013.pdf (accessed on 18 August 2024).
- Gomiero, T. Soil Degradation, Land Scarcity and Food Security: Reviewing a Complex Challenge. Sustainability 2016, 8, 281. [Google Scholar] [CrossRef]
- Broca, S. Food Insecurity, Poverty and Agriculture: A Concept Paper. Agric. Econ. Dev. Anal. Div. 2002, 2, 87. [Google Scholar]
- FAO; IFAD; UNICEF; WEP; WHO. The State of Food Security and Nutrition in the World 2023: Urbanization, Agrifood Systems Transformation and Healthy Diets across the Rural–Urban Continuum; FAO: Rome, Italy, 2023; ISBN 9789251372265. [Google Scholar]
- The State of Food Insecurity in the World Addressing Food Insecurity in Protracted Crises 2010 Key Messages; Food and Agriculture Organization of the United Nations: Remo, Italy, 2010; ISBN 9789251066102.
- Ivers, L.C.; Cullen, K.A. Food Insecurity: Special Considerations for Women. Am. J. Clin. Nutr. 2011, 94, 1740S–1744S. [Google Scholar] [CrossRef]
- Sardare, M.D.; Admane, S.V. A Review on Plant Without Soil-Hydroponics. Int. J. Res. Eng. Technol. 2013, 02, 299–304. [Google Scholar] [CrossRef]
- Lakhiar, I.A.; Gao, J.; Syed, T.N.; Ali Chandio, F.; Tunio, M.H.; Ahmad, F.; Ali Solangi, K. Overview of the Aeroponic Agriculture—An Emerging Technology for Global Food Security. Int. J. Agric. Biol. Eng. 2020, 13, 1–10. [Google Scholar] [CrossRef]
- Feltman, R. The Nine-Story Tokyo Office Building That’s Also a Farm. 2013. Available online: https://qz.com/115226/photos-the-nine-story-tokyo-office-building-thats-also-a-farm (accessed on 18 August 2024).
- Vertical Container Farms in Israel: Illuminating the Future of Hydroponic Agriculture Development. 2023. Available online: https://www.hydroponicsfactory.com/blog/vertical-container-farms-in-israel-illuminating-the-future-of-hydroponic-agriculture-development.html (accessed on 19 August 2024).
- Tyson, R.V.; Hochmuth, R.C.; Lamb, E.M.; Hochmuth, G.J.; Sweat, M.S. A Decade of Change in Florida’S Greenhouse Vegetable Industry: 1991–2001. Proc. Fla. State Hort. Soc. 2001, 114, 280–283. [Google Scholar]
- Touliatos, D.; Dodd, I.C.; Mcainsh, M. Vertical Farming Increases Lettuce Yield per Unit Area Compared to Conventional Horizontal Hydroponics. Food Energy Secur. 2016, 5, 184–191. [Google Scholar] [CrossRef]
- Resh, H.M. A Definitive Guidebook for the Advanced Home Gardener and the Commercial Hydroponic Grower; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Abingdon, UK, 2013; ISBN 9781439878675. [Google Scholar]
- van Delden, S.H.; SharathKumar, M.; Butturini, M.; Graamans, L.J.A.; Heuvelink, E.; Kacira, M.; Kaiser, E.; Klamer, R.S.; Klerkx, L.; Kootstra, G.; et al. Current Status and Future Challenges in Implementing and Upscaling Vertical Farming Systems. Nat. Food 2021, 2, 944–956. [Google Scholar] [CrossRef]
- Kozai, T.; Niu, G.; Takagaki, M. Plant Factory: An Indoor Vertical Farming System for Efficient Quality Food Production; Academic Press: Cambridge, MA, USA, 2015; pp. 1–405. [Google Scholar]
- Michell, K.A.; Isweiri, H.; Newman, S.E.; Bunning, M.; Bellows, L.L.; Dinges, M.M.; Grabos, L.E.; Rao, S.; Foster, M.T.; Heuberger, A.L.; et al. Microgreens: Consumer Sensory Perception and Acceptance of an Emerging Functional Food Crop. J. Food Sci. 2020, 85, 926–935. [Google Scholar] [CrossRef] [PubMed]
- Guffanti, D.; Cocetta, G.; Franchetti, B.M.; Ferrante, A. The Effect of Flushing on the Nitrate Content and Postharvest Quality of Lettuce (Lactuca sativa L. Var. Acephala) and Rocket (Eruca Sativa Mill.) Grown in a Vertical Farm. Horticulturae 2022, 8, 604. [Google Scholar] [CrossRef]
- Barbieri, F.; Barbi, S.; Bertacchini, A.; Montorsi, M. Combined Effects of Different LED Light Recipes and Slow-Release Fertilizers on Baby Leaf Lettuce Growth for Vertical Farming: Modeling through DoE. Appl. Sci. 2023, 13, 8687. [Google Scholar] [CrossRef]
- Boros, I.F.; Székely, G.; Balázs, L.; Csambalik, L.; Sipos, L. Effects of LED Lighting Environments on Lettuce (Lactuca sativa L.) in PFAL Systems—A Review. Sci. Hortic. 2023, 321, 112351. [Google Scholar] [CrossRef]
- Lee, M.J.; Son, K.H.; Oh, M.M. Increase in Biomass and Bioactive Compounds in Lettuce under Various Ratios of Red to Far-Red LED Light Supplemented with Blue LED Light. Hortic. Environ. Biotechnol. 2016, 57, 139–147. [Google Scholar] [CrossRef]
- Li, Q.; Kubota, C. Effects of Supplemental Light Quality on Growth and Phytochemicals of Baby Leaf Lettuce. Environ. Exp. Bot. 2009, 67, 59–64. [Google Scholar] [CrossRef]
- Park, Y.; Runkle, E.S. Far-Red Radiation Promotes Growth of Seedlings by Increasing Leaf Expansion and Whole-Plant Net Assimilation. Environ. Exp. Bot. 2017, 136, 41–49. [Google Scholar] [CrossRef]
- Baroli, I.; Price, G.D.; Badger, M.R.; Von Caemmerer, S. The Contribution of Photosynthesis to the Red Light Response of Stomatal Conductance. Plant Physiol. 2008, 146, 737–747. [Google Scholar] [CrossRef]
- Hogewoning, S.W.; Trouwborst, G.; Maljaars, H.; Poorter, H.; van Ieperen, W.; Harbinson, J. Blue Light Dose-Responses of Leaf Photosynthesis, Morphology, and Chemical Composition of Cucumis Sativus Grown under Different Combinations of Red and Blue Light. J. Exp. Bot. 2010, 61, 3107–3117. [Google Scholar] [CrossRef]
- Hosotani, S.; Yamauchi, S.; Kobayashi, H.; Fuji, S.; Koya, S.; Shimazaki, K.I.; Takemiya, A. A BLUS1 Kinase Signal and a Decrease in Intercellular CO2 Concentration Are Necessary for Stomatal Opening in Response to Blue Light. Plant Cell 2021, 33, 1813–1827. [Google Scholar] [CrossRef] [PubMed]
- Darko, E.; Heydarizadeh, P.; Schoefs, B.; Sabzalian, M.R. Photosynthesis under Artificial Light: The Shift in Primary and Secondary Metabolism. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130243. [Google Scholar] [CrossRef] [PubMed]
- Kozai, T.; Zhang, G. Some Aspects of the Light Environment. In LED Lighting Urban Agriculture; Kozai, T., Fujiwara, K., Runkle, E.S., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 49–55. [Google Scholar]
- Dutta, G.S. Light Emitting Diodes for Agriculture; Springer: Berlin/Heidelberg, Germany, 2017; ISBN 9780123694010. [Google Scholar]
- Liu, X.Y.; Jiao, X.L.; Chang, T.T.; Guo, S.R.; Xu, Z.G. Photosynthesis and Leaf Development of Cherry Tomato Seedlings under Different LED-Based Blue and Red Photon Flux Ratios. Photosynthetica 2018, 56, 1212–1217. [Google Scholar] [CrossRef]
- Ali, A.; Santoro, P.; Ferrante, A.; Cocetta, G. Investigating Pulsed LED Effectiveness as an Alternative to Continuous LED through Morpho-Physiological Evaluation of Baby Leaf Lettuce (Lactuca sativa L. Var. Acephala). S. Afr. J. Bot. 2023, 160, 560–570. [Google Scholar] [CrossRef]
- Wojciechowska, R.; Kołton, A.; Długosz-Grochowska, O.; Żupnik, M.; Grzesiak, W. The Effect of LED Lighting on Photosynthetic Parameters and Weight of Lamb’s Lettuce (Valerianella Locusta). Folia Hortic. 2013, 25, 41–47. [Google Scholar] [CrossRef]
- Ying, Q.; Jones-Baumgardt, C.; Zheng, Y.; Bozzo, G. The Proportion of Blue Light from Light-Emitting Diodes Alters Microgreen Phytochemical Profiles in a Species-Specific Manner. HortScience 2021, 56, 13–20. [Google Scholar] [CrossRef]
- Putra, P.A.; Yuliando, H. Soilless Culture System to Support Water Use Efficiency and Product Quality: A Review. Agric. Agric. Sci. Procedia 2015, 3, 283–288. [Google Scholar] [CrossRef]
- Jensen, M.H. Hydroponics. HortScience 1997, 32, 1018–1021. [Google Scholar] [CrossRef]
- Schnitzler, W.H. Pest and Disease Management of Soilless Culture. Acta Hortic. 2004, 648, 191–203. [Google Scholar] [CrossRef]
- Ehret, D.; Alsanius, B.; Wohanka, W.; Menzies, J.; Utkhede, R.; Ehret, D.; Alsanius, B.; Wohanka, W.; Menzies, J.; Utkhede, R. Disinfestation of Recirculating Nutrient Solutions in Greenhouse Horticulture to Cite This Version: HAL Id: Hal-00886118 Disinfestation of Recirculating Nutrient Solutions in Greenhouse Horticulture. Agronomie 2001, 21, 323–339. [Google Scholar] [CrossRef]
- Van Os, E.A. Recent Advances in Soilless Culture in Europe. Acta Hortic. 2017, 1176, 1–8. [Google Scholar] [CrossRef]
- Vallance, J.; Franck, D.; Le Floch, G.; Guérin-Dubrana, L. Pathogenic and Beneficial Microorganisms in Soilless Cultures. Agron. Sustain. Dev. 2011, 31, 191–203. [Google Scholar] [CrossRef]
- Mohammed, S.B.; Sookoo, R. Nutrient Film Technique for Commercial Production. Agric. Sci. Res. J. 2016, 6, 269–274. [Google Scholar]
- Sharma, N.; Acharya, S.; Kumar, K.; Singh, N.; Chaurasia, O.P. Hydroponics as an Advanced Technique for Vegetable Production: An Overview. J. Soil. Water Conserv. 2018, 17, 364. [Google Scholar] [CrossRef]
- Sulma, V.; Gimenes, R.; Erlaine, B. Economic Viabilility for Deploying Hydroponic System in Emerging Countries: A Differentiated Risk Adjustment Proposal. Land Use Policy 2019, 1, 357–369. [Google Scholar] [CrossRef]
- Khatri, L.; Kunwar, A.; Bist, D.R. Hydroponics: Advantages and Challenges in Soilless Farming. Big Data Agric. (BDA) 2024, 6, 81–88. [Google Scholar]
- Tran, Q.D.; Galiana, E.; Thomen, P.; Cohen, C.; Orange, F.; Peruani, F.; Noblin, X. Coordination of Two Opposite Flagella Allows High-Speed Swimming and Active Turning of Individual Zoospores. eLife 2022, 11, 71227. [Google Scholar] [CrossRef]
- Somerville, C.; Cohen, M.; Pantanella, E.; Stankus, A.; Lovatelli, A. Small Scale Aquaponic Food Production: Integrated Fish and Plant Farming; Food and Agriculture Organization of the United Nations: Remo, Italy, 2014; ISBN 978-92-5-108532-5. [Google Scholar]
- Rosberg, A.K. Dynamics of Root Microorganisms in Closed Hydroponic Cropping Systems; Department of Biosystems and Technology, Swedish University of Agricultural Sciences: Uppsala, Sweden, 2014; ISBN 9789157680501. [Google Scholar]
- Favrin, R.J. Pythium Spp. Associated with Crown Rot of Cucumbers in British Columbia Greenhouses. Plant Dis. 1988, 72, 683. [Google Scholar] [CrossRef]
- Orlikowski, L.B.; Treder, W.; Ptaszek, M. Necessity of Disinfecting Water for Crop Irrigation. Infrastruct. Ecol. Rural Areas 2017, 4, 1387–1400. [Google Scholar]
- Deniel, F.; Vallance, J.; Barbier, G.; Quillec, S.L.; Benhamou, N.; Rey, P. Control of Pythium Spp. Root Colonization in Tomato Soilless Culture through Chlorination of Water Storage Tank. Acta Hortic. 2011, 893, 1293–1300. [Google Scholar] [CrossRef]
- Sela Saldinger, S.; Rodov, V.; Kenigsbuch, D.; Bar-Tal, A. Hydroponic Agriculture and Microbial Safety of Vegetables: Promises, Challenges, and Solutions. Horticulturae 2023, 9, 51. [Google Scholar] [CrossRef]
- Riggio, G.M.; Wang, Q.; Kniel, K.E.; Gibson, K.E. Microgreens—A Review of Food Safety Considerations along the Farm to Fork Continuum. Int. J. Food Microbiol. 2019, 290, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Ko, E.Y.; Keum, Y.S. Minimally Processed Ready-to-Eat Baby-Leaf Vegetables: Production, Processing, Storage, Microbial Safety, and Nutritional Potential. Food Rev. Int. 2017, 33, 644–663. Available online: https://www.researchgate.net/deref/http%3A%2F%2Fdx.doi.org%2F10.1080%2F87559129.2016.1204614?_tp=eyJjb250ZXh0Ijp7ImZpcnN0UGFnZSI6InB1YmxpY2F0aW9uIiwicGFnZSI6InB1YmxpY2F0aW9uIiwicG9zaXRpb24iOiJwYWdlQ29udGVudCJ9fQ (accessed on 28 August 2024).
- Love, D.C.; Fry, J.P.; Genello, L.; Hill, E.S.; Frederick, J.A.; Li, X.; Semmens, K. An International Survey of Aquaponics Practitioners. PLoS ONE 2014, 9, 102662. [Google Scholar] [CrossRef]
- Calone, R.; Pennisi, G.; Morgenstern, R.; Sanyé-Mengual, E.; Lorleberg, W.; Dapprich, P.; Winkler, P.; Orsini, F.; Gianquinto, G. Improving Water Management in European Catfish Recirculating Aquaculture Systems through Catfish-Lettuce Aquaponics. Sci. Total Environ. 2019, 687, 759–767. [Google Scholar] [CrossRef]
- Endo, M. Aquaponics in Plant Factory. In Plant Factory Using Artifical Light; Anpo, M., Fukuda, H., Wada, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 339–352. ISBN 9780128139738. [Google Scholar]
- Eldridge, B.M.; Manzoni, L.R.; Graham, C.A.; Rodgers, B.; Farmer, J.R.; Dodd, A.N. Getting to the Roots of Aeroponic Indoor Farming. New Phytol. 2020, 228, 1183–1192. [Google Scholar] [CrossRef]
- Kozai, T.; Niu, G.; Takagaki, M. Plant Factory; Elsevier: Amsterdam, The Netherlands, 2020; ISBN 9780128166918. [Google Scholar]
- Anpo, M.; Fukuda, H.; Wada, T. (Eds.) Plant Factory Using Artificial Light; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 9785984520973. [Google Scholar]
- Graamans, L.; Baeza, E.; van den Dobbelsteen, A.; Tsafaras, I.; Stanghellini, C. Plant Factories versus Greenhouses: Comparison of Resource Use Efficiency. Agric. Syst. 2018, 160, 31–43. [Google Scholar] [CrossRef]
- Ragaveena, S.; Shirly Edward, A.; Surendran, U. Smart Controlled Environment Agriculture Methods: A Holistic Review. Rev. Environ. Sci. Biotechnol. 2021, 20, 887–913. [Google Scholar] [CrossRef]
- Jovicich, E.; Cantliffe, D.J.; Stoffella, P.J. “Spanish” Pepper Trellis System and High Plant Density Can Increase Fruit Yield, Fruit Quality, and Reduce Labor in a Hydroponic, Passive-Ventilated Greenhouse. In Proceedings of the Acta Horticulturae; International Society for Horticultural Science (ISHS), Leuven, Belgium, 30 September 2003; pp. 255–262. [Google Scholar]
- Sharma, S.; Dhingra, P.; Koranne, S. Microgreens: Exciting New Food for 21st Century. Ecol. Environ. Conserv. 2020, 26, S248–S251. [Google Scholar]
- Orsini, F.; Pennisi, G.; Zulfiqar, F.; Gianquinto, G. Sustainable Use of Resources in Plant Factories with Artificial Lighting (PFALs). Eur. J. Hortic. Sci. 2020, 85, 297–309. [Google Scholar] [CrossRef]
- Ooshima, T.; Hagiya, K.; Yamaguchi, T.; Endo, T. Commercialization of LED Used Plant. Nishimatsu Constr. Ind. Tech. Rep. 2010, 36, 1–6. [Google Scholar]
- Kozai, T. Plant Factory in Japan-Current Situation and Perspectives. Chron. Hortic. 2007, 53, 8–11. [Google Scholar]
- Polycarpou, P.; Neokleous, D.; Chimonidou, D.; Papadopoulos, I. A Closed System for Soil Less Culture Adapted to the Cyprus Conditions. Non-Conv. Water Use 2005, 241, 237–241. [Google Scholar]
- Kikuchi, Y.; Kanematsu, Y.; Yoshikawa, N.; Okubo, T.; Takagaki, M. Environmental and Resource Use Analysis of Plant Factories with Energy Technology Options: A Case Study in Japan. J. Clean. Prod. 2018, 186, 703–717. [Google Scholar] [CrossRef]
- Choubchilangroudi, A.; Zarei, A. Investigation the Effectiveness of Light Reflectors in Transmitting Sunlight into the Vertical Farm Depth to Reduce Electricity Consumption. Clean. Eng. Technol. 2022, 7, 100421. [Google Scholar] [CrossRef]
- Specht, K.; Siebert, R.; Hartmann, I.; Freisinger, U.B.; Sawicka, M.; Werner, A.; Thomaier, S.; Henckel, D.; Walk, H.; Dierich, A. Urban Agriculture of the Future: An Overview of Sustainability Aspects of Food Production in and on Buildings. Agric. Hum. Values 2014, 31, 33–51. [Google Scholar] [CrossRef]
- Caplow, T. Building Integrated Agriculture: Philosophy and Practice. In Urban Futures 2030; Heinrich Böll Stiftung, Publication Series of Ecology 2009; ISBN 9783869280080. Available online: https://www.researchgate.net/publication/312442295_Building_integrated_agriculture_philosophy_and_practice_Urban_Futures_2030 (accessed on 28 August 2024).
- Cathey, H.; Campbell, L. Light and Lighting Systems for Horticultural Plants. In Horticultural Reviews; American Society for Horticultural Science. 2011, Volume 2, pp. 491–537, ISBN 9781118060759. Available online: https://www.researchgate.net/publication/230215879_Light_and_Lighting_Systems_for_Horticultural_Plants (accessed on 28 August 2024).
- Cocetta, G.; Casciani, D.; Bulgari, R.; Musante, F.; Kołton, A.; Rossi, M.; Ferrante, A. Light Use Efficiency for Vegetables Production in Protected and Indoor Environments. Eur. Phys. J. Plus 2017, 132, 43. [Google Scholar] [CrossRef]
- Bantis, F.; Smirnakou, S.; Ouzounis, T.; Koukounaras, A.; Ntagkas, N.; Radoglou, K. Current Status and Recent Achievements in the Field of Horticulture with the Use of Light-Emitting Diodes (LEDs). Sci. Hortic. 2018, 235, 437–451. [Google Scholar] [CrossRef]
- Kozai, T. Resource Use Efficiency of Closed Plant Production System with Artificial Light: Concept, Estimation and Application to Plant Factory. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2013, 89, 447–461. [Google Scholar] [CrossRef] [PubMed]
- Kelly, N.; Choe, D.; Meng, Q.; Runkle, E.S. Promotion of Lettuce Growth under an Increasing Daily Light Integral Depends on the Combination of the Photosynthetic Photon Flux Density and Photoperiod. Sci. Hortic. 2020, 272, 109565. [Google Scholar] [CrossRef]
- Martin, M.; Orsini, F. Life Cycle Assessment of Indoor Vertical Farms. In Advances in Plant Factories: New Technologies in Indoor Vertical Farming; Kozai, T., Hayashi, E., Eds.; Burleigh Dodds Science Publishing: London, UK, 2023; pp. 75–96. [Google Scholar] [CrossRef]
- Martin, M.; Elnour, M.; Siñol, A.C. Environmental Life Cycle Assessment of a Large-Scale Commercial Vertical Farm. Sustain. Prod. Consum. 2023, 40, 182–193. [Google Scholar] [CrossRef]
- Yano, A.; Kadowaki, M.; Furue, A.; Tamaki, N.; Tanaka, T.; Hiraki, E.; Kato, Y.; Ishizu, F.; Noda, S. Shading and Electrical Features of a Photovoltaic Array Mounted inside the Roof of an East-West Oriented Greenhouse. Biosyst. Eng. 2010, 106, 367–377. [Google Scholar] [CrossRef]
- Ureña-Sánchez, R.; Callejón-Ferre, Á.J.; Pérez-Alonso, J.; Carreño-Ortega, Á. Greenhouse Tomato Production with Electricity Generation by Roof-Mounted Flexible Solar Panels. Sci. Agric. 2012, 69, 233–239. [Google Scholar] [CrossRef]
- Kavga, A.; Strati, I.F.; Sinanoglou, V.J.; Fotakis, C.; Sotiroudis, G.; Christodoulou, P.; Zoumpoulakis, P. Evaluating the Experimental Cultivation of Peppers in Low-Energy-Demand Greenhouses. An. Interdiscip. Study. J. Sci. Food Agric. 2019, 99, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Despommier, D. Farming up the City: The Rise of Urban Vertical Farms. Trends Biotechnol. 2013, 31, 388–389. [Google Scholar] [CrossRef]
- Baselice, A.; Colantuoni, F.; Lass, D.A.; Nardone, G.; Stasi, A. Trends in EU Consumers’ Attitude towards Fresh-Cut Fruit and Vegetables. Food Qual. Prefer. 2017, 59, 87–96. [Google Scholar] [CrossRef]
- Sharma, S.; Shree, B.; Sharma, D.; Kumar, S.; Kumar, V.; Sharma, R.; Saini, R. Vegetable Microgreens: The Gleam of next Generation Super Foods, Their Genetic Enhancement, Health Benefits and Processing Approaches. Food Res. Int. 2022, 155, 111038. [Google Scholar] [CrossRef]
- Harakotr, B.; Srijunteuk, S.; Rithichai, P.; Tabunhan, S. Effects of Light-Emitting Diode Light Irradiance Levels on Yield, Antioxidants and Antioxidant Capacities of Indigenous Vegetable Microgreens. Sci. Technol. Asia 2019, 24, 59–66. [Google Scholar] [CrossRef]
- Di Gioia, F.; De Bellis, P.; Mininni, C.; Santamaria, P.; Serio, F. Physicochemical, Agronomical and Microbiological Evaluation of Alternative Growing Media for the Production of Rapini (Brassica rapa L.) Microgreens. J. Sci. Food Agric. 2017, 97, 1212–1219. [Google Scholar] [CrossRef] [PubMed]
- Ying, Q.; Kong, Y.; Zheng, Y. Applying Blue Light Alone, or in Combination with Far-Red Light, during Nighttime Increases Elongation without Compromising Yield and Quality of Indoor-Grown Microgreens. HortScience 2020, 55, 876–881. [Google Scholar] [CrossRef]
- Choe, U.; Yu, L.L.; Wang, T.T.Y. The Science behind Microgreens as an Exciting New Food for the 21st Century. J. Agric. Food Chem. 2018, 66, 11519–11530. [Google Scholar] [CrossRef]
- Sun, J.; Kou, L.; Geng, P.; Huang, H.; Yang, T.; Luo, Y.; Chen, P. Metabolomic Assessment Reveals an Elevated Level of Glucosinolate Content in CaCl₂ Treated Broccoli Microgreens. J. Agric. Food Chem. 2015, 63, 1863–1868. [Google Scholar] [CrossRef]
- Lu, Y.; Dong, W.; Alcazar, J.; Yang, T.; Luo, Y.; Wang, Q.; Chen, P. Effect of Preharvest CaCl2 Spray and Postharvest UV-B Radiation on Storage Quality of Broccoli Microgreens, a Richer Source of Glucosinolates. J. Food Compos. Anal. 2018, 67, 55–62. [Google Scholar] [CrossRef]
- Manjula, D. Ghoora Effect of Packaging and Coating Technique on Postharvest Quality and Shelf Life of Raphanus sativus L. and Hibiscus sabdariffa L. microgreens. Foods 2020, 9, 653. [Google Scholar]
- Xiao, Z.; Lester, G.E.; Luo, Y.; Wang, Q. Assessment of Vitamin and Carotenoid Concentrations of Emerging Food Products: Edible Microgreens. J. Agric. Food Chem. 2012, 60, 7644–7651. [Google Scholar] [CrossRef] [PubMed]
- Bergquist, S.Å.M.; Gertsson, U.E.; Olsson, M.E. Influence of Growth Stage and Postharvest Storage on Ascorbic Acid and Carotenoid Content and Visual Quality of Baby Spinach (Spinacia oleracea L.). J. Sci. Food Agric. 2006, 86, 346–355. [Google Scholar] [CrossRef]
- Kou, L.; Yang, T.; Liu, X.; Luo, Y. Effects of Pre- and Postharvest Calcium Treatments on Shelf Life and Postharvest Quality of Broccoli Microgreens. HortScience 2015, 50, 1801–1808. [Google Scholar] [CrossRef]
- Berba, K.J.; Uchanski, M.E. Post-Harvest Physiology of Microgreens. J. Young Investig. 2012, 24, 1–5. [Google Scholar]
- Di Gioia, F.; Renna, M.; Santamaria, P. Sprouts, Microgreens and “Baby Leaf” Vegetables. In Minimally Processed Refrigerated Fruits and Vegetables; Yildiz, F., Wiley, R.C., Eds.; Springer: Boston, MA, USA, 2017; pp. 403–432. ISBN 978-1-4939-7018-6. [Google Scholar]
- Du, M.; Xiao, Z.; Luo, Y. Advances and Emerging Trends in Cultivation Substrates for Growing Sprouts and Microgreens toward Safe and Sustainable Agriculture. Curr. Opin. Food Sci. 2022, 46, 100863. [Google Scholar] [CrossRef]
- Wong, C.E.; Teo, Z.W.N.; Shen, L.; Yu, H. Seeing the Lights for Leafy Greens in Indoor Vertical Farming. Trends Food Sci. Technol. 2020, 106, 48–63. [Google Scholar] [CrossRef]
- Swartz, T.E.; Corchnoy, S.B.; Christie, J.M.; Lewis, J.W.; Szundi, I.; Briggs, W.R.; Bogomolni, R.A. The Photocycle of a Flavin-Binding Domain of the Blue Light Photoreceptor Phototropin. J. Biol. Chem. 2001, 276, 36493–36500. [Google Scholar] [CrossRef]
- Eguchi, T.; Hernández, R.; Kubota, C. End-of-Day Far-Red Lighting Combined with Blue-Rich Light Environment to Mitigate Intumescence Injury of Two Interspecific Tomato Rootstocks. Acta Hortic. 2016, 1134, 163–170. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, Z.; Ager, E.; Kong, L.; Tan, L. Nutritional Quality and Health Benefits of Microgreens, a Crop of Modern Agriculture. J. Future Foods 2021, 1, 58–66. [Google Scholar] [CrossRef]
- Davis, P.A.; Burns, C. Photobiology in Protected Horticulture. Food Energy Secur. 2016, 5, 223–238. [Google Scholar] [CrossRef]
- Wilson, P.E.; Morrison, S.C.; Hedges, L.J.; Kerkhofs, N.S.; Lister, C.E. Phenolics Contribute Significantly to Higher Antioxidant Activity of Red Lettuce Compared to Green Lettuce. In Proceedings of the XXII International Conference on Polyphenols, Helsinki, Finland, 25–28 August 2004. [Google Scholar]
- Turkmen, N.; Poyrazoglu, E.S.; Sari, F.; Sedat Velioglu, Y. Effects of Cooking Methods on Chlorophylls, Pheophytins and Colour of Selected Green Vegetables. Int. J. Food Sci. Technol. 2006, 41, 281–288. [Google Scholar] [CrossRef]
- Nicolle, C.; Carnat, A.; Fraisse, D.; Lamaison, J.L.; Rock, E.; Michel, H.; Amoureux, P.; Remesy, C. Characterisation and Variation of Antioxidant Micronutrients in Lettuce (Lactuca sativa Folium). J. Sci. Food Agric. 2004, 84, 2061–2069. [Google Scholar] [CrossRef]
- Mou, B. Genetic Variation of Beta-Carotene and Lutein Contents in Lettuce. J. Am. Soc. Hortic. Sci. 2005, 130, 870–876. [Google Scholar] [CrossRef]
- Viršile, A.; Brazaityte, A.; Sirtautas, R.; Duchovskis, P. Light Spectral Effects on Phenolic Compounds in Perilla Frutescens Leaves as Related to the Leaf Age, Color and Duration of Exposure. Acta Hortic. 2017, 1170, 981–988. [Google Scholar] [CrossRef]
- Signore, A.; Bell, L.; Santamaria, P.; Wagstaff, C.; Van Labeke, M.C. Red Light Is Effective in Reducing Nitrate Concentration in Rocket by Increasing Nitrate Reductase Activity, and Contributes to Increased Total Glucosinolates Content. Front. Plant Sci. 2020, 11, 604. [Google Scholar] [CrossRef]
- Son, K.H.; Lee, S.R.; Oh, M.M. Comparison of Lettuce Growth under Continuous and Pulsed Irradiation Using Light-Emitting Diodes. Hortic. Sci. Technol. 2018, 36, 542–551. [Google Scholar] [CrossRef]
- Kozai, T.; Oki, H.; Fujiwara, K. Photosynthetic Characteristics of Cymbidium Plantlet in Vitro. Plant Cell Tissue Organ. Cult. 1990, 22, 205–211. [Google Scholar] [CrossRef]
- Miliauskienė, J.; Karlicek, R.F.; Kolmos, E. Effect of Multispectral Pulsed Light-Emitting Diodes on the Growth, Photosynthetic and Antioxidant Response of Baby Leaf Lettuce (Lactuca sativa L.). Plants 2021, 10, 762. [Google Scholar] [CrossRef] [PubMed]
- Son, K.H.; Oh, M.M. Leaf Shape, Growth, and Antioxidant Phenolic Compounds of Two Lettuce Cultivars Grown under Various Combinations of Blue and Red Light-Emitting Diodes. HortScience 2013, 48, 988–995. [Google Scholar] [CrossRef]
- Wang, J.; Lu, W.; Tong, Y.; Yang, Q. Leaf Morphology, Photosynthetic Performance, Chlorophyll Fluorescence, Stomatal Development of Lettuce (Lactuca sativa L.) Exposed to Different Ratios of Red Light to Blue Light. Front. Plant Sci. 2016, 7, 250. [Google Scholar] [CrossRef]
- Viršilė, A.; Brazaitytė, A.; Vaštakaitė-Kairienė, V.; Miliauskienė, J.; Jankauskienė, J.; Novičkovas, A.; Samuolienė, G. Lighting Intensity and Photoperiod Serves Tailoring Nitrate Assimilation Indices in Red and Green Baby Leaf Lettuce. J. Sci. Food Agric. 2019, 99, 6608–6619. [Google Scholar] [CrossRef]
- Zhen, S.; Bugbee, B. Far-Red Photons Have Equivalent Efficiency to Traditional Photosynthetic Photons: Implications for Redefining Photosynthetically Active Radiation. Plant Cell Environ. 2020, 43, 1259–1272. [Google Scholar] [CrossRef]
- Spalholz, H.; Perkins-Veazie, P.; Hernández, R. Impact of Sun-Simulated White Light and Varied Blue:Red Spectrums on the Growth, Morphology, Development, and Phytochemical Content of Green- and Red-Leaf Lettuce at Different Growth Stages. Sci. Hortic. 2020, 264, 109195. [Google Scholar] [CrossRef]
- Cossu, M.; Tiloca, M.T.; Cossu, A.; Deligios, P.A.; Pala, T.; Ledda, L. Increasing the Agricultural Sustainability of Closed Agrivoltaic Systems with the Integration of Vertical Farming: A Case Study on Baby-Leaf Lettuce. Appl. Energy 2023, 344, 121278. [Google Scholar] [CrossRef]
- Kim, H.H.; Goins, G.D.; Wheeler, R.M.; Sager, J.C. Green-Light Supplementation for Enhanced Lettuce Growth under Red-and Blue-Light-Emitting Diodes. HortScience 2004, 39, 1617–1622. [Google Scholar] [CrossRef]
- Snowden, M.C.; Cope, K.R.; Bugbee, B. Sensitivity of Seven Diverse Species to Blue and Green Light: Interactions with Photon Flux. PLoS ONE 2016, 11, e0163121. [Google Scholar] [CrossRef] [PubMed]
- Samuolienė, G.; Viršilė, A.; Haimi, P.; Miliauskienė, J. Photoresponse to Different Lighting Strategies during Red Leaf Lettuce Growth. J. Photochem. Photobiol. B 2020, 202, 111726. [Google Scholar] [CrossRef]
- Frutos-Totosa, A.; Hernández-Adasme, C.; Martínez, V.; Mestre, T.; Díaz-Mula, H.M.; Botella, M.A.; Flores, P.; Martínez-Moreno, A. Light Spectrum Effects on Rocket and Lamb’s Lettuce Cultivated in a Vertical Indoor Farming System. Sci. Hortic. 2023, 321, 112221. [Google Scholar] [CrossRef]
- Proietti, S.; Moscatello, S.; Riccio, F.; Downey, P.; Battistelli, A. Continuous Lighting Promotes Plant Growth, Light Conversion Efficiency, and Nutritional Quality of Eruca Vesicaria (L.) Cav. in Controlled Environment With Minor Effects Due to Light Quality. Front. Plant Sci. 2021, 12, 730119. [Google Scholar] [CrossRef]
- Shamshiri, R.R.; Kalantari, F.; Ting, K.C.; Thorp, K.R.; Hameed, I.A.; Weltzien, C.; Ahmad, D.; Shad, Z. Advances in Greenhouse Automation and Controlled Environment Agriculture: A Transition to Plant Factories and Urban Agriculture. Int. J. Agric. Biol. Eng. 2018, 11, 1–22. [Google Scholar] [CrossRef]
- Jin, W.; Formiga Lopez, D.; Heuvelink, E.; Marcelis, L.F.M. Light Use Efficiency of Lettuce Cultivation in Vertical Farms Compared with Greenhouse and Field. Food Energy Secur. 2023, 12, 391. [Google Scholar] [CrossRef]
- Carotti, L.; Pistillo, A.; Zauli, I.; Meneghello, D.; Martin, M.; Pennisi, G.; Gianquinto, G.; Orsini, F. Improving Water Use Efficiency in Vertical Farming: Effects of Growing Systems, Far-Red Radiation and Planting Density on Lettuce Cultivation. Agric. Water Manag. 2023, 285, 108365. [Google Scholar] [CrossRef]
- Li, Q.; Li, X.; Tang, B.; Gu, M. Growth Responses and Root Characteristics of Lettuce Grown in Aeroponics, Hydroponics, and Substrate Culture. Horticulturae 2018, 4, 35. [Google Scholar] [CrossRef]
- Zou, J.; Zhang, Y.; Zhang, Y.; Bian, Z.; Fanourakis, D.; Yang, Q.; Li, T. Morphological and Physiological Properties of Indoor Cultivated Lettuce in Response to Additional Far-Red Light. Sci. Hortic. 2019, 257, 108725. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).