Abstract
Improving plant regeneration ability and shortening regeneration time can promote the development of genetic transformation breeding technology for horticultural crops. We optimized several culture conditions, including explant type, mother plant genotype, and medium, to improve shoot formation in winter squash (Cucurbita maxima Duch.). Histological analysis of the occurrence of shoots was also carried out. The results indicate that cotyledon was the most suitable explant for inducing the shoot regeneration of winter squash. We found that ‘Jin-li’ had a shorter shoot induction time and a higher average number of shoots. The highest induction rate of 95.23% among the five lines. The average shoot induction rate of five lines was the highest (84.85%) on Murashige and Skoog (MS) medium supplemented with 2.0 mg/L 6-benzylaminopurine (6-BA) and 0.2 mg/L indole-3-acetic acid (IAA). We also found that there was an interaction between genotypes and induction media, and their interaction had a greater impact on the shoot induction rate than individual effects. Histological observation revealed that the induced shoots of winter squash cotyledons originated from subepidermal cells. We also found that the optimal medium for de novo root regeneration was 1/2 MS. We acclimatized and cultivated regenerated plants and harvested their fruits, which maintained the characteristics of mother plants. These findings lay an important foundation for further research on direct shoot regeneration and accelerate its application in winter squash genetic transformation.
1. Introduction
Winter squash (Cucurbita maxima Duch.) is a fragrant, sweet, nutritious fruit with certain medicinal and health-promoting properties and is very popular among food manufacturers and consumers [1,2,3,4]. Traditional crop breeding methods, such as interspecific hybridization and intraspecific hybridization for C. maxima, have a long cycle and low efficiency and can no longer meet market demand. With the development of genome sequencing and genome editing technologies, the precise improvement of plant traits through genetic transformation has become a new approach for many species of the Cucurbitaceae. According to previous reports, cucumber [5,6,7,8], melon [9,10,11,12,13], and watermelon [14,15,16,17] have successfully undergone gene transformation and genome editing.
The genus Cucurbita is recalcitrant to transformation, and there are few related reports on Cucurbita moschata Duch. In 2011, Nanasato et al. first used wounding with aluminum borate whiskers to induce Agrobacterium-mediated transformation of C. moschata, and the average transgenic efficiency was approximately 2.7% [18,19]. In 2022, Xin et al. utilized CRISPR/Cas9 technology to create compact structural mutants in C. moschata by optimizing genetic transformation programs [20]. A transient transformation system for studying gene function in C. moschata has also been described [21]. However, due to differences in genotype and regeneration pathways, the current transformation methods used for the genus Cucurbita are not universally applicable. Notably, there are no reports of transformation in C. maxima, possibly because of the lack of stable and efficient regeneration systems.
Previous studies have demonstrated that organogenesis in the genus Cucurbita is influenced by explant type, genotype, induction media, and other factors [22,23,24,25]. Lee et al. reported the cultivation of cotyledon explants from two winter squash varieties, both of which successfully produced complete plants, and investigated several factors affecting organogenesis, such as the size of the explants and the type of culture media [26]. The critical factor in inducing plant organogenesis is the use of hormones on explants with regenerative potential at a certain growth stage [27]. Since the organogenesis of winter squash is also influenced by various factors, improving the regeneration rate of winter squash solely through explants is insufficient. More attention should be given to the mutual influence of these factors. A long organogenesis time is also a vital matter that must be addressed in the regeneration of the genus Cucurbita. Additionally, a critical understanding of the timing and site of shoot formation will also be helpful for the development of genetic transformation methods. However, there is currently limited research on organogenesis in winter squash.
To address the aforementioned issues, we evaluated the effects of explant type, genotype, culture media, and their interactions on the de novo organogenesis of winter squash and observed histological events related to the initiation and development of shoots. The aim of this study was to optimize the de novo organogenesis systems of five winter squash lines, determine the timing and origin site of shoots, and obtain complete plants in a short period, providing efficient and stable de novo organogenesis system support for the genetic transformation of winter squash.
2. Materials and Methods
2.1. Plant Materials
In this study, five winter squash lines were used as mother plants (Table 1). Except for L-4 and L-5, all the other lines were the first filial generations of commercial varieties in China. We chose evenly sized, plump seeds and soaked them in tap water for 5 h before peeling off the testae to ensure that the seeds were intact. The seeds were immersed in 75% (v/v) ethanol for 30 s, rinsed with sterile water three times, immersed in a 2% (v/v) sodium hypochlorite solution for 10 min and gently shaken 2–3 times. Next, the seeds were rinsed five times with sterile water. Finally, the seed surfaces were dried with sterile paper, inoculated in culture bottles (6 cm in diameter, 9 cm tall) containing MS media [28] with 10 seeds per bottle, and placed in a 28 °C incubator for dark cultivation for 4 days.

Table 1.
The seed supplier on the varieties that are used for in vitro culture of winter squash.
2.2. Explant Preparation and Plantlet Regeneration
After 4 days of cultivation, the hypocotyls of the plants extended to 2–3 cm, and 2–4 roots were generated. Half of the cotyledons farthest from the hypocotyl were cut off and gently separated from the hypocotyl to form two cotyledons (Figure 1(a1)). Then, the cotyledon was cut in two segments along the main vein to obtain two 1/2 cotyledons (Figure 1(c1)). Half of the cotyledons farthest from the hypocotyl were cut off, 2 mm of the hypocotyl was collected and divided in half, and the main bud was scraped off with a blade, resulting in a cotyledon +2 mm hypocotyl explant (Figure 1(b1)). Two cotyledons were cut off, and a 5 mm hypocotyl was taken from the junction between the cotyledons and hypocotyl (Figure 1(d1)).

Figure 1.
Shoot induction and plant regeneration of winter squash: (a1), cotyledon on the day of culture establishment; (a2), cotyledon regenerated shoot on Murashige and Skoog (MS) medium supplemented with 2.0 mg/L 6-BA and 0.2 mg/L IAA (M8) after 14 days; (a3), cotyledon regenerated roots on MS medium after 14 days; (b1), cotyledon + 2 mm hypocotyl on the day of culture establishment; (b2), cotyledon + 2 mm hypocotyl regenerated shoot on M8 medium after 14 days; (b3), cotyledon + 2 mm hypocotyl regenerated roots on MS medium after 14 days; (c1), 1/2 cotyledon on the day of culture establishment; (c2), 1/2 cotyledon regenerated shoot on M8 medium after 14 days; (c3), 1/2 cotyledon regenerated roots on MS medium after 14 days, (d1), 5 mm hypocotyl on the day of culture establishment; (d2), 5 mm hypocotyl regenerated shoot on M8 medium after 14 days; (d3), 5 mm hypocotyl regenerated roots on MS medium after 14 days; (e), shoot cultured on M8 medium for 4 weeks; (f), rooted plantlet on 1/2 MS (R2) medium after 15 days; (g), rooted plantlet on MS supplemented with 0.1 mg/L NAA (R3) medium after 15 days; arrows, non-embryonic callus tissue; bars = 1.0 cm.
Pre-experiments showed that cotyledon explants curled when they were placed flat on some of the media, causing the backs of the cotyledons to lose contact with the media, thereby affecting the induction rate and shoot quality. In this study, the hypocotyl was placed flat on culture media, while other types of explants were inserted at a 45° angle into the media to address these issues. L-5 (JP) was used for the screening of explant types. The culture was maintained at 25 °C with a 16/8 h (light/dark) photoperiod and a light intensity of 2000 lux, and the same medium was replaced every 14 days until shoots appeared. After about 28 days of cultivation, the shoot reached a height of 2.0–3.0 cm or 2–3 leaves were growing. The shoot was separated from the explant and transferred to R media to induce rooting under the same cultivation conditions.
2.3. Media Composition and Preparation
The previously mentioned MS culture medium contained Murashige and Skoog basal salt and vitamins (all the materials were purchased from Beijing Coolaber Technology Co., Ltd.; Beijing, China). The media compositions are shown in Table 2. Before dividing the culture media into 40 mL portions that were placed into culture bottles, the pH of all culture media was adjusted to 5.8 ± 0.1, and then the media were autoclaved at 121 °C for 20 min.

Table 2.
The composition of culture medium for the induction of shoots and roots of winter squash.
2.4. Histological Analysis
For histological observations, cotyledons were placed on shoot-inducing medium M8 and sampled after 0, 3, 6, 8, and 12 days of culture. The cultivation conditions were the same as those in Section 2.2. Three small pieces (2 mm × 2 mm) were taken at each cultivation stage of cotyledons. Small pieces cut from the junction between cotyledons and hypocotyls were immersed in formaldehyde-acetic acid-ethanol (FAA) fixative (provided by Wuhan Servicebio Technology Co., Ltd., Wuhan, China) for 24 h. The fixed sample was washed twice with 50% ethanol absolute and then dehydrated. It was sequentially treated with ethanol absolute at concentrations of 75%, 85%, 95%, and 100%, followed by 2/3 ethanol absolute +1/3 xylene,1/2 ethanol absolute +1/2 xylene,1/3 ethanol absolute +2/3 xylene, and xylene twice, and each step lasted for 1–2 h. The dehydrated sample was immersed in paraffin at room temperature (20–30 °C) until saturated, and then transferred to a constant temperature box at 30–40 °C. When the volume ratio of xylene to paraffin reached 1:1, it was transferred to a constant temperature box at 58–60 °C for complete volatilization, and it was embedded after 6 h. The cross-sectional and longitudinal sections were cut to 5–7 μm thickness using an ultramicrotome (Leica RM2016, Leica Microsystems Trading (Shanghai) Co., Ltd., Shanghai, China) and stained with hematoxylin-eosin. The slices were dehydrated and cleared before they were sealed with gum and observed under an optical microscope (Nikon Eclipse Ci, Nikon Precision Shanghai Co.,Ltd., Shanghai, China). Images were recorded using a charge-coupled device (CCD) connected to a microscope, and analyzed using image processing software (CaseViewer 2.4, The Digital Pathology Company, Budapest, Hungary).
2.5. Propagation, Acclimatization, and Transplantation
Under sterile conditions, the regenerated plant, cultured for 4 weeks to be propagated, was cut into stem segments of about 3 cm (including a mature leaf and lateral bud) and inserted into R2 medium (Table 2). The cultivation conditions were the same as those in Section 2.2. After about 4 weeks, it has grown 5–7 leaves and can continue to propagate in a circular manner or wash the culture medium of its roots before transplanting to the seedling substrate and acclimatizing in an artificial climate box. The humidity of the artificial climate box was set to 90%, the temperature was 25 °C, the light cycle was 16/8 h (light/dark), and the light intensity was 2000 lux. After one week, the humidity of the artificial climate box was adjusted to 80%, the temperature cycle was 25/18 °C (light/dark), the light cycle was 16/8 h (light/dark), and the light intensity was 2000 lux. After two to three weeks, the acclimatization was completed, and the plants were transplanted into the greenhouse with a spacing of 50–60 cm.
2.6. Statistical Analysis
The experiments were performed at least three times. (i) The shoot induction rate (number of explants induced for shoots/30 explants), (ii) average number of shoots (number of shoots/30 explants), (iii) rooting induction rate (number of rooted plantlets/30 shoots), and (iv) average number of roots (number of roots/30 shoots). Each treatment consisted of 30 explants, with a set of three replicates for each experiment. The data were processed using SPSS 26.0 (IBM, Chicago, IL, USA). One-way analysis of variance (ANOVA) was used to evaluate the effects of genotype and shoot induction media. The shoot regeneration data were analyzed using a two-way ANOVA factorial experimental design to test the significance of genotype (lines) and induction media (media). The initial data were collected in 2021 and 2023.
3. Results
3.1. Effects of Explant Type on Shoot Induction
Generally, the explants enlarged considerably, and the color changed from light yellow to light green and then to dark green during the first few days in culture (Figure 1). Subsequently, sporadic white callus tissues, which were fragile nonembryonic callus tissues, appeared at the contact points between the explants and culture medium (Figure 1, arrows). The explants regenerated buds on the induction medium (Figure 1(a2,b2,c2,d2)), and regenerated roots on MS medium (Figure 1(a3,b3,c3,d3)). At 2–3 weeks, the explants no longer grew, and their area was two to three times larger than that before cultivation, while the shoots continued to grow. L-5 (JP) was used for the screening of explant types. The regeneration results after the fourth week showed that all four types of explants induced shoot formation, and there was a significant difference in the shoot induction rate (Figure 2). When the four types of explants were induced on media containing 6-BA (M1~M4), the shoot induction rates of the four types of explants tended to first increase and then decrease with increasing 6-BA concentration, and their shoot induction rates were higher on media supplemented with 2 mg/L 6-BA (M2). Among the six media combi-nations of NAA,2,4-D, IAA and 6-BA, M8 (2.0 mg/L 6-BA + 0.2 mg/L IAA) ranged from 40.51% to 90.50% had a higher shoot induction rate ranged from 40.51% to 90.50% among the four types of explants than did the other media. An evaluation of the regeneration of four types of explants on ten induction media showed that the induction rate of cotyledon regeneration was higher than that of the other three types of explants, which was the most suitable explant for inducing winter squash shoot regeneration.
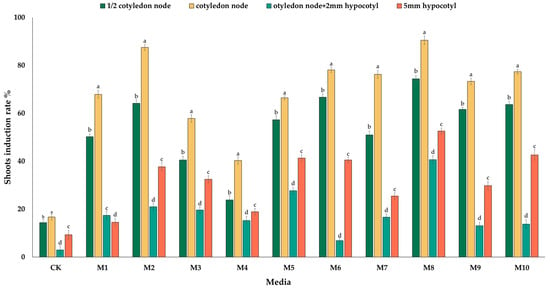
Figure 2.
Four types of explants of L-5 (JP) induced shoot formation in ten media: means followed by the same letter in a column are not significantly different (±SD) at the 0.05 level based on Duncan’s multiple range test (DMRT); CK is MS media.
3.2. Effects of Genotype on De Novo Shoot Regeneration
The purpose of this experiment was to evaluate the effect of genotype on shoot regeneration through the regeneration of cotyledons from five lines, and the results are shown in Table 3. Based on previous experiments, four media were selected, and all five lines were able to induce shoot regeneration on these four media. Shoot production was quantified for each genotype, revealing that the five lines had different shoot induction rates on the same media. L-2 (85.67%), L-3 (95.23%), L-4 (80.33%), and L-5 (90.50%) had the highest shoot induction rates on M8, whereas L-1 (91.16%) had the highest shoot induction rates on M6. L-2 and L-3 shoots appeared earlier (8.33–10.33 days), while L-4 and L-1 shoots appeared later (13.33–15.67 days). The average shoot induction rates of L-2 and L-3 are higher than the other three lines. L-3 had the shortest number of days required for shoot regeneration, as well as the highest regeneration rate and average number of shoots among the five genotypes (Table 3).

Table 3.
Shoots induction through cotyledons culture with different lines.
3.3. Effects of Media on De Novo Shoot Regeneration
The results for shoot induction through cotyledons on the four media are shown in Table 4. All media clearly induced shoot formation, but there were significant differences in the shoot induction rate of the same line among the different media. For example, the shoot induction rates and average numbers of shoots of L-3 on M2 and M8 media were significantly greater than those on the other four media, while the shoot regeneration rates on M6 media were significantly lower than those on the other media. The evaluation of the average shoot induction rate showed that M8 medium (2 mg/L 6-BA + 0.2 mg/L IAA) had the highest average shoot induction rate (84.85%) of the five lines, suggesting that M8 is a more suitable induction medium for the de novo regeneration of winter squash shoots.

Table 4.
Shoots induction through cotyledons culture with different media.
3.4. Effects of Genotype and Induction Medium on De Novo Shoot Regeneration
We used marginal mean estimation to create a model graph to infer the average values for the genotype, culture medium, and genotype * culture media (Figure 3). The p values for genotype, media, and lines * media were all less than 0.05, as shown in Table 5, clearly demonstrating that genotype, media, and their interaction significantly impacted shoot induction. The largest difference in the partial eta squared (ηp2) value was observed for genotype * media (ηp2 = 0.993), indicating that there was an interaction between them and that their interaction effect was greater than their individual effects.
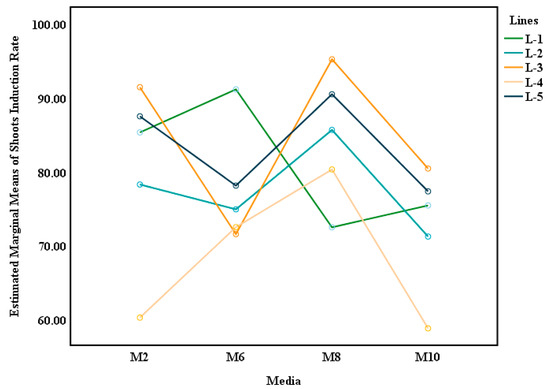
Figure 3.
Interaction effect of genotype and media on shoot induction: the lines are the mother plants; the x-axis shows the four types of shoot induction media, and the y-axis shows the average shoot induction rates. The intersecting lines indicate the interactions between two factors.

Table 5.
Tests of Between-Subjects Effects: Effects of genotype, induction media and their interaction on shoots induction.
3.5. Histological Observation of Adventitious Shoot Formation
To facilitate the identification of specific cells that can respond to external stimuli to produce shoots, we first determined the anatomical structure of the cotyledons (Figure 4a,b). As shown in the adaxial cross section, the winter squash cotyledon is composed of epidermis, mesophyll, and conductive tissue (Figure 4b). On the third day of cultivation, significant cellular changes occurred in the subepidermal area. First, subepidermal cells were activated and rapidly divided under induction in culture medium (Figure 4c). These cell masses continue to proliferate, leading to rapid expansion of the total volume and surface area (Figure 4d). On the eighth day of explant establishment and culture, regenerated shoots appeared (Figure 4e). After 12 days of cultivation on induction media, the buds exhibited visible conductive tissue connections (Figure 4f). Histological observation and analysis indicated that the origin of de novo shoot regeneration was from subepidermal cells, and the shoots induced from winter squash explants had the same structure as those produced during seed development (Figure 4g,h).
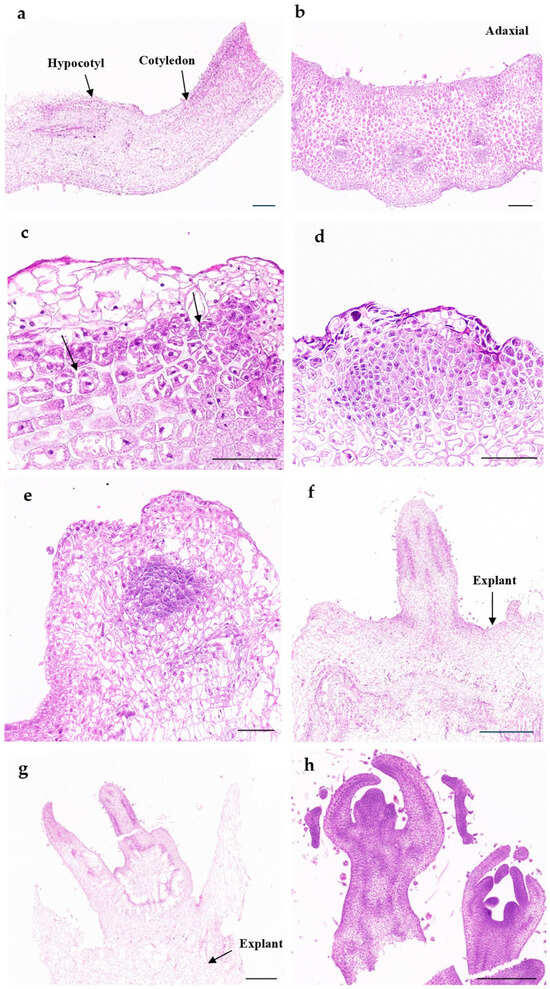
Figure 4.
Histological observation of de novo shoot regeneration in cotyledon explants: (a) longitudinal section of explant on the day of culture establishment, bar = 300 μm; (b) cross section of explant on the day of culture establishment, bar = 200 μm; (c) after 3 days on induction medium, subepidermal cells divided; arrows indicate cells after division, bar = 100μm; (d) after 6 days of cultivation on induction medium, subepidermal cells proliferate and form protrusions, bar = 50 μm; (e) young adventitious shoot (the precursor of the shoots) grows on the explant after 8 days of cultivation, bar = 100 μm; (f) shoot with connection of conductive tissue of explant after 12 days of cultivation, bar = 500 μm; (g) regenerated shoot after 3 weeks of cultivation, bar = 500μm; (h) shoots developed from seeds, bar = 500 μm.
3.6. Root Regeneration and Character Observation
After four weeks of cultivation, the shoots had grown to a height of 2.0–3.0 cm and had two to three leaves (Figure 1e). At this time, the shoot was removed from the explant and transferred to R media to induce rooting and form a complete plant (Figure 1f,g). As shown in Table 6, the average number of days required for winter squash (L-1–L-5) to root on the five R media was 8.67–13.33 days. Adding NAA (0.1~0.3 mg/L) relatively reduced the number of days of root induction and increased the number of roots. The R media were ranked according to the root induction rate as R3 < R2 < R1 < R4 < R5. The shoots were induced to have multiple thick roots in R3 medium, with an average number of 4.73 roots and a rooting rate of 97.77%. However, the internodes of the plants were long, and the leaves were small, exhibiting a phenomenon similar to excessive growth (Figure 1g). With increasing NAA concentration (0.1~0.5 mg/L), both root growth and number of roots decreased. Shoots were grown in R2 media (1/2 MS) for 12.33 days, at a rooting rate of 95.55% and an average of 3.54 roots, after which the plants grew vigorously (Figure 1f). Based on the root induction indicators in Table 6 and plant growth, R2 media was more suitable for root induction.

Table 6.
Effect of different concentration of MS and NAA on root induction of five winter squash.
Three lines of regenerated plants were selected for acclimatization and cultivation (L-1, L-3, and L5). After 2 weeks of acclimatization (Figure 5a), these plants were transplanted to greenhouses and single vine pruning was performed (Figure 5b). The regenerated plants of three lines all harvested fruits (Figure 5c–e), and their growth periods and fruit characteristics are shown in Table 7. These plants maintain the characteristics of mother plants from the first female flower node, fruit development period, growth period, and average single fruit weight.
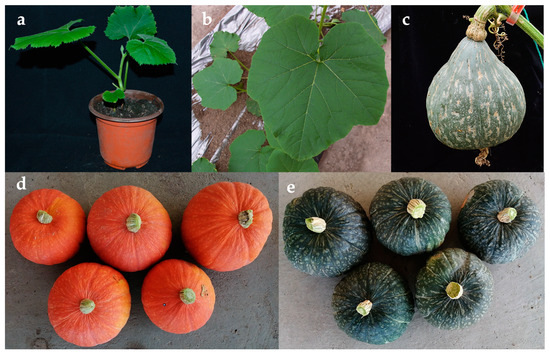
Figure 5.
Observation of characteristics of regenerated plants: (a) regenerated plant after two weeks of acclimatization; (b) regenerated plant during the nutritional growth period; (c) the fruit of regenerated plants of L-5; (d) the fruit of regenerated plants of L-1; (e) the fruit of regenerated plants of L-3.

Table 7.
Observation on the growth period and fruit characteristics of regenerated plants from L-1, L-3, and L5.
4. Discussion
Whether or not winter squash explants will begin to regenerate will largely depend on the extent of their limitations in relation to their original tissue capacity. In this study, we designed four explants with regeneration potential, and the shoot induction rates showed that the plants could produce shoots (Figure 2). The shoot regeneration rates observed in the cotyledons were greater than those in the half cotyledons (1/2 cotyledons). Similarly, Kim et al. reported that smaller cotyledons of figleaf gourd (C. ficifolia Bouche) produced fewer adventitious shoots, which indicated that the shoot induction rate is greater when the area adjacent to the explant containing the cotyledon and hypocotyl is greater [29]. We found that the shoots appeared at the junction between the cotyledon and hypocotyl, which is consistent with previous findings for C. pepo [30]. We achieved a regeneration rate of 52.62% for the 5 mm long hypocotyl. However, Lee et al. reported that shoots cannot be regenerated from winter squash hypocotyls [26]. We speculate that differences in the specific location of hypocotyl sampling and genotype may account for this discrepancy in results. The shoot regeneration rate of cotyledons was significantly greater than that of the other three types of explants on ten different media (Figure 2). Previous studies have shown that plant regeneration from cotyledons is an immensely reliable reproductive process that maintains genetic similarity. Cotyledons can serve as explants for de novo shoot regeneration in winter squash.
Genotype is an important factor affecting cell division, differentiation, and morphogenesis in the organogenesis of the family Cucurbitaceae [31]. Obtaining effective regenerated plants from commercial genotypes is a prerequisite for applying genetic transformation techniques for crop improvement [32]. Differences in embryo and shoot induction rates have been observed even when cultured on the same medium in plants such as summer squash (C. pepo) [25] and pumpkin (C. moschata) varieties [33]. This experiment evaluated the effect of genotype on regeneration using three widely cultivated varieties (L-1, L-2, and L-3) in China and two inbred lines (L-4 and L-5) from Hunan Province. The results showed that the induction rate of regenerated shoots was the highest for L-3 (Jin-Li), followed by L-2 (Hong-li No. 2) and L-5 (JP) (Table 3). In addition, the number of regeneration days and average number of shoots were closely related to the tested genotypes. Overall, under the same cultivation conditions, the shoot regeneration rate of the commercial varieties was greater than that of the inbred lines.
The induction medium components had a strong impact on the regeneration of winter squash. It is thought that cytokinin activity in explants can be effectively triggered by lower doses of auxin to produce more shoots [34]. However, Zhang et al. concluded that 6-BA is necessary for C. moschata to induce shoot formation in cotyledons and that there was no difference in the concentration of 6-BA between 0.50~2.00 mg/L [35]. Similarly, Samuel Aworunse et al. reported a lack of shoot response in C. pepo explants cultured on media supplemented with both 6-BA and 2,4-D, whereas lower concentrations of 6-BA alone achieved better outcomes [36]. In this paper, shoot induction was assessed using four media supplemented with 6-BA and six combinations of media, and the results indicated that on culture media supplemented with only 6-BA (1.0 mg/L-4 mg/L), the shoot induction rate tended to increase and then decrease with increasing 6-BA concentration (Figure 2). Although 6-BA influences cotyledon regeneration, the addition of 2.0 mg/L6-BA and 0.2 mg/L IAA media resulted in a greater shoot induction rate than did the addition of media supplemented with only 2.0 mg/L6-BA (Table 4). Previous studies have also reported similar phenomena [37,38,39]. A further finding was the production of fragile white embryogenic callus tissue at the contact point between the explant and culture medium, which increased with increasing hormone content. This phenomenon also occurs in C. moschata [35]. Ananthakrishnan et al. suggested that rapid callus growth in C. pepo reduces shoot regeneration [30]. However, our results showed that this fact did not affect the regeneration of shoots. This difference may be due to the difference in the ratio of auxin to cytokinin in the media.
Several factors can affect the induction of Cucurbitaceae shoot growth, the two most important of which are genotype and medium composition [40,41]. The mutual influence between these factors leads to increased complexity of this process. To date, many studies have focused on single-factor effects, but evaluations of the interactions between factors in the genus Cucurbita have not yet been performed. However, we can still draw some insights from previous research. Lee et al. induced shoot formation from two genotypes of winter squash on 6-BA media supplemented with four different concentrations of salt [26]. The results showed that the different genotypes had distinct regeneration rates on the same medium and that the same genotype had varying regeneration rates on different media, indicating an interaction between these two factors. A similar conclusion was reached in another study on shoot regeneration in C. pepo [25]. In this study, we determined the impact of genotype and induction medium interactions on shoot induction through two-way ANOVA and found that their interactions had a greater impact on shoot induction than did their individual effects. This result indicates that mutual interactions among factors represent the main stimuli for inducing shoot growth and should receive increased attention.
MS, 1/2 MS, or medium supplemented with the hormones IAA and NAA are commonly used to induce root growth in Cucurbita. Kurtar et al. rooted C. maxima and C. moschata plants on MS media supplemented with 0.01 mg/l IAA [42]. Kabir et al. also used this medium to induce root growth in sweet gourd (C. moschata) [33]. We evaluated whether the rooting duration, rooting rate, and average rooting number of the winter squash plants were variable in the five different culture media (Table 6). The highest rooting rate was observed for R3 (97.77%), followed by R2 (95.55%), and the lowest rooting rate was observed for R5 (66.87%). From the perspective of plant growth status, R2 (1/2 MS) is the most suitable as a root induction medium for winter squash. We also found that the rooting time of shoots in culture medium with 0.1–0.3 mg/L NAA added was reduced compared to that in MS medium.
The development of plants is largely determined by their ability to form new organs [43]. Somatic cell regeneration in the family Cucurbitaceae can occur from cotyledons or through calli. Although genetic transformation plants through cotyledon regeneration may exhibit chimerism, de novo organogenesis is more suitable for the genus Cucurbita due to its minimal potential for somaclonal variation and capacity for a shorter regeneration time [20]. Histological observations revealed that cell division occurred in the subepidermal region before the appearance of meristem tissue (Figure 4). These cells, which have perpetual proliferative abilities, continuously proliferate through mitosis and differentiate into shoots. However, during the normal growth process of plants, cells with potential meristematic ability usually have little or no meristematic function [27]. The anatomical structures of the de novo regenerated shoots and the vegetative buds produced on intact plants are similar. We believe that these proliferating cells under the epidermis may be targets for direct gene transfer in winter squash cotyledon explants. If the intensity of the Agrobacterium infection can be controlled at these sites, the probability of obtaining transformed plants will greatly increase.
5. Conclusions
In this study, we successfully established an effective protocol for the de novo organogenesis of five winter squash over approximately 50 days. First, we evaluated the shoot induction rates of four types of explants. The shoot induction rate was highest in the cotyledons, making them the most suitable explants for winter squash. Second, the effects of genotype and medium on regeneration were quantified. The commercial variety L-3 (Jin-Li) had the shortest shoot regeneration period and the highest regeneration rate and average number of shoots among the five genotypes. M8 medium (2 mg/L 6-BA + 0.2 mg/L IAA) had the highest average induction rate (84.85%) for the five strains of shoots and can be used as a medium for winter squash de novo shoot regeneration. Moreover, we first found that the interaction effect between genotype and induction medium was greater than the effect of each factor individually. Third, we conducted histological observations of the de novo shoot regeneration process and determined that the induced shoots of cotyledons originated from subepidermal cells, establishing this report as the first to describe the origin of shoot regeneration in winter squash. Fourth, we confirmed that 1/2 MS is suitable for root regeneration. Finally, we acclimatized and cultivated the regenerated plants, which maintained the characteristics of mother plants in their growth period and fruit. This study provides an important foundation for future de novo shoots regeneration research on the family Cucurbitaceae and accelerates the application of cotyledon culture systems in genetic transformation breeding technology for winter squash.
Author Contributions
Conceptualization, H.S. and X.S.; Data curation, H.J.; Formal analysis, H.Y.; Funding acquisition, X.S.; Investigation, S.D.; Methodology, X.S.; Project administration, X.S.; Resources, L.S.; Software, Z.C.; Supervision, X.S.; Validation, H.S., J.Y. and H.J.; Visualization, Z.C.; Writing—original draft, H.S.; Writing—review and editing, H.S. and X.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Department Of Science And Technology Of Hunan Province, grant number 2022NK2007.
Data Availability Statement
Data are contained within the article, further inquiries can be directed to the corresponding author.
Acknowledgments
This work was supported by the Engineering Research Center for Germplasm Innovation and New Varieties Breeding of Horticultural Crops, Key Laboratory for Vegetable Biology of Hunan Province, College of Horticulture, Hunan Agricultural University, Changsha, China. In addition, we thank Yan Weidong from the School of Mechanical Engineering and Automation at Fuzhou University for his assistance in statistical analysis, and Yusong Luo from the Horticultural College of Hunan Agricultural University for her profound comments on the previous version of this manuscript. We thank the editors and reviewers for their valuable insights and constructive suggestions.
Conflicts of Interest
Longjun Sun was employed by the company Hunan Xuefeng Seed Industry Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Miljić, M.; Rocchetti, G.; Krstić, S.; Mišan, A.; Brdar-Jokanović, M.; Marcheggiani, F.; Martinelli, E.; Lucini, L.; Damiani, E. Comparative In Vitro Antioxidant Capacity and Terpenoid Profiling of Pumpkin Fruit Pulps from a Serbian Cucurbita maxima and Cucurbita moschata Breeding Collection. Antioxidants 2021, 10, 1580. [Google Scholar] [CrossRef] [PubMed]
- El-Gendi, H.; Abu-Serie, M.M.; Kamoun, E.A.; Saleh, A.K.; El-Fakharany, E.M. Statistical Optimization and Characterization of Fucose-Rich Polysaccharides Extracted from Pumpkin (Cucurbita maxima) along with Antioxidant and Antiviral Activities. Int. J. Biol. Macromol. 2023, 232, 123372. [Google Scholar] [CrossRef] [PubMed]
- Abbas, H.M.K.; Huang, H.X.; Huang, W.J.; Xue, S.D.; Yan, S.J.; Wu, T.Q.; Li, J.X.; Zhong, Y.J. Evaluation of metabolites and antioxidant activity in pumpkin species. Nat. Prod. Commun. 2020, 15, 1934578X20920983. [Google Scholar] [CrossRef]
- Adams, G.G.; Imran, S.; Wang, S.; Mohammad, A.; Kok, M.S.; Gray, D.A.; Channell, G.A.; Harding, S.E. The Hypoglycemic Effect of Pumpkin Seeds, Trigonelline (TRG), Nicotinic Acid (NA), and D-Chiro-Inositol (DCI) in Controlling Glycemic Levels in Diabetes Mellitus. Crit. Rev. Food Sci. Nutr. 2014, 54, 1322–1329. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.V.; Hoang, T.T.-H.; Le, N.T.; Tran, H.T.; Nguyen, C.X.; Moon, Y.-H.; Chu, H.H.; Do, P.T. An Efficient Hairy Root System for Validation of Plant Transformation Vector and CRISPR/Cas Construct Activities in Cucumber (Cucumis sativus L.). Front. Plant Sci. 2022, 12, 770062. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Li, D.; Liu, X.; Qi, J.; Gao, D.; Zhao, S.; Huang, S.; Sun, J.; Yang, L. Engineering Non-Transgenic Gynoecious Cucumber Using an Improved Transformation Protocol and Optimized CRISPR/Cas9 System. Mol. Plant 2017, 10, 1575–1578. [Google Scholar] [CrossRef]
- Baskaran, P.; Soós, V.; Balázs, E.; Van Staden, J. Shoot Apical Meristem Injection: A Novel and Efficient Method to Obtain Transformed Cucumber Plants. S. Afr. J. Bot. 2016, 103, 210–215. [Google Scholar] [CrossRef]
- Nanasato, Y.; Konagaya, K.; Okuzaki, A.; Tsuda, M.; Tabei, Y. Improvement of Agrobacterium-Mediated Transformation of Cucumber (Cucumis sativus L.) by Combination of Vacuum Infiltration and Co-Cultivation on Filter Paper Wicks. Plant Biotechnol. Rep. 2013, 7, 267–276. [Google Scholar] [CrossRef]
- Valls, M.P.; Lasa, J.M. Agrobacterium-Mediated Transformation of Commercial Melon (Cucumis Melo L., Cv. Amarillo Ore). Plant Cell Rep. 1994, 13, 145–148. [Google Scholar]
- Grumet, R. Agrobacterium Tumefaciens Mediated Transformation and Regeneration of Muskmelon Plants. Plant Cell Rep. 1990, 9, 160–164. [Google Scholar]
- Hooghvorst, I.; López-Cristoffanini, C.; Nogués, S. Efficient Knockout of Phytoene Desaturase Gene Using CRISPR/Cas9 in Melon. Sci. Rep. 2019, 9, 17077. [Google Scholar] [CrossRef]
- Akasaka-Kennedy, Y.; Tomita, K.; Ezura, H. Efficient Plant Regeneration and Agrobacterium-Mediated Transformation via Somatic Embryogenesis in Melon (Cucumis melo L.). Plant Sci. 2004, 166, 763–769. [Google Scholar] [CrossRef]
- Nizan, S.; Amitzur, A.; Dahan-Meir, T.; Benichou, J.I.C.; Bar-Ziv, A.; Perl-Treves, R. Mutagenesis of the Melon Prv Gene by CRISPR/Cas9 Breaks Papaya Ringspot Virus Resistance and Generates an Autoimmune Allele with Constitutive Defense Responses. J. Exp. Bot. 2023, 74, 4579–4596. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, J.; Guo, S.; Tian, S.; Zhang, J.; Ren, Y.; Li, M.; Gong, G.; Zhang, H.; Xu, Y. CRISPR/Cas9-Mediated Mutagenesis of ClBG1 Decreased Seed Size and Promoted Seed Germination in Watermelon. Hortic. Res. 2021, 8, 70. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Jiang, L.; Gao, Q.; Zhang, J.; Zong, M.; Zhang, H.; Ren, Y.; Guo, S.; Gong, G.; Liu, F.; et al. Efficient CRISPR/Cas9-Based Gene Knockout in Watermelon. Plant Cell Rep. 2017, 36, 399–406. [Google Scholar] [CrossRef]
- Tian, S.; Jiang, L.; Cui, X.; Zhang, J.; Guo, S.; Li, M.; Zhang, H.; Ren, Y.; Gong, G.; Zong, M.; et al. Engineering Herbicide-Resistant Watermelon Variety through CRISPR/Cas9-Mediated Base-Editing. Plant Cell Rep. 2018, 37, 1353–1356. [Google Scholar] [CrossRef]
- Feng, Q.; Xiao, L.; He, Y.; Liu, M.; Wang, J.; Tian, S.; Zhang, X.; Yuan, L. Highly Efficient, Genotype-independent Transformation and Gene Editing in Watermelon (Citrullus Lanatus) Using a Chimeric ClGRF4-GIF1 Gene. Integr. Plant Biol. 2021, 63, 2038–2042. [Google Scholar] [CrossRef]
- Nanasato, Y.; Tabei, Y. A Method of Transformation and Current Progress in Transgenic Research on Cucumbers and Cucurbita Species. Plant Biotechnol. 2020, 37, 141–146. [Google Scholar] [CrossRef]
- Nanasato, Y.; Konagaya, K.; Okuzaki, A.; Tsuda, M.; Tabei, Y. Agrobacterium-Mediated Transformation of Kabocha Squash (Cucurbita Moschata Duch) Induced by Wounding with Aluminum Borate Whiskers. Plant Cell Rep. 2011, 30, 1455–1464. [Google Scholar] [CrossRef][Green Version]
- Xin, T.; Tian, H.; Ma, Y.; Wang, S.; Yang, L.; Li, X.; Zhang, M.; Chen, C.; Wang, H.; Li, H.; et al. Targeted Creation of New Mutants with Compact Plant Architecture Using CRISPR/Cas9 Genome Editing by an Optimized Genetic Transformation Procedure in Cucurbit Plants. Hortic. Res. 2022, 9, uhab086. [Google Scholar] [CrossRef]
- Chen, X.; He, S.; Jiang, L.; Li, X.; Guo, W.; Chen, B.; Zhou, J.; Skliar, V. An Efficient Transient Transformation System for Gene Function Studies in Pumpkin (Cucurbita moschata D.). Sci. Hortic. 2021, 282, 110028. [Google Scholar] [CrossRef]
- Ananthakrishnan, G.; Xia, X.; Amutha, S.; Singer, S.; Muruganantham, M.; Yablonsky, S.; Fischer, E.; Gaba, V. Ultrasonic Treatment Stimulates Multiple Shoot Regeneration and Explant Enlargement in Recalcitrant Squash Cotyledon Explants in Vitro. Plant Cell Rep. 2007, 26, 267–276. [Google Scholar] [CrossRef]
- Jelaska, S. Embryogenesis and Organogenesis in Pumpkin Explants. Physiol. Plant 1974, 31, 257–261. [Google Scholar] [CrossRef]
- Zou, T.; Song, H.; Chu, X.; Tong, L.; Liang, S.; Gong, S.; Yang, H.; Sun, X. Efficient Induction of Gynogenesis through Unfertilized Ovary Culture with Winter Squash (Cucurbita maxima Duch.) and Pumpkin (Cucurbita moschata Duch.). Sci. Hortic. 2020, 264, 109152. [Google Scholar] [CrossRef]
- Gonsalves, C.; Xue, B.; Gonsalves, D. Somatic Embryogenesis and Regeneration from Cotyledon Explants of Six Squash Cultivars. HortScience 1995, 30, 1295–1297. [Google Scholar] [CrossRef]
- Lee, Y.K.; Chung, W.I.; Ezura, H. Efficient Plant Regeneration via Organogenesis in Winter Squash (Cucurbita maxima Duch.). Plant Sci. 2003, 164, 413–418. [Google Scholar] [CrossRef]
- Sugimoto, K.; Temman, H.; Kadokura, S.; Matsunaga, S. To Regenerate or Not to Regenerate: Factors That Drive Plant Regeneration. Curr. Opin. Plant Biol. 2019, 47, 138–150. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Kim, K.-M.; Kim, C.K.; Han, J.-S. In Vitro Regeneration from Cotyledon Explants in Figleaf Gourd (Cucurbita ficifolia Bouché), a Rootstock for Cucurbitaceae. Plant Biotechnol. Rep. 2010, 4, 101–107. [Google Scholar] [CrossRef]
- Ananthakrishnan, G.; Xia, X.; Elman, C.; Singer, S.; Paris, H.S.; Gal-On, A.; Gaba, V. Shoot Production in Squash (Cucurbita Pepo) by in Vitro Organogenesis. Plant Cell Rep. 2003, 21, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Kathiravan, K.; Vengedesan, G.; Singer, S.; Steinitz, B.; Paris, H.S.; Gaba, V. Adventitious Regeneration in Vitro Occurs across a Wide Spectrum of Squash (Cucurbita pepo) Genotypes. Plant Cell Tiss. Organ Cult. 2006, 85, 285–295. [Google Scholar] [CrossRef]
- Raza, G.; Singh, M.B.; Bhalla, P.L. Somatic Embryogenesis and Plant Regeneration from Commercial Soybean Cultivars. Plants 2019, 9, 38. [Google Scholar] [CrossRef] [PubMed]
- Kabir, M.R.; Akhond, M.Y.; Amin, M.A.; Haque, M.S. In Vitro Regeneration of Sweetgourd (Cucurbita moschata Duch.). Plant Tissue Cult. Biotech. 2016, 26, 67–75. [Google Scholar] [CrossRef]
- Venkatachalam, P.; Jinu, U.; Sangeetha, P.; Geetha, N.; Sahi, S.V. High Frequency Plant Regeneration from Cotyledonary Node Explants of Cucumis sativus L. Cultivar ‘Green Long’ via Adventitious Shoot Organogenesis and Assessment of Genetic Fidelity by RAPD-PCR Technology. 3 Biotech 2018, 8, 60. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, J.; Wu, T.; Cao, J. Shoot Regeneration and the Relationship between Organogenic Capacity and Endogenous Hormonal Contents in Pumpkin. Plant Cell Tiss. Organ Cult. 2008, 93, 323. [Google Scholar] [CrossRef]
- Samuel Aworunse, O.; Voke Omasoro, R.; Soneye, B.; Odun Obembe, O. Effect of Low BAP Levels on Multiple Shoots Induction in Indigenous Nigerian Pumpkin (Cucurbita pepo Linn.). J. Phys. Conf. Ser. 2019, 1299, 012100. [Google Scholar] [CrossRef]
- Zhang, H.; Peng, G.; Feishi, L. Efficient Plant Regeneration from Cotyledonary Node Explants of Cucumis melo L. Afr. J. Biotechnol. 2011, 10, 6757–6761. [Google Scholar]
- Ficcadenti, N.; Rotino, G.L. Genotype and Medium Affect Shoot Regeneration of Melon. Plant Cell Tiss. Organ Cult. 1995, 40, 293–295. [Google Scholar] [CrossRef]
- Ren, Y.; Bang, H.; Gould, J.; Rathore, K.S.; Patil, B.S.; Crosby, K.M. Shoot Regeneration and Ploidy Variation in Tissue Culture of Honeydew Melon (Cucumis melo L. Inodorus). In Vitro Cell. Dev. Biol.-Plant 2013, 49, 223–229. [Google Scholar] [CrossRef]
- Urbanek, A.; Zechmann, B.; Muller, M. Plant Regeneration via Somatic Embryogenesis in Styrian Pumpkin: Cytological and Biochemical Investigations. Plant Cell Tiss. Organ Cult. 2004, 79, 329–340. [Google Scholar] [CrossRef]
- Parvin, S.; Kausar, M.; Haque, M.E.; Khalekuzzaman, M.; Sikdar, B.; Islam, M.A. In Vitro Propagation of Muskmelon (Cucumis melo L.) from Nodal Segments, Shoot Tips and Cotyledonary Nodes. Rajshahi Univ. J. Life Earth Agric. Sci. 2015, 41, 71–77. [Google Scholar] [CrossRef][Green Version]
- Kurtar, E.S.; Balkaya, A.; Ozbakir Ozer, M. Production of Callus Mediated Gynogenic Haploids in Winter Squash (Cucurbita maxima Duch.) and Pumpkin (Cucurbita moschata Duch.). Czech J. Genet. Plant Breed. 2018, 54, 9–16. [Google Scholar] [CrossRef]
- Benková, E.; Michniewicz, M.; Sauer, M.; Teichmann, T.; Seifertová, D.; Jürgens, G.; Friml, J. Local, Efflux-Dependent Auxin Gradients as a Common Module for Plant Organ Formation. Cell 2003, 115, 591–602. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).