Abstract
The genus Sorbus has maintained an extremely relevant role over time from a landscape and environmental perspective in many countries in the Mediterranean and Central Europe. Based on the requirements coming from the environmental policies provided in the European strategy Next Generation EU, Sorbus has been considered a valuable species to be introduced in urban and peri-urban areas. The purpose of this study was to propagate four Sorbus accessions selected in the Sicilian territory, Southern Italy, using the liquid substrate in temporary immersion bioreactors Plantform™. The results obtained showed that the presence of 1 mg L−1 mT in the substrate in combination with IBA 0.05 mg L−1 produced a significant number of shoots (4.7) and a greater length (2.2 cm). Among the accessions, there were statistically significant differences; the accession SN2 and SN1 produced more shoots (respectively, 4.0 and 3.6), and a greater length of the shoots was observed in the selections SN4 and SN3 (respectively, 2.4 cm and 2.3 cm). The relative growth rate (RGR) was significantly influenced by the presence of the culture substrate of the combination of cytokines and auxin; SN4 selection showed the best RGR results of 8.3 mg−1 d−1. The use of the bioreactor Plantform™ in Sorbus domestica L. has favored a better development of plants obtained in vitro, demonstrating that this system is a valid alternative for the micropropagation of Sorbus.
1. Introduction
The genus Sorbus L. belongs to the Rosaceae family and is native to Central and Southern Europe, Asia Minor, and North Africa [1]. It is a frequent species throughout its natural range, almost never cultivated in specialized plantations but common in an agroforestry ecosystem. Most species are grown as ornamental plants, with only a few cultivated for their fruits [2]. One such economically valuable fruit-bearing species is the common sorbs (Sorbus domestica L.). In the Mediterranean region, S. domestica trees have been cultivated for their fruits for over two thousand years [3]. Italy has always been a significant center of differentiation of agricultural biodiversity, exhibiting high biological diversity in terms of genes, species, populations, and ecosystems. However, with increasing globalization, there is a gradual reduction in this variability due to intensified fruit plantations and the abandonment of old cultivars in favor of new ones. This leads to a loss of the once-rich plant heritage that formed the basis of Italian fruit cultivation [4].
Moreover, it is well known that the climate crisis that is sweeping the planet requires a considerable contribution in terms of ecological transition. As confirmed by a wide body of literature [5,6], an increased tree presence is able to foster greater carbon sequestration for the purpose of reducing the concentration of climate-altering gases in the atmosphere.
As a consequence, the policies of the postpandemic Next Generation EU program [7], as well as the summary of the last Conference of Parties (COP) meetings [8], have provided specific actions in each EU member state for the spread of forested areas in both suburban and urban areas. Agroforestry and urban forestry have, therefore, become important objectives within the agricultural European policy [9], and recent initiatives developed to strengthen the spread of trees and economically supported by Next Regeneration EU funds have highlighted a concrete shortage of tree availability in nurseries and the need to identify efficient propagation methods for species of interest for carbon sequestration purposes. These include Sorbus L., a group of species capable of living at different altitudes, with broad Mediterranean adaptation and able to contribute to ecological transition.
Besides the ecological interest, there is a growing appreciation for local varieties characterized by high organoleptic and nutritional qualities that were prevalent in the past. The sorb is part of the underutilized or forgotten fruit species, known as “minor fruits”, defined from biological, socioeconomic–cultural, productive, and commercial perspectives [10]. These fruits represent an important resource for study, conservation, and promotion as they contribute significantly to the recovery and safeguarding of the genetic resources of the region. For instance, sorb trees were historically used as medicinal plants, and their fruits were valuable sources of vitamins and sugars for winter consumption [11]. The most commonly used propagation method for S. domestica L. is by seed [12], resulting in a wide genetic variability of seedlings in various aspects of the plant, fruit, and wood. On the other hand, asexual propagation methods like cuttings have not been adequately effective [13], while vegetative propagation by grafting onto quince is seldom used due to compatibility issues [14], with respect to studies on in vitro approaches [15,16,17,18,19,20,21,22]. In vitro propagation is a useful tool for both propagation and safeguarding genotypes with high genetic erosion, using methods that enhance the efficiency of materials and significantly reduce environmental impact [23,24,25]. The efficiency of the regeneration of plant shoots is affected by the type of explant, composition of the medium, and mixture of phytohormones and growth additives [26,27,28]. The presence of cytokinin in the cultural substrate is necessary for the formation of new shoots in woody species, and very often, the combination of cytokinin with low concentration auxin can increase the number of shoots. One widely used cytokinin is 6-benzylaminopurine (BAP), as it has greater efficacy in the induction of sprouts in many plant species, including Sorbus species. On the other hand, it can cause growth abnormalities in some plant species, reducing rooting and worsening the subsequent acclimatization of plants [29,30,31]. For these reasons, new aromatic derivatives of cytokinin have been the subject of intense research [32]. One of these is meta-Topolin (mT), a highly active natural aromatic cytokinin found in poplar leaves [33]. However, literature on Sorbus micropropagation as a fruit plant is limited, mainly due to minimal cultivar selection, and research on clonal propagation is sporadic [34]. While the solid substrate culture system has been successful, there is a growing need to reduce propagation time and improve productivity [35]. In recent years, temporary immersion systems (TIS) by using bioreactors with liquid substrates are considered more effective than solid culture systems because the plant material has greater accessibility to the substrate components, allowing for increased biomass gains and reduced propagation times. Additionally, it offers cost reduction possibilities in terms of management, scalability, and automation of the propagation process [36,37,38,39]. Among liquid substrate systems, TISs allows temporary contact between plants and the liquid substrate, avoiding continuous immersions that negatively affect plant growth and morphogenesis while providing adequate oxygen supply to the cultured plants [40]. Tree species of great economic interest require high-efficiency in vitro multiplication to obtain a sufficient number of plants for production purposes. Although well-established protocols exist for solid culture systems, liquid culture using a TIS is now considered an alternative method that has shown advantages for many of these species [41,42,43,44]. Various types of TIS bioreactors exist, with the automated temporary immersion system (RITA®) introduced by Alvard [42], being used in the micropropagation of various species, including Malus sp. [45], Eucalyptus spp. [46], Rubus sp. [47], Camptotheca acuminata [48], and many others. The Twin Flasks system (BIT®), described by Escalona [49], has been reported in numerous studies [50]. However, all these bioreactors are often too small, with a limited internal bottom size that can lead to competitive growth disturbances due to high density. A new bioreactor has recently been developed by PlantformTM (www.plantform.se (accessed on 5 January 2024), offering larger dimensions that promote adequate crop growth. The forced ventilation in the temporary immersion system enhances the complete renewal of the culture atmosphere, preventing the accumulation of carbon dioxide and ethylene, which generally occurs in semisolid culture and has a negative effect on morphogenesis [51]. The use of forced ventilation improves the propagation of many species by promoting photoautotrophic behaviors [52].
The aim of this study is to determine suitable conditions to achieve the best performance using the PlantformTM bioreactor in the in vitro propagation of four accessions of S. domestica L. (69SRB001S; 69SRB003S; 69SRB004P; 69SRB002S), each represented by a single specimen. These genotypes were preliminarily described in a study characterizing and enhancing the biodiversity of minor fruit trees in a particularly suitable area of the Mediterranean Basin [53]. Moreover, the use of the PlantformTM bioreactor can provide a valuable tool for the long-term conservation of the genetic resources of this species and the production of uniform plant materials for possible reintroduction into collection fields and cultivation. In particular, the role of different levels of plant growth regulators (PGR) in in vitro multiplication through the use of temporary immersion bioreactors in liquid substrates was studied with this objective in mind.
2. Materials and Methods
2.1. Plant Material and Cultural Environment
The plant material was collected from S. domestica L. adult trees located in the province of Messina, Sicily, South Italy. Four genotypes were selected, namely, 69SRB001S (SN1), 69SRB003S (SN2), and 69SRB004P (SN3), identified in the municipality of Reitano (38°00′38.8″ N 14°19′48.5″ E), and 69SRB002S (SN4) identified in the municipality of Pettineo (37°57′10.6″ N 14°17′31.2″ E). The starting vegetative material was collected in mid-April as cuttings, 25–30 cm long and 0.5–0.8 cm thick. After leaf removal, they were washed in running water and sectioned into uninodal segments of 1 cm in length. The explants with one node each were sterilized by immersion in a 70% ethanol solution for 5 min, followed by a 2% sodium hypochlorite solution with a drop of Tween-20 for 15 min, and then three rinses of 5 min each in sterile distilled water. The initial explants, used for tests in the Plantform™ bioreactor, were originated from nodal explants and initially stabilized in Microbox containers containing 25 mL of MS Murashige and Skoog [54], vitamins Nitch and Nitch [55], supplemented with 8 g L−1 sucrose, and plant agar (Duchefa, Haarlem, The Netherlands) as a gelling agent. The pH of the media was adjusted to 5.8. The cultures were placed in a climate growth chamber at 25 ± 1 °C under a photoperiod of 16 h per day with a light intensity of 50 µmol m−2 s−1, and subcultures were performed every 28 days. After approximately 12 weeks, stabilized explants were taken for testing proliferation in the Plantform™ bioreactor. Before introducing the culture, all parts of the bioreactors were sterilized in an autoclave for 20 min at 120 °C. Each container contained 500 mL of substrate. The liquid substrate consisted of MS Murashige and Skoog (MS) and vitamins Nitch and Nitch. In order to evaluate the effect of different levels of plant growth regulators (PGR), the TIS substrate was integrated with different cytokinins: 6-benzylaminopurine (BAP) (Sigma B-4308) and meta-Topolin (mT), at the same concentration of 1 mg L−1 individually and in combination with an auxin, indole-3-butyric acid (IBA) at 0.5 mg L−1 (Table 1). The substrate with Murashige and Skoog (MS) with vitamins Nitch and Nitch was used as a control. Each treatment included 30 explants. Cultures placed in the Plantform™ bioreactor were maintained in a climate growth chamber at 25 ± 1 °C under a photoperiod of 16 h per day with a light intensity of 50 µmol m−2 s−1. The immersion and aeration periods of the Plantform™ culture were controlled using air pumps and a timer connected to the bioreactor and positioned in the climate chamber; the immersion frequency was 5 min every 12 h with 4 min of aeration every 8 h.

Table 1.
Plant growth regulators integrated into the culture medium for shoot stimulation in S. domestica L.
2.2. Data Collection and Statistical Analysis
After 4 weeks of culture in the TIS, the number and length (cm) of shoots were measured, and the relative growth rate (RGR) index was calculated based on the initial and final fresh weight of the plant as [(Wf − Wi)/(tf − ti)] × 100/days of culture [56]. Each treatment included 30 explants. Statistical analysis was performed using SYSTAT 13 (Systat Intelligent Software). To highlight statistically significant differences and potential interactions between the culture system and plant growth regulators (PGR), a two-way analysis of variance (ANOVA) was performed (p ≤ 0.05). One-way ANOVA was conducted when the interaction between two factors was not significant; each factor was analyzed individually, and mean separation was carried out using the Tukey test (p ≤ 0.05).
3. Results
The effects of the Plantform™ bioreactor (TIS) were evaluated for four studied accessions of S. domestica. The results obtained after 4 weeks of culture showed that the culture substrates, type of hormone used, and concentration significantly influenced the number and length (cm) of shoots and the RGR. The interaction between the four Sorbus accessions and the treatment with different PGR in the culture substrate exhibited a varied response pattern in average shoot production. In all four accessions, when the culture substrate contained meta-Topolin (mT) at 1 mg L−1 and IBA at 0.5 mg L−1, there was a higher shoot production, respectively, in SN1 (4.8), SN2 (5.9), and SN4 (4.4), and lower in SN3 (3.5). When mT was replaced with BAP at the same concentration and in combination with IBA, the number of shoots produced by the four accessions was lower (SN1 3.6; SN2 4.4; SN3 2.4; SN4 3.3). Overall, shoot formation was higher when the culture substrate contained cytokinin alone or in combination with auxin compared to the hormone-free substrate (Table 2).

Table 2.
Effect of interaction between S. domestica accessions and cultural media on the average number of shoots obtained per explant.
Statistically significant differences (p value ≤ 0.05) among the Sorbus accessions were found in terms of the average number of shoots per explant. Selections SN2 (4.0) and SN1 (3.6) produced significantly more shoots than SN4 (3.2) and SN3 (2.4) (Figure 1).
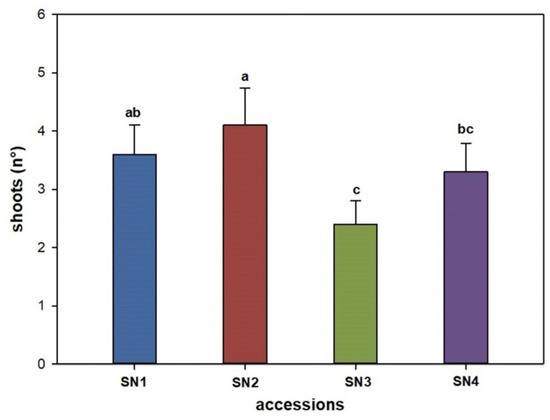
Figure 1.
Effect of Sorbus accessions on the number of shoots produced per explant. Bars represent standard error, and the different letters above grouped for each individual treatment indicate statistically significant differences (Tukey’s test, p ≤ 0.05).
The presence of the combination of cytokinins and auxins in the multiplication substrate significantly increased shoot regeneration compared with the control and with the substrate in which cytokinin alone was present. A higher shoot production of 4.7 was observed in the substrate containing mT 1 mg L−1 in combination with indol-3-butyric acid (IBA) 0.5 mg L−1 (Figure 2).
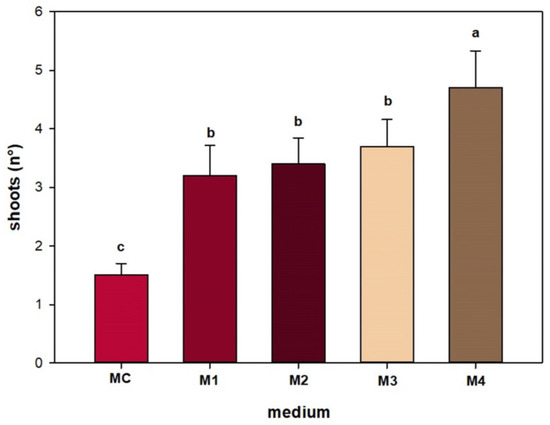
Figure 2.
Effect of different PGRs on the number of Sorbus shoots obtained per explant. The bars represent the standard error, and the different letters above grouped for each individual treatment indicate statistically significant differences (Tukey’s test, p ≤ 0.05).
Regarding the interaction between accessions and PGR treatment on shoot length, statistically significant differences were observed. In all four accessions, shoot elongation was significantly better in the treatment with only mT or when mT was in combination with IBA. In SN1 and SN2, shoot elongation was significantly better in the mT + IBA treatment (1.5 cm and 1.1 cm) compared to the combined BAP + IBA treatment (1.08 cm and 0.93 cm), as well as the mT treatment (1.3 cm and 1.2 cm) and the BAP treatment (0.89 cm and 0.94 cm). In SN3 and SN4, shoot elongation was significantly better in the mT + IBA treatment (3.1 cm and 3.3 cm) compared to the combined BAP + IBA treatment (2.4 cm and 2.5 cm), as well as the mT treatment (2.8 cm and 3.1 cm) and the BAP treatment (2.0 cm and 2.1 cm). In the hormone-free substrate, a lower shoot length was obtained in all four selections (Table 3).

Table 3.
Effect of interaction between Sorbus accessions and PGR treatment on the average number of shoots per explant in different Sorbus accessions.
A longer shoot length per explant was almost similar for selections SN4 (2.4 cm) and SN3 (2.3 cm). Meanwhile, selections SN1 and SN2 produced significantly shorter shoots (Figure 3).
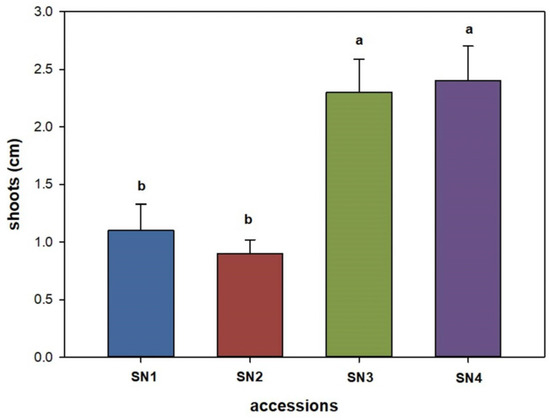
Figure 3.
Effect of Sorbus accessions on the length of shoots produced per explant. Bars represent standard error, and the different letters above grouped for each individual treatment indicate statistically significant differences (Tukey’s test, p ≤ 0.05).
The presence of PGRs in the culture substrate also affected the total shoot length. The combination of the two cytokinins (BAP and mT) with auxin (IBA) had a greater effect on shoot length than the substrate without hormones (Figure 4).

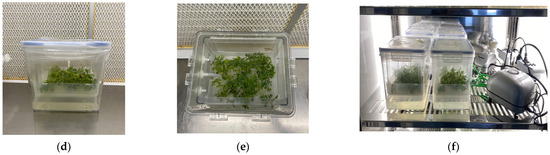
Figure 4.
In vitro regeneration of Sorbus accessions: (a) initial explants for in vitro establishment; (b) shoot regeneration of Sorbus accession (SN3) in the Plantform™; (c) shoot regeneration of Sorbus accession (SN4) in Plantform™; (d,e) Sorbus accessions (SN4) placed in the Plantform™ bioreactor; (f) Sorbus accessions placed in the Plantform™ bioreactor in a climate growth chamber. Bars (a–c) = 1 cm.
Shoots in substrates containing mT in combination with IBA or alone were longer (2.2 and 2.1) than in substrates containing BAP in combination with IBA or alone (1.7 and 1.5) (Figure 5).
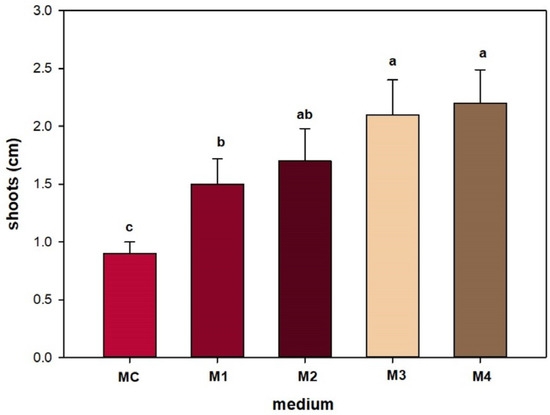
Figure 5.
Effect of different PGRs on shoot length of Sorbus per explant. Bars represent standard error, and the different letters above grouped for each individual treatment indicate statistically significant differences (Tukey’s test, p ≤ 0.05).
The relative growth rate (RGR) was highest when mT was used as the cytokinin source. The SN4 selection showed the best RGR results of 8.3 mg g−1 d−1 in the substrate containing mT + IBA, whereas when mT was present in the culture substrate, an RGR of 5.4 mg g−1 d−1 was obtained. In contrast, all Sorbus selections showed the lowest RGR in the absence of PGR (Table 4).

Table 4.
Relative growth rate (RGR) of different accessions of S. domestica L.
4. Discussion
The use of liquid substrate in bioreactors for plant production is a complementary strategy to overcome some limitations present in systems based on traditional in vitro cultivation techniques on solid substrates. The increased absorption of nutrients and hormones through the liquid substrate, as well as the air exchange within bioreactors, contribute to improving the physiological status of the explants [57]. In solid substrates, explants absorb nutrients through the cutting surface and translocate components from the xylem to the phloem. In contrast, in a liquid substrate, substrate components are absorbed through the leaves via stomata and aqueous pores [58]. This allows nutrients to reach the growth areas much more efficiently [59], and may explain why the TIS leads to a significant increase in shoot proliferation compared to solid substrate cultivation systems. The use of temporary immersion bioreactors should be optimized for each species. The Plantform™ bioreactor has been utilized in the propagation of various species. In Olea europaea L. [60] and Capparis spinosa L. [56], the use of Plantform™ bioreactors resulted in increased shoot proliferation in terms of shoot length and vigor. Regueira et al. [61], comparing two cultivation systems, RITA® or Plantform™, in Salix viminalis, found a greater number and length in the Plantform™ system. In Quercus robur, Plantform™ promotes biomass production in terms of shoot growth and proliferation [62]. The use of Plantform™ temporary immersion bioreactors has also yielded the best results in chestnut in vitro propagation [63], as well as in Phoenix dactylifera [64]. The results obtained in this study showed that the four selected Sorbus accessions responded well to the TIS, and the use of PGR positively influenced proliferation. Plant growth regulators play a key role in adventitious shoot regeneration in various plants [65]. The presence of cytokinin is necessary for the formation of new shoots in woody species, and often, cytokinin is combined with a low concentration of auxin to increase the number of shoots. BAP is an important aromatic cytokinin that is very effective in inducing shoots in many plant species, including Sorbus species and is cost-effective compared to other cytokinins. However, it can cause growth abnormalities in some plant species, reducing rooting and worsening subsequent plant acclimatization [32,66,67]. For these reasons, new aromatic derivatives of cytokinin have been intensively researched [66]. One such derivative is mT, a highly active natural aromatic cytokinin widely used in the micropropagation of various species [56,68,69].
In this study, mT positively influenced shoot length, the number of shoots, and the RGR. In fact, the highest RGR was observed in the substrate containing mT alone as well as in combination with IBA. This behavior has been confirmed in other studies where mT has been successfully used in the micropropagation of many plant species. Similar results were obtained in Capparis spinosa L. [56], using the same Plantform™ bioreactors, where an increasing RGR was observed depending on the hormone concentration in the substrate. In our study on Sorbus, comparing the effectiveness of two cytokinins (BAP and mT), either individually or in combination with auxin (IBA), mT had a significantly higher effect on shoot regeneration, both in terms of the number and total length of shoots, with a greater shoot production in different genotypes. This demonstrates that mT enhances shoot potential more effectively than BAP. A similar response was observed in ribes, where a greater shoot elongation was observed in the presence of mT [70]. Many authors, for the Sorbus genus, commonly used a combination of cytokinin and auxin for proliferation without further analysis [21,71,72,73,74]. In contrast to our results, in studies conducted on the in vitro culture of S. domestica L. on solid substrates, it was demonstrated that the presence of cytokinin was essential for the proliferation of shoots; in particular, the use of BAP significantly increased the number of shoots [15,16,17,18,19,20,21,22]. Also, it has a higher proliferation rate compared to a solid medium including BAP [75]. A study on Sorbus commixta Hedl. reported that the combination of cytokinin (BA) and auxin (IAA or NAA) had a synergistic effect on shoot multiplication.
5. Conclusions
A successful temporary immersion in vitro propagation protocol has been developed using Plantform™ bioreactors for four Sorbus accessions. The results obtained in this study highlight how the use of the TIS led to a higher relative growth rate (RGR) as well as an increased number and length of shoots. The use of mT in the culture substrate has shown a positive effect in increasing the quality of Sorbus micropropagated plants. Our data have shown that certain genotypes are more adaptable to in vitro multiplication techniques in liquid culture, confirming that developing a single protocol for each species is not straightforward, necessitating a broader evaluation of adaptation. In comparison to other micropropagation protocols reported on Sorbus, the use of this technique of cultivation in liquid substrates, through the temporary immersion in Plantfrom™ bioreactor, is significantly more effective as it has allowed substantial increases in the number of shoots formed in a shorter period of time, with the advantage of reducing production costs by avoiding subcultures in the multiplication process. Moreover, the possibility of starting an efficient nursery production of valuable genotypes through TISs is promoted, effectively contributing to a wider availability of trees of a valuable species able to provide a significative ecological contribution to climate crisis mitigation. The final stage of the in vitro propagation needs to be completed, but for this aspect, an efficient methodology has already been set up. In addition, the conservation and reproduction of genetic resources of rare and endemic species of Sorbus biodiversity is a favorable outcome always in order to ensure adequate compensation for climate alterations. These species, hence, are significant not only taxonomically but also in terms of primary biodiversity conservation for agro-environmental purposes.
Author Contributions
Conceptualization, V.G. and F.S.; methodology, V.G. and F.S.; investigation, V.G.; data curation, V.G.; writing—original draft preparation, V.G.; writing—review and editing, F.S.; supervision, F.S.; funding acquisition, F.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was carried out as part of the activities funded by Slow Food, grant number CON-0626/2023 Agricultural biodiversity preservation.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Li, M.; Tetsuo, O.T.; Gao, Y.-D.; Xu, B.; Zhu, Z.-M.; Ju, W.-B.; Gao, X.-F. Molecular phylogenetics and historical biogeography of Sorbus sensu stricto (Rosaceae). Mol. Phylogenetics Evol. 2017, 111, 76–86. [Google Scholar] [CrossRef]
- Špíšek, Z.; Uherková, A.; Svitok, M.; Vašut, R.J. Sorbus domestica L. at its northern Pannonian distribution limits: Distribution of individuals, fruit shapes and dendrometric characteristics. Dendrobiology 2018, 80, 37–47. [Google Scholar] [CrossRef]
- Brütsh, U.; Rotach, P. Der Speierling (Sorbus domestica L.) in der Schweiz: Verbreitung, Ökologie, Standsortsanspruche, Konkurrenzkraft und Waldbauliche Eignung. Schweiz. Z. Forstwes. 1993, 144, 967–991. [Google Scholar]
- Sottile, F.; Girgenti, V.; Del Signore, M.B.; Peano, C. Quality of autochthonous Sicilian plums. Ital. J. Food Sci. 2015, 27, 320–329. [Google Scholar]
- Hobbie, S.E.; Grimm, N.B. Nature-based approaches to managing climate change impacts in cities. Philos. Trans. R. Soc. B 2020, 375, 20190124. [Google Scholar] [CrossRef] [PubMed]
- Pereira, P.; Wang, F.; Inacio, M.; Kalinauskas, M.; Bogdzevič, K.; Bogunovic, I.; Zhao, W.; Barcelo, D. Nature-based solutions for carbon sequestration in urban environments. Curr. Opin. Environ. Sci. Health 2024, 37, 100536. [Google Scholar] [CrossRef]
- Paleari, S. The EU policy on climate change, biodiversity and circular economy: Moving towards a Nexus approach. Environ. Sci. Policy 2024, 151, 103603. [Google Scholar] [CrossRef]
- Salik, A.N. COP21 Paris. Strateg. Stud. 2017, 37, 74–89. [Google Scholar] [CrossRef]
- Golicz, K.; Bellingrath-Kimura, S.; Breuer, L.; Wartenberg, A.C. Carbon accounting in European agroforestry systems—Key research gaps and data needs. Curr. Res. Environ. Sustain. 2022, 4, 100134. [Google Scholar] [CrossRef]
- Bellini, E.; Giordani, E.; La Malfa, S. I fruttiferi minori in Italia, una risorsa tradizionale per l’innovazione frutticola: Il kaki e il melograno come casi di studio. Italus Hortus 2010, 17, 75–90. [Google Scholar]
- Piagnani, M.C.; Debellini, C.; Lo Scalzo, R. Phyllometry and carpometry, chemical and functional characterization of fruits of Sorbus domestica L. (service tree) selections. J. Berry Res. 2012, 2, 7–22. [Google Scholar] [CrossRef]
- Bignami, C. Il sorbo. In Conservazione dei Fruttiferi Minori, Proceedings of the National Workshop on EC Project GENRES 29, Firenze 27–28 November 1998; Department of Horticulture, University of Florence: Florence, Italy, 1998. [Google Scholar]
- Pant, M.; Bhandari, A.; Husen, A. Adventitious root formation and clonal propagation of forest-based tree species. In Environmental, Physiological and Chemical Controls of Adventitious Rooting in Cuttings; Husen, A., Ed.; Academic Press: Cambridge, MA, USA, 2022; Chapter 22; pp. 471–490. [Google Scholar]
- Lall, S.; Mandegaran, Z.; Roberts, A. Shoot multiplication and adventitious regeneration in Sorbus aucuparia. Plant Cell Tissue Organ Cult. 2006, 85, 23–29. [Google Scholar] [CrossRef]
- Arrillaga, I.; Marzo, T.; Segura, J. Micropropagation of juvenile and adult Sorbus domestica L. Plant Cell Tissue Organ Cult. 1991, 27, 341–348. [Google Scholar] [CrossRef]
- Meier-Dinkel, A. In vitro Vermehrung von Speierling (Sorbus domestica L.). Corminaria 1998, 9, 9–13. [Google Scholar]
- Miko, M.; Gažo, J.; Biroščíková, M. In vitro klonové množenie genetických zdrojov jarabiny oskorušovej (Sorbus domestica L.) zúzemia Slovenska. Acta Fytotech. Zootech. 2004, 7, 85–89. [Google Scholar]
- Nikolaou, P.; Zagas, D.; Scaltsoyiannes, V.; Balas, E.; Xilogianni, V.; Tsoulpha, P.; Tsaktsira, M.; Voulgaridou, E.; Iliev, I.; Triantafyllou, K.; et al. Advances in the micropropagation of service tree (Sorbus domestica L.). Propag. Ornam. Plants 2008, 8, 154–157. [Google Scholar]
- Durkovič, J.; Mišalová, A. Wood formation during ex vitro acclimatisation in micropropagated true service tree (Sorbus domestica L.). Plant Cell Tissue Organ Cult. 2009, 96, 343–348. [Google Scholar] [CrossRef]
- Malá, J.; Cvrčková, H.; Máchová, P.; Dostál, J. Mikropropagace Jeřábu Oskeruše (Sorbus domestica L.); Certified Methodology 4/2011; Forestry and Game Management Research Institute: Strnady, Czech Republic, 2011; pp. 1–17. (In Czech) [Google Scholar]
- Ördögh, M.; Jàmbor-Benczùr, E.; Tilly-Màndy, A.; Lelik, L. The effects of growth regulators in proliferation of Sorbus rediliana “Burokvolgy”. Int. J. Hortic. Sci. 2006, 12, 77–83. [Google Scholar] [CrossRef]
- Jana, Š.; Jiří, V.; Daniel, Z. Micropropagation as a Tool for the Conservation of Autochthonous Sorbus Species of Czechia. Plants 2023, 12, 488. [Google Scholar] [CrossRef]
- Bengi, E.; Yeald, E. In vitro micropropagation of Anthemis xylopoda O. Schwarz, a Critically Endangered Species from Turkey. Pak. J. Biol. Sci. 2005, 8, 691–695. [Google Scholar]
- Trejgell, A.; Michalska, M.; Tretyn, A. Micropropagation of Senecio Macrophyllus M. Bieb. Acta Biol. Cracoviensia Ser. Bot. 2010, 52, 67–72. [Google Scholar] [CrossRef]
- Gianguzzi, V.; Barone, G.; Di Gristina, E.; Sottile, F.; Domina, G. Micropropagation of Endemic Endangered Taxa of the Italian Flora: Adenostyles alpina subsp. macrocephala (Asteraceae), as a Case Study. Plants 2023, 12, 1530. [Google Scholar] [CrossRef] [PubMed]
- Iliev, I.A. Factors affecting the axillary and adventitious shoot formation in woody plants in vitro (Conference Paper). Acta Horticulturae 2017, 1155, 15–28. [Google Scholar] [CrossRef]
- Liu, C.; Callow, P.; Rowland, L.J.; Hancock, J.F.; Song, G.Q. Adventitious shoot regeneration from leaf explants of southern highbush blueberry cultivars. Plant Cell Tissue Organ Cult. 2010, 103, 137–144. [Google Scholar] [CrossRef]
- Durkovič, J.; Mišalová, A. Micropropagation of temperate noble hardwoods: An overview. Funct. Plant Sci. Bio-technol. 2008, 2, 1–19. [Google Scholar]
- Gianguzzi, V.; Di Gristina, E.; Barone, G.; Sottile, F.; Domina, G. Seed germination and vegetative and in vitro propagation of Hieracium lucidum subsp. lucidum(Asteraceae), a critically endangered endemic taxon of the Sicilian flora. PeerJ 2024, 12, e16839. [Google Scholar] [CrossRef]
- Werbrouck, S.P.O.; Van der Jeugt, B.; Dewitte, W.; Prinsen, E.; Van Onckelen, H.A.; Debergh, P.C. The metabolism of benzyladenine in Spathiphyllum floribundum ‘Schott Petite’ in relation to acclimatisation problems. Plant Cell Tissue Organ Cult. 1995, 14, 662–665. [Google Scholar] [CrossRef]
- Centeno, M.L.; Rodrígue, A.; Feito, I.; Fernández, B. Relationship between endogenous auxin and cytokinin levels and morphogenic responses in Actinidia deliciosa tissue cultures. Plant Cell Rep. 1996, 16, 58–62. [Google Scholar] [CrossRef]
- Tarkowská, D.; Doležal, K.; Tarkowski, P.; Astot, C.; Holub, J.; Fuksová, K.; Schmülling, T.; Sandberg, G.; Strnad, M. Identification of new aromatic cytokinins in Arabidopsis thaliana and Populus × canadensis leaves by LC-(+)ESI-MS and capillary liquid chromatography/frit-fast atom bombardment mass spektrometry. Physiol. Plant. 2003, 117, 579–590. [Google Scholar] [CrossRef]
- Strnad, M.; Hanuš, J.; Vanˇek, T.; Kamínek, M.; Ballantine, J.A.; Fussell, B.; Hanke, D.E. Meta-topolin, a highly active aromatic cytokinin from poplar leaves (Populus × canadensis Moench., cv. Robusta). Phytochemistry 1997, 45, 213–218. [Google Scholar] [CrossRef]
- Chalupa, V. European hardwoods. In Cell and Tissue Culture in Forestry; Bonga, J.M., Durzan, D.J., Eds.; Martinus Nijhoff Publishers: Dordrecht, The Netherlands, 1987; Volume 3, pp. 224–246. [Google Scholar]
- Phillips, G.C.; Garda, M. Plant tissue culture media and practices: An overview. Vitr. Cell. Dev. Biol.-Plant 2019, 55, 242–257. [Google Scholar] [CrossRef]
- Dutra, L.F.; Wendling, I.; Brondani, G.E. A micropropagação de eucalipto [The micropropagation of eucalyptus]. Pesqui. Florest. Bras. 2009, 58, 49–59. [Google Scholar]
- Oliveira, M.L.; Xavier, A.; Penchel, R.M.; Santos, A.F. Multiplicação in vitro de Eucalyptus grandis x E. urophylla cultivado em meio semissólido e em biorreator de imersão temporária [In vitro multiplication of Eucalyptus grandis × E. urophylla grown in semisolidù medium and in temporary immersion bioreactor]. Sci. For. 2011, 39, 309–315. [Google Scholar]
- Welander, M.; Persson, J.; Asp, H.; Zhu, L.H. Evaluation of a new vessel system based on temporary immersion system for micropropagation. Sci. Hortic. 2014, 179, 227–232. [Google Scholar] [CrossRef]
- Businge, E.; Trifonova, A.; Schneider, C.; Rödel, P.; Egertsdotter, U. Evaluation of a new temporary immersion bioreactor system for micropropagation of cultivars of eucalyptus, birch and fir. Forests 2017, 8, 196. [Google Scholar] [CrossRef]
- Etienne, H.; Berthouly, M. Temporary immersion systems in plant micropropagation. Plant Cell Tissue Organ Cult. 2002, 69, 215–231. [Google Scholar] [CrossRef]
- Sottile, F.; Caltagirone, C.; Peano, C.; Del Signore, M.B.; Barone, E. Can the Caper (Capparis spinosa L.) Still Be Considered a Difficult-to-Propagate Crop? Horticulturae 2021, 7, 316. [Google Scholar] [CrossRef]
- Alvard, D.; Cote, F.; Teisson, C. Comparison of methods of liquid medium culture for banana micropropagation. Effects of temporary immersion of explants. Plant Cell Tissue Organ Cult. 1993, 32, 55–60. [Google Scholar] [CrossRef]
- Akdemir, H.; Süzerer, V.; Onay, A.; Tilkat, E.; Ersali, Y.; Çiftçi, Y.O. Micropropagation of the pistachio and its rootstocks by temporary immersion system. Plant Cell Tissue Organ Cult. 2014, 117, 65–76. [Google Scholar] [CrossRef]
- Albarran, J.; Bertrand, B.; Lartaud, M.; Etienne, H. Cycle characteristics in a temporary immersion bioreactors affects regeneration, morphology, water and mineral status of coffee (Coffea arabica) somatic embryos. Plant Cell Tissue Organ Cult. 2005, 81, 27–36. [Google Scholar] [CrossRef]
- Zhu, L.H.; Li, X.Y.; Welander, M. Optimisation of growing conditions of the apple rootstock M26 grown in RITA® container using temporary immersion principle. Plant Cell Tissue Organ Cult. 2005, 81, 313–318. [Google Scholar] [CrossRef]
- McAlister, B.; Finnie, J.; Watt, M.P.; Blakeway, F. Use of temporary immersion system (RITA®) for production of commercial Eucalyptus clones in Mondi Forests (SA). Plant Cell Tissue Organ Cult. 2005, 81, 347–358. [Google Scholar] [CrossRef]
- Debnath, S.C. A two-step procedure for in vitro multiplication of cloudberry (Rubus chamaemorus L.) shoots using bioreactor. Plant Cell Tissue Organ Cult. 2007, 88, 185–191. [Google Scholar] [CrossRef]
- Sankar-Thomas, Y.D.; Surminski, K.S.; Lieberi, R. Plant regeneration via somatic embryogenesis of Camptotheca acuminata in temporary immersion system (TIS). Plant Cell Tissue Organ Cult. 2008, 95, 163–173. [Google Scholar] [CrossRef]
- Escalona, M.; Lorenzo, J.C.; Gonzales, B.; Daquinta, M.; Gonzalez, J.L.; Desjardins, Y.; Borroto, C.G. Pineapple (Ananas comosus L. Merr) micropropagation in temporary immersion systems. Plant Cell Rep. 1999, 18, 743–748. [Google Scholar] [CrossRef]
- Welander, M.; Zhu, L.H.; Li, X.Y. Factors influencing conventional and semi-automated micropropagation. Propag. Ornam. Plants 2007, 7, 103–111. [Google Scholar]
- Roels, S.; Noceda, C.; Escalona, M.; Sandoval, J.; Canal, M.J.; Rodríguez, R.; Debergh, P. The effect of headspace renewal in a temporary immersion bioreactor of plantain (Musa AAB) shoot proliferation and quality. Plant Cell Tissue Organ Cult. 2006, 84, 155–163. [Google Scholar] [CrossRef]
- Xiao, Y.; Niu, G.; Kozai, T. Development and application of photoautotrophic micropropagation plant system. Plant Cell Tissue Organ Cult. 2011, 105, 149–158. [Google Scholar] [CrossRef]
- Sottile, F.; Del Signore, M.B.; Giuggioli, N.; Peano, C. The potential of the Sorb (Sorbus domestica L.) as a minor fruit species in the Mediterranean areas: Description and quality traits of underutilized accessions. Prog. Nutr. 2017, 19, 41–48. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F.A. Revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Nitsch, J.P.; Nitsch, C. Haploid plants from pollen grains. Science 1969, 163, 85–87. [Google Scholar] [CrossRef]
- Gianguzzi, V.; Inglese, P.; Barone, E.; Sottile, F. In Vitro Regeneration of Capparis spinosa L. by Using a Temporary Immersion System. Plants 2019, 8, 177. [Google Scholar] [CrossRef]
- Vidal, N.; Sánchez, C. Use of bioreactor systems in the propagation of forest trees. Eng. Life Sci. 2019, 19, 896–915. [Google Scholar] [CrossRef] [PubMed]
- Schönherr, J. Characterization of aqueous pores in plant cuticles and permeation of ionic solutes. J. Exp. Bot. 2006, 57, 2471–2491. [Google Scholar] [CrossRef] [PubMed]
- De Klerk, G.J.; Ter Brugge, J. Micropropagation of dahlia in static liquid medium using slow-release tools of medium ingredients. Sci. Hortic. 2011, 127, 542–547. [Google Scholar] [CrossRef]
- Benelli, C.; De Carlo, A. In vitro multiplication and growth improvement of Olea europea L. cv Canino with temporary immersion system (Plantform™). 3 Biotech 2018, 8, 317. [Google Scholar] [CrossRef] [PubMed]
- Regueira, M.; Rial, E.; Blanco, B.; Bogo, B.; Aldrey, A.; Correa, B.; Varas, E.; Sánchez, C.; Vidal, N. Micropropagation of axillary shoots of Salix viminalis using a temporary immersion system. Trees 2018, 32, 61–71. [Google Scholar] [CrossRef]
- Gatti, E.; Sgarbi, E.; Ozudogru, E.A.; Lambardi, M. The effect of Plantform™ bioreactor on micropropagation of Quercus robur in comparison to a conventional in vitro culture on gelled medium, and assessment of the microenvironment influence on leaf structure. Plant Biosyst. 2017, 151, 1129–1136. [Google Scholar] [CrossRef]
- Vidal, N.; Blanco, B.; Cuenca, B. A temporary immersion system for micropropagation of axillary shoots of hybrid chestnut. Plant Cell Tissue Organ Cult. 2015, 126, 229–243. [Google Scholar] [CrossRef]
- Nayyef, M.N.; Ali Abdulhussein, M.A.; Ali Almusawi, A.H. The efficiency of plant form as temporary immersion system in bud and multiplication shoot via direct organogenesis of date palm under LED light. Plant Arch. 2019, 19, 1419–1426. [Google Scholar]
- Bairu, M.W.; Stirk, W.A.; Doležal, K.; Van Staden, J. Optimizing the micropropagation protocol for the endangered Aloe polyphylla: Can meta-topolin and its derivatives serve as replacement for benzyladenine and zeatin? Plant Cell Tissue Organ Cult. 2007, 90, 15–23. [Google Scholar] [CrossRef]
- Werbrouck, S.P.O.; Strnad, M.; Van Onckelen, H.A.; Debergh, P.C. Meta-topolin, an alternative to benzyladenine in tissue culture. Physiol. Plant. 2008, 98, 291–297. [Google Scholar] [CrossRef]
- Kubalakova, M.; Strnad, M. The effects of aromatic cytokinins on micropropagation and regeneration of sugar beet in vitro. Biol. Plant 1992, 34, 578–579. [Google Scholar]
- Chiancone, B.; Karasawa, M.M.G.; Gianguzzi, V.; Abdelgalel, A.M.; Bárány, I.; Testillano, D.; Marinoni, D.; Botta, D.; Germanà, M.A. Early embryo achievement through isolated microspore culture in Citrus clementina Hort. ex Tan., cvs. ‘Monreal Rosso’ and ‘Nules’. Front. Plant Sci. 2015, 6, 413. [Google Scholar] [CrossRef]
- Chiancone, B.; Martorana, L.; Gianguzzi, V.; Germanà, M.A. The effects of meta-Topolin and benzyladenine on in vitro organogenesis from epicotyl cuttings of Troyer citrange (Citrus sinensis [L.] Osbeck × Poncirus trifoliata [L.] Raf.). Acta Hortic. 2017, 1155, 185–192. [Google Scholar] [CrossRef]
- Kucharska, D.; Orlikowska, T.; Maciorowski, R.; Kunka, M.; Wójcik, D.; Pluta, S. Application of meta-Topolin for improving micropropagation of gooseberry (Ribes grossularia). Sci. Hortic. 2020, 272, 109529. [Google Scholar] [CrossRef]
- Malá, J.; Máchová, P.; Cvrčková, H.; Karady, M.; Novák, O.; Mikulík, J.; Hauserová, E.; Greplová, J.; Strnad, M.; Doležal, K. Micropropagation of wild service tree (Sorbus torminalis (L.) Crantz): The regulative role of different aromatic cytokinins during organogenesis. J. Plant Growth Regul. 2009, 28, 341–348. [Google Scholar] [CrossRef]
- Martini, A.N.; Papafotiou, M. Season and explant origin affect phenolic content, browning of explants, and micropropagation of ×Malosorbus florentina (Zucc.) Browicz. HortScience 2013, 48, 102–107. [Google Scholar] [CrossRef]
- Chalupa, V. In vitro propagation of mature trees of Sorbus aucuparia L. and field performance of micropropagated tree. J. For. Sci. 2002, 48, 529–535. [Google Scholar] [CrossRef]
- Ördögh, M.; Jambor-Benczúr, E.; Tilly-Mándy, A.; Lelik, L. Effects of different cytokinins on proliferation of Sorbus borbasii ‘Herkulesfürdö’. Propag. Ornam. Plants 2009, 9, 43–46. [Google Scholar]
- Jeong, B.R.; Sivanesan, I. Micropropagation of Sorbus commixta Hedl. Propag. Ornam. Plants 2015, 15, 142–146. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).