Abstract
To counter the harmful impacts of agricultural chemicals on the environment and human health, there is an increasing demand for safe, eco-friendly, and potent plant-based biopesticides. In this study, we aimed to investigate the antimicrobial effects of ginger essential oil and selected volatile compounds (linalool, eugenol, citral, and cinnamaldehyde [CA]) against Fusarium oxysporum FOX-1. Minimum inhibitory concentrations (MICs) were determined using the mycelium growth inhibition method. The compound CA exhibited the most potent antifungal effect against F. oxysporum FOX-1 and was selected for further investigation. After treatment with CA at 1/2 MIC or MIC, the spore germination of F. oxysporum FOX-1 was significantly inhibited at 12 h. Furthermore, microscopic observation revealed that CA treatment resulted in the morphological degradation of F. oxysporum FOX-1. CA destroyed the cell membrane integrity of F. oxysporum FOX-1, increasing the relative conductivity and the leakage of intracellular protein, nucleic acids, and malondialdehyde, affecting the integrity and metabolism of the cell membrane. The effects were positively related to CA concentration. Additionally, in vivo experiments with rhizome sections showed that CA significantly reduced the pathogenicity of F. oxysporum FOX-1. Overall, these findings provide evidence for the potential of using ginger essential oil components as fungicides, offering a basis for future research to develop robust and eco-friendly plant-derived fungicides that serve as a sustainable means to reduce fungus-driven agricultural losses.
1. Introduction
Fusarium oxysporum is a soil-borne fungus responsible for major global losses in agricultural yields. The fungus causes wilt and root rot in many crops, including ginger (Zingiber officinale Roscoe), cucumber (Cucumis sativus L.), and cotton (Gossypium hirsutum L.) [1]. Generally, F. oxysporum invades the root hair zone of a plant and moves through the xylem towards the tip; its presence in the vascular tissues disrupts water transport, resulting in the yellowing, wilting, and death of the plant [2]. The fungus survives in the soil for extended periods and is spread through rainfall and irrigation. Once introduced into a field, the fungus is almost impossible to eradicate [3]. Currently, chemical control is the main method for treating Fusarium wilt disease [4]. However, in line with the ever-increasing understanding of the deleterious effects of chemicals used in the agricultural sector on ecological integrity and human health, there is a growing need for the development of safe, environmentally friendly, and highly efficient plant-derived biopesticides.
Plant essential oils (EOs) include a wide range of volatile compounds characterised by a strong odour [5]. These compounds are garnering the attention of researchers worldwide owing to their plant origin and useful biological activities [6]. Various plant EOs have been reported to exhibit broad-spectrum antimicrobial activity against bacteria and fungi [7].
Ginger, cloves, peppercorns, and cinnamon have medicinal and dietary value [8], and EOs derived from these agricultural products have been shown to be effective fungicides [9,10,11,12,13]. Ginger, gingerene, linalool, and citral have been reported to be the chemical constituents of ginger-derived EOs [9]; these products exhibit antimicrobial activity against Fusarium solani, Pyricularia oryzae, Colletotrichum falcatum, Ganoderma boninense, and Rigidoporus microporus [10]. The main chemical component of clove EO is eugenol, which has strong inhibitory effects against various food-borne bacteria [11]. Linalool and limonene are components of peppercorn oil, which have a significant inhibitory effect against Malassezia restricta [12]. Badr et al. [13] reported that in cinnamon, cinnamon EO and its main component cinnamaldehyde (CA) exhibited antimicrobial activity against Aspergillus flavus, Candida albicans, Staphylococcus aureus, and Aspergillus niger.
Although the fungistatic activities of various plant EOs have been reported, their fungistatic activities and mechanisms against F. oxysporum FOX-1 have rarely been reported. Screening out natural products that can effectively prevent and control phytopathogenic microorganisms and other pathogens can help lay the foundation for the next step of developing fungicides of plant origin. Accordingly, we investigated the antimicrobial effects of ginger EO and selected volatile compounds (linalool, eugenol, citral, and CA) against F. oxysporum FOX-1, and the most effective volatile compound, CA, was selected. CA was then made the target of the study, and its effects on mycelial growth and spore germination were analysed, along with scanning electron microscopy, cell membrane integrity, and permeability, and changes in the pathogenicity of F. oxysporum FOX-1.
2. Materials and Methods
2.1. Reagents and Fungus Strains
The Fusarium oxysporum strain (F. oxysporum FOX-1) was provided by the Institute of Special Plants, Chongqing University of Arts and Sciences [14], and stored at 4 °C. The preserved strains were transferred to potato dextrose agar (PDA) plates for activation and placed in a constant-temperature incubator (ICHE260, Aiam Scientific Instruments Co., Beijing, China) at 28 °C for 5 days. Then, a perforator (d = 6 mm) was used to perforate the outer edge of the colony (for spare use). Ginger EO (CAS-No. 8007-08-7; purity > 99%), linalool (CAS-No. 78-70-6; purity > 98%), eugenol (CAS-No. 97-53-0; purity > 99%), citral (CAS-No. 5392-40-5; purity > 97%), and CA (CAS-No. 104-55-2; purity > 99.5%) were purchased from Aladdin Chemical Reagent Co., Ltd. (Shanghai, China).
2.2. Antifungal Activity Assay of Ginger EO and Selected Volatile Compounds
Fungal inhibitory activity was assessed using the mycelial growth inhibition assay: the samples (ginger EO, linalool, eugenol, citral, and CA) were dissolved into 4 g/L solutions; emulsified in 30% ethanol; diluted to 0.125 g/L, 0.5 g/L, 1 g/L, 2 g/L, and 4 g/L with sterilised PDA medium; poured into Petri dishes; and allowed to solidify. The experiment was performed using three replicates of the samples. Equal volumes of 30% ethanol solvent and sterile water served as the blank control, and chlorothalonil (8 g/L) was used as the positive control [15]. The samples were incubated in a constant-temperature incubator at 28 °C for 5 days. The diameter of F. oxysporum FOX-1 colonies was measured every 24 h after inoculation. The mass concentration at which no mycelial growth was observed within 48 h was designated as the minimum inhibitory concentration (MIC) [16].
2.3. Influence of Ginger EO and Selected Volatile Compounds on the Germination of Spores
The effect of ginger EO and selected volatile compounds (linalool, eugenol, citral, and CA) on spore germination was observed under a laser confocal microscope (Lasertec Corp., Yokohama, Japan). Briefly, F. oxysporum FOX-1 (d = 6 mm) was inoculated on PDA plates and incubated in a constant-temperature incubator at 28 °C for 5 days. After numerous conidia were produced, the spores were eluted with sterile water to form a spore suspension. The suspension of F. oxysporum FOX-1 at a concentration of 1 × 104 spores/mL was incubated in the dark at 28 °C for 6 h with the addition of ginger EO and selected volatile compounds (linalool, eugenol, citral, and CA) at different concentrations (1/2 MIC, and MIC), respectively; sterile water was used as the blank control. Spores were counted using a haematocrit plate. Germination was confirmed when the length of the germ tube was longer than half of the diameter of the spore. The germination rate was calculated based on the number of germinated spores; this was repeated three times.
2.4. Determination of Cell Membrane Integrity in CA-Treated F. oxysporum FOX-1 Cells
2.4.1. Observation of F. oxysporum FOX-1 Morphology Using Scanning Electron Microscopy
Micromorphological changes of F. oxysporum FOX-1 mycelium after CA treatment were observed using SEM (Ion Sputter JFC-1100, Tokyo, Japan) [17]. A sample suspension of 1 × 106 spores/mL of F. oxysporum FOX-1 was added to 50 mL of potato dextrose broth medium. CA was added to the final concentrations of 1/2 MIC or MIC. Sterile water with 30% ethanol was added as a blank control. The mixture was then incubated in darkness in a shaking incubator (IST-3075, Jeio Tech Co., Ltd., Shanghai, China) at 28 °C and 150 rpm for 3 days. The mycelia were collected and dehydrated in different ethanol concentrations (30%, 50%, 70%, 80%, 90%, or 100%) at 15 min intervals for each concentration. The samples were fixed and dried using a vacuum freeze dryer and coated using an ion sputter coater for 120 s. The morphology of F. oxysporum FOX-1 mycelia was observed using SEM operating at 20 kV at 5000× magnification. Five measurements were performed per treatment, and each measurement consisted of 10–20 microscopic fields.
2.4.2. Propidium Iodide Staining Analysis
Propidium iodide (PI) staining was used to determine the damage to the cell membrane of F. oxysporum FOX-1 induced by CA treatment [18]. The mycelium preparation and collection methods used are described in Section 2.4.1. A small sample of mycelia was added to a 2 mL centrifuge tube, to which 500 μL of PI (Biosharp Life Science, Shanghai, China) solution was added. The mycelia were stained for 10 min at 37 °C in a water bath. The fungal suspension was centrifuged at 4000× g for 5 min and then washed with PBS (pH 7.2) to remove the residual dye. Five replicated experiments were performed for each treatment. The experimental outcomes were observed and photographed under a fluorescence microscope.
2.4.3. Cellular Leakage Assay
The mycelium preparation and collection method used was based on a previously reported method [18] and is described in Section 2.4.1. Nine grammes of mycelium (fresh weight) were divided into three parts and added to deionised water with different concentrations of CA (1/2 MIC or MIC). A blank control group (CK, added to an equal amount of deionised water) was also prepared. The conductivity was measured at 0, 2, 3, 4, 6, 8, 9, 10, and 12 h under ambient conditions of 25 °C. The effect of CA on the permeability of F. oxysporum FOX-1 cell membranes was observed according to the conductivity changes. The experiment was independently repeated three times.
To further investigate cellular leakage, the mycelia were treated as described above, and the nucleic acid and protein concentrations were assessed by measuring the absorbance of the supernatant at 260 nm and 280 nm, respectively, using a UV spectrophotometer (UV-2800A, Mapada, Shanghai, China). The malondialdehyde (MDA) contents of F. oxysporum FOX-1 mycelia were determined using the thiobarbituric acid test. The experiment was independently repeated three times.
2.4.4. Determination of Ergosterol Content in Cell Membranes
The ergosterol content of F. oxysporum FOX-1 treated with different CA concentrations was measured using the method described by Bao [19]. As described in Section 2.4.1, after incubation for 3 days at 28 °C, the culture was centrifuged at 12,000× g for 10 min at 4 °C. Then, 1.0 g of mycelium was collected, and the mycelium was saponified with 10% NaOH mixed with methanol in a water bath at 90 °C. After 2 h, 2 mL of sterile water and 5 mL of n-heptane were added. After vigorous vortex agitation and centrifugation at 8000× g for 15 min, the supernatant was diluted five-fold with 95% ethanol and analysed using a spectrophotometer at wavelengths of 281.5 nm and 230 nm. Finally, the ergosterol content and 24(28)-dehydroergosterol [24(28) DHE] were calculated and expressed as a percentage of mycelium by dry weight using the following equations:
Ergosterol (%) + 24(28) DHE (%) = [(A281.5/290) × 5]/0.1,
24(28) DHE (%) = [(A230/518) × 5]/0.1,
Ergosterol (%) = [Ergosterol (%) + 24(28) DHE (%)] − 24(28) DHE (%).
The assays were performed in triplicate, and the results are expressed as means ± standard deviation. The experiment was independently repeated three times.
2.5. Determination of the Effect of CA on the Pathogenicity of F. oxysporum FOX-1
Mycelial cake samples (d = 6 mm) were retrieved from the PDA plate treated with CA for 3 days at 1/2 MIC or MIC, and the mycelium was placed face down on rhizome sections (3 cm × 4 cm × 1 cm). Mycelial cake grown for 3 days on PDA plates without any additions was used as the blank control. After 3 days of humidified incubation at 28 °C in a light incubator (12 h light, 12 h dark), the lesion area (area of mycelial growth) was measured and photographed. Eight replicates were set up for each treatment, and the experiment was repeated three times. The following equation was used to determine the control effect:
control effect (%) = (sterile distilled water treated lesion area − drug-treated lesion area)/sterile distilled water treated lesion area × 100.
2.6. Statistical Analyses
MS Excel 2016 (Microsoft Corp., Redmond, WA, USA) was used to process the data, and IBM SPSS Statistics 26 (IBM Corp., Armonk, NY, USA) was used for data analysis, with Duncan’s test (p < 0.05).
3. Results
3.1. Ginger EO and Selected Volatile Compounds Inhibited the Growth of F. oxysporum FOX-1
The inhibitory effects of ginger EO and selected volatile compounds on the mycelial growth of F. oxysporum FOX-1 were evaluated. Results showed that all the samples exhibited significant antifungal activity (p < 0.05) throughout the 5 day incubation period (Table 1). However, these samples still exhibited significant differences in terms of their fungistatic activity, and among the five tested samples, CA showed the best fungistatic effect (Figure 1(a5)), with an EC50 value for F. oxysporum FOX-1 of 0.242 g/L and MIC of 0.25 g/L. Additionally, the results showed that 30% (mass v/v) ethanol had almost no effect on colony growth (p > 0.05).

Table 1.
Inhibitory effects of ginger EO and selected volatile compounds on F. oxysporum FOX-1.
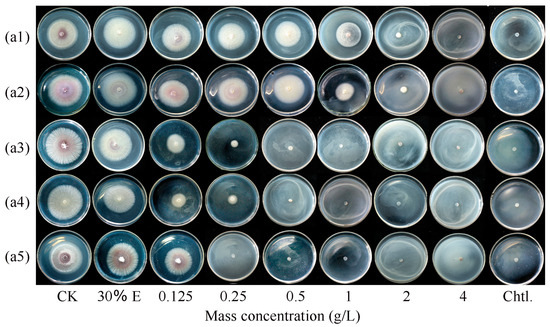
Figure 1.
Effects of different concentrations of ginger essential oil (a1), linalool (a2), eugenol (a3), citral (a4), and CA (a5) on F. oxysporum FOX-1 growth after 5 days of incubation; 30% E, 30% ethanol; CK, control (essential oils and ethanol are not included); Chtl, chlorothalonil (8 g/L).
3.2. Ginger EO and Selected Volatile Compounds Affected Spore Germination in F. oxysporum FOX-1
Table 2 shows that ginger EO and selected volatile compounds significantly inhibited the spore germination of F. oxysporum FOX-1 after the 12 h treatment; the larger the mass concentration, the higher the inhibitory effect. Specifically, when ginger EO and selected volatile compounds were used at the MIC, the spore germination rate of F. oxysporum FOX-1 was the lowest, and the spore germination rate with 8 g/L of chlorothalonil treatment was 8.98%. The spore germination rate of CK was above 88%, and the spore germination inhibition rate of CA-MIC treatment was as high as 96.20%. This result suggests that among ginger EO and the selected volatile compounds, the sensitivity of F. oxysporum FOX-1 to CA was the highest.

Table 2.
Effect of ginger EO and selected volatile compound concentrations on spore germination of F. oxysporum FOX-1.
3.3. CA Altered F. oxysporum FOX-1 Morphology
The effects of CA (1/2 MIC or MIC) treatments on the morphology of F. oxysporum FOX-1 mycelium were observed using SEM. The mycelium of the CK group grew normally and had a smooth, plump appearance devoid of cracks and wrinkles (Figure 2a). In contrast, 1/2 MIC CA treatment resulted in obvious wrinkles and depressions on the mycelial surface (Figure 2b). In addition, MIC-CA-treated mycelium exhibited severe shrinkage, collapse, deformation, surface roughness, and other structural abnormalities (Figure 2c), indicating that CA disrupts the permeability of the cell membrane and promotes the entry of CA into the cell, leading to cell shrinkage or atrophy.

Figure 2.
Effects of CA on the mycelial morphology of F. oxysporum FOX-1. (a) CK, 0 g/L CA. (b) 1/2 MIC, treatment with 0.125 g/L CA. (c) MIC, treatment with 0.25 g/L CA. MIC, minimum inhibitory concentration. SEM MAG: 5.00 kV; SEM HV: 20.0 kV; and SEM MAG: 5.00 kV.
3.4. CA-Induced Cell Membrane Injury of F. oxysporum FOX-1
The effect of CA on the permeability of F. oxysporum FOX-1 cell membranes was determined using the extracellular conductivity method. As shown in Figure 3a, the extracellular conductivity of the fungal sap without CA treatment (CK group) was unchanged from 0 to 12 h of action, whereas the conductivity of the fungal sap treated with CA changed to different degrees. When the treatment time of CA exceeded 4 h, the extracellular conductivity increased with the increase in CA concentration. When the treatment time was the same, a higher CA concentration led to a higher conductivity of the corresponding fungal solution. This indicates that the cell membrane of F. oxysporum FOX-1 mycelium treated with CA was damaged, and small intracellular molecules such as Na+, K+, and Ca2+ ions leaked out, resulting in a change in conductivity.
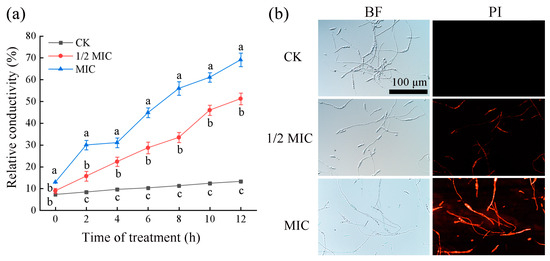
Figure 3.
CA damaged the cell membrane of F. oxysporum FOX-1. (a) Relative conductivity of mycelia; and (b) PI staining of mycelia. PI represents PI staining; and BF represents the bright field. The bars on the curves represent standard deviations, and different letters at the same time point represent significant differences (p < 0.05).
The effect of CA on the cell membrane of F. oxysporum FOX-1 was further explored using PI staining. As shown in Figure 3b, after PI staining, the CK group mycelium had no observable fluorescence, while a few mycelia emitted sporadic red fluorescence after treatment with 1/2 MIC CA. Moreover, the number of mycelia increased in the MIC treatment group (see image that shows mycelia emitting red fluorescence). This indicated that the degree of damage to the mycelial cell membrane by the agent was concentration-dependent. When the pathogen was exposed to a high concentration of CA (MIC), almost all mycelia observed appeared to display bright red fluorescence, indicating that the mycelial cell membrane was destroyed. The PI staining pictures reflected that CA treatment would damage the F. oxysporum FOX-1 mycelial cell membrane, increasing the permeability of the cell membrane, resulting in the efflux of cytoplasm, an imbalance of homeostasis within the fungus, and fungal death.
3.5. CA-Induced Cellular Leakage of F. oxysporum FOX-1
As seen in Figure 4a,b, the leakage of soluble protein and nucleic acid in the CK group was almost unchanged. Conversely, the cellular leakage of soluble proteins and nucleic acids in F. oxysporum FOX-1 mycelia significantly increased after 2 h of CA treatment, which lasted for more than 8 h. The amount of soluble protein and nucleic acid measured after 12 h of MIC CA treatment were 3.14-fold and 8.11-fold higher, respectively, than those of CK, which indicated that CA could induce cellular leakage by increasing the permeability of the cellular membranes of F. oxysporum FOX-1.
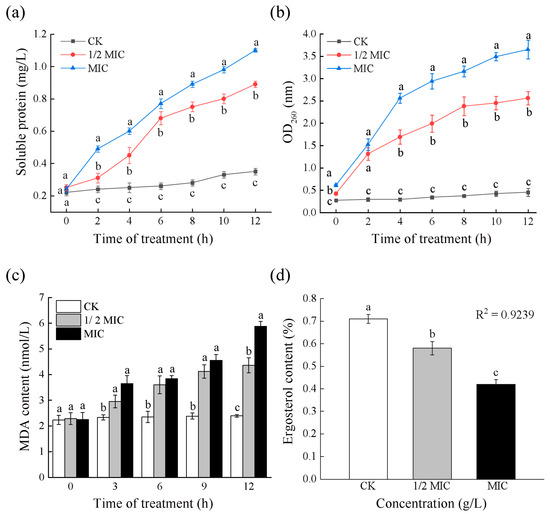
Figure 4.
Effects of different CA concentrations on the extracellular leakage of (a) soluble proteins, (b) nucleic acids, (c) malondialdehyde, and (d) ergosterol contents. The bars on the curves represent standard deviations, and different letters at the same time point represent significant differences (p < 0.05).
3.6. Changes in Malondialdehyde and Ergosterol Content
Malondialdehyde (MDA) concentration is often used as an indicator of lipid peroxidation or the oxidative damage of cytoplasmic membranes. The MDA content significantly increased by 1.82- and 2.46-fold under the 1/2 MIC and MIC CA treatments, respectively, compared to that for CK (Figure 4c). Ergosterol is the major sterol component of fungal cell membranes and is important in maintaining cell membrane integrity and cellular function. Compared with CK, the 1/2 MIC and MIC treatments reduced ergosterol content in F. oxysporum FOX-1 by 19.15% and 41.58%, respectively (Figure 4d). These results suggest that CA treatment induces cell membrane damage in F. oxysporum FOX-1.
3.7. Changes in the Infectivity of F. oxysporum FOX-1 after Treatment with Different Mass Concentrations of CA
As shown in Figure 5, 3 days after inoculation, obvious differences were observed in the development of fungal growth on rhizome sections among different treatments. Among these, the negative control (Figure 5(a1)) did not develop any disease, while the positive control showed obvious mycelial growth radiating from the fungal cake (Figure 5(a2)). The 1/2 MIC treated ginger piece (Figure 5(a3)) inhibited fungal growth compared with the positive control, and no mycelial growth was observed on the rhizome sections after CA treatment at the MIC (Figure 5(a4)). The 1/2 MIC and MIC CA treatments significantly reduced the infectivity of F. oxysporum FOX-1.
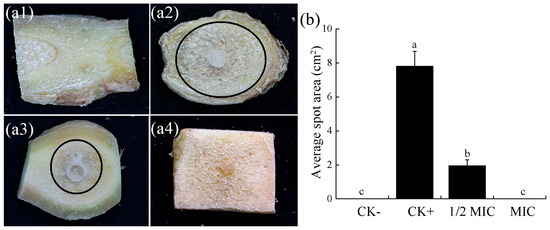
Figure 5.
Variation in the infectivity of F. oxysporum FOX-1 on rhizome sections after CA treatment. (a1) CK−, negative control, no inoculation; (a2) CK+, positive control, inoculated with F. oxysporum FOX-1; (a3) 1/2 MIC, inoculated with F. oxysporum FOX-1 and treated with 0.125 g/L CA; (a4) MIC, inoculated with F. oxysporum FOX-1 and treated with 0.25 g/L CA. (b) Graphical representation of the fungal lesion area. Different small letters meant significant difference among treatments at 0.05 level.
4. Discussion
In this study, we investigated the inhibitory effects of ginger EO and selected volatile compounds with activity against F. oxysporum FOX-1 using the mycelial growth rate method and the spore germination method. The results showed that both ginger EO and the selected volatile compounds had inhibitory effects on F. oxysporum FOX-1. Their antifungal activities were listed in the following order: CA > citral > eugenol > linalool ≥ ginger EO. Among the five commercial EOs studied, CA showed robust antifungal effects. Specifically, the MIC of CA on F. oxysporum FOX-1 was 0.25 g/L. The inhibitory effect of plant EO on pathogenic fungi was in line with those reported in previous studies. For example, Kong et al. [20] found that camphor has an inhibitory effect on Fusarium spp.; a 2 mg/mL mass concentration of PDA plate (camphor) increased the absolute inhibition of four Fusarium phytopathogens (F. oxysporum G5, F. solani G9, F. verticillioides, and F. graminearum) by >80%. Similarly, we found that a concentration of 2 g/L in PDA plates (ginger EO, linalool, eugenol, citral, and CA) could significantly inhibit the mycelial growth of F. oxysporum FOX-1 and that this effect was concentration-dependent, except for CA. Spores are important in the secondary infection of F. oxysporum FOX-1 in hosts. Therefore, any fungicide that inhibits spore production and germination should be useful for controlling diseases caused by F. oxysporum FOX-1 [21]. Therefore, we further investigated the effects of five commercial EO components on F. oxysporum FOX-1 spore germination in this study. Results revealed that these EO components (ginger EO, linalool, eugenol, citral, and CA) significantly inhibited the spore germination of F. oxysporum FOX-1. Thus, ginger EO and selected volatile compounds can reduce disease incidence by directly inhibiting the mycelial and reproductive growth of the pathogen. Among the examined ginger EO and the selected volatile compounds, CA was revealed to demonstrate the most robust antimicrobial effects; therefore, we further investigated the mechanisms underlying its effectiveness.
Previous studies have suggested that the antifungal activity of plant EOs is partly due to the damage they cause to the cell membrane [18]. SEM analysis showed that CA treatment caused irregular contractions and the obvious concave collapse of the F. oxysporum FOX-1 mycelia. Similarly, Xing et al. [22] found that SEM and transmission electron microscopy of Fusarium verticillioides exposed to the MIC of CA showed irreversible deleterious morphological and ultrastructural alterations, such as lack of cytoplasmic contents, plasma membrane disruption, mitochondrial destruction, and folding of the cell. Wang et al. [23] found that A. ochraceus exposed to 0.4 mmol/L of CA exhibited irreversible and harmful morphological and ultrastructural modifications such as cell folding, disruption of the plasma membrane, destruction of the mitochondria, and loss of intracellular organelles. In the present study, the relative conductivity content gradually increased with the increase in the experimental treatment time and concentration, indicating that 1/2 MIC or MIC CA treatments disrupted the cell membrane integrity of F. oxysporum FOX-1 and increased the K+ and Na+ content within the sap, thereby increasing the relative conductivity content. The PI staining assay further confirmed the membrane-disrupting effect of CA. Additionally, we observed that the MIC of CA emitted a continuous red fluorescence, indicating that the integrity of the cell membrane was severely disrupted. Similarly, Xu et al. [24] demonstrated that CA destroyed the membrane integrity of Alternaria alternata, as demonstrated by the release of intracellular components, leakage of electrolytes, and the intracellular presence of PI staining. The present study showed that CA treatment resulted in severe cell membrane integrity impairment in F. oxysporum FOX-1, suggesting that the cell membrane is the target of CA.
Damage to the cell membrane usually leads to leakage of soluble proteins and nucleic acids into the extracellular space. Therefore, the effect of CA on the cellular leakage of F. oxysporum FOX-1 mycelia was studied. Results confirmed that protein and nucleic acid leakage from the mycelium significantly increased after CA treatment, suggesting that CA treatment disrupts the cell membranes of the apical dome. This is both the zone of fungal growth and where the synthesis of new plasma membranes and cell walls occurs. Due to its high metabolic activity, the apical dome area is vulnerable to CA damage, leading to cellular leakage of contents and resulting in the cell death of F. oxysporum FOX-1. In the present study, the disruption of apical dome cell membranes was the key mechanism underlying the antimicrobial activity of EOs in different plants. Xi et al. [25] reported that ginger rhizome extract could disrupt the cell membrane of F. solani. Jing et al. [26] found that eugenol was effective in controlling black stock tobacco by inducing disruption of the cell membrane of F. nicotianae. Recently, the damaging effect of CA on cell membranes has been suggested as a mechanism for antifungal activity against F. solanacearum. In the present study, CA also affected the integrity of cell membranes, but its specific site of action on cell membranes should be further investigated.
Ergosterol is the major sterol component of the yeast and filamentous fungal cell membrane and is responsible for maintaining normal growth, viability, cell integrity, and function [27]. Malondialdehyde, a product of membrane lipid peroxidation, could indicate the degree of membrane lipid peroxidation [28]. Studies have shown that numerous fungicides exert an inhibitory effect by destroying the integrity of the cell membrane [28,29]. Niu et al. [28] found that CA vapour could inhibit ergosterol synthesis in A. niger HY2, promote MDA production, and cause plasma membrane damage, which would further lead to the leakage of intracellular contents. In the present study, when exposed to CA, especially at higher concentrations, ergosterol biosynthesis was interrupted and MDA biosynthesis was promoted.
We tested for changes in F. oxysporum FOX-1 pathogenicity by performing in vivo inoculation. Perina et al. [30] showed that Cinnamomum zeylanicum oil and trans-CA could reduce the pathogenicity of Alternaria brown spot significantly in tangerine via direct effects and induced resistance. Naserzadeh et al. [31] showed that emulsions and nanoemulsions of cinnamon EO could significantly inhibit Rhizopus rot and grey mould in strawberry fruits. Similarly, the results of the present study revealed that treatment of rhizome sections with CA solution at various concentrations attenuated the infectivity of F. oxysporum FOX-1 significantly, suggesting that CA inhibits the growth of F. oxysporum FOX-1 mycelia and spores on rhizome sections. Moreover, CA was demonstrated to reduce the capacity of F. oxysporum FOX-1 to decompose the rhizome, thus mitigating the occurrence of the disease.
Further studies are warranted to improve our understanding of the inhibitory effects of EOs on Fusarium wilt. Antifungal drug development has identified numerous targets, such as the cell membrane, cell wall, ergosterol, cytoskeleton, and tubulin [32,33]. In the present study, the cell membrane was the main target used to investigate the inhibitory effect of CA on FOX, and further studies can be conducted to validate the inhibitory mechanisms of different targets to find the best target against F. oxysporum FOX-1. In addition, molecular-level studies should be conducted and verified by field experiments, which could facilitate commercial applications of CA.
5. Conclusions
Due to their eco-friendly and health-promoting properties, plant-derived fungicides are a desirable alternative to many of the commercial fungicides currently in use. Screening plants for active compounds that can be used as fungicides is currently an active area of research. The present study investigated the in vitro antifungal effects of five commercial EOs on F. oxysporum FOX-1. SEM observations, PI staining, release of intracellular components, leakage of electrolytes, and reduction in ergosterol content in apical dome cell membranes indicated that the integrity of the F. oxysporum FOX-1 cell membrane was damaged after CA treatment. In vivo experiments also confirmed that CA could reduce its pathogenicity. In conclusion, CA can inhibit FOA by disrupting the cell membrane. The findings could facilitate the development of highly efficient and environmentally friendly plant-derived antifungal agents.
Author Contributions
Conducting experiments and writing—original draft preparation, L.-R.Z. and H.-J.H.; data collection and data analysis, J.W.; literature search and supervision, X.-D.Z.; writing—review and editing, Y.-X.Z.; conception and designing, funding acquisition, resources, supervision, writing—review and editing, J.-W.M. and Y.-Q.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Flavouring Industry System Innovation Team Project in Chongqing, China (2023CQMAITS007), the Key R&D Projects in Hubei Province, China (2021BBA096), the Key R&D Projects in Hubei Province, China (2022BBA0061), and the Chongqing Outstanding Scientist Programme (CQYC20220101514).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zhou, J.; Liu, X.; Sun, C.; Li, G.; Yang, P.; Jia, Q.; Cai, X.; Zhu, Y.; Yin, J.; Liu, Y. Silica Nanoparticles Enhance the Disease Resistance of Ginger to Rhizome Rot during Postharvest Storage. Nanomaterials 2022, 12, 1418. [Google Scholar] [CrossRef] [PubMed]
- Park, C.J.; Kim, H.S.; Lee, D.W.; Kim, J.; Choi, Y.H. Identification of antifungal constituents of essential oils extracted from Boesenbergia pulcherrima against Fusarium wilt (Fusarium oxysporum). Appl. Biol. Chem. 2020, 63, 34. [Google Scholar] [CrossRef]
- Peng, H.; Hu, H.; Xi, K.; Zhu, X.; Zhou, J.; Yin, J.; Guo, F.; Liu, Y.; Zhu, Y. Silicon Nanoparticles Enhance Ginger Rhizomes Tolerance to Postharvest Deterioration and Resistance to Fusarium solani. Front. Plant Sci. 2022, 13, 816143. [Google Scholar] [CrossRef] [PubMed]
- Şesan, T.E.; Enache, E.; Iacomi, B.M.; Oprea, M.; Oancea, F.; Iacomi, C. In vitro antifungal activity of some plant extracts against Fusarium oxysporum in blackcurrant (Ribes nigrum L.). Acta Sci. Pol. Hortorum Cultus 2017, 16, 167–176. [Google Scholar] [CrossRef]
- Wang, Q.F.; Wang, X.Y.; Li, H.S.; Yang, X.Y.; Zhang, R.M.; Gong, B.; Shi, Q.H. Effects of linalool on Botrytis cinerea growth and control of tomato gray mold. J. Appl. Ecol. 2023, 34, 213–220. (In Chinese) [Google Scholar] [CrossRef]
- El Khetabi, A.; Ezrari, S.; El Ghadraoui, L.; Tahiri, A.; Ait Haddou, L.; Belabess, Z.; Lahlali, R. In vitro and in vivo antifungal activities of nine commercial essential oils against brown rot in apples. Horticulturae 2021, 7, 545. [Google Scholar] [CrossRef]
- Ju, J.; Xie, Y.; Yu, H.; Guo, Y.; Cheng, Y.; Qian, H.; Yao, W. Synergistic interactions of plant essential oils with antimicrobial agents: A new antimicrobial therapy. Crit. Rev. Food Sci. Nutr. 2022, 62, 1740–1751. (In Chinese) [Google Scholar] [CrossRef] [PubMed]
- Reddy, D.N. Essential oils extracted from medicinal plants and their applications. In Natural Bio-Active Compounds: Volume 1: Production and Applications; Springer: Berlin/Heidelberg, Germany, 2019; pp. 237–283. [Google Scholar] [CrossRef]
- Cheny, R.; Luol, M.; Liuz, Y. Comparison and analysis of chemical constituents of volatile oil from ginger from different habitats. Jiangxi J. Tradit. Chin. Med. 2022, 53, 67–70. [Google Scholar]
- Abdullahi, A.; Khairulmazmi, A.; Yasmeen, S.; Ismail, I.S.; Norhayu, A.; Sulaiman, M.R.; Ahmed, O.H.; Ismail, M.R. Phytochemical profiling and antimicrobial activity of ginger (Zingiber officinale) essential oils against important phytopathogens. Arab. J. Chem. 2020, 13, 8012–8025. [Google Scholar] [CrossRef]
- Ma, Q.; Zhang, Y.; Wang, Z.G. Antimicrobial activity of the essential oils from five plants. Plant Prot. 2020, 46, 161–167. (In Chinese) [Google Scholar] [CrossRef]
- Liao, S.; Yang, G.; Huang, S.; Li, B.; Li, A.; Kan, J. Chemical composition of Zanthoxylum schinifolium Siebold & Zucc. essential oil and evaluation of its antifungal activity and potential modes of action on Malassezia Restricta. Ind. Crops Prod. 2022, 180, 114698. [Google Scholar] [CrossRef]
- Badr, M.M.; Badawy, M.E.; Taktak, N.E. Preparation, characterization, and antimicrobial activity of cinnamon essential oil and cinnamaldehyde nanoemulsions. J. Essent. Oil Res. 2022, 34, 544–558. [Google Scholar] [CrossRef]
- Liu, Y.; Wisniewski, M.; Kennedy, J.F.; Jiang, Y.; Tang, J.; Liu, J. Chitosan and oligochitosan enhance ginger (Zingiber officinale Roscoe) resistance to rhizome rot caused by Fusarium oxysporum in storage. Carbohydr. Polym. 2016, 151, 474–479. [Google Scholar] [CrossRef]
- Ma, X.Y.; Yu, C. Bacteriostatic Ability and Field Control Effect of Eight Fungicides against Fusarium oxysporum. J. Eest China For. Sci. 2023, 52, 69–74. (In Chinese) [Google Scholar] [CrossRef]
- Weng, T.; Wang, Y.; Long, C. Study on Mechanism of Geraniol against Geotrichum citri-aurantii of Citrus. Food Sci. 2023, 44, 14–21. (In Chinese). Available online: https://kns.cnki.net/kcms/detail/11.2206.TS.20220425.1801.064.html (accessed on 12 October 2023).
- Huang, J.; Liu, S.; Liu, R.; Yi, Y.; Li, C.; Xiao, Z.; Tu, J.; Xiao, J. Mechanisms of Litsea cubeba essential oil in the control of Colletotrichum scovillei in pepper (Capsicum annuum L.): Cell membrane/wall perspective. Physiol. Mol. Plant Pathol. 2023, 127, 102103. [Google Scholar] [CrossRef]
- Wang, B.; Li, P.; Yang, J.; Yong, X.; Yin, M.; Chen, Y.; Wang, Q. Inhibition efficacy of Tetradium glabrifolium fruit essential oil against Phytophthora capsici and potential mechanism. Ind. Crops Prod. 2022, 176, 114310. [Google Scholar] [CrossRef]
- Bao, Z.; Fan, M.; Hannachi, K.; Li, T.; Zhao, J.; Li, Y.; Qian, H.; Wang, L. Antifungal activity of star anise extract against Penicillium roqueforti and Aspergillus niger for bread shelf life. Food Res. Int. 2023, 172, 113225. [Google Scholar] [CrossRef]
- Kong, W.; Huo, H.; Gu, Y.; Cao, Y.; Wang, J.; Liang, J.; Niu, S. Antifungal activity of camphor against four phytopathogens of Fusarium. S. Afr. J. Bot. 2022, 148, 437–445. [Google Scholar] [CrossRef]
- Matheron, M.E.; Porchas, M. Impact of azoxystrobin, dimethomorph, fluazinam, fosetyl-Al, and metalaxyl on growth, sporulation and zoospore cyst germination of Three Phytophthora spp. Plant Dis. 2000, 84, 454–458. [Google Scholar] [CrossRef]
- Xing, F.; Hua, H.; Selvaraj, J.N.; Zhao, Y.; Zhou, L.; Liu, X.; Liu, Y. Growth inhibition and morphological alterations of Fusarium verticillioides by cinnamon oil and cinnamaldehyde. Food Control 2014, 46, 343–350. [Google Scholar] [CrossRef]
- Wang, L.; Jin, J.; Liu, X.; Wang, Y.; Liu, Y.; Zhao, Y.; Xing, F. Effect of cinnamaldehyde on morphological alterations of Aspergillus ochraceus and expression of key genes involved in ochratoxin A biosynthesis. Toxins 2018, 10, 340. [Google Scholar] [CrossRef]
- Xu, L.; Tao, N.; Yang, W.; Jing, G. Cinnamaldehyde damaged the cell membrane of Alternaria alternata and induced the degradation of mycotoxins in vivo. Ind. Crops Prod. 2018, 112, 427–433. [Google Scholar] [CrossRef]
- Xi, K.Y.; Xiong, S.J.; Li, G.; Guo, C.Q.; Zhou, J.; Ma, J.W.; Yin, J.L.; Liu, Y.Q.; Zhu, Y.X. Antifungal Activity of Ginger Rhizome Extract against Fusarium solani. Horticulturae 2022, 8, 983. [Google Scholar] [CrossRef]
- Jing, C.; Gou, J.; Han, X.; Wu, Q.; Zhang, C. In vitro and in vivo activities of eugenol against tobacco black shank caused by Phytophthora nicotianae. Pestic. Biochem. Physiol. 2017, 142, 148–154. [Google Scholar] [CrossRef]
- Wei, J.; Bi, Y.; Xue, H.; Wang, Y.; Zong, Y.; Prusky, D. Antifungal activity of cinnamaldehyde against Fusarium sambucinum involves inhibition of ergosterol biosynthesis. J. Appl. Microbiol. 2020, 129, 256–265. [Google Scholar] [CrossRef]
- Niu, A.; Wu, H.; Ma, F.; Tan, S.; Wang, G.; Qiu, W. The antifungal activity of cinnamaldehyde in vapor phase against Aspergillus niger isolated from spoiled paddy. LWT 2022, 159, 113181. [Google Scholar] [CrossRef]
- Sun, Q.; Li, J.; Sun, Y.; Chen, Q.; Zhang, L.; Le, T. The antifungal effects of cinnamaldehyde against Aspergillus niger and its application in bread preservation. Food Chem. 2020, 317, 126405. [Google Scholar] [CrossRef]
- Perina, F.J.; de Andrade, C.C.L.; Moreira, S.I.; Nery, E.M.; Ogoshi, C.; Alves, E. Cinnamomun zeylanicum oil and trans-cinnamaldehyde against Alternaria brown spot in tangerine: Direct effects and induced resistance. Phytoparasitica 2019, 47, 575–589. [Google Scholar] [CrossRef]
- Yousef, N.; Niloufar, M.; Elena, P. Antipathogenic effects of emulsion and nanoemulsion of cinnamon essential oil against Rhizopus rot and grey mold on strawberry fruits. Foods Raw Mater. 2019, 7, 210–216. [Google Scholar] [CrossRef]
- Gow, N.A.; Latge, J.P.; Munro, C.A. The fungal cell wall: Structure, biosynthesis, and function. Microbiol. Spectr. 2017, 5, 1–25. [Google Scholar] [CrossRef]
- Ishii, H. Target sites of tubulin-binding fungicides. In Target Sites of Fungicide Action; CRC Press: Boca Raton, FL, USA, 2018; pp. 43–52. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).