Polyploid Induction and Identification of Begonia × benariensis
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Explant Preparation
2.2. Induction of Leaf Callus
2.3. Induction of Polyploidy in Begonia × benariensis by Colchicine
2.4. Ploidy Identification of Polyploid Begonia × benariensis Plants
2.4.1. Preparation of Nuclei Suspension
2.4.2. Fluorescence Staining
2.4.3. Loading Test
2.4.4. Stomatal Identification
2.5. Determination of Morphophysiological Indexes in Polyploid Begonia × benariensis Plants
2.5.1. Measurement of Plant Height
2.5.2. Measurement of Crown Width
2.5.3. Measurement of Leaves
2.5.4. Determination of Chlorophyll Content
2.6. Data Analysis
3. Results
3.1. Effects of Different Concentrations of Colchicine on the Induction Efficiency of Begonia × benariensis
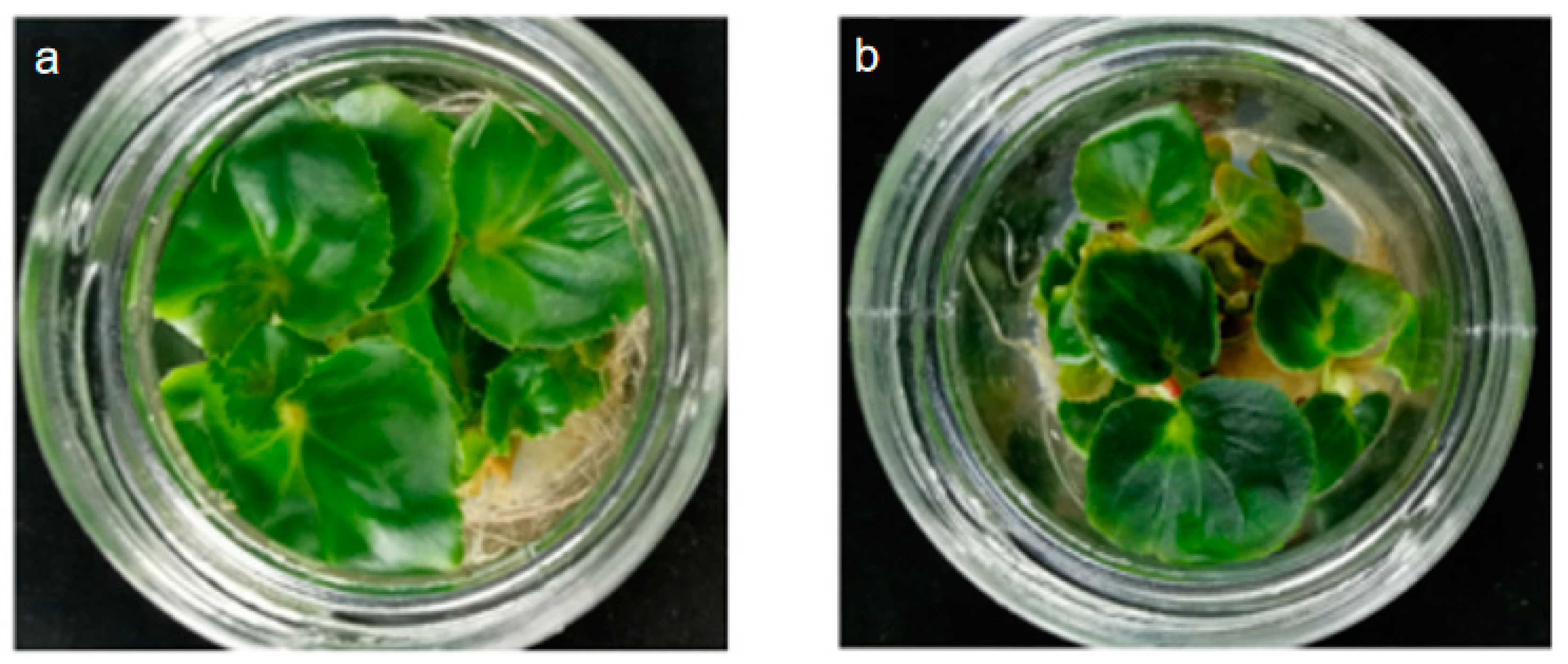
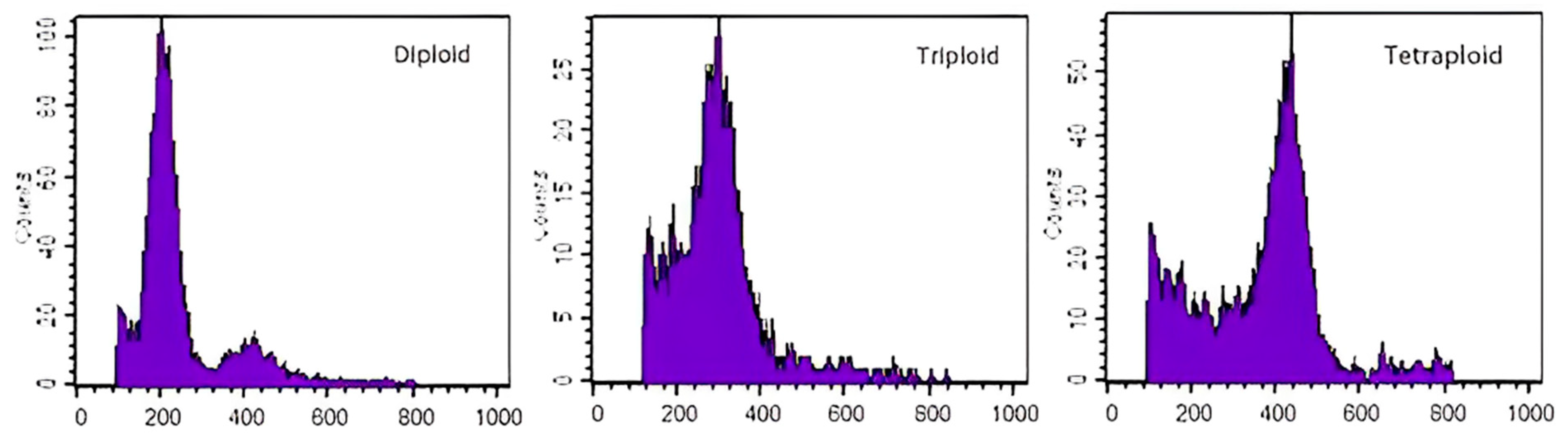
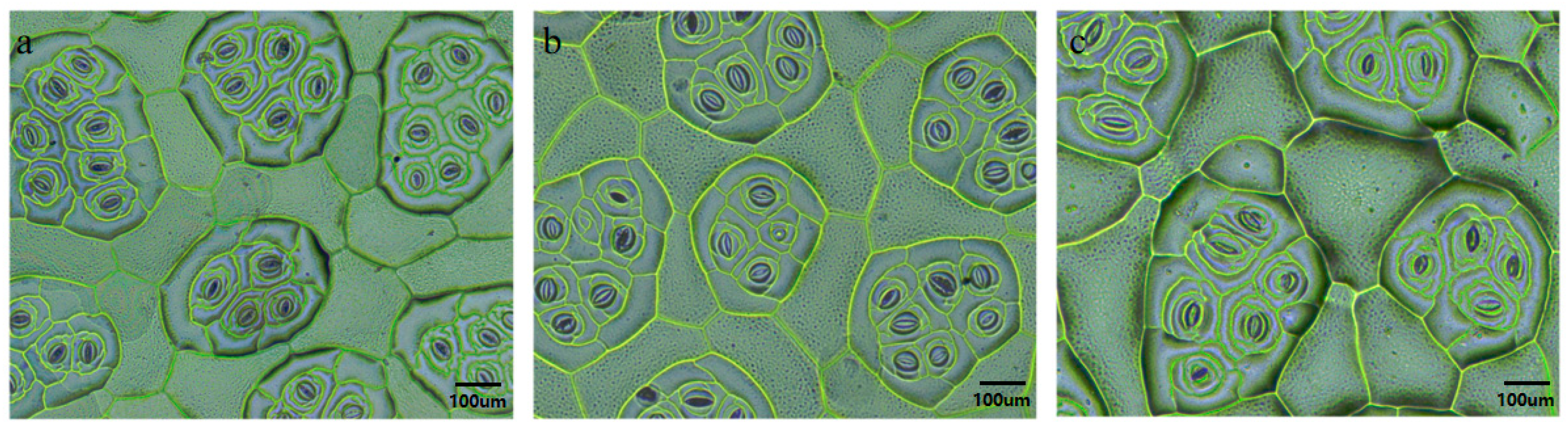
3.2. Identification of Polyploid Plants of Begonia × benariensis
3.2.1. Morphological Identification
3.2.2. Flow Cytometry Identification
3.2.3. Stomatal Identification
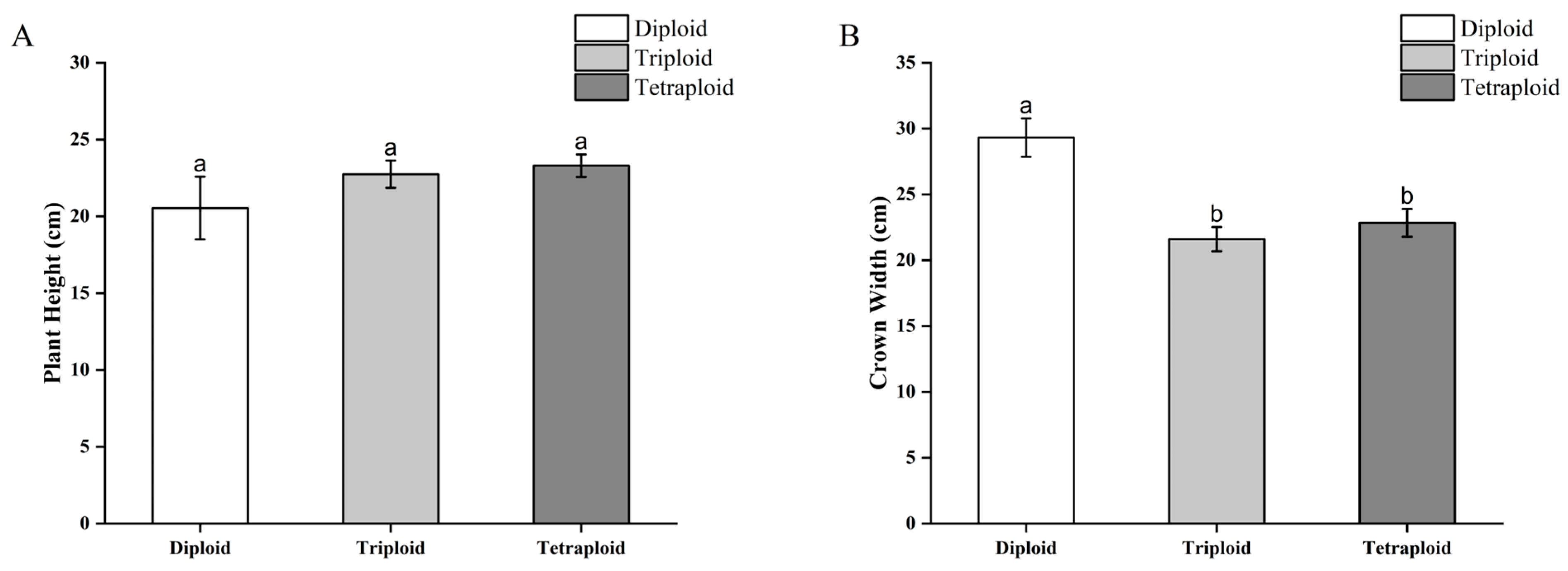
3.3. Comparison of Morphophysiological Indexes in Doubled Begonia × benariensis Plants
3.3.1. Morphological Differences in Begonia × benariensis Plants with Different Ploidies
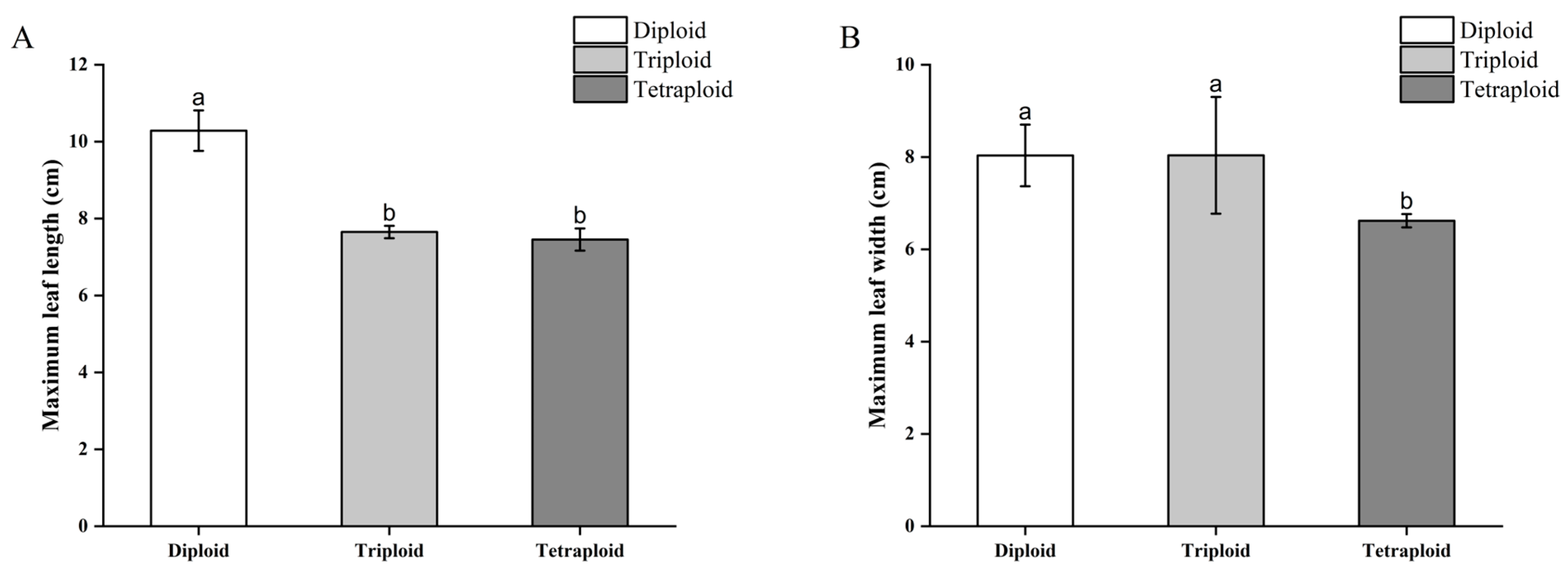
3.3.2. Morphological Differences in Begonia × benariensis Leaf Blades of Different Ploidies
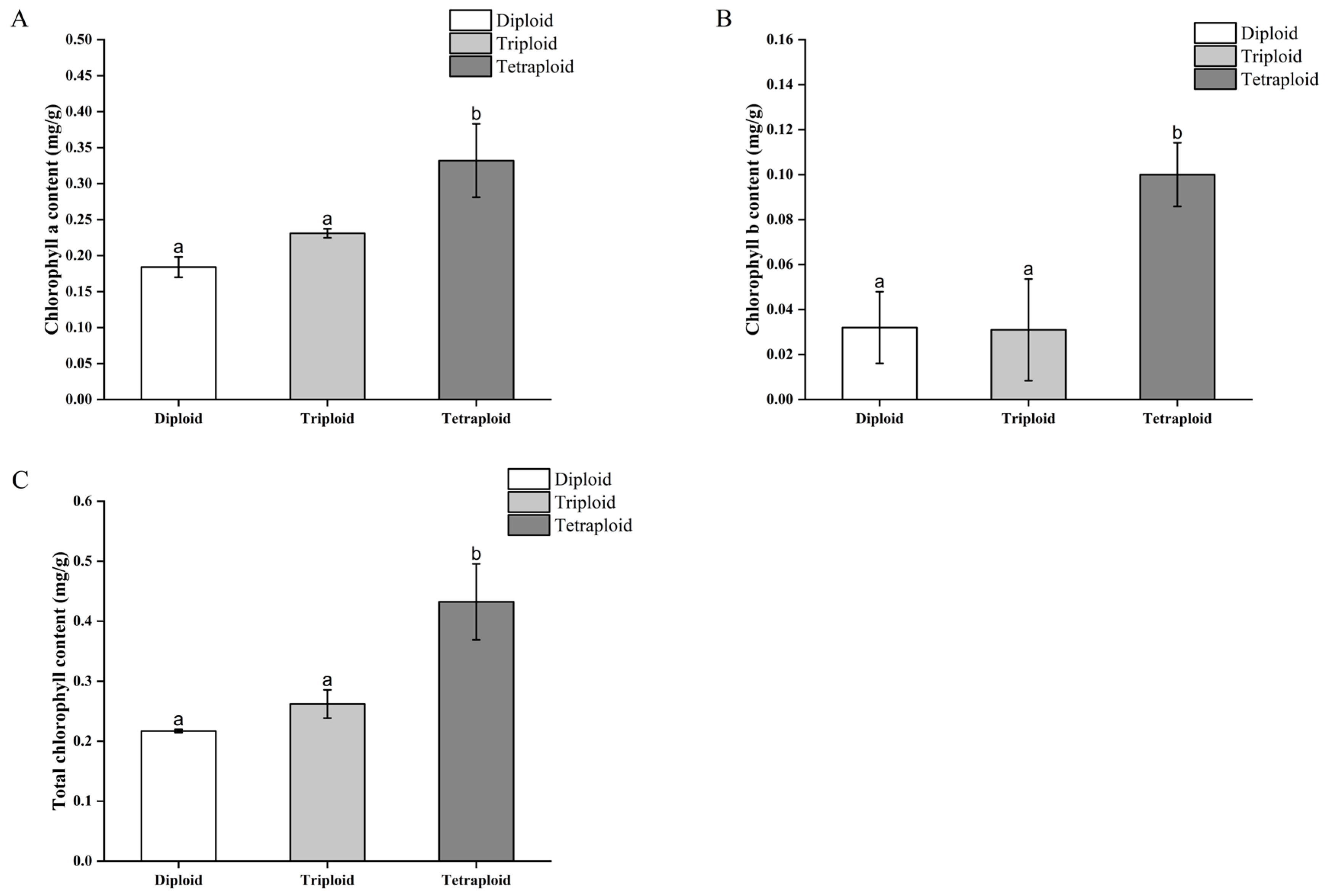
3.3.3. Differences in Chlorophyll Content of Begonia × benariensis Leaf Blades of Different Ploidies
4. Discussion
4.1. Induction of Polyploidy by Colchicine Impregnation
4.2. Ploidy Identification of Begonia × benariensis Polyploids
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhou, H.; Li, M.; Shi, Z.; Yang, J.; Zhang, S. Selection of formulation of nutrient solutions in soilless culture for Begonia × benariensis. J. Anhui Agri. Sci. 2014, 42, 2565–2567. [Google Scholar] [CrossRef]
- Zhai, Y.; Yan, R.; Li, L.; Zhang, L. Optimization of adventitious bud induction technology of Begonia Benariensis based on response surface methodology. Seed 2022, 41, 137–144. [Google Scholar] [CrossRef]
- Zhong, W.; Ba, W.; Zhu, M.; Wu, Y.; Liang, M.; Zhang, L. Polyploid watermelon induction breeding techniques. Contemp. Hortic. 2022, 45, 25–27. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, X.; Li, M. Research progress on colchicine-induced polyploidy in medicinal plants. Jiangsu Agric. Sci. 2014, 42, 178–181. [Google Scholar] [CrossRef]
- Wu, T.; Jia, R.; Yang, S.; Zhao, X.; Yu, X.; Guo, Y.; Ge, H. Research advances and prospects on Phalaenopsis polyploid breeding. Acta Hortic. Sin. 2022, 49, 448–462. [Google Scholar] [CrossRef]
- Sun, Q.; Sun, H.; Zhu, E.; Li, L. Polyploid induction in pear in vitro treatment with gamma-rays. Acta Hortic. Sin. 2009, 36, 257–260. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Q.; Yang, L.; Wu, C. Effect of N+ implantation on the inducement of polyploid Astragalus membranaceus (Fisch.) Bge. Var. mongholicus (Bge.) Hsiao. Bull. Bot. Res. 2011, 31, 563–568. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Z.; Li, Y.; Wu, L.; Liu, H. Study on polyploid induction of blueberry in vitro with colchicine treatment. J. Northeast. Agric. Univ. 2010, 41, 38–42. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, H.; Yue, S.; Yang, T.; Bao, Q.; Lu, G.; Xie, Y. Effect of colchicine on blueberry chromosome doubling and preliminary identification. J. Kunming Univ. 2015, 37, 72–76. [Google Scholar] [CrossRef]
- Cheng, Z.; Sun, C.; Sun, P.; Guo, S.; Wu, Q. Optimization of polyploidy induction system in dragon fruit. J. Fruit Sci. 2020, 37, 1089–1097. [Google Scholar] [CrossRef]
- Wei, Z.; Zhang, X.; Zhang, Y.; Liu, X.; Ye, Q.; Li, Y.; Zhang, H. Polyploid induction and identification of Yellow-flesh Actinidia Chinensis. Mol. Plant Breed. 2020, 18, 4036–4040. [Google Scholar] [CrossRef]
- Premjet, D.; Obeng, A.K.; Kongbangkerd, A.; Premjet, S. Intergeneric hybrid from Jatropha curcas L. and Ricinus communis L.: Characterization and polyploid induction. Biology 2019, 8, 50. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, K.; Zhao, Q.; Yu, J.; Gongga, Y.; Hu, B. Induction of polyploid Malus sieversii (Ledeb.) Roem. and characteristic analysis of its leaves. J. Tianjin Agric. Univ. 2021, 28, 6–10. [Google Scholar] [CrossRef]
- Xi, Y.; Dong, X.; Yang, M.; Meng, Q.; Huang, T. In Vitro polyploid induction and establishment of a clone for Cyclocodon lancifolius (Roxb.) Kurz. Cytologia 2021, 86, 367–374. [Google Scholar] [CrossRef]
- Pachakkil, B.; Midori, I.; Yukiko, K.; Ryo, M.; Hidehiko, K.; Antonio, L.; Hironobu, S. Somatic polyploidization and characterization of induced polyploids of Dioscorea rotundata and Dioscorea cayenensis. Afr. J. Biotechnol. 2016, 15, 2098–2105. [Google Scholar] [CrossRef][Green Version]
- Liu, Z.; Zheng, Y.; Li, M.; Niu, W.; Liu, D. Polyploid induction and identification of Clematis fruticose. For. Ecol. Sci. 2023, 38, 338–344. [Google Scholar] [CrossRef]
- Huang, L.; Yin, X.; Yang, Y.; Li, W.; Yi, Z. Polyploid induction on the hybrid of miscanthus lutarioriparia × Miscanthus sinensis. Acta Bot. Boreali-Occident. Sin. 2015, 35, 50–56. [Google Scholar] [CrossRef]
- Cruz, V.; Lynch, A.; Ray, D.; Niaura, W.; Purdy, P.; Dierig, D. Analysis of mode of reproduction of guayule (Parthenium argentatum A. Gray) using flow cytometry and identification of polyhaploids for breeding. Ind. Crops Prod. 2017, 107, 618–623. [Google Scholar] [CrossRef]
- Liu, Z.; Zheng, Y.; Niu, W.; Wang, X.; Li, M.; Liu, D. Polyploid induction and identification of two wild Clematis. North. Hortic. 2022, 18, 52–59. [Google Scholar]
- Mo, L.; Chen, J.; Lou, X.; Xu, Q.; Dong, R.; Tong, Z.; Huang, H.; Lin, E. Colchicine-induced Polyploidy in Rhododendron fortunei Lindl. Plants 2020, 9, 424. [Google Scholar] [CrossRef]
- Hassan, J.; Miyajima, I.; Ozaki, Y.; Mizunoe, Y.; Sakai, K.; Zaland, W. Tetraploid induction by colchicine treatment and crossing with a diploid reveals less-seeded fruit production in pointed gourd (Trichosanthes dioica Roxb.). Plants 2020, 9, 370. [Google Scholar] [CrossRef]
- Sun, Q.; Sun, H.; Li, L.; Bell, R.L. In vitro colchicine-induced polyploid plantlet production and regeneration from leaf explants of the diploid pear (Pyrus communis L.) cultivar, ‘Fertility’. J. Hortic. Sci. Biotechnol. 2009, 84, 548–552. [Google Scholar] [CrossRef]
- Huy, N.P.; Tam, D.T.T.; Luan, V.Q.; Tung, H.T.; Hien, V.U.; Ngan, H.T.M.; Duy, P.N.; Nhut, D.T. In vitro polyploid induction of Paphiopedilum villosum using colchicine. Sci. Hortic. 2019, 252, 283–290. [Google Scholar] [CrossRef]
- Luo, Z.; Brian, J.; Katrina, C. Colchicine-induced polyploidy has the potential to improve rubber yield in Taraxacum kok-saghyz. Ind. Crops Prod. 2018, 112, 75–81. [Google Scholar] [CrossRef]
- Mahpara, K.; Sabbi, J.; Aijaz, A.; Manoj, K. Induction of polyploidy in saffron (Crocus sativus L.) using colchicine. J. Crop Improv. 2021, 36, 555–581. [Google Scholar] [CrossRef]
- Mo, L.; Chen, J.; Chen, F.; Xu, Q.; Tong, Z.; Huang, H.; Dong, R.; Lou, X.; Lin, E. Induction and characterization of polyploids from seeds of Rhododendron fortunei Lindl. J. Integr. Agric. 2020, 19, 2016–2026. [Google Scholar] [CrossRef]
- Liu, Y.; Duan, S.; Jia, Y.; Hao, L.; Xiang, D.; Chen, D.; Niu, S. Polyploid induction and karyotype analysis of Dendrobium officinale. Horticulturae 2023, 9, 329. [Google Scholar] [CrossRef]
- Ren, X.; Wang, X.; Xiao, F.; Zhou, Y.; Wei, L. Polyploidy induction and identification of Hibiscus syriacus. J. West China For. Sci. 2019, 48, 119–125. [Google Scholar] [CrossRef]
- Li, J.; Deng, W.; Xu, Z.; Zhang, Z.; Lu, B. Induction and identification of polyploid from Pistacia chinensis. J. Northeast. For. Univ. 2020, 50, 18–22. [Google Scholar] [CrossRef]


| Treatment Concentration (%) | Treatment Time (h) | Treatment Quantity | Survival Number | Survival Rate (%) | Mutagenic Number | Mutagenic Rate (%) |
|---|---|---|---|---|---|---|
| 0 | 2 | 60 | 48 | 80.00% | 0 | 0% |
| 4 | 60 | 44 | 73.33% | 0 | 0% | |
| 8 | 60 | 43 | 71.67% | 0 | 0% | |
| 0.01% | 2 | 60 | 41 | 68.33% | 0 | 0% |
| 4 | 60 | 39 | 65.00% | 2 | 3.33% | |
| 8 | 60 | 37 | 61.67% | 0 | 0% | |
| 0.05% | 2 | 60 | 38 | 63.33% | 4 | 6.67% |
| 4 | 60 | 36 | 60.00% | 28 | 46.67% | |
| 8 | 60 | 26 | 43.33% | 0 | 0% | |
| 0.1% | 2 | 60 | 25 | 41.67% | 1 | 1.67% |
| 4 | 60 | 19 | 31.67% | 0 | 0% | |
| 8 | 60 | 11 | 18.33% | 0 | 0% |
| Ploidy | Growth State of Seedlings | Leaf Character |
|---|---|---|
| CK | Normal | Thin, oily, smooth, larger, bright green leaves |
| Variant plant | Stocky and slow growing | Leaf blades thickened and curled, leaf color deepened, leaf blades smaller and darker green |
| Ploidy | Leaf Character | Flower Character |
|---|---|---|
| CK | Thin, oily, smooth, larger, bright green leaves | Thinner and smaller petals |
| Variant plant | Leaf blades thickened and curled, leaf color deepened, leaf blades smaller and darker green | Thickened and enlarged petals, more reddish flower color |
| Ploidy | Defense Cell Length (µm) | Defense Cell Width (µm) | Stomatal Density (No./mm2) |
|---|---|---|---|
| CK | 58.98 ± 5.87 b | 14.35 ± 2.70 b | 112.37 ± 12.89 a |
| Triploid | 63.12 ± 4.78 b | 14.39 ± 1.10 b | 102.67 ± 11.39 a |
| Tetraploid | 85.42 ± 8.48 a | 20.89 ± 2.44 a | 67.11 ± 6.21 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, N.; Zhao, Y.; Huang, M.; Chen, C.; Cao, C.; Wang, J.; Shi, Z.; Gao, J. Polyploid Induction and Identification of Begonia × benariensis. Horticulturae 2024, 10, 47. https://doi.org/10.3390/horticulturae10010047
Xie N, Zhao Y, Huang M, Chen C, Cao C, Wang J, Shi Z, Gao J. Polyploid Induction and Identification of Begonia × benariensis. Horticulturae. 2024; 10(1):47. https://doi.org/10.3390/horticulturae10010047
Chicago/Turabian StyleXie, Ninghao, Yi Zhao, Min Huang, Caixia Chen, Chuanqu Cao, Jisheng Wang, Zhihua Shi, and Junshan Gao. 2024. "Polyploid Induction and Identification of Begonia × benariensis" Horticulturae 10, no. 1: 47. https://doi.org/10.3390/horticulturae10010047
APA StyleXie, N., Zhao, Y., Huang, M., Chen, C., Cao, C., Wang, J., Shi, Z., & Gao, J. (2024). Polyploid Induction and Identification of Begonia × benariensis. Horticulturae, 10(1), 47. https://doi.org/10.3390/horticulturae10010047







