Enterococcus faecalis-Aided Fermentation to Facilitate Edible Properties and Bioactive Transformation of Underutilized Cyathea dregei Leaves
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Collection of Plant Leaves
2.3. Handling of Lactic Acid Bacteria for the Experimentation
2.4. Fermentation Procedure Setup to Harness the Edible Properties of Plant Leaves
2.5. Monitoring of Quality Parameters after Fermentation
2.5.1. Quantitative Chemical and Phytochemical Constituent Determination
2.5.2. Assay for Antioxidant Qualities of the Extracts
2.5.3. Procedure for Preparing the Sample Leaves and Micronutrient Analysis Using Atomic Absorption Spectrometric Technique
2.5.4. HPLC Procedure for the Determination of Vitamins in the Sample Leaves
2.5.5. Preparation and HPLC Procedure for Quantitative Determination of Individual Phytochemical Constituents of the Sample Leaves
2.5.6. Procedure for Lactate Dehydrogenase Activity Analysis
2.6. Method of Statistical Analysis
3. Results
3.1. Effect of Fermentation on the Acidity of the Leaves of C. dregei
3.2. The Nutritional Attributes of the Fermented Leaves of C. dregei
3.3. Antioxidant Attributes in the Fermented Leaves of C. dregei
3.4. Antioxidant Attributes of the Fermented C. dregei Leaves
3.5. Qualitative Screening of the Phytochemicals Present in the Fermented Leaves of C. dregei
3.6. Role of Lactic Acid Dehydrogenate in the Fermented Leaves of C. dregei
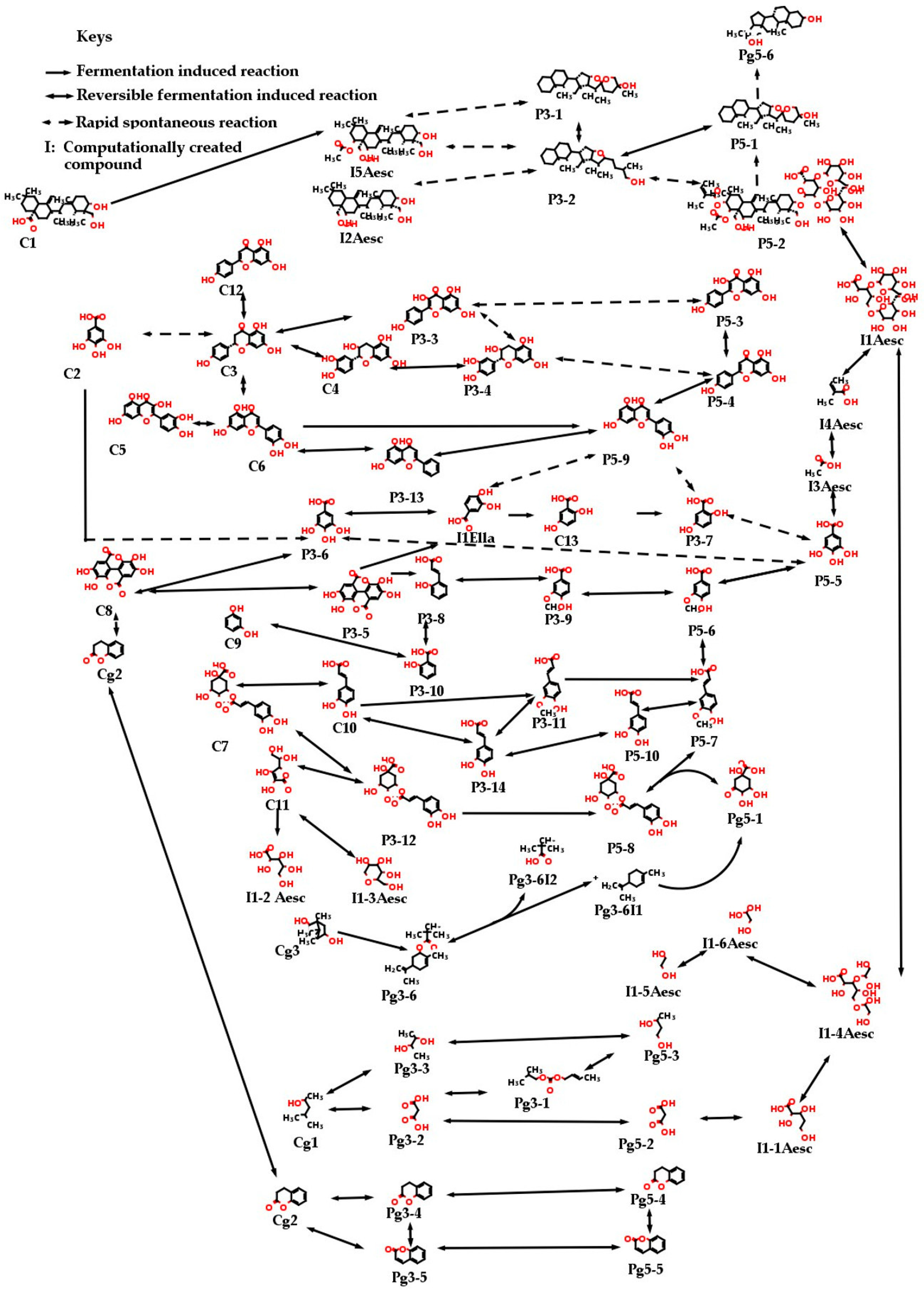
4. Discussion
4.1. Nutritional Constituents in the Fermented C. dregei Leaves
4.2. Influence of Fermentation on the Antioxidants Qualities of C. dregei
4.3. Phytochemical Constituents in the Leaves of the Fermented C. dregei
4.4. Influence of Lactic Acid Dehydrogenase on the Fermentation of the Leaves of C. dregei
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Misci, C.; Taskin, E.; Dall′Asta, M.; Fontanella, M.C.; Bandini, F.; Imathiu, S.; Sila, D.; Bertuzzi, T.; Cocconcelli, P.S.; Puglisi, E. Fermentation as a tool for increasing food security and nutritional quality of indigenous African leafy vegetables: The case of Cucurbita sp. Food Microbiol. 2021, 99, 103820. [Google Scholar] [CrossRef] [PubMed]
- Codjoe, S.N.; Okutu, D.; Abu, M. Urban household characteristics and dietary diversity: An analysis of food security in Accra, Ghana. Food Nutr. Bull. 2016, 37, 202–218. [Google Scholar] [CrossRef] [PubMed]
- Afolabi, I.S.; Ahuekwe, E.F.; Garuba, P.A.; Adigun, A.J.; Odutayo, O.E.; Adeyemi, A.O. Enterococcus faecalis-induced biochemical transformation during fermentation of underutilized Solenostemon monostachyus leaves. Fermentation 2023, 9, 33. [Google Scholar] [CrossRef]
- Seenivasan, S.; Talukdar, D.; Nagpal, A. National income and macro-economic correlates of the double burden of malnutrition: An ecological study of adult populations in 188 countries over 42 years. Lancet Planet Health 2023, 7, e469–e477. [Google Scholar] [CrossRef]
- Nyanhanda, T.; Mwanri, L.; Mude, W. Double burden of malnutrition: A population level comparative cross-sectional study across three sub-saharan African countries-Malawi, Namibia and Zimbabwe. Int. J. Environ. Res. Public Health 2023, 20, 5860. [Google Scholar] [CrossRef]
- Saunders, J.; Smith, T. Malnutrition: Causes and consequences. Clin. Med. 2010, 10, 624–627. [Google Scholar] [CrossRef] [PubMed]
- De, P.; Chattopadhyay, N. Effects of malnutrition on child development: Evidence from a backward district of India. Clin. Epidemiol. Glob. Health 2019, 7, 439–445. [Google Scholar] [CrossRef]
- Matemilola, S.; Elegbede, I. The challenges of food security in Nigeria. OALib 2017, 4, 1–22. [Google Scholar] [CrossRef]
- Ayed, L.; M’hir, S.; Hamdi, M. Microbiological, biochemical, and functional aspects of fermented vegetable and fruit beverages. J. Chem. 2020, 2020, 1–12. [Google Scholar] [CrossRef]
- Takahata, M.; Frémont, M.; Desreumaux, P.; Rousseaux, C.; Dubuquoy, C.; Shimomiya, Y.; Nakamura, Y.; Miyake, Y. Evaluation of therapeutic properties of fermented vegetables extract (OM-X®) in the model of colitis induced by Citrobacter rodentium in mice. J. Funct. Foods 2014, 10, 117–127. [Google Scholar] [CrossRef]
- Odutayo, O.E.; Adegboye, B.E.; Omonigbehin, E.A.; Olawole, T.D.; Ogunlana, O.O.; Afolabi, I.S. Structural transformation and creativity induced by biological agents during fermentation of edible nuts from Terminalia catappa. Molecules 2021, 26, 5874. [Google Scholar] [CrossRef]
- Odutayo, O.E.; Adegboye, B.E.; Omonigbehin, E.A.; Ogunlana, O.O.; Afolabi, I.S. Characterization of potential probiotics isolated from fermented under-utilized Chrysophyllum albidum Linn kernels using microbiological, biochemical and molecular techniques. J. Phys. Conf. Ser. 2021, 2070, 012036. [Google Scholar] [CrossRef]
- AOAC. AOAC Official Methods of Analysis; Association of Official Analytical Chemists, Inc.: Fairfax, VA, USA, 1990. [Google Scholar]
- Kalaydzhiev, H.; Ivanova, P.; Uzunova, G.; Manolov, I.; Chalova, V. Biuret and Bradford methods suitability for protein quantification in rapeseed meal. Contemp. Agric. 2018, 67, 87–92. [Google Scholar] [CrossRef]
- Ibrahim, M.H.; Jaafar, H.Z.E. Involvement of carbohydrate, protein and phenylanine ammonia lyase in up-regulation of secondary metabolites in Labisia pumila under various CO2 and N2 level. Molecules 2011, 16, 4172–4190. [Google Scholar] [CrossRef]
- Olawole, T.D.; Olalere, A.T.; Adeyemi, O.A.; Okwumabua, O.; Afolabi, I.S. Tannin and antioxidant status of fermented and dried Sorghum bicolor. Rasāyan J. Chem. 2019, 12, 523–530. [Google Scholar] [CrossRef]
- Sharma, D.C.; Shukla, R.; Ali, J.; Sharma, S.; Bajpai, P.; Pathak, N. Phytochemical evaluation, antioxidant assay, antibacterial activity and determination of cell viability (J774 and THP1 alpha cell lines) of P. sylvestris leaf crude and methanol purified fractions. EXCLI J. 2016, 15, 85–94. [Google Scholar] [CrossRef]
- Sakat, S.; Juvekar, A. Comparative study of Erythrina indica Lam. (Febaceae) leaves extracts for antioxidant activity. J. Young Pharm. 2010, 2, 63–67. [Google Scholar] [CrossRef]
- Boyer, K.W. Metals and Other Elements at Trace Levels in Foods; Association of Official Analytical Chemists, Inc.: Fairfax, VA, USA, 1984; pp. 444–476. [Google Scholar]
- Afolabi, I.S.; Nwachukwu, I.C.; Ezeoke, C.S.; Woke, R.C.; Adegbite, O.A.; Olawole, T.D.; Martins, O.C. Production of a new plant-based milk from Adenanthera pavonina seed and evaluation of its nutritional and health benefits. Front. Nutr. 2018, 5, 9. [Google Scholar] [CrossRef]
- Odutayo, O.E.; Omonigbehin, E.A.; Olawole, T.D.; Ogunlana, O.O.; Afolabi, I.S. Fermentation enhanced biotransformation of compounds in the kernel of Chrysophyllum albidum. Molecules 2020, 25, 6021. [Google Scholar] [CrossRef]
- Baccouri, O.; Boukerb, A.M.; Farhat, L.B.; Zebre, A.; Zimmermann, K.; Domann, E.; Cambronel, M.; Barreau, M.; Maillot, O.; Rince, I.; et al. Probiotic potential and safety evaluation of Enterococcus faecalis OB14 and OB15, isolated from traditional Tunisian testouri cheese and rigouta, using physiological and genomic analysis. Front. Microbiol. 2019, 10, 881. [Google Scholar] [CrossRef]
- Jiao, Y.; Yang, H.; Shigwedha, N.; Zhang, S.; Liu, F.; Zhang, L. Probiotic effects and metabolic products of Enterococcus faecalis LD33 with respiration capacity. Foods 2022, 11, 606. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Gu, J.; Wu, H.; Rauf, A.; Emran, T.B.; Khan, Z.; Mitra, S.; Aljohani, A.S.M.; Alhumaydhi, F.A.; Al-Awthan, Y.S.; et al. Phytochemicals, nutrition, metabolism, bioavailability, and health benefits in lettuce-A comprehensive review. Antioxidants 2022, 11, 1158. [Google Scholar] [CrossRef] [PubMed]
- Yakubu, C.M.; Sharma, R.; Sharma, S.; Singh, B. Influence of alkaline fermentation time on in vitro nutrient digestibility, bio- & techno-functionality, secondary protein structure and macromolecular morphology of locust bean (Parkia biglobosa) flour. LWT 2022, 161, 113295. [Google Scholar] [CrossRef]
- Nkhata, S.G.; Ayua, E.; Kamau, E.H.; Shingiro, J.B. Fermentation and germination improve nutritional value of cereals and legumes through activation of endogenous enzymes. Food Sci. Nutr. 2018, 6, 2446–2458. [Google Scholar] [CrossRef] [PubMed]
- Bautista-Exposito, S.; Penas, E.; Silvan, J.M.; Frias, J.; Martinez-Villaluenga, C. pH-controlled fermentation in mild alkaline conditions enhances bioactive compounds and functional features of lentil to ameliorate metabolic disturbances. Food Chem. 2018, 248, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Navarrete del Toro, M.d.l.A.; García-Carreño, F.L.; Córdova-Murueta, J.H. Comparison of digestive proteinases in three penaeids. Aquaculture 2011, 317, 99–106. [Google Scholar] [CrossRef]
- Bayala, B.; Coulibaly, A.Y.; Djigma, F.W.; Nagalo, B.M.; Baron, S.; Figueredo, G.; Lobaccaro, J.-M.A.; Simpore, J. Chemical composition, antioxidant, anti-inflammatory and antiproliferative activities of the essential oil of Cymbopogon nardus, a plant used in traditional medicine. Biomol. Concepts 2020, 11, 86–96. [Google Scholar] [CrossRef]
- Lu, Y.; Zhou, Y.; Nakai, S.; Hosomi, M.; Zhang, H.; Kronzucker, H.J.; Shi, W. Stimulation of nitrogen removal in the rhizosphere of aquatic duckweed by root exudate components. Planta 2014, 239, 591–603. [Google Scholar] [CrossRef]
- Ahmad, M.; Dwivedy, A.; Mariadasse, R.; Tiwari, S.; Kar, D.; Jeyakanthan, J.; Biswal, B.K. Prediction of small molecule inhibitors targeting the severe acute respiratory syndrome coronavirus-2 RNA-dependent RNA polymerase. ACS Omega 2020, 5, 18356–18366. [Google Scholar] [CrossRef]
- Maltini, E.; Torreggiani, D.; Venir, E.; Bertolo, G. Water activity and the preservation of plant foods. Food Chem. 2003, 82, 79–86. [Google Scholar] [CrossRef]
- Worland, A.M.; Czajka, J.J.; Xing, Y.; Harper, W.F., Jr.; Moore, A.; Xiao, Z.; Han, Z.; Wang, Y.; Su, W.W.; Tang, Y.J. Analysis of Yarrowia lipolytica growth, catabolism, and terpenoid biosynthesis during utilization of lipid-derived feedstock. Metab. Eng. Commun. 2020, 11, e00130. [Google Scholar] [CrossRef]
- Makwe, C.C.; Soibi-Harry, A.P.; Rimi, G.S.; Ugwu, O.A.; Ajayi, A.T.; Adesina, T.A.; Okunade, K.S.; Oluwole, A.A.; Anorlu, R.I. Micronutrient and trace element levels in serum of women with uterine fibroids in Lagos. Cureus 2021, 13, e18638. [Google Scholar] [CrossRef]
- Miller, B.D.D.; Welch, R.M. Food system strategies for preventing micronutrient malnutrition. Food Policy 2013, 42, 115–128. [Google Scholar] [CrossRef]
- Afolabi, I.S.; Osikoya, I.O.; Fajimi, O.D.; Usoro, P.I.; Ogunleye, D.O.; Bisi-Adeniyi, T.; Adeyemi, O.A.; Adekeye, B.T. Solenostemon monostachyus, Ipomoea involucrata and Carica papaya seed oil versus Glutathione, or Vernonia amygdalina: Methanolic extracts of novel plants for the management of sickle cell anemia disease. BMC Complement. Altern. Med. 2012, 12, 262. [Google Scholar] [CrossRef]
- Corguinha, A.P.B.; Souza, G.A.d.; Gonçalves, V.C.; Carvalho, C.d.A.; Lima, W.E.A.d.; Martins, F.A.D.; Yamanaka, C.H.; Francisco, E.A.B.; Guilherme, L.R.G. Assessing arsenic, cadmium, and lead contents in major crops in Brazil for food safety purposes. J. Food Compos. Anal. 2015, 37, 143–150. [Google Scholar] [CrossRef]
- Committee on Nutrition Standards for Foods in Schools. Nutrition Standards for Foods in Schools: Leading the Way toward Healthier Youth; The National Academies Press: Washington, DC, USA, 2018; pp. 1–296. [Google Scholar]
- Saxena, M.; Saxena, J.; Nema, R.; Singh, D.; Gupta, A. Phytochemistry of medicinal plants. J. Pharmacogn. Phytochem. 2013, 1, 168–182. [Google Scholar]
- Ayoola, G.A.; Coker, H.A.; Adesegun, S.A.; Adepoju-Bello, A.A.; Obaweya, K.; Ezennia, E.C.; Atangbayila, T.O. Phytochemical screening and antioxidant activities of some selected medicinal plants used for malaria therapy in Southwestern Nigeria. Trop. J. Pharm. Res. 2008, 7, 1019–1024. [Google Scholar] [CrossRef]
- Bertoni, G.; Minuti, A.; Trevisi, E. Immune system, inflammation and nutrition in dairy cattle. Anim. Prod. Sci. 2015, 55, 943. [Google Scholar] [CrossRef]
- Sharma, R.; Diwan, B.; Singh, B.P.; Kulshrestha, S. Probiotic fermentation of polyphenols: Potential sources of novel functional foods. Food Prod. Process. Nutr. 2022, 4, 21. [Google Scholar] [CrossRef]
- Axten, L.G.; Wohlers, M.W.; Wegrzyn, T. Using phytochemicals to enhance health benefits of milk: Impact of polyphenols on flavor profile. J. Food Sci. 2008, 73, H122–H126. [Google Scholar] [CrossRef]
- Khubber, S.; Hashemifesharaki, R.; Mohammadi, M.; Gharibzahedi, S.M.T. Garlic (Allium sativum L.): A potential unique therapeutic food rich in organosulfur and flavonoid compounds to fight with COVID-19. Nutr. J. 2020, 19, 124. [Google Scholar] [CrossRef]
- Pandey, P.; Khan, F.; Kumar, A.; Srivastava, A.; Jha, N.K. Screening of potent inhibitors against 2019 novel coronavirus (COVID-19) from Allium sativum and Allium cepa: An in silico approach. Biointerface Res. Appl. Chem. 2020, 11, 7981–7993. [Google Scholar] [CrossRef]
- Subramanian, M.S.; Nandagopal Ms, G.; Amin Nordin, S.; Thilakavathy, K.; Joseph, N. Prevailing knowledge on the bioavailability and biological activities of sulphur compounds from alliums: A potential drug candidate. Molecules 2020, 25, 4111. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Scientific opinion on the use of resorcinol as a food additive. EFSA J. 2010, 8, 1411. [Google Scholar] [CrossRef]
- Irakoze, M.L.; Wafula, E.N.; Owaga, E. Potential role of African fermented indigenous vegetables in maternal and child nutrition in sub-saharan Africa. Int. J. Food Sci. 2021, 2021, 3400329. [Google Scholar] [CrossRef]
- Kang, I.; Buckner, T.; Shay, N.F.; Gu, L.; Chung, S. Improvements in metabolic health with consumption of ellagic acid and subsequent conversion into urolithins: Evidence and mechanisms. Adv. Nutr. 2016, 7, 961–972. [Google Scholar] [CrossRef]
- Bruck, W.M.; Diaz Escobar, V.D.; Droz-Dit-Busset, L.; Baudin, M.; Nicolet, N.; Andlauer, W. Fermentative liberation of ellagic acid from walnut press cake ellagitannins. Foods 2022, 11, 3102. [Google Scholar] [CrossRef]
- Moccia, F.; Flores-Gallegos, A.C.; Chavez-Gonzalez, M.L.; Sepulveda, L.; Marzorati, S.; Verotta, L.; Panzella, L.; Ascacio-Valdes, J.A.; Aguilar, C.N.; Napolitano, A. Ellagic acid recovery by solid state fermentation of pomegranate wastes by Aspergillus niger and saccharomyces cerevisiae: A comparison. Molecules 2019, 24, 3689. [Google Scholar] [CrossRef]
- Cao, J.-X.; Wang, T.; Li, Y.; Cao, S.; Fan, T.-T.; Pareek, S. Ellagic acid treatment improves postharvest quality of tomato fruits by enhancing the antioxidant defense system. J. Food Biochem. 2023, 2023, 8375867. [Google Scholar] [CrossRef]
- Pang, X.; Huang, H.Z.; Zhao, Y.; Xiong, C.-Q.; Yu, L.Y.; Ma, B.-P. Conversion of furostanol saponins into spirostanol saponins improves the yield of diosgenin from Dioscorea zingiberensis by acid hydrolysis. RSC Adv. 2015, 5, 4831–4837. [Google Scholar] [CrossRef]
- Harmatha, J.; Budesinsky, M.; Zidek, Z.; Kmonickova, E. Spirostanol saponins from flowers of Allium porrum and related compounds indicating cytotoxic activity and affecting nitric oxide production inhibitory effect in peritoneal macrophages. Molecules 2021, 26, 6533. [Google Scholar] [CrossRef]
- Wang, C.; Zheng, M.; Wu, S.; Zou, X.; Chen, X.; Ge, L.; Zhang, Q. Effects of gallic acid on fermentation parameters, protein fraction, and bacterial community of whole plant soybean silage. Front. Microbiol. 2021, 12, 662966. [Google Scholar] [CrossRef]
- Sharma, R.; Garg, P.; Kumar, P.; Bhatia, S.K.; Kulshrestha, S. Microbial fermentation and its role in quality improvement of fermented foods. Fermentation 2020, 6, 106. [Google Scholar] [CrossRef]
- Minervini, F.; Missaoui, J.; Celano, G.; Calasso, M.; Achour, L.; Saidane, D.; Gobbetti, M.; De Angelis, M. Use of autochthonous lactobacilli to increase the safety of zgougou. Microorganisms 2019, 8, 29. [Google Scholar] [CrossRef] [PubMed]
- Chen, W. Demystification of fermented foods by omics technologies. Curr. Opin. Food Sci. 2022, 46, 100845. [Google Scholar] [CrossRef]
- Jin, G.; Zhu, Y.; Xu, Y. Mystery behind Chinese liquor fermentation. Trends Food Sci. Technol. 2017, 63, 18–28. [Google Scholar] [CrossRef]
- Montet, D.; Ray, R.C. Fermented Foods: Part 1: Biochemistry and Biotechnology; CRC Press: Boca Raton, FL, USA, 2016; p. 413. [Google Scholar] [CrossRef]
- Alba-Lois, L.; Segal-Kischinevsky, C. Yeast fermentation and the making of beer and wine. Nat. Educ. 2010, 3, 17. [Google Scholar]
- Basan, M.; Hui, S.; Okano, H.; Zhang, Z.; Shen, Y.; Williamson, J.R.; Hwa, T. Overflow metabolism in Escherichia coli results from efficient proteome allocation. Nature 2015, 528, 99–104. [Google Scholar] [CrossRef]
- Petersen, M.; Abdullah, Y.; Benner, J.; Eberle, D.; Gehlen, K.; Hucherig, S.; Janiak, V.; Kim, K.H.; Sander, M.; Weitzel, C.; et al. Evolution of rosmarinic acid biosynthesis. Phytochemistry 2009, 70, 1663–1679. [Google Scholar] [CrossRef]
- Biancur, D.E.; Kapner, K.S.; Yamamoto, K.; Banh, R.S.; Neggers, J.E.; Sohn, A.S.W.; Wu, W.; Manguso, R.T.; Brown, A.; Root, D.E.; et al. Functional genomics identifies metabolic vulnerabilities in pancreatic cancer. Cell Metab. 2021, 33, 199–210.e198. [Google Scholar] [CrossRef]
- Vacanti, N.M.; Metallo, C.M. Exploring metabolic pathways that contribute to the stem cell phenotype. Biochim. Biophys. Acta 2013, 1830, 2361–2369. [Google Scholar] [CrossRef] [PubMed]
- Minami, T.; Oomura, Y.; Nabekura, J.; Fukuda, A. Direct effects of 3,4-dihydroxybutanoic acid gamma-lactone and 2,4,5-trihydroxypentanoic acid gamma-lactone on lateral and ventromedial hypothalamic neurons. Brain Res. 1988, 462, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Elliot, S.G.; Andersen, C.; Tolborg, S.; Meier, S.; Sádaba, I.; Daugaard, A.E.; Taarning, E. Synthesis of a novel polyester building block from pentoses by tin-containing silicates. RSC Adv. 2017, 7, 985–996. [Google Scholar] [CrossRef]
- Dosedel, M.; Jirkovsky, E.; Macakova, K.; Krcmova, L.K.; Javorska, L.; Pourova, J.; Mercolini, L.; Remiao, F.; Novakova, L.; Mladenka, P.; et al. Vitamin C-sources, physiological role, kinetics, deficiency, use, toxicity, and determination. Nutrients 2021, 13, 615. [Google Scholar] [CrossRef] [PubMed]
- Yussif, N.M. Vitamin C. In Vitamin C—An Update on Current Uses and Functions; LeBlanc, J.G., Ed.; IntechOpen: Rijeka, Croatia, 2018; pp. 1–28. [Google Scholar]
- Holland, H.L.; Riemla, E. The mechanism of enzymic hydroxyl group removal from C-21 of tetrahydrodeoxycorticosterone. Can. J. Chem. 1985, 63, 981–983. [Google Scholar] [CrossRef]
- Szpara, R.; Goyder, A.; Porter, M.J.; Hailes, H.C.; Sheppard, T.D. Regioselective dehydration of sugar thioacetals under mild conditions. Org. Lett. 2021, 23, 2488–2492. [Google Scholar] [CrossRef]
- Leesong, M.; Henderson, B.S.; Gillig, J.R.; Schwab, J.M.; Smith, J.L. Structure of a dehydratase-isomerase from the bacterial pathway for biosynthesis of unsaturated fatty acids: Two catalytic activities in one active site. Structure 1996, 4, 253–264. [Google Scholar] [CrossRef]
- Ren, Y.; Eronen, V.; Blomster Andberg, M.; Koivula, A.; Hakulinen, N. Structure and function of aldopentose catabolism enzymes involved in oxidative non-phosphorylative pathways. Biotechnol. Biofuels Bioprod. 2022, 15, 147. [Google Scholar] [CrossRef]
- Trader, D.J.; Carlson, E.E. Chemoselective hydroxyl group transformation: An elusive target. Mol. Biosyst. 2012, 8, 2484–2493. [Google Scholar] [CrossRef]
- Danon, B.; Marcotullio, G.; de Jong, W. Mechanistic and kinetic aspects of pentose dehydration towards furfural in aqueous media employing homogeneous catalysis. Green Chem. 2014, 16, 39–54. [Google Scholar] [CrossRef]
- Hiasa, M.; Kurokawa, M.; Ohta, K.; Esumi, T.; Akita, H.; Niki, K.; Yagi, Y.; Echigo, N.; Hatakeyama, D.; Kuzuhara, T. Identification and purification of resorcinol, an antioxidant specific to Awa-ban (pickled and anaerobically fermented) tea. Food Res. Int. 2013, 54, 72–80. [Google Scholar] [CrossRef]
- Arnold, P.K.; Finley, L.W.S. Regulation and function of the mammalian tricarboxylic acid cycle. J. Biol. Chem. 2023, 299, 102838. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, M.N.; Aslankoohi, E.; Verstrepen, K.J.; Courtin, C.M. Contribution of the tricarboxylic acid (TCA) cycle and the glyoxylate shunt in Saccharomyces cerevisiae to succinic acid production during dough fermentation. Int. J. Food Microbiol. 2015, 204, 24–32. [Google Scholar] [CrossRef] [PubMed]
- López-Vázquez, E.; Prieto-García, F.; Gayosso-Canales, M.; Sánchez, E.M.O.; Ibarra, J.R.V. Phenolic acids, flavonoids, ascorbic acid, β-glucans and antioxidant activity in Mexican wild edible mushrooms. Int. J. Food Sci. 2017, 29, 767–774. [Google Scholar]
- Engelhardt, L.; Pohnl, T.; Neugart, S. Interactions of ascorbic acid, 5-caffeoylquinic Acid, and quercetin-3-rutinoside in the presence and absence of iron during thermal processing and the influence on antioxidant activity. Molecules 2021, 26, 7698. [Google Scholar] [CrossRef] [PubMed]
- Gramazio, P.; Prohens, J.; Plazas, M.; Andujar, I.; Herraiz, F.J.; Castillo, E.; Knapp, S.; Meyer, R.S.; Vilanova, S. Location of chlorogenic acid biosynthesis pathway and polyphenol oxidase genes in a new interspecific anchored linkage map of eggplant. BMC Plant Biol. 2014, 14, 350. [Google Scholar] [CrossRef]
- Afolabi, I.S.; Oloyede, O.B. Biochemical response of sweet potato to bemul-wax coating combined with calcium chloride treatment during ambient storage. Afr. J. Biotechnol. 2011, 10, 2724–2732. [Google Scholar] [CrossRef]
- Alcazar Magana, A.; Kamimura, N.; Soumyanath, A.; Stevens, J.F.; Maier, C.S. Caffeoylquinic acids: Chemistry, biosynthesis, occurrence, analytical challenges, and bioactivity. Plant J. 2021, 107, 1299–1319. [Google Scholar] [CrossRef]
- Debolt, S.; Melino, V.; Ford, C.M. Ascorbate as a biosynthetic precursor in plants. Ann. Bot. 2007, 99, 3–8. [Google Scholar] [CrossRef]
- Sassa, T.; Kihara, A. Metabolism of very long-chain Fatty acids: Genes and pathophysiology. Biomol. Ther. 2014, 22, 83–92. [Google Scholar] [CrossRef]
- Chen, Q.; Kong, B.; Han, Q.; Xia, X.; Xu, L. The role of bacterial fermentation in lipolysis and lipid oxidation in Harbin dry sausages and its flavour development. LWT 2017, 77, 389–396. [Google Scholar] [CrossRef]
- Abedi, E.; Sahari, M.A. Long-chain polyunsaturated fatty acid sources and evaluation of their nutritional and functional properties. Food Sci. Nutr. 2014, 2, 443–463. [Google Scholar] [CrossRef] [PubMed]
- Kihara, A. Very long-chain fatty acids: Elongation, physiology and related disorders. J. Biochem. 2012, 152, 387–395. [Google Scholar] [CrossRef]
- Wang, B.; Wu, L.; Chen, J.; Dong, L.; Chen, C.; Wen, Z.; Hu, J.; Fleming, I.; Wang, D.W. Metabolism pathways of arachidonic acids: Mechanisms and potential therapeutic targets. Signal Transduct. Target 2021, 6, 94. [Google Scholar] [CrossRef] [PubMed]
- Monroig, Ó.; Kabeya, N. Desaturases and elongases involved in polyunsaturated fatty acid biosynthesis in aquatic invertebrates: A comprehensive review. Fish. Sci. 2018, 84, 911–928. [Google Scholar] [CrossRef]
- Zhuang, X.Y.; Zhang, Y.H.; Xiao, A.F.; Zhang, A.H.; Fang, B.S. Key enzymes in fatty acid synthesis pathway for bioactive lipids biosynthesis. Front. Nutr. 2022, 9, 851402. [Google Scholar] [CrossRef]
- Hashimoto, K.; Yoshizawa, A.C.; Okuda, S.; Kuma, K.; Goto, S.; Kanehisa, M. The repertoire of desaturases and elongases reveals fatty acid variations in 56 eukaryotic genomes. J. Lipid Res. 2008, 49, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Oboh, A.; Betancor, M.B.; Tocher, D.R.; Monroig, O. Biosynthesis of long-chain polyunsaturated fatty acids in the African catfish Clarias gariepinus: Molecular cloning and functional characterisation of fatty acyl desaturase (fads2) and elongase (elovl2) cDNAs7. Aquaculture 2016, 462, 70–79. [Google Scholar] [CrossRef]
- McDonald, A.G.; Tipton, K.F. Parameter reliability and understanding enzyme function. Molecules 2022, 27, 263. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Liu, J.; Xu, H.; Li, W.; Zhang, J. L-Lactic acid fermentation by Enterococcus faecium: A new isolate from bovine rumen. Biotechnol. Lett. 2015, 37, 1379–1383. [Google Scholar] [CrossRef]

| Parameters | Leaf Types | Duration of Fermentation (Days) | ||
|---|---|---|---|---|
| 0 | 3 | 5 | ||
| pH | WL Ϯ | 4.31 ± 0.02 a | 7.50 ± 0.00 b | 8.00 ± 0.00 c |
| CD | 3.98 ± 0.04 a | 8.49 ± 0.01 b | 9.00 ± 0.00 c | |
| Moisture (%) | WL Ϯ | 11.62 ± 0.03 a | 20.77 ± 0.06 b | 23.49 ± 0.00 c |
| CD | 16.32 ±0.00 a | 10.39 ±0.01 b | 10.69 ±0.00 b | |
| Protein (%) | WL Ϯ | 12.20 ± 0.10 a | 21.40 ± 0.10 b | 12.13 ± 0.06 c |
| CD | 14.23± 0.05 a | 24.07 ± 0.05 b | 15.43 ± 0.05 b | |
| Fats (%) | WL Ϯ | 14.34 ± 0.11 a | 19.10 ± 0.17 b | 29.90 ± 0.09 c |
| CD | 2.57 ± 0.05 a | 1.78 ± 0.10 b | 20.60 ± 0.04 c | |
| Carbohydrates (%) | WL Ϯ | 17.54 ± 0.04 a | 18.36 ± 0.13 b | 16.98 ± 0.04 c |
| CD | 31.02± 0.09 a | 18.12 ± 0.06 b | 12.41 ± 0.09 c | |
| Crude fiber (%) | WL Ϯ | 32.56 ± 0.01 a | 10.01 ± 0.01 b | 11.22 ± 0.01 c |
| CD | 15.54 ± 0.00 a | 19.01 ± 0.00 b | 11.22 ± 0.00 c | |
| Ash (%) | WL Ϯ | 11.73 ± 0.01 a | 10.35 ± 0.01 b | 6.27 ± 0.01 c |
| CD | 20.30 ±0.00 a | 27.11 ± 0.00 b | 25.42 ± 0.00 c | |
| Mineral | Leaf Type | Duration of Fermentation (Days) | |||
|---|---|---|---|---|---|
| 0 | 3 | 5 | Recommended Daily Intake † | ||
| Ca (mg/L) | WL ¥ | 0.51 ± 0.00 | NA | NA | 1000 mg |
| CD | 0.81 ± 0.01 a | 0.87 ± 0.02 b | 0.85 ± 0.02 c | ||
| Se (mg/L) | WL ¥ | 0.23 ± 0.00 | NA | NA | 25–34 μg |
| CD | 0.28 ± 0.02 a | 0.25 ± 0.00 b | 0.23 ± 0.00 c | ||
| Mg (mg/L) | WL ¥ | 0.69 ± 0.00 | NA | NA | 400 mg |
| CD | 0.87 ± 0.02 a | 0.84 ± 0.00 b | 0.83 ± 0.02 c | ||
| K (mg/L) | WL ¥ | 1.93 ± 0.00 | NA | NA | 3500 mg |
| CD | 2.18 ± 0.02 a | 2.31 ± 0.02 a | 2.26 ± 0.02 a | ||
| Na (mg/L) | WL ¥ | 0.49 ± 0.00 | NA | NA | 2400 mg |
| CD | 0.57 ± 0.00 a | 0.54 ± 0.00 b | 0.51 ± 0.01 c | ||
| Zn (mg/L) | WL ¥ | 0.80 ± 0.00 | NA | NA | 15 mg |
| CD | 0.84 ± 0.00 a | 0.91 ± 0.01 b | 0.87 ± 0.01 c | ||
| Cu (mg/L) | WL ¥ | 0.19 ± 0.00 a | 0.28 ± 0.16 | 0.00 ± 0.00 b | 2 mg |
| CD | 0.30 ± 0.00 a | 0.25 ± 0.00 a | 0.23 ± 0.00 b | ||
| Fe (mg/L) | WL ¥ | 8.73 ± 0.07 a | 16.52 ± 0.11 b | 0.00 ± 0.00 c | 18 mg |
| CD | 18.75 ± 0.00 a | 11.91 ± 0.00 b | 14.94 ± 0.00 c | ||
| Cd (mg/L) | WL ¥ | 0.05 ± 0.00 a | 0.05 ± 0.00 | 0.00 ± 0.00 b | 3.6 μg/kg bw |
| CD | 0.07 ± 0.03 a | 0.05 ± 0.00 b | 0.05 ± 0.00 b | ||
| Mn (mg/L) | WL ¥ | 1.24 ± 0.00 a | 0.91 ± 0.01 | 0.00 ± 0.00 b | 2 mg |
| CD | 4.00 ± 0.00 a | 2.18 ± 0.00 b | 2.54 ± 0.00 b | ||
| Pb (mg/L) | WL ¥ | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 1.0 μg/kg bw |
| CD | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | ||
| Vit. A (mg/mL) | WL ¥ | 0.13 ± 0.00 | NA | NA | 5000 (I.U.) |
| CD | 0.08 ± 0.00 a | 0.05 ± 0.01 b | 0.06 ± 0.01 c | ||
| Vit. C (mg/mL) | WL ¥ | 0.61 ± 0.00 | NA | NA | 60 mg |
| CD | 2.12 ± 0.00 a | 1.85 ± 0.06 b | 0.20 ± 0.01 c | ||
| Vit. E (mg/mL) | WL ¥ | 0.82 ± 0.00 | NA | NA | 30 (I.U.) |
| CD | 0.57 ± 0.00 a | 0.33 ± 0.01 b | 0.03 ± 0.00 c | ||
| Days | Phenol (mgGAE/g) × 10−2 | Saponin (mgGAE/g) | Flavonoids (mgGAE/g) × 10−1 | TAC (µg/mL) | FRAP (µg/mL) × 10−5 | DPPH Inhibition (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WL ¥ | CD | WL ¥ | CD | WL ¥ | CD | WL ¥ | CD | WL ¥ | CD | Vit. C | WL ¥ | CD | |
| 0 | 1.88 ± 0.01 a | 1.70 ± 0.01 a | 1.66 ± 0.08 a | 2.93 ± 0.25 a | 0.49 ± 0.00 a | 1.21 ± 0.00 a | 26.38 ± 0.01 a | 27.01 ± 0.01 a | 24.10 ± 0.00 a | 5.32 ± 0.02 a | 95.58 ± 0.00 a | 75.92 ± 0.25 a | 69.29 ± 0.25 a |
| 3 | 4.73 ± 0.01 b | 6.92 ± 0.01 b | 0.90 ± 0.02 b | 2.30 ± 0.01 b | 0.56 ± 0.01 b | 1.42 ± 0.00 b | 25.89 ± 0.02 b | 27.15 ± 0.00 b | 8.49 ± 0.00 b | 8.52 ± 0.00 b | NA | 70.52 ± 0.00 b | 88.86 ± 0.14 b |
| 5 | 6.90 ± 0.00 c | 8.20 ± 0.01 c | 3.23 ± 0.01 b | 3.55 ± 0.01 b | 0.59 ± 0.01 c | 1.56 ± 0.03 c | 26.51 ± 0.02 c | 26.63 ± 0.02 c | 6.72 ± 0.01 c | 8.55 ± 0.00 c | NA | 80.02 ± 0.14 c | 94.60 ± 0.25 c |
| S/N | Identified Compound (Tr (min)Peak nos.) | Concentration (mg/10 g Extract) | ||
|---|---|---|---|---|
| Saponin | Control (Day 0) | 3-Day-Fermented | 5-Day-Fermented | |
| 1 | Spirostanol (1.4658–1.5154) | - | 0.01 | 60.24 |
| 2 | Furostanol (2.3828) | - | 47.86 | - |
| 3 | Aescin (2.0235) | - | - | 39.72 |
| 4 | P-Scd (3.00710) | - | 52.05 | - |
| 5 | Hederagenin (4.37320) | 99.77 | - | - |
| Phenolic | Control (Day 0) | 3-Day-Fermented | 5-Day-Fermented | |
| 6 | Caffeic acid (1.1982) | 21.75 | - | - |
| 7 | Gallic acid (1.3403–1.4482–1.5732) | 44.83 | 29.81 | 10.44 |
| 8 | Ferulic acid (1.6323–1.7983) | - | 56.13 | 48.35 |
| 9 | Ellagic acid (2.2324–2.5324) | 19.11 | 6.04 | - |
| 10 | Chlorogenic acid (2.9325–3.0734) | - | 5.04 | 11.58 |
| 11 | S-Pcd (3.3235–3.1656) | 3.52 | 0.09 | - |
| 12 | Luteolin (3.3736–3.3825) | 3.97 | - | 20.54 |
| 13 | Naringenin (4.0077) | 6.74 | - | - |
| 14 | Apigenin (4.9908–5.1987) | 0.03 | - | 5.33 |
| 15 | Kaempferol (4.9987–5.0486) | - | 2.74 | 3.53 |
| 16 | Chrysin (6.3658) | - | 0.14 | - |
| 17 | P-Pcd (6.5738) | - | - | 0.23 |
| Bioflavonoid | Control (Day 0) | 3-Day-Fermented | 5-Day-Fermented | |
| 18 | 2,5-dihydroxybenzoic acid (0.3152–0.3402) | 0.26 | 0.31 | - |
| 19 | Ascorbic Acid (1.3903) | 96.26 | - | - |
| 20 | Caffeic acid (1.4985–1.4231) | - | 15.19 | 61.71 |
| 21 | O-Coumaric acid (1.5656) | - | 2.68 | - |
| 22 | Salicylic acid (1.6237) | - | 19.48 | - |
| 23 | Vanillic acid (2.0908–2.4822) | - | 44.60 | 11.65 |
| 24 | Chlorogenic acid (4.1485–4.24010–4.2574) | 1.27 | 12.58 | 0.18 |
| 25 | S-Bcd (4.3236–4.1829) | 0.12 | 5.08 | - |
| 26 | Gallic acid (4.4487) | 0.65 | - | - |
| 27 | Quercetin (4.9488) | 0.87 | - | - |
| 28 | Resorcinol (5.63210) | 0.21 | - | - |
| 29 | Catechin (6.81513–6.64012) | 0.01 | 0.02 | - |
| 30 | Apigenin (2.5323) | - | - | 26.47 |
| S/N | Peak | Tr | Area (%) | Similarity Index (%) | Class of Compound | IUPAC Name | Common Name |
|---|---|---|---|---|---|---|---|
| Unfermented—Day 0 | |||||||
| 1 | 1 | 4.017 | 15.05 | 55 | Fluoro-amino acids | 4,4,4-Trifluorothreonine | 4,4,4-Trifluorothreonine |
| 2 | 2 | 6.059 | 2.52 | 89 | Secondary alcohols | 2-Pentanol, 4-methyl- | Isobutylmethylmethanol |
| 3 | 3 | 12.492 | 22.65 | 75 | Hydroxycoumarins | 2H-1-Benzopyran-2-one, 3,4-dihydro- | Hydrocoumarin |
| 4 | 4 | 13.666 | 53.34 | 77 | Terpene ether | 1,7,7-Trimethylbicyclo[2.2.1]heptane-2,5-diol | (1S,4S)-Bornane-2alpha,5beta-diol |
| 5 | 5 | 15.032 | 5.44 | 64 | Fatty acid esters | Ethyl (5E,8E,11E,14E,17E)-5,8,11,14,17-icosapentaenoate | Ethyl 5,8,11,14,17-icosapentaenoate |
| Fermented—Day 3 | |||||||
| 1 | 1 | 4.033 | 4.4 | 62 | Carboxylic acid | (E)-But-2-enyl isobutyl carbonate | Carbonic acid 2-butenyl=ethyl |
| 2 | 2 | 5.213 | 4.53 | 81 | Fatty acid | Propanedioic acid | Malonic acid |
| 3 | 3 | 5.542 | 13.68 | 96 | Alcohol | 2,3-Butanediol | Dimethylethylene glycol |
| 4 | 4 | 11.375 | 11.30 | 90 | Hydroxycoumarins | 2H-1-Benzopyran-2-one, 3,4-dihydro- | Hydrocoumarin |
| 5 | 5 | 12.224 | 63.28 | 93 | Benzopyrones | o-Hydroxycinnamic acid lactone | Coumarin |
| 6 | 6 | 14.996 | 1.21 | 52 | Heterocyclic | N-Phenyl-2-cyclohexene-1-carboxamide | 2-Cyclohexenecarboxanilide |
| 7 | 7 | 15.479 | 1.62 | 60 | Dihydrocarvone | 5-Isopropenyl-2-methyl-2-cyclohexen-1-yl pivalate | Limonen-6-ol, pivalate |
| Fermented—Day 5 | |||||||
| 1 | 1 | 4.025 | 1.42 | 83 | Carboxylic acid | 1,3,4-Trihydroxy-5-oxocyclohexanecarboxylic acid | Cyclohexan-1,4,5-triol-3-one-1-carboxylic acid |
| 2 | 2 | 4.043 | 0.54 | 83 | Carboxylic acid | 1,3,4-Trihydroxy-5-oxocyclohexanecarboxylic acid | Cyclohexan-1,4,5-triol-3-one-1-carboxylic acid |
| 3 | 3 | 5.076 | 4.94 | 79 | Fatty acid | Propanedioic acid | Malonic acid |
| 4 | 4 | 5.498 | 9.30 | 89 | Secondary alcohols | 1,3-Butanediol | Methyltrimethylene glycol |
| 5 | 5 | 11.333 | 18.96 | 87 | Hydroxycoumarins | 2H-1-Benzopyran-2-one, 3,4-dihydro- | Hydrocoumarin |
| 6 | 6 | 12.235 | 64.13 | 93 | Benzopyrones | o-Hydroxycinnamic acid lactone | Coumarin |
| 7 | 7 | 14.999 | 0.70 | 61 | Sulfated steroids | 5.alpha.-Pregnan-3.beta.,20.beta.-diol | Pregnane-3,20-diol |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afolabi, I.S.; Adigun, A.J.; Garuba, P.A.; Ahuekwe, E.F.; Odutayo, O.E.; Adeyemi, A.O. Enterococcus faecalis-Aided Fermentation to Facilitate Edible Properties and Bioactive Transformation of Underutilized Cyathea dregei Leaves. Fermentation 2023, 9, 707. https://doi.org/10.3390/fermentation9080707
Afolabi IS, Adigun AJ, Garuba PA, Ahuekwe EF, Odutayo OE, Adeyemi AO. Enterococcus faecalis-Aided Fermentation to Facilitate Edible Properties and Bioactive Transformation of Underutilized Cyathea dregei Leaves. Fermentation. 2023; 9(8):707. https://doi.org/10.3390/fermentation9080707
Chicago/Turabian StyleAfolabi, Israel Sunmola, Aderinsola Jumai Adigun, Precious Amaneshi Garuba, Eze Frank Ahuekwe, Oluwatofunmi E. Odutayo, and Alaba Oladipupo Adeyemi. 2023. "Enterococcus faecalis-Aided Fermentation to Facilitate Edible Properties and Bioactive Transformation of Underutilized Cyathea dregei Leaves" Fermentation 9, no. 8: 707. https://doi.org/10.3390/fermentation9080707
APA StyleAfolabi, I. S., Adigun, A. J., Garuba, P. A., Ahuekwe, E. F., Odutayo, O. E., & Adeyemi, A. O. (2023). Enterococcus faecalis-Aided Fermentation to Facilitate Edible Properties and Bioactive Transformation of Underutilized Cyathea dregei Leaves. Fermentation, 9(8), 707. https://doi.org/10.3390/fermentation9080707






