Abstract
Promising green technologies that can overcome the challenges associated with the use of fossil fuels require microorganisms that can effectively ferment lignocellulosic hydrolysate for biochemical production with reduced sensitivity to toxic chemicals derived from the pretreatment process. In this study, a sequential adaptation approach was developed to obtain new bacterial lines from Basfia (B.) succiniciproducens strains, which are adapted to inhibitory compounds of the Arundo (A.) donax hydrolysate or those that accumulate during the fermentation process. The early adaptation stages resulted in newly adapted B. succiniciproducens bacterial lines that can tolerate fermentation end-products such as acetic, lactic, and succinic acids, as well as toxic compounds such as furfural and hydroxymethylfurfural. These adapted bacterial lines were further investigated to assess their ability to produce succinic acid in an MHM medium supplemented with a filtrate of A. donax hydrolysate. Batch growth tests on a small laboratory scale showed that bacterial lines 2E and 4D produced 5.80 ± 0.56 g L−1 and 5.81 ± 0.39 g L−1 of succinic acid, respectively, after 24 h of fermentation. Based also on its growth rate, the adapted bacterial line B. succiniciproducens 4D was selected for tests in a lab-scale fermenter, where it was able to synthesize up to 17.24 ± 0.39 g L−1 of succinate (corresponding to YSA/gluc 0.96 ± 0.02 g g−1 and to YSA/(G + X) 0.48 ± 0.01 g g−1) from MHM medium added with A. donax hydrolysate. Experiments showed an increase of ~17% compared to the control strain. The overall results demonstrate the potential of adapted bacterial lines for succinate production from A. donax hydrolysate and the development of improved technologies for bio-based succinic acid production.
1. Introduction
Succinic acid (SA) is a four-carbon dicarboxylic acid produced by many microorganisms as an intermediate of the tricarboxylic acid cycle [1,2]. It is a crucial precursor for various industrial applications, including biodegradable plastics, green solvents, plant growth promotion ingredients [3], as well as food and pharmaceutical [4,5]. Traditionally, SA is mainly produced from petrochemical-derived maleic anhydride. The production process can be replaced by applying microbial fermentations that employ cheaper renewable resources while also increasing the environmental benefit by utilizing CO2 fixation as a substrate [5,6]. Nevertheless, the biotechnological pathway for producing succinic acid faces challenges, particularly concerning the expensive nature of the required raw materials [7]. Furthermore, succinic acid was recognized by the US Department of Energy as an important building block that can be produced from biomass in biorefineries. As a result, several companies have tried to produce it on an industrial scale, with varying degrees of success [8]. Bio-based succinic acid production is currently estimated at 36,600 tonnes using various bacterial strains [9,10].
Several succinic acid-producing microorganisms were isolated from the rumen and other environments. Among these are fungi such as Aspergillus spp. and Byssochlamys nivea, yeasts such as Saccharomyces cerevisiae, and bacteria such as Actinobacillus (A.) succinogenes, Anaerobiospirillum succiniciproducens Bacteroides amylophilus, Bacteroides fragilis, Basfia (B.) succiniciproducens, Fibrobacter succinogenes, Mannheimia succiniciproducens, Prevotella ruminicola, and Ruminococcus albus [11,12,13]. Metabolically engineered Escherichia coli has also been used for succinic acid production [14].
B. succiniciproducens is a Gram-negative, facultatively anaerobic, capnophilic bacterium that belongs to the family Pasteurellaceae [15], and it is one of the most efficient wild bacteria known for producing succinic acid [16]. Several B. succiniciproducens strains were widely investigated for SA production [16,17,18], as well as for lactic acid (LA), acetic acid (AA), and formic acid (FA) productions using different carbon sources such as glucose, xylose, fructose, sucrose, arabinose, galactose, mannose, and glycerol [18]. LA, SA, and AA are the major organic acid end-products (or by-products) of the fermentation process carried out by strains belonging to B. succiniciproducens species [15]. These strains may utilize several carbon sources as crude renewable resources (such as organic fractions of municipal solid wastes, non-food dedicated energy crops, and corn stover hydrolysate) supplemented with various nutrients to produce organic acids [15,19,20,21,22].
The investigation and utilization of low-cost materials have been encouraged by the increased focus on environmental protection and the circular economy; the use of alternative carbon sources is considered the most promising biotechnological strategy to reduce costs for producing microbial biomass or organic acids by fermentation [23,24,25,26,27]. This approach emphasizes the principles of recycling, reusing, and manufacturing by making efficient use of biological resources derived from diverse alternative sources [28]. Lignocellulosic biomass, derived from dedicated energy crops such as Arundo (A.) donax, Populus nigra, and Eucalyptus camaldulensis, is the most abundant and sustainable carbon source, which has the greatest potential to replace fossil fuels thanks to its low-cost conversion [6]. These crops can grow in poor, degraded, and polluted soils that are not suitable for food crops as well as in soils subjected to accelerated erosion [29,30]. Furthermore, the abundance and low cost of lignocellulosic biomass make it an attractive and sustainable alternative feedstock for SA production, resulting in green technologies that are cheaper than petroleum-based ones [24,31]. To meet the growing demand for bioplastics and reduce the dependence on fossil fuels, the development of biopolymers and bio-based plastics from renewable resources is essential [32]. Different types of lignocellulosic biomasses have been employed in small-scale fermentation processes using natural succinic acid producers. However, the extraction of fermentable sugars from these complex materials is often accompanied by low sugar yields and the release of substances that impede microbial growth during hydrolysis [33].
Furthermore, although hydrolysis of plant biomass produces sugar mixtures that are rich in glucose and xylose, fermentation inhibitors are generated during the pretreatment required to break down lignocellulosic biomass and expose cellulose and hemicellulose to enzymatic attack [34,35]. Consequently, several toxic products, such as acids (formic, acetic, and levulinic acids) and furan aldehydes (furfural and 5-hydroxymethylfurfural; 5-HMF), are formed during pretreatment, which can significantly affect subsequent microbial growth and fermentation performance [22,34]. To advance SA production, research and technologies must focus on bacterial strains that can tolerate inhibitory compounds released during lignocellulosic biomass fermentation, as well as efficiently utilize both hexose and pentose sugars [13,34,35].
Microbial fermentation for SA production is a cost-effective and efficient technology. However, the use of wild-type strains poses several limitations to yield and productivity. To enhance SA yield and productivity, numerous research groups are actively involved in the metabolic engineering of Basfia strains. This technology has significant potential for improving microbial production performance [13,36]. To the best of our knowledge, only Roquette, BASF, and PTT-MCC Biochem continue to operate their bio-succinic acid production facilities, primarily using metabolically engineered strains [8]. Genetic engineering is widely employed to develop new bacterial strains, known as genetically engineered microorganisms (GEMs), with unique characteristics compared to the wild type, such as the ability to produce more succinic acid or to tolerate the presence of inhibitors in the growth medium [36].
Furthermore, it has been widely reported that the presence of inhibitors from lignocellulosic biomass [15], secondary metabolites, auxotrophy, pH sensitivity, and product inhibition have affected various engineered hosts [8].
In this context, a sequential adaptation protocol for SA-producing bacteria was designed to promote tolerance to inhibitory substances without resorting to genetic engineering. The two selected strains, B. succiniciproducens BPP7 and BPP8, were exposed to increasing concentrations of toxic by-products and fermentation end-products such as furfural (F), hydroxymethylfurfural (HMF), AA, LA, and SA to optimize production performances during fermentation. The experiment involved exposing the B. succiniciproducens strains to a single or a mixture of typical inhibitor(s) released during the pretreatment and fermentation of lignocellulosic biomass. The most promising adapted and resistant bacterial lines were tested on a small laboratory scale, followed by a lab-scale fermenter, to evaluate their ability to produce succinic acid from A. donax hydrolysate.
2. Materials and Methods
2.1. Experimental Design
The two SA-producing strains, B. succiniciproducens BPP7 and BPP8, previously isolated from the rumen ecosystem and tested for the ability to synthesize SA from renewable lignocellulosic biomass [13], were selected for a sequential adaptation procedure. They were grown with increasing concentrations of toxic by-products and fermentation end-products such as F, HMF, AA, LA, and SA to enhance bacterial performance during fermentation. The adaptation process was conducted in several stages. The two strains were adapted to grow in tube using a synthetic liquid medium, firstly added with individual growth-limiting compounds (F, HMF, AA, LA, and SA) at increasing concentrations. Line cells able to grow at the highest limiting concentrations of SA and AA were selected for testing in synthetic liquid medium with a mix of all inhibiting substances. Then, adapted bacterial lines able to grow in presence of all inhibiting compounds were evaluated on a small laboratory scale by adding the synthetic medium with A. donax hydrolysate. Finally, the best-adapted line cell was selected to test in a lab-scale fermenter.
2.2. Sequential Adaptation of B. succiniciproducens Strains
B. succiniciproducens BPP7 and BPP8 were activated in 10 mL of Brain-Heart Infusion (BHI, Oxoid, Milan, Italy) by incubation at 37 °C for 24 h in an incubator (Pbi International CO2 Incubator, Milan, Italy) at 5% CO2. Adaptation process to toxic by-products was performed in a working volume of 10 mL of MH medium (10 g L−1 polypeptone, 5 g L−1 yeast extract, 3 g L−1 K2HPO4, 2 g L−1 NaCl, 2 g L−1 (NH4)SO4, 0.2 g L−1 CaCl2, 0.2 g L−1 MgCl2, 0.001 g L−1 Na2S, without MgCO3 and supplemented with 20 g L−1 of glucose, pH 6.5- MHM) [16], inoculated with approximately 3.55 × 107 ± 2.14 CFU mL−1 of bacterial strains. MHM medium was modified with increasing amounts of single inhibitory compounds released by lignocellulosic biomass pretreatment process as follows: from 1.8 to 3.0 g L−1 of F (≥99% purity, Sigma-Aldrich, Milan, Italy), from 3.0 to 3.6 g L−1 of HMF (≥97% purity, Carbosynth, Compton, Berkshire, UK), from 1.0 to 5.0 g L−1 of AA (≥99.7% purity, Sigma-Aldrich, Milan, Italy). The two strains were also adapted to grow in presence of increasing concentrations of fermentation end-products as SA (≥99% purity, Sigma-Aldrich, Milan, Italy) from 40 to 60 g L−1, and LA from 5.0 to 7.5 g L−1 (≥85% purity, Sigma-Aldrich, Milan, Italy). Bacterial growth was determined by observing the development of turbidity of cultures and comparing them with McFarland Turbidity Standard (0.5–1, about 5 × 107 cells mL−1). Adapted bacterial lines were stored at −20 °C in 2 mL cryotubes containing BHI liquid medium with 10% glycerol until further analysis.
2.3. Growth Tolerance Tests to Different Inhibitory Mixtures
Resistant bacterial lines of B. succiniciproducens BPP7 and BPP8 to the single inhibitory compounds, able to tolerate high concentrations of AA and SA, were inoculated in 10 mL MHM medium [12] using a microbial concentration of approximately 2.82 × 107 ± 1.62 CFU mL−1 at 37 °C for 24 h in incubator (Pbi International CO2 Incubator) at 5% CO2. MHM medium was supplemented with different mixtures of the growth-inhibiting compounds (F, HMF, AA, SA, LA), as reported in Table 1. In detail, the inhibiting substances were supplied considering two different concentrations: mid of the maximum concentration and the maximum concentration achieved in the first selection phase. Microbial growth was evaluated by comparing turbidity to McFarland Turbidity Standard (0.5–1, about 5 × 107 cells mL−1). B. succiniciproducens BPP7 and BPP8 wild-type strains were used as negative controls. Tolerant bacterial lines were stored at −20 °C in cryotubes (BHI liquid medium with 10% glycerol) until further analysis.

Table 1.
Growth of B. succiniciproducens BPP7 and BPP8 adapted bacterial lines in MHM medium supplemented with mixtures of inhibitory compounds (SA, AA, LA, F, HMF).
2.4. Growth in Inhibitory Mixture Containing Fermentation End-Products and Toxic Compounds
Selected bacterial lines adapted to different growth conditions and controls (B. succiniciproducens BPP7 and BPP8 wild type) were re-inoculated into the MHM + Mix 7 containing 30 g L−1 of SA, 3.75 g L−1 of LA, 2 g L−1 of AA, 1 g L−1 of F and, 1.8 g L−1 of HMF (Table 1). Microbial growth was investigated by comparing bacterial growth with McFarland Turbidity Standard (0.5–1, about 5 × 107 cells mL−1). Obtained bacterial lines were stored at −20 °C in 2 mL cryotubes as described above.
2.5. Study of Succinic Acid Production in Batch Fermentation
Seven adapted strains (1F, 2D, 2E, 2F, 4C, 4D, 4E) resulting from the previous selective adaptation process were subjected to preliminary batch growth tests to assess their ability to produce SA in the presence of increasing concentrations of SA, LA, and AA. In detail, 500 mL flasks were filled with MHM medium modified with the addition of A. donax hydrolysate, obtained as previously described [16]. Briefly, hydrolysis was performed with 20% (w/v) of pretreated A. donax biomass in sodium acetate buffer (0.05 M, pH 5.0) and adding the commercial cellulase mixture Cellic® CTec2 Novozymes (Bagsvaerd, Denmark), with a concentration of 70 FPU/g of pretreated biomass solids. The filter paper cellulase units (FPUs) of the enzyme Cellic® CTec2 were determined according to the standard procedure recommended by the International Union of Pure and Applied Chemistry (IUPAC). Hydrolysis was performed at 37 °C with shaking (120× g) for 72 h. Clarified hydrolysate, including only the liquid fraction, was obtained by filtering the supernatant after centrifugation (11,180× g for 20 min). The clarified hydrolysate was supplemented with MHM components medium in 500 mL flask (pH 6.5). After sterilization, liquid medium was inoculated with 2% bacterial cells suspension (3.16 × 106 ± 1.07 CFU mL−1) and incubated at 37 °C for 72 h under static anaerobic conditions (Whitley DG250 Anaerobic Workstation, Don Whitley Scientific, Shipley, UK). Wild-type strains B. succiniciproducens BPP7 and BPP8 were used as controls. During fermentation, samples were withdrawn after 24, 48, and 72 h, and bacterial growth was determined by viable counting on BHI solid medium. SA, LA, and AA production, as well as glucose and xylose consumption, were determined by high-performance liquid chromatography (HPLC, refractive index detector 133; Gilson system; pump 307, column Metacarb 67 h-Varian with flow 0.4 mL/min of H2SO4 0.01 N, Gilson, Villiers le Bel, France). All tests were performed in triplicate.
2.6. Optimization of Culture Conditions in Fermentation Processes
Selected bacterial line B. succiniciproducens 4D was used in a batch experiment in a 10 L fermenter (New Brunswick BioFlo®/CelliGen® 115, Eppendorf, Hamburg, Germany) with a working volume of 3 L. Strain B. succiniciproducens BPP8 grown under optimal conditions was used as control. MH-modified medium with A. donax hydrolysate was prepared as described above. Liquid medium was inoculated with 2% bacterial cell suspension (~4.68 × 106 ± 1.17 CFU mL−1). The pH was automatically maintained at 6.5 by adding a solution of 4M NaOH, and the initial pH was corrected by the addition of around 10 mL of a solution of 5% v/v H2SO4. A solution of 3% v/v Antifoam 204 (Sigma-Aldrich, Milan, Italy) was added at the beginning of the process and after 30 h of fermentation. The experiment was performed at 37 °C for 48 h, under agitation (150 rpm) and CO2 sparging at 0.1 vvm. Samples were withdrawn after 24 and 48 h of fermentation to determine cell growth by counting on BHI medium. SA, LA, and AA production and glucose and xylose consumption were determined by HPLC as described above. The trial was conducted in triplicate.
2.7. Calculation of Yields and Molar Ratios
SA production (g L−1) and yield per gram of products divided by glucose consumption (g) (YSA/G, g g−1), or per gram of total sugars consumed (glucose + xylose, YSA/(G + X), g g−1)t at each sample point of fermentation, were determined. Molar production yields were calculated for SA, LA, and AA (mol of products mol−1 glucose).
2.8. Statistical Analysis and Data Visualization
Data from batch fermentation process were analyzed by linear mixed model Univariate analysis followed by Duncan’s HSD post hoc for pairwise comparison of means (at p < 0.05) using SPSS 19.0 statistical software package (SPSS Inc., Cary, NC, USA). Results were visualized in RStudio (2023.03.0) by ggplot2 (3.4.1). Comparison of data from the lab scale-up experiment in the fermenter was performed with the Student’s t-test.
3. Results and Discussion
3.1. Sequential Adaptation with Single Inhibiting Compound
This work aimed to generate new B. succiniciproducens bacterial lines capable of tolerating inhibitory compounds released during the pretreatment and fermentation processes of lignocellulosic biomass. To achieve this, native B. succiniciproducens strains BPP7 and BPP8 were tested at growth-limiting concentrations of inhibitor substances (F and HMF) released by pretreatment and fermentation end-products (AA, SA, and LA) deriving from hydrolysate of A. donax.
Direct inoculation at 60 g L−1 of SA, 7.5 g L−1 of LA, 4 g L−1 of AA, 2 g L−1 of F, and 3.6 g L−1 of HMF, showed that the strains were unable to tolerate these concentrations (Figure 1). Sequential inoculation of native B. succiniciproducens BPP7 and BPP8 strains into MHM medium containing increasing concentrations of inhibitory substances (F and HMF) and fermentation products (AA, SA, and LA) resulted in 13 adapted B. succiniciproducens bacterial lines capable of tolerating high concentration of toxic compounds. B. succiniciproducens BPP7 and BPP8 bacterial lines were able to grow up to 4 g L−1 of AA, starting from an initial concentration of 1 g L−1. The concentration of AA was increased by 1 g L−1 in each subsequent experimental step of adaptation. However, growth was completely inhibited when the concentration of AA was further increased to 5 g L−1 (Figure 1A). B. succiniciproducens BPP7 and BPP8 strains were unable to grow in the presence of 60 g L−1 of succinic acid. However, through sequential adaptation with increasing concentrations of succinate (ranging from 40 g L−1 to 70 g L−1, with an increment of 10 g L−1 for each step), bacterial lines able to tolerate 60 g L−1 of SA were obtained. The final step of 70 g L−1 of SA resulted in a complete inhibition of growth (Figure 1B). Concerning lactic acid tolerance, the adaptation strategy employed resulted in the growth of B. succiniciproducens BPP7 and BPP8 bacterial lines capable of tolerating 7.5 g L−1 of LA (Figure 1C). Sequential adaptation was also carried out for inhibitory substances such as F and HMF. The applied procedure resulted in bacterial lines from B. succiniciproducens BPP7 and BPP8 that could grow with 3.6 g L−1 of HMF and 2 g L−1 of F (Figure 1D, E).
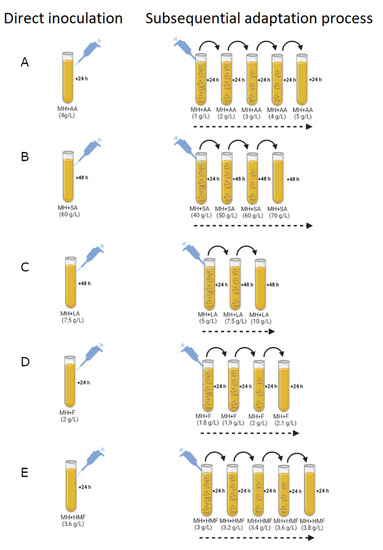
Figure 1.
B. succiniciproducens BPP7 and BPP8 response to direct inoculation in MHM medium with the highest concentration of inhibitory compounds and to sequential adaptation in MHM medium supplemented with increasing concentration of by-products and end-products inhibitors as (A) AA (up to 5.0 g L−1), (B) SA (up to 60 g L−1), (C) LA (up to 7.5 g L−1), (D) F (up to 3.0 g L−1), and (E) HMF (up to 3.6 g L−1) at 37 °C in an atmosphere with 5% CO2 and pH 6.5.
The release of inhibitors during biomass pretreatment can significantly affect the microbial ability to ferment sugars for succinic acid production in engineered hosts, too [15]. Hydrolysates can contain inhibiting substances such as AA, F, HMF, and phenolic compounds derived from lignin or xylo-oligosaccharides, that can have detrimental effects on the fermentation process, strongly delaying the growth of B. succiniciproducens [17,33,37]. Previous studies demonstrated that inhibiting substances can increase the duration of the lag phase, resulting in decreased SA production [17,33]. These fermentation end-products have a wide range of applications ranging from traditional ones as food additives or pharmaceutical intermediates to building blocks for biopolymers and bioplastics [38]. Nevertheless, recent studies suggest the ability of some strains of B. succiniciproducens to detoxify A. donax hydrolysate [15,17,33], a key feature considered in this paper, as it could potentially enable the use of A. donax hydrolysate as a feedstock for succinic acid production.
3.2. Sequential Adaptation in a Mixture of Inhibitory Compounds
Two B. succiniciproducens BPP7 and BPP8 bacterial lines, capable of growing with the highest concentrations of succinic and acetic acid (60 g L−1 of SA and 4 g L−1 of AA), were inoculated into MHM medium supplemented with different mixtures of 30 g L−1 of SA, 3.75 g L−1 of LA, 2 g L−1 of AA, 1 g L−1 of F, and 1.8 g L−1 of HMF (Table 1).
B. succiniciproducens BPP7 and BPP8 bacterial lines adapted to 4 g L−1 of AA grew in the mixtures 2, 4, and 6, resulting in the new bacterial lines 2C, 4C, and 6C, and 2D, 4D, and 6D for B. succiniciproducens BPP7 and BPP8, respectively. Similarly, B. succiniciproducens BPP7 and BPP8 bacterial lines adapted to 60 g L−1 of SA also showed growth in mixtures 2, 4, and 6, resulting in three new bacterial lines for each one (2E, 4E, 6E, or 2F, 4F, 6F for B. succiniciproducens BPP7 and BPP8, respectively). Only bacterial line B. succiniciproducens BPP8 adapted to 60 g L−1 of SA grew in mixture 1 (adapted bacterial line 1F). B. succiniciproducens BPP7 and BPP8 bacterial lines adapted to grow with 60 g L−1 of SA and 4 g L−1 of AA were not able to grow in MHM medium supplemented with maximum concentrations of inhibitory compounds (mixture 8-14 described in Table 1).
The 13 microbial lines obtained (2C, 4C, 6C, 2D, 4D, 6D, 2E, 4E, 6E, 1F, 2F, 4F, 6F) were tested in MHM medium added with 1 g L−1 of F and 1.8 g L−1 of HMF 30 g L−1 of SA, 3.75 g L−1 of LA, 2 g L−1 of AA. This increasingly selective growth condition allowed us to obtain seven new bacterial lines (4C, 2D, 4D, 2E, 4E, 1F, 2F) that tolerated all the inhibitors considered in this study (Table 2). Microbial strains highly tolerant to inhibiting substances, whether isolated from natural environments and adapted in vitro or specifically engineered for that purpose, represent a unique reservoir of information and biotechnological applications. Improving tolerance to inhibiting substances is relevant to enhance their utilization of biomass-derived sugars [39]. Adaptation procedures allow bacteria to develop the necessary genetic regulatory networks, which not only enable bacteria to survive under stress conditions but also maintain the ability to multiply [35].

Table 2.
Growth of B. succiniciproducens BPP7 and BPP8 adapted bacterial lines in MHM medium supplemented with a mixture of all inhibitory substances (30 g L−1 SA, 2 g L−1 AA, 3.75 g L−1 LA, 1 g L−1 F, 1.8 g L−1 HMF).
3.3. Performance of B. succiniciproducens BPP7 and BPP8 Adapted Bacterial Lines on A. donax Hydrolysate in Batch Fermentation Trial
The new bacterial lines 4C, 2D, 4D, 2E, 4E, 1F, and 2F of B. succiniciproducens BPP7 and BPP8 tested in batch experiments on the MHM medium added with A. donax hydrolysate were able to synthesize different levels of succinate (Figure 2A). After 24 h of incubation, the concentration of SA recovered from B. succiniciproducens 2E and 4D bacterial lines was significantly higher (p < 0.05) than BPP7 and BPP8 control strains, reaching values of 5.80 ± 0.56 g L−1 and 5.81 ± 0.39 g L−1, respectively (Figure 2A). Similarly, the molar ratio of SA productions of bacterial lines 2E and 4D had higher values than control strains BPP7 and BPP8 after 24 h of fermentation (p < 0.05, Table 3). Extending the fermentation to 48 and 72 h resulted in a reduction of the differences in acid production among bacterial lines and controls (Figure 2; Table 3). Moreover, bacterial lines 4D and 2E showed a YSA/gluc, 0.50 ± 0.07 g g−1 and YSA/gluc + xyl, 0.42 ± 0.06 g g−1, and YSA/gluc, 0.50 ± 0.05 g g−1 and YSA/gluc + xyl, 0.42 ± 0.06 g g−1, respectively (Table 3). Similar yields (from 0.48 to 0.71 g g−1) were achieved by an engineered strain of B. succiniciproducens fermenting corn stover hydrolysate at different concentrations [17].
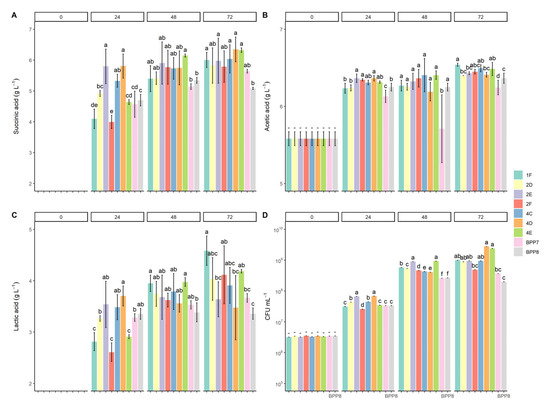
Figure 2.
B. succiniciproducens adapted bacterial lines (from B. succiniciproducens BPP7: 2E, 4E, and 4C; from B. succiniciproducens BPP8: 1F, 2F, 2D, and 4D) and control type strains B. succiniciproducens strains BPP7 and BPP8, grown in batch fermentation experiments with MHM modified medium fortified with A. donax hydrolysate. Experiments were conducted at 37 °C with continuous sparging of CO2 at 0.5 vvm. (A) Productions of succinic acid (SA); (B) productions of acetic acid (AA); (C) productions of lactic acid (LA); (D) viable count of adapted bacterial lines and controls (CFU mL−1) after 24, 48, and 72 h of fermentation. Bars represent the means ± SD of three replicates. Different letters on bars indicate significant differences (p < 0.05).

Table 3.
Molar ratios (mol mol−1) of fermentation end-products SA, LA, and AA and succinic acid (SA) yields (YSA/gluc, g g−1) and for both glucose and xylose (YSA/gluc + xyl, g g−1) of B. succiniciproducens adapted bacterial lines (4C, 2D, 4D, 2E, 4E, 1F, 2F) and control strains BPP7 and BPP8 after 24, 48, and 72 h of fermentation.
The well-known toxic effect of A. donax hydrolysate on microbial metabolism, due to the presence of specific compounds (e.g., furfural, HMF, phydroxybenzoic aldehyde, and vaniline), limits the efficiency of conversion of fermentable sugars in biochemicals and biofuels [40]. These findings demonstrated the suitability of the sequential adaptation strategy, highlighting that B. succiniciproducens adapted bacterial lines can promote the fermentation of A. donax hydrolysate, exhibiting an increase in succinate production and yield.
At the beginning of the fermentation, the amount of AA present in the hydrolysate of A. donax was 5.58 ± 0.08 g L−1 (Figure 2B). During the fermentation process, all adapted bacterial lines and B. succiniciproducens BPP8 produced similar amounts of AA, ranging from 6.19 ± 0.03 g L−1 to 6.54 ± 0.03 g L−1 (Figure 2B). The strain B. succiniciproducens BPP7 showed the significantly lowest AA values in all sampling times (Figure 2B). Consequently, no significant differences were recovered in the Molar Ratio of AA among samples, except for BPP7, which showed the lowest value (0.01 ± 0.00) in Table 3. Acetate is the second most important fermentation end-product after succinic acid [15,16]. Acetic acid represents a hydrolysis by-product that is present in the hydrolysate due to the pretreatment of the biomass in sodium acetate buffer [16], and its concentration increases during fermentation. Concentration levels of acetic acid below the critical threshold of 12 g L−1 in the medium can improve succinic acid production without inhibiting the fermentation process [15,41].
Lactic acid is one of the major organic acid by-products of A. donax hydrolysate fermentation by B. succiniciproducens strains [15]. Its production showed significant differences between the adapted bacterial lines (Figure 2C). Cell line 4D had the highest production (3.71 ± 0.20 g L−1) in 24 h, followed by 2E (3.54 ± 0.15 g L−1), 4C (3.49 ± 0.08 g L−1), and the control BPP8 (3.36 ± 0.03 g L−1) (Figure 2C). LA production ranged from 3.39 ± 0.19 to 3.98 ± 0.09 g L−1 and from 3.36 ± 0.04 to 4.59 ± 0.10 g L−1 after 48 and 72 h of fermentation, although there were no major differences (Figure 2C). Finally, after 72 h of fermentation, the adapted bacterial line 1F resulted in the highest molar ratio value (0.21 ± 0.01, p < 0.05; Table 3). Although this work mainly focused on SA, LA production was also considered due to the relevance of this by-product. The market of LA is expanding due to its importance in the food, pharmaceutical, textile, leather, and chemical industries. Its production through microbial fermentation has gained more attention, especially thanks to the possible use of lignocellulosic biomass as an attractive approach to minimizing the production costs of lactic acid [42].
Glucose consumption was approximately 63% after 24 h of fermentation. All adapted bacterial lines consumed similar amounts of glucose after 24, 48, and 72 h of fermentation, starting from a concentration of 17.44 g L−1 until reaching approximately 6.32 g L−1. Xylose consumption in the first 24 h of fermentation was around 20% (from 13.01 g L−1 to ~10.39 g L−1), although a significant slowdown in its consumption was detected towards the end of the process. At the end of the trial (72 h), the xylose residues ranged from a minimum of ~9.25 ± 0.05 g L−1 to a maximum of ~10.11 ± 0.39 g L−1. Co-consumption of the main sugars, glucose, and xylose by B. succiniciproducens native strains BPP7 and BPP8 was previously demonstrated in controlled 48 h batch processes with constant CO2 sparging using A. donax hydrolysate [15,16,40]. Glucose and xylose were never depleted from the medium after 72 h of fermentation).
The comparison of viabilities showed that the adapted bacterial lines 2E and 4D achieved the highest viability after 24 h of fermentation (~6.31 × 107 CFU mL−1, p < 0.05), whereas, at the end of the process, the highest viability values (p < 0.05) was reached by the adapted bacterial lines 4D and 4E (2.83 × 109 ± 1.05 and 2.40 × 109 ± 1.05 CFU mL−1, respectively).
Batch experiments showed that the adapted bacterial lines had improved characteristics, particularly tolerance to A. donax inhibitory compounds and higher succinic yields. The 4D bacterial line was selected for a laboratory scale-up, as it was among the best producers of succinic acid (5.81 ± 0.13 g L−1 of SA and YSA/gluc 0.50 ± 0.05 g g−1 after 24 h) and high viability value (2.83 × 109 ± 1.05 CFU mL−1).
This research work is particularly important in the study and optimization of SA production. This is particularly important due to the increasing interest in utilizing lignocellulosic biomass as a renewable and cost-effective source of fermentable sugars for biofuels, biochemicals, and other valuable products [3,15,16,33,38]. Therefore, a better understanding of the potential of B. succiniciproducens for lignocellulosic hydrolysate fermentation could lead to the development of more efficient and sustainable bioprocesses [12,14,15,16].
3.4. Fermentation Trial
Based on these observations, B. succiniciproducens 4D was used in a 3 L fermenter scale trial with MHM-modified medium supplemented with A. donax hydrolysate under agitation speed set to 150 rpm and CO2 sparging at 0.1 vvm. The experiment ran for 48 h at 37 °C to determine production rates, sugar consumption, and growth. Curtailing the experiment’s duration, compared to the batch trial, was necessary to reduce the production cost and make it easier for potential commercialization.
The adapted strain B. succiniciproducens 4D produced a final concentration of 17.24 ± 0.39 g L−1 of SA (final yields 0.96 ± 0.02 SA/gluc (g g−1) and 0.48 ± 0.01 SA/(gluc + xyl) g g−1 (Table 4)). This result represents a 6.49 % increase compared to the control BPP8 (14.80 ± 0.12 g L−1) after 48h of incubation. Moreover, considering the glucan and xylan content of A. donax biomass used as the raw material [43], it is possible to calculate the overall yield of fermentation. The 4D strain exhibited a yield of approximately 31%, whereas the native strain achieved a yield of 25% per kg of dry A. donax. The total amount of succinic acid produced by B. succiniciproducens 4D from A. donax hydrolysate depends on the type of feedstock, which is characterized by low initial sugar concentration and is a limiting factor in obtaining higher succinic acid values [8]. The use of new biocatalysts, which are more effective in the hydrolyzed phase, as well as new bacterial strains adapted to the toxic chemicals, can make the bioconversion processes of lignocellulosic-rich biomass more competitive. During fermentation of A. donax hydrolysate by B. succiniciproducens 4D, sugar consumption was delayed because residual glucose was still present after 24 h (4.58 ± 0.09 g), even though it was completely depleted after 48 h. Similarly, after 24 h of fermentation, B. succiniciproducens 4D consumed less xylose than the native strain (Table 4). Nonetheless, the biotechnological performance of the B. succiniciproducens 4D adapted bacterial line is comparable to that achieved in other studies using genetically modified B. succiniciproducens strains in synthetic media added with glucose as a carbon source [17,23,36]. Moreover, no significant differences were recorded between B. succiniciproducens 4D and BPP8 in LA and AA production (Table 4).

Table 4.
Rates of SA, LA, and AA productions, glucose and xylose consumption, SA yields for both glucose (YSA/gluc, g g−1) and xylose (YSA/gluc + xyl, g g−1) of B. succiniciproducens 4D, and control strain BPP8 in 3 L batch fermenter after 24 and 48 h of fermentation.
Microbial growth of B. succiniciproducens 4D increased by roughly three orders of magnitude (from 4.17 × 106 ± 1.41 to 1.86 × 1010 ± 1.35 CFU mL−1) after 24 h of fermentation. In contrast, the control strain B. succiniciproducens BPP8 increased by only about two orders (from 5.25 × 106 ± 1.58 to 5.89 × 109 ± 1.29 CFU mL−1). This finding suggests that the sequentially adapted B. succiniciproducens 4D is more resistant to the inhibiting compounds contained in the liquid medium added with filtrate of A. donax hydrolysate. B. succiniciproducens 4D showed higher growth speed and less metabolic activity in the first 24 h, while at the end of the trial, it had similar viability to the control (~1.91 × 109 CFU mL−1).
The performances of the bacterial line B. succiniciproducens 4D observed in this study were advantageous compared to the native strain BPP8, as well as also to those described by other studies. The adapted bacterial line 4D showed promising results in lab-scale fermenter trials compared to the BPP7 strain mentioned in a prior study, which produced approximately 9.4 ± 0.4 g L−1 of succinic acid within 24 h in a working volume of 250 mL [16]. Becker et al. [36] reported the natural capacity of the wild-type strain B. succiniciproducens DD1, which has the ability to synthesize SA with a metabolic yield of 0.49 g g−1, starting from 45 g L−1 of glucose, which is significantly better than the starting concentration employed in this study (~16.80 g L−1). This promising result demonstrates how natural adaptation can be a valuable biotechnology for enhancing the effectiveness and tolerance of SA-producing strains to lignocellulosic biomasses. The adapted bacterial lines obtained may be an interesting starting point for genetic engineering to develop strains with enhanced ability to withstand inhibitors found in lignocellulosic biomass. Moreover, the obtained adapted bacterial strain 4D could be further evaluated in fed-batch trials, following the methodology described by Cimini et al. [33]. In their study, B. succiniciproducens BPP7 was tested on a pre-pilot scale of 70–80 L, resulting in the production of 37 g/L of succinic acid by supplementing pure glucose during the fed-batch phase [33].
Recent research reported the ability to synthesize SA using xylose-rich hemicellulosic fractions from olive pits and sugarcane bagasse of A. succinogenes (yield from 28 to 34 g L−1 and 0.27 g g−1) [28]. Oil palm biomass has been studied as a carbon source for SA production by various strains of A. succinogenes [7]. However, the yields can vary depending on the treatments applied to the biomass and the fermentation conditions [7]. These recent studies highlighted the potential of additional low-cost sources for succinic acid production. Considering the adaptability of strain 4D and its resistance to stress conditions, it could be potentially applied for alternative sources.
4. Conclusions
In conclusion, the subsequential adaptation strategy employed in this study enabled the development of new bacterial lines that can tolerate toxic compounds from lignocellulosic biomass while also synthesizing succinic acid. The new B. succiniciproducens 4D strain, obtained through subsequential adaptation, shows promising biotechnological applications by efficiently fermenting A. donax hydrolysate and producing succinate. Although further development is required to achieve industrially appealing values, based on these findings, the strain B. succiniciproducens 4D represents an important microbiological source capable of utilizing sugars derived from lignocellulosic feedstock to develop ameliorative technologies for bio-based succinic acid production.
Author Contributions
Conceptualization, O.P., A.R. and I.R.; formal analysis, A.R. and I.R.; investigation, A.R. and I.R.; writing—original draft preparation, I.R., A.R. and O.P.; writing—review and editing, O.P., V.V. and V.F.; visualization, I.R.; supervision and funding acquisition, O.P. All authors have read and agreed to the published version of the manuscript.
Funding
This study was carried out within the Agritech National Research Center and received funding from the European Union NextGenerationEU (PIANO NAZIONALE DI RIPRESA E RESILIENZA (PNRR)—MISSIONE 4 COMPONENTE 2, INVESTIMENTO 1.4—D.D. 1032 17/06/2022, CN00000022). This manuscript reflects only the authors’ views and opinions; neither the European Union nor the European Commission can be considered responsible for them.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kim, W.J.; Ahn, J.H.; Kim, H.U.; Kim, T.Y.; Lee, S.Y. Metabolic Engineering of Mannheimia Succiniciproducens for Succinic Acid Production Based on Elementary Mode Analysis with Clustering. Biotechnol. J. 2017, 12, 1600701. [Google Scholar] [CrossRef] [PubMed]
- Pateraki, C.; Ladakis, D.; Stragier, L.; Verstraete, W.; Kookos, I.; Papanikolaou, S.; Koutinas, A. Pretreatment of Spent Sulphite Liquor via Ultrafiltration and Nanofiltration for Bio-Based Succinic Acid Production. J. Biotechnol. 2016, 233, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, J.; Idris, A.; Aziz, R.A. Recent Advances in Production of Succinic Acid from Lignocellulosic Biomass. Appl. Microbiol. Biotechnol. 2014, 98, 987–1000. [Google Scholar] [CrossRef] [PubMed]
- Saxena, R.K.; Saran, S.; Isar, J.; Kaushik, R. 27-Production and Applications of Succinic Acid. In Current Developments in Biotechnology and Bioengineering, Production, Isolation and Purification of Industrial Products; Pandey, A., Negi, S., Soccol, C.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 601–630. ISBN 978-0-444-63662-1. [Google Scholar]
- Nghiem, N.P.; Kleff, S.; Schwegmann, S. Succinic Acid: Technology Development and Commercialization. Fermentation 2017, 3, 26. [Google Scholar] [CrossRef]
- Liu, Y.-J.; Li, B.; Feng, Y.; Cui, Q. Consolidated Bio-Saccharification: Leading Lignocellulose Bioconversion into the Real World. Biotechnol. Adv. 2020, 40, 107535. [Google Scholar] [CrossRef]
- Hariz, H.B.; Zaidi, S.A.S.; Luthfi, A.A.I.; Bukhari, N.A.; Sajab, M.S.; Markom, M.; Harun, S.; Tan, J.-P.; Ding, G.-T.; Abdul, P.M. Succinic Acid Production from Oil Palm Biomass: A Prospective Plastic Pollution Solution. Fermentation 2023, 9, 46. [Google Scholar] [CrossRef]
- Hijosa-Valsero, M.; Paniagua-García, A.I.; Díez-Antolínez, R. Assessment of Vine Shoots and Surplus Grape Must for Succinic Acid Bioproduction. Appl. Microbiol. Biotechnol. 2022, 106, 4977–4994. [Google Scholar] [CrossRef]
- Kuhnert, P.; Scholten, E.; Haefner, S.; Mayor, D.; Frey, J. Basfia Succiniciproducens Gen. Nov., Sp. Nov., a New Member of the Family Pasteurellaceae Isolated from Bovine Rumen. Int. J. Syst. Evol. Microbiol. 2010, 60, 44–50. [Google Scholar] [CrossRef]
- Dickson, R.; Mancini, E.; Garg, N.; Woodley, J.M.; Gernaey, K.V.; Pinelo, M.; Liu, J.; Mansouri, S.S. Sustainable Bio-Succinic Acid Production: Superstructure Optimization, Techno-Economic, and Lifecycle Assessment. Energy Environ. Sci. 2021, 14, 3542–3558. [Google Scholar] [CrossRef]
- Van der Werf, M.J.; Guettler, M.V.; Jain, M.K.; Zeikus, J.G. Environmental and Physiological Factors Affecting the Succinate Product Ratio during Carbohydrate Fermentation by Actinobacillus Sp. 130Z. Arch. Microbiol. 1997, 167, 332–342. [Google Scholar] [CrossRef]
- Lee, P.; Lee, S.; Hong, S.; Chang, H. Isolation and Characterization of a New Succinic Acid-Producing Bacterium, Mannheimia Succiniciproducens MBEL55E, from Bovine Rumen. Appl. Microbiol. Biotechnol. 2002, 58, 663–668. [Google Scholar] [CrossRef]
- Ahn, J.H.; Jang, Y.-S.; Lee, S.Y. Production of Succinic Acid by Metabolically Engineered Microorganisms. Curr. Opin. Biotechnol. 2016, 42, 54–66. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Z.; Zhu, N.; Wang, B.; Chen, T.; Zhao, X. Metabolic Engineering of Escherichia coli and in Silico Comparing of Carboxylation Pathways for High Succinate Productivity under Aerobic Conditions. Microbiol. Res. 2014, 169, 432–440. [Google Scholar] [CrossRef]
- Cimini, D.; Argenzio, O.; D’Ambrosio, S.; Lama, L.; Finore, I.; Finamore, R.; Pepe, O.; Faraco, V.; Schiraldi, C. Production of Succinic Acid from Basfia Succiniciproducens up to the Pilot Scale from Arundo Donax Hydrolysate. Bioresour. Technol. 2016, 222, 355–360. [Google Scholar] [CrossRef]
- Ventorino, V.; Robertiello, A.; Cimini, D.; Argenzio, O.; Schiraldi, C.; Montella, S.; Faraco, V.; Ambrosanio, A.; Viscardi, S.; Pepe, O. Bio-Based Succinate Production from Arundo Donax Hydrolysate with the New Natural Succinic Acid-Producing Strain Basfia Succiniciproducens BPP7. BioEnergy Res. 2017, 10, 488–498. [Google Scholar] [CrossRef]
- Salvachúa, D.; Smith, H.; John, P.C.S.; Mohagheghi, A.; Peterson, D.J.; Black, B.A.; Dowe, N.; Beckham, G.T. Succinic Acid Production from Lignocellulosic Hydrolysate by Basfia Succiniciproducens. Bioresour. Technol. 2016, 214, 558–566. [Google Scholar] [CrossRef]
- Scholten, E.; Dägele, D. Succinic Acid Production by a Newly Isolated Bacterium. Biotechnol. Lett. 2008, 30, 2143–2146. [Google Scholar] [CrossRef]
- Salvachúa, D.; Mohagheghi, A.; Smith, H.; Bradfield, M.F.A.; Nicol, W.; Black, B.A.; Biddy, M.J.; Dowe, N.; Beckham, G.T. Succinic Acid Production on Xylose-Enriched Biorefinery Streams by Actinobacillus Succinogenes in Batch Fermentation. Biotechnol. Biofuels 2016, 9, 28. [Google Scholar] [CrossRef]
- Ladakis, D.; Michailidi, K.; Vlysidis, A.; Koutinas, A.; Kookos, I.K. Valorization of Spent Sulphite Liquor for Succinic Acid Production via Continuous Fermentation System. Biochem. Eng. J. 2018, 137, 262–272. [Google Scholar] [CrossRef]
- Stylianou, E.; Pateraki, C.; Ladakis, D.; Cruz-Fernández, M.; Latorre-Sánchez, M.; Coll, C.; Koutinas, A. Evaluation of Organic Fractions of Municipal Solid Waste as Renewable Feedstock for Succinic Acid Production. Biotechnol. Biofuels 2020, 13, 72. [Google Scholar] [CrossRef]
- Pennacchio, A.; Ventorino, V.; Cimini, D.; Pepe, O.; Schiraldi, C.; Inverso, M.; Faraco, V. Isolation of New Cellulase and Xylanase Producing Strains and Application to Lignocellulosic Biomasses Hydrolysis and Succinic Acid Production. Bioresour. Technol. 2018, 259, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Nielsen, J. Biobased Organic Acids Production by Metabolically Engineered Microorganisms. Curr. Opin. Biotechnol. 2016, 37, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Varriale, S.; Houbraken, J.; Granchi, Z.; Pepe, O.; Cerullo, G.; Ventorino, V.; Chin-A-Woeng, T.; Meijer, M.; Riley, R.; Grigoriev, I.V.; et al. Talaromyces Borbonicus, Sp. Nov., a Novel Fungus from Biodegraded Arundo Donax with Potential Abilities in Lignocellulose Conversion. Mycologia 2018, 110, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Romano, I.; Ventorino, V.; Ambrosino, P.; Testa, A.; Chouyia, F.E.; Pepe, O. Development and Application of Low-Cost and Eco-Sustainable Bio-Stimulant Containing a New Plant Growth-Promoting Strain Kosakonia Pseudosacchari TL13. Front. Microbiol. 2020, 11, 2044. [Google Scholar] [CrossRef] [PubMed]
- Ventorino, V.; Robertiello, A.; Viscardi, S.; Ambrosanio, A.; Faraco, V.; Pepe, O. Bio-Based Chemical Production from Arundo Donax Feedstock Fermentation Using Cosenzaea Myxofaciens BPM1. BioResources 2016, 11, 6566–6581. [Google Scholar] [CrossRef]
- Nolasco, A.; Squillante, J.; Velotto, S.; D’Auria, G.; Ferranti, P.; Mamone, G.; Errico, M.E.; Avolio, R.; Castaldo, R.; Cirillo, T.; et al. Valorization of Coffee Industry Wastes: Comprehensive Physicochemical Characterization of Coffee Silverskin and Multipurpose Recycling Applications. J. Clean. Prod. 2022, 370, 133520. [Google Scholar] [CrossRef]
- Oreoluwa Jokodola, E.; Narisetty, V.; Castro, E.; Durgapal, S.; Coulon, F.; Sindhu, R.; Binod, P.; Rajesh Banu, J.; Kumar, G.; Kumar, V. Process Optimisation for Production and Recovery of Succinic Acid Using Xylose-Rich Hydrolysates by Actinobacillus Succinogenes. Bioresour. Technol. 2022, 344, 126224. [Google Scholar] [CrossRef]
- Fagnano, M.; Impagliazzo, A.; Mori, M.; Fiorentino, N. Agronomic and Environmental Impacts of Giant Reed (Arundo donax L.): Results from a Long-Term Field Experiment in Hilly Areas Subject to Soil Erosion. BioEnergy Res. 2015, 8, 415–422. [Google Scholar] [CrossRef]
- Ventorino, V.; Ionata, E.; Birolo, L.; Montella, S.; Marcolongo, L.; de Chiaro, A.; Espresso, F.; Faraco, V.; Pepe, O. Lignocellulose-Adapted Endo-Cellulase Producing Streptomyces Strains for Bioconversion of Cellulose-Based Materials. Front. Microbiol. 2016, 7, 2061. [Google Scholar] [CrossRef]
- Kawaguchi, H.; Hasunuma, T.; Ogino, C.; Kondo, A. Bioprocessing of Bio-Based Chemicals Produced from Lignocellulosic Feedstocks. Curr. Opin. Biotechnol. 2016, 42, 30–39. [Google Scholar] [CrossRef]
- Coppola, G.; Gaudio, M.T.; Lopresto, C.G.; Calabro, V.; Curcio, S.; Chakraborty, S. Bioplastic from Renewable Biomass: A Facile Solution for a Greener Environment. Earth Syst. Environ. 2021, 5, 231–251. [Google Scholar] [CrossRef]
- Cimini, D.; Zaccariello, L.; D’Ambrosio, S.; Lama, L.; Ruoppolo, G.; Pepe, O.; Faraco, V.; Schiraldi, C. Improved Production of Succinic Acid from Basfia Succiniciproducens Growing on A. Donax and Process Evaluation through Material Flow Analysis. Biotechnol. Biofuels 2019, 12, 22. [Google Scholar] [CrossRef]
- Binder, J.; Raines, R. Fermentable Sugars by Chemical Hydrolysis of Biomass. Proc. Natl. Acad. Sci. USA 2010, 107, 4516–4521. [Google Scholar] [CrossRef]
- Zou, L.; Jin, X.; Tao, Y.; Zheng, Z.; Ouyang, J. Unraveling the Mechanism of Furfural Tolerance in Engineered Pseudomonas Putida by Genomics. Front. Microbiol. 2022, 13, 1035263. [Google Scholar] [CrossRef]
- Becker, J.; Reinefeld, J.; Stellmacher, R.; Schäfer, R.; Lange, A.; Meyer, H.; Lalk, M.; Zelder, O.; von Abendroth, G.; Schröder, H.; et al. Systems-Wide Analysis and Engineering of Metabolic Pathway Fluxes in Bio-Succinate Producing Basfia Succiniciproducens. Biotechnol. Bioeng. 2013, 110, 3013–3023. [Google Scholar] [CrossRef]
- Abatenh, E.; Gizaw, B.; Tsegaye, Z.; Wassie, M. The Role of Microorganisms in Bioremediation-A Review. Open J. Environ. Biol. 2017, 2, 38–46. [Google Scholar] [CrossRef]
- D’ambrosio, S.; Alfano, A.; Cimini, D. Production of Succinic Acid From Basfia Succiniciproducens. Front. Chem. Eng. 2021, 3, 785691. [Google Scholar] [CrossRef]
- Ladakis, D.; Papapostolou, H.; Vlysidis, A.; Koutinas, A. Chapter 9—Inventory of Food Processing Side Streams in European Union and Prospects for Biorefinery Development. In Food Industry Wastes, Assessment and Recuperation of Commodities, 2nd ed.; Kosseva, M.R., Webb, C., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 181–199. ISBN 978-0-12-817121-9. [Google Scholar]
- Palmqvist, E.; Hahn-Hägerdal, B. Fermentation of Lignocellulosic Hydrolysates. I: Inhibition and Detoxification. Bioresour. Technol. 2000, 74, 17–24. [Google Scholar] [CrossRef]
- Trček, J.; Mira, N.P.; Jarboe, L.R. Adaptation and Tolerance of Bacteria against Acetic Acid. Appl. Microbiol. Biotechnol. 2015, 99, 6215–6229. [Google Scholar] [CrossRef]
- Chauhan, S.; Mitra, S.; Yadav, M.; Kumar, A. Microbial Production of Lactic Acid Using Organic Wastes as Low-Cost Substrates. Phys. Sci. Rev. 2023, 3, 4. [Google Scholar] [CrossRef]
- Giacobbe, S.; Balan, V.; Montella, S.; Fagnano, M.; Mori, M.; Faraco, V. Assessment of Bacterial and Fungal (Hemi)Cellulose-Degrading Enzymes in Saccharification of Ammonia Fibre Expansion-Pretreated Arundo donax. Appl. Microbiol. Biotechnol. 2016, 100, 2213–2224. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).