Abstract
Seaweeds (macroalgae) are gaining attention as potential sustainable feedstock for the production of fuels and chemicals. This comparative study focuses on the characterization of the microbial production of alcohols from fermentable carbohydrates in the hydrolysate of the macroalgae Laminaria digitata as raw material. The potential of a hydrolysate as a carbon source for the production of selected alcohols was tested, using three physiologically different fermentative microbes, in two main types of processes. For the production of ethanol, Saccharomyces cerevisiae was used as a benchmark microorganism and compared with the strictly anaerobic thermophile Thermoanaerobacterium strain AK17. For mixed production of acetone/isopropanol, butanol, and ethanol (A/IBE), three strictly anaerobic Clostridium strains were compared. All strains grew well on the hydrolysate, and toxicity constraints were not observed, but fermentation performance and product profiles were shown to be both condition- and strain-specific. S. cerevisiae utilized only glucose for ethanol formation, while strain AK17 utilized glucose, mannitol, and parts of the glucan oligosaccharides. The clostridia strains tested showed different nutrient requirements, and were able to utilize glucan, mannitol, and organic acids in the hydrolysate. The novelty of this study embodies the application of different inoculates for fermenting a common brown seaweed found in the northern Atlantic Ocean. It provides important information on the fermentation properties of different microorganisms and pinpoints the value of carbon source utilization when selecting microbes for efficient bioconversion into biofuel and chemical products of interest.
1. Introduction
There is an increased worldwide emphasis to find and utilize new sources of sustainable biomass that can be converted into biobased chemicals and energy carriers to supplant fossil fuels for transportation vehicles [1,2]. Seaweed (macroalgal) biomass is a largely underexploited feedstock in Europe, and biorefining applications are at an early stage [3,4]. Brown algae (Phaeophyta) neither contain lignin nor crystalline cellulose, which are major obstacles for the utilization of lignocellulosic feedstocks in biorefineries [5]. Due to their high photosynthetic ability, they have the potential to generate and store sufficient carbon resources needed for biorefinery applications. It has also been reported that seaweeds have higher productivity rates than terrestrial biomass such as corn and switchgrass [6]. Furthermore, they do not compete with arable land used for agricultural crops and do not require the use of fertilizer or other chemicals [7,8]. Brown macroalgae, however, have a complex carbohydrate composition containing cell wall polysaccharides, alginate, and fucoidan, and the storage carbohydrates mannitol and laminarin, and possibly also cell wall structural components, such as 1,4-β-glucan or mixed linkage glucans in smaller quantities [9,10]. Mannitol and laminarin are reserve carbohydrates that can account for up to 20% and 50%, respectively, of the dry weight [11].
Brown macroalgae are harvested commercially in large quantities in the world, especially in East Asia [12]. From 2005, the production has more than doubled with over 30 million tons of macroalgae harvested globally in 2015, indicating that they have a large potential for industrial exploitation [13].
However, the complex carbohydrate composition of macroalgae presents challenges for economic fuel and chemical production through conventional fermentation methods [14]. This has led to a focus on the use of macroalgae in microbial anaerobic digestion for biogas production [15], and the production of bio-oil through thermochemical processes such as pyrolysis or hydrothermal liquefaction [16,17]. In order to utilize macroalgae as a feedstock in biorefineries, there is a need for efficient and cost-effective pre-treatment methodologies [18], polysaccharide degrading enzymes [19,20], and versatile fermentative microorganisms that can utilize mixed carbon sources [21,22]. In recent years, there have been efforts to identify natural strains or genetically engineer microorganisms with the ability to ferment sugars from seaweed into biofuels, including ethanol [23,24,25] and butanol [26,27]. For example, two brown seaweed species (L. japonica and S. fulvellum) have been shown to be a potential feedstock for the production of bioethanol through simultaneous saccharification and fermentation using a genetically engineered strain of Escherichia coli [24]. Similarly, L. digitata has been evaluated for its potential to produce butanol through fermentation using Clostridium beijerinckii [26]. With such challenges in mind, the choice of microorganisms for the conversion of macroalgal feedstock is the first step in the development of a viable biorefinery platform. Non-conventional strains could be good candidates for the utilization of second and third-generation biomass (such as seaweeds), having the required substrate utilization range.
In this study, a comparative approach has been used to evaluate the potential of different microorganisms. Saccharomyces cerevisiae was used as the benchmark organism for ethanol production. It is the conventional fermentative organism for the production of ethanol for the consumer as well as industrial usage and is widely used on inexpensive glucose derived from first-generation biomass. Fermentation technologies are well established and advanced. S. cerevisiae utilizes glucose very efficiently, up to 90–95% of maximum theoretical yields are typically achieved under optimal conditions and it has a relatively high tolerance to ethanol. The drawback is its limited substrate utilization range.
The strictly anaerobic A/IBE-fermentation by clostridia is a benchmark process for the production of acetone/isopropanol, n-butanol, and ethanol from carbohydrates and ABE-fermentation was widely used on an industrial scale during World War I, using, e.g., molasses as feedstock. Clostridium strains are today potentially, highly versatile production organisms for second/third generation biomass utilization in the emerging seaweed biorefineries. Clostridia have a wide substrate utilization range, including C6 and C5 sugars as well as sugar alcohols, such as mannitol and organic acids [28,29,30].
Less established, non-conventional, but promising biorefinery organisms are thermoanaerobes. In this study, the strain Thermoanaerobacterium strain AK17 was evaluated for ethanol production from brown seaweed hydrolysates. It grows at 60 °C and has a wide substrate range, growing on all the lignocellulose sugars, and has been shown to be a good producer of ethanol [31] on defined media and second-generation lignocellulosic feedstocks [32]. Cultivation at high temperature (60–70 °C) has advantages as it reduces the costs of cooling, and higher temperatures protect the cultures from mesophilic spoilage bacteria. It increases the solubility of polysaccharides and leads to the reduced viscosity of fermentation broth, which may alleviate scale-up problems of mixing and enable greater substrate loadings. Elevated temperatures may also enable the cost-effective recovery of volatile products by distillation or gas stripping, which would reduce product inhibition and prolong the production phase of the culture.
The focus of this study was hence set to investigate the fermentability of a sugar-rich hydrolysate from the brown seaweed Laminaria digitata in the selected microbial conversion processes with reference to potential biorefinery applications. Cultivation of conventional and non-conventional organisms was compared, for the production of ethanol or mixes of acetone/butanol/ethanol/isopropanol (ABE/IBE).
2. Materials and Methods
2.1. Hydrolysate
The preparation of the hydrolysate used in this study was previously described by Hou et al. [26]. Briefly, it was obtained from wild-grown sugar-rich Laminaria digitata collected in August 2014 from the coast of the Danish North Sea. The dried seaweed was then processed by enzymatic hydrolysis at the DTI pilot scale (600L) facility, as described [26], and due to incomplete hydrolysis of the biomass, a substantial quantity of glucans (laminarin) remained in the hydrolysate. At the end of the process, the concentrations of the different sugars were measured by high-performance liquid chromatography (HPLC).
2.2. Ethanol Fermentation
2.2.1. Fermentation by Saccharomyces cerevisiae
Ethanol fermentation trials were performed as triplicates in 100 mL fermentation flasks sealed by air locks (filled with glycerol), with 60 mL working volume of hydrolysate as sole component. The fermentation flasks containing the hydrolysate were autoclaved at 121 °C for 10 min and cooled down to room temperature before inoculation. A strain of commercial Saccharomyces cerevisiae (Quick Yeast, dry form, Doves Farm Foods Ltd., Hungerford, UK) was then inoculated at 1 g/L, and the fermentation flasks were incubated at 30 °C in an orbital shaker-incubator (Grant Bio, ES-20) at 120 rpm. Samples for analysis were taken under sterile conditions before and after fermentation to measure the sugar consumption and the ethanol production.
2.2.2. Fermentation by Thermoanaerobacterium Strain AK17
The Thermoanaerobacterium AK17 inoculum was cultured in BM medium [32] containing (in g L−1): NaH2PO4, 5.5; Na2HPO4, 0.6; KH2PO4, 0.6; NH4Cl, 0.3; NaCl, 0.3; CaCl2 2H2O, 0.1; MgCl2 6H2O, 0.1. Then was added micronutrients (in mg L−1: FeCl2 4H2O, 2; H3BO3, 0.05; ZnCl2, 0.05; CuCl2 2H2O, 0.038; MnCl2·2H2O, 0.041; (NH4)6Mo7O24 4H2O, 0.05; AlCl3, 0.05; CoCl2·6H2O, 0.05; NiCl2·6H2O, 0.05; EDTA, 0.5; Na2SeO3·5H2O, 0.026; NaWO4·2H2O, 0.033) and vitamins (DSMZ medium No141, German Collection of Microorganisms and Cell Cultures). The medium was supplemented with 20 mM glucose as carbon source. An exponentially grown inoculum culture, corresponding to 2% total culture volume was then transferred into flasks with BM medium containing 50 to 100% (v/v) seaweed hydrolysate. Prior to inoculation, the pH was adjusted to 6.5 with 1M NaOH, and the cultures were supplemented with 0.2% yeast extract (Bacto™ Yeast Extract, BD biosciences, San Jose, CA, USA). Micronutrients, vitamins, and reducing agent (3 mM Cysteine hydrochloride + 3 mM Na2S) were also added prior to inoculation. Cultivations were carried out in anaerobic conditions, using closed flasks (118 mL) with 50 mL working volume, at 60 °C for 4 days. Samples for analysis were taken under sterile conditions before and after fermentation to measure the sugar consumption and the ethanol production.
2.3. ABE and IBE Fermentation
The microbial strains used in the ABE/IBE fermentations include Clostridium acetobutylicum ATCC824 and C. beijerinckii strains NCIMB 8052 and DSM6423. Strains were stored at −20 °C as spore suspension in 20% glycerol. The suspension was heat-shocked for 1 min at 95 °C (C. beijerinckii) or for 10 min at 80 °C (C. acetobutylicum) before inoculation. Control fermentations on pure sugar (glucose and mannitol) were performed in CM2 medium containing (in g L−1): yeast extract, 1.00; KH2PO4, 1.00; K2HPO4, 0.61; MgSO4·7 H2O, 1.00; FeSO4·7 H2O, 0.0066; para-aminobenzoic acid (pABA), 0.10; and ammonium acetate, 2.90. Stock solutions of D-glucose and mannitol were autoclaved separately and added after autoclaving of the medium to a final concentration of 40 g L−1. All liquid media were made anaerobic by flushing with nitrogen gas. The Laminaria digitata hydrolysate was tested as such (H), or supplemented with salts and nutrients (HSN, that is, all nutrients in CM2). Samples for analysis were taken under sterile conditions before and after fermentation to measure the sugar consumption and the ethanol production.
2.4. Analysis of Free Organic Acids and Monosaccharides by HPLC
Samples for high-performance liquid chromatography (HPLC) analysis were filtered through 0.2 µm filter (Phenomenex) prior injection. Glucose, mannitol, acetic acid, lactic acid, and ethanol were quantified using a Dionex 2000 HPLC system (Dionex, Idstein, Germany) with a Rezex ROA-Organic Acid H + (8%, Phenomenex, Aschaffenburg, Germany) and a RI-101 detector (Shodex, München, Germany). Separation was performed at a column temperature of 60 °C with 0.2 mM sulfuric acid (Carl Roth, Karlsruhe, Germany) as eluent at a flow rate of 600 μL/min for 30 min. Quantification was carried out using external standards with HPLC grade (Merck, Darmstadt, Germany, Sigma-Aldrich, St. Louis, MO, USA) and the Chromeleon evaluation software version 6.80 (Dionex, Idstein, Germany).
2.5. Total Monosaccharides Analysis by HPAEC-PAD
Polysaccharides were hydrolyzed to monosaccharides by a two-step acid hydrolysis process [33]. Twenty-five milligrams of the samples were mixed with 250 µL of sulfuric acid 72% (w/w) (Thermo Scientific™) in pyrex tubes with screw caps. The tubes were incubated in an incubator at 30 °C and 150 rpm shaking for 1 h. Afterward, 7 mL of milliQ-water was added to the tubes to reach the acid concentration of 4% (w/w). The samples were vortexed and autoclaved for 1 h. After cooling down, the samples were centrifuged at 3000× g (Sigma 3-16PK centrifuge, Germany) for 10 min to remove the solid particles. The hydrolysate was then neutralized by Barium hydroxide (0.1 M).
To analyze the monosaccharides, the hydrolysate was diluted with milliQ-water, and filtered through a 0.2 µm syringe filter. Monosaccharides were analyzed by an anion exchange chromatography (HPAEC-PAD) system (Thermo Fisher Scientific, Sunnyvale, CA, USA) using a Carbopac PA-20 column coupled with a guard column (Thermo Fisher Scientific, Sunnyvale, CA, USA). Neutral sugars were separated under isocratic condition using Milli-Q-water (A), 1 mM sodium hydroxide (B), and 200 mM sodium hydroxide (C) as eluents. Elution was performed using an eluent mixture of 62.5% (A) and 37.5% (B) for 25 min. Uronic acids were analyzed using the same column but with different eluents, Milli-Q-water (A), 1M sodium acetate (B), and 200 mM sodium hydroxide (C). Uronic acids were eluted by an eluent mixture of 55% (A), 15% (B), and 30% (C) for 18 min. For both neutral sugars and uronic acids, separation was performed at a flowrate of 0.5 mL/min, and column and compartment temperatures were kept at 30 °C [34]. The concentrations of glucans were then estimated by subtracting the quantity of free monomeric glucose from the total glucose content.
2.6. Yields Calculation Formulas
Two different calculations for the ethanol yields were considered to highlight either the added amount of substrate (total) or the substrate utilization profile (partial). First, the ethanol yield (total) was calculated as:
where [ethanol]p represents the ethanol produced during the fermentation in g/L, and [substrate]i represent the initial total concentrations of the different substrates.
Then, the ethanol yield (partial) was calculated as:
where [ethanol]p represents the ethanol produced during the fermentation in g/L, and [substrate]c represents the concentrations of the different substrates effectively consumed during the fermentation.
In addition, the calculated yields could be compared to the theoretical maximum yields of 0.511, which corresponds to the maximum obtainable yield of ethanol from glucose. The assumption was made that the glucans were solemnly composed of glucose units, and that the small molecular weight difference between mannitol (182.172 g/mol) and glucose (180.156 g/mol) could be omitted. The yields were then divided by 0.511 and shown as percentage (%) of the theoretical maximum yield.
For the calculation of the yields of butanol or A/IBE, the above formula for the [ethanol]p was used, where ethanol was replaced by butanol or A/IBE as indicated in the text.
2.7. Determination of Extracellular and Intracellular Enzymatic Activity in the Thermoanaerobacterium Strain AK17 Culture
To elucidate the apparent consumption of glucans/glucooligosaccharides in the L. digitata hydrolysate by strain AK17, the glucan hydrolyzing activity, both intracellular and extracellular, was investigated. Strain AK17 was grown on cellobiose in 10 mL volume in 25 mL flasks following conditions previously described. After cultivation, cells were harvested by centrifugation (5000 g/10 min) and the supernatant was collected. The supernatant was concentrated 10-fold using Amicon centrifugal filters with a 10 kDa cut off to retain the enzymes and would constitute the extracellular enzyme fraction. The pelleted cells were resuspended in 1 mL 0.1 M phosphate buffer pH 7 and lyzed by sonication (Branson Sonifier 250, 40% duty cycle, output control 4, 5 min). After centrifugation (5000 g/20 min), the supernatant was kept and would constitute the intracellular enzyme fraction. The glucosidase activity of the two fractions was then tested against oligo-laminarin and oligo-cellulose (pentose, triose, and biose). The experiment was carried out in 200 µL volume, with 1 mg/mL oligosaccharides and 10 µL extract (either concentrated culture supernatant or intracellular fraction) and incubated for 12 h at 60 °C. Samples were analyzed using Thin Layer Chromatography (TLC). Four microliters of each reaction mixture were applied to a TLC silica plate. The reaction product was developed with 1-butanol:acetic acid:water = 2:1:1 (v:v:v) and detected by heating at 130 °C for 10 min after spraying 10% (w/v) sulfuric acid–90% (w/v) ethanol.
3. Results
3.1. L. digitata Hydrolysate Utilization by S. cerevisiae and Thermoanaerobacterium Strain AK17 for Production of Ethanol
The carbohydrate content of the L. digitata hydrolysate was analyzed as described in the Material and Methods, and was composed of 9.5 g/L glucose, 5 g/L mannitol, 10 g/L glucans, and 1.2 g/L lactic acid. The lactic acid most probably originated from early contamination of the seaweeds, which occurred during the enzymatic processing, as described by Hou et al., 2017 [26], and which stopped the enzymatic digestion earlier than planned.
Fermentation of L. digitata hydrolysate by S. cerevisiae was used as a benchmark, in this study, for fermentative glucose utilization and ethanol production. The results show that S. cerevisiae in principle utilized all the soluble monomeric glucose (9.3 g/L) in the hydrolysate, indicating no inhibition by hydrolysate components. Neither mannitol (4.6 g/L) nor oligo-/polymeric glucans (11.4 g/L) were consumed (Table 1), resulting in the conversion of 38% of the total carbohydrate source in the hydrolysate by S. cerevisiae (Table 2). Based on the use of free monomeric glucose in the cultivation medium the ethanol yield (partial) was 0.37 g ethanol/gram free glucose which corresponds to 72% of the maximum obtainable yield. However, if the whole carbon source is considered (free glucose + mannitol + glucan), then the ethanol yield (total) represents only 0.14 g ethanol/gram of available substrate, which corresponds to 27% of the maximum obtainable yield.

Table 1.
Substrate concentration, utilization, and produced product by S. cerevisiae and Thermoanaerobacterium AK17 in cultivations using different amounts of L. digitata hydrolysate as raw material. Data represent the average of three replicate experiments. ND, not detectable, below the detection limit.

Table 2.
Substrate consumptions and ethanol yields of S. cerevisiae and Thermoanaerobacterium AK17 in cultivations using different amounts of L. digitata hydrolysate as raw material. Data represent the average of three replicate experiments.
The fermentative capability of Thermoanaerobacterium strain AK17 on the hydrolysate was tested at two conditions (using 50 and 100% hydrolysate, respectively) to investigate possible inhibitory effects on the fermentation. Strain AK17 was able to grow at both conditions, in 24 h in the 50% hydrolysate with no lag-phase, and in 48 h in the 100% hydrolysate but with ~24 h lag-phase before the exponential phase started (Figure 1). In cultivations using diluted hydrolysate (50%), all the monomeric glucose, 20% of the glucan, and 50% of the mannitol were consumed, resulting in the conversion of 56% of the total carbon sources (50% hydrolysate, Table 2). On the undiluted hydrolysate (100% hydrolysate, Table 1), strain AK17 used 82% of the glucose, 20% of the glucan, and 47% of the mannitol. At this condition, a slightly reduced amount of the carbohydrates was converted (49% of the carbon sources in the hydrolysate), due to the lower conversion of the available glucose. Interestingly, strain AK17 utilized ~20% of the glucans. Regarding fermentation products, strain AK17 produced ethanol as the main fermentation product, but, in addition to this, both lactic and acetic acids were also produced. The ethanol yield (partial) at both conditions was around 0.30 g ethanol/g consumed substrate, which is around 59% of the maximum theoretical yield (considering ethanol as a single product). Remarkably, a higher ethanol concentration (3.82 g/L) was reached using strain AK17, compared to S. cerevisiae (3.47 g/L) despite producing organic acids as well. In addition, the ethanol yields (total) were also slightly higher than S. cerevisae (27%) on 50% and 100% hydrolysate, reaching 32% and 30% of the maximum obtainable yield (again considering ethanol as a single product in the calculation), respectively.
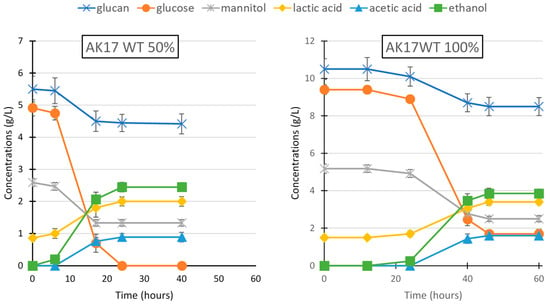
Figure 1.
Fermentation of L. digitata hydrolysate by Thermoanaerobacterium strain AK17 either diluted (left) or pure (right). Substrates and fermentation products were measured at a different time during the fermentation. Data represent the average of three replicate experiments.
3.2. Oligosaccharide Degradation and Uptake by Thermoanaerobacterium Strain AK17
The utilization of glucan indicates extracellular degradation and/or import and utilization of 1,3-β-oligosaccharides. To verify that strain, AK17 produces the enzymes to convert glucan and/or glucan oligosaccharides into glucose, and the presence of relevant activities was investigated, both intracellularly and extracellularly. Thermoanaerobacterium strain AK17 was subsequently grown on cellobiose to induce the expression of genes encoding β-glucanases (extracellularly) and/or β-glucosidases (extracellularly or intracellularly). After extraction and separation of the intracellular and extracellular fractions, the respective fraction was analyzed for the presence of glucanase/glucosidase activity using laminari-oligosaccharides and cello-oligosaccharides as substrates. The results are presented in Figure 2. The intracellular enzyme fraction of strain AK17 had clear β-glucosidase activity and was able to degrade pentaose, triose, and biose from laminarin and cellulose (and a small amount of the gentobiose) to glucose. Intracellular glucosidase activity was hence verified. The extracellular enzyme fraction could clearly hydrolyze pentaose and triose from laminarin to mainly biose, but with weak activity on cellooligosaccharides. These results show that strain AK17 expresses glucanase(s) active on oligosaccharides with β-1,3 linkages and glucosidase(s) active on β-1,3 and β-1,4 linkages (with minor activity on the gentobiose β-1,6 linkage). The data also show that the main extracellular product is biose, indicating an uptake system for bioses in strain AK17.
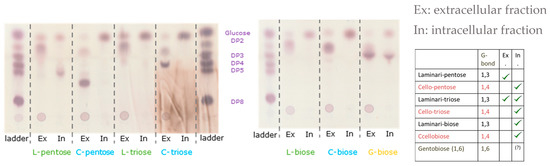
Figure 2.
Glucosidase activity of intracellular (In) and extracellular (Ex) fraction of AK17 on different oligo-laminarin and oligo-cellulose. From left to right, different substrates were tested: laminaripentose (L-pentose), cellopentose (C-pentose), laminaritriose (L-triose), cellotriose (C-triose), laminaribiose (L-biose), cellobiose (C-biose), and gentobiose (G-biose).
3.3. L. digitata Hydrolysate Utilization by Clostridial Strains for Production of A/IBE
The hydrolysate was also tested for ABE and IBE production by Clostridium species. The strains selected for this study are characterized by a wide substrate range. Fermentation studies were carried out on a small scale using media containing pure sugars (glucose and mannitol) as control, and hydrolysate alone (H) or supplemented with salts and nutrients as in CM2 medium (HSN) as described in the material and methods section. The results of the fermentations on the hydrolysate-based media are shown in Table 3 and Figure 3. In addition, all the fermentation data have been compiled in Supplementary Table S1.

Table 3.
Fermentation yields on L. digitata hydrolysate by C. acetobutylicum ATCC 824 and C. beijerinckii strains NCIMB 8052 and DSM6423 on different conditions: raw hydrolysate (H), hydrolysate with addition of salts and nutrients (HSN), compared to fermentation on pure sugars mixtures containing mannitol (4 g/L) and glucose (22 g/L) (control). Substrate concentration was 24–26 g/L. Data from 6 days of cultivation. Data represent the average of two replicate experiments.
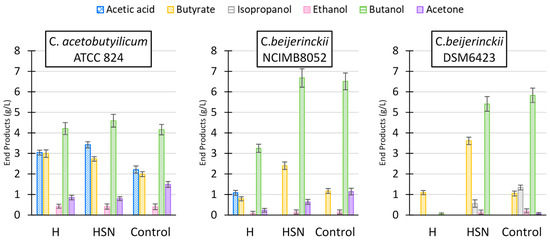
Figure 3.
Fermentation products of L. digitata hydrolysate by C. acetobutylicum ATCC 824 and C. beijerinckii strains NCIMB 8052 and DSM 6423 at different conditions: raw hydrolysate (H), hydrolysate with addition of salts and nutrients (HSN), compared to fermentation on pure sugars (control). Substrate concentration was 24–26 g/L. Data from 6 days of cultivation. Data represent the average of two replicate experiments.
For C. beijerinckii strain DSM 6423, the ratio of butanol compared to the other products of the fermentation was significantly higher in cultures grown on hydrolysate compared to the ratios determined in a culture grown on pure sugars (Figure 3). The percentage of butanol in ABE or IBE in the clostridial cultures varied between 91% in C. beijerinckii NCIMB 8052 cultures and 77% found in C. acetobutylicum cultures, in both cases grown on hydrolysate (H). The percentage of butanol found in the hydrolysate-based media (H, HSN) is approximately 10% higher compared to cultures grown on pure sugars (Control). In the case of the culture described by Hou et al., butanol in the ABE total products in the hydrolysate culture corresponded to 89% in weight, while on the culture grown on sugars, the butanol represented 72% of the total ABE. For all three strains studied, the ratios of butanol in ABE or IBE observed in cultures grown on mannitol and glucose mixes were higher than the butanol ratios determined in control cultures grown on glucose as only sugar (Table S1).
C. acetobutylicum ATCC 824 reached an ABE yield of ~0.22 g/g on all conditions, for both total and partial yields, as it consumed almost all the carbon sources available (Table 3). On the other hand, on conditions with added nutrients, C. beijerinckii strain NCIMB 8052 reached a 50% higher ABE partial yield (0.34 g/g), and a slightly lower total yield (0.30 g/g), as it did not use all the available carbon source. In comparison, C. beijerinckii strain DSM6422 was reported to reach an ABE partial yield of 0.53 g/g [24], but a total yield of 0.33 g/g, closer to what was observed with the strain NCIMB 8052. Regarding IBE fermentation by C. beijerinckii strain DSM 6423 on conditions where nutrients were added to the hydrolysate, the partial IBE yield reached 0.29 g/g, with a total IBE yield of 0.23 g/g, as this strain used even less of the carbon source available (Table 3).
4. Discussion
4.1. Strain AK17 and Ethanol Production
The L. digitata hydrolysate obtained after enzymatic hydrolysis of the biomass using cellulases is a complex feedstock that contains soluble free monomeric sugars (glucose and mannitol) and a mixture of soluble glucose-containing oligosaccharides and laminarin. Uronic acids were not quantified using the methodology by Hou et al., 2017 [26], and alginate content was not determined. When feeding the hydrolysate as a substrate to a standard industrial strain such as S. cerevisae, only the free glucose was consumed, which represents 38% of all the carbon sources available. The ethanol yield was also lower (72%) than the one usually seen in Saccharomyces, which could be explained by the harsh conditions of the hydrolysate (presence of ash, salt, antioxidants, or other inhibiting substances). Comparable yields have been obtained in other studies. For instance, bioethanol yields of 71–76% were achieved by S. cerevisiae PE-2 using Sargassum spp as biomass [35,36]. Similarly, bioethanol yields of 74–84% were achieved by S. cerevisiae KCTC 7906 using Gelidium amansii [37]. In addition, these studies are focused on yeast, which only utilizes the glucose. This highlights the need to use more versatile organisms for the conversion of complex biomass-derived hydrolysates, where a variety of (oligomeric or polymeric) sugars are usually present in mixtures with other components, such as organic acids. Several species of anaerobic bacteria from the genus Clostridium or Thermoanaerobacterium show interesting properties for the utilization of mixed sugar streams for the production of bioalcohols, such as ethanol or butanol [27,38,39,40]. The thermophilic strain Thermoanaerobacterium AK17 was able to use up to 56% of the carbon sources in the L. digitata hydrolysate when this hydrolysate was twofold diluted, which represents a 47% intake increase compared to the yeast, using all the glucose and part of the mannitol and glucans. The limited utilization of mannitol (~50%) compared to glucose, could be explained by the higher reduced form of mannitol, which can lead to a redox imbalance, compared to the more oxidized glucose. Such redox imbalance is common but can be overcome by genetic engineering, for instance, by deleting a transcriptional repressor such as Rex, which has been shown to increase the performance in T. aotearoense SCUT27 [41]. Other Thermoanaerobacterium strains have been reported to utilize mannitol, but not from macroalgal extracts [42], which shows that strain AK17 could be a good candidate for this purpose from the genus Thermoanaerobacterium.
On the pure hydrolysate, strain AK17 did not use all the glucose (82%). The strain has previously been shown to be sensitive to glucose load higher than 5 g/L [38], which could explain the reduced conversion of free glucose. Strain AK17 was also capable of metabolizing the carbon source of a higher degree of polymerization, using part (~20%) of the glucans under both conditions. The unchanged glucan utilization at a higher concentration of hydrolysate indicates hydrolysis of polysaccharides, and an uptake of oligosaccharides that is independent of the mechanism controlling glucose uptake. This is further strengthened by the data, which showed that strain AK17 was able to express extracellular glucanases that can specifically degrade laminari-oligosaccharides to laminaribiose, which means that it could partially use the remaining laminari-oligosaccharides present in the hydrolysate, followed by uptake of the produced biose. Extracellular β-glucanases active on cello-oligosaccharides appeared to not be present (or displaying very low activity on this linkage) in the extracellular fraction. However, strain AK17 was able to grow on cellobiose (data not shown), which indicates presence of the required transporter, which is likely responsible for the uptake of both cellobiose and laminaribiose, and may also import, e.g., laminari-oligosaccharides, up to a degree of polymerization of five (DP5). Intracellularly, such oligosaccharides are then hydrolyzed to glucose and flux into the glycolysis. This is the first time a Thermoanaerobacterium strain has been shown to be using β-1,3 glucans as a carbohydrate source.
Regarding the fermentation products, strain AK17 produces both acetic and lactic acid in addition to ethanol, which decreases the overall ethanol yield of the fermentation, as around 40% of the carbon sources are fluxed into these organic acids. Nonetheless, the ethanol yields (partial) reached ~60% of the theoretical maximum yield, which is close to what has been previously reported [38]. However, for industrial purposes, the strain AK17 would need to be engineered to increase ethanol production and decrease the production of residues (unused substrates) and side products (acetic acid, lactic acid).
4.2. Butanol Production by Mesophilic Clostridia
Strains of C. acetobutylicum and C. beijerinckii grown in control media with mixed glucose and mannitol at similar levels as those in the L. digitata hydrolysate showed the utilization of both sugars and production of butanol as the main product. The utilization of mannitol as a substrate for solventogenic clostridial strains is not well studied, but there are several studies that show the ability of clostridial strains to utilize mannitol as the sole carbon source for the production of butanol [43]. Mannitol as co-substrate with glucose or acetate has also been reported to enhance the production of butyrate to high yields [44,45] and the production of acetone, butanol, and ethanol has been reported as well [30,46] in control media. The C. beijerinckii strains showed increased mannitol utilization when grown on lower glucose starting concentrations (Table S1), which suggests that glucose plays a role in the regulation of the mannitol utilization pathway; however, this needs to be further studied.
The Clostridial strains tested utilized the soluble glucans in the hydrolysate. This is in agreement with the findings of Hou et al. (2017) [26] using a different solventogenic strain on the same hydrolysate, and with a previous study from Hueseman and co-workers using a hydrolysate from Saccharina latissima, a brown seaweed, where partial glucan consumption was observed [47]. Growth of C. beijerinckii on purified laminarin as the sole carbon source and production of butanol has also been confirmed in another experiment (data not shown). The ability of Clostridial strains to use laminarin or laminarin-derived oligosaccharides directly for growth and butanol production opens possibilities for the processing of brown seaweeds without the need of enzymes for the hydrolysis of this polymer into monomeric sugars.
On hydrolysate media, the substrate consumption in cultures of C. acetobutylicum was higher than that observed in C. beijerinckii cultures. However, the ABE yields reached in C. acetobutylicum cultures was lower than the yields observed in the cultures by the C beijerinckii strains, due to the formation of butyrate as a by-product. In addition, the nutrient requirement of the strains appears to be different, and the C. beijerinckii strains grew better on hydrolysate supplemented with nutrients as in the control medium, compared to when cultivated on hydrolysate alone.
The three Clostridial strains used in this study utilized glucan present in the hydrolysate, as was observed earlier for strain C. beijerinckii 6422 [26]. It is well known that a number of solventogenic strains have the capacity to degrade some of the polymers present in plant biomasses, such as starch, or xylan [48,49,50]. In addition, the utilization of lichenan, a complex glucan present in lichens, and of laminarin by C. acetobutylicum has been reported previously [47,49], which makes the use of these strains for conversion of brown seaweed biomasses very interesting and, as stated above, removes the need of enzymes for hydrolysis of this polymer.
In the hydrolysate, a low concentration of lactic acid is present (approx. 1.2 g/L). Interestingly, all Clostridial strains tested were able to consume the lactic acid, in accordance with the observation by Hou et al. for strain DSM6422 [26]. This is an interesting result in relation to the possibilities for using ensiled seaweed as feedstock, since, during ensiling, some of the sugars in the biomass are fermented into lactic acid with the resultant drop in pH that protects the seaweed biomass from decay. If this lactic acid can be converted into ABE or IBE, then the sugars are not completely lost for bioconversion. It has been previously reported that lactic acid utilization by solventogenic Clostridia results in butanol production, contributing to reaching higher ratios of butanol compared to when grown on sugars [51].
The fermentation of mannitol and glucose mixtures resulted as well in higher butanol ratios compared to the ratios obtained on cultures grown on glucose as only sugar. Mannitol fermentation by some Clostridium strains have been reported to result in higher butanol production [43,52] compared to growth on glucose. In these studies, the higher butanol levels could be explained by the use of the butanol production pathway as a sink for reducing equivalents generated by the mannitol metabolism. Higher butanol ratios on mannitol/glucose mixtures compared to glucose-only cultures have been also reported on hydrolysates of the brown seaweed S. latissima hydrolysates fermented by C. acetobutylicum [30,47]. In addition to the different butanol ratios in the solvent mix, in all mannitol-containing cultures, higher production of butyric acid was observed. This higher formation of butyric acid has been associated with the need of disposing of the NADH generated during the conversion step of mannitol-1-phosphate to fructose 6-phosphate by mannitol 1-phosphate dehydrogenase, and the use of acetate as co-substrate. This NADH could be utilized in the conversion of acetate to butyrate via aceto-acetyl-CoA as a hydrogen acceptor [52,53]. When fermenting glucose/mannitol mixtures, a higher butyrate/acetate ratio can be expected compared to glucose-only conditions, as we observe in this study. As an example, the butyrate concentrations reached in glucose/mannitol control cultures by C. beijerinckii NCIMB 8052 were of approx. 1.2 g/L, higher than the 0.7 g/L of butyrate produced in the control cultures on glucose by this strain (Table S1). The product levels and ratios of butanol and organic acids expected to be produced on hydrolysates of biomasses in general, including hydrolysates of seaweeds, depend not only on the sugar content, but also on the interactions of the bacteria with the components of the hydrolysate. In this study, the L. digitata hydrolysate used as a substrate is relatively well fermentable without purification steps by the clostridial strains tested. This is not always the case, and a similar hydrolysate from the brown seaweed S. latissima required extensive purification steps, including desalting and removal of hydrophobic compounds, to allow efficient fermentation by C. acetobutylicum [30].
5. Conclusions
Compared to S. cerevisiae, Thermoanaerobacterium AK17 was able to utilize a wider range or a greater part of the soluble carbohydrates after enzymatic hydrolysis of L. digitata biomass. A substantial part of the carbohydrate substrate was catabolically converted to acetic acid and lactic acid by strain AK17, but still, the ethanol yields on total sugars were comparable to the yields from S. cerevisiae fermentation. The Clostridium strains are other examples of microorganisms that efficiently use the carbohydrate substrate of the seaweed material and produce several products of interest. This utilization of the seaweed feedstock shows that strain AK17, as well as the Clostridia, is a promising microorganism for the biorefining of this marine resource. Further improvement in the yield of individual products from these microorganisms may also be reached by metabolic engineering, directing the carbon flux from the catabolic conversion of organic acids and alcohol mixtures, towards single products. Although seaweed cultivation techniques still need to be optimized to be more cost-effective and more sustainable, we have shown here that the biotechnological processes are developing and could contribute to the valorization of this promising biomass feedstock for biorefinery.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/fermentation9010059/s1, Table S1: Fermentation data on L. digitata hydrolysate by C. acetobutylicum ATCC 824 and C. beijerinckii strains NCIMB 8052 and DSM 6423.
Author Contributions
Conceptualization, A.M.L.-C., G.Ó.H. and Ó.H.F.; methodology, A.M., M.B., X.H., J.Ö. and A.-B.B.; analysis, A.M., L.A. and E.N.K.; investigation, Ó.H.F. and A.M.L.-C.; resources, G.Ó.H., A.M.L.-C. and A.-B.B.; writing—original draft preparation, A.M.; writing—review and editing, A.M., Ó.H.F., G.Ó.H., E.N.K. and A.M.L.-C.; supervision, G.Ó.H.; project administration, A.-B.B. and G.Ó.H.; funding acquisition, A.-B.B., G.Ó.H. and A.M.L.-C. All authors have read and agreed to the published version of the manuscript.
Funding
This project is by the European Union’s Horizon 2020 research and innovation program under grant agreement No 654010 (EU MACROFUELS project). A doctoral student grant from RANNÍS (No 185126-051) was also attributed to A.M.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The author would like to thank Truus de Vrije for fermentation assistance and useful discussions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- BP Statistical Review of World Energy Globally Consistent Data on World Energy Markets and Authoritative Publications in the Field of Energy The Statistical Review World of World Energy and Data on World Energy Markets from Is The Review Has Been Providing. 2020, 66. Available online: https://www.bp.com/content/dam/bp/business-sites/en/global/corporate/pdfs/energy-economics/statistical-review/bp-stats-review-2020-full-report.pdf (accessed on 1 November 2022).
- Bentsen, N.S.; Felby, C. Biomass for energy in the European Union—A review of bioenergy resource assessments. Biotechnol. Biofuels 2012, 5, 25. [Google Scholar] [CrossRef]
- Kelly, M.S.; Dworjanyn, S. The Potential of Marine Biomass for Anaerobic Biogas Production; Crown Estate: London, UK, 2008; ISBN 978-1-906410-05-6. [Google Scholar]
- Tedesco, S.; Stokes, J. Valorisation to biogas of macroalgal waste streams: A circular approach to bioproducts and bioenergy in Ireland. Chem. Pap. 2017, 71, 721–728. [Google Scholar] [CrossRef]
- Zabed, H.; Sahu, J.N.; Boyce, A.N.; Faruq, G. Fuel ethanol production from lignocellulosic biomass: An overview on feedstocks and technological approaches. Renew. Sustain. Energy Rev. 2016, 66, 751–774. [Google Scholar] [CrossRef]
- Chung, I.K.; Beardall, J.; Mehta, S.; Sahoo, D.; Stojkovic, S. Using marine macroalgae for carbon sequestration: A critical appraisal. J. Appl. Phycol. 2011, 23, 877–886. [Google Scholar] [CrossRef]
- Adams, J.M.; Gallagher, J.A.; Donnison, I. Fermentation study on Saccharina latissima for bioethanol production considering variable pre-treatments. J. Appl. Phycol. 2009, 21, 569–574. [Google Scholar] [CrossRef]
- Minh, T.H.; Hanh, V. Bioethanol production from marine algae biomass: Prospect and troubles. J. Vietnam. Environ. 2012, 3, 25–29. [Google Scholar] [CrossRef]
- Kawai, S.; Murata, K. Biofuel Production Based on Carbohydrates from Both Brown and Red Macroalgae: Recent Developments in Key Biotechnologies. Int. J. Mol. Sci. 2016, 17, 145. [Google Scholar] [CrossRef]
- Manns, D.; Deutschle, A.L.; Saake, B.; Meyer, A.S. Methodology for quantitative determination of the carbohydrate composition of brown seaweeds (Laminariaceae). RSC Adv. 2014, 4, 25736–25746. [Google Scholar] [CrossRef]
- Manns, D.; Nielsen, M.M.; Bruhn, A.; Saake, B.; Meyer, A.S. Compositional variations of brown seaweeds Laminaria digitata and Saccharina latissima in Danish waters. J. Appl. Phycol. 2017, 29, 1493–1506. [Google Scholar] [CrossRef]
- Ferdouse, F.; Løvstad Holdt, S.; Smith, R.; Murúa, P.; Yang, Z. The Global Status of Seaweed Production, Trade and Utilization. FAO Globefish Res. Program. 2018, 124, 120. [Google Scholar]
- Radulovich, R.; Neori, A.; Valderrama, D.; Reddy, C.R.K.; Cronin, H.; Forster, J. Farming of Seaweeds; Elsevier Inc.: Amsterdam, The Netherlands, 2015; ISBN 9780124199583. [Google Scholar]
- Kostas, E.T.; Adams, J.M.M.; Ruiz, H.A.; Durán-Jiménez, G.; Lye, G.J. Macroalgal biorefinery concepts for the circular bioeconomy: A review on biotechnological developments and future perspectives. Renew. Sustain. Energy Rev. 2021, 151, 111553. [Google Scholar] [CrossRef]
- Montingelli, M.E.; Tedesco, S.; Olabi, A.G. Biogas production from algal biomass: A review. Renew. Sustain. Energy Rev. 2015, 43, 961–972. [Google Scholar] [CrossRef]
- Yan, L.; Wang, Y.; Li, J.; Zhang, Y.; Ma, L.; Fu, F.; Chen, B.; Liu, H. Hydrothermal liquefaction of Ulva prolifera macroalgae and the influence of base catalysts on products. Bioresour. Technol. 2019, 292, 121286. [Google Scholar] [CrossRef]
- Yuan, C.; Wang, S.; Cao, B.; Hu, Y.; Abomohra, A.E.-F.; Wang, Q.; Qian, L.; Liu, L.; Liu, X.; He, Z.; et al. Optimization of hydrothermal co-liquefaction of seaweeds with lignocellulosic biomass: Merging 2nd and 3rd generation feedstocks for enhanced bio-oil production. Energy 2019, 173, 413–422. [Google Scholar] [CrossRef]
- Maneein, S.; Milledge, J.J.; Nielsen, B.V.; Harvey, P.J. A Review of Seaweed Pre-Treatment Methods for Enhanced Biofuel Production by Anaerobic Digestion or Fermentation. Fermentation 2018, 4, 100. [Google Scholar] [CrossRef]
- Li, J.; He, Z.; Liang, Y.; Peng, T.; Hu, Z. Insights into Algal Polysaccharides: A Review of Their Structure, Depolymerases, and Metabolic Pathways. J. Agric. Food Chem. 2022, 70, 1749–1765. [Google Scholar] [CrossRef]
- Hreggviðsson, G.; Nordberg-Karlsson, E.M.; Tøndervik, A.; Aachmann, F.L.; Dobruchowska, J.M.; Linares-Pastén, J.; Daugbjerg-Christensen, M.; Moenaert, A.; Kristjansdottir, T.; Sletta, H.; et al. Biocatalytic refining of polysaccharides from brown seaweeds. Sustain. Seaweed Technol. 2020, 2020, 447–504. [Google Scholar] [CrossRef]
- Lee, O.K.; Lee, E.Y. Sustainable production of bioethanol from renewable brown algae biomass. Biomass Bioenergy 2016, 92, 70–75. [Google Scholar] [CrossRef]
- Jiang, R.; Ingle, K.N.; Golberg, A. Macroalgae (seaweed) for liquid transportation biofuel production: What is next? Algal Res. 2016, 14, 48–57. [Google Scholar] [CrossRef]
- Ji, S.-Q.; Wang, B.; Lu, M.; Li, F.-L. Direct bioconversion of brown algae into ethanol by thermophilic bacterium Defluviitalea phaphyphila. Biotechnol. Biofuels 2016, 9, 81. [Google Scholar] [CrossRef]
- Kim, N.-J.; Li, H.; Jung, K.; Chang, H.N.; Lee, P.C. Ethanol production from marine algal hydrolysates using Escherichia coli KO11. Bioresour. Technol. 2011, 102, 7466–7469. [Google Scholar] [CrossRef]
- Enquist-Newman, M.; Faust, A.M.E.; Bravo, D.D.; Santos, C.N.S.; Raisner, R.M.; Hanel, A.; Sarvabhowman, P.; Le, C.; Regitsky, D.D.; Cooper, S.R.; et al. Efficient ethanol production from brown macroalgae sugars by a synthetic yeast platform. Nature 2013, 505, 239–243. [Google Scholar] [CrossRef]
- Hou, X.; From, N.; Angelidaki, I.; Huijgen, W.J.J.; Bjerre, A.-B. Butanol fermentation of the brown seaweed Laminaria digitata by Clostridium beijerinckii DSM-6422. Bioresour. Technol. 2017, 238, 16–21. [Google Scholar] [CrossRef]
- Xue, C.; Cheng, C. Butanol production by Clostridium. Adv. Bioenergy 2019, 4, 35–77. [Google Scholar] [CrossRef]
- Contreras, A.M.L.; Kuit, W.; Siemerink, M.A.J.; Kengen, S.W.M.; Springer, J.; Claassen, P.A.M. Production of longer-chain alcohols from lignocellulosic biomass: Butanol, isopropanol and 2,3-butanediol. Bioalcohol. Prod. Biochem. Convers. Lignocellul. Biomass 2010, 415–460. [Google Scholar] [CrossRef]
- Hon, S.; Olson, D.G.; Holwerda, E.K.; Lanahan, A.A.; Murphy, S.J.L.; Maloney, M.I.; Zheng, T.; Papanek, B.; Guss, A.M.; Lynd, L.R. The ethanol pathway from Thermoanaerobacterium saccharolyticum improves ethanol production in Clostridium thermocellum. Metab. Eng. 2017, 42, 175–184. [Google Scholar] [CrossRef]
- Schultze-Jena, A.; Vroon, R.C.; Macleod, A.K.A.; Hreggviðsson, G.; Adalsteinsson, B.T.; Engelen-Smit, N.P.E.; de Vrije, T.; Budde, M.A.W.; van der Wal, H.; López-Contreras, A.M.; et al. Production of acetone, butanol, and ethanol by fermentation of Saccharina latissima: Cultivation, enzymatic hydrolysis, inhibitor removal, and fermentation. Algal Res. 2022, 62, 102618. [Google Scholar] [CrossRef]
- Koskinen, P.E.P.; Beck, S.R.; Örlygsson, J.; Puhakka, J.A. Ethanol and hydrogen production by two thermophilic, anaerobic bacteria isolated from Icelandic geothermal areas. Biotechnol. Bioeng. 2008, 101, 679–690. [Google Scholar] [CrossRef]
- Sveinsdottir, M.; Beck, S.R.; Orlygsson, J. Ethanol Production from Monosugars and Lignocellulosic Biomass by Thermophilic Bacteria Isolated from Icelandic Hot Springs. Icel. Agric. Sci. 2009, 22, 45–58. [Google Scholar]
- Van Wychen, S.; Laurens, L.M.L. Determination of Total Carbohydrates in Algal Biomass: Laboratory Analytical Procedure (LAP); National Renewable Energy Laboratory: Golden, CO, USA, 2015; p. 18.
- Allahgholi, L.; Sardari, R.R.R.; Hakvåg, S.; Ara, K.Z.G.; Kristjansdottir, T.; Aasen, I.M.; Fridjonsson, O.H.; Brautaset, T.; Hreggvidsson, G.O.; Karlsson, E.N. Composition analysis and minimal treatments to solubilize polysaccharides from the brown seaweed Laminaria digitata for microbial growth of thermophiles. J. Appl. Phycol. 2020, 32, 1933–1947. [Google Scholar] [CrossRef]
- Aparicio, E.; Rodríguez-Jasso, R.M.; Pinales-Márquez, C.D.; Loredo-Treviño, A.; Robledo-Olivo, A.; Aguilar, C.N.; Kostas, E.T.; Ruiz, H.A. High-pressure technology for Sargassum spp biomass pretreatment and fractionation in the third generation of bioethanol production. Bioresour. Technol. 2021, 329, 124935. [Google Scholar] [CrossRef] [PubMed]
- González-Gloria, K.D.; Rodríguez-Jasso, R.M.; Rosero-Chasoy, G.; Shiva; Kostas, E.T.; Aparicio, E.; Sanchez, A.; López-Sandin, I.; Ruiz, H.A. Scale-up of hydrothermal processing: Liquid hot water and pilot-scale tubular steam explosion batch reactor for bioethanol production using macroalgae Sargassum spp biomass. Bioresour. Technol. 2023, 369, 128448. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Wi, S.G.; Jung, S.; Song, Y.; Bae, H.-J. Efficient approach for bioethanol production from red seaweed Gelidium amansii. Bioresour. Technol. 2015, 175, 128–134. [Google Scholar] [CrossRef]
- Almarsdottir, A.R.; Sigurbjornsdottir, M.A.; Orlygsson, J. Effect of various factors on ethanol yields from lignocellulosic biomass by Thermoanaerobacterium AK17. Biotechnol. Bioeng. 2012, 109, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Di Donato, P.; Finore, I.; Poli, A.; Nicolaus, B.; Lama, L. The production of second generation bioethanol: The biotechnology potential of thermophilic bacteria. J. Clean. Prod. 2019, 233, 1410–1417. [Google Scholar] [CrossRef]
- Taylor, M.P.; Eley, K.L.; Martin, S.; Tuffin, M.I.; Burton, S.G.; Cowan, D.A. Thermophilic ethanologenesis: Future prospects for second-generation bioethanol production. Trends Biotechnol. 2009, 27, 398–405. [Google Scholar] [CrossRef]
- Qu, C.; Chen, L.; Li, Y.; Fu, H.; Wang, J. The redox-sensing transcriptional repressor Rex is important for regulating the products distribution in Thermoanaerobacterium aotearoense SCUT27. Appl. Microbiol. Biotechnol. 2020, 104, 5605–5617. [Google Scholar] [CrossRef]
- Chades, T.; Scully, S.M.; Ingvadottir, E.M.; Orlygsson, J. Fermentation of Mannitol Extracts From Brown Macro Algae by Thermophilic Clostridia. Front. Microbiol. 2018, 9, 1931. [Google Scholar] [CrossRef]
- Heyndrickx, M.; De Vos, P.; Ley, J. De Fermentation Characteristics of Clostridium Pasteurianum LMG 3285 Grown on Glucose and Mannitol. J. Appl. Microbiol. 1991, 70, 52–58. [Google Scholar]
- Fu, H.; Hu, J.; Guo, X.; Feng, J.; Zhang, Y.; Wang, J. High-Selectivity Butyric Acid Production from Saccharina japonica Hydrolysate by Clostridium tyrobutyricum. Ind. Eng. Chem. Res. 2020, 59, 17147–17155. [Google Scholar] [CrossRef]
- Oh, H.J.; Kim, K.-Y.; Lee, K.M.; Lee, S.-M.; Gong, G.; Oh, M.-K.; Um, Y. Enhanced butyric acid production using mixed biomass of brown algae and rice straw by Clostridium tyrobutyricum ATCC25755. Bioresour. Technol. 2019, 273, 446–453. [Google Scholar] [CrossRef] [PubMed]
- López-Contreras, A.M.; Harmsen, P.F.H.; Blaauw, R.; Houweling-Tan, B.; Van Der Wal, H.; Huijgen, W.J.J.; Van Hal, J.W. Biorefinery of the Brown Seaweed Saccharina Latissima for Fuels and Chemicals. In Proceedings of the Mie Bioforum on lignocellulose degradation and biorefinery, Mie Prefecture, Japan, 18–21 November 2014; pp. 7–8. [Google Scholar]
- Huesemann, M.H.; Kuo, L.-J.; Urquhart, L.; Gill, G.; Roesijadi, G. Acetone-butanol fermentation of marine macroalgae. Bioresour. Technol. 2012, 108, 305–309. [Google Scholar] [CrossRef]
- Mitchell, W.J.; Albasheri, K.A.; Yazdanian, M. Factors affecting utilization of carbohydrates by clostridia. FEMS Microbiol. Rev. 1995, 17, 317–329. [Google Scholar] [CrossRef]
- López-Contreras, A.M.; Claassen, P.A.M.; Mooibroek, H.; De Vos, W.M. Utilisation of Saccharides in Extruded Domestic Organic Waste by Clostridium Acetobutylicum ATCC 824 for Production of Acetone, Butanol and Ethanol. Appl. Microbiol. Biotechnol. 2000, 54, 162–167. [Google Scholar] [CrossRef]
- Madihah, M.S.; Ariff, A.B.; Sahaid, K.M.; Suraini, A.A.; Karim, M.I.A. Direct fermentation of gelatinized sago starch to acetone–butanol–ethanol by Clostridium acetobutylicum. World J. Microbiol. Biotechnol. 2001, 17, 567–576. [Google Scholar] [CrossRef]
- Yoshida, T.; Tashiro, Y.; Sonomoto, K. Novel high butanol production from lactic acid and pentose by Clostridium saccharoperbutylacetonicum. J. Biosci. Bioeng. 2012, 114, 526–530. [Google Scholar] [CrossRef]
- Heyndrickx, M.; De Vos, P.; Speybrouck, A.; De Ley, J. Fermentation of mannitol by Clostridium butyricum: Role of acetate as an external hydrogen acceptor. Appl. Microbiol. Biotechnol. 1989, 31, 323–328. [Google Scholar] [CrossRef]
- Crabbendam, P.M.; Neijssel, O.M.; Tempest, D.W. Metabolic and energetic aspects of the growth of Clostridium butyricum on glucose in chemostat culture. Arch. Microbiol. 1985, 142, 375–382. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).