Abstract
Kefir, a traditional probiotic beverage with significant cultural, social, and health relevance, has garnered increasing scientific interest for its functional properties. Here, we synthesized findings from 14 studies investigating the bacterial and fungal diversity in artisanal cow’s milk kefir through metagenomic analysis. Following the PRISMA guidelines (Preferred Reporting Items for Systematic Reviews and Meta-Analyses), a comprehensive search was conducted in databases including Portal BVS, Scopus, Scielo, and Web of Science. From an initial pool of 522 articles, 14 were selected based on stringent inclusion and exclusion criteria, focusing on English-written studies. Key terms such as “kefir milk”, “artisanal kefir”, “milk”, “metagenomics”, and “cow” were identified through Boolean searches over the last five years. This review addresses the growing need for research on the microbial diversity of artisanal cow’s milk kefir from various global regions. The results indicate a remarkable diversity in microbial communities, primarily dominated by bacteria from the phylum Firmicutes (notably Lactobacillus) and yeasts from the genera Saccharomyces and Kluyveromyces. These microbial compositions are shaped by factors such as milk type, production methods, and grain handling practices, reflecting regional adaptations and influencing kefir’s sensory, probiotic, and functional properties. We argue that a full understanding of these microbial dynamics is critical for standardizing production processes and enhancing quality control measures, ultimately ensuring artisanal kefir’s consistency and health benefits.
1. Introduction
Kefir is a traditional fermented beverage obtained from kefir grains, a symbiotic matrix of bacteria, yeasts, and polysaccharides [1]. The grains, which originated in regions such as the Caucasus Mountains, Tibet, and Mongolia, harbor a complex microbial diversity that varies significantly across different geographical cultures [2,3] and maintaining the stability of their microbiome is crucial for ensuring the quality and functional properties of the final product, especially on an industrial scale [4].
Kefir is widely recognized for its probiotic and therapeutic properties, offering a variety of health benefits, such as modulation of the intestinal microbiota, regulation of the immune system, and anti-allergic, antimicrobial, antitumor, anti-inflammatory and antioxidant effects. In addition, it can help prevent conditions such as carcinogenesis, diabetes and inflammation and also has curative effects, making it a functional food with considerable therapeutic potential [2,5,6,7,8,9,10,11,12,13,14].
According to the definition of the Food and Agriculture Organization (FAO) and the World Health Organization (WHO), kefir is classified as a natural probiotic beverage due to its microbial composition, which includes several species of lactic acid bacteria (LAB) with recognized health-promoting properties [15,16]. Therefore, the characterization of the microorganisms present in artisanal kefir is essential for an in-depth understanding of its potential therapeutic effects.
The metabolites produced during kefir fermentation, such as organic acids, ethanol, carbon dioxide and bioactive peptides, play a crucial role in defining the functional and sensory properties of the drink. Organic acids such as lactic acid and acetic acid are responsible for reducing the pH of kefir, as well as exerting antimicrobial action, inhibiting pathogens. Ethanol and carbon dioxide, products of the yeast’s metabolic activity, are responsible for the drink’s effervescent characteristic and distinctive texture. Bioactive peptides resulting from the degradation of milk proteins by bacteria, in turn, have antioxidant, antimicrobial and immunomodulatory activities, with the potential to regulate the body’s inflammatory response [17,18].
In addition, lactic fermentation not only improves the digestibility of the proteins present in milk, but also favors the production of exopolysaccharides, including kefiran, which gives kefir prebiotic properties [19]. The conversion of lactose into lactic acid, promoted by lactic acid bacteria, makes kefir a suitable food alternative for individuals with lactose intolerance, relieving gastrointestinal symptoms [20]. These secondary metabolites, derived from the joint action of bacteria and yeasts, also play an important role in modulating the intestinal microbiota, consolidating kefir as a functional food with therapeutic potential and enhanced benefits for human health [21].
Although our study provides valuable insights into the microbial diversity of artisanal kefir, further research is needed to directly evaluate the probiotic properties of the identified microorganisms and their metabolic outputs. Future studies focusing on the functional characterization of these microbes and their bioactive compounds will be essential to fully understand kefir’s therapeutic potential.
Despite kefir grains being fundamental to traditional kefir production, they are not typically used in commercial manufacturing due to the challenges of maintaining them. Industrial kefir is usually produced using starter cultures, which, while offering health benefits, may not fully replicate the microbial diversity of artisanal kefir [22]. Artisanal kefir, made on a smaller scale using traditional grains, exhibits considerable variation in flavor, texture, and nutritional composition, influenced by local practices and techniques specific to each region [23,24].
Several factors influence the microbial composition and diversity of kefir grains, including the type of milk used, fermentation conditions, geographical origin of the grains, and processing methods. For example, milk from different sources, such as cow’s milk or goat’s milk, significantly alters the microbial communities within kefir grains [25,26]. These factors are critical when assessing microbial dynamics during kefir grain production and revitalization. Furthermore, microbial interactions may vary throughout the storage time of kefir. Ströher et al. [27] analyzed the microbiota of artisanal cow’s milk kefir in southern Brazil for 30 days, observing a reduction in Enterococcus and Acetobacter, while Lactobacillus, Lactococcus, Lentilactobacillus and Leuconostoc remained stable. Among the fungi, Kazachstania initially predominated, but was replaced by Penicillium and Aspergillus. These findings highlight temporal variations in microbial composition, reinforcing the importance of longitudinal studies for a more comprehensive understanding of kefir fermentation dynamics. The analyses confirm the potential for standardizing and marketing artisanal kefir, guaranteeing its safety for up to 30 days.
In recent decades, metagenomic analysis has emerged as a powerful tool for characterizing microbial communities in kefir grains and beverages. This approach not only reveals microbial diversity but also provides precise identification of species and strains, uncovering aspects that traditional culture-dependent methods often miss [28,29,30]. Advanced sequencing technologies have been instrumental in detecting both dominant microbial groups and minority populations that play crucial roles in kefir’s microbial ecology [16]. These insights open up new possibilities for health applications and innovation in the food industry [31,32].
Expanding research on the microbiota of kefir grains and artisanal kefir is vital, as current studies remain limited. Understanding how microbial composition is shaped by regional adaptations to environmental and processing conditions is key to advancing scientific knowledge, improving production processes, and standardizing artisanal kefir production. These efforts will not only enhance quality control strategies but also maximize the health benefits of this probiotic beverage.
2. Materials and Methods
The present study was conducted following the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines to ensure a systematic and trans-parent selection process for included articles [33]. Eligibility criteria were established to guarantee the relevance and quality of the selected studies.
2.1. Eligibility Criteria
Only articles published within the last five years were included, ensuring that the data reflected the latest advancements, technological developments, and scientific trends in metagenomic studies applied to artisanal kefir. The inclusion was further restricted to studies written in English, allowing access to high-quality publications with international reach and peer-reviewed standards.
Additionally, only studies with full-text availability were considered, ensuring a thorough examination of the methodologies and results reported, thereby avoiding potential interpretative gaps due to incomplete data. The studies selected had to specifically investigate the bacterial and fungal diversity of artisanal cow’s milk kefir using metagenomic approaches, which were chosen for their high precision in identifying microbial compositions, including those that conventional methods may overlook.
Exclusion criteria were applied to eliminate studies lacking detailed methodological descriptions, as this would hinder the evaluation of scientific validity. Furthermore, studies focusing solely on commercial kefir were excluded, as the analysis was centered on the unique characteristics of artisanal kefir, which is traditionally produced and exhibits greater microbial variability. These stringent criteria ensured that the final sample of analyzed studies consistently aligned with the research objectives, contributing to a robust and reliable systematic review.
2.2. Search Strategy
The search strategy for this study involved a comprehensive approach designed to identify relevant literature systematically. Searches were conducted in well-established scientific databases, including Portal BVS, Scopus, SciELO, and Web of Science. These databases were chosen for their broad coverage of high-quality, peer-reviewed articles across multiple disciplines, ensuring access to the most relevant studies within the field of microbial diversity and metagenomics in artisanal cow’s milk kefir. However, some databases were excluded from the review due to limitations such as restricted access to full-text articles, lack of relevant indexing for metagenomic studies, or low representation of publications on artisanal kefir.
To maximize the relevance and specificity of the retrieved studies, a carefully curated list of keywords was developed. Key terms such as “kefir milk”, “artisanal kefir”, “milk”, “metagenomics” and “cow” were selected based on their direct relation to the study’s scope. These terms were refined and tested iteratively to ensure their effectiveness in capturing a comprehensive dataset of potential articles. Boolean operators were used strategically to enhance the search process, allowing for the combination of terms and the exclusion of unrelated studies. For example, terms were linked using logical connectors like AND, OR, and NOT to create targeted search strings that could navigate the vast datasets within each database efficiently.
A Boolean search is a method that combines keywords using logical operators (AND, OR, NOT) to refine search results effectively. This approach allows the inclusion of synonyms and related terms while excluding irrelevant studies. Some examples are presented below:
- (“kefir milk” OR “artisanal kefir”) AND (“metagenomics” AND “cow”)—Retrieves studies that mention artisanal kefir milk and metagenomics of cow’s milk.
- “bacterial diversity” OR “fungal diversity” AND “artisanal cow’s milk kefir”—Expands the search to include studies on microbial diversity in artisanal kefir.
The search was further refined by applying filters to exclude irrelevant results. These included restricting the time frame to the past five years, limiting the language to English, and focusing on studies with full-text availability. Additional techniques, such as truncation and wildcard operators, were employed to account for variations in spelling and terminology, ensuring no relevant studies were overlooked.
2.3. Study Selection
The study selection process was conducted systematically in three distinct stages to ensure the inclusion of only the most relevant and high-quality articles. Initially, a comprehensive database search yielded 522 articles. These results were then screened to refine the pool of studies, adhering strictly to the predefined eligibility criteria.
In the first stage, the titles and abstracts of all retrieved articles were thoroughly re-viewed. This step aimed to eliminate studies that were clearly outside the scope of the re-view, such as those unrelated to artisanal cow’s milk kefir, microbial diversity, or metagenomic analysis. Additionally, articles that did not meet the inclusion requirements, such as those published in languages other than English or beyond the established five-year time frame, were excluded. Many studies at this stage were disqualified for focusing solely on commercial kefir or lacking relevance to the specific objectives of the re-search.
The second stage involved an in-depth evaluation of the full texts of the remaining articles. At this point, the methodological rigor of each study was carefully assessed to ensure alignment with the review’s goals. Articles that failed to provide sufficient methodological detail or did not explicitly employ metagenomic techniques to analyze the microbial diversity of artisanal kefir were excluded. This stage was crucial in ensuring that only scientifically robust and reliable studies were retained.
In the final stage, duplicate entries resulting from searches across multiple databases were identified and removed. Additionally, articles with incomplete or insufficient data that precluded a thorough analysis were excluded. Since searches were conducted across multiple databases, some articles appeared more than once. To ensure that each study was considered only once, a systematic approach was used for duplicate identification and removal. The retrieved references were imported into Mendeley Reference Manager, which automatically detected and flagged duplicate entries based on matching titles, authors and DOIs. A manual verification was then performed to resolve discrepancies, ensuring that different versions of the same study (e.g., preprints and final published versions) were not counted twice.
After this meticulous process, the final selection comprised 14 studies that fully met the inclusion criteria and were directly aligned with the aims of the systematic review. Figure 1 shows the flow diagram for the identification and selection of articles for the study.
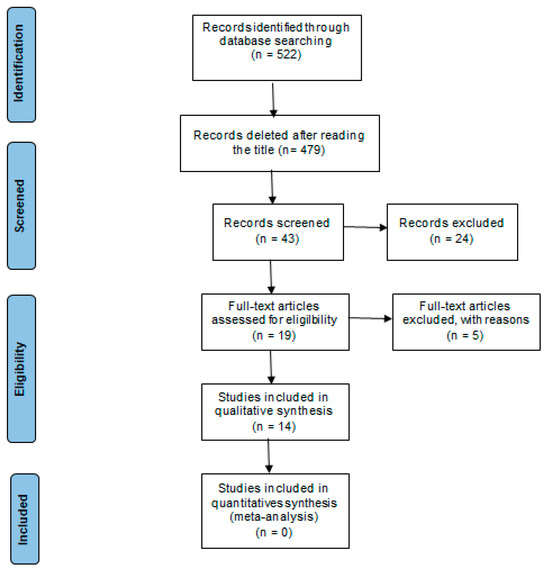
Figure 1.
Flow diagram for the identification and selection of articles for the study, according to the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) methodology.
3. Results and Discussion
This systematic review provides a comprehensive analysis of microbial diversity of artisanal kefir from a diverse range of countries and continents, including South Korea, Ireland, Lithuania, the United Kingdom, the Caucasus region, the United States, Brazil, Bosnia and Herzegovina, Australia, Turkey, Indonesia, Greece, Tibet (China), and Mexico (Figure 2). Recent research has revealed significant variations in the microbial communities of kefir across different geographic regions, highlighting the influence of local environmental, climatic, and cultural factors [34,35,36]. The predominant bacteria identified in kefir grains include genera of lactic acid bacteria such as Lactobacillus (recently reclassified) [37], Lactococcus, Leuconostoc, and Streptococcus. Additionally, common yeast genera found in these grains comprise Kluyveromyces, Candida, Saccharomyces, Kazachstania, and Pichia [38,39]. Notably, the prevalence of these microorganisms can vary significantly depending on local production practices and the specific characteristics of the fermentation substrate [22].
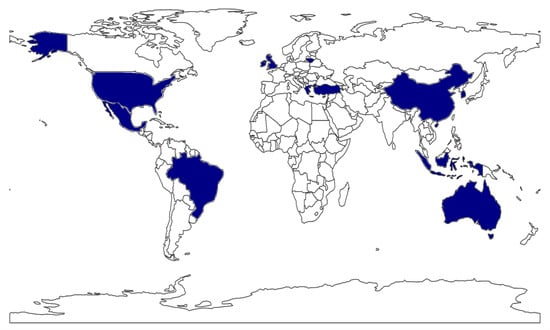
Figure 2.
Global distribution of the kefir studies reviewed in this paper. Countries highlighted in blue represent those with studies included in the review.
The reviewed studies highlight the intricate complexity and diversity of the microbiota present in artisanal kefir and its grains, sourced from various regions around the world. Each study offers a distinct perspective on microbial composition, influenced by geographical factors, production methods, and the types of milk used. The observed diversity within kefir’s bacterial microbiota underscores the complexity of this fermentative system, as evidenced by the various studies analyzed. This diversity not only contributes to the unique sensory qualities of kefir but also influences its potential health benefits, emphasizing the need for further investigation into the relationships between microbial composition and the functional properties of kefir (Table 1).

Table 1.
Analysis of the microbiota of artisanal kefir based on studies selected using the Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) methodology.
Overall, these findings underscore the importance of understanding the microbiological landscape of artisanal kefir. Such knowledge is essential for optimizing production practices, enhancing the health-promoting attributes of kefir, and preserving its traditional character across different cultures.
3.1. Bacterial Analysis of Kefir and Its Grains
A comprehensive comparative analysis of recent literature on kefir microbiota underscores the consistent predominance of the Firmicutes phylum across diverse geographical regions and kefir types. This phylum has been frequently identified in milk kefir grains, as evidenced in several studies [40,41,42,43,44,46,47,51]. The prevalence of Firmicutes in milk kefir grains suggests a level of stability conferred by its constituent lactic acid bacteria, which are crucial for kefir fermentation and contribute significantly to its sensory and probiotic attributes [46].
In addition to Firmicutes, Sindi et al. [40] reported the presence of other phyla, including Proteobacteria, Actinobacteria, Verrucomicrobia, Planctomycetes, and Nitrospirae, in varying proportions across kefir grain samples from South Korea, Ireland, Lithuania, Great Britain, and the Caucasus region. Conversely, Tenorio-Salgado et al. [29] noted that the predominant bacterial phyla in their samples were Actinobacteria and Proteobacteria, with Firmicutes following closely. The study by Ilıkkan and Bagdat [47] corroborated the presence of both Firmicutes and Proteobacteria in all kefir grain samples analyzed. In a regional study, De Almeida Brasiel et al. [41] characterized the bacterial composition of artisanal kefir grains from Viçosa, Brazil, identifying Bacteroidetes and Proteobacteria as the most abundant phyla alongside Firmicutes.
3.2. Dominant Bacterial Families and Genera
Among the bacterial families analyzed, Lactobacillaceae emerged as the most prevalent in the studies by González-Orozco et al., Garofalo et al., and Sumarmono et al. [9,42,45].
In contrast, Kalamaki and Angelidis [46] documented a more diverse family profile, including Lactobacillaceae, Ruminococcaceae, Lachnospiraceae, Bacteroidaceae. Notably, Ilıkkan and Bagdat [47] identified Bifidobacteriaceae as the dominant family, illustrating the complexity of kefir microbiota.
The primary bacterial genera identified across the studies included Lactobacillus, Lentilactobacillus, Lactococcus, Leuconostoc, and Acetobacter, with Lactobacillus being the most abundant. Sindi et al. [40] emphasized the richness of bacterial diversity in kefir, highlighting genera such as Lacticaseibacillus and Acetobacter. Gao and Zhang [49] observed distinct stratification in kefir grains, with rod-shaped Lactobacillus dominating the inner layers, while cocci and yeasts predominated in the outer layers, thus influencing the microbial composition of the final kefir products.
3.3. Bacteria During Fermentation
In a longitudinal study, De Almeida Brasiel et al. [41] reported significant shifts in the bacterial composition of Brazilian milk kefir over 30 days of fermentation. The microbial community was predominantly composed of the phyla Firmicutes, Bacteroidetes, and Proteobacteria. At the family level, Streptococcaceae emerged as the most prevalent group. Furthermore, the genera Leuconostoc, Lactococcus, and Acetobacter were identified as the most abundant within the analyzed samples.
González-Orozco et al. and Liu et al. [9,48] observed a rise in Pseudomonas, raising concerns about food spoilage and potential public health risks, as certain species within this genus can act as opportunistic pathogens [24,52,53,54,55]. Given the widespread domestic consumption of artisanal fermented beverages, monitoring kefir microbiota throughout its shelf life is crucial [56].
Alraddadi et al. [43] noted that Lactococcus emerged as the most prevalent genus in milk kefir, with a concomitant decline in the dominance of Lactobacillus during fermentation. Tenorio-Salgado et al. [29] identified 15 distinct genera in their kefir samples, affirming Lactobacillus and Acetobacter as the most abundant. Gao and Zhang [49] identified several genera in their kefir analyses, while Biçer et al. [51] observed that Lactococcus was the most prevalent in commercial samples, in contrast to the dominance of Streptococcus in artisanal kefir.
3.4. Species Diversity and Probiotic Potential
The diversity of bacterial species in kefir grains and beverages is noteworthy. Sindi et al. [40] reported species such as L. kefiranofaciens, Lentilactobacillus kefiri, L. ultunensis, L. apis, L. gigeriorum, Gluconobacter morbifer, Acetobacter orleanensis, Acetobacter pasteurianus, Acidocella aluminiidurans, and L. helveticus, underscoring the presence of bacteriocin-producing species with potential probiotic and antimicrobial properties [57].
Lentilactobacillus kefiri, a heterofermentative lactic acid bacterium, is recognized for its probiotic capabilities, including the production of lactic acid, acetic acid, ethanol, and carbon dioxide. Its health-promoting potential includes adherence to intestinal mucus and the ability to assimilate cholesterol, with studies indicating therapeutic benefits in gastric cancers and immunomodulatory effects on gut microbiota [58,59,60].
Lactic acid bacteria are known for their production of various antimicrobial substances, including organic acids, free fatty acids, diacetyl, hydrogen peroxide, and bacteriocins. Bacteriocins are a broad class of ribosomally synthesized peptides, predominantly active against bacteria that are phylogenetically close to the producing strain. However, recent evidence indicates the existence of bacteriocins with a broader antimicrobial spectrum [61,62].
3.5. Metagenomic Insights into Kefir Microbiota
Using metagenomic approaches, González-Orozco et al. [9] analyzed kefir grains and beverages from the United States, identifying dominant species such as L. kefiranofaciens, L. helveticus, and Lentilactobacillus kefiri in grains, while L. helveticus, L. kefiranofaciens, Stenotrophomonas maltophilia, and Pseudomonas luteola were predominant in the kefir beverages. Similarly, Garofalo et al. [42] documented the presence of L. kefiranofaciens, Acetobacter syzygii, and Lactococcus lactis, among other species, illustrating significant differences between traditional kefir, re-inoculated kefir, and the grains themselves.
Species such as L. parakefiri and L. kefiranofaciens are frequently associated with kefir, indicating a specific adaptation to the kefir environment. L. parakefiri, first described by Takizawa et al. [63], remains prevalent in milk kefir grains [34,64]. L. helveticus is particularly notable for its complex proteolytic system that enhances the flavor and maturation of kefir. In addition to its probiotic benefits, such as the production of angiotensin-converting enzyme (ACE) inhibitor peptides, L. helveticus demonstrates resilience in challenging industrial conditions while conferring gastrointestinal health benefits [65]. The genetic diversity of L. helveticus facilitates adaptation to varying substrates, which is crucial for consistent kefir production. Furthermore, it modulates bioactive compounds in milk, yielding antimicrobial and antioxidant peptides, thereby positively influencing gut microbiota and immune responses [66].
Cufaoglu and Erdinc [44] highlighted L. parakefiri as the most abundant species in their kefir samples, followed by Ileibacterium valens and L. kefiranofaciens. However, the identification of Ileibacterium valens in kefir raised concerns about contamination, as it had not been previously reported in kefir. While it is known to inhabit fermented dairy products, its presence might be associated with adverse sensory changes in kefir due to the production of undesirable compounds [67,68].
The environmental conditions surrounding kefir production and storage significantly affect microbial composition. For instance, temperature, humidity, and the substrate used for fermentation can influence the proliferation of specific bacterial genera, as seen in the study by Alraddadi et al. [43], which demonstrated that varying these factors could selectively promote or inhibit the growth of certain bacteria within kefir grains.
3.6. Health Implications of Kefir Consumption
The health-promoting properties of kefir are largely attributed to its diverse microbiota, which offers probiotic benefits, enhances gut health, and may play a role in preventing certain diseases [69,70]. The presence of antimicrobial compounds and metabolic products produced by the dominant bacteria can inhibit pathogenic microorganisms, contributing to the overall health benefits associated with regular kefir consumption [52].
In summary, the multifaceted bacterial dynamics within kefir grains and beverages underscore the importance of understanding the specific microbial populations and their contributions to the sensory, nutritional, and health-promoting attributes of kefir. Future research focusing on the interaction between bacterial species, the impact of environmental factors, and the exploration of novel strains may further elucidate the complex nature of kefir microbiota and its potential applications in health and nutrition. In addition, advances in metagenomic and metabolomic analysis will allow for a more precise characterization of the kefir microbiota, paving the way for its application in personalized nutrition, functional foods and next-generation probiotics.
3.7. Fungal Analysis of Kefir and Its Grains
The analysis of fungal communities within kefir and its grains reveals significant variability in species composition, reflecting the intricate and diverse nature of these microbial ecosystems. A common method for identifying fungal species in food products is the examination of the internal transcribed spacer (ITS) region [71].
3.7.1. Composition of Fungal Phyla
A recurring theme across multiple studies is the predominance of the phylum Ascomycota within kefir grains and milk kefir. However, the dominant species within this phylum differ markedly among regions and raw materials. For instance, Cufaoglu and Erdinc [44] identified the family Dipodascaceae as dominant in the kefir grains analyzed, while González-Orozco et al. [9] and Sumarmono et al. [45] also recognized Ascomycota as the most prevalent phylum in their respective studies, with the latter specifically noting the abundance of Saccharomycetaceae, Kazachstania, and Kluyveromyces.
Ilıkkan and Bagdat [47] corroborated this finding, identifying Ascomycota as the predominant fungal phylum and highlighting Saccharomycetaceae as particularly abundant along with several other families, including Pichiaceae, Trichocomaceae, and Schizosaccharomycetaceae. Alraddadi et al. [43] in Tasmania identified Ascomycota, Basidiomycota and Zygomycota, with Ascomycota being the most prevalent.
3.7.2. Dominant Fungal Genera
Within the identified fungal genera, Kazachstania and Torulaspora emerged as prominent species. Gao and Zhang [49] indicated that Saccharomycetaceae, Pichiaceae, and Trichocomaceae consistently dominated their analyzed community, particularly emphasizing Kazachstania. In another study by Zeng et al. [50], conducted in Lhasa, Tibet, Saccharomycetaceae was also identified as the most prevalent family.
Investigating fungal diversity over time, De Almeida Brasiel et al. [41] reported a greater variety in the fungal community after 30 days of fermentation, identifying Aspergillus sp., Cordyceps sp., and Saccharomyces sp. as the most prevalent genera. Similarly, Sumarmono et al. [45] highlighted Kazachstania and Kluyveromyces as dominant, while minor genera such as Aspergillus spp. and Botryotrichum were also detected. The genus Aspergillus, although less prevalent in kefir, is known for its enzyme-producing capabilities, essential for fermentation processes [52,53].
González-Orozco et al. [9] identified Kluyveromyces marxianus, Kazachstania africana, and Naumovozyma dairenensis as the dominant species in kefir grains, with Naumovozyma dairenensis being particularly common in milk kefir. The repeated identification of Kluyveromyces marxianus across studies underscores its significance in kefir ecology, likely due to its biotechnological potential, including various enzymatic activities [64,72,73].
Garofalo et al. [42] expanded on this discussion by identifying Kazachstania unispora and Saccharomyces cerevisiae, which were not dominant in the studies by González-Orozco et al. [9] and Ilıkkan and Bagdat [47]. Their findings indicated that Kazachstania unispora is frequently found in dairy products, suggesting its adaptation to this environment. The study concluded that variations exist in bacterial composition between different types of kefir, noting that cow’s milk kefir exhibited lower yeast diversity compared to goat’s milk kefir while still showing a broader distribution of yeast species.
The discrepancies in findings among studies, such as Garofalo et al. [42], indicate significant diversity within the Ascomycota phylum, highlighting minor species like Alternaria tenuissima and Cladosporium cladosporioides, which suggest environmental contamination in uncontrolled samples. Furthermore, they noted that fungal species prevalent in kefir grains often decreased in the final beverages, while those initially less abundant became dominant. This shift can be attributed to the location and adhesion of species to the grains during fermentation, directly influencing the quality of kefir.
3.7.3. Temporal Dynamics and Environmental Influences
The non-lactose-fermenting yeasts Saccharomyces cerevisiae, Torulaspora delbrueckii, and Pichia fermentans are important contributors to the fermentation process, utilizing galactose and glucose released by lactose hydrolysis [73,74,75]. Notably, the presence of Saccharomyces is significant due to its probiotic properties, which include immune modulation, antioxidant effects, gastrointestinal health benefits, and the production of essential nutrients [76,77].
Alraddadi et al. [43] noted temporal dynamics in fungal communities, with Kazachstania turicensis dominating the early stages and being replaced by Torulaspora delbrueckii as fermentation progressed. This observation emphasizes the importance of considering fermentation stages when assessing kefir’s microbial composition, an aspect less explored in other studies.
Cufaoglu and Erdinc [44] reported the presence of Geotrichum silvicola, a species absent in other investigations, suggesting geographical or methodological differences that underscore the necessity for standardized analytical techniques to enhance the comparability of results. Geotrichum spp. are recognized as yeast-like fungi inhabiting diverse environments, including soil, milk, and water [78].
In the study by Zeng et al. [50], the most abundant yeast species varied among grains: Saccharomyces cerevisiae was most prevalent in grain 1, while Kazachstania africana and Kluyveromyces marxianus dominated grains 2 and 3, respectively. The ability of Kluyveromyces marxianus to metabolize lactose as a carbon source contributes significantly to the production of ethanol, CO2, and the characteristic fermented flavor of kefir [22,42,79]. Additionally, Kluyveromyces marxianus shows potential for nisin production when co-cultured with Lactococcus lactis, alongside its β-galactosidase activity and antioxidant properties [64,72,73].
Ilıkkan and Bagdat et al. [47] further elucidated fungal diversity by identifying Naumovozyma dairenensis as the dominant species in kefir grain A, followed by Kluyveromyces marxianus, Zygosaccharomyces rouxii, Kazachstania africana, and Tetrapisispora phaffi. In contrast, kefir grain G displayed higher diversity, with Zygosaccharomyces rouxii being predominant alongside other species. Notably, Zygosaccharomyces rouxii is recognized for its role in flavor development during fermentation, particularly in soy sauce production [80]. Kefir A was yellowish, with a cauliflower-like appearance and a slightly rigid texture. In contrast, kefir G was whiter and softer, with elastic characteristics that contribute to the formation of gel in the kefir grains. Both types were collected in the Ankara province of Turkey.
The microbial composition of artisanal kefir is influenced by a multitude of factors, including geographical origin, production methods, fermentation conditions, environmental factors, maintenance practices, and the type of milk utilized [40,48,81]. Key elements such as milk type, incubation temperature and duration, and kefir grain-to-milk ratio play crucial roles in shaping the microbial community [82,83,84]. Understanding this microbial complexity is essential for identifying the beneficial properties of kefir, guiding its applications, and ensuring the quality of fermented beverages [41].
However, studies by Kazou et al. [85] and Blasche et al. [86] indicate that milk kefir may harbor a more diverse microbiota compared to the grains themselves, highlighting a dynamic population shift during fermentation. This underscores the necessity for further research to elucidate the differences in microbial composition and their implications for sensory characteristics and microbial interactions in the final kefir products [87].
4. Conclusions
The systematic review of studies on the microbial composition of artisanal kefir reveals a remarkable diversity within the bacterial and fungal communities of these fermented products. This variation reflects adaptations to the specific environmental and processing conditions of each region, influencing both sensory characteristics and potential probiotic benefits. The complex interactions among these microorganisms during fermentation not only shape the sensory and nutritional properties of kefir but also contribute to its health benefits.
Comparative studies across different locales have extensively documented the influence of variables such as milk type, production methods and grain handling. These findings highlight the regional adaptations of microbial communities. However, there is a pressing need to expand research on the microbiota of kefir grains and artisanal kefir in Brazil, especially given the growing popularity of this probiotic beverage and the lack of updated studies in the country. Such advancements are crucial not only for academic purposes but also for the commercial application of kefir as a functional food.
Metagenomic approaches have provided valuable insights into the microbial structure of kefir, allowing for the identification and quantification of previously undetected microorganisms. Understanding the complexity of the artisanal kefir microbiota is fundamental for establishing production standards and ensuring the consistency of these fermented products’ quality.
Functional studies involving simulated gastrointestinal analyses, physicochemical assessments and sensory evaluations throughout the shelf life of Brazilian artisanal kefir are essential. Although metagenomics is a powerful tool, it has limitations. Some microbial species may not be represented in existing databases, especially non-culturable or novel microorganisms. Consequently, important microbial members of the kefir microbiota might be overlooked, impacting the study’s comprehensiveness. However, advancements in metagenomic databases and sequencing technologies may help mitigate this limitation in future research.
In summary, the review of 14 articles confirms a consistent microbial composition in kefir grains and milk kefir, predominantly characterized by the phylum Firmicutes and the genus Lactobacillus. Species such as L. kefiranofaciens and L. helveticus, recognized for their probiotic properties and bacteriocin production, stand out due to their potential health benefits and industrial applications. Regional and methodological variations significantly influence this composition, underscoring the need for rigorous practices in the artisanal production of kefir to ensure its food safety and probiotic efficacy.
Author Contributions
Conceptualization, J.A.S.; Validation, W.d.C.O. and S.H.F.; Formal analysis, A.S.d.F.; Investigation, J.A.S., L.d.F.F.d.S. and L.B.; Writing—original draft, J.A.S.; Writing—review and editing, W.d.C.O., A.S.d.F., M.M.S. and S.H.F.; Visualization, M.M.S.; Project administration, P.d.S.M.; Funding acquisition, P.d.S.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) grant number 140557/2024-5.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article and further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Oliveira Leite, A.M.; Miguel, M.A.; Peixoto, R.S.; Rosado, A.S.; Silva, J.T.; Paschoalin, V.M. Microbiological, technological and therapeutic properties of kefir: A natural probiotic beverage. Braz. J. Microbiol. 2013, 44, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Rosa, D.D.; Dias, M.M.S.; Grześkowiak, Ł.M.; Reis, S.A.; Conceição, L.L.; Peluzio, M.D.C.G. Milk kefir: Nutritional, microbiological and health benefits. Nutr. Res. Rev. 2017, 30, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Bengoa, A.A.; Iraporda, C.; Garrote, G.L.; Abraham, A.G. Kefir micro-organisms: Their role in grain assembly and health properties of fermented milk. J. Appl. Microbiol. 2019, 126, 686–700. [Google Scholar] [CrossRef]
- Vardjan, T.; Mohar Lorbeg, P.; Rogelj, I.; Čanžek Majhenič, A. Characterization and stability of lactobacilli and yeast microbiota in kefir grains. J. Dairy Sci. 2013, 96, 2729–2736. [Google Scholar] [CrossRef]
- Klippel, B.F.; Duemke, L.B.; Leal, M.A.; Friques, A.G.; Dantas, E.M.; Dalvi, R.F.; Gava, A.L.; Pereira, T.M.; Andrade, T.U.; Meyrelles, S.S.; et al. Effects of Kefir on the Cardiac Autonomic Tones and Baroreflex Sensitivity in Spontaneously Hypertensive Rats. Front. Physiol. 2016, 7, 211. [Google Scholar] [CrossRef]
- De Montijo-Prieto, S.; Moreno, E.; Bergillos-Meca, T.; Lasserrot, A.; Ruiz-López, M.D.; Ruiz-Bravo, A.; Jiménez-Valera, M. A Lactobacillus plantarum strain isolated from kefir protects against intestinal infection with Yersinia enterocolitica O9 and modulates immunity in mice. Res. Microbiol. 2015, 166, 626–632. [Google Scholar] [CrossRef]
- Arena, M.P.; Russo, P.; Spano, G.; Capozzi, V. Exploration of the Microbial Biodiversity Associated with North Apulian Sourdoughs and the Effect of the Increasing Number of Inoculated Lactic Acid Bacteria Strains on the Biocontrol against Fungal Spoilage. Fermentation 2019, 5, 97. [Google Scholar] [CrossRef]
- Brasil, G.A.; Silva-Cutini, M.A.; Moraes, F.S.A.; Pereira, T.M.C.; Vasquez, E.C.; Lenz, D.; Andrade, T.U. The Benefits of Soluble Nonbacterial Fraction of Kefir on Blood Pressure and Cardiac Hypertrophy in Hypertensive Rats Are Mediated by an Increase in Baroreflex Sensitivity and Decrease in Angiotensin-Converting Enzyme Activity. Nutrition 2018, 51–52, 66–72. [Google Scholar] [CrossRef] [PubMed]
- González-Orozco, B.D.; García-Cano, I.; Escobar-Zepeda, A.; Jiménez-Flores, R.; Álvarez, V.B. Metagenomic analysis and antibacterial activity of kefir microorganisms. J. Food Sci. 2023, 88, 2933–2949. [Google Scholar] [CrossRef]
- Yılmaz, B.; Sharma, H.; Melekoglu, E.; Ozogul, F. Recent Developments in Dairy Kefir-Derived Lactic Acid Bacteria and Their Health Benefits. Food Biosci. 2022, 46, 101592. [Google Scholar] [CrossRef]
- Youn, H.; Seo, K.; Kim, H.; Kim, Y.; Kim, H. Effect of Postbiotics Derived from Kefir Lactic Acid Bacteria-Mediated Bioconversion of Citrus Pomace Extract and Whey on High-Fat Diet-Induced Obesity and Gut Dysbiosis. Food Res. Int. 2022, 162, 112345. [Google Scholar] [CrossRef]
- Chen, H.L.; Hung, K.F.; Yen, C.C.; Liao, C.H.; Wang, J.L.; Lan, Y.W.; Chong, K.Y.; Fan, H.C.; Chen, C.M. Kefir Peptides Alleviate Particulate Matter <4 μm (PM4.0)-Induced Pulmonary Inflammation by Inhibiting the NF-κB Pathway Using Luciferase Transgenic Mice. Sci. Rep. 2019, 9, 11529. [Google Scholar]
- Wang, Y.; Xu, N.; Xi, A.; Ahmed, Z. Effects of Lactobacillus plantarum MA2 Isolated from Tibet Kefir on Lipid Metabolism and Intestinal Microflora of Rats Fed on High-Cholesterol Diet. Appl. Microbiol. Biotechnol. 2009, 84, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Hurtado-Romero, A.; Toro-Barbosa, D.; Gradilla-Hernández, M.S.; Garcia-Amezquita, L.E.; García-Cayuela, T. Probiotic Properties, Prebiotic Fermentability, and GABA-Producing Capacity of Microorganisms Isolated from Mexican Milk Kefir Grains: A Clustering Evaluation for Functional Dairy Food Applications. Foods 2021, 10, 2275. [Google Scholar] [CrossRef] [PubMed]
- Miguel, M.G.d.C.P.; Cardoso, P.G.; Lago, L.d.A.; Schwan, R.F. Diversity of bacteria present in milk kefir grains using culture-dependent and culture-independent methods. Food Res. Int. 2010, 43, 1523–1528. [Google Scholar] [CrossRef]
- Nejati, F.; Junne, S.; Neubauer, P. A Big World in Small Grain: A Review of Natural Milk Kefir Starters. Microorganisms 2020, 8, 192. [Google Scholar] [CrossRef] [PubMed]
- Farnworth, E.R. Kefir: A Complex Probiotic. Food Sci. Technol. Bull. Funct. Foods 2005, 2, 1–17. [Google Scholar] [CrossRef]
- Leeuwendaal, N.K.; Stanton, C.; O’Toole, P.W.; Beresford, T.P. Fermented Foods, Health and the Gut Microbiome. Nutrients 2022, 14, 1527. [Google Scholar] [CrossRef]
- Garbers, I.M.; Britz, T.J.; Witthuhn, R.C. PCR-Based Denaturing Gradient Gel Electrophoretic Typification and Identification of the Microbial Consortium Present in Kefir Grains. World J. Microbiol. Biotechnol. 2004, 20, 687–693. [Google Scholar] [CrossRef]
- Fan, D.; Stoyanova, L.G.; Netrusov, A.I. Microbiome and Metabiotic Properties of Kefir Grains and Kefirs Based on Them. Microbiology 2022, 91, 339–355. [Google Scholar]
- Yamane, T.; Sakamoto, T.; Nakagaki, T.; Nakano, Y. Lactic Acid Bacteria from Kefir Increase Cytotoxicity of Natural Killer Cells to Tumor Cells. Foods 2018, 7, 1–9. [Google Scholar] [CrossRef]
- Bourrie, B.C.; Willing, B.P.; Cotter, P.D. The Microbiota and Health Promoting Characteristics of the Fermented Beverage Kefir. Front. Microbiol. 2016, 7, 647. [Google Scholar] [CrossRef] [PubMed]
- Farnworth, E.R.; Mainville, I. Kefir: A fermented milk product. In Handbook of Fermented Functional Foods; CRC Press: Boca Raton, FL, USA, 2003; p. 89. [Google Scholar]
- Chen, H.C.; Wang, S.Y.; Chen, M.J. Microbiological study of lactic acid bacteria in kefir grains by culture-dependent and culture-independent methods. Food Microbiol. 2008, 25, 492–501. [Google Scholar] [CrossRef]
- Kumar, H.; Yadav, D.; Kumar, N.; Seth, R.; Goyal, A. Nutritional and nutraceutical properties of goat milk-A review. Indian J. Dairy Sci. 2016, 69, 513–518. [Google Scholar]
- Nayik, G.A.; Jagdale, Y.D.; Gaikwad, S.A.; Devkatte, A.N.; Dar, A.H.; Dezmirean, D.S.; Bobis, O.; Ranjha, M.M.A.N.; Ansari, M.J.; Hemeg, H.A.; et al. Recent Insights into Processing Approaches and Potential Health Benefits of Goat Milk and Its Products: A Review. Front. Nutr. 2021, 8, 789117. [Google Scholar] [CrossRef] [PubMed]
- Ströher, J.A.; Oliveira, W.D.C.; Freitas, A.S.D.; Salazar, M.M.; Flôres, S.H.; Malheiros, P.D.S. Microbial Dynamics and Volatile Compound Profiles in Artisanal Kefir During Storage. Fermentation 2025, 11, 105. [Google Scholar] [CrossRef]
- You, L.; Yang, C.; Jin, H.; Kwok, L.-Y.; Sun, Z.; Zhang, H. Metagenomic features of traditional fermented milk products. LWT Food Sci. Technol. 2022, 155, 112945. [Google Scholar] [CrossRef]
- Tenorio-Salgado, S.; Castelán-Sánchez, H.G.; Dávila-Ramos, S.; Huerta-Saquero, A.; Rodríguez-Morales, S.; Merino-Pérez, E.; Roa de la Fuente, L.F.; Solis-Pereira, S.E.; Pérez-Rueda, E.; Lizama-Uc, G. Metagenomic analysis and antimicrobial activity of two fermented milk kefir samples. MicrobiologyOpen 2021, 10, e1183. [Google Scholar] [CrossRef]
- Bresciani, L.; Ströher, J.A.; da Silva, L.D.; Lehn, D.N.; Rama, G.R.; de Souza, E.M.; Wilsmann, L.M.; Salazar, M.M. Kefir artesanal: Análise dos atributos de qualidade em relação aos requisitos da legislação brasileira. Obs. La Econ. Latinoam. 2024, 22, 1–17. [Google Scholar] [CrossRef]
- Castellanos-Rozo, J.; Pérez Pulido, R.; Grande, M.J.; Lucas, R.; Gálvez, A. Analysis of the Bacterial Diversity of Paipa Cheese (a Traditional Raw Cow’s Milk Cheese from Colombia) by High-Throughput Sequencing. Microorganisms 2020, 8, 218. [Google Scholar] [CrossRef]
- Kamilari, E.; Anagnostopoulos, D.A.; Papademas, P.; Kamilaris, A.; Tsaltas, D. Characterizing Halloumi cheese’s bacterial communities through metagenomic analysis. LWT 2020, 126, 109298. [Google Scholar] [CrossRef]
- Salameh, J.P.; Bossuyt, P.M.; McGrath, T.A.; Thombs, B.D.; Hyde, C.J.; Macaskill, P.; Deeks, J.J.; Leeflang, M.; Korevaar, D.A.; Whiting, P.; et al. Preferred reporting items for systematic review and meta-analysis of diagnostic test accuracy studies (PRISMA-DTA): Explanation, elaboration, and checklist. BMJ (Clin. Res. Ed.) 2020, 370, m2632. [Google Scholar] [CrossRef]
- Leite, A.M.; Mayo, B.; Rachid, C.T.; Peixoto, R.S.; Silva, J.T.; Paschoalin, V.M.; Delgado, S. Assessment of the microbial diversity of Brazilian kefir grains by PCR-DGGE and pyrosequencing analysis. Food Microbiol. 2012, 31, 215–221. [Google Scholar] [CrossRef]
- Zanirati, D.F.; Abatemarco, M., Jr.; Sandes, S.H.C.; Nicoli, J.R.; Nunes, Á.C.; Neumann, E. Selection of lactic acid bacteria from Brazilian kefir grains for potential use as starter or probiotic cultures. Anaerobe 2015, 32, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Ströher, J.A.; da Silva, L.D.; Lehn, D.N.; Bresciani, L.; Rama, G.R.; de Souza, E.M.; Wilsmann, L.M.; Salazar, M.M. A influência das boas práticas de fabricação nos atributos físico-químicos do kefir artesanal ao longo de sua vida útil. Obs. La Econ. Latinoam. 2024, 22, 1–22. [Google Scholar] [CrossRef]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harris, H.M.B.; Mattarelli, P.; Lebeer, S. A Taxonomic Note on the Genus Lactobacillus: Description of 23 Novel Genera, Emended Description of the Genus Lactobacillus Beijerinck 1901, and Union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Li, B. Chemical and microbiological characteristics of kefir grains and their fermented dairy products: A review. Cogent Food Agric. 2016, 2, 1272152. [Google Scholar] [CrossRef]
- Prado, M.R.; Blandón, L.M.; Vandenberghe, L.P.; Rodrigues, C.; Castro, G.R.; Thomaz-Soccol, V.; Soccol, C.R. Milk kefir: Composition, microbial cultures, biological activities, and related products. Front. Microbiol. 2015, 6, 1177. [Google Scholar] [CrossRef] [PubMed]
- Sindi, A.; Badsha, M.B.; Ünlü, G. Bacterial Populations in International Artisanal Kefirs. Microorganisms 2020, 8, 1318. [Google Scholar] [CrossRef]
- De Almeida Brasiel, P.G.; Dutra Medeiros, J.; Barbosa Ferreira Machado, A.; Schuchter Ferreira, M.; Gouveia Peluzio, M.d.C.; Potente Dutra Luquetti, S.C. Microbial community dynamics of fermented kefir beverages changes over time. Int. J. Dairy Technol. 2021, 74, 324–331. [Google Scholar] [CrossRef]
- Garofalo, C.; Ferrocino, I.; Reale, A.; Sabbatini, R.; Milanović, V.; Alkić-Subašić, M.; Boscaino, F.; Aquilanti, L.; Pasquini, M.; Trombetta, M.F.; et al. Study of kefir drinks produced by backslopping method using kefir grains from Bosnia and Herzegovina: Microbial dynamics and volatilome profile. Food Res. Int. 2020, 137, 109369. [Google Scholar] [CrossRef]
- Alraddadi, F.A.J.; Ross, T.; Powell, S.M. Evaluation of the microbial communities in kefir grains and kefir over time. Int. Dairy J. 2023, 136, 105490. [Google Scholar] [CrossRef]
- Cufaoglu, G.; Erdinc, A.N. Comparative analyses of milk and water kefir: Fermentation temperature, physicochemical properties, sensory qualities, and metagenomic composition. Food Biosci. 2023, 55, 103079. [Google Scholar] [CrossRef]
- Sumarmono, J.; Kusuma, R.J.; Rahayu, N.; Sukarno, A.S.; Wulansari, P.D. Metagenomic analysis of the microbial community in kefir grains from different milk sources. Biodiversitas J. Biol. Divers. 2023, 24, 5302–5308. [Google Scholar] [CrossRef]
- Kalamaki, M.S.; Angelidis, A.S. High-Throughput, Sequence-Based Analysis of the Microbiota of Greek Kefir Grains from Two Geographic Regions. Food Technol. Biotechnol. 2020, 58, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Ilıkkan, Ö.K.; Bağdat, E.Ş. Comparison of bacterial and fungal biodiversity of Turkish kefir grains with high-throughput metagenomic analysis. LWT 2021, 152, 112375. [Google Scholar] [CrossRef]
- Liu, S.; Lu, S.Y.; Qureshi, N.; Enshasy, H.A.E.; Skory, C.D. Antibacterial Property and Metagenomic Analysis of Milk Kefir. Probiotics Antimicrob. Proteins 2022, 14, 1170–1183. [Google Scholar] [CrossRef]
- Gao, W.; Zhang, L. Comparative analysis of the microbial community composition between Tibetan kefir grains and milks. Food Res. Int. 2019, 116, 137–144. [Google Scholar] [CrossRef]
- Zeng, X.; Wang, Y.; Jia, H.; Wang, Z.; Gao, Z.; Luo, Y.; Sheng, Q.; Yuan, Y.; Yue, T. Metagenomic analysis of microflora structure and functional capacity in probiotic Tibetan kefir grains. Food Res. Int. 2022, 151, 110849. [Google Scholar] [CrossRef]
- Biçer, Y.; Telli, A.E.; Sönmez, G.; Turkal, G.; Telli, N.; Uçar, G. Comparison of commercial and traditional kefir microbiota using metagenomic analysis. Int. J. Dairy Technol. 2021, 74, 528–534. [Google Scholar] [CrossRef]
- Tamang, J.P.; Shin, D.H.; Jung, S.J.; Chae, S.W. Functional Properties of Microorganisms in Fermented Foods. Front. Microbiol. 2016, 7, 578. [Google Scholar] [CrossRef] [PubMed]
- Tamang, J.P.; Watanabe, K.; Holzapfel, W.H. Review: Diversity of Microorganisms in Global Fermented Foods and Beverages. Front. Microbiol. 2016, 7, 377. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Chen, K.N.; Lo, Y.M.; Chiang, M.L.; Chen, H.C.; Liu, J.R.; Chen, M.J. Investigation of microorganisms involved in biosynthesis of the kefir grain. Food Microbiol. 2012, 32, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Raposo, A.; Pérez, E.; de Faria, C.T.; Ferrús, M.A.; Carrascosa, C. Food Spoilage by Pseudomonas spp.—An Overview. In Foodborne Pathogens and Antibiotic Resistance; John Wiley & Sons: Hoboken, NJ, USA, 2016; pp. 41–71. [Google Scholar]
- Ströher, J.; Salazar, M.; Lehn, D.; Silva, L.; Flôres, S.; Malheiros, P. Percepções sobre a produção artesanal de kefir em um grupo focal. Rev. Conex. UEPG 2024, 20, 1–21. [Google Scholar] [CrossRef]
- Mokoena, M.P. Lactic Acid Bacteria and Their Bacteriocins: Classification, Biosynthesis and Applications against Uropathogens: A Mini-Review. Molecules 2017, 22, 1255. [Google Scholar] [CrossRef]
- Zheng, Y.; Lu, Y.; Wang, J.; Yang, L.; Pan, C.; Huang, Y. Probiotic properties of Lactobacillus strains isolated from Tibetan kefir grains. PLoS ONE 2013, 8, e69868. [Google Scholar] [CrossRef]
- Slattery, C.; Cotter, P.D.; O’Toole, P.W. Analysis of Health Benefits Conferred by Lactobacillus Species from Kefir. Nutrients 2019, 11, 1252. [Google Scholar] [CrossRef]
- Ghoneum, M.; Felo, N. Selective induction of apoptosis in human gastric cancer cells by Lactobacillus kefiri (PFT), a novel kefir product. Oncol. Rep. 2015, 34, 1659–1666. [Google Scholar] [CrossRef]
- Carasi, P.; Díaz, M.; Racedo, S.M.; De Antoni, G.; Urdaci, M.C.; Serradell, M. de L. Safety characterization and antimicrobial properties of kefir-isolated Lactobacillus kefiri. BioMed Res. Int. 2014, 2014, 208974. [Google Scholar] [CrossRef]
- Perez, R.H.; Zendo, T.; Sonomoto, K. Novel bacteriocins from lactic acid bacteria (LAB): Various structures and applications. Microb. Cell Factories 2014, 13, 1–13. [Google Scholar] [CrossRef]
- Takizawa, S.; Kojima, S.; Tamura, S.; Fujinaga, S.; Benno, Y.; Nakase, T. Lactobacillus kefirgranum sp. nov. and Lactobacillus parakefir sp. nov., Two New Species from Kefir Grains. Int. J. Syst. Evol. Microbiol. 1994, 44, 435–439. [Google Scholar] [CrossRef]
- Korsak, N.; Taminiau, B.; Leclercq, M.; Nezer, C.; Crevecoeur, S.; Ferauche, C.; Detry, E.; Delcenserie, V.; Daube, G. Short communication: Evaluation of the microbiota of kefir samples using metagenetic analysis targeting the 16S and 26S ribosomal DNA fragments. J. Dairy Sci. 2015, 98, 3684–3689. [Google Scholar] [CrossRef] [PubMed]
- Taverniti, V.; Guglielmetti, S. Health-Promoting Properties of Lactobacillus helveticus. Front. Microbiol. 2012, 3, 392. [Google Scholar] [CrossRef]
- FitzGerald, R.J.; Murray, B.A.; Walsh, D.J. Hypotensive peptides from milk proteins. J. Nutr. 2004, 134, 980S–988S. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, T.; Fang, X.; Min, W.; Yang, Z. Characterization and immunomodulatory activity of an exopolysaccharide produced by Lactobacillus plantarum JLK0142 isolated from fermented dairy tofu. Int. J. Biol. Macromol. 2018, 115, 985–993. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Dang, N.; Ren, D.; Zhao, F.; Lv, R.; Ma, T.; Bao, Q.; Menghe, B.; Liu, W. Comparison of Bacterial Microbiota in Raw Mare’s Milk and Koumiss Using PacBio Single Molecule Real-Time Sequencing Technology. Front. Microbiol. 2020, 11, 581610. [Google Scholar] [CrossRef]
- Bengmark, S. Bio-ecological control of chronic liver disease and encephalopathy. Metab. Brain Dis. 2008, 24, 223–236. [Google Scholar] [CrossRef]
- O’Flaherty, M.; Buchan, I.; Capewell, S. Contributions of treatment and lifestyle to declining CVD mortality: Why have CVD mortality rates declined so much since the 1960s? Heart (Br. Card. Soc.) 2013, 99, 159–162. [Google Scholar] [CrossRef]
- Thangardurai, D.; Nollet, L.M.L.; Islam, S.; Sangeetha, J. Sequencing Technologies in Microbial Food Safety and Quality; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar]
- Hsu, S.-A.; Chou, J.-Y. Yeasts in fermented food and kefir: In vitro characterization of probiotic traits. JAPS J. Anim. Plant Sci. 2021, 31, 567–582. [Google Scholar]
- Johansen, P.G.; Owusu-Kwarteng, J.; Parkouda, C.; Padonou, S.W.; Jespersen, L. Occurrence and Importance of Yeasts in Indigenous Fermented Food and Beverages Produced in Sub-Saharan Africa. Front. Microbiol. 2019, 10, 1789. [Google Scholar] [CrossRef]
- Garofalo, C.; Osimani, A.; Milanović, V.; Aquilanti, L.; De Filippis, F.; Stellato, G.; Di Mauro, S.; Turchetti, B.; Buzzini, P.; Ercolini, D.; et al. Bacteria and yeast microbiota in milk kefir grains from different Italian regions. Food Microbiol. 2015, 49, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Diosma, G.; Romanin, D.E.; Rey-Burusco, M.F.; Londero, A.; Garrote, G.L. Yeasts from kefir grains: Isolation, identification, and probiotic characterization. World J. Microbiol. Biotechnol. 2014, 30, 43–53. [Google Scholar] [CrossRef]
- Fakruddin, M.; Hossain, M.N.; Ahmed, M.M. Antimicrobial and antioxidant activities of Saccharomyces cerevisiae IFST062013, a potential probiotic. BMC Complement. Altern. Med. 2017, 17, 64. [Google Scholar] [CrossRef]
- Hong, J.Y.; Lee, N.K.; Yi, S.H.; Hong, S.P.; Paik, H.D. Short communication: Physicochemical features and microbial community of milk kefir using a potential probiotic Saccharomyces cerevisiae KU200284. J. Dairy Sci. 2019, 102, 10845–10849. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, M.; O’Kiely, P.; Forristal, P.D.; Fuller, H.T. Fungi isolated from contaminated baled grass silage on farms in the Irish Midlands. FEMS Microbiol. Lett. 2005, 247, 131–135. [Google Scholar] [CrossRef]
- Bengoa, A.A.; Llamas, M.G.; Iraporda, C.; Dueñas, M.T.; Abraham, A.G.; Garrote, G.L. Impact of growth temperature on exopolysaccharide production and probiotic properties of Lactobacillus paracasei strains isolated from kefir grains. Food Microbiol. 2018, 69, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Suezawa, Y.; Suzuki, M.; Mori, H. Genotyping of a miso and soy sauce fermentation yeast, Zygosaccharomyces rouxii, based on sequence analysis of the partial 26S ribosomal RNA gene and two internal transcribed spacers. Biosci. Biotechnol. Biochem. 2008, 72, 2452–2455. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, L.; Zhou, H.; Yuan, M.; Hu, D.; Wang, Y.; Sun, H.; Xu, J.; Lan, R. Antimicrobial Resistance and Molecular Characterization of Citrobacter spp. Causing Extraintestinal Infections. Front. Cell. Infect. Microbiol. 2021, 11, 737636. [Google Scholar] [CrossRef]
- Nejati, F.; Junne, S.; Kurreck, J.; Neubauer, P. Quantification of Major Bacteria and Yeast Species in Kefir Consortia by Multiplex TaqMan qPCR. Front. Microbiol. 2020, 11, 1291. [Google Scholar] [CrossRef]
- Barao, C.E.; Klososki, S.J.; Pinheiro, K.H.; Marcolino, V.; Junior, O.V.; Cruz, A.G.; Silva, T.T.D.; Pimentel, T. Growth Kinetics of Kefir Biomass: Influence of the Incubation Temperature in Milk. Chem. Eng. Trans. 2019, 75, 499–504. [Google Scholar]
- Gul, O.; Mortas, M.; Atalar, I.; Dervisoglu, M.; Kahyaoglu, T. Manufacture and characterization of kefir made from cow and buffalo milk, using kefir grain and starter culture. J. Dairy Sci. 2015, 98, 1517–1525. [Google Scholar] [CrossRef] [PubMed]
- Kazou, M.; Grafakou, A.; Tsakalidou, E.; Georgalaki, M. Zooming into the Microbiota of Home-Made and Industrial Kefir Produced in Greece Using Classical Microbiological and Amplicon-Based Metagenomics Analyses. Front. Microbiol. 2021, 12, 621069. [Google Scholar] [CrossRef] [PubMed]
- Blasche, S.; Kim, Y.; Mars, R.A.T.; Machado, D.; Maansson, M.; Kafkia, E.; Milanese, A.; Zeller, G.; Teusink, B.; Nielsen, J.; et al. Metabolic cooperation and spatiotemporal niche partitioning in a kefir microbial community. Nat. Microbiol. 2021, 6, 196–208. [Google Scholar] [CrossRef]
- Dobson, A.; O’Sullivan, O.; Cotter, P.D.; Ross, P.; Hill, C. High-throughput sequence-based analysis of the bacterial composition of kefir and an associated kefir grain. FEMS Microbiol. Lett. 2011, 320, 56–62. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).