Abstract
Chronic pain is a multifactorial and complex condition that significantly affects individuals’ quality of life. The underlying mechanisms of chronic pain involve complex alterations in neural circuits, gene expression, and cellular signaling pathways. Recently, ncRNAs, such as miRNAs, lncRNAs, circRNAs, and siRNAs, have been identified as crucial regulators in the pathophysiology of chronic pain. These ncRNAs modulate gene expression at both the transcriptional and post-transcriptional levels, affecting pain-related pathways like inflammation, neuronal plasticity, and sensory processing. miRNAs have been shown to control genes involved in pain perception and nociceptive signaling, while lncRNAs interact with chromatin remodeling factors and transcription factors to modify pain-related gene expression. CircRNAs act as sponges for miRNAs, thereby influencing pain mechanisms. siRNAs, recognized for their gene-silencing capabilities, also participate in regulating the expression of pain-related genes. This review examines the diverse roles of ncRNAs in chronic pain, emphasizing their potential as biomarkers for pain assessment and as targets for novel therapeutic strategies. A profound understanding of the ncRNA-mediated regulatory networks involved in chronic pain could result in more effective and personalized pain management solutions.
1. Introduction
The International Association for the Study of Pain (IASP) describes pain as “an unpleasant sensory and emotional experience often associated with actual or potential tissue damage” [1,2]. This definition highlights the multifaceted nature of pain, encompassing physical and emotional dimensions, and frequently indicating health issues that necessitate medical intervention [3,4,5]. The process of pain perception is initiated by the activation of nociceptors, which are specialized sensory neurons placed in peripheral tissues, including the skin, muscles, and internal organs [6]. These sensory neurons detect harmful stimuli, including mechanical pressure, extreme temperatures, and chemical changes indicative of potential tissue damage [7]. Upon stimulation, nociceptors create electrical signals that travel along afferent nerve fibers to the central nervous system (CNS), where they are processed and interpreted as pain [8]. However, it is important to note that pain involves some pathways within both the peripheral nervous system (PNS) and the CNS, allowing for multiple levels of modulation [9].
The characteristics of pain differ depending on its duration and etiology [10]. Acute pain is commonly brief and has a protective function, indicating immediate tissue injury and inducing behavioral responses to prevent further damages [11]. Conversely, chronic pain persists beyond the usual healing period, usually for years, and can become a debilitating condition affecting both physical and psychological well-being [12]. Chronic pain may arise from conditions including osteoarthritis [13], lower back pain [14], fibromyalgia [15], and cancer [16]. Unlike acute pain, which has a well-defined cause [11], chronic pain can develop into a distinct pathological condition, sometimes in the absence of tissue damage [17].
Chronic pain is particularly prevalent in older adults, as aging is associated with an increased incidence of pain-related conditions, including osteoarthritis, neuropathy and degenerative diseases [18]. Several epidemiological studies in Europe estimate that 38% to 60% of people aged 65 years and older experience chronic pain [19,20]. With advancing age, cumulative health conditions often exacerbate chronic pain, further impairing overall health and quality of life [21]. Several factors contribute to the risk of developing chronic pain, including age, gender, lifestyle and socioeconomic status [12]. Older people are particularly vulnerable due to physiological changes such as reduced tissue elasticity, muscle mass and bone density, all of which increase pain sensitivity and risk of injuries [22]. Gender differences in pain prevalence have also been observed, with women more likely to have chronic pain conditions [23]. In addition, modifiable lifestyle factors including smoking, alcohol consumption, obesity, and sedentary behavior can increase the likelihood of developing or worsening chronic pain [24,25,26].
These factors can influence gene expression and the regulation of pain-related pathways. In recent years, research has increasingly focused on the role of epigenetics in the modulation of chronic pain. Epigenetic modifications, such as DNA methylation [27], histone modifications [28] and non-coding RNAs (ncRNAs) [29], can modify gene expression without altering the underlying DNA sequence. ncRNAs have attracted considerable attention as regulators in the complex mechanisms underlying pain pathways. These molecules play a key role in regulating many biological processes essential for pain perception and response [30]. Unlike traditional protein-coding genes that directly encode proteins, ncRNAs exert their effects through regulatory functions that influence the expression of genes involved in some processes such as inflammation, neuronal plasticity and pain sensitivity [31,32,33].
Specifically, ncRNAs regulate gene expression by interacting with mRNA, chromatin, and other regulatory molecules, thereby influencing the activity of those genes implicated in pain pathways [34]. In the context of inflammation, ncRNAs can either promote or suppress the expression of pro-inflammatory cytokines and other signaling molecules, thus modulating the inflammatory response, which is a crucial contributor to pain [31,35]. Furthermore, it has been demonstrated that ncRNAs play a pivotal role in neuronal plasticity, a key factor in the development of chronic pain [32,36]. By modulating those genes that regulate synaptic strength, receptor sensitivity, and the formation of new neural connections, ncRNAs influence the persistence and exacerbation of pain following injury [37].
In conclusion, this article emphasizes the significant role of ncRNAs in the complex mechanisms that contribute to the onset and development of chronic pain. The following sections will explore the various types of ncRNAs and their roles in pain signaling, neural plasticity, and the regulation of pain-associated genes. A deeper understanding of the molecular interactions and regulatory functions of ncRNAs provides important insights into potential therapeutic approaches for chronic pain.
2. MicroRNAs (miRNAs)
MicroRNAs (miRNAs) constitute a subtype of small ncRNA molecules, usually ranging from 21 to 25 nucleotides in length, that act as key regulators of gene expression at the post-transcriptional level across a wide range of organisms, including plants, animals, and viruses [38,39,40]. These ncRNAs regulate gene expression by binding to complementary mRNA sequences, resulting in translational repression or mRNA degradation, ensuring precise and adaptable control of gene activity [41].
The biogenesis of miRNAs (Figure 1) is a multistep process that starts in the cell nucleus, where they are transcribed as primary miRNAs (pri-miRNAs) by RNA polymerase II, or in some cases, RNA polymerase III [42,43]. pri-miRNAs are further recognized and processed by the Drosha–DGCR8 microprocessor complex, which cleaves them into precursor miRNAs (pre-miRNAs) that are transported to the cytoplasm via exportin-5 (XPO5) in a RanGTP-dependent manner [44,45]. In the cytoplasm, pre-miRNAs are further cleaved by the endoribonuclease Dicer, resulting in the formation of mature miRNA duplexes [46]. From miRNA duplexes, only one strand (guide strand) is selectively incorporated into the RNA-induced silencing complex (RISC), while the passenger strand is commonly degraded [47]. Once integrated into the RISC, the miRNA directs the complex to specific mRNAs based on sequence complementarity, with binding primarily occurring in the 3′ untranslated region (3′ UTR) of the target gene [48], leading to either mRNA cleavage and degradation [49] or translation repression [50].
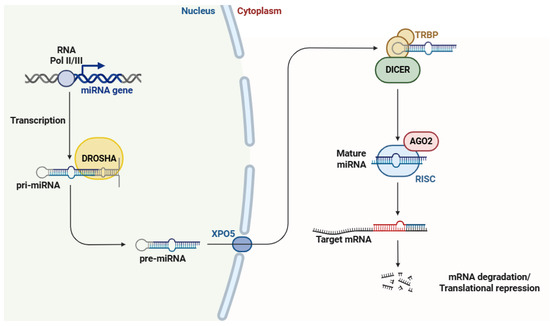
Figure 1.
The biosynthesis of miRNAs begins in the nucleus with the transcription of miRNA genes, generating pri-miRNAs. These pri-miRNAs are then processed by the Drosha protein, which enzymatically cleaves them to generate pre-miRNAs. Subsequently, pre-miRNAs are exported to the cytoplasm via the XPO5 protein. Once in the cytoplasm, pre-miRNAs undergo further maturation through the coordinated action of TRBP, Dicer, AGO2, and the RISC protein. This final step yields mature miRNAs, which are responsible for targeting mRNAs. Abbreviations: RNA Pol II/III (RNA polymerases II and III), miRNA (microRNA), pri-miRNA (primary microRNA), pre-miRNA (precursor microRNA), XPO5 (exportin 5), TRBP (transactivation response element RNA-binding protein), AGO2 (argonaute 2), RISC (RNA-induced silencing complex), and mRNA (messenger RNA). Figure adapted from Ref. [51].
This regulatory mechanism allows miRNAs to modulate gene networks with exceptional specificity and versatility, impacting a wide array of biological processes, including cell proliferation and differentiation [52], apoptosis [53], stress response [54], and immune function [55]. Furthermore, miRNAs are essential in intercellular communication by being released into extracellular fluids like blood, saliva, and urine. They are either encapsulated within exosomes and microvesicles [56,57,58] or associated with RNA-binding proteins such as argonaute (AGO), thereby functioning as systemic signaling molecules [59]. Given their crucial role in maintaining cellular homeostasis, disruptions in miRNA function are associated with numerous diseases, such as cancer [60], neurodegenerative disorders [61], cardiovascular diseases [62], and autoimmune conditions [63], positioning them as promising candidates for therapeutic intervention and biomarker discovery [64].
Advancements in bioinformatics and high-throughput sequencing technologies, like microarrays [65], RNA sequencing [66], and cross-linking immunoprecipitation (CLIP) assays [67], have enabled the identification of miRNA targets and expression patterns under several biological conditions, providing a more profound understanding of their biological functions [68]. As research continues to elucidate the complexities of miRNA networks, it has become increasingly clear that these small molecules exert remarkable control over gene expression, functioning as master regulators with significant implications for precision medicine, regenerative therapies, and disease intervention strategies [69].
Numerous studies have shown that certain miRNAs are upregulated in response to inflammatory mediators, playing a role in the enhancement of pain signaling by regulating the expression of cytokines, receptors, and ion channels involved in pain transmission [70,71]. In contrast, other miRNAs have been shown to attenuate excessive pain responses and promote various analgesic pathways [72,73]. Furthermore, miRNAs are implicated in the modulation of synaptic plasticity, a key process in the transition from acute to chronic pain, by influencing the expression of biomolecules linked to long-term potentiation (LTP) and long-term depression (LTD) in pain-processing areas including the spinal cord and brain [74]. Finally, recent studies have highlighted the potential of miRNAs as diagnostic biomarkers for chronic pain, as their expression profiles reflect the underlying pathological changes in pain pathways [75].
Table 1 outlines the altered expression of miRNAs in various preclinical chronic pain conditions, including inflammatory, neuropathic, and cancer-related pain. These miRNAs could serve as potential biomarkers or therapeutic targets.

Table 1.
List of dysregulated miRNAs across chronic pain disorders. Abbreviations: SNL (spinal nerve ligation), CCI (chronic constriction injury), CIPN (chemotherapy-induced neuropathic pain), CFA (complete Freund’s adjuvant), SNI (spared nerve injury), CIVP (chronic inflammatory visceral pain), DPN (diabetic peripheral neuropathy), SCI (spinal cord injury), BCP (bone cancer pain), CRC (colorectal cancer), bCCI (bilateral chronic constriction injury), CCI-IoN (chronic constriction injury of the infraorbital nerve), CCD (chronic compression of the DRG), L5-VRT (L5 ventral root transection), AIA (adjuvant-induced arthritis), and BTZ (bortezomib).
3. Small Interfering RNAs (siRNAs)
Small interfering RNAs (siRNAs) are fundamental elements of the RNA interference (RNAi) pathway, a highly conserved regulatory mechanism that facilitates post-transcriptional gene silencing [149]. The biosynthesis of siRNAs, characterized as double-stranded RNA (dsRNA) molecules, initiates in the cytoplasm with the cleavage of endogenous or exogenous dsRNA substrates by the enzyme Dicer (Figure 2) [150]. Dicer, in combination with some cofactors (such as TRBP) in mammals, recognizes and cleaves the dsRNA into siRNA duplexes with characteristic two-nucleotide 3′ overhangs [151]. Subsequently, the siRNA duplexes are loaded into the RISC, where they undergo strand selection, a process mediated by the helicase activity of AGO proteins [152].
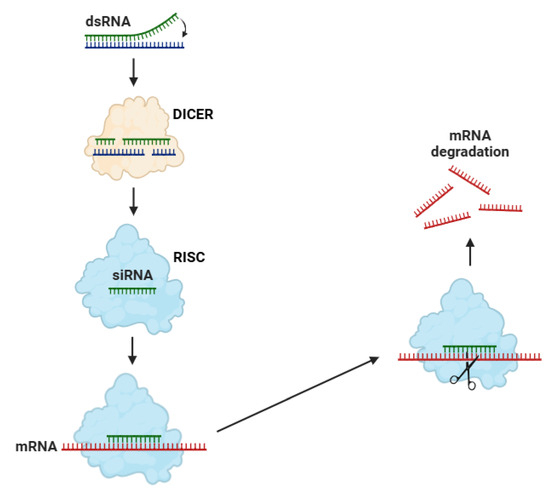
Figure 2.
The biosynthesis of siRNAs begins with the formation of dsRNA, which is recognized and cleaved by the enzyme Dicer into small interfering siRNAs. One strand of the siRNA is then incorporated into the RISC, guiding it to a complementary mRNA. RISC binds to the target mRNA and cleaves it at a specific site, contributing to its degradation and preventing protein biosynthesis. Abbreviations: dsRNA (double-strand RNA), RISC (RNA-induced silencing complex), and mRNA (messenger RNA). Figure adapted from Ref. [153].
The strand with the thermodynamically less stable 5′ end is preferentially retained as the guide strand, while the passenger strand is degraded [154,155]. The guide strand, incorporated into the mature RISC, facilitates sequence-specific gene silencing by recognizing and binding to complementary target mRNAs [155]. This binding enables the AGO-mediated endonucleolytic cleavage of the target transcript, leading to its degradation and preventing translation [156]. In certain organisms, amplification of the RNAi response occurs through RNA-dependent RNA polymerases (RdRPs), which generate secondary siRNAs from cleaved target mRNAs, thereby enhancing gene silencing [157,158].
A key feature of siRNAs is their antiviral function, where they specifically recognize and degrade viral RNA, thereby preventing viral replication [159]. Their ability to disrupt viral genomic material limits the progression of infection and enhances the host’s capacity to initiate an immune response [160]. In addition to their antiviral properties, siRNAs are crucial for maintaining genomic stability. siRNAs are crucial in silencing transposable elements, which are mobile genetic components capable of moving within the genome, potentially causing mutations and genomic instability [161], or contributing to the development of diseases such as cancer [149], neurodegenerative disorders [162], and genetic syndromes [163]. Conversely, siRNAs are involved in modulating gene expression, influencing the activation or repression of specific genes in response to various internal and external stimuli [164]. This function extends to cellular differentiation, where siRNAs contribute to the determination of cell fate during development, ensuring the proper identity and function of cells within tissues [165]. Moreover, siRNAs mediate many cellular responses to environmental stressors, including alterations in temperature, nutrient availability, and exposure to toxins [164].
Recent studies have highlighted the potential of siRNAs in pain management by selectively modulating the expression of genes involved in pain pathways. Through the targeted silencing of key molecules such as inflammatory cytokines [166], ion channels [167], and neurotransmitters [168], siRNAs offer a promising approach to developing effective and personalized therapies for chronic pain. Table 2 provides an overview of the siRNAs used for treating inflammatory, neuropathic, and cancer-related pain.

Table 2.
List of siRNAs employed in the treatment of several chronic pain disorders. Abbreviations: CCI (chronic constriction injury), ERK (extracellular signal-regulated kinase), STAT3 (signal transducer and activator of transcription 3), CFA (complete Freund’s adjuvant), NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells), IL-1β (interleukin 1 beta), DPN (diabetic peripheral neuropathy), PARP1 (poly-ADP-ribose- polymerase 1), CIPN (chemotherapy-induced neuropathic pain), SNL (spinal nerve ligation), BCP (bone cancer pain), PDN (painful diabetic neuropathy), TLR4 (Toll-like receptor 4), PANX1 (pannexin 1), NLRP3 (NOD-like receptor family pyrin domain containing 3), SDH (spinal dorsal horn), IBA-1 (ionized calcium binding adapter molecule 1), PKC (protein kinase C), PI3K (phosphoinositide 3-kinase), PKB (protein kinase B), AAV (adeno-associated virus), SCI (spinal cord injury), LPS (lipopolysaccharide), CCL2 (C-C motif chemokine ligand 2), KCC2 (potassium chloride cotransporter 2), CACNA1H (calcium voltage-gated channel subunit alpha1 H), p-ERK (phosphorylated extracellular signal-regulated kinase), GFAP (glial fibrillary acidic protein), and OX42 (OX42 antigen/CD11b).
4. Long Non-Coding RNAs (lncRNAs)
Long non-coding RNAs (lncRNAs) represent a complex class of non-protein-coding transcripts that exceed 200 nucleotides in length [201]. lncRNAs have emerged as key regulators of gene expression at multiple levels, including chromatin remodeling [202], transcriptional and post-transcriptional modulation [203,204] across many organisms. Unlike miRNAs and siRNAs, which mainly function through base-pairing interactions with target mRNAs [41,154], lncRNAs regulate gene expression via a broad spectrum of mechanisms, acting as molecular scaffolds, decoys, sponges, and guides [205]. By facilitating the recruitment of specific protein complexes to genomic loci, they regulate gene expression in a context-dependent manner. However, developments in technology have shown that numerous lncRNAs contain small open reading frames (sORFs) capable of encoding micropeptides. These micropeptides mediate key biological functions, including the regulation of homeostasis, inflammation, immune responses, metabolism, and tumor progression [206].
The biogenesis of lncRNAs (Figure 3) shares similarities with that of protein-coding mRNAs. They are transcribed by RNA polymerase II and undergo several post-transcriptional modifications, including the addition of a 5′ cap, polyadenylation at the 3′ end, and splicing [207]. Despite these similarities, lncRNAs lack protein-coding potential due to the absence of long open reading frames (ORFs) and ribosomal translation initiation signals [208]. While some lncRNAs are polyadenylated, others remain non-polyadenylated, adding another layer of complexity to their function [209,210]. The expression of lncRNAs is often highly cell-type-specific and tightly regulated, with distinct spatial localization patterns that contribute to their functional diversity [211].
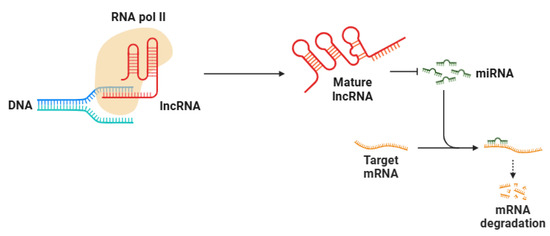
Figure 3.
lncRNAs are mainly transcribed by RNA polymerase II from non-coding genomic regions. Their biosynthesis closely resembles mRNA processing, encompassing transcription initiation, elongation, splicing, 5′ capping, and 3′ polyadenylation. lncRNAs act as molecular sponges for miRNAs, inhibiting miRNA-mediated repression of target genes. Abbreviations: RNA pol II (RNA polymerase II), lncRNA (long non-coding RNA), miRNA (microRNA), and mRNA (messenger RNA). Figure created with BioRender 23.
Depending on their localization, lncRNAs can exert their effects either within the nucleus or the cytoplasm. Nuclear lncRNAs play crucial roles in chromatin architecture and epigenetic modifications, often interacting with chromatin-modifying complexes such as Polycomb Repressive Complex 2 (PRC2) to induce gene silencing through histone modifications [212,213]. Furthermore, lncRNAs can recruit transcription factors to specific genomic loci, regulating transcription in a gene-specific fashion [214]. Conversely, in the cytoplasm, lncRNAs affect mRNA stability, translation, and post-transcriptional regulation by interacting with RNA-binding proteins, miRNAs, and ribosomes [215]. One intriguing mechanism is their ability to function as competing endogenous RNAs (ceRNAs), wherein lncRNAs act as molecular sponges for miRNAs, thereby preventing miRNA-mediated repression of target genes [216]. This ceRNA network adds another regulatory layer to gene expression and has significant implications in various biological processes [217].
Functionally, lncRNAs are deeply embedded in numerous physiological processes, including cell differentiation [218], proliferation [219], apoptosis [220], immune responses [221], and genomic imprinting [222]. Furthermore, recent evidence indicates that lncRNAs can be involved in intercellular communication by being selectively incorporated into extracellular vesicles, such as exosomes [223]. Once secreted, these lncRNA-containing vesicles can be taken up by recipient cells, where they influence gene expression and cellular behavior, further highlighting their role in systemic regulation [223].
Given their regulatory roles, dysregulation of lncRNAs has been strongly associated with various diseases, including cancer [224], neurodegenerative disorders [225], cardiovascular diseases [226], and metabolic syndromes [227]. In cancer, dysregulated lncRNA expression can play a role in tumor initiation, progression, metastasis, and resistance to therapy by modulating oncogenic and tumor-suppressor pathways [224]. Some lncRNAs function as oncogenes, promoting tumor growth and invasion, while others act as tumor suppressors, inhibiting malignancy [228]. In neurodegenerative disorders, lncRNAs have been implicated in neuroinflammation, synaptic dysfunction, and protein aggregation, suggesting their potential as therapeutic targets [225]. On the other hand, in cardiovascular diseases, lncRNAs participate in cardiac hypertrophy and atherosclerosis, further emphasizing their broad biological significance [226]. Techniques such as RNA sequencing (RNA-seq) [229], chromatin isolation by RNA purification (ChIRP) [230], and CLIP [231] have provided valuable insights into lncRNA functions and their molecular interactions. These methodologies have provided a substantial toolkit for dissecting the complex regulatory networks mediated by lncRNAs. By mapping the interactions between lncRNAs and the DNA-RNA-protein complex, researchers can construct molecular interaction networks that reveal the roles of lncRNAs in gene regulation.
In the field of chronic pain research, increasing evidence suggests that lncRNAs play a significant role in modulating pain by regulating the expression of pro-nociceptive and anti-nociceptive genes, influencing neuroinflammatory pathways, and affecting synaptic plasticity in critical pain-processing regions such as the spinal cord and brain [232]. Some lncRNAs have been shown to contribute to pain sensitization through interactions with inflammatory cytokines, ion channels, and intracellular signaling pathways involved in pain transmission [233].
Finally, lncRNAs have emerged as valuable diagnostic biomarkers for chronic pain conditions, as their expression patterns reflect underlying pathological changes in pain-processing circuits. The detection of several lncRNAs in biofluids including blood [234], cerebrospinal fluid [235], and saliva [236] makes them promising candidates for non-invasive pain diagnostics. Identifying distinct lncRNA signatures associated with various pain conditions could support the development of precision medicine approaches, enabling personalized therapies tailored to individual patients [237]. Table 3 summarizes the alterations in the expression of lncRNAs observed across various preclinical models of chronic pain, including those related to inflammation, nerve injury, and cancer. These changes in lncRNA expression are significant as they could serve as biomarkers for the diagnosis of chronic pain conditions or as targets for the development of novel therapeutic approaches aimed at pain relief. Targeting these lncRNAs could facilitate the development of effective treatments for individuals afflicted with different types of chronic pain.

Table 3.
List of dysregulated lncRNAs across chronic pain disorders. Abbreviations: CFA (complete Freund’s adjuvant), lncRNA (long non-coding RNA), MEG3 (maternally expressed gene 3), TRPV1 (transient receptor potential vanilloid 1), SNL (spinal nerve ligation), DRG (dorsal root ganglion), NEAT1 (nuclear enriched abundant transcript 1), mRNA (messenger RNA), TNFAIP1 (tumor necrosis factor alpha induced protein 1), AKT (protein kinase B), CREB (cAMP response element binding protein), BCP (bone cancer pain), siRNA (small interfering RNA), miRNA (microRNA), CXCL13 (C-X-C motif chemokine ligand 13), SCI (spinal cord injury), CXCR5 (C-X-C chemokine receptor type 5), CCI (chronic constriction injury), WNT5A (Wnt family member 5A), JMJD1A (Jumonji domain containing 1A), bCCI (bilateral chronic constriction injury), SOCS3 (suppressor of cytokine signaling 3), JAK2 (Janus kinase 2), STAT3 (signal transducer and activator of transcription 3), SNL (spinal nerve ligation), SNHG1 (small nucleolar RNA host gene 1), pSNL (partial spinal nerve ligation), BAI1 (brain-specific angiogenesis inhibitor 1), EZH2 (enhancer of zeste homolog 2), CXCL9 (C-X-C motif chemokine ligand 9), and c-Fos (FBJ murine osteosarcoma viral oncogene homolog).
5. Circular RNAs (circRNAs)
Circular RNAs (circRNAs) have attracted significant attention owing to their distinctive and remarkable structural characteristics, which differentiate them from conventional linear RNAs. Unlike linear RNAs that possess a 5′ cap and a 3′ poly(A) tail [252], circRNAs are distinguished by their covalently closed loop structures, which render them more stable and resistant to exonuclease-mediated degradation [253]. This stability contributes to the prolonged presence of circRNAs in various bodily fluids, positioning them in a wide range of physiological and pathological processes [254].
CircRNAs arise from pre-mRNAs via a non-canonical splicing mechanism known as back-splicing (Figure 4). In this process, a downstream splice donor site is connected to an upstream splice acceptor site, leading to the formation of a circRNA [255]. This back-splicing event contrasts with the typical linear splicing processes involved in the production of conventional mRNA molecules [256]. The different structural forms of circRNAs, including exonic circRNAs (ecircRNAs), circular intronic RNAs (ciRNAs), and exon-intron circRNAs (EIciRNAs), further support their roles in gene expression regulation and cellular functions [257,258,259]. The biogenesis of circRNAs is regulated by a host of factors, such as RNA-binding proteins (RBPs), spliceosome components, and cis-regulatory elements [260,261]. These factors help facilitate the back-splicing process, ensuring that circRNAs are produced in a controlled and context-specific manner. Key RBPs like MBL (mannose-binding lectin) and FUS (fused in sarcoma) are crucial for stabilizing circRNA formation by either aiding intronic base-pairing or recruiting the splicing machinery [262,263]. Furthermore, the complementary sequences within intronic regions play a critical role in determining the type and diversity of circRNAs that are expressed across different tissues and developmental stages [264].
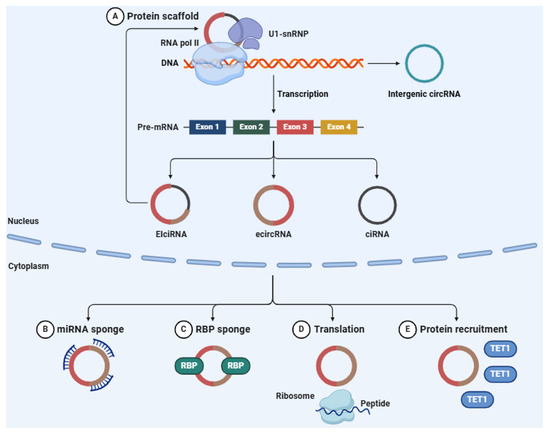
Figure 4.
circRNAs are generated via the back-splicing of precursor mRNAs, resulting in RNA molecules that are covalently closed. These circRNAs participate in the regulation of gene expression by functioning as molecular sponges for miRNAs. Through their interaction with miRNAs, circRNAs inhibit their ability to target mRNAs, thereby modulating gene expression. In a similar fashion, circRNAs can sequester RBPs, influencing several processes such as translation, protein recruitment, and RNA processing. Abbreviations: U1-snRNP (U1 small nuclear ribonucleoprotein), RNA pol II (RNA polymerase II), DNA (deoxyribonucleic acid), circRNA (circular RNA), pre-mRNA precursor messenger RNA), EIciRNA (exon-intron circRNA), ecircRNA (exonic circRNA), ciRNA (circular intronic RNA), miRNA (microRNA), RBP (RNA-binding protein), TET1 (ten-eleven translocation methylcytosine dioxygenase 1). Figure adapted from an original illustration created with BioRender 23 (Authorship: Martina Maritan).
CircRNAs have been identified as important regulators of gene expression, particularly through their interactions with different miRNAs. One of the most documented roles of circRNAs is their capacity to function as molecular sponges for miRNAs [265]. By sequestering miRNAs, circRNAs prevent them from interacting with their target mRNAs, thereby modulating gene expression at the post-transcriptional level [266]. In addition to acting as miRNA sponges, circRNAs have also been implicated in modulating transcription, adjusting alternative splicing events, and even regulating translation [267,268,269]. Certain circRNAs have been shown to harbor internal ribosome entry sites (IRESs) or post-transcriptional modifications, such as N6-methyladenosine (m6A), which enable them to serve as templates for translation into functional peptides [270,271]. These functions underscore the versatility of circRNAs in cellular regulation and their potential involvement in many cellular processes, from proliferation to apoptosis [272]. The dysregulation of circRNAs has been implicated in a diverse array of pathological conditions, including cancer [273], neurodegenerative diseases [274], and cardiovascular disorders [275].
The role of circRNAs in sensory processing represents an energizing area of research, with emerging evidence suggesting that these ncRNAs may contribute significantly to the pathophysiology of pain. Within the context of chronic pain, specific circRNAs have been shown to be upregulated in pain-associated tissues, including the DRG (dorsal root ganglion) and spinal cord [276,277]. These circRNAs interact with miRNAs, thereby influencing the expression of genes that regulate pain signaling pathways. For instance, circRNAs regulate the expression of ion channels, like ASICs (acid-sensing ion channels), that are essential for the transmission of pain signals [278]. Moreover, several circRNAs have been shown to modulate the activity of pain-related transcription factors, such as AP-1 (activator protein 1) and NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells), as well as signaling molecules including cytokines and neurotrophins, which are pivotal in the sensitization of nociceptors [279,280,281,282]. Through their ability to influence the expression of those genes involved in inflammation, neuronal excitability, and synaptic plasticity, circRNAs contribute to the complex network that regulates many chronic pain responses [283,284,285]. Especially, circRNAs that modulate numerous inflammatory responses have been shown to influence the onset and persistence of inflammatory pain. The dysregulation of circRNAs may contribute to the maintenance of chronic pain states by altering the balance between pro-inflammatory and anti-inflammatory signaling [286].
Finally, given their role in regulating gene expression and several cellular processes, circRNAs hold immense potential as both diagnostic biomarkers and therapeutic targets for diseases associated with chronic pain [287]. The implications of circRNAs in pain represent a promising frontier in both basic research and clinical applications. By understanding how circRNAs contribute to pain modulation and chronic pain conditions, researchers may develop novel therapeutic strategies aimed at alleviating pain through the manipulation of circRNA function [288,289]. In this way, circRNAs may emerge as key players in the next generation of pain management therapies, potentially offering new hope for patients suffering from chronic pain disorders.
Table 4 presents a comprehensive overview of circRNA expression alterations identified in various preclinical models of chronic pain, including those linked to inflammation, neuropathies, and cancer. These alterations are highly relevant, as they could be used as biomarkers for the diagnosis of chronic pain or as targets for the development of novel therapeutic approaches in pain management.

Table 4.
List of dysregulated circRNAs in chronic pain conditions. Abbreviations: CIVP (chronic inflammatory visceral pain), GFAP (glial fibrillary acidic protein), CFA (complete Freund’s adjuvant), DPN (diabetic peripheral neuropathy), DRG (dorsal root ganglion), STZ (streptozotocin), CCI (chronic constriction injury), NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells), IL-1β (interleukin 1 beta), IL-6 (interleukin 6), TNF-α (tumor necrosis factor alpha), SLICK (sequence like an intermediate conductance K channel), ENO1 (enolase 1), DHX9 (DEAH-box helicase 9), UBR5 (ubiquitin protein ligase E3 component N-recognin 5), ALB (albumin), COX-2 (cyclooxygenase 2), IL-10 (interleukin 10), CCI-IoN (chronic constriction injury of the infraorbital nerve), IST1 (increased sodium tolerance 1), LC3-II (microtubule-associated protein 1 light chain 3-II), p62 (Sequestosome 1-SQSTM1-), 3′-UTR (3′-untranslated region), KCNK1 (potassium channel, two-pore domain subfamily K, member 1), SNI (spared nerve injury), GAD65 (glutamate decarboxylase 65), NK1R (neurokinin 1 receptor), SNL (spinal nerve ligation), VEGFB (vascular endothelial growth factor B), Ybx1 (Y-Box binding protein 1), Wnt5a (Wnt family member 5A), LPAR3 (lysophosphatidic acid receptor 3), and BCP (bone cancer pain).
6. Conclusions
The role of ncRNAs in the development of chronic pain is increasingly recognized as a key area of research with significant therapeutic potential (Table 5). ncRNAs, including miRNAs, siRNAs, lncRNAs, and circRNAs, have emerged as pivotal regulators of gene expression, impacting numerous cellular processes linked to pain perception and chronic pain pathophysiology. These biomolecules regulate the expression of pain-related genes, influence neuroinflammation, alter ion channel function, and affect the plasticity of neural circuits involved in pain processing. Dysregulation of ncRNA has been associated with some chronic pain conditions, underscoring their role as potential biomarkers for diagnosis and targets for treatment.

Table 5.
Comparative overview of ncRNA types involved in chronic pain: stability, specificity, and translational challenges. Abbreviations: ncRNA (non-coding RNA), miRNA (microRNA), siRNA (small interfering RNA), circRNA (circular RNA), and lncRNA (long non-coding RNA).
A growing body of evidence supports the differential expression of numerous classes of ncRNAs in both animal models and human patients with chronic pain conditions, including neuropathic, inflammatory, and cancer-related pain. These results emphasize the prospective utility of ncRNAs as minimally invasive diagnostic biomarkers, detectable in biofluids including blood and cerebrospinal fluid, offering new promising possibilities for early detection, pain subtyping, and monitoring of treatment responses [307]. Additionally, recent research highlights that miRNAs function as suppressors of mRNA translation, leading to reduced protein levels of pain modulators, while lncRNAs and circRNAs act as molecular sponges, sequestering miRNAs and influencing their regulatory activities. siRNAs also play a crucial role in this regulatory network by promoting the degradation of specific mRNA. The complex interactions between these ncRNAs and their target molecules suggest a sophisticated regulatory network that contributes to both the persistence and intensification of chronic pain states. Understanding these complex interactions opens new avenues for the development of ncRNA-based therapeutics, such as miRNA mimics [308], antagomirs [309], lncRNA modulators [310], and siRNA-based treatments [311], aiming to restore normal gene expression patterns and alleviate pain.
However, despite these advances, significant barriers hinder the clinical translation of ncRNA-based diagnostics and therapeutics. These include challenges related to the stability, specificity, and immunogenicity of RNA-targeting agents, efficient delivery across biological barriers (particularly the blood–brain barrier), and the risk of off-target effects due to the pleiotropic and context-dependent nature of ncRNA function [312]. Interindividual variability in ncRNA expression profiles, differences across pain etiologies, and the current lack of standardized detection and quantification methods further complicate clinical implementation [313]. To overcome these limitations, future research should prioritize the development of targeted delivery systems (e.g., nanoparticle-based platforms), refine computational tools for predicting ncRNA-target interactions, and conduct clinical studies to validate candidate ncRNAs across diverse patient populations [314,315,316]. In parallel, regulatory frameworks must adapt to the unique characteristics of RNA-based therapeutics. Through the resolution of these challenges, the field can advance toward the clinical integration of ncRNA-based strategies, ultimately contributing to more precise, personalized, and effective approaches to chronic pain management.
In conclusion, ncRNAs show significant potential for elucidating the complex mechanisms underlying chronic pain and developing innovative strategies. Continued exploration of ncRNA biology will be essential to overcoming current limitations and harnessing their full therapeutic potential in the management of chronic pain.
Funding
This research received no external funding.
Data Availability Statement
Not applicable. No new data were generated.
Acknowledgments
I gratefully acknowledge BioRender for providing a professional and scientifically rigorous platform that enabled the creation of high-quality graphical illustrations presented in this review.
Conflicts of Interest
The author declares no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| 3′-UTR | 3′-untranslated region |
| AAV | Adeno-associated virus |
| AGO | Argonaute |
| AGO2 | Argonaute 2 |
| AIA | Adjuvant-induced arthritis |
| AKT | Protein kinase B |
| ALB | Albumin |
| AP-1 | Activator protein 1 |
| ASIC | Acid-sensing ion channel |
| BAI1 | Brain-specific angiogenesis inhibitor 1 |
| bCCI | Bilateral chronic constriction injury |
| BCP | Bone cancer pain |
| BTZ | Bortezomib |
| CACNA1H | Calcium voltage-gated channel subunit alpha1 H |
| CCD | Chronic compression of the DRG |
| CCI | Chronic constriction injury |
| CCI-IoN | Chronic constriction injury of the infraorbital nerve |
| CCL2 | C-C motif chemokine ligand 2 |
| ceRNA | Competing endogenous RNA |
| CFA | Complete Freund’s adjuvant |
| c-Fos | FBJ murine osteosarcoma viral oncogene homolog |
| ChIRP | Chromatin isolation by RNA purification |
| ciRNA | Circular intronic RNA |
| circRNA | Circular RNA |
| CIPN | Chemotherapy-induced neuropathic pain |
| CIVP | Chronic inflammatory visceral pain |
| CLIP | Class II-associated invariant chain peptide |
| CNS | Central nervous system |
| COX-2 | Cyclooxygenase 2 |
| CRC | Colorectal cancer |
| CREB | cAMP response element binding protein |
| CXCL13 | C-X-C motif chemokine ligand 13 |
| CXCL9 | C-X-C motif chemokine ligand 9 |
| CXCR5 | C-X-C chemokine receptor type 5 |
| DGCR8 | DiGeorge Syndrome critical region 8 |
| DNA | Deoxyribonucleic acid |
| DPN | Diabetic peripheral neuropathy |
| DRG | Dorsal root ganglion |
| DHX9 | DEAH-box helicase 9 |
| dsRNA | Double-strand RNA |
| ecircRNA | Exonic circRNA |
| EIciRNA | Exon-intron circRNA |
| ENO1 | Enolase 1 |
| ERK | Extracellular signal-regulated kinase |
| EZH2 | Enhancer of zeste homolog 2 |
| FUS | Fused in sarcoma |
| GAD65 | Glutamate decarboxylase 65 |
| GFAP | Glial fibrillary acidic protein |
| GTP | Guanosine triphosphate |
| IASP | International Association for the Study of Pain |
| IBA-1 | Ionized calcium binding adapter molecule 1 |
| IFT52 | Intraflagellar transport 52 |
| IFT88 | Intraflagellar transport 88 |
| IKBKB | Inhibitor of NF-κB |
| IL-1β | Interleukin 1 beta |
| IL-10 | Interleukin 10 |
| IL-6 | Interleukin 6 |
| IRES | Internal ribosome entry site |
| IST1 | Increased sodium tolerance 1 |
| JAK2 | Janus kinase 2 |
| JMJD1A | Jumonji domain containing 1A |
| KCC2 | Potassium chloride cotransporter 2 |
| KCNK1 | Potassium channel, two-pore domain subfamily K, member 1 |
| L5-VRT | L5 ventral root transection |
| lncRNA | Long non-coding RNA |
| LC3-II | Microtubule-associated protein 1 light chain 3-II |
| LPAR3 | Lysophosphatidic acid receptor 3 |
| LPS | Lipopolysaccharide |
| LTD | Long-term depression |
| LTP | Long-term potentiation |
| m6A | N6-methyladenosine |
| MBL | Mannose-binding lectin |
| MEG3 | Maternally expressed gene 3 |
| miRNA | MicroRNA |
| mRNA | Messenger RNA |
| NAMPT | Nicotinamide phosphoribosyltransferase |
| ncRNA | Non-coding RNA |
| NEAT1 | Nuclear enriched abundant transcript 1 |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NK1R | Neurokinin 1 receptor |
| NLRP3 | NOD-like receptor family pyrin domain containing 3 |
| NR2B | N-methyl-D-aspartate receptor subunit 2B |
| ORF | Open reading frame |
| OX42 | OX42 antigen/CD11b |
| p62 | Sequestosome 1 (SQSTM1) |
| PANX1 | Pannexin 1 |
| PARP1 | Poly(ADP-ribose) polymerase 1 |
| p-ERK | Phosphorylated extracellular signal-regulated kinase |
| PDN | Painful diabetic neuropathy |
| PI3K | Phosphoinositide 3-kinase |
| PI3KCB | Phosphoinositide 3-kinase catalytic subunit beta |
| PKB | Protein kinase B |
| PKC | Protein kinase C |
| PKM2 | Pyruvate kinase M2 |
| PNS | Peripheral nervous system |
| PRC2 | Polycomb repressive complex 2 |
| pre-mRNA | Precursor messenger RNA |
| pre-miRNA | Precursor microRNA |
| pri-miRNA | Primary microRNA |
| pSNL | Partial spinal nerve ligation |
| PVT1 | Plasmacytoma variant translocation 1 |
| Ran | Ras-related nuclear protein |
| RBP | RNA-binding protein |
| RdRPs | RNA-dependent RNA polymerase |
| RISC | RNA-induced silencing complex |
| RNA | Ribonucleic acid |
| RNA Pol II | RNA polymerase II |
| RNA Pol III | RNA polymerase III |
| RNA-seq | RNA sequencing |
| SCI | Spinal cord injury |
| SDH | Spinal dorsal horn |
| SGK3 | Serum/glucocorticoid regulated kinase family member 3 |
| siRNA | Small interfering RNA |
| SLICK | Sequence like an intermediate conductance K channel |
| SNHG1 | Small nucleolar RNA host gene 1 |
| SNI | Spared nerve injury |
| SNL | Spinal nerve ligation |
| SOCS3 | Suppressor of cytokine signaling 3 |
| STAT3 | Signal transducer and activator of transcription 3 |
| STZ | Streptozotocin |
| TET1 | Ten-eleven translocation methylcytosine dioxygenase 1 |
| TLR4 | Toll-like receptor 4 |
| TNFAIP1 | Tumor necrosis factor alpha induced protein 1 |
| TNF-α | Tumor necrosis factor alpha |
| TRBP | Transactivation response element RNA-binding protein |
| TRPV1 | Transient receptor potential vanilloid 1 |
| U1-snNRP | U1 small nuclear ribonucleoprotein |
| UBR5 | Ubiquitin protein ligase E3 component N-recognin 5 |
| VEGFB | Vascular endothelial growth factor B |
| WNT5A | Wnt family member 5A |
| Ybx1 | Y-Box binding protein 1 |
| XPO5 | Exportin 5 |
References
- Raja, S.N.; Carr, D.B.; Cohen, M.; Finnerup, N.B.; Flor, H.; Gibson, S.; Keefe, F.J.; Mogil, J.S.; Ringkamp, M.; Sluka, K.A.; et al. The revised International Association for the Study of Pain definition of pain: Concepts, challenges, and compromises. Pain 2020, 161, 1976–1982. [Google Scholar] [CrossRef] [PubMed]
- Vader, K.; Bostick, G.P.; Carlesso, L.C.; Hunter, J.; Mesaroli, G.; Perreault, K.; Tousignant-Laflamme, Y.; Tupper, S.; Walton, D.M.; Wideman, T.H.; et al. The Revised IASP Definition of Pain and Accompanying Notes: Considerations for the Physiotherapy Profession. Physiother. Can. 2021, 73, 103–106. [Google Scholar] [CrossRef]
- Malfliet, A.; Coppieters, I.; Van Wilgen, P.; Kregel, J.; De Pauw, R.; Dolphens, M.; Ickmans, K. Brain changes associated with cognitive and emotional factors in chronic pain: A systematic review. Eur. J. Pain 2017, 21, 769–786. [Google Scholar] [CrossRef]
- Lee, G.I.; Neumeister, M.W. Pain: Pathways and physiology. Clin. Plast. Surg. 2020, 47, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, P.; Hu, Y.; Meng, J. Pain Modulates Responses to Emotional Stimuli. Front. Psychol. 2020, 11, 595987. [Google Scholar] [CrossRef]
- Nikolenko, V.N.; Shelomentseva, E.M.; Tsvetkova, M.M.; Abdeeva, E.I.; Giller, D.B.; Babayeva, J.V.; Achkasov, E.E.; Gavryushova, L.V.; Sinelnikov, M.Y. Nociceptors: Their Role in Body’s Defenses, Tissue Specific Variations and Anatomical Update. J. Pain Res. 2022, 15, 867–877. [Google Scholar] [CrossRef]
- Middleton, S.J.; Barry, A.M.; Comini, M.; Li, Y.; Ray, P.R.; Shiers, S.; Themistocleous, A.C.; Uhelski, M.L.; Yang, X.; Dougherty, P.M.; et al. Studying human nociceptors: From fundamentals to clinic. Brain 2021, 144, 1312–1335. [Google Scholar] [CrossRef] [PubMed]
- Dubin, A.E.; Patapoutian, A. Nociceptors: The sensors of the pain pathway. J. Clin. Investig. 2010, 120, 3760–3772. [Google Scholar] [CrossRef]
- De Ridder, D.; Adhia, D.; Vanneste, S. The anatomy of pain and suffering in the brain and its clinical implications. Neurosci. Biobehav. Rev. 2021, 130, 125–146. [Google Scholar] [CrossRef]
- Orr, P.M.; Shank, B.C.; Black, A.C. The Role of Pain Classification Systems in Pain Management. Crit. Care Nurs. Clin. N. Am. 2017, 29, 407–418. [Google Scholar] [CrossRef]
- Mears, L.; Mears, J. The pathophysiology, assessment, and management of acute pain. Br. J. Nurs. 2023, 32, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Mills, S.E.E.; Nicolson, K.P.; Smith, B.H. Chronic pain: A review of its epidemiology and associated factors in population-based studies. Br. J. Anaesth. 2019, 123, e273–e283. [Google Scholar] [CrossRef] [PubMed]
- Fu, K.; Robbins, S.R.; McDougall, J.J. Osteoarthritis: The genesis of pain. Rheumatology 2018, 57, iv43–iv50. [Google Scholar] [CrossRef]
- Nicol, V.; Verdaguer, C.; Daste, C.; Bisseriex, H.; Lapeyre, É.; Lefèvre-Colau, M.M.; Rannou, F.; Rören, A.; Facione, J.; Nguyen, C. Chronic Low Back Pain: A Narrative Review of Recent International Guidelines for Diagnosis and Conservative Treatment. J. Clin. Med. 2023, 12, 1685. [Google Scholar] [CrossRef]
- García-Domínguez, M. A Comprehensive Analysis of Fibromyalgia and the Role of the Endogenous Opioid System. Biomedicines 2025, 13, 165. [Google Scholar] [CrossRef] [PubMed]
- Mestdagh, F.; Steyaert, A.; Lavand’homme, P. Cancer Pain Management: A Narrative Review of Current Concepts, Strategies, and Techniques. Curr. Oncol. 2023, 30, 6838–6858. [Google Scholar] [CrossRef]
- Crofford, L.J. Chronic Pain: Where the Body Meets the Brain. Trans. Am. Clin. Climatol. Assoc. 2015, 126, 167–183. [Google Scholar]
- Dagnino, A.P.A.; Campos, M.M. Chronic Pain in the Elderly: Mechanisms and Perspectives. Front. Hum. Neurosci. 2022, 16, 736688. [Google Scholar] [CrossRef]
- Larsson, C.; Hansson, E.E.; Sundquist, K.; Jakobsson, U. Chronic pain in older adults: Prevalence, incidence, and risk factors. Scand. J. Rheumatol. 2017, 46, 317–325. [Google Scholar] [CrossRef]
- Zimmer, Z.; Fraser, K.; Grol-Prokopczyk, H.; Zajacova, A. A global study of pain prevalence across 52 countries: Examining the role of country-level contextual factors. Pain 2022, 163, 1740–1750. [Google Scholar] [CrossRef]
- Tinnirello, A.; Mazzoleni, S.; Santi, C. Chronic Pain in the Elderly: Mechanisms and Distinctive Features. Biomolecules 2021, 11, 1256. [Google Scholar] [CrossRef] [PubMed]
- García-Domínguez, M. Chronic pain in the elderly: Exploring cellular and molecular mechanisms and therapeutic perspectives. Front. Aging 2024, 5, 1477017. [Google Scholar] [CrossRef] [PubMed]
- Osborne, N.R.; Davis, K.D. Sex and gender differences in pain. Int. Rev. Neurobiol. 2022, 164, 277–307. [Google Scholar]
- Khan, J.S.; Hah, J.M.; Mackey, S.C. Effects of smoking on patients with chronic pain: A propensity-weighted analysis on the Collaborative Health Outcomes Information Registry. Pain 2019, 160, 2374–2379. [Google Scholar] [CrossRef]
- Karimi, R.; Mallah, N.; Nedjat, S.; Beasley, M.J.; Takkouche, B. Association between alcohol consumption and chronic pain: A systematic review and meta-analysis. Br. J. Anaesth. 2022, 129, 355–365. [Google Scholar] [CrossRef]
- Okifuji, A.; Hare, B.D. The association between chronic pain and obesity. J. Pain Res. 2015, 8, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Mattei, A.L.; Bailly, N.; Meissner, A. DNA methylation: A historical perspective. Trends Genet. 2022, 38, 676–707. [Google Scholar] [CrossRef]
- Liu, R.; Wu, J.; Guo, H.; Yao, W.; Li, S.; Lu, Y.; Jia, Y.; Liang, X.; Tang, J.; Zhang, H. Post-translational modifications of histones: Mechanisms, biological functions, and therapeutic targets. MedComm. 2023, 4, e292. [Google Scholar] [CrossRef]
- Nemeth, K.; Bayraktar, R.; Ferracin, M.; Calin, G.A. Non-coding RNAs in disease: From mechanisms to therapeutics. Nat. Rev. Genet. 2024, 25, 211–232. [Google Scholar] [CrossRef]
- Nirvanie-Persaud, L.; Millis, R.M. Epigenetics and Pain: New Insights to an Old Problem. Cureus 2022, 14, e29353. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Zarrabi, A.; Mostafavi, E.; Aref, A.R.; Sethi, G.; Wang, L.; Tergaonkar, V. Non-coding RNA-based regulation of inflammation. Semin. Immunol. 2022, 59, 101606. [Google Scholar] [CrossRef]
- Nayak, M.; Das, D.; Pradhan, J.; Ahmed, R.G.; Laureano-Melo, R.; Dandapat, J. Epigenetic signature in neural plasticity: The journey so far and journey ahead. Heliyon 2022, 8, e12292. [Google Scholar] [CrossRef]
- Giordano, R.; Kjær-Staal Petersen, K.; Arendt-Nielsen, L. The link between epigenetics, pain sensitivity and chronic pain. Scand. J. Pain 2022, 22, 664–666. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Gao, R.; Zhou, R.; Chen, H.; Liu, C.; Zhu, T.; Chen, C. The emerging power and promise of non-coding RNAs in chronic pain. Front. Mol. Neurosci. 2022, 15, 1037929. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Sahoo, S.S.; Chauss, D.; Kazemian, M.; Afzali, B. Non-coding RNAs in immunoregulation and autoimmunity: Technological advances and critical limitations. J. Autoimmun. 2023, 134, 102982. [Google Scholar] [CrossRef] [PubMed]
- Musgrove, M.R.B.; Mikhaylova, M.; Bredy, T.W. Fundamental Neurochemistry Review: At the intersection between the brain and the immune system: Non-coding RNAs spanning learning, memory and adaptive immunity. J. Neurochem. 2024, J168, 961–976. [Google Scholar] [CrossRef]
- Li, X.; Jin, D.S.; Eadara, S.; Caterina, M.J.; Meffert, M.K. Regulation by noncoding RNAs of local translation, injury responses, and pain in the peripheral nervous system. Neurobiol. Pain 2023, 13, 100119. [Google Scholar] [CrossRef]
- Wang, J.; Mei, J.; Ren, G. Plant microRNAs: Biogenesis, Homeostasis, and Degradation. Front. Plant Sci. 2019, 10, 360. [Google Scholar] [CrossRef]
- Gebert, L.F.R.; MacRae, I.J. Regulation of microRNA function in animals. Nat. Rev. Mol. Cell Biol. 2019, 20, 21–37. [Google Scholar] [CrossRef]
- Mishra, R.; Kumar, A.; Ingle, H.; Kumar, H. The Interplay Between Viral-Derived miRNAs and Host Immunity During Infection. Front. Immunol. 2020, 10, 3079. [Google Scholar] [CrossRef]
- Shang, R.; Lee, S.; Senavirathne, G.; Lai, E.C. microRNAs in action: Biogenesis, function and regulation. Nat. Rev. Genet. 2023, 24, 816–833. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, M.; Han, J.; Yeom, K.H.; Lee, S.; Baek, S.H.; Kim, V.N. MicroRNA genes are transcribed by RNA polymerase II. EMBO J. 2004, 23, 4051–4060. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Huang, C.; Xia, X.G. A tightly regulated Pol III promoter for synthesis of miRNA genes in tandem. Biochim. Biophys. Acta 2008, 1779, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Yi, R.; Qin, Y.; Macara, I.G.; Cullen, B.R. Exportin-5 mediates the nuclear export of pre-microRNAs and short hairpin RNAs. Genes Dev. 2003, 17, 3011–3016. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Wang, J.; Liu, C.P.; Wang, H.W.; Xu, R.M. Structural Basis for pri-miRNA Recognition by Drosha. Mol. Cell 2020, 78, 423–433.e5. [Google Scholar] [CrossRef]
- Lee, Y.Y.; Lee, H.; Kim, H.; Kim, V.N.; Roh, S.H. Structure of the human DICER-pre-miRNA complex in a dicing state. Nature 2023, 615, 331–338. [Google Scholar] [CrossRef]
- Iwakawa, H.O.; Tomari, Y. Life of RISC: Formation, action, and degradation of RNA-induced silencing complex. Mol. Cell 2022, 82, 30–43. [Google Scholar] [CrossRef]
- Gu, S.; Jin, L.; Zhang, F.; Sarnow, P.; Kay, M.A. Biological basis for restriction of microRNA targets to the 3′ untranslated region in mammalian mRNAs. Nat. Struct. Mol. Biol. 2009, 16, 144–150. [Google Scholar] [CrossRef]
- Buhagiar, A.F.; Kleaveland, B. To kill a microRNA: Emerging concepts in target-directed microRNA degradation. Nucleic Acids Res. 2024, 52, 1558–1574. [Google Scholar] [CrossRef]
- Naeli, P.; Winter, T.; Hackett, A.P.; Alboushi, L.; Jafarnejad, S.M. The intricate balance between microRNA-induced mRNA decay and translational repression. FEBS J. 2023, 290, 2508–2524. [Google Scholar] [CrossRef]
- Tuli, H.S.; Garg, V.K.; Bhushan, S.; Uttam, V.; Sharma, U.; Jain, A.; Sak, K.; Yadav, V.; Lorenzo, J.M.; Dhama, K.; et al. Natural flavonoids exhibit potent anticancer activity by targeting microRNAs in cancer: A signature step hinting towards clinical perfection. Transl. Oncol. 2023, 27, 101596. [Google Scholar] [CrossRef]
- Galagali, H.; Kim, J.K. The multifaceted roles of microRNAs in differentiation. Curr. Opin. Cell Biol. 2020, 67, 118–140. [Google Scholar] [CrossRef] [PubMed]
- Hajizadeh, M.; Hajizadeh, F.; Ghaffarei, S.; Amin Doustvandi, M.; Hajizadeh, K.; Yaghoubi, S.M.; Mohammadnejad, F.; Khiabani, N.A.; Mousavi, P.; Baradaran, B. MicroRNAs and their vital role in apoptosis in hepatocellular carcinoma: miRNA-based diagnostic and treatment methods. Gene 2023, 888, 147803. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.K.; Sharp, P.A. MicroRNA functions in stress responses. Mol. Cell 2010, 40, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Gaál, Z. Role of microRNAs in Immune Regulation with Translational and Clinical Applications. Int. J. Mol. Sci. 2024, 25, 1942. [Google Scholar] [CrossRef]
- Rahimian, N.; Nahand, J.S.; Hamblin, M.R.; Mirzaei, H. Exosomal MicroRNA Profiling. Methods Mol. Biol. 2023, 2595, 13–47. [Google Scholar]
- Shu, Z.; Tan, J.; Miao, Y.; Zhang, Q. The role of microvesicles containing microRNAs in vascular endothelial dysfunction. J. Cell. Mol. Med. 2019, 23, 7933–7945. [Google Scholar] [CrossRef]
- Layne, T.R.; Green, R.A.; Lewis, C.A.; Nogales, F.; Dawson Cruz, T.C.; Zehner, Z.E.; Seashols-Williams, S.J. microRNA Detection in Blood, Urine, Semen, and Saliva Stains After Compromising Treatments. J. Forensic Sci. 2019, 64, 1831–1837. [Google Scholar] [CrossRef]
- Klein, M.; Chandradoss, S.D.; Depken, M.; Joo, C. Why Argonaute is needed to make microRNA target search fast and reliable. Semin. Cell. Dev. Biol. 2017, 65, 20–28. [Google Scholar] [CrossRef]
- Smolarz, B.; Durczyński, A.; Romanowicz, H.; Szyłło, K.; Hogendorf, P. miRNAs in Cancer (Review of Literature). Int. J. Mol. Sci. 2022, 23, 2805. [Google Scholar] [CrossRef]
- Li, S.; Lei, Z.; Sun, T. The role of microRNAs in neurodegenerative diseases: A review. Cell. Biol. Toxicol. 2023, 39, 53–83. [Google Scholar] [CrossRef] [PubMed]
- Searles, C.D. MicroRNAs and Cardiovascular Disease Risk. Curr. Cardiol. Rep. 2024, 26, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wu, H.; Zhao, M.; Chang, C.; Lu, Q. Clinical significance of miRNAs in autoimmunity. J. Autoimmun. 2020, 109, 102438. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Q.; Zhang, R.; Dai, X.; Chen, W.; Xing, D. Circulating microRNAs: Biomarkers of disease. Clin. Chim. Acta 2021, 516, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Ekiz Kanik, F.; Celebi, I.; Sevenler, D.; Tanriverdi, K.; Lortlar Ünlü, N.; Freedman, J.E.; Ünlü, M.S. Attomolar sensitivity microRNA detection using real-time digital microarrays. Sci. Rep. 2022, 12, 16220. [Google Scholar] [CrossRef]
- Androvic, P.; Benesova, S.; Rohlova, E.; Kubista, M.; Valihrach, L. Small RNA-Sequencing for Analysis of Circulating miRNAs: Benchmark Study. J. Mol. Diagn. 2022, 24, 386–394. [Google Scholar] [CrossRef]
- Stebel, S.; Breuer, J.; Rossbach, O. Studying miRNA-mRNA Interactions: An Optimized CLIP-Protocol for Endogenous Ago2-Protein. Methods Protoc. 2022, 5, 96. [Google Scholar] [CrossRef]
- Riolo, G.; Cantara, S.; Marzocchi, C.; Ricci, C. miRNA Targets: From Prediction Tools to Experimental Validation. Methods Protoc. 2020, 4, 1. [Google Scholar] [CrossRef]
- Martino, E.; D’Onofrio, N.; Anastasio, C.; Abate, M.; Zappavigna, S.; Caraglia, M.; Balestrieri, M.L. MicroRNA-nanoparticles against cancer: Opportunities and challenges for personalized medicine. Mol. Ther. Nucleic Acids 2023, 32, 371–384. [Google Scholar] [CrossRef]
- Sabina, S.; Panico, A.; Mincarone, P.; Leo, C.G.; Garbarino, S.; Grassi, T.; Bagordo, F.; De Donno, A.; Scoditti, E.; Tumolo, M.R. Expression and Biological Functions of miRNAs in Chronic Pain: A Review on Human Studies. Int. J. Mol. Sci. 2022, 23, 6016. [Google Scholar] [CrossRef]
- López-González, M.J.; Landry, M.; Favereaux, A. MicroRNA and chronic pain: From mechanisms to therapeutic potential. Pharmacol. Ther. 2017, 180, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kiyosawa, N.; Watanabe, K.; Toyama, K.; Ishizuka, H. Circulating miRNA Signature as a Potential Biomarker for the Prediction of Analgesic Efficacy of Hydromorphone. Int. J. Mol. Sci. 2019, 20, 1665. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, S.; Shenoda, B.B.; Ajit, S.K. Overview of microRNA Modulation in Analgesic Research. Curr. Protoc. Pharmacol. 2017, 79, 9.25.1–9.25.10. [Google Scholar] [CrossRef]
- Luo, M.; Li, L.; Ding, M.; Niu, Y.; Xu, X.; Shi, X.; Shan, N.; Qiu, Z.; Piao, F.; Zhang, C. Long-term potentiation and depression regulatory microRNAs were highlighted in Bisphenol A induced learning and memory impairment by microRNA sequencing and bioinformatics analysis. PLoS ONE 2023, 18, e0279029. [Google Scholar] [CrossRef]
- Ramanathan, S.; Ajit, S.K. MicroRNA-Based Biomarkers in Pain. Adv. Pharmacol. 2016, 75, 5–62. [Google Scholar]
- Sakai, A.; Saitow, F.; Miyake, N.; Miyake, K.; Shimada, T.; Suzuki, H. miR-7a alleviates the maintenance of neuropathic pain through regulation of neuronal excitability. Brain 2013, 136, 2738–2750. [Google Scholar] [CrossRef]
- Cai, L.; Liu, X.; Guo, Q.; Huang, Q.; Zhang, Q.; Cao, Z. MiR-15a attenuates peripheral nerve injury-induced neuropathic pain by targeting AKT3 to regulate autophagy. Genes Genom. 2020, 42, 77–85. [Google Scholar] [CrossRef]
- Ito, N.; Sakai, A.; Miyake, N.; Maruyama, M.; Iwasaki, H.; Miyake, K.; Okada, T.; Sakamoto, A.; Suzuki, H. miR-15b mediates oxaliplatin-induced chronic neuropathic pain through BACE1 down-regulation. Br. J. Pharmacol. 2017, 174, 386–395. [Google Scholar] [CrossRef]
- Chen, W.; Guo, S.; Wang, S. MicroRNA-16 Alleviates Inflammatory Pain by Targeting Ras-Related Protein 23 (RAB23) and Inhibiting p38 MAPK Activation. Med. Sci. Monit. 2016, 22, 3894–3901. [Google Scholar] [CrossRef]
- Li, T.; Wan, Y.; Sun, L.; Tao, S.; Chen, P.; Liu, C.; Wang, K.; Zhou, C.; Zhao, G. Inhibition of MicroRNA-15a/16 Expression Alleviates Neuropathic Pain Development through Upregulation of G Protein-Coupled Receptor Kinase 2. Biomol. Ther. 2019, 27, 414–422. [Google Scholar] [CrossRef]
- You, H.; Zhang, L.; Chen, Z.; Liu, W.; Wang, H.; He, H. MiR-20b-5p relieves neuropathic pain by targeting Akt3 in a chronic constriction injury rat model. Synapse 2019, 73, e22125. [Google Scholar] [CrossRef] [PubMed]
- Zeboudj, L.; Sideris-Lampretsas, G.; Silva, R.; Al-Mudaris, S.; Picco, F.; Fox, S.; Chambers, D.; Malcangio, M. Silencing miR-21-5p in sensory neurons reverses neuropathic allodynia via activation of TGF-β-related pathway in macrophages. J. Clin. Investig. 2023, 133, e164472. [Google Scholar] [CrossRef]
- Reinhold, A.K.; Krug, S.M.; Salvador, E.; Sauer, R.S.; Karl-Schöller, F.; Malcangio, M.; Sommer, C.; Rittner, H.L. MicroRNA-21-5p functions via RECK/MMP9 as a proalgesic regulator of the blood nerve barrier in nerve injury. Ann. N. Y. Acad. Sci. 2022, 1515, 184–195. [Google Scholar] [CrossRef]
- Zhang, Y.; Su, Z.; Liu, H.L.; Li, L.; Wei, M.; Ge, D.J.; Zhang, Z.J. Effects of miR-26a-5p on neuropathic pain development by targeting MAPK6 in in CCI rat models. Biomed. Pharmacother. 2018, 107, 644–649. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Liu, M.; Guo, X.; Wang, P.; Zeng, F.; Wang, H.; Tang, J.; Qin, Z.; Tao, T. miR-26a-5p alleviates CFA-induced chronic inflammatory hyperalgesia through Wnt5a/CaMKII/NFAT signaling in mice. CNS Neurosci. Ther. 2023, 29, 1254–1271. [Google Scholar] [CrossRef]
- Liu, C.C.; Hung, K.C.; Li, Y.Y.; Yi-Kung Huang, E.; Chu, C.C.; Chow, L.H.; Tan, P.H. The concerted actions of microRNA-29a and interferon-β modulate complete Freund’s adjuvant-induced inflammatory pain by regulating the expression of type 1 interferon receptor, interferon-stimulated gene 15, and p-extracellular signal-regulated kinase. BJA Open 2025, 13, 100376. [Google Scholar] [CrossRef]
- Li, C.; Wang, X.; Zhang, G.; Zhang, Y.; Xia, F.; Xu, S.; Shen, X. Downregulation of microRNA-29c reduces pain after child delivery by activating the oxytocin-GABA pathway. Mol. Med. Rep. 2020, 22, 1921–1931. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Yao, Y.; Ma, Y.; Chen, Y. MiR-30a-5p ameliorates LPS-induced inflammatory injury in human A549 cells and mice via targeting RUNX2. Innate Immun. 2021, 27, 41–49. [Google Scholar] [CrossRef]
- Liao, J.; Liu, J.; Long, G.; Lv, X. MiR-30b-5p attenuates neuropathic pain by the CYP24A1-Wnt/β-catenin signaling in CCI rats. Exp. Brain Res. 2022, 240, 263–277. [Google Scholar] [CrossRef]
- Tramullas, M.; Francés, R.; de la Fuente, R.; Velategui, S.; Carcelén, M.; García, R.; Llorca, J.; Hurlé, M.A. MicroRNA-30c-5p modulates neuropathic pain in rodents. Sci. Transl. Med. 2018, 10, eaao6299. [Google Scholar] [CrossRef]
- Brandenburger, T.; Johannsen, L.; Prassek, V.; Kuebart, A.; Raile, J.; Wohlfromm, S.; Köhrer, K.; Huhn, R.; Hollmann, M.W.; Hermanns, H. MiR-34a is differentially expressed in dorsal root ganglia in a rat model of chronic neuropathic pain. Neurosci. Lett. 2019, 708, 134365. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wang, Q.; Jiang, W.; Yu, S.; Zhang, S. MiR-34c Ameliorates Neuropathic Pain by Targeting NLRP3 in a Mouse Model of Chronic Constriction Injury. Neuroscience 2019, 399, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Gu, Y.; Dai, Q.; He, Y.; Wang, J. Spinal miR-34a regulates inflammatory pain by targeting SIRT1 in complete Freund’s adjuvant mice. Biochem. Biophys. Res. Commun. 2019, 516, 1196–1203. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Shao, A.; Tang, X.; Feng, M.; Wang, J.; Qiu, Y. MiR-34a affects dexmedetomidine-inhibited chronic inflammatory visceral pain by targeting to HDAC2. BMC Anesthesiol. 2019, 19, 131. [Google Scholar] [CrossRef]
- Qiu, S.; Liu, B.; Mo, Y.; Wang, X.; Zhong, L.; Han, X.; Mi, F. MiR-101 promotes pain hypersensitivity in rats with chronic constriction injury via the MKP-1 mediated MAPK pathway. J. Cell. Mol. Med. 2020, 24, 8986–8997. [Google Scholar] [CrossRef]
- Xie, T.; Zhang, J.; Kang, Z.; Liu, F.; Lin, Z. miR-101 down-regulates mTOR expression and attenuates neuropathic pain in chronic constriction injury rat models. Neurosci. Res. 2020, 158, 30–36. [Google Scholar] [CrossRef]
- Favereaux, A.; Thoumine, O.; Bouali-Benazzouz, R.; Roques, V.; Papon, M.A.; Salam, S.A.; Drutel, G.; Léger, C.; Calas, A.; Nagy, F.; et al. Bidirectional integrative regulation of Cav1.2 calcium channel by microRNA miR-103: Role in pain. EMBO J. 2011, 30, 3830–3841. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, R.; Xu, M.J.; Sha, J.; Xu, G.Y.; Wu, J.; Zhang, P.A. MiRNA-107 contributes to inflammatory pain by down-regulating GLT-1 expression in rat spinal dorsal horn. Eur. J. Pain 2021, 25, 1254–1263. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, H.L.; An, L.J.; Li, L.; Wei, M.; Ge, D.J.; Su, Z. miR-124-3p attenuates neuropathic pain induced by chronic sciatic nerve injury in rats via targeting EZH2. J. Cell. Biochem. 2019, 120, 5747–5755. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, X.; Wang, X.; Xu, F.; Zhang, J.; Li, L.; Xie, X.; Wang, L.; Yang, Y.; Xu, J.T. MicroRNA-124-3p attenuates the development of nerve injury-induced neuropathic pain by targeting early growth response 1 in the dorsal root ganglia and spinal dorsal horn. J. Neurochem. 2021, 158, 928–942. [Google Scholar] [CrossRef]
- Liu, C.C.; Cheng, J.T.; Li, T.Y.; Tan, P.H. Integrated analysis of microRNA and mRNA expression profiles in the rat spinal cord under inflammatory pain conditions. Eur. J. Neurosci. 2017, 46, 2713–2728. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Li, P.; Ni, Y.; Zhao, J.; Liu, Z. Decreased microRNA-125a-3p contributes to upregulation of p38 MAPK in rat trigeminal ganglions with orofacial inflammatory pain. PLoS ONE 2014, 9, e111594. [Google Scholar] [CrossRef] [PubMed]
- Kasimu, A.; Apizi, X.; Talifujiang, D.; Ma, X.; Fang, L.; Zhou, X. miR-125a-5p in astrocytes attenuates peripheral neuropathy in type 2 diabetic mice through targeting TRAF6. Endocrinol. Diabetes Nutr. 2022, 69, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, B.; Geng, X.; Guo, X.; Wang, T.; Xu, J.; Jiang, L.; Zhen, H. microRNA-125b-5p alleviated CCI-induced neuropathic pain and modulated neuroinflammation via targeting SOX11. Synapse 2024, 78, e22306. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Cai, W.; Liu, Y.; Liu, H.; Zhang, Z.; Su, Z. MicroRNA-128-3p Alleviates Neuropathic Pain Through Targeting ZEB1. Neurosci. Lett. 2020, 729, 134946. [Google Scholar] [CrossRef]
- Xian, S.; Ding, R.; Li, M.; Chen, F. LncRNA NEAT1/miR-128-3p/AQP4 axis regulating spinal cord injury-induced neuropathic pain progression. J. Neuroimmunol. 2021, 351, 577457. [Google Scholar] [CrossRef]
- Yao, L.; Guo, Y.; Wang, L.; Li, G.; Qian, X.; Zhang, J.; Liu, H.; Liu, G. Knockdown of miR-130a-3p alleviates spinal cord injury induced neuropathic pain by activating IGF-1/IGF-1R pathway. J. Neuroimmunol. 2021, 351, 577458. [Google Scholar] [CrossRef]
- Dong, J.; Xu, C.; Xia, R.; Zhang, Z. Upregulating miR-130a-5p relieves astrocyte over activation-induced neuropathic pain through targeting C-X-C motif chemokine receptor 12/C-X-C motif chemokine receptor 4 axis. NeuroReport 2021, 32, 135–143. [Google Scholar] [CrossRef]
- Leinders, M.; Üçeyler, N.; Pritchard, R.A.; Sommer, C.; Sorkin, L.S. Increased miR-132-3p expression is associated with chronic neuropathic pain. Exp. Neurol. 2016, 283, 276–286. [Google Scholar] [CrossRef]
- Ji, L.J.; Su, J.; Xu, A.L.; Pang, B.; Huang, Q.M. MiR-134-5p attenuates neuropathic pain progression through targeting Twist1. J. Cell Biochem. 2019, 120, 1694–1701. [Google Scholar] [CrossRef]
- Ni, J.; Gao, Y.; Gong, S.; Guo, S.; Hisamitsu, T.; Jiang, X. Regulation of μ-opioid type 1 receptors by microRNA134 in dorsal root ganglion neurons following peripheral inflammation. Eur. J. Pain 2013, 17, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.G.; He, H.; Wang, P.J. A critical role for miR-135a-5p-mediated regulation of SLC24A2 in neuropathic pain. Mol. Med. Rep. 2020, 22, 2115–2122. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Zhang, Y.; Su, Z. ciRS-7 targeting miR-135a-5p promotes neuropathic pain in CCI rats via inflammation and autophagy. Gene 2020, 736, 144386. [Google Scholar] [CrossRef]
- Liu, M.; Cheng, X.; Yan, H.; Chen, J.; Liu, C.; Chen, Z. MiR-135-5p Alleviates Bone Cancer Pain by Regulating Astrocyte-Mediated Neuroinflammation in Spinal Cord through JAK2/STAT3 Signaling Pathway. Mol. Neurobiol. 2021, 58, 4802–4815. [Google Scholar] [CrossRef]
- Zhang, Y.; Mou, J.; Cao, L.; Zhen, S.; Huang, H.; Bao, H. MicroRNA-142-3p relieves neuropathic pain by targeting high mobility group box 1. Int. J. Mol. Med. 2018, 41, 501–510. [Google Scholar] [CrossRef]
- Li, J.; Guo, Y.; Zhu, C.; Wang, D.; Li, Y.; Hao, X.; Cao, L.; Fan, Y.; Fang, B. Biosynthesis inhibition of miR-142-5p in a N6-methyladenosine-dependent manner induces neuropathic pain through CDK5/TRPV1 signaling. Cell. Mol. Biol. Lett. 2025, 30, 16. [Google Scholar] [CrossRef]
- Lu, Y.; Cao, D.L.; Jiang, B.C.; Yang, T.; Gao, Y.J. MicroRNA-146a-5p attenuates neuropathic pain via suppressing TRAF6 signaling in the spinal cord. Brain Behav. Immun. 2015, 49, 119–129. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, F.; Wei, M.; Qiu, Y.; Ma, C.; Shen, L.; Huang, Y. Chronic constriction injury-induced microRNA-146a-5p alleviates neuropathic pain through suppression of IRAK1/TRAF6 signaling pathway. J. Neuroinflamm. 2018, 15, 179. [Google Scholar] [CrossRef] [PubMed]
- Garo, L.P.; Ajay, A.K.; Fujiwara, M.; Gabriely, G.; Raheja, R.; Kuhn, C.; Kenyon, B.; Skillin, N.; Kadowaki-Saga, R.; Saxena, S.; et al. MicroRNA-146a limits tumorigenic inflammation in colorectal cancer. Nat. Commun. 2021, 12, 2419. [Google Scholar] [CrossRef]
- Dou, L.; Lin, H.; Wang, K.; Zhu, G.; Zou, X.; Chang, E.; Zhu, Y. Long non-coding RNA CCAT1 modulates neuropathic pain progression through sponging miR-155. Oncotarget 2017, 8, 89949–89957. [Google Scholar] [CrossRef]
- Chen, J.; Li, C.; Liu, W.; Yan, B.; Hu, X.; Yang, F. miRNA-155 silencing reduces sciatic nerve injury in diabetic peripheral neuropathy. J. Mol. Endocrinol. 2019, 63, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sun, H.; Sun, Y. LncRNA p21, downregulating miR-181b, aggravates neuropathic pain by upregulating Tnfaip1 and inhibit the AKT/CREB axis. Brain Res. Bull. 2021, 171, 150–161. [Google Scholar] [CrossRef]
- Zhang, Y.U.; Ye, G.; Zhao, J.; Chen, Y.; Kong, L.; Sheng, C.; Yuan, L. Exosomes carried miR-181c-5p alleviates neuropathic pain in CCI rat models. An. Acad. Bras. Cienc. 2022, 94, e20210564. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Ma, L.; Xi, K.; Zhang, W.; Fan, D. MicroRNA-183 Suppresses Neuropathic Pain and Expression of AMPA Receptors by Targeting mTOR/VEGF Signaling Pathway. Cell Physiol. Biochem. 2017, 41, 181–192. [Google Scholar] [CrossRef]
- Huang, L.; Wang, L. Upregulation of miR-183 represses neuropathic pain through inhibiton of MAP3K4 in CCI rat models. J. Cell Physiol. 2020, 235, 3815–3822. [Google Scholar] [CrossRef]
- Yang, D.; Yang, Q.; Wei, X.; Liu, Y.; Ma, D.; Li, J.; Wan, Y.; Luo, Y. The role of miR-190a-5p contributes to diabetic neuropathic pain via targeting SLC17A6. J. Pain Res. 2017, 10, 2395–2403. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.; Shi, J.; Liu, K.; Liu, N.; Wang, Y.; Fu, Z.; Ding, J.; Jia, L.; Yuan, W. Increased miR-195 aggravates neuropathic pain by inhibiting autophagy following peripheral nerve injury. Glia 2013, 61, 504–512. [Google Scholar] [CrossRef]
- Wang, X.; Wang, H.; Zhang, T.; He, M.; Liang, H.; Wang, H.; Xu, L.; Chen, S.; Xu, M. Inhibition of MicroRNA-195 Alleviates Neuropathic Pain by Targeting Patched1 and Inhibiting SHH Signaling Pathway Activation. Neurochem. Res. 2019, 44, 1690–1702. [Google Scholar] [CrossRef]
- Li, Z.; Li, Y.; Li, Z. Low-Level miR-199 Contribute to Neuropathic Low Back Pain via TRPV1 by Regulating the Production of Pro-Inflammatory Cytokines on Macrophage. Turk. Neurosurg. 2024, 34, 299–307. [Google Scholar]
- Saadh, M.J.; Rashed, A.B.; Jamal, A.; Castillo-Acobo, R.Y.; Kamal, M.A.; Cotrina-Aliaga, J.C.; Gonzáles, J.L.A.; Alothaim, A.S.; Alhoqail, W.A.; Ahmad, F.; et al. miR-199a-3p suppresses neuroinflammation by directly targeting MyD88 in a mouse model of bone cancer pain. Life Sci. 2023, 333, 122139. [Google Scholar] [CrossRef]
- Li, H.; Huang, Y.; Ma, C.; Yu, X.; Zhang, Z.; Shen, L. MiR-203 involves in neuropathic pain development and represses Rap1a expression in nerve growth factor differentiated neuronal PC12 cells. Clin. J. Pain 2015, 31, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, X.; Fu, Z.; Zhou, Q. MicroRNA-212-3p Attenuates Neuropathic Pain via Targeting Sodium Voltage-gated Channel Alpha Subunit 3 (NaV 1.3). Curr. Neurovasc. Res. 2019, 16, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Guan, C.; Luan, L.; Li, J.; Yang, L. MiR-212-3p improves rat functional recovery and inhibits neurocyte apoptosis in spinal cord injury models via PTEN downregulation-mediated activation of AKT/mTOR pathway. Brain Res. 2021, 1768, 147576. [Google Scholar] [CrossRef]
- Pan, Z.; Zhu, L.J.; Li, Y.Q.; Hao, L.Y.; Yin, C.; Yang, J.X.; Guo, Y.; Zhang, S.; Hua, L.; Xue, Z.Y.; et al. Epigenetic modification of spinal miR-219 expression regulates chronic inflammation pain by targeting CaMKIIγ. J. Neurosci. 2014, 34, 9476–9483. [Google Scholar] [CrossRef]
- Zhu, J.; Yang, J.; Xu, J. miR-223 Inhibits the Polarization and Recruitment of Macrophages via NLRP3/IL-1β Pathway to Meliorate Neuropathic pain. Pain Res. Manag. 2021, 2021, 6674028. [Google Scholar] [CrossRef]
- Huang, B.; Guo, S.; Zhang, Y.; Lin, P.; Lin, C.; Chen, M.; Zhu, S.; Huang, L.; He, J.; Zhang, L.; et al. MiR-223-3p alleviates trigeminal neuropathic pain in the male mouse by targeting MKNK2 and MAPK/ERK signaling. Brain Behav. 2022, 12, e2634. [Google Scholar] [CrossRef] [PubMed]
- Manners, M.T.; Tian, Y.; Zhou, Z.; Ajit, S.K. MicroRNAs downregulated in neuropathic pain regulate MeCP2 and BDNF related to pain sensitivity. FEBS Open Bio 2015, 5, 733–740. [Google Scholar] [CrossRef]
- Lu, Z.; Zhang, Y.; Li, Y. LncRNA FTX ameliorates neuropathic pain by targeting miR-320a in a rat model of chronic constriction injury. Folia Neuropathol. 2023, 61, 291–300. [Google Scholar] [CrossRef]
- Peng, C.; Zhang, C.; Su, Z.; Lin, D. DGCR5 attenuates neuropathic pain through sponging miR-330-3p and regulating PDCD4 in CCI rat models. J. Cell. Physiol. 2019, 234, 7292–7300. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, Y.; Peng, Y.; Xu, H.; Zhou, X. METTL3 regulates inflammatory pain by modulating m6A-dependent pri-miR-365-3p processing. FASEB J. 2020, 34, 122–132. [Google Scholar] [CrossRef]
- Xiang, W.; Jiang, L.; Zhou, Y.; Li, Z.; Zhao, Q.; Wu, T.; Cao, Y.; Zhou, J. The lncRNA Ftx/miR-382-5p/Nrg1 axis improves the inflammation response of microglia and spinal cord injury repair. Neurochem. Int. 2021, 143, 104929. [Google Scholar] [CrossRef] [PubMed]
- Xu, A.; Shen, H.; Mei, S.; Wang, Z.; Xie, Q.; Cui, H.; Chu, Y.; Feng, B. Down-regulation of microRNA-382-5p reduces neuropathic pain by targeting regulation of dual specificity phosphatase-1. Korean J. Pain 2024, 37, 320–331. [Google Scholar] [CrossRef]
- Huang, Z.Z.; Wei, J.Y.; Ou-Yang, H.D.; Li, D.; Xu, T.; Wu, S.L.; Zhang, X.L.; Liu, C.C.; Ma, C.; Xin, W.J. mir-500-Mediated GAD67 Downregulation Contributes to Neuropathic Pain. J. Neurosci. 2016, 36, 6321–6331. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Shen, W.; Hu, Y. The Role of miR-539 in the Anterior Cingulate Cortex in Chronic Neuropathic pain. Pain Med. 2017, 18, 2433–2442. [Google Scholar] [CrossRef]
- Li, H.; Shen, L.; Ma, C.; Huang, Y. Differential expression of miRNAs in the nervous system of a rat model of bilateral sciatic nerve chronic constriction injury. Int. J. Mol. Med. 2013, 32, 219–226. [Google Scholar] [CrossRef]
- Liu, Y.; Jeon, S.M.; Caterina, M.J.; Qu, L. miR-544-3p mediates arthritis pain through regulation of FcγRI. Pain 2022, 163, 1497–1510. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Du, X.J.; Zhao, Y.; Xia, D.L. XIST/miR-544 axis induces neuropathic pain by activating STAT3 in a rat model. J. Cell Physiol. 2018, 233, 5847–5855. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, L.; Xu, T.; Gou, B.; Mai, J.W.; Luo, D.X.; Xin, W.J.; Wu, J.Y. MiR-672-5p-Mediated Upregulation of REEP6 in Spinal Dorsal Horn Participates in Bortezomib-Induced Neuropathic Pain in Rats. Neurochem. Res. 2023, 48, 229–237. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, B.; Gan, C.; Sun, H.; Zhang, J.; Feng, L. A Comprehensive Review of Small Interfering RNAs (siRNAs): Mechanism, Therapeutic Targets, and Delivery Strategies for Cancer Therapy. Int. J. Nanomed. 2023, 18, 7605–7635. [Google Scholar] [CrossRef]
- Wang, Q.; Xue, Y.; Zhang, L.; Zhong, Z.; Feng, S.; Wang, C.; Xiao, L.; Yang, Z.; Harris, C.J.; Wu, Z.; et al. Mechanism of siRNA production by a plant Dicer-RNA complex in dicing-competent conformation. Science 2021, 374, 1152–1157. [Google Scholar] [CrossRef]
- Lee, H.Y.; Zhou, K.; Smith, A.M.; Noland, C.L.; Doudna, J.A. Differential roles of human Dicer-binding proteins TRBP and PACT in small RNA processing. Nucleic Acids Res. 2013, 41, 6568–6576. [Google Scholar] [CrossRef]
- Xu, R.; Njumbe Ediage, E.; Verhaeghe, T.; Snoeys, J.; Dillen, L. Therapeutic siRNA Loaded to RISC as Single and Double Strands Requires an Appropriate Quantitative Assay for RISC PK Assessment. Nucleic Acid. Ther. 2024, 34, 199–210. [Google Scholar] [CrossRef]
- Saddique, M.N.; Qadri, M.; Ain, N.U.; Farhan, E.; Shahid, F.; Benyamin, J.; Bashir, M.A.; Jain, H.; Iqbal, J. Safety and effectiveness of interference RNA (RNAi) based therapeutics in cardiac failure: A systematic review. Heart Lung 2024, 68, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Leuschner, P.J.; Ameres, S.L.; Kueng, S.; Martinez, J. Cleavage of the siRNA passenger strand during RISC assembly in human cells. EMBO Rep. 2006, 7, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, R.A.; Onizuka, K.; Ball-Jones, A.A.; Hu, T.; Suter, S.R.; Beal, P.A. Guide Strand 3′-End Modifications Regulate siRNA Specificity. Chembiochem. 2016, 17, 2340–2345. [Google Scholar] [CrossRef] [PubMed]
- Meister, G.; Landthaler, M.; Patkaniowska, A.; Dorsett, Y.; Teng, G.; Tuschl, T. Human Argonaute2 mediates RNA cleavage targeted by miRNAs and siRNAs. Mol. Cell 2004, 15, 185–197. [Google Scholar] [CrossRef]
- Qi, X.; Bao, F.S.; Xie, Z. Small RNA deep sequencing reveals role for Arabidopsis thaliana RNA-dependent RNA polymerases in viral siRNA biogenesis. PLoS ONE 2009, 4, e4971. [Google Scholar] [CrossRef]
- Marker, S.; Le Mouël, A.; Meyer, E.; Simon, M. Distinct RNA-dependent RNA polymerases are required for RNAi triggered by double-stranded RNA versus truncated transgenes in Paramecium tetraurelia. Nucleic Acids Res. 2010, 38, 4092–4107. [Google Scholar] [CrossRef]
- Qureshi, A.; Tantray, V.G.; Kirmani, A.R.; Ahangar, A.G. A review on current status of antiviral siRNA. Rev. Med. Virol. 2018, 28, e1976. [Google Scholar] [CrossRef]
- Wallen, M.; Aqil, F.; Kandimalla, R.; Jeyabalan, J.; Auwardt, S.; Tyagi, N.; Schultz, D.J.; Spencer, W.; Gupta, R.C. A model system for antiviral siRNA therapeutics using exosome-based delivery. Mol. Ther. Nucleic Acids 2022, 29, 691–704. [Google Scholar] [CrossRef]
- Yang, Q.; Ye, Q.A.; Liu, Y. Mechanism of siRNA production from repetitive DNA. Genes Dev. 2015, 29, 526–537. [Google Scholar] [CrossRef] [PubMed]
- Amiri, A.; Barreto, G.; Sathyapalan, T.; Sahebkar, A. siRNA Therapeutics: Future Promise for Neurodegenerative Diseases. Curr. Neuropharmacol. 2021, 19, 1896–1911. [Google Scholar] [CrossRef] [PubMed]
- Wittrup, A.; Lieberman, J. Knocking down disease: A progress report on siRNA therapeutics. Nat. Rev. Genet. 2015, 16, 543–552. [Google Scholar] [CrossRef]
- Dalmay, T. Short RNAs in environmental adaptation. Proc. Biol. Sci. 2006, 273, 1579–1585. [Google Scholar] [CrossRef] [PubMed]
- Takada, T.; Nemoto, K.; Yamashita, A.; Kato, M.; Kondo, Y.; Torii, R. Efficient gene silencing and cell differentiation using siRNA in mouse and monkey ES cells. Biochem. Biophys. Res. Commun. 2005, 331, 1039–1044. [Google Scholar] [CrossRef]
- Wu, C.; Zhao, J.; Zhu, G.; Huang, Y.; Jin, L. SiRNA directed against NF-κB inhibits mononuclear macrophage cells releasing proinflammatory cytokines in vitro. Mol. Med. Rep. 2017, 16, 9060–9066. [Google Scholar] [CrossRef]
- Dong, X.W.; Goregoaker, S.; Engler, H.; Zhou, X.; Mark, L.; Crona, J.; Terry, R.; Hunter, J.; Priestley, T. Small interfering RNA-mediated selective knockdown of Na(V)1.8 tetrodotoxin-resistant sodium channel reverses mechanical allodynia in neuropathic rats. Neuroscience 2007, 146, 812–821. [Google Scholar] [CrossRef]
- Ganju, P.; Hall, J. Potential applications of siRNA for pain therapy. Expert Opin. Biol. Ther. 2004, 4, 531–542. [Google Scholar] [CrossRef]
- Wang, B.; Liu, S.; Fan, B.; Xu, X.; Chen, Y.; Lu, R.; Xu, Z.; Liu, X. PKM2 is involved in neuropathic pain by regulating ERK and STAT3 activation in rat spinal cord. J. Headache Pain 2018, 19, 7. [Google Scholar] [CrossRef]
- Peng, J.; Ma, J.; Yang, X.; He, H.; Wu, H.; Ma, T.; Lu, J. Water-Soluble Polymer Assists N-Methyl-D-Aspartic Acid Receptor 2B siRNA Delivery to Relieve Chronic Inflammatory Pain In Vitro and In Vivo. Pain Res. Manag. 2018, 2018, 7436060. [Google Scholar] [CrossRef]
- Dai, Y.; Lin, J.; Chen, X.; Ren, J.; Wu, C.; Shen, H.; Li, X.; Yu, J.; Jiang, B.; Yu, L. NAMPT/NAD+/PARP1 Pathway Regulates CFA-Induced Inflammatory Pain via NF-κB Signaling in Rodents. Adv. Biol. 2024, 8, e2400028. [Google Scholar] [CrossRef] [PubMed]
- Moqbel Redhwan, M.A.; Hariprasad, M.G.; Samaddar, S.; Bafail, D.; Hard, S.A.A.A.; Guha, S.; Dhavale, A. siRNA targeting PARP-1 alleviates diabetic peripheral neuropathy in a streptozotocin-induced rat model. J. Drug Target. 2025, 33, 424–435. [Google Scholar] [CrossRef]
- Fitzsimons, L.A.; Staurengo-Ferrari, L.; Khomula, E.V.; Bogen, O.; Araldi, D.; Bonet, I.J.M.; Green, P.G.; Jordan, E.E.; Sclafani, F.; Nowak, C.E.; et al. The Nociceptor Primary Cilium Contributes to Mechanical Nociceptive Threshold and Inflammatory and Neuropathic Pain. J. Neurosci. 2024, 44, e1265242024. [Google Scholar] [CrossRef]
- Lee, S.; Shin, H.J.; Noh, C.; Kim, S.I.; Ko, Y.K.; Lee, S.Y.; Lim, C.; Hong, B.; Yang, S.Y.; Kim, D.W.; et al. IKBKB siRNA-Encapsulated Poly (Lactic-co-Glycolic Acid) Nanoparticles Diminish Neuropathic Pain by Inhibiting Microglial Activation. Int. J. Mol. Sci. 2021, 22, 5657. [Google Scholar] [CrossRef]
- Pan, R.; Di, H.; Zhang, J.; Huang, Z.; Sun, Y.; Yu, W.; Wu, F. Inducible Lentivirus-Mediated siRNA against TLR4 Reduces Nociception in a Rat Model of Bone Cancer Pain. Mediat. Inflamm. 2015, 2015, 523896. [Google Scholar] [CrossRef]
- Wang, Q.; Li, H.Y.; Ling, Z.M.; Chen, G.; Wei, Z.Y. Inhibition of Schwann cell pannexin 1 attenuates neuropathic pain through the suppression of inflammatory responses. J. Neuroinflamm. 2022, 19, 244. [Google Scholar] [CrossRef] [PubMed]
- Liao, Q.; Yang, Y.; Li, Y.; Zhang, J.; Fan, K.; Guo, Y.; Chen, J.; Chen, Y.; Zhu, P.; Huang, L.; et al. Targeting TANK-binding kinase 1 attenuates painful diabetic neuropathy via inhibiting microglia pyroptosis. Cell. Commun. Signal. 2024, 22, 368. [Google Scholar] [CrossRef]
- Xu, L.; Feng, Q.; Deng, H.; Zhang, X.; Ni, H.; Yao, M. Neurexin-2 is a potential regulator of inflammatory pain in the spinal dorsal horn of rats. J. Cell. Mol. Med. 2020, 24, 13623–13633. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Cao, D.L.; Ma, L.J.; Gao, Y.J. TRAF6 Contributes to CFA-Induced Spinal Microglial Activation and Chronic Inflammatory Pain in Mice. Cell. Mol. Neurobiol. 2022, 42, 1543–1555. [Google Scholar] [CrossRef]
- Peng, S.; Lu, Y.; Li, P.; Liu, P.; Shi, X.; Liu, C.; Zhang, Y.; Liu, S.; Wang, J. The short interference RNA (siRNA) targeting NMUR2 relieves nociception in a bone cancer pain model of rat through PKC-ERK and PI3K-AKT pathways. Biochem. Biophys. Res. Commun. 2019, 512, 616–622. [Google Scholar] [CrossRef]
- Kamata, Y.; Kambe, T.; Chiba, T.; Yamamoto, K.; Kawakami, K.; Abe, K.; Taguchi, K. Paclitaxel Induces Upregulation of Transient Receptor Potential Vanilloid 1 Expression in the Rat Spinal Cord. Int. J. Mol. Sci. 2020, 21, 4341. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhao, J.; Meng, Q. AAV-mediated siRNA against TRPV1 reduces nociception in a rat model of bone cancer pain. Neurol. Res. 2019, 41, 972–979. [Google Scholar] [CrossRef]
- Zhang, K.; Xu, Y. Suppressing BRD4 exhibits protective effects against vincristine-induced peripheral neuropathy by alleviating inflammation and oxidative stress. Biochem. Biophys. Res. Commun. 2020, 532, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Tan, P.H.; Chia, Y.Y.; Chow, L.H.; Chen, J.J.; Yang, L.C.; Hung, K.C.; Chen, H.S.; Kuo, C.H. Gene knockdown of the N-methyl-D-aspartate receptor NR1 subunit with subcutaneous small interfering RNA reduces inflammation-induced nociception in rats. Anesthesiology 2010, 112, 1482–1493. [Google Scholar] [CrossRef]
- Liu, X.; Tian, Y.; Lu, N.; Gin, T.; Cheng, C.H.; Chan, M.T. Stat3 inhibition attenuates mechanical allodynia through transcriptional regulation of chemokine expression in spinal astrocytes. PLoS ONE 2013, 8, e75804. [Google Scholar] [CrossRef]
- Hang, L.H.; Yang, J.P.; Yin, W.; Wang, L.N.; Guo, F.; Ji, F.H.; Shao, D.H.; Xu, Q.N.; Wang, X.Y.; Zuo, J.L. Activation of spinal TDAG8 and its downstream PKA signaling pathway contribute to bone cancer pain in rats. Eur. J. Neurosci. 2012, 36, 2107–2117. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.X.; Wang, J.; Ma, F.J.; Liu, M.M.; Chen, S.H.; Wei, Y.; Xiao, Y.F.; Lv, P.Y.; Liu, X.; Qu, J.Q.; et al. Rab11a in the spinal cord: An essential contributor to complete Freund’s adjuvant-induced inflammatory pain in mice. Mol. Brain 2023, 16, 70. [Google Scholar] [CrossRef]
- Yang, L.; Bai, H.H.; Zhang, Z.Y.; Liu, J.P.; Suo, Z.W.; Yang, X.; Hu, X.D. Disruption of SHP1/NMDA receptor signaling in spinal cord dorsal horn alleviated inflammatory pain. Neuropharmacology 2018, 137, 104–113. [Google Scholar] [CrossRef]
- Li, Q.; Liu, S.; Li, L.; Ji, X.; Wang, M.; Zhou, J. Spinal IL-36γ/IL-36R participates in the maintenance of chronic inflammatory pain through astroglial JNK pathway. Glia 2019, 67, 438–451. [Google Scholar] [CrossRef]
- Li, Q.; Yang, Z.; Wang, K.; Chen, Z.; Shen, H. Suppression of microglial Ccl2 reduces neuropathic pain associated with chronic spinal compression. Front. Immunol. 2023, 14, 1191188. [Google Scholar] [CrossRef]
- Lin, C.R.; Cheng, J.K.; Wu, C.H.; Chen, K.H.; Liu, C.K. Epigenetic suppression of potassium-chloride co-transporter 2 expression in inflammatory pain induced by complete Freund’s adjuvant (CFA). Eur. J. Pain 2017, 21, 309–321. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.L.; Qian, B.; Zhang, Z.J.; Gao, Y.J.; Wu, X.B. Chemokine receptor CXCR2 in dorsal root ganglion contributes to the maintenance of inflammatory pain. Brain Res. Bull. 2016, 127, 219–225. [Google Scholar] [CrossRef]
- Kwon, A.; Jeon, S.M.; Hwang, S.H.; Kim, J.H.; Cho, H.J. Expression and functional role of metallothioneins I and II in the spinal cord in inflammatory and neuropathic pain models. Brain Res. 2013, 1523, 37–48. [Google Scholar] [CrossRef]
- Wang, H.; Song, T.; Wang, W.; Zhang, Z. TRPM2 participates the transformation of acute pain to chronic pain during injury-induced neuropathic pain. Synapse 2019, 73, e22117. [Google Scholar] [CrossRef]
- Deval, E.; Noël, J.; Lay, N.; Alloui, A.; Diochot, S.; Friend, V.; Jodar, M.; Lazdunski, M.; Lingueglia, E. ASIC3, a sensor of acidic and primary inflammatory pain. EMBO J. 2008, 27, 3047–3055. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Qian, Z.; Jiang, D.; Sun, Y.; Gao, S.; Jiang, X.; Wang, H.; Tao, J. Neuromedin B receptor stimulation of Cav3.2 T-type Ca2+ channels in primary sensory neurons mediates peripheral pain hypersensitivity. Theranostics 2021, 11, 9342–9357. [Google Scholar] [CrossRef]
- Noh, C.; Shin, H.J.; Lee, S.; Kim, S.I.; Kim, Y.H.; Lee, W.H.; Kim, D.W.; Lee, S.Y.; Ko, Y.K. CX3CR1-Targeted PLGA Nanoparticles Reduce Microglia Activation and Pain Behavior in Rats with Spinal Nerve Ligation. Int. J. Mol. Sci. 2020, 21, 3469. [Google Scholar] [CrossRef] [PubMed]
- Xue, P.; Chen, L.; Lu, X.; Zhang, J.; Bao, G.; Xu, G.; Sun, Y.; Guo, X.; Jiang, J.; Gu, H.; et al. Vimentin Promotes Astrocyte Activation After Chronic Constriction Injury. J. Mol. Neurosci. 2017, 63, 91–99. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, J.; He, M.; Liu, R.; Belegu, V.; Dai, P.; Liu, W.; Wang, W.; Xia, Q.J.; Shang, F.F.; et al. Mechanisms of PDGF siRNA-mediated inhibition of bone cancer pain in the spinal cord. Sci. Rep. 2016, 6, 27512. [Google Scholar] [CrossRef]
- Huang, H.J.; Zhang, M. Downregulation of PI3Kcb utilizing adenovirus-mediated transfer of siRNA attenuates bone cancer pain. Int. J. Clin. Exp. Pathol. 2014, 7, 8127–8135. [Google Scholar]
- Gao, N.; Li, Y.; Li, J.; Gao, Z.; Yang, Z.; Li, Y.; Liu, H.; Fan, T. Long Non-Coding RNAs: The Regulatory Mechanisms, Research Strategies, and Future Directions in Cancers. Front. Oncol. 2020, 10, 598817. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Chang, C.P. Long non-coding RNA and chromatin remodeling. RNA Biol. 2015, 12, 1094–1098. [Google Scholar] [CrossRef]
- Vance, K.W.; Ponting, C.P. Transcriptional regulatory functions of nuclear long noncoding RNAs. Trends. Genet. 2014, 30, 348–355. [Google Scholar] [CrossRef]
- He, R.Z.; Luo, D.X.; Mo, Y.Y. Emerging roles of lncRNAs in the post-transcriptional regulation in cancer. Genes Dis. 2019, 6, 6–15. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, W.; Zhu, W.; Dong, J.; Cheng, Y.; Yin, Z.; Shen, F. Mechanisms and Functions of Long Non-Coding RNAs at Multiple Regulatory Levels. Int. J. Mol. Sci. 2019, 20, 5573. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Ren, Y.; Hu, W.; Paliouras, A.R.; Zhang, W.; Zhong, L.; Yang, K.; Su, L.; Wang, P.; Li, Y.; et al. Long non-coding RNA-encoded micropeptides: Functions, mechanisms and implications. Cell Death Discov. 2024, 10, 450. [Google Scholar] [CrossRef]
- Nojima, T.; Proudfoot, N.J. Mechanisms of lncRNA biogenesis as revealed by nascent transcriptomics. Nat. Rev. Mol. Cell Biol. 2022, 23, 389–406. [Google Scholar] [CrossRef] [PubMed]
- Ransohoff, J.D.; Wei, Y.; Khavari, P.A. The functions and unique features of long intergenic non-coding RNA. Nat. Rev. Mol. Cell Biol. 2018, 19, 143–157. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, L.; Chen, L.L. Life without A tail: New formats of long noncoding RNAs. Int. J. Biochem. Cell. Biol. 2014, 54, 338–349. [Google Scholar] [CrossRef]
- Xu, S.M.; Curry-Hyde, A.; Sytnyk, V.; Janitz, M. RNA polyadenylation patterns in the human transcriptome. Gene 2022, 816, 146133. [Google Scholar] [CrossRef]
- Jiang, C.; Li, Y.; Zhao, Z.; Lu, J.; Chen, H.; Ding, N.; Wang, G.; Xu, J.; Li, X. Identifying and functionally characterizing tissue-specific and ubiquitously expressed human lncRNAs. Oncotarget. 2016, 7, 7120–7133. [Google Scholar] [CrossRef] [PubMed]
- Davidovich, C.; Cech, T.R. The recruitment of chromatin modifiers by long noncoding RNAs: Lessons from PRC2. RNA 2015, 21, 2007–2022. [Google Scholar] [CrossRef]
- Teo, W.W.; Cao, X.; Wu, C.S.; Tan, H.K.; Zhou, Q.; Gao, C.; Vanuytsel, K.; Kumar, S.S.; Murphy, G.J.; Yang, H.; et al. Non-coding RNA LEVER sequestration of PRC2 can mediate long range gene regulation. Commun. Biol. 2022, 5, 343. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, J.; Dimitrova, N. Transcription regulation by long non-coding RNAs: Mechanisms and disease relevance. Nat. Rev. Mol. Cell Biol. 2024, 25, 396–415. [Google Scholar] [CrossRef] [PubMed]
- Noh, J.H.; Kim, K.M.; McClusky, W.G.; Abdelmohsen, K.; Gorospe, M. Cytoplasmic functions of long noncoding RNAs. Wiley Interdiscip. Rev. RNA 2018, 9, e1471. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, H.; Li, L.; Gao, Y.; Yu, B.; Ma, G.; Jin, X.; Sun, Y. New mechanism of LncRNA: In addition to act as a ceRNA. Noncoding RNA Res. 2024, 9, 1050–1060. [Google Scholar] [CrossRef]
- Peng, Y.; Long, X.D. The role of the ceRNA network mediated by lncRNA SNHG3 in the progression of cancer. Discov. Oncol. 2024, 15, 514. [Google Scholar] [CrossRef]
- Mirzadeh Azad, F.; Polignano, I.L.; Proserpio, V.; Oliviero, S. Long Noncoding RNAs in Human Stemness and Differentiation. Trends Cell. Biol. 2021, 31, 542–555. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Zhang, X.; Gu, X.; Li, X.; Shang, L. Progress in understanding the role of lncRNA in programmed cell death. Cell Death Discov. 2021, 7, 30. [Google Scholar] [CrossRef]
- Tüncel, Ö.; Kara, M.; Yaylak, B.; Erdoğan, İ.; Akgül, B. Noncoding RNAs in apoptosis: Identification and function. Turk. J. Biol. 2021, 46, 1–40. [Google Scholar]
- Bocchetti, M.; Scrima, M.; Melisi, F.; Luce, A.; Sperlongano, R.; Caraglia, M.; Zappavigna, S.; Cossu, A.M. LncRNAs and Immunity: Coding the Immune System with Noncoding Oligonucleotides. Int. J. Mol. Sci. 2021, 22, 1741. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Li, J.; Yang, L.; Wu, M.; Ma, Q. The Role of Long Non-coding RNAs in Human Imprinting Disorders: Prospective Therapeutic Targets. Front. Cell. Dev. Biol. 2021, 9, 730014. [Google Scholar] [CrossRef]
- Dragomir, M.; Chen, B.; Calin, G.A. Exosomal lncRNAs as new players in cell-to-cell communication. Transl. Cancer Res. 2018, 7, S243–S252. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Weiswald, L.B.; Poulain, L.; Denoyelle, C.; Meryet-Figuiere, M. Involvement of lncRNAs in cancer cells migration, invasion and metastasis: Cytoskeleton and ECM crosstalk. J. Exp. Clin. Cancer Res. 2023, 42, 173. [Google Scholar] [CrossRef]
- Anilkumar, A.K.; Vij, P.; Lopez, S.; Leslie, S.M.; Doxtater, K.; Khan, M.M.; Yallapu, M.M.; Chauhan, S.C.; Maestre, G.E.; Tripathi, M.K. Long Non-Coding RNAs: New Insights in Neurodegenerative Diseases. Int. J. Mol. Sci. 2024, 25, 2268. [Google Scholar] [CrossRef] [PubMed]
- Kohlmaier, A.; Holdt, L.M.; Teupser, D. Long noncoding RNAs in cardiovascular disease. Curr. Opin. Cardiol. 2023, 38, 179–192. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Lin, J.D. Long Noncoding RNAs: A New Regulatory Code in Metabolic Control. Trends Biochem. Sci. 2015, 40, 586–596. [Google Scholar] [CrossRef] [PubMed]
- Nadhan, R.; Isidoro, C.; Song, Y.S.; Dhanasekaran, D.N. LncRNAs and the cancer epigenome: Mechanisms and therapeutic potential. Cancer Lett. 2024, 605, 217297. [Google Scholar] [CrossRef]
- Arrigoni, A.; Ranzani, V.; Rossetti, G.; Panzeri, I.; Abrignani, S.; Bonnal, R.J.; Pagani, M. Analysis RNA-seq and Noncoding RNA. Methods Mol. Biol. 2016, 1480, 125–135. [Google Scholar]
- Chu, C.; Qu, K.; Zhong, F.L.; Artandi, S.E.; Chang, H.Y. Genomic maps of long noncoding RNA occupancy reveal principles of RNA-chromatin interactions. Mol. Cell 2011, 44, 667–678. [Google Scholar] [CrossRef]
- Zhao, K.; Wang, X.; Hu, Y. Identification of lncRNA-Protein Interactions by CLIP and RNA Pull-Down Assays. Methods Mol. Biol. 2021, 2348, 231–242. [Google Scholar] [PubMed]
- Habib, A.M.; Cox, J.J.; Okorokov, A.L. Out of the dark: The emerging roles of lncRNAs in pain. Trends Genet. 2024, 40, 694–705. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, X.; Jian, W.; Xue, Q.; Liu, Z. Roles of Long Non-coding RNAs in the Development of Chronic pain. Front. Mol. Neurosci. 2021, 14, 760964. [Google Scholar] [CrossRef]
- Videira, R.F.; da Costa Martins, P.A.; Falcão-Pires, I. Non-Coding RNAs as Blood-Based Biomarkers in Cardiovascular Disease. Int. J. Mol. Sci. 2020, 21, 9285. [Google Scholar] [CrossRef]
- Garofalo, M.; Pandini, C.; Sproviero, D.; Pansarasa, O.; Cereda, C.; Gagliardi, S. Advances with Long Non-Coding RNAs in Alzheimer’s Disease as Peripheral Biomarker. Genes 2021, 12, 1124. [Google Scholar] [CrossRef]
- Wong, D.T. Salivary extracellular noncoding RNA: Emerging biomarkers for molecular diagnostics. Clin. Ther. 2015, 37, 540–551. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, X.; Han, X.; Pandey, V.; Lobie, P.E.; Zhu, T. The potential of long noncoding RNAs for precision medicine in human cancer. Cancer Lett. 2021, 501, 12–19. [Google Scholar] [CrossRef]
- Peng, J.W.; Gu, Y.Y.; Wei, J.; Sun, Y.; Zhu, C.L.; Zhang, L.; Song, Y.; Chen, L.; Chen, X.; Wang, Q.; et al. LncRNA MEG3-TRPV1 signaling regulates chronic inflammatory pain in rats. Mol. Pain 2022, 18, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, M.; Sakai, A.; Fukunaga, T.; Miyagawa, Y.; Okada, T.; Hamada, M.; Suzuki, H. Neat1 lncRNA organizes the inflammatory gene expressions in the dorsal root ganglion in neuropathic pain caused by nerve injury. Front. Immunol. 2023, 14, 1185322. [Google Scholar] [CrossRef]
- Sun, W.; Ma, M.; Yu, H.; Yu, H. Inhibition of lncRNA X inactivate-specific transcript ameliorates inflammatory pain by suppressing satellite glial cell activation and inflammation by acting as a sponge of miR-146a to inhibit Nav 1.7. J. Cell. Biochem. 2018, 119, 9888–9898. [Google Scholar] [CrossRef]
- Dou, Q.; Ba, F.; Hu, S.; Xu, G.Y.; Wei, J.; Jiang, G.Q. LncRNA NONRATT014888.2 contributes to cancer-induced bone pain through downregulation of natriuretic peptide receptor 3 in rats. Biochem. Biophys. Res. Commun. 2023, 683, 149114. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lu, C.; Wang, X.; Wang, L.; Chen, J.; Ji, F. LncRNA NONRATT009773.2 promotes bone cancer pain progression through the miR-708-5p/CXCL13 axis. Eur. J. Neurosci. 2022, 55, 661–674. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Sun, H.; Ji, Z. Downregulating lncRNA PVT1 Relieves Astrocyte Overactivation Induced Neuropathic Pain Through Targeting miR-186-5p/CXCL13/CXCR5 Axis. Neurochem. Res. 2021, 46, 1457–1469. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhu, D.; Li, Q. LncRNA CRNDE exacerbates neuropathic pain in chronic constriction injury-induced(CCI) rats through regulating miR-146a-5p/WNT5A pathway. Bioengineered 2021, 12, 7348–7359. [Google Scholar] [CrossRef]
- Huo, M.; Zheng, X.; Bai, N.; Xu, R.; Yang, G.; Zhao, Z. LncRNA PCAT19 Regulates Neuropathic Pain via Regulation of miR-182-5p/JMJD1A in a Rat Model of Chronic Constriction Injury. Neuroimmunomodulation 2022, 29, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Feng, L.; Ren, S.; Zhang, Y.; Xue, J. Inhibition of lncRNA DILC attenuates neuropathic pain via the SOCS3/JAK2/STAT3 pathway. Biosci. Rep. 2020, 40, BSR20194486. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Lv, D.B.; Su, Y.N.; Wang, X.L.; Sheng, W.C.; Yang, G.; Li, L.X.; Gao, X.; Gao, Y.Z.; Li, J.T. LncRNA SNHG1 attenuates neuropathic pain following spinal cord injury by regulating CDK4 level. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 12034–12040. [Google Scholar]
- Zhang, Z.; Sun, X.; Zhao, G.; Ma, Y.; Zeng, G. LncRNA embryonic stem cells expressed 1 (Lncenc1) is identified as a novel regulator in neuropathic pain by interacting with EZH2 and downregulating the expression of Bai1 in mouse microglia. Exp. Cell Res. 2021, 399, 112435. [Google Scholar] [CrossRef]
- Sun, R.M.; Wei, J.; Wang, S.S.; Xu, G.Y.; Jiang, G.Q. Upregulation of lncRNA-NONRATT021203.2 in the dorsal root ganglion contributes to cancer-induced pain via CXCL9 in rats. Biochem. Biophys. Res. Commun. 2020, 524, 983–989. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, C.; Liu, P.; He, Q.; Zhang, S.; Liu, Z.; Ni, C.; Chen, L.; Zhi, T.; Xu, L.; et al. LncRNA 51325 Alleviates Bone Cancer Induced Hyperalgesia Through Inhibition of Pum2. J. Pain Res. 2024, 17, 265–284. [Google Scholar] [CrossRef]
- Ni, H.; Xu, M.; Kuang, J.; Xu, C.; He, Q.; Luo, G.; Fu, J.; Zhu, J.; Ni, C.; Zhao, B.; et al. Upregulation of LncRNA71132 in the spinal cord regulates hypersensitivity in a rat model of bone cancer pain. Pain 2023, 164, 180–196. [Google Scholar] [CrossRef] [PubMed]
- Qin, T.; Li, J.; Zhang, K.Q. Structure, Regulation, and Function of Linear and Circular Long Non-Coding RNAs. Front. Genet. 2020, 11, 150. [Google Scholar] [CrossRef]
- Liu, L.; Wang, J.; Khanabdali, R.; Kalionis, B.; Tai, X.; Xia, S. Circular RNAs: Isolation, characterization and their potential role in diseases. RNA Biol. 2017, 14, 1715–1721. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, K.; Tan, S.; Xin, J.; Yuan, Q.; Xu, H.; Xu, X.; Liang, Q.; Christiani, D.C.; Wang, M.; et al. Circular RNAs in body fluids as cancer biomarkers: The new frontier of liquid biopsies. Mol. Cancer 2021, 20, 13. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wilusz, J.E.; Chen, L.L. Biogenesis and Regulatory Roles of Circular RNAs. Annu. Rev. Cell. Dev. Biol. 2022, 38, 263–289. [Google Scholar] [CrossRef]
- Rogalska, M.E.; Vivori, C.; Valcárcel, J. Regulation of pre-mRNA splicing: Roles in physiology and disease, and therapeutic prospects. Nat. Rev. Genet. 2023, 24, 251–269. [Google Scholar] [CrossRef]
- Chen, I.; Chen, C.Y.; Chuang, T.J. Biogenesis, identification, and function of exonic circular RNAs. Wiley Interdiscip. Rev. RNA 2015, 6, 563–579. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.O.; Chen, T.; Xiang, J.F.; Yin, Q.F.; Xing, Y.H.; Zhu, S.; Yang, L.; Chen, L.L. Circular intronic long noncoding RNAs. Mol. Cell 2013, 51, 792–806. [Google Scholar] [CrossRef]
- Yang, Y.; Zhong, Y.; Chen, L. EIciRNAs in focus: Current understanding and future perspectives. RNA Biol. 2025, 22, 1–12. [Google Scholar] [CrossRef]
- Das, A.; Sinha, T.; Shyamal, S.; Panda, A.C. Emerging Role of Circular RNA-Protein Interactions. Noncoding RNA 2021, 7, 48. [Google Scholar] [CrossRef]
- Eger, N.; Schoppe, L.; Schuster, S.; Laufs, U.; Boeckel, J.N. Circular RNA Splicing. Adv. Exp. Med. Biol. 2018, 1087, 41–52. [Google Scholar] [PubMed]
- Pamudurti, N.R.; Patop, I.L.; Krishnamoorthy, A.; Bartok, O.; Maya, R.; Lerner, N.; Ashwall-Fluss, R.; Konakondla, J.V.V.; Beatus, T.; Kadener, S. circMbl functions in cis and in trans to regulate gene expression and physiology in a tissue-specific fashion. Cell Rep. 2022, 39, 110740. [Google Scholar] [CrossRef] [PubMed]
- Colantoni, A.; Capauto, D.; Alfano, V.; D’Ambra, E.; D’Uva, S.; Tartaglia, G.G.; Morlando, M. FUS Alters circRNA Metabolism in Human Motor Neurons Carrying the ALS-Linked P525L Mutation. Int. J. Mol. Sci. 2023, 24, 3181. [Google Scholar] [CrossRef]
- Misir, S.; Wu, N.; Yang, B.B. Specific expression and functions of circular RNAs. Cell Death Differ. 2022, 39, 81–491. [Google Scholar] [CrossRef] [PubMed]
- Panda, A.C. Circular RNAs Act as miRNA Sponges. Adv. Exp. Med. Biol. 2018, 1087, 67–79. [Google Scholar]
- Xiao, J.; Joseph, S.; Xia, M.; Teng, F.; Chen, X.; Huang, R.; Zhai, L.; Deng, W. Circular RNAs Acting as miRNAs’ Sponges and Their Roles in Stem Cells. J. Clin. Med. 2022, 11, 2909. [Google Scholar] [CrossRef]
- Shao, T.; Pan, Y.H.; Xiong, X.D. Circular RNA: An important player with multiple facets to regulate its parental gene expression. Mol. Ther. Nucleic Acids 2020, 23, 369–376. [Google Scholar] [CrossRef]
- Li, X.; Zhang, B.; Li, F.; Yu, K.; Bai, Y. The mechanism and detection of alternative splicing events in circular RNAs. PeerJ. 2020, 8, e10032. [Google Scholar] [CrossRef]
- Sinha, T.; Panigrahi, C.; Das, D.; Chandra Panda, A. Circular RNA translation, a path to hidden proteome. Wiley Interdiscip. Rev. RNA 2022, 13, e1685. [Google Scholar] [CrossRef]
- Chen, C.K.; Cheng, R.; Demeter, J.; Chen, J.; Weingarten-Gabbay, S.; Jiang, L.; Snyder, M.P.; Weissman, J.S.; Segal, E.; Jackson, P.K.; et al. Structured elements drive extensive circular RNA translation. Mol. Cell 2021, 81, 4300–4318.e13. [Google Scholar] [CrossRef]
- Di Timoteo, G.; Dattilo, D.; Centrón-Broco, A.; Colantoni, A.; Guarnacci, M.; Rossi, F.; Incarnato, D.; Oliviero, S.; Fatica, A.; Morlando, M.; et al. Modulation of circRNA Metabolism by m6A Modification. Cell Rep. 2020, 31, 107641. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.X.; Chen, L.L. Circular RNAs: Characterization, cellular roles, and applications. Cell 2022, 185, 2016–2034. [Google Scholar] [CrossRef] [PubMed]
- Conn, V.M.; Chinnaiyan, A.M.; Conn, S.J. Circular RNA in cancer. Nat. Rev. Cancer 2024, 24, 597–613. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, P.; Qi, S.; Zhou, J.; Amalraj, J.; Wang, J.; Ding, Z. The clinical perspective of circular RNAs in neurodegenerative diseases: Potential diagnostic tools and therapeutic targets. Front. Cell. Neurosci. 2024, 18, 1470641. [Google Scholar] [CrossRef]
- Long, Q.; Lv, B.; Jiang, S.; Lin, J. The Landscape of Circular RNAs in Cardiovascular Diseases. Int. J. Mol. Sci. 2023, 24, 4571. [Google Scholar] [CrossRef]
- Kurtović, Z.; Sandor, K.; Ter Heegde, F.; Rudjito, R.; Svensson, C.I.; Palada, V. circRNA landscape in dorsal root ganglia from mice with collagen antibody-induced arthritis. Neurobiol. Pain 2023, 14, 100142. [Google Scholar] [CrossRef]
- Siddiq, M.M.; Toro, C.A.; Johnson, N.P.; Hansen, J.; Xiong, Y.; Mellado, W.; Tolentino, R.E.; Johnson, K.; Jayaraman, G.; Suhail, Z.; et al. Spinal cord injury regulates circular RNA expression in axons. Front. Mol. Neurosci. 2023, 16, 1183315. [Google Scholar] [CrossRef]
- Chen, X.; Song, Y.; Chen, G.; Zhang, B.; Bai, Y.; Sun, C.; Fan, D.; Chen, Z. Circular RNA CircFOXO3 Functions as a Competitive Endogenous RNA for Acid-Sensing Ion Channel Subunit 1 Mediating Oxeiptosis in Nucleus Pulposus. Biomedicines 2024, 12, 678. [Google Scholar] [CrossRef]
- Benitez, M.B.M.; Navarro, Y.P.; Azuara-Liceaga, E.; Cruz, A.T.; Flores, J.V.; Lopez-Canovas, L. Circular RNAs and the regulation of gene expression in diabetic nephropathy (Review). Int. J. Mol. Med. 2024, 53, 44. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, S.; Liao, X.; Li, M.; Chen, S.; Li, X.; Wu, X.; Yang, M.; Tang, M.; Hu, Y.; et al. Circular RNA circIKBKB promotes breast cancer bone metastasis through sustaining NF-κB/bone remodeling factors signaling. Mol. Cancer 2021, 20, 98. [Google Scholar] [CrossRef]
- Wang, Z.; Deng, C.; Zheng, Y. Involvement of circRNAs in Proinflammatory Cytokines-Mediated β-Cell Dysfunction. Mediat. Inflamm. 2021, 2021, 5566453. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Xiao, D.; Wu, C.; Yang, J.; Peng, X.; Chen, L.; Zhang, J.; Zha, G.; Li, W.; Ju, R.; et al. Circular RNA-based therapy provides sustained and robust neuroprotection for retinal ganglion cells. Mol. Ther. Nucleic Acids 2024, 35, 102258. [Google Scholar] [CrossRef]
- Qadir, J.; Wen, S.Y.; Yuan, H.; Yang, B.B. CircRNAs regulate the crosstalk between inflammation and tumorigenesis: The bilateral association and molecular mechanisms. Mol. Ther. 2023, 31, 1514–1532. [Google Scholar] [CrossRef] [PubMed]
- Giusti, S.A.; Pino, N.S.; Pannunzio, C.; Ogando, M.B.; Armando, N.G.; Garrett, L.; Zimprich, A.; Becker, L.; Gimeno, M.L.; Lukin, J.; et al. A brain-enriched circular RNA controls excitatory neurotransmission and restricts sensitivity to aversive stimuli. Sci. Adv. 2024, 10, eadj8769. [Google Scholar] [CrossRef]
- You, X.; Vlatkovic, I.; Babic, A.; Will, T.; Epstein, I.; Tushev, G.; Akbalik, G.; Wang, M.; Glock, C.; Quedenau, C.; et al. Neural circular RNAs are derived from synaptic genes and regulated by development and plasticity. Nat. Neurosci. 2015, 18, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Dong, X.; Li, X.; Yang, Y.; Li, H.; Hong, Y.; Yang, G.; Kong, X.; Wang, X.; Ma, X. Moxibustion ameliorates chronic inflammatory visceral pain via spinal circRNA-miRNA-mRNA networks: A central mechanism study. Mol. Brain 2024, 17, 23. [Google Scholar] [CrossRef]
- Yang, S.Q.; Peng, L.; Lin, L.D.; Chen, Y.Z.; Liu, M.Z.; Zhang, C.; Chen, J.W.; Luo, D.Y. Identification of circRNA-miRNA-mRNA network as biomarkers for interstitial cystitis/bladder pain syndrome. Aging 2023, 15, 12155–12170. [Google Scholar] [CrossRef]
- He, A.T.; Liu, J.; Li, F.; Yang, B.B. Targeting circular RNAs as a therapeutic approach: Current strategies and challenges. Signal Transduct. Target. Ther. 2021, 6, 185. [Google Scholar] [CrossRef]
- Qi, D.; Ke, R.; Huang, J.H.; Wu, E. Forging the future of circRNA therapeutics: Unleashing synthetic potential and conquering challenges. Mol. Ther. Nucleic Acids 2023, 33, 42–43. [Google Scholar] [CrossRef]
- Pan, Z.; Li, G.F.; Sun, M.L.; Xie, L.; Liu, D.; Zhang, Q.; Yang, X.X.; Xia, S.; Liu, X.; Zhou, H.; et al. MicroRNA-1224 Splicing CircularRNA-Filip1l in an Ago2-Dependent Manner Regulates Chronic Inflammatory Pain via Targeting Ubr5. J. Neurosci. 2019, 39, 2125–2143. [Google Scholar] [CrossRef]
- Wang, L.; Luo, T.; Bao, Z.; Li, Y.; Bu, W. Intrathecal circHIPK3 shRNA alleviates neuropathic pain in diabetic rats. Biochem. Biophys. Res. Commun. 2018, 505, 644–650. [Google Scholar] [CrossRef]
- Wang, K.; Shen, Z.; Peng, X.; Wu, X.; Mao, L. Circular RNA-GRIN2B Suppresses Neuropathic Pain by Targeting the NF-κB/SLICK Pathway. Neuromol. Med. 2024, 26, 12. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Song, X.; Ge, Q. Circular RNA SMEK1 promotes neuropathic pain in rats through targeting microRNA-216a-5p to mediate Thioredoxin Interacting Protein (TXNIP) expression. Bioengineered 2021, 12, 5540–5551. [Google Scholar] [CrossRef]
- Li, L.; Luo, Y.; Zhang, Y.; Wei, M.; Zhang, M.; Liu, H.; Su, Z. CircZNF609 aggravates neuropathic pain via miR-22-3p/ENO1 axis in CCI rat models. Gene 2020, 763, 145069. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.D.; Jin, T.; Tao, Y.; Zhang, M.; Zheng, H.L.; Liu, Q.Q.; Yang, K.H.; Wei, R.N.; Li, S.Y.; Huang, Y.; et al. DHX9/DNA-tandem repeat-dependent downregulation of ciRNA-Fmn1 in the dorsal horn is required for neuropathic pain. Acta Pharmacol. Sin. 2023, 44, 1748–1767. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gao, T.; Li, X.; Wen, C.C.; Yan, X.T.; Peng, C.; Xiao, Y. Circ_0005075 targeting miR-151a-3p promotes neuropathic pain in CCI rats via inducing NOTCH2 expression. Gene 2021, 767, 145079. [Google Scholar] [CrossRef]
- Tang, Q.; Fang, Z.; Liao, H.; Zhang, Y.; Li, C.; Zhou, C.; Liu, F.; Shen, J. Reduced circ_lrrc49 in trigeminal ganglion contributes to neuropathic pain in mice by downregulating Ist1 and impairing autophagy. J. Neurochem. 2024, 168, 1265–1280. [Google Scholar] [CrossRef]
- Xie, L.; Zhang, M.; Liu, Q.; Wei, R.; Sun, M.; Zhang, Q.; Hao, L.; Xue, Z.; Wang, Q.; Yang, L.; et al. Downregulation of ciRNA-Kat6b in dorsal spinal horn is required for neuropathic pain by regulating Kcnk1 in miRNA-26a-dependent manner. CNS Neurosci. Ther. 2023, 29, 2955–2971. [Google Scholar] [CrossRef]
- Xu, T.; Li, Z.Y.; Liu, M.; Zhang, S.B.; Ding, H.H.; Wu, J.Y.; Lin, S.Y.; Liu, J.; Wei, J.Y.; Zhang, X.Q.; et al. CircFhit Modulates GABAergic Synaptic Transmission via Regulating the Parental Gene Fhit Expression in the Spinal Dorsal Horn in a Rat Model of Neuropathic pain. Neurosci. Bull. 2023, 39, 947–961. [Google Scholar] [CrossRef]
- Zhang, S.B.; Lin, S.Y.; Liu, M.; Liu, C.C.; Ding, H.H.; Sun, Y.; Ma, C.; Guo, R.X.; Lv, Y.Y.; Wu, S.L.; et al. CircAnks1a in the spinal cord regulates hypersensitivity in a rodent model of neuropathic pain. Nat. Commun. 2019, 10, 4119. [Google Scholar] [CrossRef]
- Wei, M.; Li, L.; Zhang, Y.; Zhang, M.; Su, Z. Downregulated circular RNA zRANB1 mediates Wnt5a/β-Catenin signaling to promote neuropathic pain via miR-24-3p/LPAR3 axis in CCI rat models. Gene 2020, 761, 145038. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.W.; Zhang, X.X.; Peng, Z.D.; Xing, Z.M.; Zhang, Y.W.; Li, Y.L. The circular RNA circSlc7a11 promotes bone cancer pain pathogenesis in rats by modulating LLC-WRC 256 cell proliferation and apoptosis. Mol. Cell Biochem. 2021, 476, 1751–1763. [Google Scholar] [CrossRef]
- Tian, H.; Cheng, L.; Liang, Y.; Lei, H.; Qin, M.; Li, X.; Ren, Y. MicroRNA therapeutic delivery strategies: A review. J. Drug Deliv. Sci. Technol. 2024, 93, 105430. [Google Scholar] [CrossRef]
- Ali Zaidi, S.S.; Fatima, F.; Ali Zaidi, S.A.; Zhou, D.; Deng, W.; Liu, S. Engineering siRNA therapeutics: Challenges and strategies. J. Nanobiotechnology 2023, 21, 381. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.Y.; Zhu, S.X.; Pu, K.J.; Huang, H.J.; Chen, Y.Q.; Wang, W.T. New insight into circRNAs: Characterization, strategies, and biomedical applications. Exp. Hematol. Oncol. 2023, 12, 91. [Google Scholar] [CrossRef]
- Han, S.; Chen, X.; Huang, L. The tumor therapeutic potential of long non-coding RNA delivery and targeting. Acta Pharm. Sin. B 2023, 13, 1371–1382. [Google Scholar] [CrossRef]
- Hossam Abdelmonem, B.; Kamal, L.T.; Wardy, L.W.; Ragheb, M.; Hanna, M.M.; Elsharkawy, M.; Abdelnaser, A. Non-coding RNAs: Emerging biomarkers and therapeutic targets in cancer and inflammatory diseases. Front. Oncol. 2025, 15, 1534862. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Chu, H.; Ma, J.; Yan, Y.; Zhang, X.; Liang, Y. The Regulatory Mechanisms and Therapeutic Potential of MicroRNAs: From Chronic Pain to Morphine Tolerance. Front. Mol. Neurosci. 2018, 11, 80. [Google Scholar] [CrossRef]
- Tao, Y.; Zhang, Y.; Jin, X.; Hua, N.; Liu, H.; Qi, R.; Huang, Z.; Sun, Y.; Jiang, D.; Snutch, T.P.; et al. Epigenetic regulation of beta-endorphin synthesis in hypothalamic arcuate nucleus neurons modulates neuropathic pain in a rodent pain model. Nat. Commun. 2023, 14, 7234. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Z.; Chen, X.; Zhang, S. Long non-coding RNAs: From disease code to drug role. Acta Pharm. Sin. B 2021, 11, 340–354. [Google Scholar] [CrossRef]
- Li, Y.Z.; Ji, R.R. Gene therapy for chronic pain management. Cell Rep. Med. 2024, 5, 101756. [Google Scholar] [CrossRef] [PubMed]
- Winkle, M.; El-Daly, S.M.; Fabbri, M.; Calin, G.A. Noncoding RNA therapeutics—Challenges and potential solutions. Nat. Rev. Drug Discov. 2021, 20, 629–651. [Google Scholar] [CrossRef] [PubMed]
- Vicentini, C.; Galuppini, F.; Corbo, V.; Fassan, M. Current role of non-coding RNAs in the clinical setting. Noncoding RNA Res. 2019, 4, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Karthik, S.; Mohan, S.; Magesh, I.; Bharathy, A.; Kolipaka, R.; Ganesamoorthi, S.; Sathiya, K.; Shanmugavadivu, A.; Gurunathan, R.; Selvamurugan, N. Chitosan nanocarriers for non-coding RNA therapeutics: A review. Int. J. Biol. Macromol. 2024, 263, 130361. [Google Scholar] [CrossRef]
- Rincón-Riveros, A.; Morales, D.; Rodríguez, J.A.; Villegas, V.E.; López-Kleine, L. Bioinformatic Tools for the Analysis and Prediction of ncRNA Interactions. Int. J. Mol. Sci. 2021, 222, 11397. [Google Scholar] [CrossRef]
- Ito, M.; Miyata, Y.; Okada, M. Current clinical trials with non-coding RNA-based therapeutics in malignant diseases: A systematic review. Transl. Oncol. 2023, 31, 101634. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).