Advances in Preparation and Biomedical Applications of Sodium Alginate-Based Electrospun Nanofibers
Abstract
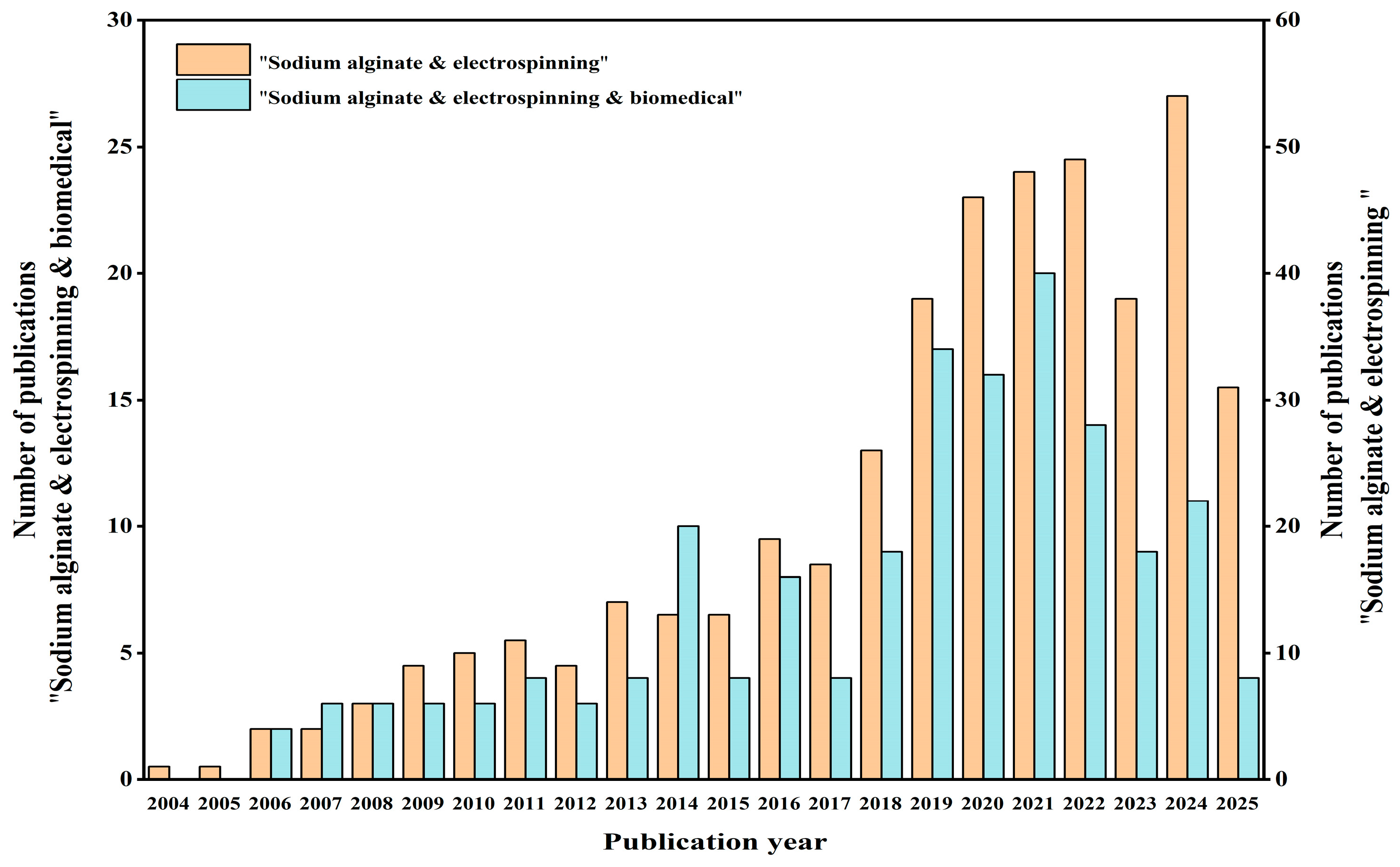
1. Introduction
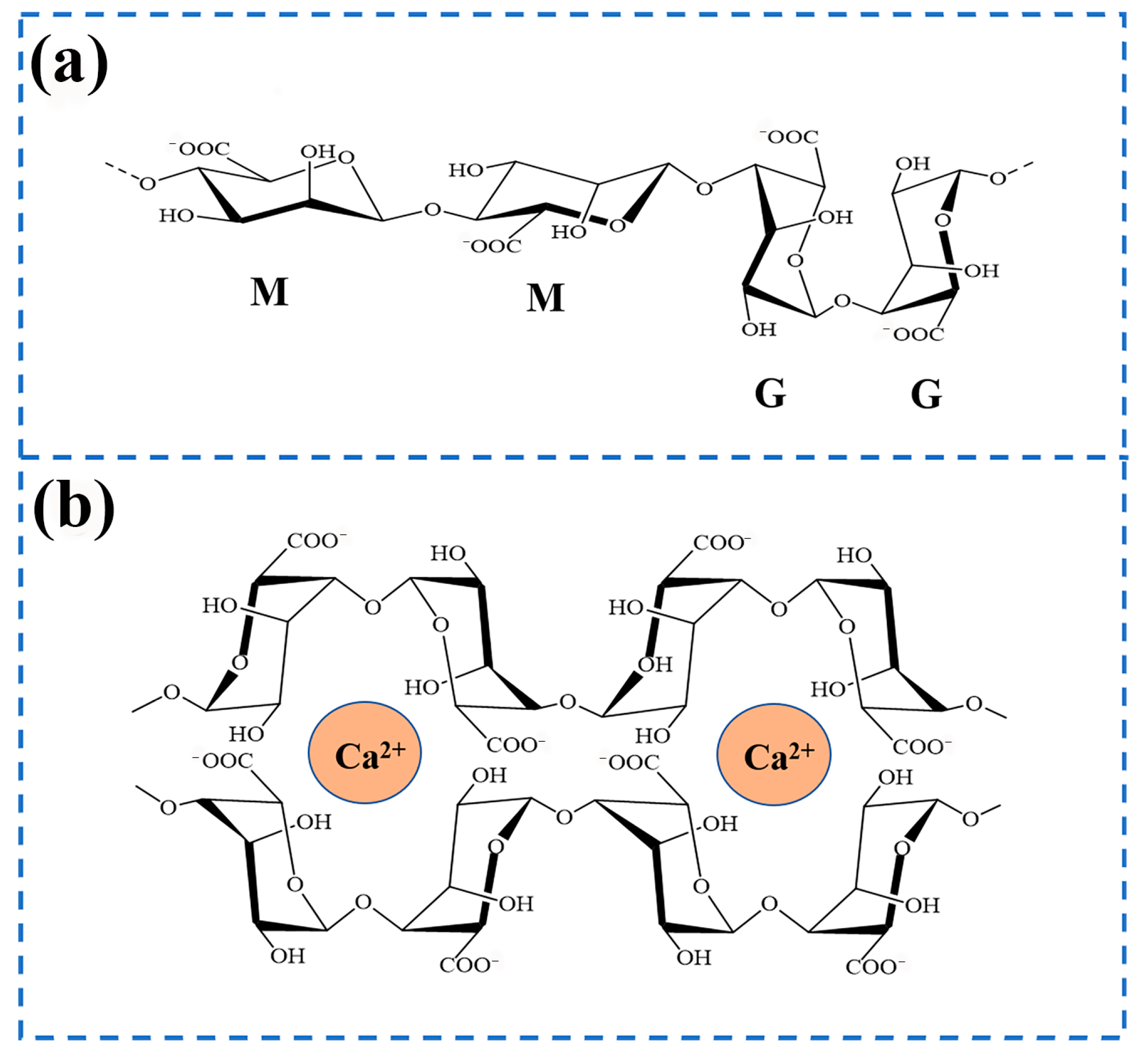
2. Sodium Alginate
3. Preparation Method of Sodium Alginate Nanofibers
3.1. Blended Electrospinning
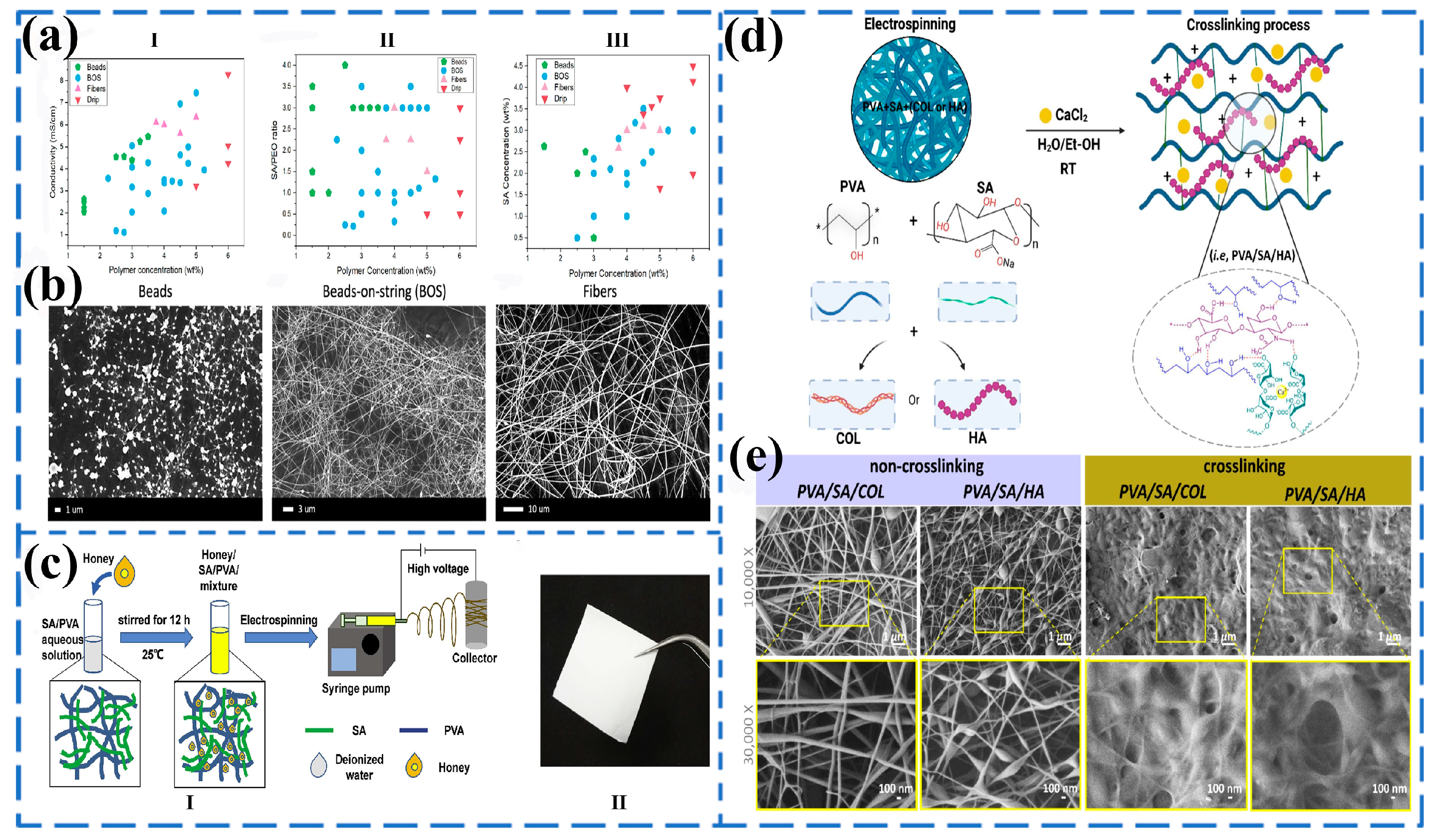
3.2. Coaxial Electrospinning
3.3. Lotion Electrospinning
4. Application of Sodium Alginate Composite Nanofibers in Biomedical Field
4.1. Tissue Engineering Scaffolds
4.2. Wound Dressings
4.3. Drug Delivery
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SA | Sodium alginate |
| PVA | Polyvinyl alcohol |
| PEO | Polyethylene oxide |
| ECM | Extracellular matrix |
| Dex | Dexpanthenol |
| ZnO NP | Zinc oxide nanoparticles |
| PLCL | Poly(L-propylcaprolactone CO)-ε-caprolactone) |
| Gel | Gelatin |
| COL | Collagen |
| HA | Hyaluronic acid |
| CP | Chlorella pyrenoidosa |
| CHI | Chitosan |
| W/O | Water-in-oil emulsion |
| O/W | Oil-in-water emulsion |
| PCL | Polycaprolactone |
| CMCS | Carboxymethyl chitosan |
| 3D | Three-dimensional |
| HGF | Human gingival fibroblast |
| SAEO | Salvia miltiorrhiza essential oil |
| EXOs | Exosomes |
| EPS | Extracellular polysaccharides |
| Bate | Betamethasone |
| PrO2 | Praseodymium oxide |
| CIO | Calophyllum inophyllum seed oil |
| DPPH | 2,2-diphenyl-2-picrylhydrazyl |
| ABTS | 2,2-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid |
| Ba | Baicalein |
| TFA | Trifluoroacetic acid |
| SDR | Sustained drug release |
| RAOA | Reductive amination of oxidized alginate derivative |
| PBS | Phosphate buffer saline |
| AD | Alginate formaldehyde |
| PS80 | Polysorbate |
| CaCO3 | Calcium carbonate |
| PDLGA | Poly(D,L-lactide-co-glycolide) |
| PCU | Polycarbonate polyurethane |
| CFAGS | A novel alginate/gelatin sponge combined with curcumin-supported electrospun fibers |
| IPN | Interosmotic polymer network |
References
- Sellitto, M.R.; Larobina, D.; De Soricellis, C.; Amante, C.; Falcone, G.; Russo, P.; Bernardes, B.G.; Oliveira, A.L.; Del Gaudio, P. Silk Fibroin–Alginate Aerogel Beads Produced by Supercritical CO2 Drying: A Dual-Function Conformable and Haemostatic Dressing. Gels 2025, 11, 603. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Hu, W.; You, W.; Huang, G.; Tian, W.; Huselstein, C.; Wu, C.-L.; Xiao, Y.; Chen, Y.; Wang, X. Antibacterial and angiogenic wound dressings for chronic persistent skin injury. Chem. Eng. J. 2021, 404, 126525. [Google Scholar] [CrossRef]
- Wang, C.; Wang, M.; Xu, T.; Zhang, X.; Lin, C.; Gao, W.; Xu, H.; Lei, B.; Mao, C. Engineering Bioactive Self-Healing Antibacterial Exosomes Hydrogel for Promoting Chronic Diabetic Wound Healing and Complete Skin Regeneration. Theranostics 2019, 9, 65–76. [Google Scholar] [CrossRef]
- Alsulami, K.A.; Bakr, A.A.; Aodah, A.H.; Almughem, F.A.; Alamer, A.A.; Alharbi, L.A.; Alsuwayeh, D.S.; Halwani, A.A.; Alamoudi, A.A.; Alfassam, H.A.; et al. Fabrication and evaluation of ribavirin-loaded electrospun nanofibers as an ntimicrobial wound dressing. Saudi Pharm. J. 2024, 32, 102058. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, L.; Ma, A.; Bai, X.; Zeng, Y.; Liu, D.; Liu, B.; Zhang, W.; Tang, S. Recent advances in coaxial electrospun nanofibers for wound healing. Mater. Today. Bio 2024, 29, 101309. [Google Scholar] [CrossRef] [PubMed]
- Brauer, E.; Lippens, E.; Klein, O.; Nebrich, G.; Schreivogel, S.; Korus, G.; Duda, G.N.; Petersen, A. Collagen Fibrils Mechanically Contribute to Tissue Contraction in an In Vitro Wound Healing Scenario. Adv. Sci. 2019, 6, 1801780. [Google Scholar] [CrossRef]
- Yu, J.; Liu, J.; Liu, Y.; Liu, S.; Su, Z.; Liang, D. Ag/TA@CNC Reinforced Hydrogel Dressing with Enhanced Adhesion and Antibacterial Activity. Gels 2025, 11, 591. [Google Scholar] [CrossRef]
- Dong, R.; Guo, B. Smart wound dressings for wound healing. Nano Today 2021, 41, 101290. [Google Scholar] [CrossRef]
- Zhou, S.; Liu, Z.; Jin, Y.; Huang, Y.; Fang, Y.; Tian, H.; Wu, H. Poly (lactic acid) electrospun nanofiber membranes: Advanced characterization for biomedical applications with drug loading performance studies. Int. J. Biol. Macromol. 2024, 281, 136188. [Google Scholar] [CrossRef]
- Kamoun, E.A.; Loutfy, S.A.; Hussein, Y.; Kenawy, E.-R.S. Recent advances in PVA-polysaccharide based hydrogels and electrospun nanofibers in biomedical applications: A review. Int. J. Biol. Macromol. 2021, 187, 755–768. [Google Scholar] [CrossRef]
- Dadashpour, M.; Kalavi, S.; Gorgzadeh, A.; Nosrati, R.; Amandi, A.F.; Mohammadikhah, M.; Sara, M.R.S.; Alizadeh, E. Preparation and in vitro evaluation of cell adhesion and long-term proliferation of stem cells cultured on silibinin co-embedded PLGA/Collagen electrospun composite nanofibers. Exp. Cell Res. 2024, 435, 113926. [Google Scholar] [CrossRef]
- Nicoară, A.-I.; Anton, A.V.; Trușcă, R.D.; Bîrcă, A.C.; Ilie, C.-I.; Dițu, L.-M. Antibacterial Composites Based on Alginate/Egg White and ZnO Nanoparticles with the Addition of Essential Oils. Gels 2025, 11, 459. [Google Scholar] [CrossRef]
- Rezaei, S.; Valipouri, A.; Hosseini Ravandi, S.A.; Kouhi, M.; Ghasemi Mobarakeh, L. Fabrication, characterization, and drug release study of vitamin C–loaded alginate/polyethylene oxide nanofibers for the treatment of a skin disorder. Polym. Adv. Technol. 2019, 30, 2447–2457. [Google Scholar] [CrossRef]
- Wu, T.; Sawut, A.; Simayi, R. Preparation and Heavy Metal Adsorption Performance of 2-Aminopyridine-Modified Sodium Alginate/Polyacrylic Acid Hydrogel. Gels 2025, 11, 224. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Li, L.; Jia, L. Fabrication of calcium/cupric crosslinked alginate electrospun nanofibers for enhancing fluoroquinolones adsorption. Microporous Mesoporous Mater. 2022, 336, 111857. [Google Scholar] [CrossRef]
- Sun, F.; Guo, J.; Liu, Y.; Yu, Y. Preparation, characterizations and properties of sodium alginate grafted acrylonitrile/polyethylene glycol electrospun nanofibers. Int. J. Biol. Macromol. 2019, 137, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Shen, R.; Yan, Y.; Gao, J. Preparation and characterization of electrospun alginate/PLA nanofibers as tissue engineering material by emulsion eletrospinning. J. Mech. Behav. Biomed. 2017, 65, 428–438. [Google Scholar] [CrossRef] [PubMed]
- Bâldea, I.; Soran, M.-L.; Stegarescu, A.; Opriș, O.; Kacso, I.; Tripon, S.; Adascalitei, A.; Fericel, I.G.; Decea, R.; Lung, I. Lilium candidum Extract Loaded in Alginate Hydrogel Beads for Chronic Wound Healing. Gels 2025, 11, 22. [Google Scholar] [CrossRef]
- Xue, S.; Huang, Q. Preparation of Novel Flaxseed Oil/Beeswax Oleogel Systems and Its Application in the Improvement of Sodium Alginate Films. Gels 2024, 10, 78. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, C.; Liang, Y.; Shi, J.; Yu, Q.; Liu, S.; Yu, D.; Liu, H. Advanced postoperative tissue antiadhesive membranes enabled with electrospun nanofibers. Biomater. Sci. 2024, 12, 1643–1661. [Google Scholar] [CrossRef]
- Khorshidi, S.; Karkhaneh, A. Particle-Coated Electrospun Scaffold: A Semi-Conductive Drug Eluted Scaffold with Layered Fiber/Particle Arrangement. J. Biomed. Mater. Res. Part A 2018, 106, 3248–3254. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Liu, Y.; Shang, B.; Geng, M.; Teng, F. Layer-by-layer self-assembled liposomes fabricated using sodium alginate and chitosan: Investigation of co-encapsulation of folic acid and vitamin E. Int. J. Biol. Macromol. 2024, 281, 136464. [Google Scholar] [CrossRef]
- Amr, M.; Dykes, I.; Counts, M.; Kernan, J.; Mallah, A.; Mendenhall, J.; Van Wie, B.; Abu-Lail, N.; Gozen, B.A. 3D printed, mechanically tunable, composite sodium alginate, gelatin and Gum Arabic (SA-GEL-GA) scaffolds. Bioprinting 2021, 22, e00133. [Google Scholar] [CrossRef]
- Chaurasia, A.S.; Jahanzad, F.; Sajjadi, S. Flexible Microfluidic Fabrication of Oil-Encapsulated Alginate Microfibers. Chem. Eng. J. 2017, 308, 1090–1097. [Google Scholar] [CrossRef]
- Wang, X.; Yuan, Y.; Huang, X.; Yue, T. Controlled release of protein from core–shell nanofibers prepared by emulsion electrospinning based on green chemical. J. Appl. Polym. Sci. 2015, 132, 41811. [Google Scholar] [CrossRef]
- Elnabawy, E.; Sun, D.; Shearer, N.; Shyha, I. The effect of electro blow spinning parameters on the characteristics of polylactic acid nanofibers: Towards green development of high-performance biodegradable membrane. Polymer 2024, 311, 127553. [Google Scholar] [CrossRef]
- Liang, Q.; Xie, H.; Huang, W.; Liufu, J.; Liao, M.; Tong, Z.; Zhao, H.; Zheng, W.; Cao, Y.; Zhou, W.; et al. Electrostatic spinning silver oxide/silver -loaded coconut fiber and tannic acid modified polylactic acid packaging film for fresh beef preservation. Int. J. Biol. Macromol. 2025, 310, 143202. [Google Scholar] [CrossRef]
- Zhang, J.; He, L.; Liu, X.; Tong, L. Polyarylene ether nitrile/boron nitride nanosheets thermally conductive films through heterogeneous multilayer electrostatic spinning. Mater. Lett. 2024, 377, 137292. [Google Scholar] [CrossRef]
- Wang, H.; Wang, L.; Liu, Z.; Luo, Y.; Kang, Z.; Che, X. Astragaloside/PVP/PLA nanofiber functional dressing prepared by coaxial electrostatic spinning technology for promoting diabetic wound healing. Eur. Polym. J. 2024, 210, 112950. [Google Scholar] [CrossRef]
- Nie, H.; He, A.; Zheng, J.; Xu, S.; Li, J.; Han, C.C. Effects of Chain Conformation and Entanglement on the Electrospinning of Pure Alginate. Biomacromolecules 2008, 9, 1362–1365. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Guan, G.; Wang, X.; Lou, T. Electrospun molecularly imprinted sodium alginate/polyethylene oxide nanofibrous membranes for selective adsorption of methylene blue. Int. J. Biol. Macromol. 2022, 207, 62–71. [Google Scholar] [CrossRef]
- Surendhiran, D.; Cui, H.; Lin, L. Encapsulation of Phlorotannin in Alginate / PEO blended nanofibers to preserve chicken meat from Salmonella contaminations. Food Packag. Shelf 2019, 21, 100346. [Google Scholar] [CrossRef]
- Li, Q.; Chen, Z.; Guo, J.; Gao, N.; Hou, M.; Cai, Z.; Zhao, K.; Lin, L. High-strength, super-hydrophilic alginate electrospun nanofibrous membranes for rapid oil-water emulsion separation. Colloid. Surf. A 2025, 705, 135637. [Google Scholar] [CrossRef]
- Najafiasl, M.; Osfouri, S.; Azin, R.; Zaeri, S. Fabrication. characterization and in vivo evaluation of dexpanthenol sustained-release nanofibers for wound healing. Polym. Test. 2020, 91, 106827. [Google Scholar] [CrossRef]
- Khan, A.U.R.; Huang, K.; Khalaji, M.S.; Yu, F.; Xie, X.; Zhu, T.; Morsi, Y.; Jinzhong, Z.; Mo, X. Multifunctional bioactive core-shell electrospun membrane capable to terminate inflammatory cycle and promote angiogenesis in diabetic wound. Bioact. Mater. 2021, 6, 2783–2800. [Google Scholar] [CrossRef] [PubMed]
- Eskandani, M.; Derakhshankhah, H.; Jahanban-Esfahlan, R.; Jaymand, M. Biomimetic alginate-based electroconductive nanofibrous scaffolds for bone tissue engineering application. Int. J. Biol. Macromol. 2023, 249, 125991. [Google Scholar] [CrossRef] [PubMed]
- Abid, S.; Wang, L.; Haider, M.K.; Mayakrishnan, G.; Lakshminarayanan, R.; Kim, K.O.; Ullah, A.; Kim, I.S. Investigating alginate and chitosan electrospun nanofibers as a potential wound dressing: An in vitro study. Nanocomposites 2024, 10, 242–255. [Google Scholar] [CrossRef]
- Norouzi, M.-R.; Ghasemi-Mobarakeh, L.; Itel, F.; Schoeller, J.; Fashandi, H.; Fortunato, G.; Rossi, R.M. Dual- Functional Antibacterial-Antioxidant Core/Shell Alginate/Poly(ε-caprolactone) Nanofiber Membrane: A Potential Wound Dressing. ACS Omega 2024, 9, 25124–25134. [Google Scholar] [CrossRef]
- Wróblewska-Krepsztul, J.; Rydzkowski, T.; Michalska-Pożoga, I.; Thakur, V.K. Biopolymers for Biomedical and Pharmaceutical Applications: Recent Advances and Overview of Alginate Electrospinning. Nanomaterials 2019, 9, 404. [Google Scholar] [CrossRef]
- Taemeh, M.A.; Shiravandi, A.; Korayem, M.A.; Daemi, H. Fabrication challenges and trends in biomedical applications of alginate electrospun nanofibers. Carbohydr. Polym. 2020, 228, 115419. [Google Scholar] [CrossRef]
- Mokhena, T.C.; Mochane, M.J.; Mtibe, A.; John, M.J.; Sadiku, E.R.; Sefadi, J.S. Electrospun Alginate Nanofibers Toward Various Applications: A Review. Materials 2020, 13, 934. [Google Scholar] [CrossRef]
- Mostafizur, R.M.; Abdus, S.M.; Tanvir, H.M.; Sohan, S.M.; Sunjidur, R.M.; Nasir, U.; Abdur, R.; Amin, K.R.; Imam, H. Sources, extractions, and applications of alginate: A review. Discov. Appl. Sci. 2024, 6, 443. [Google Scholar] [CrossRef]
- Chowdhury, S.; Chakraborty, S.; Maity, M.; Hasnain, M.S.; Nayak, A.K. Biocomposites of alginates in drug delivery. In Alginates in Drug Delivery; Nayak, A.K., Hasnain, M.S., Eds.; Academic Press: New York, NY, USA, 2020; pp. 153–185. [Google Scholar] [CrossRef]
- Pawar, S.N.; Edgar, K.J. Alginate Derivatization: A Review of Chemistry, Properties and Applications. Biomaterials 2012, 33, 3279–3305. [Google Scholar] [CrossRef]
- Chavez-Granados, P.A.; Garcia-Contreras, R.; Reyes-Lopez, C.A.S.; Correa-Basurto, J.; Hernandez-Rojas, I.E.; Hernandez-Gomez, G.; Jurado, C.A.; Alhotan, A. Green Synthesis of Silver Nanoparticles with Roasted Green Tea: Applications in Alginate–Gelatin Hydrogels for Bone Regeneration. Gels 2024, 10, 706. [Google Scholar] [CrossRef]
- Sakai, S.; Kawakami, K. Synthesis and characterization of both ionically and enzymatically cross-linkable alginate. Acta Biomater. 2007, 3, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Jadach, B.; Kowalczyk, M.; Froelich, A. Assessment of Alginate Gel Films as the Orodispersible Dosage Form for Meloxicam. Gels 2024, 10, 379. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhao, D.; Su, L.; Ding, M.; Zhang, M.; He, H.; Li, C. Encapsulation and release of salidroside in myofibrillar protein-sodium alginate gel: Effects of different M/G ratios of sodium alginate. Int. J. Biol. Macromol. 2024, 282, 136811. [Google Scholar] [CrossRef]
- Tan, J.; Wen, M.; Zhang, Y.; Zhang, S.; Fang, M.; Xiang, J.; Liu, X.; Tian, J.; Lu, L.; Luo, B.; et al. Development of an Asymmetric Alginate Hydrogel Loaded with S-Nitrosoglutathione and Its Application in Chronic Wound Healing. Gels 2025, 11, 354. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Chen, H.S.; Wang, X.L.; Qiu, F.X.; Liu, H.H.; Lu, J.W.; Tong, L.; Yang, Y.M.; Wang, X.S.; Wu, H. Degradable and Bioadhesive Alginate-Based Composites: An Effective Hemostatic Agent. ACS Biomater. Sci. Eng. 2019, 5, 5498–5505. [Google Scholar] [CrossRef]
- Zhang, H.; Cheng, J.; Ao, Q. Preparation of Alginate-Based Biomaterials and Their Applications in Biomedicine. Mar. Drugs 2021, 19, 264. [Google Scholar] [CrossRef]
- Lee, S.; Eun, J. n-Octadecane/alginate composites of varying mannuronate/guluronate ratios. Mater. Chem. Phys. 2025, 343, 131027. [Google Scholar] [CrossRef]
- Dekamin, M.G.; Peyman, S.Z.; Karimi, Z.; Javanshir, S.; Naimi-Jamal, M.R.; Barikani, M. Sodium Alginate: An Efficient Biopolymeric Catalyst for Green Synthesis of 2-Amino-4H-Pyran Derivatives. Int. J. Biol. Macromol. 2016, 87, 172–179. [Google Scholar] [CrossRef]
- Otterlei, M.; Ostgaard, K.; Skjåk-Braek, G.; Smidsrød, O.; Soon-Shiong, P.; Espevik, T. Induction of cytokine production from human monocytes stimulated with alginate. J. Immunother. 1991, 10, 286–291. [Google Scholar] [CrossRef]
- He, J.; Chen, Y.; Wang, J.; Chen, S.; Lan, X.; Liu, M.; Chen, X.; Ou, W.; Wang, H. Lavender Essential Oil-Loaded Composite Fiber Membrane Prepared by Coaxial Electrospinning for High-Performance, Antibacterial, and Lasting-Fragrance Air Filtration. J. Appl. Polym. Sci. 2025, 142, e57000. [Google Scholar] [CrossRef]
- Wang, T.; Su, E. Electrospinning meets food packaging: A promising pathway towards novel opportunities in food preservation. Food Packag. Shelf 2024, 41, 101234. [Google Scholar] [CrossRef]
- Dede, S.; Sadak, O.; Didin, M.; Gunasekaran, S. Basil oil-loaded electrospun biofibers: Edible food packaging material. J. Food Eng. 2022, 319, 110914. [Google Scholar] [CrossRef]
- Orisawayi, A.O.; Koziol, K.; Hao, S.; Tiwari, S.; Rahatekar, S.S. Development of hybrid electrospun alginate-pulverized moringa composites. RSC Adv. 2024, 14, 8502–8512. [Google Scholar] [CrossRef]
- Talebi, M.; Abbasizadeh, S.; Keshtkar, A.R. Evaluation of single and simultaneous thorium and uranium sorption from water systems by an electrospun PVA/SA/PEO/HZSM5 nanofiber. Process Saf. Environ. 2017, 109, 340–356. [Google Scholar] [CrossRef]
- Lian, S.; Zhao, M.; Lamprou, D.A. One-Step fabrication method of insulin-loaded polycaprolactone nanofibers by blend electrospinning for diabetic wound care. Int. J. Pharm. 2025, 683, 126032. [Google Scholar] [CrossRef]
- Li, M.; Zhang, P.; Wang, Q.; Yu, N.; Zhang, X.; Su, S. Electrospinning Novel Sodium Alginate/MXene Nanofiber Membranes for Effective Adsorption of Methylene Blue. Polymers 2023, 15, 2110. [Google Scholar] [CrossRef] [PubMed]
- Henning, S.; Lach, R.; Schönherr, H.; Longo, R.; Catauro, M.; Vertuccio, L.; Guadagno, L. Comparison between Morphological and Mechanical Properties of Membranes Produced via Coaxial and Monoaxial Electrospinning. Macromol. Symp. 2023, 409, 2200155. [Google Scholar] [CrossRef]
- Carroll-Bassham, H.; Brettmann, B.K. The interplay between conductivity and viscosity in electrospinning charged biopolymers. MRS Commun. 2024, 14, 1416–1423. [Google Scholar] [CrossRef]
- Haghbin, M.; Sadeghi-Avalshahr, A.; Hassanzadeh, H.; Moloodi, A.; Harati, Z. Preparation of porous alginate-based smart dressings used in real-time monitoring of pH in chronic wounds by evaluating two fabrication routes: Freeze-drying vs. electrospinning. J. Porous Mater. 2023, 30, 1953–1963. [Google Scholar] [CrossRef]
- Mathew, J.T.; Jose, E.T.; Philip PMohan, M.G.; Cherian, S.K. Preparation and Characterization of Sodium Alginate-Polyvinyl Alcohol Electrospun Nanofibres Using Green Solvents for Biomedical Applications. Polym. Sci. Ser. A 2022, 64, 724–732. [Google Scholar] [CrossRef]
- Khatun, M.R.; Bhattacharyya, A.; Gunbayar, M.; Jo, Y.O.; Noh, I. Gelatin-alginate hydrogel for near-field electrospinning assisted 3D and 4-axis bioprinting. Carbohyd. Polym. 2025, 348, 122853. [Google Scholar] [CrossRef]
- Tang, Y.; Lan, X.; Liang, C.; Zhong, Z.; Xie, R.; Zhou, Y.; Miao, X.; Wang, H.; Wang, W. Honey loaded alginate/PVA nanofibrous membrane as potential bioactive wound dressing. Carbohyd. Polym. 2019, 219, 113–120. [Google Scholar] [CrossRef]
- Soto-Quintero, A.; Castillo, E.I.G.; Lizárraga, K.G.; Barba-Pingarrón, A.; Hernández, M. Enhancing the performance of nanostructured PVA/SA scaffolds through incorporation of macromolecules: From synergistic effects to advanced multifunctionalities. Mater. Lett. 2024, 376, 137251. [Google Scholar] [CrossRef]
- Wang, H.; Ren, R.; Li, M.; Gao, Z.; Li, H.; Li, S. Coaxial emulsion electrospinning fiber films loaded with OEO for active packaging of pork. Food Res. Int. 2025, 214, 116655. [Google Scholar] [CrossRef]
- Chen, X.; Wu, J.; Zhang, T.; Li, H.; Zhang, R.; Lu, W.; Guo, Y. Multifunctional coaxial gelatin/polylactic acid three-dimensional nanofibrous scaffolds for diabetic wounds. Int. J. Biol. Macromol. 2025, 306, 141525. [Google Scholar] [CrossRef]
- Ji, E.; Song, Y.H.; Lee, J.K.; Kim, Y.; Lee, E.; Joo, K.I.; Seo, J.H. Bioadhesive levan-based coaxial nanofibrous membranes with enhanced cell adhesion and mesenchymal stem cell differentiation. Carbohyd. Polym. 2025, 354, 123337. [Google Scholar] [CrossRef]
- Dai, T.; Liu, Y.; Wang, L.; Yao, J.; Zhu, G.; Guo, B.; Militk, J.; Kremenakova, D.; Zhang, M. PVA@tea tree oil-emulsion core–shell microfiber membranes for extending the shelf life of fruits. J. Appl. Polym. Sci. 2024, 141, e55038. [Google Scholar] [CrossRef]
- Sun, Z.; Zussman, E.; Yarin, A.L.; Wendorff, J.H.; Greiner, A. Compound Core–Shell Polymer Nanofibers by Co-Electrospinning. Adv. Mater. 2003, 15, 1929–1932. [Google Scholar] [CrossRef]
- Wang, K.; Chen, E.; Lin, X.; Tian, X.; Wang, L.; Huang, K.; Skirtach, A.G.; Tan, M.; Su, W. Core-shell nanofibers based on microalgae proteins/alginate complexes for enhancing survivability of probiotics. Int. J. Biol. Macromol. 2024, 271, 132461. [Google Scholar] [CrossRef]
- Nista, S.V.G.; Bettini, J.; Mei, L.H.I. Coaxial nanofibers of chitosan–alginate–PEO polycomplex obtained by electrospinning. Carbohyd. Polym. 2015, 127, 222–228. [Google Scholar] [CrossRef]
- Hou, W.; Guo, W.; Dai, Z.; Ren, H.; Luo, X.; Fu, J. Baicalin nanofiber membranes: A comprehensive study on preparation, characterization, and antimicrobial pharmacological activities. J. Mol. Struct. 2025, 1322, 140628. [Google Scholar] [CrossRef]
- Walden, R.; Aazem, I.; Hinder, S.; Brennan, B.; Goswami, A.; McGranaghan, G.; Pillai, S.C. Parametric optimisation of PDMS/PMMA nanofibers prepared using emulsion electrospinning technique. Results Mater. 2024, 22, 100576. [Google Scholar] [CrossRef]
- Lv, H.; Wang, C.; Xu, E.; Jin, Z.; Zhao, H.; Yuan, C.; Zhao, M.; Yu, B.; Wu, Z.; He, D.; et al. Preparation of starch-based oral fast-disintegrating nanofiber mats for astaxanthin encapsulation and delivery via emulsion electrospinning. Int. J. Biol. Macromol. 2025, 289, 136466. [Google Scholar] [CrossRef]
- Zhang, C.; Li, S.; Chen, D.; Lu, W.; Xiao, C. Emulsion-coaxial electrospinning: The role of zein as a shell layer in multicore-shell structured nanofibers for bioactive delivery. Int. J. Biol. Macromol. 2025, 306, 141432. [Google Scholar] [CrossRef] [PubMed]
- Negahdari, N.; Alizadeh, S.; Majidi, J.; Saeed, M.; Ghadimi, T.; Tahermanesh, K.; Arabsorkhi-Mishabi, A.; Pezeshki-Modaress, M. Heat-treated alginate-polycaprolactone core-shell nanofibers by emulsion electrospinning process for biomedical applications. Int. J. Biol. Macromol. 2024, 275, 133709. [Google Scholar] [CrossRef]
- Tao, F.; Cheng, Y.; Tao, H.; Jin, L.; Wan, Z.; Dai, F.; Xiang, W.; Deng, H. Carboxymethyl chitosan/sodium alginate-based micron-fibers fabricated by emulsion electrospinning for periosteal tissue engineering. Mater. Des. 2020, 194, 108849. [Google Scholar] [CrossRef]
- Yan, X.; Xu, B.; Xia, C.; Xu, M.; Zeng, B.; Zhang, R.; Zhu, L.; Zhang, C. Dual drug-loaded core-shell nanofibers membranes via emulsion electrospinning and their controllable sustained release property. J. Drug Deliv. Sci. Technol. 2023, 88, 104909. [Google Scholar] [CrossRef]
- Bruni, G.P.; de Oliveira, J.P.; Gómez-Mascaraque, L.G.; Fabra, M.J.; Martins, V.G.; da Rosa Zavareze, E.; López-Rubio, A. Electrospun β-carotene–loaded SPI: PVA fiber mats produced by emulsion-electrospinning as bioactive coatings for food packaging. Food Packag. Shelf 2020, 23, 100426. [Google Scholar] [CrossRef]
- Norouzi, M.-R.; Ghasemi-Mobarakeh, L.; Itel, F.; Schoeller, J.; Fashandi, H.; Borzi, A.; Neels, A.; Fortunato, G.; Rossi, R.M. Emulsion electrospinning of sodium alginate/poly(ε-caprolactone) core/shell nanofibers for biomedical applications. Nanoscale Adv. 2022, 4, 2929–2941. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Feng, F.; Zhang, H. Emulsion electrospinning: Fundamentals, food applications and prospects. Trends Food Sci. Technol. 2018, 80, 175–186. [Google Scholar] [CrossRef]
- Xuan, L. 3D Printing Pharmaceutical Preparation Technology Based on Digital Light Processing. Ph.D. Thesis, Sichuan University, Chengdu, China, 2021. [Google Scholar] [CrossRef]
- Lou, W.; Qiu, X.; Qin, Y.; Lu, Y.; Cao, Y.; Lu, H. 3D-printed advanced scaffold armed with exosomes derived from human skeletal stem cell identified by single-cell RNA sequencing enhances osteochondral regeneration. Bioact. Mater. 2025, 51, 231–256. [Google Scholar] [CrossRef]
- Jiang, S.; Zhang, A.; Akhavan, B.; Whitelock, J.; Bilek, M.M.; Wise, S.G.; Lord, M.S.; Rnjak-Kovacina, J. Biofunctionalization of electrospun silk scaffolds with perlecan for vascular tissue engineering. Biomater. Sci. 2025, 19, 3598–3616. [Google Scholar] [CrossRef]
- Umemori, K.; Pourdeyhimi, B.; Little, D. Three-dimensional meltblowing as a high-speed fabrication process for tendon tissue engineered scaffolds. Bioprinting 2025, 48, e00409. [Google Scholar] [CrossRef]
- Sasan, S.; Molavi, A.M.; Moqadam, K.H.; Farrokhi, N.; Oroojalian, F. Enhanced wound healing properties of biodegradable PCL/alginate core-shell nanofibers containing Salvia abrotanoides essential oil and ZnO nanoparticles. Int. J. Biol. Macromol. 2024, 279, 135152. [Google Scholar] [CrossRef]
- Ashrafi, F.; Emami, A.; Sefidbakht, S.; Aghayan, H.; Soleimani, F.; Omidfar, K. Accelerated healing of full-thickness skin wounds by multifunctional exosome-loaded scaffolds of alginate hydrogel/PCL nanofibers with hemostatic efficacy. Int. J. Biol. Macromol. 2025, 307, 142271. [Google Scholar] [CrossRef]
- Ashraf, S.S.; Parivar, K.; Roodbari, N.H.; Mashayekhan, S.; Amini, N. Fabrication and characterization of biaxially electrospun collagen/alginate nanofibers, improved with Rhodotorula mucilaginosa sp. GUMS16 produced exopolysaccharides for wound healing applications. Int. J. Biol. Macromol. 2022, 196, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Hernández, M.; Álvarez-Pérez, M.; Genesca, J.; Gómez, K.; Covelo, A. Evaluation of the biocompatibility of a PVA/SA scaffold with a human gingival fibroblast (HGF) by using electrochemical impedance spectroscopy. Bioelectrochemistry 2020, 131, 107386. [Google Scholar] [CrossRef]
- Hernández, M.; González-Alva, P.; Martínez-Moreno, A.; Bañuelos, G.; Álvarez-Pérez, M.; Gómez, K. Study and characterization of the cellular viability of non-fractured and fractured PVA/SA scaffolds with cardiomyocyte cells using electrochemical impedance and biological measurements. Mater. Lett. 2024, 376, 137271. [Google Scholar] [CrossRef]
- Zattarin, E.; Sotra, Z.; Wiman, E.; Bas, Y.; Rakar, J.; Berglund, L.; Starkenberg, A.; Björk, E.M.; Khalaf, H.; Oksman, K.; et al. Controlled release of antimicrobial peptides from nanocellulose wound dressings for treatment of wound infections. Mater. Today Bio. 2025, 32, 101756. [Google Scholar] [CrossRef]
- Moshfeghi, T.; Najmoddin, N.; Arkan, E.; Hosseinzadeh, L. A novel bilayer hydrogel/ fibrous wound dressing with Falcaria vulgaris extract and green synthesized ZnO nanoparticles for enhanced wound healing. J. Sci. Adv. Mater. Dev. 2025, 10, 100896. [Google Scholar] [CrossRef]
- Mavrokefalou, E.; Monou, P.K.; Tzetzis, D.; Bouropoulos, N.; Vizirianakis, I.S.; Fatouros, D.G. Preparation and in vitro evaluation of electrospun sodium alginate fiber films for wound healing applications. J. Drug Deliv. Sci. Technol. 2023, 81, 104298. [Google Scholar] [CrossRef]
- Hiytesh, V.K.; Sivalingam, D.; Prakash, J.; Marvaan, M.S.; Sundar, M.; Pannerselvam, B.; Venkatasubbu, G.D. Accelerated wound healing by PrO2 incorporated PVA/SA fibers. J. Ind. Eng. Chem. 2024, 137, 301–316. [Google Scholar] [CrossRef]
- Luong, A.H.; Lin, W.-C. Enhancement of wound healing by a bilayer hydrogel and nanofiber scaffold infused with Calophyllum inophyllum oil and Platostoma palustre aqueous extract. Biomater. Adv. 2025, 172, 214247. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi, S.; Chamchangi, M.A.; Raoufi, Z.; Heidari, F. Dual-layer alginate hydrogel dressings with chitosan nanofibers for enhanced wound healing, infection prevention, and controlled drug release. Int. J. Biol. Macromol. 2025, 307, 142033. [Google Scholar] [CrossRef]
- Qi, Y.; Wang, P.; Lu, Q.; Zhou, Z.; Hu, Y. Preparation and properties of Baicalein loaded sodium alginate/polyvinyl alcohol nanofiber antibacterial wound dressing. Mater. Lett. 2025, 389, 138408. [Google Scholar] [CrossRef]
- Maikovych, O.; Pasetto, P.; Nosova, N.; Kudina, O.; Ostapiv, D.; Samaryk, V.; Varvarenko, S. Functional Properties of Gelatin–Alginate Hydrogels for Use in Chronic Wound Healing Applications. Gels 2025, 11, 174. [Google Scholar] [CrossRef]
- Hajiali, H.; Heredia-Guerrero, J.A.; Liakos, I.; Athanassiou, A.; Mele, E. Alginate Nanofibrous Mats with Adjustable Degradation Rate for Regenerative Medicine. Biomacromolecules 2015, 16, 936–943. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, H.; Yu, D.-G.; Bligh, S.-W.A. Alginate-Based Electrospun Nanofibers and the Enabled Drug Controlled Release Profiles: A Review. Biomolecules 2024, 14, 789. [Google Scholar] [CrossRef]
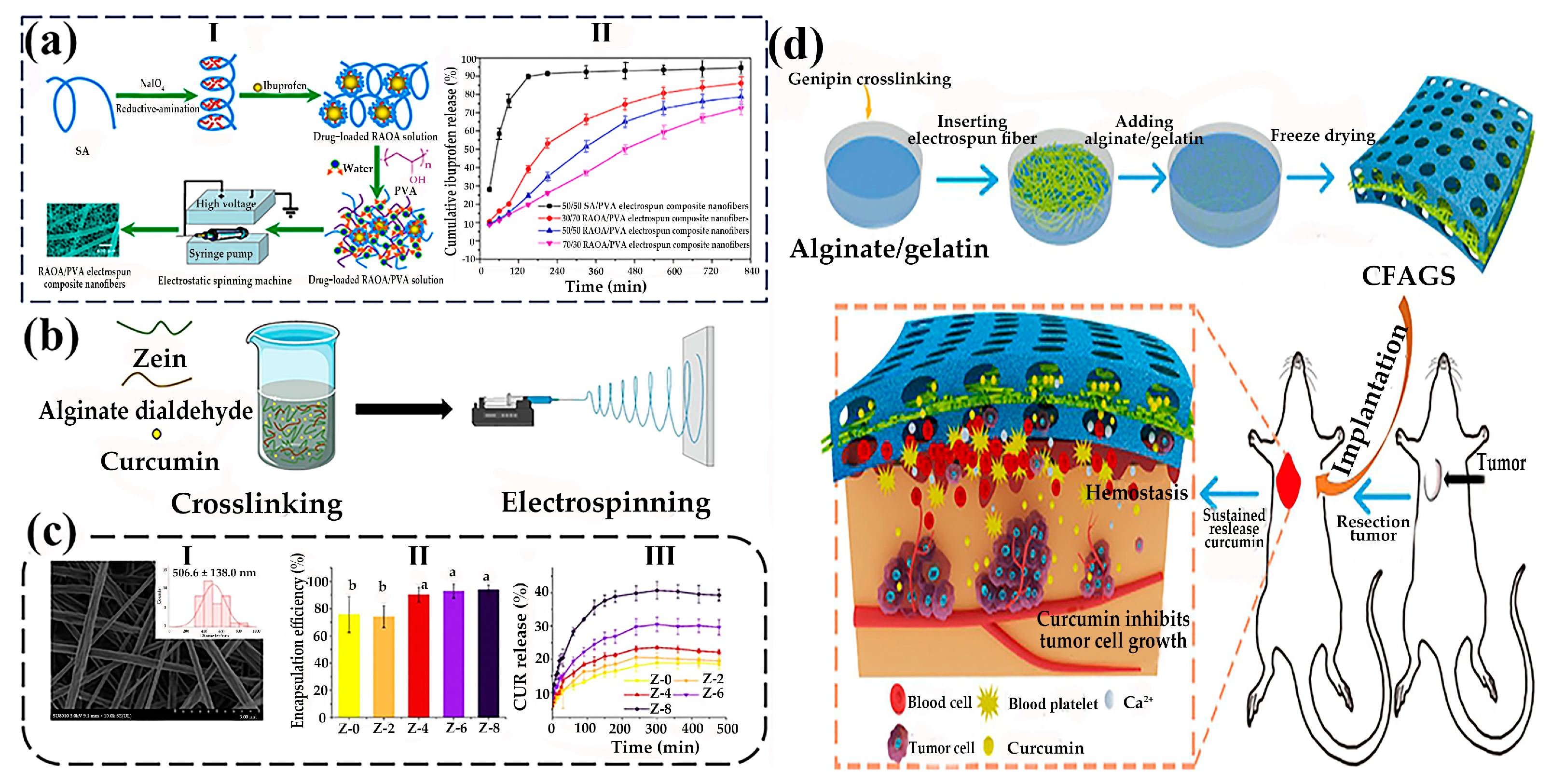
- Chen, X.; Zhu, Q.; Wen, Y.; Li, Z.; Cao, S.; Yan, H.; Lin, Q. Chemical modification of alginate via the oxidation-reductive amination reaction for the development of alginate derivative electrospun composite nanofibers. J. Drug Deliv. Sci. Technol. 2022, 68, 103113. [Google Scholar] [CrossRef]
- Li, J.; Zheng, Y.; Wang, P.; Zhang, H. The alginate dialdehyde crosslinking on curcumin-loaded zein nanofibers for controllable release. Food Res. Int. 2024, 178, 113944. [Google Scholar] [CrossRef]
- Chen, K.; Pan, H.; Yan, Z.; Li, Y.; Ji, D.; Yun, K.; Su, Y.; Liu, D.; Pan, W. A novel alginate/gelatin sponge combined with curcumin-loaded electrospun fibers for postoperative rapid hemostasis and prevention of tumor recurrence. Int. J. Biol. Macromol. 2021, 182, 1339–1350. [Google Scholar] [CrossRef] [PubMed]
- Diep, E.; Schiffman, J.D. Targeted release of live probiotics from alginate-based nanofibers in a simulated gastrointestinal tract. Electronic supplementary information (ESI) available. RSC Appl. Polym. 2024, 2, 719–725. [Google Scholar] [CrossRef]

 and 5.0%wt
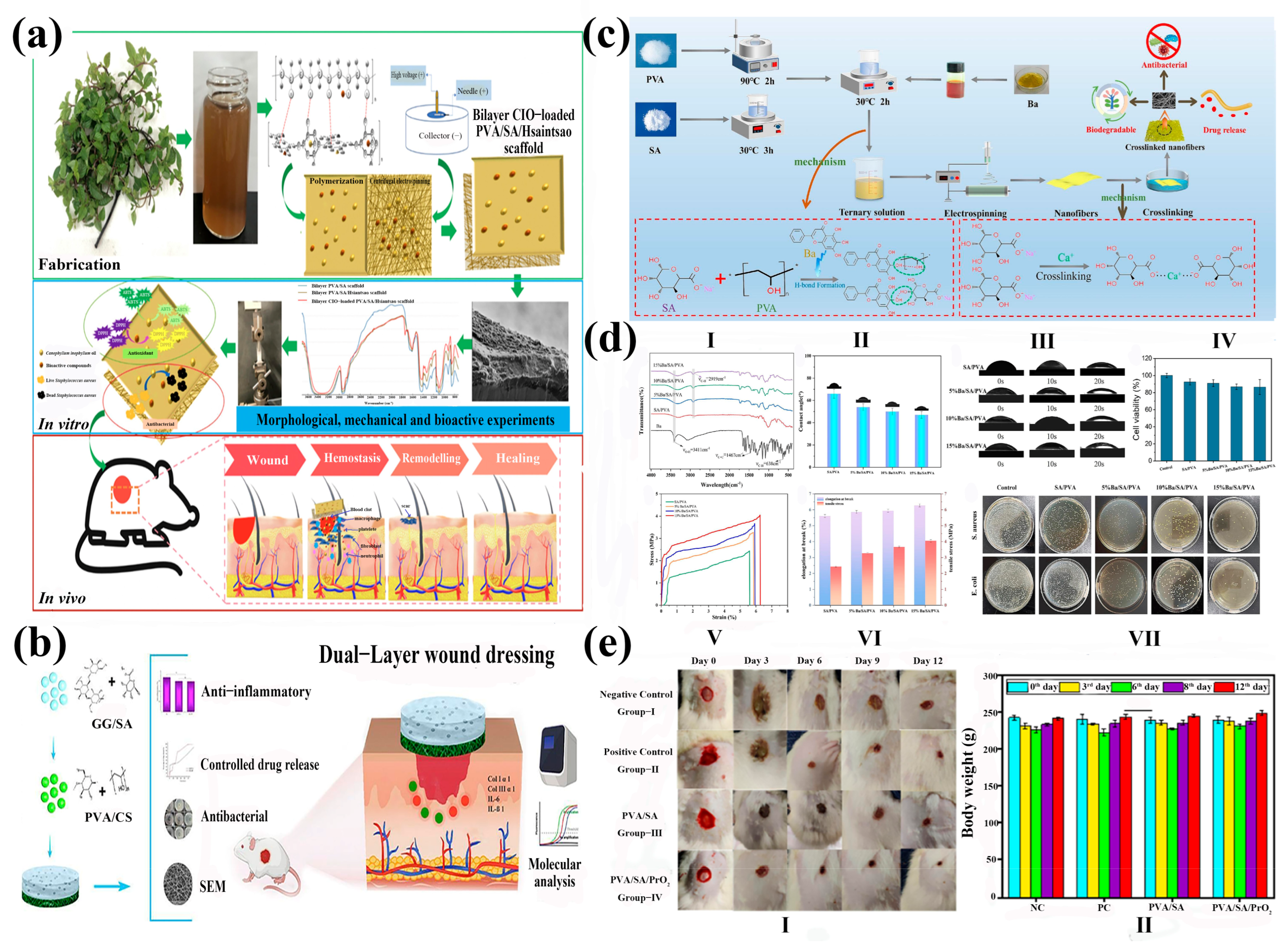
and 5.0%wt  , respectively). In Bode plots the filled symbols represent the impedance modulus, |Z| and the non-filled ones represent the phase shift, θ, against frequency, υ. (b) Fluorescence assessments of HGF systems (I) 3.5 wt%, (II) 4 wt%, and (III) 5 wt% after 24 incubation time [93]. Published by [Elsevier], (2020). (c) Fabrication process of biaxially electrospun collagen/alginate nanofibers, improved with Rhodotorula mucilaginosa sp. GUMS16-produced exopolysaccharides for wound healing applications [92]. Published by [Elsevier], (2022). (d) Fabrication procedure of multifunctional hybrid scaffolds loaded with exosomes, and their application for the healing of a full-thickness wound in a rat model [91]. Published by [Elsevier], (2025) (e) Scaffold architecture/cell seeding/viability on PVA/SA scaffolds [94]. Published by [Elsevier], (2024).
, respectively). In Bode plots the filled symbols represent the impedance modulus, |Z| and the non-filled ones represent the phase shift, θ, against frequency, υ. (b) Fluorescence assessments of HGF systems (I) 3.5 wt%, (II) 4 wt%, and (III) 5 wt% after 24 incubation time [93]. Published by [Elsevier], (2020). (c) Fabrication process of biaxially electrospun collagen/alginate nanofibers, improved with Rhodotorula mucilaginosa sp. GUMS16-produced exopolysaccharides for wound healing applications [92]. Published by [Elsevier], (2022). (d) Fabrication procedure of multifunctional hybrid scaffolds loaded with exosomes, and their application for the healing of a full-thickness wound in a rat model [91]. Published by [Elsevier], (2025) (e) Scaffold architecture/cell seeding/viability on PVA/SA scaffolds [94]. Published by [Elsevier], (2024).
 and 5.0%wt
and 5.0%wt  , respectively). In Bode plots the filled symbols represent the impedance modulus, |Z| and the non-filled ones represent the phase shift, θ, against frequency, υ. (b) Fluorescence assessments of HGF systems (I) 3.5 wt%, (II) 4 wt%, and (III) 5 wt% after 24 incubation time [93]. Published by [Elsevier], (2020). (c) Fabrication process of biaxially electrospun collagen/alginate nanofibers, improved with Rhodotorula mucilaginosa sp. GUMS16-produced exopolysaccharides for wound healing applications [92]. Published by [Elsevier], (2022). (d) Fabrication procedure of multifunctional hybrid scaffolds loaded with exosomes, and their application for the healing of a full-thickness wound in a rat model [91]. Published by [Elsevier], (2025) (e) Scaffold architecture/cell seeding/viability on PVA/SA scaffolds [94]. Published by [Elsevier], (2024).
, respectively). In Bode plots the filled symbols represent the impedance modulus, |Z| and the non-filled ones represent the phase shift, θ, against frequency, υ. (b) Fluorescence assessments of HGF systems (I) 3.5 wt%, (II) 4 wt%, and (III) 5 wt% after 24 incubation time [93]. Published by [Elsevier], (2020). (c) Fabrication process of biaxially electrospun collagen/alginate nanofibers, improved with Rhodotorula mucilaginosa sp. GUMS16-produced exopolysaccharides for wound healing applications [92]. Published by [Elsevier], (2022). (d) Fabrication procedure of multifunctional hybrid scaffolds loaded with exosomes, and their application for the healing of a full-thickness wound in a rat model [91]. Published by [Elsevier], (2025) (e) Scaffold architecture/cell seeding/viability on PVA/SA scaffolds [94]. Published by [Elsevier], (2024).
| Method | Advantages | Disadvantages |
|---|---|---|
| Blended electrospinning | One-step molding, easy to process, low equipment requirements [60] | Limited functionality, easy to disperse unevenly, and initial burst release [60] |
| Coaxial electrospinning | Core–shell structure nanofibers can be prepared, functional partitioning can be implemented, sustained release of the drug can be achieved [74,75] | Low efficiency, complex operation, and high requirement of process variables [78] |
| Emulsion electrospinning | The core–shell structure can be prepared on a single axis, and it can contain both hydrophilic and lipophilic drugs, simplifying the process flow and reducing the complexity of equipment and parameter control [84] | Emulsion instability, relatively low productivity [85] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, X.; Wang, Y.; Ji, C. Advances in Preparation and Biomedical Applications of Sodium Alginate-Based Electrospun Nanofibers. Gels 2025, 11, 704. https://doi.org/10.3390/gels11090704
Zhou X, Wang Y, Ji C. Advances in Preparation and Biomedical Applications of Sodium Alginate-Based Electrospun Nanofibers. Gels. 2025; 11(9):704. https://doi.org/10.3390/gels11090704
Chicago/Turabian StyleZhou, Xuan, Yudong Wang, and Changchun Ji. 2025. "Advances in Preparation and Biomedical Applications of Sodium Alginate-Based Electrospun Nanofibers" Gels 11, no. 9: 704. https://doi.org/10.3390/gels11090704
APA StyleZhou, X., Wang, Y., & Ji, C. (2025). Advances in Preparation and Biomedical Applications of Sodium Alginate-Based Electrospun Nanofibers. Gels, 11(9), 704. https://doi.org/10.3390/gels11090704






