Tailoring Therapy: Hydrogels as Tunable Platforms for Regenerative Medicine and Cancer Intervention
Abstract
1. Introduction
1.1. Hydrogels: Enabling Precision Medicine
1.2. The Aim of the Review
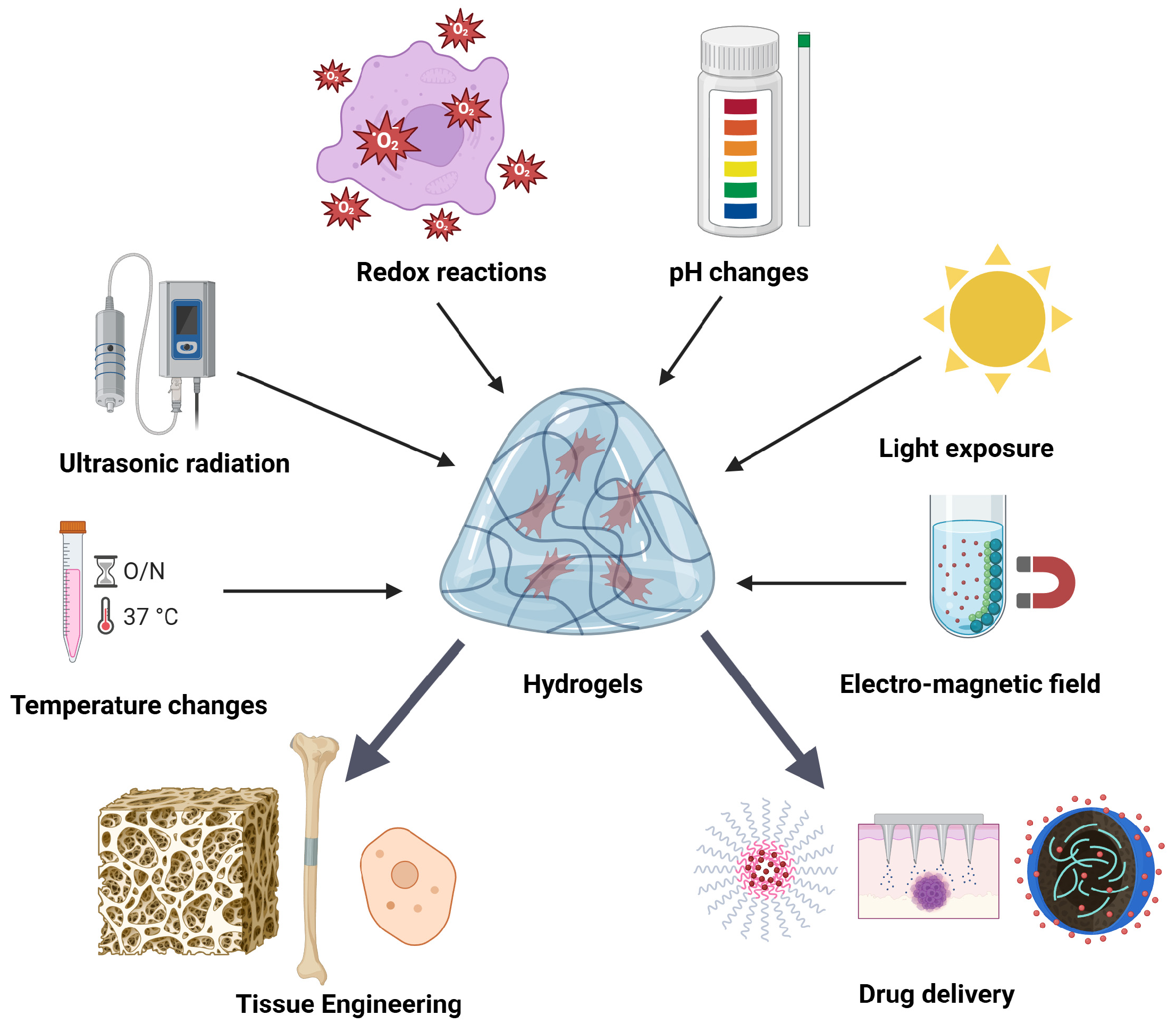
2. Tunable Hydrogel Fundamentals
2.1. Material Selection and Crosslinking
2.2. Physico-Chemical and Mechanical Control
- Rubber-like hydrogels—often modeled by classical rubber elasticity and viscoelasticity theories; they are characterized by significant extensibility under low stress and full recovery driven primarily by entropic elasticity [39]. Examples include polyacrylamide, PEG (polyethylene glycol)-based, and PDMS (polydimethylsiloxane)-based hydrogels;
- Fibrillar hydrogels—such as collagen, fibrin, silk fibroin, or synthetic fiber-forming systems, where the mechanical response is dominated by enthalpic elasticity. In these materials, deformation resistance is due to stretching of semi-flexible fibers rather than entropy-driven chain retraction [40,41]. Fibrillar networks often exhibit strain stiffening, a nonlinear behavior crucial in mimicking extracellular matrix mechanics [36,42].
- Crosslinking density—higher density increases rigidity but may reduce extensibility [43];
- Monomer molecular weight—larger monomers or longer polymer chains yield stronger, more resilient networks [44];
- Monomer concentration—higher concentrations create denser networks, enhancing strength and viscoelasticity [45];
- Monomer composition—specific chemistries (e.g., hydrophobic domains or ionic groups) can improve toughness, energy dissipation, or responsiveness [46].
2.3. Smart and Bio-Functional Hydrogels
2.4. Limitations of Conventional Hydrogels
3. Tunable Hydrogels in Regenerative Medicine
- Tailored mechanical properties: the mechanical properties of the body’s tissues vary greatly, ranging from tough bone to soft brain matter;
- Controlled degradation rates: the hydrogel scaffold must break down at a rate that precisely corresponds to the rate at which new tissue is growing for successful tissue regeneration [8];
- Accurate biochemical signaling: hydrogels can be designed to contain and release specific bioactive substances, including peptides, growth factors, or cytokines, in a controlled and prolonged manner;
- Cell delivery and encapsulation: hydrogels offer a supportive and safe environment for the delivery and encapsulation of cells, including stem cells;
- Architectural customization: hydrogel scaffolds with incredibly complex and accurate designs that replicate the intricate structures of natural tissues and organs can be created using advanced fabrication techniques, such as 3D bioprinting [68].
3.1. Tissue Engineering Scaffolds
3.2. Controlled Release Systems/Polyethylene Glycol (PEG)
3.3. Cell Microenvironments & Bioprinting
- Accurate spatial management of cell positioning: By simulating the highly ordered multicellular structures found in natural tissues, bioprinting enables the precise placement of various cell types within a scaffold. To replicate intricate organ architecture, this is essential [109];
- Creation of complex architectures: bioprinting enables the design and construction of intricate geometries, such as internal vascular channels, as well as the production of various material distributions that replicate the subtle structural differences found in organs [110];
- Localized microenvironmental control: It is possible to design sections of a single bio-printed construct with different hydrogel characteristics, such as different growth factor presentations or stiffness gradients. This allows for localized regulation of cell behavior, mirroring the minute changes in the surrounding environment that cells experience in a natural tissue [111].
4. Tunable Hydrogels in Cancer Intervention
4.1. Precision Drug Delivery
| Method | Precision | Effects | References |
|---|---|---|---|
| Tunable hydrogels as drug carriers | Designed to deliver medication specifically to tumor sites based on local pathological conditions | Enhances treatment effectiveness while minimizing harm to healthy tissue | [119,120] |
| Stimuli-responsive hydrogels (e.g., light, pH, temperature) | React to specific tumor microenvironment signals to trigger drug release | Allows controlled and targeted release of therapeutic agents; improves drug efficiency | [123,127] |
| Biodegradable and injectable hydrogel systems | Enable localized administration with built-in degradation over time | Improve patient comfort, reduce systemic exposure, and simplify administration | [127] |
| Multinetwork and porous hydrogel structures | Provide channels for uniform drug distribution and adjustable loading capacity | Enhance versatility for various treatments, including wound healing, biosensing, and cancer therapy | [122,123,124] |
| Hydrogels for combination therapy (e.g., loaded with chemo- and immunodrugs) | Can carry multiple types of drugs for sequential or simultaneous release | Enable integrated cancer treatment strategies with better coordination of therapy modalities | [129] |
| Hydrogels replacing conventional chemotherapy methods | Offer localized, sustained, and responsive release versus passive, non-targeted chemotherapy | Avoid systemic side effects and ensure higher drug concentration at the tumor site | [119,120,121] |
4.2. Immunotherapy Enhancement
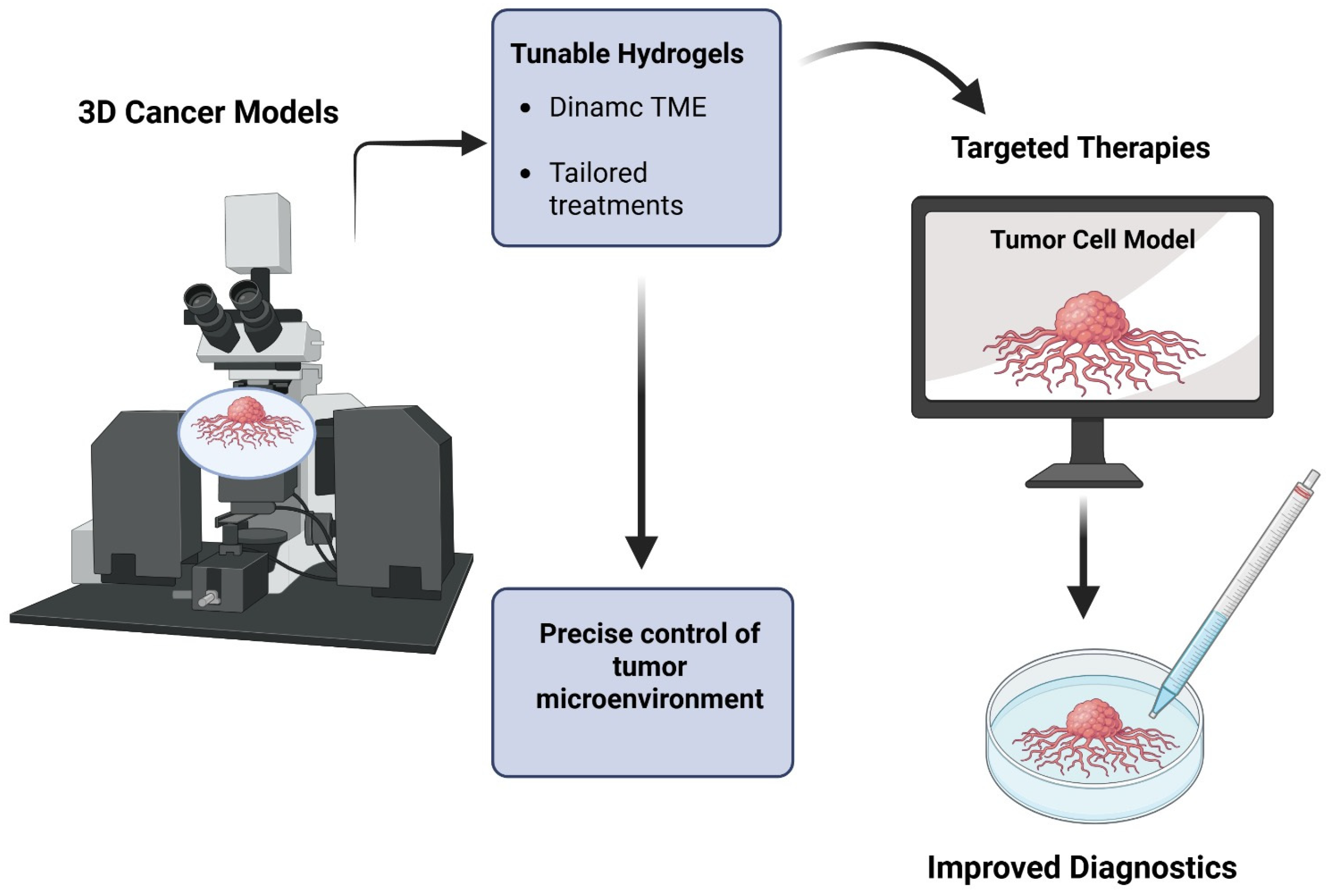
4.3. Three-Dimensional Cancer Models and Diagnostics
5. Comparative Analysis Between Hydrogels
5.1. Quantitative Discussion of Trade-Offs and Limitations
5.2. Critical Evaluation of Clinical Translation Challenges
5.3. Identification of Knowledge Gaps and Research Priorities
5.4. Challenges and Future Outlook
6. Conclusions
7. Materials and Methods
Author Contributions
Funding
Conflicts of Interest
References
- Marques, L.; Costa, B.; Pereira, M.; Silva, A.; Santos, J.; Saldanha, L.; Silva, I.; Magalhães, P.; Schmidt, S.; Vale, N. Advancing precision medicine: A review of innovative in silico approaches for drug development, clinical pharmacology and personalized healthcare. Pharmaceutics 2024, 16, 332. [Google Scholar] [CrossRef]
- Camacho-Cardenosa, M.; Pulido-Escribano, V.; Estrella-Guisado, G.; Dorado, G.; Herrera-Martínez, A.D.; Gálvez-Moreno, M.Á.; Casado-Díaz, A. Bioprinted Hydrogels as Vehicles for the Application of Extracellular Vesicles in Regenerative Medicine. Gels 2025, 11, 191. [Google Scholar] [CrossRef]
- El-Tanani, M.; Satyam, S.M.; Rabbani, S.A.; El-Tanani, Y.; Aljabali, A.A.; Al Faouri, I.; Rehman, A. Revolutionizing drug delivery: The impact of advanced materials science and technology on precision medicine. Pharmaceutics 2025, 17, 375. [Google Scholar] [CrossRef] [PubMed]
- Revathi, D.; Panda, S.; Deshmukh, K.; Khotele, N.; Murthy, V.; Pasha, S.K. Smart Hydrogels for Sensing and Biosensing: Preparation, Smart Behaviours, and Emerging Applications: A Comprehensive Review. Polym. Test. 2025, 150, 108912. [Google Scholar] [CrossRef]
- Wu, J.; Xue, W.; Yun, Z.; Liu, Q.; Sun, X. Biomedical applications of stimuli-responsive “smart” interpenetrating polymer network hydrogels. Mater. Today Bio 2024, 25, 100998. [Google Scholar] [CrossRef]
- Li, G.; Wang, Q.; Liu, G.; Yao, M.; Wang, Y.; Li, Y.; Lin, K.; Liu, X. Hydrogel extinguishants. Nanomaterials 2024, 14, 1128. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.-M.; Liu, X. Advancing biomaterials of human origin for tissue engineering. Prog. Polym. Sci. 2016, 53, 86–168. [Google Scholar] [CrossRef] [PubMed]
- Pablos, J.L.; Lozano, D.; Manzano, M.; Vallet-Regí, M. Regenerative medicine: Hydrogels and mesoporous silica nanoparticles. Mater. Today Bio 2024, 29, 101342. [Google Scholar] [CrossRef]
- Bu, Y.; Yang, Y.; Sun, F. Editorial for the Special Issue “Hydrogels with Appropriate/Tunable Properties for Biomedical Applications”. Gels 2025, 11, 277. [Google Scholar] [CrossRef]
- Ullah, A.; Lim, S.I. Bioinspired tunable hydrogels: An update on methods of preparation, classification, and biomedical and therapeutic applications. Int. J. Pharm. 2022, 612, 121368. [Google Scholar] [CrossRef]
- Andrade, F.; Roca-Melendres, M.M.; Durán-Lara, E.F.; Rafael, D.; Schwartz, S., Jr. Stimuli-responsive hydrogels for cancer treatment: The role of pH, light, ionic strength and magnetic field. Cancers 2021, 13, 1164. [Google Scholar] [CrossRef] [PubMed]
- Vegad, U.; Patel, M.; Khunt, D.; Zupančič, O.; Chauhan, S.; Paudel, A. pH stimuli-responsive hydrogels from non-cellulosic biopolymers for drug delivery. Front. Bioeng. Biotechnol. 2023, 11, 1270364. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, M.A.; Xavier, C.P.; Pereira, R.F.; Petrikaitė, V.; Vasconcelos, M.H. 3D cell culture models as recapitulators of the tumor microenvironment for the screening of anti-cancer drugs. Cancers 2021, 14, 190. [Google Scholar] [CrossRef]
- Rao, T.; Chvs, P.; Yamini, M.; Prasad, C. Hydrogels the three dimensional networks: A review. Int. J. Curr. Pharm. Res. 2021, 13, 12–17. [Google Scholar] [CrossRef]
- Xing, Y.; Zeng, B.; Yang, W. Light responsive hydrogels for controlled drug delivery. Front. Bioeng. Biotechnol. 2022, 10, 1075670. [Google Scholar] [CrossRef]
- Dell, A.C.; Wagner, G.; Own, J.; Geibel, J.P. 3D bioprinting using hydrogels: Cell inks and tissue engineering applications. Pharmaceutics 2022, 14, 2596. [Google Scholar] [CrossRef]
- Luo, T.; Tan, B.; Zhu, L.; Wang, Y.; Liao, J. A review on the design of hydrogels with different stiffness and their effects on tissue repair. Front. Bioeng. Biotechnol. 2022, 10, 817391. [Google Scholar] [CrossRef]
- Wu, J.; Yun, Z.; Song, W.; Yu, T.; Xue, W.; Liu, Q.; Sun, X. Highly oriented hydrogels for tissue regeneration: Design strategies, cellular mechanisms, and biomedical applications. Theranostics 2024, 14, 1982. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, B.M. Current advances in stimuli-responsive hydrogels as smart drug delivery carriers. Gels 2023, 9, 838. [Google Scholar] [CrossRef]
- Peppas, N.A.; Bures, P.; Leobandung, W.; Ichikawa, H. Hydrogels in pharmaceutical formulations. Eur. J. Pharm. Biopharm. 2000, 50, 27–46. [Google Scholar] [CrossRef]
- Thakur, G.; Rousseau, D. Hydrogels: Characterization, drug delivery, and tissue engineering applications. In Encyclopedia of Biomedical Polymers and Polymeric Biomaterials; Taylor and Francis: New York, NY, USA, 2016; pp. 3853–3878. [Google Scholar]
- Ghazizadeh, E.; Sadeghi, M.; Deigner, H.-P.; Neshastehriz, A. Engineered Sustainable Mxene-PVA Hydrogel as an Inspiring Co-Delivery Carrier for Targeting Solid Tumors. Pharmaceutics 2025, 17, 823. [Google Scholar] [CrossRef]
- Protsak, I.S.; Morozov, Y.M. Fundamentals and advances in stimuli-responsive hydrogels and their applications: A review. Gels 2025, 11, 30. [Google Scholar] [CrossRef]
- Nicodemus, G.D.; Bryant, S.J. Cell encapsulation in biodegradable hydrogels for tissue engineering applications. Tissue Eng. Part B Rev. 2008, 14, 149–165. [Google Scholar] [CrossRef]
- Martinet, A.; Miebach, L.; Weltmann, K.D.; Emmert, S.; Bekeschus, S. Biomimetic Hydrogels–Tools for Regenerative Medicine, Oncology, and Understanding Medical Gas Plasma Therapy. Small 2025, 21, 2403856. [Google Scholar] [CrossRef]
- Mishra, M. Encyclopedia of Polymer Applications, 3 Volume Set; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Hashemi-Afzal, F.; Fallahi, H.; Bagheri, F.; Collins, M.N.; Eslaminejad, M.B.; Seitz, H. Advancements in hydrogel design for articular cartilage regeneration: A comprehensive review. Bioact. Mater. 2025, 43, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, X.; Xu, M.; Geng, Z.; Ji, P.; Liu, Y. Hydrogel systems for targeted cancer therapy. Front. Bioeng. Biotechnol. 2023, 11, 1140436. [Google Scholar] [CrossRef]
- Ashley, G.W.; Henise, J.; Reid, R.; Santi, D.V. Hydrogel drug delivery system with predictable and tunable drug release and degradation rates. Proc. Natl. Acad. Sci. USA 2013, 110, 2318–2323. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Ruan, D.; Huang, M.; Tian, M.; Zhu, K.; Gan, Z.; Xiao, Z. Harnessing the potential of hydrogels for advanced therapeutic applications: Current achievements and future directions. Signal Transduct. Target. Ther. 2024, 9, 166. [Google Scholar] [CrossRef]
- Cheng, H.; Guo, Q.; Zhao, H.; Liu, K.; Kang, H.; Gao, F.; Guo, J.; Yuan, X.; Hu, S.; Li, F. An injectable hydrogel scaffold loaded with dual-drug/sustained-release PLGA microspheres for the regulation of macrophage polarization in the treatment of intervertebral disc degeneration. Int. J. Mol. Sci. 2022, 24, 390. [Google Scholar] [CrossRef] [PubMed]
- Thoniyot, P.; Tan, M.J.; Karim, A.A.; Young, D.J.; Loh, X.J. Nanoparticle–hydrogel composites: Concept, design, and applications of these promising, multi-functional materials. Adv. Sci. 2015, 2, 1400010. [Google Scholar] [CrossRef]
- Par, S.; Vaides, S.; VanderVere-Carozza, P.S.; Pawelczak, K.S.; Stewart, J.; Turchi, J.J. OB-folds and genome maintenance: Targeting protein–DNA interactions for cancer therapy. Cancers 2021, 13, 3346. [Google Scholar] [CrossRef]
- Jiang, L.; Fan, F.; Wang, X.; Ali, S.; Zhou, F.; Zhang, J. Multi-Level Drug Delivery System Integrated with Injectable Hydrogels and ZIF-8 for Sustained Release of Lidocaine. J. Pharm. BioTech Ind. 2025, 2, 3. [Google Scholar] [CrossRef]
- Mishra, M. Concise Encyclopedia of Biomedical Polymers and Polymeric Biomaterials; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Pritchard, R.H.; Huang, Y.Y.S.; Terentjev, E.M. Mechanics of biological networks: From the cell cytoskeleton to connective tissue. Soft Matter 2014, 10, 1864–1884. [Google Scholar] [CrossRef] [PubMed]
- Janmey, P.A.; Winer, J.P.; Weisel, J.W. Fibrin gels and their clinical and bioengineering applications. J. R. Soc. Interface 2009, 6, 1–10. [Google Scholar] [CrossRef]
- Hu, C.; Xia, F.; Zhou, C.; Wang, H.; Zhou, C.; Tao, Q.; Zhang, Y.; Meng, Y. Hierarchical Hydrogels Induced by Tuning Crystalline and Secondary Ordered Structures. ACS Appl. Polym. Mater. 2025, 7, 8607–8618. [Google Scholar] [CrossRef]
- Treloar, L.G. The Physics of Rubber Elasticity; Oxford University Press: Oxford, UK, 1975. [Google Scholar]
- MacKintosh, F.; Käs, J.; Janmey, P. Elasticity of semiflexible biopolymer networks. Phys. Rev. Lett. 1995, 75, 4425. [Google Scholar] [CrossRef]
- Kouwer, P.H.; Koepf, M.; Le Sage, V.A.; Jaspers, M.; Van Buul, A.M.; Eksteen-Akeroyd, Z.H.; Woltinge, T.; Schwartz, E.; Kitto, H.J.; Hoogenboom, R. Responsive biomimetic networks from polyisocyanopeptide hydrogels. Nature 2013, 493, 651–655. [Google Scholar] [CrossRef]
- Arevalo, R.C.; Urbach, J.S.; Blair, D.L. Size-dependent rheology of type-I collagen networks. Biophys. J. 2010, 99, L65–L67. [Google Scholar] [CrossRef]
- Calvert, P. Hydrogels for soft machines. Adv. Mater. 2009, 21, 743–756. [Google Scholar] [CrossRef]
- Gong, J.P.; Katsuyama, Y.; Kurokawa, T.; Osada, Y. Double-network hydrogels with extremely high mechanical strength. Adv. Mater. 2003, 15, 1155–1158. [Google Scholar] [CrossRef]
- Peppas, N.A.; Hilt, J.Z.; Khademhosseini, A.; Langer, R. Hydrogels in biology and medicine: From molecular principles to bionanotechnology. Adv. Mater. 2006, 18, 1345–1360. [Google Scholar] [CrossRef]
- Haraguchi, K.; Takehisa, T.; Fan, S. Effects of clay content on the properties of nanocomposite hydrogels composed of poly (N-isopropylacrylamide) and clay. Macromolecules 2002, 35, 10162–10171. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Ganji, F.; Vasheghani, F.S.; Vasheghani, F.E. Theoretical description of hydrogel swelling: A review. Iran. Polym. J. 2010, 19, 375–398. [Google Scholar]
- Kharkar, P.M.; Kiick, K.L.; Kloxin, A.M. Designing degradable hydrogels for orthogonal control of cell microenvironments. Chem. Soc. Rev. 2013, 42, 7335–7372. [Google Scholar] [CrossRef]
- Collins, M.N.; Birkinshaw, C. Hyaluronic acid based scaffolds for tissue engineering—A review. Carbohydr. Polym. 2013, 92, 1262–1279. [Google Scholar] [CrossRef] [PubMed]
- Thai, V.L.; Ramos-Rodriguez, D.H.; Mesfin, M.; Leach, J.K. Hydrogel degradation promotes angiogenic and regenerative potential of cell spheroids for wound healing. Mater. Today Bio 2023, 22, 100769. [Google Scholar] [CrossRef]
- Choi, H.; Choi, W.-S.; Jeong, J.-O. A review of advanced hydrogel applications for tissue engineering and drug delivery systems as biomaterials. Gels 2024, 10, 693. [Google Scholar] [CrossRef] [PubMed]
- Annabi, N.; Nichol, J.W.; Zhong, X.; Ji, C.; Koshy, S.; Khademhosseini, A.; Dehghani, F. Controlling the porosity and microarchitecture of hydrogels for tissue engineering. Tissue Eng. Part B Rev. 2010, 16, 371–383. [Google Scholar] [CrossRef]
- Tsou, Y.-H.; Khoneisser, J.; Huang, P.-C.; Xu, X. Hydrogel as a bioactive material to regulate stem cell fate. Bioact. Mater. 2016, 1, 39–55. [Google Scholar] [CrossRef] [PubMed]
- Mohite, P.; Puri, A.; Munde, S.; Ade, N.; Kumar, A.; Jantrawut, P.; Singh, S.; Chittasupho, C. Hydrogel-forming microneedles in the management of dermal disorders through a non-invasive process: A review. Gels 2024, 10, 719. [Google Scholar] [CrossRef] [PubMed]
- Guvendiren, M.; Molde, J.; Soares, R.M.; Kohn, J. Designing biomaterials for 3D printing. ACS Biomater. Sci. Eng. 2016, 2, 1679–1693. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Liu, J. Smart stimuli-responsive chitosan hydrogel for drug delivery: A review. Int. J. Biol. Macromol. 2023, 235, 123902. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Zhu, Z.; Xia, P.; Wang, Z.; Zhao, X.; Jiang, X.; Wang, T.; Gao, Q.; Xu, J.; Shan, D. Tough gelatin hydrogel for tissue engineering. Adv. Sci. 2023, 10, 2301665. [Google Scholar] [CrossRef]
- Gu, Z.; Huang, K.; Luo, Y.; Zhang, L.; Kuang, T.; Chen, Z.; Liao, G. Double network hydrogel for tissue engineering. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2018, 10, e1520. [Google Scholar] [CrossRef]
- Ouyang, L.; Dan, Y.; Shao, Z.; Yang, S.; Yang, C.; Liu, G.; Duan, D. MMP-sensitive PEG hydrogel modified with RGD promotes bFGF, VEGF and EPC-mediated angiogenesis. Exp. Ther. Med. 2019, 18, 2933–2941. [Google Scholar] [CrossRef]
- Geckil, H.; Xu, F.; Zhang, X.; Moon, S.; Demirci, U. Engineering hydrogels as extracellular matrix mimics. Nanomedicine 2010, 5, 469–484. [Google Scholar] [CrossRef]
- Lee, K.K.; Go, K.; Lee, E.; Kim, H.; Kim, S.; Kim, J.-H.; Chae, M.S.; Jeong, J.-O. Multifunctional hydrogels for advanced cancer treatment: Diagnostic imaging and therapeutic modalities. Gels 2025, 11, 426. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, Y.; Li, T.; Zhang, J.; Tian, H. Stimuli-responsive hydrogels: Fabrication and biomedical applications. View 2022, 3, 20200112. [Google Scholar] [CrossRef]
- Caló, E.; Khutoryanskiy, V.V. Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef]
- Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef]
- Kamata, H.; Akagi, Y.; Kayasuga-Kariya, Y.; Chung, U.-i.; Sakai, T. “Nonswellable” hydrogel without mechanical hysteresis. Science 2014, 343, 873–875. [Google Scholar] [CrossRef]
- Thirumalaivasan, N.; Kanagaraj, K.; Nangan, S.; Pothu, R.; Rajendra, S.P.; Karuppiah, P.; Boddula, R. Bioactive Hydrogels (Bio-HyGs): Emerging Trends in Drug Delivery and Wound Healing Applications. Polym. Adv. Technol. 2025, 36, e70132. [Google Scholar] [CrossRef]
- Slaughter, B.V.; Khurshid, S.S.; Fisher, O.Z.; Khademhosseini, A.; Peppas, N.A. Hydrogels in regenerative medicine. Adv. Mater. 2009, 21, 3307–3329. [Google Scholar] [CrossRef]
- Huebsch, N.; Arany, P.R.; Mao, A.S.; Shvartsman, D.; Ali, O.A.; Bencherif, S.A.; Rivera-Feliciano, J.; Mooney, D.J. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nat. Mater. 2010, 9, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Guvendiren, M.; Burdick, J.A. Stiffening hydrogels to probe short-and long-term cellular responses to dynamic mechanics. Nat. Commun. 2012, 3, 792. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; DelRio, F.W.; Ma, H.; Killaars, A.R.; Basta, L.P.; Kyburz, K.A.; Anseth, K.S. Spatially patterned matrix elasticity directs stem cell fate. Proc. Natl. Acad. Sci. 2016, 113, E4439–E4445. [Google Scholar] [CrossRef]
- Phelps, E.A.; Enemchukwu, M.N.O.; Fiore, M.V.F.; Sy, J.C.; Murthy, N.; Sulchek, T.A.; Barker, T.H.; García, A.J. Maleimide cross-linked bioactive PEG hydrogel exhibits improved reaction kinetics and cross-linking for cell encapsulation and in-situ delivery. Adv. Mater. 2011, 24, 64. [Google Scholar] [CrossRef]
- Wade, R.J.; Bassin, E.J.; Gramlich, W.M.; Burdick, J.A. Nanofibrous hydrogels with spatially patterned biochemical signals to control cell behavior. Adv. Mater. 2015, 27, 1356. [Google Scholar] [CrossRef]
- Baker, B.M.; Trappmann, B.; Wang, W.Y.; Sakar, M.S.; Kim, I.L.; Shenoy, V.B.; Burdick, J.A.; Chen, C.S. Cell-mediated fibre recruitment drives extracellular matrix mechanosensing in engineered fibrillar microenvironments. Nat. Mater. 2015, 14, 1262–1268. [Google Scholar] [CrossRef]
- Kuo, K.-C.; Lin, R.-Z.; Tien, H.-W.; Wu, P.-Y.; Li, Y.-C.; Melero-Martin, J.M.; Chen, Y.-C. Bioengineering vascularized tissue constructs using an injectable cell-laden enzymatically crosslinked collagen hydrogel derived from dermal extracellular matrix. Acta Biomater. 2015, 27, 151–166. [Google Scholar] [CrossRef]
- Lotz, C.; Schmid, F.F.; Oechsle, E.; Monaghan, M.G.; Walles, H.; Groeber-Becker, F. Cross-linked collagen hydrogel matrix resisting contraction to facilitate full-thickness skin equivalents. ACS Appl. Mater. Interfaces 2017, 9, 20417–20425. [Google Scholar] [CrossRef]
- Yang, S.-X.; Chen, S.; Xu, Y.; Zhu, Z.-L.; Su, X.-Y.; Wang, Y.-T.; Liu, Y.-X.; Zhang, J.-T.; Wei, B.-M.; Zhu, L. The regulatory effect of grass carp collagen configuration on the migration behavior of stem cells. New J. Chem. 2025, 49, 3166–3173. [Google Scholar] [CrossRef]
- Mori, N.; Morimoto, Y.; Takeuchi, S. Skin integrated with perfusable vascular channels on a chip. Biomaterials 2017, 116, 48–56. [Google Scholar] [CrossRef]
- Kim, B.S.; Kwon, Y.W.; Kong, J.-S.; Park, G.T.; Gao, G.; Han, W.; Kim, M.-B.; Lee, H.; Kim, J.H.; Cho, D.-W. 3D cell printing of in vitro stabilized skin model and in vivo pre-vascularized skin patch using tissue-specific extracellular matrix bioink: A step towards advanced skin tissue engineering. Biomaterials 2018, 168, 38–53. [Google Scholar] [CrossRef] [PubMed]
- Law, J.X.; Liau, L.L.; Saim, A.; Yang, Y.; Idrus, R. Electrospun collagen nanofibers and their applications in skin tissue engineering. Tissue Eng. Regen. Med. 2017, 14, 699–718. [Google Scholar] [CrossRef]
- Caprio, N.D.; Davidson, M.D.; Daly, A.C.; Burdick, J.A. Injectable MSC spheroid and microgel granular composites for engineering tissue. Adv. Mater. 2024, 36, 2312226. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Zhang, J.; Liu, J.; Yu, Z. Granular hydrogels for 3D bioprinting applications. View 2020, 1, 20200060. [Google Scholar] [CrossRef]
- Cao, H.; Duan, L.; Zhang, Y.; Cao, J.; Zhang, K. Current hydrogel advances in physicochemical and biological response-driven biomedical application diversity. Signal Transduct. Target. Ther. 2021, 6, 426. [Google Scholar] [CrossRef]
- Xu, T.; Hu, J.; Fang, C.; Luo, T.; Liu, J.; Zhang, K. Composite hemostat spray seals post-surgical blood burst and ameliorates bacteria-arised inflammation for expediting wound healing. ACS Mater. Lett. 2023, 5, 1892–1901. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, Y.; Fang, C.; Song, L.; Wang, Y.; Lu, L.; Yang, R.; Bu, Z.; Liang, X.; Zhang, K. Urine-Microenvironment-Initiated Composite Hydrogel Patch Reconfiguration Propels Scarless Memory Repair and Reinvigoration of the Urethra. Adv. Mater. 2022, 34, 2109522. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, T.; Zehnder, S.M.; Rowe, K.G.; Jain, S.; Nixon, R.M.; Sawyer, W.G.; Angelini, T.E. Writing in the granular gel medium. Sci. Adv. 2015, 1, e1500655. [Google Scholar] [CrossRef]
- Hua, W.; Mitchell, K.; Raymond, L.; Godina, B.; Zhao, D.; Zhou, W.; Jin, Y. Fluid bath-assisted 3D printing for biomedical applications: From pre-to postprinting stages. ACS Biomater. Sci. Eng. 2021, 7, 4736–4756. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Sun, X.; Heng, Y.; Zeng, Y.; Wang, Y.; Shen, Y.; Peng, A.; Tang, W.; Zeng, M.; Yu, Z. Transforming cell–drug interaction through granular hydrogel-mediated delivery of polyplex nanoparticles for enhanced safety and extended efficacy in gene therapy. ACS Appl. Mater. Interfaces 2024, 16, 39784–39795. [Google Scholar] [CrossRef]
- Wu, G.; Wang, Y.; Shen, Y.; Zheng, H.; Zeng, Y.; Zhang, C.; Zhang, X.; Zhang, S.; Zhang, J.; Yu, Z. Improving Liposome Delivery with Macroporous Granular Hydrogels Synthesized through Freezing-Facilitated Secondary Crosslinking of Macromonomers. Adv. Mater. Interfaces 2023, 10, 2300262. [Google Scholar] [CrossRef]
- De Giorgio, G.; Matera, B.; Vurro, D.; Manfredi, E.; Galstyan, V.; Tarabella, G.; Ghezzi, B.; D’Angelo, P. Silk fibroin materials: Biomedical applications and perspectives. Bioengineering 2024, 11, 167. [Google Scholar] [CrossRef]
- Bovone, G.; Dudaryeva, O.Y.; Marco-Dufort, B.; Tibbitt, M.W. Engineering hydrogel adhesion for biomedical applications via chemical design of the junction. ACS Biomater. Sci. Eng. 2021, 7, 4048–4076. [Google Scholar] [CrossRef]
- Neumann, M.; di Marco, G.; Iudin, D.; Viola, M.; van Nostrum, C.F.; van Ravensteijn, B.G.; Vermonden, T. Stimuli-responsive hydrogels: The dynamic smart biomaterials of tomorrow. Macromolecules 2023, 56, 8377–8392. [Google Scholar] [CrossRef]
- Farasati Far, B.; Safaei, M.; Nahavandi, R.; Gholami, A.; Naimi-Jamal, M.R.; Tamang, S.; Ahn, J.E.; Ramezani Farani, M.; Huh, Y.S. Hydrogel encapsulation techniques and its clinical applications in drug delivery and regenerative medicine: A systematic review. ACS Omega 2024, 9, 29139–29158. [Google Scholar] [CrossRef]
- Wong, P.T.; Choi, S.K. Mechanisms of drug release in nanotherapeutic delivery systems. Chem. Rev. 2015, 115, 3388–3432. [Google Scholar] [CrossRef]
- Correa, S.; Grosskopf, A.K.; Lopez Hernandez, H.; Chan, D.; Yu, A.C.; Stapleton, L.M.; Appel, E.A. Translational applications of hydrogels. Chem. Rev. 2021, 121, 11385–11457. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, J. Current status and prospects of gelatin and its derivatives in oncological applications. Int. J. Biol. Macromol. 2024, 274, 133590. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.A.; Keys, K.B.; Torres-Lugo, M.; Lowman, A.M. Poly (ethylene glycol)-containing hydrogels in drug delivery. J. Control. Release 1999, 62, 81–87. [Google Scholar] [CrossRef]
- Lin, C.-C.; Metters, A.T. Hydrogels in controlled release formulations: Network design and mathematical modeling. Adv. Drug Deliv. Rev. 2006, 58, 1379–1408. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.; Kim, B. Stimuli-sensitive protein delivery systems. J. Drug Deliv. Sci. Technol. 2006, 16, 11–18. [Google Scholar] [CrossRef]
- El-Sayed, M.E.; Hoffman, A.S.; Stayton, P.S. Smart polymeric carriers for enhanced intracellular delivery of therapeutic macromolecules. Expert Opin. Biol. Ther. 2005, 5, 23–32. [Google Scholar] [CrossRef]
- Hoffman, A.S.; Stayton, P.S. Conjugates of stimuli-responsive polymers and proteins. Prog. Polym. Sci. 2007, 32, 922–932. [Google Scholar] [CrossRef]
- Benoit, D.S.; Collins, S.D.; Anseth, K.S. Multifunctional hydrogels that promote osteogenic human mesenchymal stem cell differentiation through stimulation and sequestering of bone morphogenic protein 2. Adv. Funct. Mater. 2007, 17, 2085–2093. [Google Scholar] [CrossRef]
- Zisch, A.H.; Lutolf, M.P.; Ehrbar, M.; Raeber, G.P.; Rizzi, S.C.; Davies, N.; Schmökel, H.; Bezuidenhout, D.; Djonov, V.; Zilla, P. Cell-demanded release of VEGF from synthetic, biointeractive cell-ingrowth matrices for vascularized tissue growth. FASEB J. 2003, 17, 2260–2262. [Google Scholar] [CrossRef]
- Khan, A.R.; Gholap, A.D.; Grewal, N.S.; Jun, Z.; Khalid, M.; Zhang, H.-J. Advances in smart hybrid scaffolds: A strategic approach for regenerative clinical applications. Eng. Regen. 2025, 6, 85–110. [Google Scholar] [CrossRef]
- Chaudhary, S.; Chakraborty, E. Hydrogel based tissue engineering and its future applications in personalized disease modeling and regenerative therapy. Beni-Suef Univ. J. Basic Appl. Sci. 2022, 11, 3. [Google Scholar] [CrossRef]
- Bashir, M.H.; Korany, N.S.; Farag, D.B.; Abbass, M.M.; Ezzat, B.A.; Hegazy, R.H.; Dörfer, C.E.; Fawzy El-Sayed, K.M. Polymeric nanocomposite hydrogel scaffolds in craniofacial bone regeneration: A comprehensive review. Biomolecules 2023, 13, 205. [Google Scholar] [CrossRef]
- Mirshafiei, M.; Rashedi, H.; Yazdian, F.; Rahdar, A.; Baino, F. Advancements in tissue and organ 3D bioprinting: Current techniques, applications, and future perspectives. Mater. Des. 2024, 240, 112853. [Google Scholar] [CrossRef]
- Chiticaru, E.A.; Ioniță, M. Commercially available bioinks and state-of-the-art lab-made formulations for bone tissue engineering: A comprehensive review. Mater. Today Bio 2024, 29, 101341. [Google Scholar] [CrossRef] [PubMed]
- Aftab, M.; Ikram, S.; Ullah, M.; Khan, N.; Naeem, M.; Khan, M.A.; Bakhtiyor o’g’li, R.B.; Qizi, K.S.S.; Erkinjon Ugli, O.O.; Abdurasulovna, B.M. Recent Trends and Future Directions in 3D Printing of Biocompatible Polymers. J. Manuf. Mater. Process. 2025, 9, 129. [Google Scholar] [CrossRef]
- Daly, A.C.; Prendergast, M.E.; Hughes, A.J.; Burdick, J.A. Bioprinting for the biologist. Cell 2021, 184, 18–32. [Google Scholar] [CrossRef]
- Kang, X.; Zhang, X.-B.; Gao, X.-D.; Hao, D.-J.; Li, T.; Xu, Z.-W. Bioprinting for bone tissue engineering. Front. Bioeng. Biotechnol. 2022, 10, 1036375. [Google Scholar] [CrossRef] [PubMed]
- Mota, C.; Camarero-Espinosa, S.; Baker, M.B.; Wieringa, P.; Moroni, L. Bioprinting: From tissue and organ development to in vitro models. Chem. Rev. 2020, 120, 10547–10607. [Google Scholar] [CrossRef]
- Wang, B.; Hu, S.; Teng, Y.; Chen, J.; Wang, H.; Xu, Y.; Wang, K.; Xu, J.; Cheng, Y.; Gao, X. Current advance of nanotechnology in diagnosis and treatment for malignant tumors. Signal Transduct. Target. Ther. 2024, 9, 200. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Li, Y.; Xiong, L.; Wang, W.; Wu, M.; Yuan, T.; Yang, W.; Tian, C.; Miao, Z.; Wang, T. Small molecules in targeted cancer therapy: Advances, challenges, and future perspectives. Signal Transduct. Target. Ther. 2021, 6, 201. [Google Scholar] [CrossRef]
- Sun, L.; Liu, H.; Ye, Y.; Lei, Y.; Islam, R.; Tan, S.; Tong, R.; Miao, Y.-B.; Cai, L. Smart nanoparticles for cancer therapy. Signal Transduct. Target. Ther. 2023, 8, 418. [Google Scholar] [CrossRef]
- Liu, W.; Ou-Yang, W.; Zhang, C.; Wang, Q.; Pan, X.; Huang, P.; Zhang, C.; Li, Y.; Kong, D.; Wang, W. Synthetic polymeric antibacterial hydrogel for methicillin-resistant staphylococcus aureus-infected wound healing: Nanoantimicrobial self-assembly, drug-and cytokine-free strategy. Acs Nano 2020, 14, 12905–12917. [Google Scholar] [CrossRef]
- You, D.; Chen, G.; Liu, C.; Ye, X.; Wang, S.; Dong, M.; Sun, M.; He, J.; Yu, X.; Ye, G. 4D printing of multi-responsive membrane for accelerated in vivo bone healing via remote regulation of stem cell fate. Adv. Funct. Mater. 2021, 31, 2103920. [Google Scholar] [CrossRef]
- Kass, L.E.; Nguyen, J. Nanocarrier-hydrogel composite delivery systems for precision drug release. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2022, 14, e1756. [Google Scholar] [CrossRef]
- Qi, Y.; Qian, Z.; Yuan, W.; Li, Z. Injectable and self-healing nanocomposite hydrogel loading needle-like nano-hydroxyapatite and graphene oxide for synergistic tumour proliferation inhibition and photothermal therapy. J. Mater. Chem. B 2021, 9, 9734–9743. [Google Scholar] [CrossRef]
- Tan, B.; Huang, L.; Wu, Y.; Liao, J. Advances and trends of hydrogel therapy platform in localized tumor treatment: A review. J. Biomed. Mater. Res. Part A 2021, 109, 404–425. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, Y.; Li, Q.; Yu, C.; Chu, W. Natural polymer-based stimuli-responsive hydrogels. Curr. Med. Chem. 2020, 27, 2631–2657. [Google Scholar] [CrossRef]
- Meng, D.; Yang, S.; Yang, Y.; Zhang, L.; Cui, L. Synergistic chemotherapy and phototherapy based on red blood cell biomimetic nanomaterials. J. Control. Release 2022, 352, 146–162. [Google Scholar] [CrossRef]
- Shatsberg, Z.; Zhang, X.; Ofek, P.; Malhotra, S.; Krivitsky, A.; Scomparin, A.; Tiram, G.; Calderón, M.; Haag, R.; Satchi-Fainaro, R. Functionalized nanogels carrying an anticancer microRNA for glioblastoma therapy. J. Control. Release 2016, 239, 159–168. [Google Scholar] [CrossRef]
- Lee, J.H.; Tachibana, T.; Yamana, K.; Kawasaki, R.; Yabuki, A. Simple formation of cancer drug-containing self-assembled hydrogels with temperature and pH-responsive release. Langmuir 2021, 37, 11269–11275. [Google Scholar] [CrossRef]
- Sun, L.; Shen, F.; Tian, L.; Tao, H.; Xiong, Z.; Xu, J.; Liu, Z. ATP-responsive smart hydrogel releasing immune adjuvant synchronized with repeated chemotherapy or radiotherapy to boost antitumor immunity. Adv. Mater. 2021, 33, 2007910. [Google Scholar] [CrossRef]
- Chen, M.; Wang, Z.; Suo, W.; Bao, Z.; Quan, H. Injectable hydrogel for synergetic low dose radiotherapy, chemodynamic therapy and photothermal therapy. Front. Bioeng. Biotechnol. 2021, 9, 757428. [Google Scholar] [CrossRef]
- Fu, Z.; Li, H.; Xue, P.; Yu, H.; Yang, S.; Tao, C.; Li, W.; Wang, Y.; Zhang, J.; Wang, Y. Implantable bioresponsive hydrogel prevents local recurrence of breast cancer by enhancing radiosensitivity. Front. Bioeng. Biotechnol. 2022, 10, 881544. [Google Scholar] [CrossRef]
- Wang, N.; Gao, Q.; Tang, J.; Jiang, Y.; Yang, L.; Shi, X.; Chen, Y.; Zhang, Y.; Fu, S.; Lin, S. Anti-tumor effect of local injectable hydrogel-loaded endostatin alone and in combination with radiotherapy for lung cancer. Drug Deliv. 2021, 28, 183–194. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, L.; Huang, F.; Zhao, C.; Liu, J.; Zhang, Y.; Liu, J. Multifunctional hybrid hydrogel enhanced antitumor therapy through multiple destroying DNA functions by a triple-combination synergistic therapy. Adv. Healthc. Mater. 2021, 10, 2101190. [Google Scholar] [CrossRef]
- Fischbach, M.A.; Bluestone, J.A.; Lim, W.A. Cell-based therapeutics: The next pillar of medicine. Sci. Transl. Med. 2013, 5, ps177–ps179. [Google Scholar] [CrossRef]
- Wang, L.L.W.; Janes, M.E.; Kumbhojkar, N.; Kapate, N.; Clegg, J.R.; Prakash, S.; Heavey, M.K.; Zhao, Z.; Anselmo, A.C.; Mitragotri, S. Cell therapies in the clinic. Bioeng. Transl. Med. 2021, 6, e10214. [Google Scholar] [CrossRef]
- Chen, D.S.; Mellman, I. Oncology meets immunology: The cancer-immunity cycle. Immunity 2013, 39, 1–10. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, G.; Liu, S.; Su, H.; Wang, Y.; Li, J.; Luo, C. Remodeling the tumor microenvironment with emerging nanotherapeutics. Trends Pharmacol. Sci. 2018, 39, 59–74. [Google Scholar] [CrossRef]
- Bader, J.E.; Voss, K.; Rathmell, J.C. Targeting metabolism to improve the tumor microenvironment for cancer immunotherapy. Mol. Cell 2020, 78, 1019–1033. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z. The history and advances in cancer immunotherapy: Understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell. Mol. Immunol. 2020, 17, 807–821. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, Z.; Zhang, L.; Li, Y.; Jain, A.; Barve, A.; Jin, W.; Liu, Y.; Fetse, J.; Cheng, K. Discovery of low-molecular weight anti-PD-L1 peptides for cancer immunotherapy. J. Immunother. Cancer 2019, 7, 270. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Geng, Y.; Yue, B.; Lo, P.-C.; Huang, J.; Jin, H. Injectable hydrogel as a unique platform for antitumor therapy targeting immunosuppressive tumor microenvironment. Front. Immunol. 2022, 12, 832942. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Li, Q.; Chen, X.; Nie, X.; Xue, F.; Xu, W.; Luan, Y. An injectable hydrogel to modulate T cells for cancer immunotherapy. Small 2022, 18, 2202663. [Google Scholar] [CrossRef]
- Passaro, A.; Attili, I.; de Marinis, F. Neoadjuvant chemotherapy plus immunotherapy in early-stage resectable non–small-cell lung cancer. J. Clin. Oncol. 2022, 40, 2871–2877. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, Z.; Qin, X.; Zhang, M.; Du, Q.; Li, Z.; Luan, Y. A checkpoint-regulatable immune niche created by injectable hydrogel for tumor therapy. Adv. Funct. Mater. 2021, 31, 2104630. [Google Scholar] [CrossRef]
- Jin, H.; Wan, C.; Zou, Z.; Zhao, G.; Zhang, L.; Geng, Y.; Chen, T.; Huang, A.; Jiang, F.; Feng, J.-P. Tumor ablation and therapeutic immunity induction by an injectable peptide hydrogel. Acs Nano 2018, 12, 3295–3310. [Google Scholar] [CrossRef]
- Dai, X.; Meng, J.; Deng, S.; Zhang, L.; Wan, C.; Lu, L.; Huang, J.; Hu, Y.; Zhang, Z.; Li, Y. Targeting CAMKII to reprogram tumor-associated macrophages and inhibit tumor cells for cancer immunotherapy with an injectable hybrid peptide hydrogel. Theranostics 2020, 10, 3049. [Google Scholar] [CrossRef]
- Rizvi, I.; Gudejko, H.; Briars, E.; Khan, S.; Chiang, C.-T.; El-Hamidi, H.; Celli, J.P.; Hasan, T. 3D cancer models on hydrogels. In GELS HANDBOOK: Fundamentals, Properties and Applications Volume 3: Application of Hydrogels in Drug Delivery and Biosensing; World Scientific: Singapore, 2016; pp. 207–256. [Google Scholar]
- Priya, A.S.; Premanand, R.; Ragupathi, I.; Bhaviripudi, V.R.; Aepuru, R.; Kannan, K.; Shanmugaraj, K. Comprehensive review of hydrogel synthesis, characterization, and emerging applications. J. Compos. Sci. 2024, 8, 457. [Google Scholar] [CrossRef]
- Abuwatfa, W.H.; Pitt, W.G.; Husseini, G.A. Scaffold-based 3D cell culture models in cancer research. J. Biomed. Sci. 2024, 31, 7. [Google Scholar] [CrossRef]
- Kloxin, A.M.; Kloxin, C.J.; Bowman, C.N.; Anseth, K.S. Mechanical properties of cellularly responsive hydrogels and their experimental determination. Adv. Mater. 2010, 22, 3484–3494. [Google Scholar] [CrossRef]
- Zhang, W.; Huang, G.; Xu, F. Engineering biomaterials and approaches for mechanical stretching of cells in three dimensions. Front. Bioeng. Biotechnol. 2020, 8, 589590. [Google Scholar] [CrossRef]
- Rojek, K.O.; Cwiklinska, M.; Kuczak, J.; Guzowski, J. Microfluidic formulation of topological hydrogels for microtissue engineering. Chem. Rev. 2022, 122, 16839–16909. [Google Scholar] [CrossRef]
- Hu, T.; Fang, J.; Shen, Y.; Li, M.; Wang, B.; Xu, Z.; Hu, W. Advances of naturally derived biomedical polymers in tissue engineering. Front. Chem. 2024, 12, 1469183. [Google Scholar] [CrossRef]
- Suba, R.B.; Raj, R.D.S.; Keerthika, K.; Vinothini, V. Chitosan-based biomaterial in wound healing: A review. Cureus 2024, 16, e55193. [Google Scholar] [CrossRef] [PubMed]
- Thang, N.H.; Chien, T.B.; Cuong, D.X. Polymer-based hydrogels applied in drug delivery: An overview. Gels 2023, 9, 523. [Google Scholar] [CrossRef] [PubMed]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable controlled-release polymers and polymeric nanoparticles: Mechanisms of controlling drug release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef] [PubMed]
- Satchanska, G.; Davidova, S.; Petrov, P.D. Natural and synthetic polymers for biomedical and environmental applications. Polymers 2024, 16, 1159. [Google Scholar] [CrossRef]
- Alizadeh Sardroud, H.; Wanlin, T.; Chen, X.; Eames, B.F. Cartilage tissue engineering approaches need to assess fibrocartilage when hydrogel constructs are mechanically loaded. Front. Bioeng. Biotechnol. 2022, 9, 787538. [Google Scholar] [CrossRef]
- Catoira, M.C.; González-Payo, J.; Fusaro, L.; Ramella, M.; Boccafoschi, F. Natural hydrogels R&D process: Technical and regulatory aspects for industrial implementation. J. Mater. Sci. Mater. Med. 2020, 31, 64. [Google Scholar] [CrossRef]
- Li, L.; Yu, F.; Zheng, L.; Wang, R.; Yan, W.; Wang, Z.; Xu, J.; Wu, J.; Shi, D.; Zhu, L. Natural hydrogels for cartilage regeneration: Modification, preparation and application. J. Orthop. Transl. 2019, 17, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Alonso, J.M.; Andrade del Olmo, J.; Perez Gonzalez, R.; Saez-Martinez, V. Injectable hydrogels: From laboratory to industrialization. Polymers 2021, 13, 650. [Google Scholar] [CrossRef] [PubMed]
- Revete, A.; Aparicio, A.; Cisterna, B.A.; Revete, J.; Luis, L.; Ibarra, E.; Segura González, E.A.; Molino, J.; Reginensi, D. Advancements in the use of hydrogels for regenerative medicine: Properties and biomedical applications. Int. J. Biomater. 2022, 2022, 3606765. [Google Scholar] [CrossRef]
- Vigata, M.; Meinert, C.; Hutmacher, D.W.; Bock, N. Hydrogels as drug delivery systems: A review of current characterization and evaluation techniques. Pharmaceutics 2020, 12, 1188. [Google Scholar] [CrossRef]
- Fareed, M.M.; Shityakov, S. Next-Generation Hydrogel Design: Computational Advances in Synthesis, Characterization, and Biomedical Applications. Polymers 2025, 17, 1373. [Google Scholar] [CrossRef]
- Bernatoniene, J.; Stabrauskiene, J.; Kazlauskaite, J.A.; Bernatonyte, U.; Kopustinskiene, D.M. The Future of Medicine: How 3D Printing Is Transforming Pharmaceuticals. Pharmaceutics 2025, 17, 390. [Google Scholar] [CrossRef]
- Alshangiti, D.M.; El-Damhougy, T.K.; Zaher, A.; Madani, M. Revolutionizing biomedicine: Advancements, applications, and prospects of nanocomposite macromolecular carbohydrate-based hydrogel biomaterials: A review. RSC Adv. 2023, 13, 35251–35291. [Google Scholar] [CrossRef]
- Negut, I.; Bita, B. Exploring the potential of artificial intelligence for hydrogel development—A short review. Gels 2023, 9, 845. [Google Scholar] [CrossRef]
- Li, Z.; Song, P.; Li, G.; Han, Y.; Ren, X.; Bai, L.; Su, J. AI energized hydrogel design, optimization and application in biomedicine. Mater. Today Bio 2024, 25, 101014. [Google Scholar] [CrossRef] [PubMed]
- Nosrati, H.; Nosrati, M. Artificial intelligence in regenerative medicine: Applications and implications. Biomimetics 2023, 8, 442. [Google Scholar] [CrossRef] [PubMed]
- Serrano, D.R.; Luciano, F.C.; Anaya, B.J.; Ongoren, B.; Kara, A.; Molina, G.; Ramirez, B.I.; Sánchez-Guirales, S.A.; Simon, J.A.; Tomietto, G. Artificial intelligence (AI) applications in drug discovery and drug delivery: Revolutionizing personalized medicine. Pharmaceutics 2024, 16, 1328. [Google Scholar] [CrossRef]
- Hindy, O.A.; Derici, U.S.; Yilgor, P.; Ashammakhi, N. Introduction: The Concept and History of Bioinspired and Biomimetic Regenerative Medicine. In Principles of Bioinspired and Biomimetic Regenerative Medicine; Springer: Berlin/Heidelberg, Germany, 2025; pp. 3–30. [Google Scholar]
- Andrgie, A.T.; Tsai, H.-C. Hydrogels as local depots for on-demand therapeutic delivery: Potential therapeutic approaches for tumor metastasis. Mater. Adv. 2024, 5, 3629–3643. [Google Scholar] [CrossRef]
- An, L.; Liu, Y.; Liu, Y. Organ-on-a-Chip applications in microfluidic platforms. Micromachines 2025, 16, 201. [Google Scholar] [CrossRef]
- Mandal, A.; Clegg, J.R.; Anselmo, A.C.; Mitragotri, S. Hydrogels in the clinic. Bioeng. Transl. Med. 2020, 5, e10158. [Google Scholar] [CrossRef]
- Ullah, A.; Kim, D.Y.; Lim, S.I.; Lim, H.-R. Hydrogel-Based Biointerfaces: Recent Advances, Challenges, and Future Directions in Human–Machine Integration. Gels 2025, 11, 232. [Google Scholar] [CrossRef] [PubMed]
- Segneanu, A.-E.; Bejenaru, L.E.; Bejenaru, C.; Blendea, A.; Mogoşanu, G.D.; Biţă, A.; Boia, E.R. Advancements in Hydrogels: A Comprehensive Review of Natural and Synthetic Innovations for Biomedical Applications. Polymers 2025, 17, 2026. [Google Scholar] [CrossRef] [PubMed]


| Hydrogel Property | Physico-Chemical Control | Mechanical Control | References |
|---|---|---|---|
| Mechanical Strength | Monomer composition, molecular weight, crosslinking chemistry, incorporation of hydrophobic/ionic domains | Crosslinking density, thermal annealing, nonsolvent quenching, hierarchical structuring | [27,28,43] |
| Elasticity and Viscoelasticity | Polymer network structure, entropic elasticity, fiber alignment | Degree of crosslinking, hierarchical fibrous networks, morphology type (rubber-like vs. fibrillar) | [26,28,36,37,43] |
| Swelling Behavior | Hydrophilic/hydrophobic balance, ionic interactions, degree of ionization | Crosslinking ratio, network stiffness, pore size | [23,26,48] |
| Degradation Rate | Degradable linkers (e.g., ester, enzyme-sensitive), pH-responsive groups, self-cleaving linkers | Crosslinking density, porosity, network structure | [22,29] |
| Porosity | Fabrication technique (e.g., solvent casting, gas foaming, freeze drying), microfabrication for channel creation | Scaffold stiffness (higher porosity generally leads to lower stiffness) | [36,37] |
| Drug Release Kinetics | Polymer–drug interactions, ionic content, degradation mechanism, nanoparticle/microsphere incorporation for staged release | Swelling capacity, porosity, crosslinking level, compartmentalized structures | [28,31,47,57] |
| Microarchitecture | Microfabrication (e.g., soft lithography, prototyping), material chemistry, hierarchical multi-scale designs | Pore design, structural alignment, integration of micro/nanoparticles | [33,34] |
| Integrated Design Trade-offs | Polymer chemistry to balance hydration, degradation, and diffusion | Trade-offs between stiffness, porosity, swelling, and support | [27,28,36] |
| Hydrogel Property | Benefit | Limitation of Conventional Materials | References |
|---|---|---|---|
| High water content | Mimics the natural extracellular matrix (ECM) and supports cell viability | Hydrophobic or have a low water content | [70] |
| Biocompatibility | Promotes safe integration with host tissues without immune rejection | Immunological responses, inflammation, or foreign body reactions | [70,77] |
| Tunable chemical and physical properties | Allows precise control of cell behavior (migration, proliferation, differentiation) | Fixed and challenging to modify | [71,72,73] |
| Spatiotemporal control of biophysical cues | Enables dynamic control of stiffness, porosity, and degradation to guide tissue formation | Unable to replicate the biological environment’s dynamic signals | [72,73] |
| Incorporation of functional ingredients | Enhances biochemical signaling for improved tissue regeneration | Embedding fragile biological molecules is challenging | [74] |
| Nano- and micropatterning capabilities | Facilitates complex multicellular designs and studies of cell–matrix interactions | Needs costly, multi-step lithography | [75] |
| Customizable architecture and mechanics | Supports development of ECM-mimetic structures tailored to specific tissue types | Conventional materials’ final mechanical stiffness and form are mostly determined during manufacture | [76] |
| Collagen-based formulations | Naturally cell-binding and supportive for tissue regeneration | Removing the natural biological cues | [77,78] |
| Composite and crosslinked systems | Improve stability, mechanical strength, and durability of hydrogel scaffolds | Non-biodegradable or brittle materials | [79,80,81,82] |
| Granular hydrogels (GHs) | Provide high porosity, injectability, and nutrient/waste exchange | Solid and monolithic rather than composed of linked microparticles | [86,87,88] |
| Customizable hydrogel microparticles (HMPs) | Allow fine-tuning of mechanical properties and improved cell migration | Stop cell integration, migration, and infiltration | [86,87,88] |
| Advanced fabrication methods (e.g., bioprinting) | Overcome limitations of traditional hydrogels and support structural complexity | Incompatible with the sensitive parts | [91,92,93,94] |
| Regulated degradation rates | Synchronizes scaffold breakdown with tissue regeneration pace | Non-biodegradable or breaking down uncontrollably | [49,50,51] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munteanu, C.; Prifti, E.; Surd, A.; Mârza, S.M. Tailoring Therapy: Hydrogels as Tunable Platforms for Regenerative Medicine and Cancer Intervention. Gels 2025, 11, 679. https://doi.org/10.3390/gels11090679
Munteanu C, Prifti E, Surd A, Mârza SM. Tailoring Therapy: Hydrogels as Tunable Platforms for Regenerative Medicine and Cancer Intervention. Gels. 2025; 11(9):679. https://doi.org/10.3390/gels11090679
Chicago/Turabian StyleMunteanu, Camelia, Eftimia Prifti, Adrian Surd, and Sorin Marian Mârza. 2025. "Tailoring Therapy: Hydrogels as Tunable Platforms for Regenerative Medicine and Cancer Intervention" Gels 11, no. 9: 679. https://doi.org/10.3390/gels11090679
APA StyleMunteanu, C., Prifti, E., Surd, A., & Mârza, S. M. (2025). Tailoring Therapy: Hydrogels as Tunable Platforms for Regenerative Medicine and Cancer Intervention. Gels, 11(9), 679. https://doi.org/10.3390/gels11090679








