Designing Stimuli-Responsive Supramolecular Gels by Tuning the Non-Covalent Interactions of the Functional Groups
Abstract
1. Introduction
2. Results and Discussion
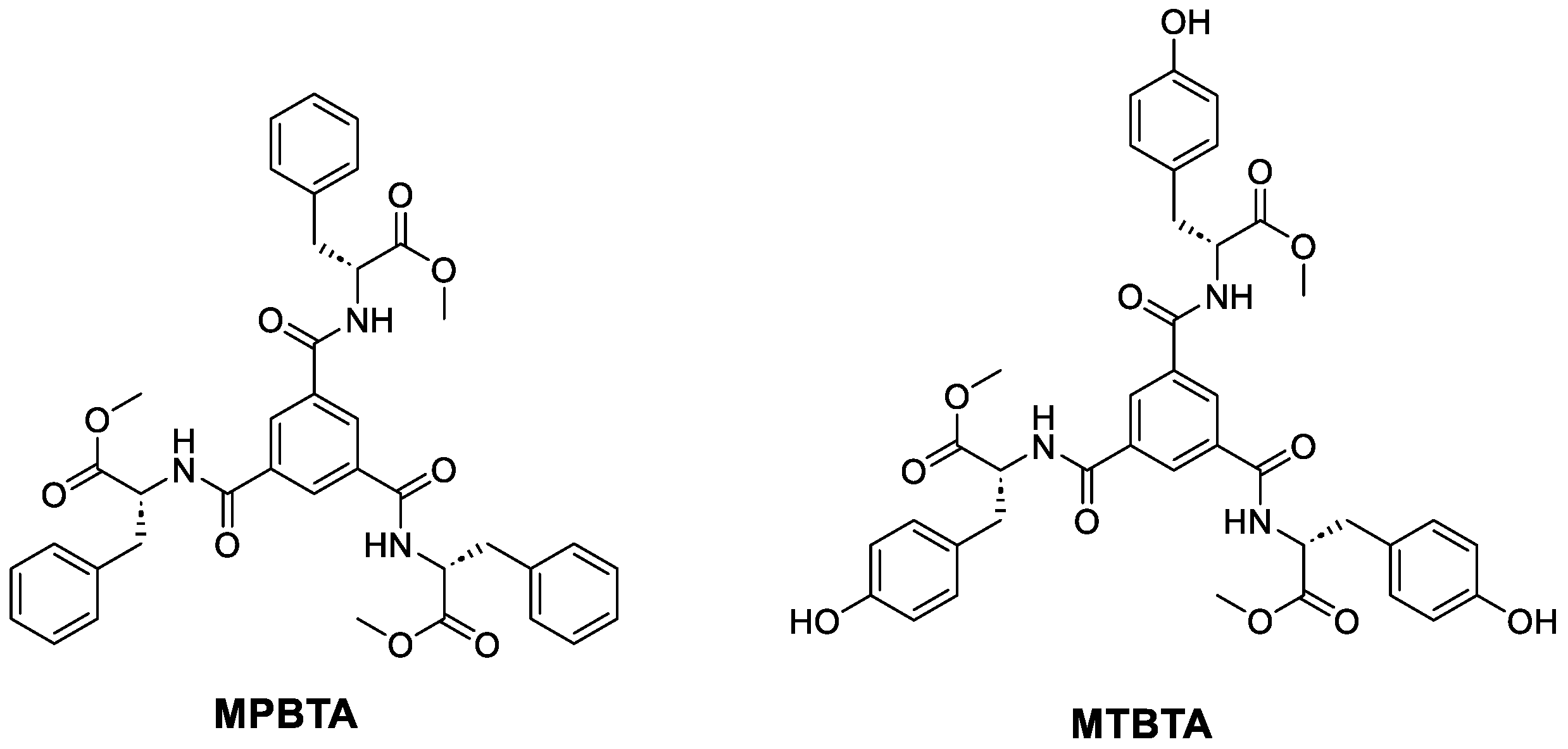
2.1. Synthesis of MPBTA and MTBTA
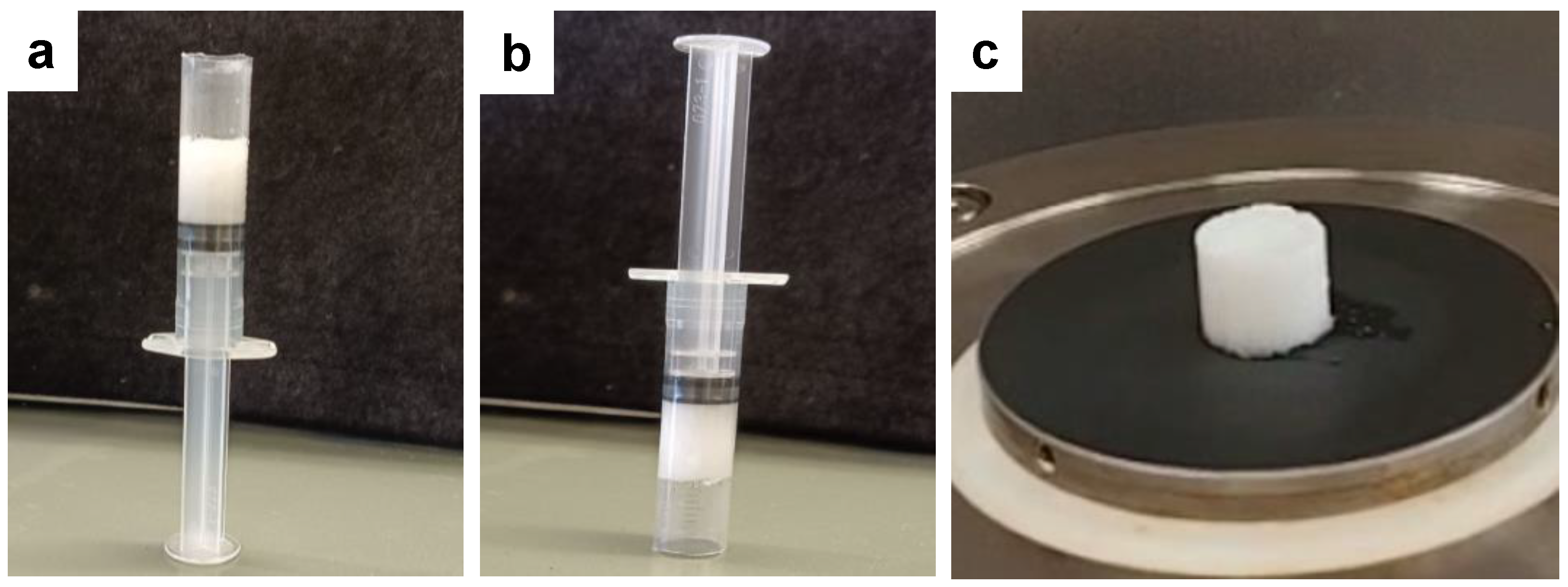
2.2. Gelation Studies
2.3. Thermal Stability
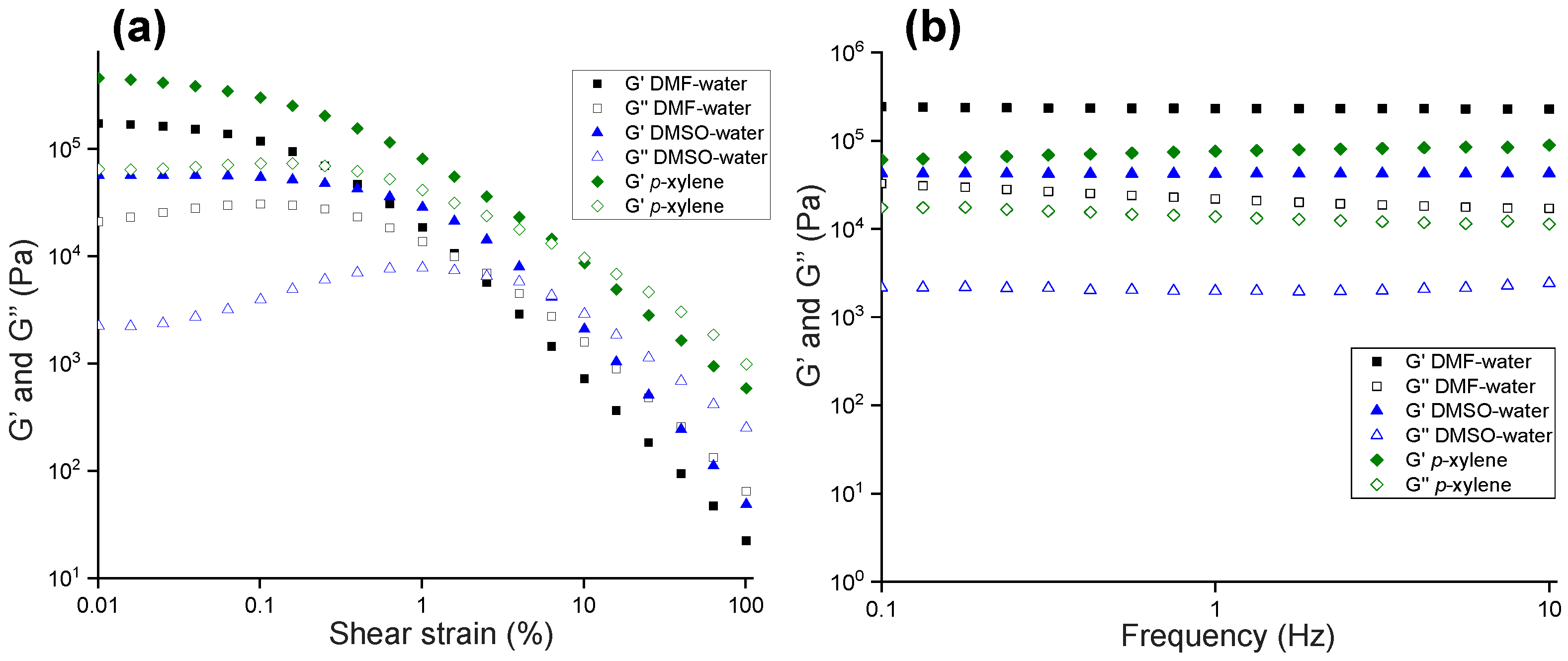
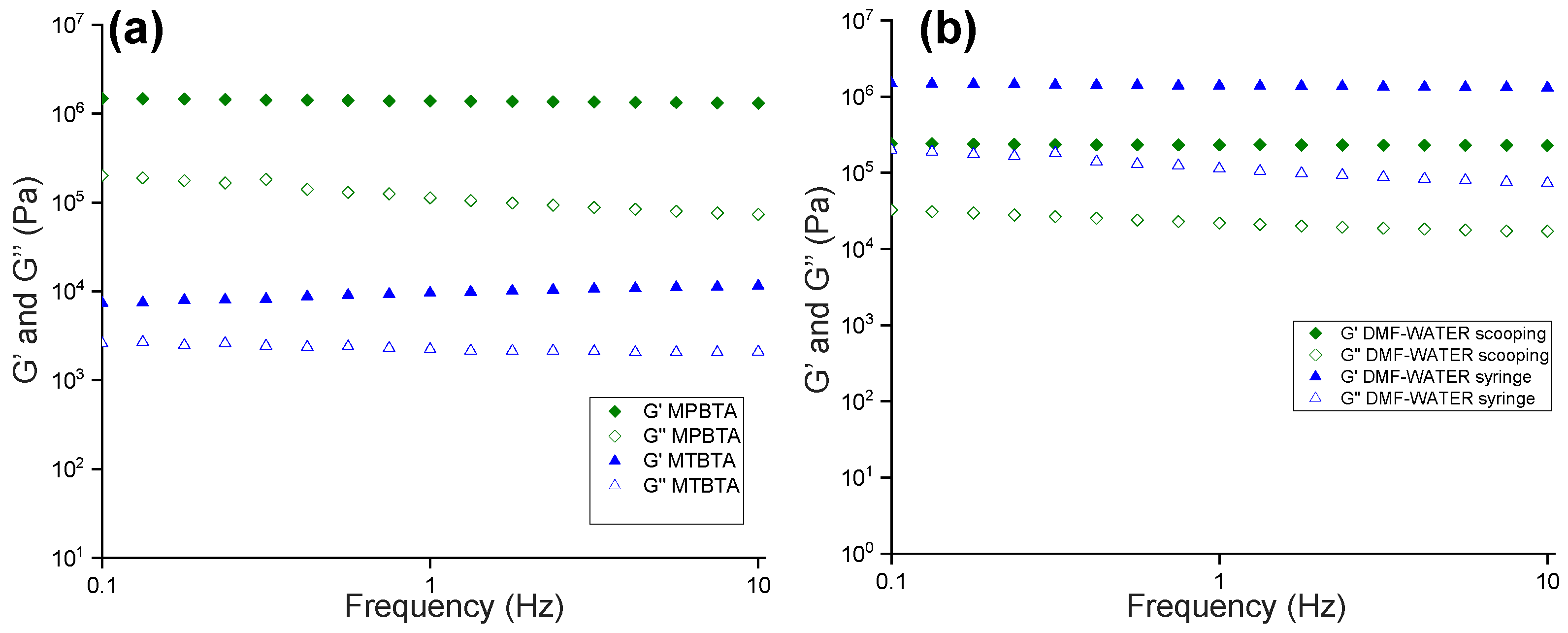
2.4. Rheology
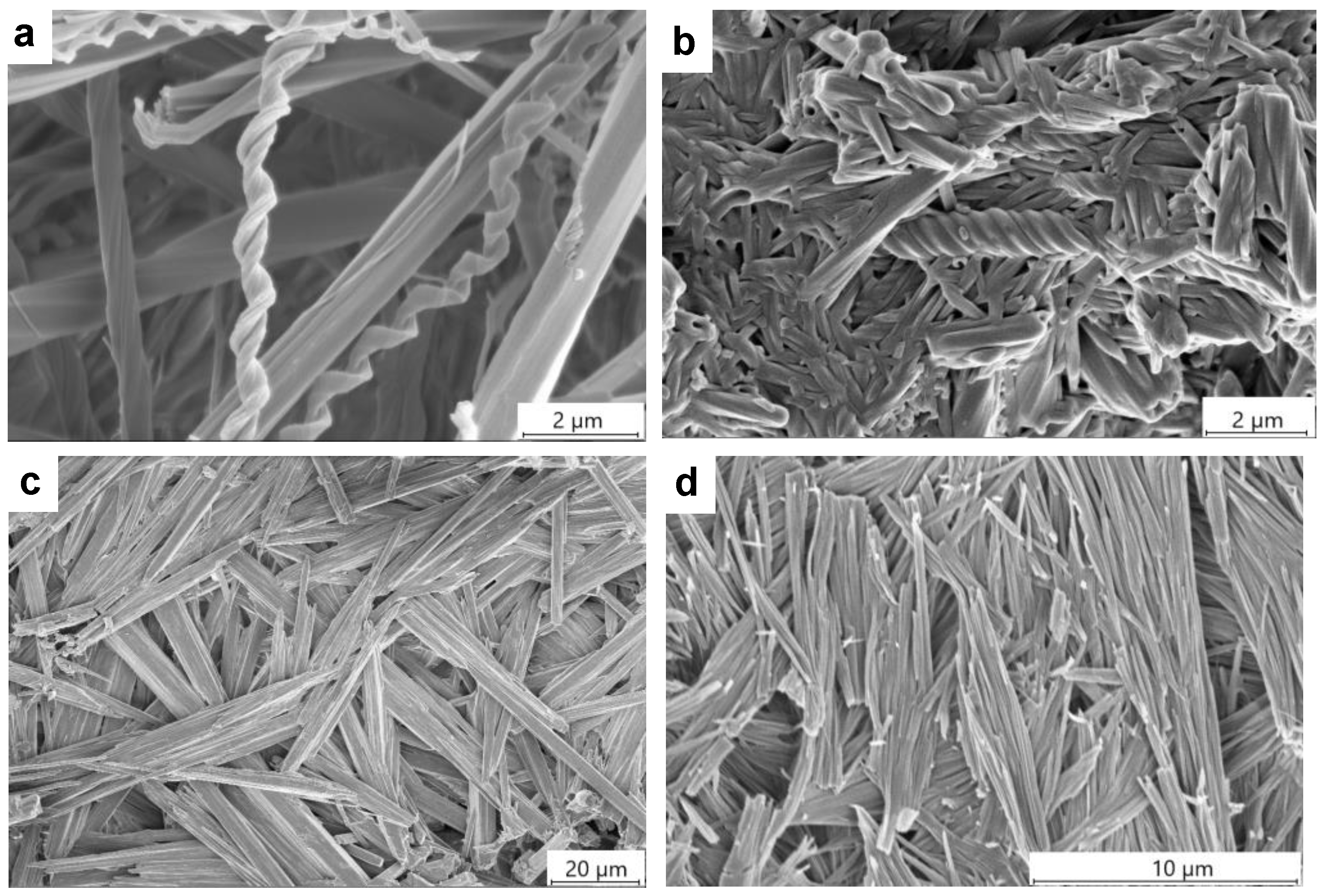
2.5. Gel Morphology
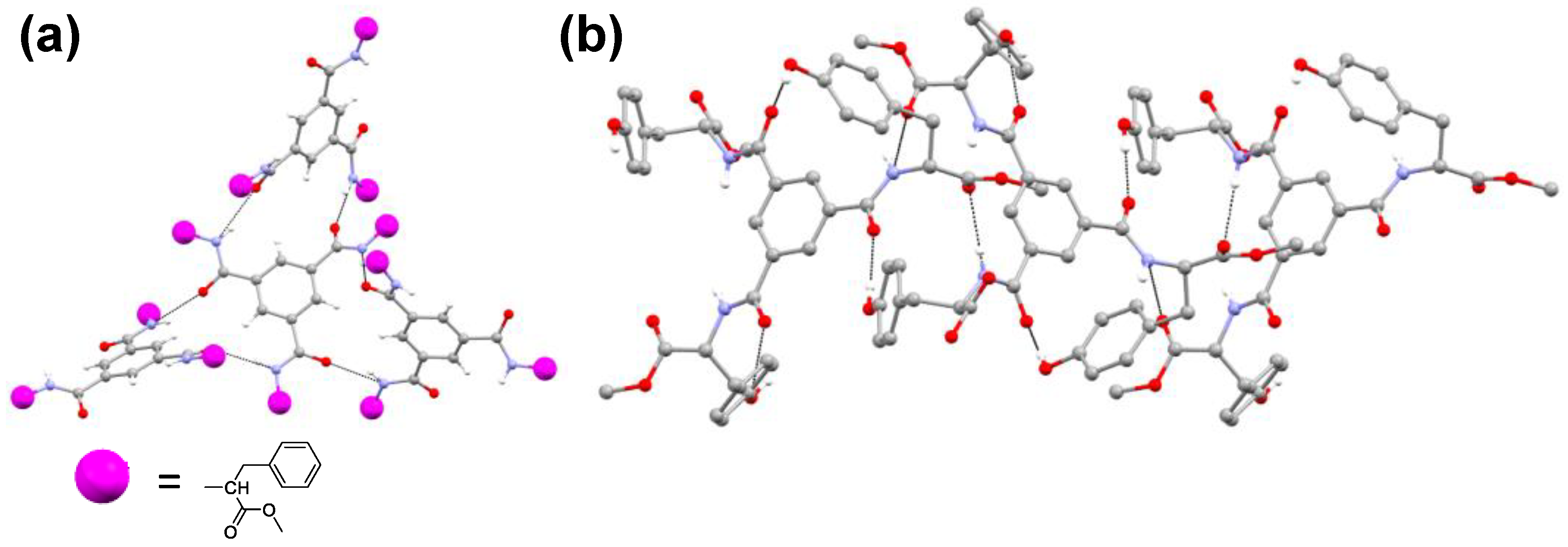
2.6. Single-Crystal X-ray Diffraction
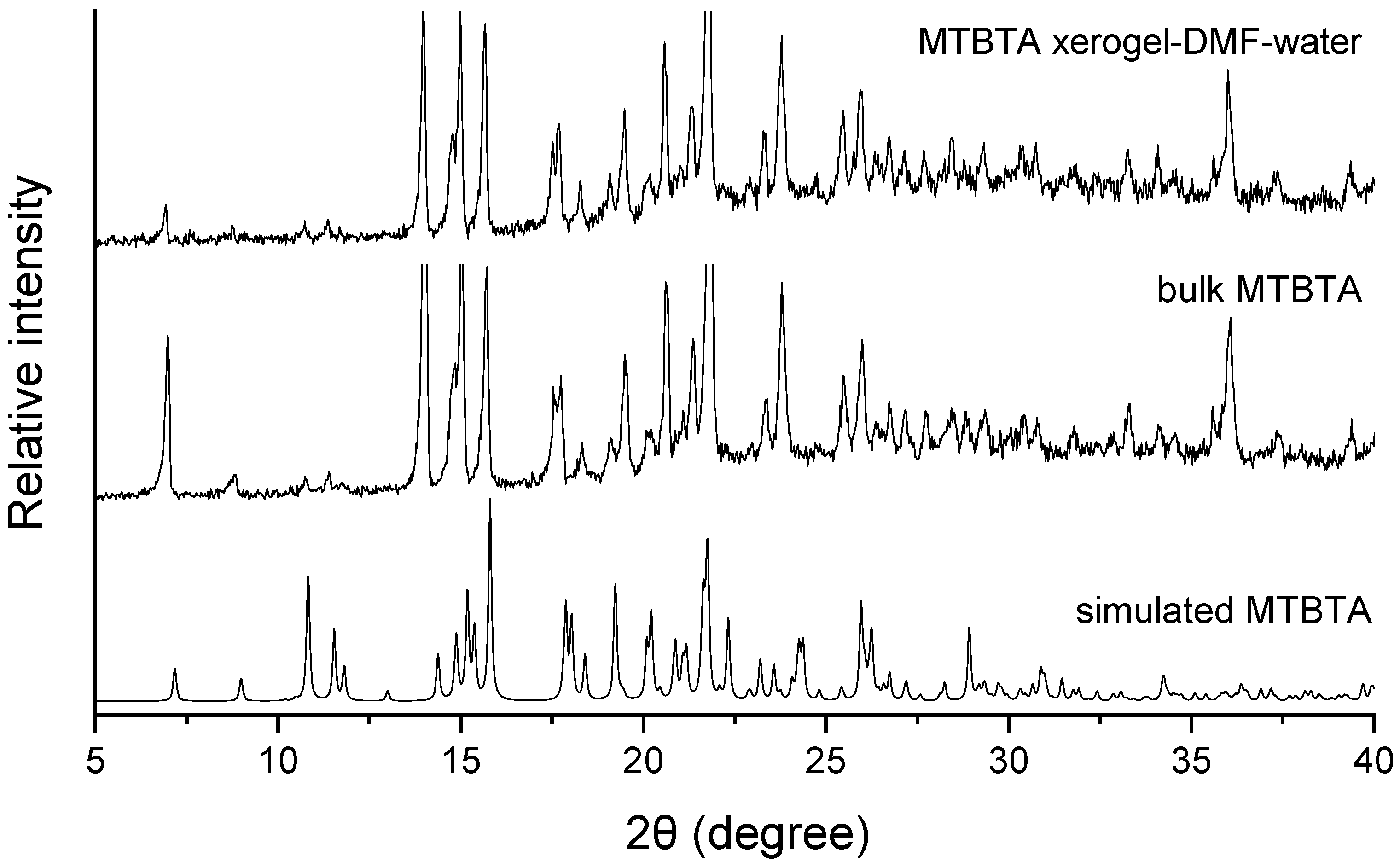
2.7. Powder X-ray Powder Diffraction (PXRD)
2.8. Infrared Spectroscopy
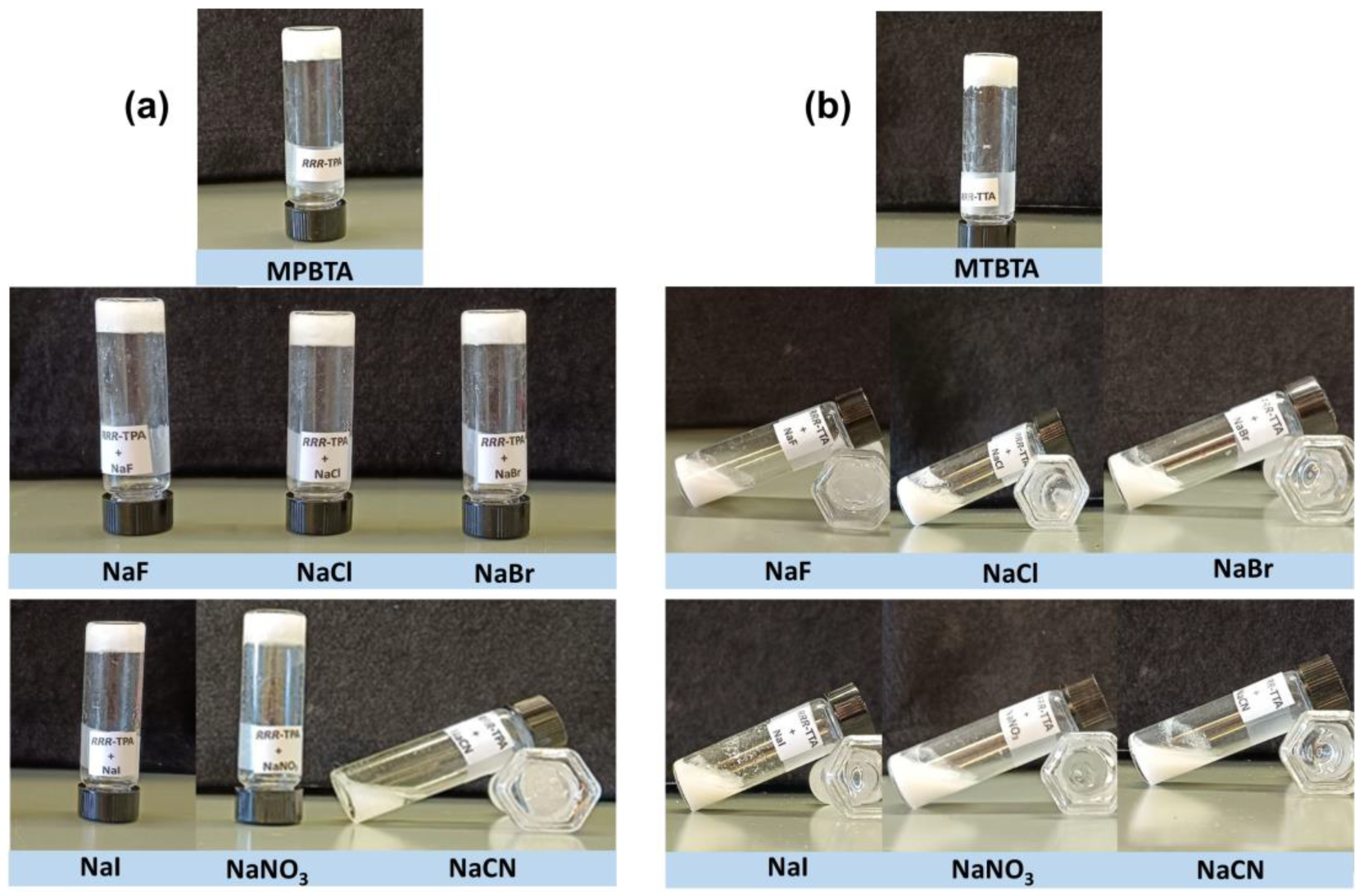
2.9. Stimuli-Responsive Properties
3. Conclusions
4. Materials and Methods
4.1. Synthesis of Ligands
4.1.1. Synthesis of R,R,R-benzene-1,3,5-tricarboxamide of Phenylalanine Methyl Ester (MPBTA)
4.1.2. Synthesis of R-tyrosine Methyl Ester Hydrochloride
4.1.3. Synthesis of R,R,R-benzene-1,3,5-tricarboxamide of Tyrosine Methyl Ester (MTBTA)
4.2. Circular Dichroism (CD)
4.3. Gelation Studies
4.3.1. Minimum Gelator Concentration (MGC)
4.3.2. Tgel Experiments
4.4. Rheology
4.5. Scanning Electron Microscopy (SEM)
4.6. Single-Crystal X-ray Diffraction (SCXRD)
4.7. Powder X-ray Diffraction
4.8. Infrared Spectroscopy
4.9. Stimuli-Responsive Properties
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Loos, M.; Feringa, B.L.; van Esch, J.H. Design and Application of Self-Assembled Low Molecular Weight Hydrogels. Eur. J. Org. Chem. 2005, 2005, 3615–3631. [Google Scholar] [CrossRef]
- Kumar, D.K.; Steed, J.W. Supramolecular gel phase crystallization: Orthogonal self-assembly under non-equilibrium conditions. Chem. Soc. Rev. 2014, 43, 2080–2088. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.K. Supramolecular gels—A panorama of low-molecular-weight gelators from ancient origins to next-generation technologies. Soft Matter 2024, 20, 10–70. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.J. Personal Perspective on Understanding Low Molecular Weight Gels. J. Am. Chem. Soc. 2022, 144, 11047–11053. [Google Scholar] [CrossRef]
- Jones, C.D.; Steed, J.W. Gels with sense: Supramolecular materials that respond to heat, light and sound. Chem. Soc. Rev. 2016, 45, 6546–6596. [Google Scholar] [CrossRef]
- Li, L.; Sun, R.; Zheng, R.; Huang, Y. Anions-responsive supramolecular gels: A review. Mater. Des. 2021, 205, 109759. [Google Scholar] [CrossRef]
- Panja, S.; Adams, D.J. Stimuli responsive dynamic transformations in supramolecular gels. Chem. Soc. Rev. 2021, 50, 5165–5200. [Google Scholar] [CrossRef]
- Chu, C.-W.; Schalley, C.A. Recent Advances on Supramolecular Gels: From Stimuli-Responsive Gels to Co-Assembled and Self-Sorted Systems. Org. Mater. 2021, 03, 025–040. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, G.; Zhang, D. Stimuli responsive gels based on low molecular weight gelators. J. Mater. Chem. 2012, 22, 38–50. [Google Scholar] [CrossRef]
- Lloyd, G.O.; Steed, J.W. Anion-tuning of supramolecular gel properties. Nat. Chem. 2009, 1, 437–442. [Google Scholar] [CrossRef]
- Yu, G.; Yan, X.; Han, C.; Huang, F. Characterization of supramolecular gels. Chem. Soc. Rev. 2013, 42, 6697–6722. [Google Scholar] [CrossRef] [PubMed]
- Draper, E.R.; Adams, D.J. Low-Molecular-Weight Gels: The State of the Art. Chem 2017, 3, 390–410. [Google Scholar] [CrossRef]
- Terech, P.; Weiss, R.G. Molecular Gels: Materials with Self-Assembled Fibrillar Networks; Springer: Dordrecht, The Netherlands, 2006. [Google Scholar]
- Fages, F.; Vögtle, F.; Žinic, M. Systematic Design of Amide- and Urea-Type Gelators with Tailored Properties. In Low Molecular Mass Gelator; Springer: Berlin/Heidelberg, Germany, 2005; pp. 77–131. [Google Scholar]
- Moulin, E.; Armao, J.J.; Giuseppone, N. Triarylamine-Based Supramolecular Polymers: Structures, Dynamics, and Functions. Acc. Chem. Res. 2019, 52, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; de Kruijff, R.M.; Lovrak, M.; Guo, X.; Eelkema, R.; van Esch, J.H. Access to Metastable Gel States Using Seeded Self-Assembly of Low-Molecular-Weight Gelators. Angew. Chem. Int. Ed. 2019, 58, 3800–3803. [Google Scholar] [CrossRef]
- Ghosh, D.; Farahani, A.D.; Martin, A.D.; Thordarson, P.; Damodaran, K.K. Unraveling the Self-Assembly Modes in Multicomponent Supramolecular Gels Using Single-Crystal X-ray Diffraction. Chem. Mater. 2020, 32, 3517–3527. [Google Scholar] [CrossRef]
- Kuppadakkath, G.; Jayabhavan, S.S.; Damodaran, K.K. Supramolecular Gels Based on C3-Symmetric Amides: Application in Anion-Sensing and Removal of Dyes from Water. Molecules 2024, 29, 2149. [Google Scholar] [CrossRef]
- Dastidar, P. Supramolecular gelling agents: Can they be designed? Chem. Soc. Rev. 2008, 37, 2699–2715. [Google Scholar] [CrossRef]
- Estroff, L.A.; Hamilton, A.D. Water Gelation by Small Organic Molecules. Chem. Rev. 2004, 104, 1201–1218. [Google Scholar] [CrossRef]
- Ghosh, D.; Chaudhary, P.; Pradeep, A.; Singh, S.; Rangasamy, J.; Damodaran, K.K. Structural modification induced hydrogelation and antibacterial properties in supramolecular gels. J. Mol. Liq. 2023, 382, 122023. [Google Scholar] [CrossRef]
- Jayabhavan, S.S.; Kristinsson, B.; Ghosh, D.; Breton, C.; Damodaran, K.K. Stimuli-Responsive Properties of Supramolecular Gels Based on Pyridyl-N-oxide Amides. Gels 2023, 9, 89. [Google Scholar] [CrossRef]
- Sudhakaran Jayabhavan, S.; Ghosh, D.; Damodaran, K.K. Making and Breaking of Gels: Stimuli-Responsive Properties of Bis(Pyridyl-N-oxide Urea) Gelators. Molecules 2021, 26, 6420. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Máñez, R.; Sancenón, F. Fluorogenic and Chromogenic Chemosensors and Reagents for Anions. Chem. Rev. 2003, 103, 4419–4476. [Google Scholar] [CrossRef] [PubMed]
- Bowman-James, K. Alfred Werner Revisited: The Coordination Chemistry of Anions. Acc. Chem. Res. 2005, 38, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Gunnlaugsson, T.; Glynn, M.; Tocci, G.M.; Kruger, P.E.; Pfeffer, F.M. Anion recognition and sensing in organic and aqueous media using luminescent and colorimetric sensors. Coord. Chem. Rev. 2006, 250, 3094–3117. [Google Scholar] [CrossRef]
- Gale, P.A.; García-Garrido, S.E.; Garric, J. Anion receptors based on organic frameworks: Highlights from 2005 and 2006. Chem. Soc. Rev. 2008, 37, 151–190. [Google Scholar] [CrossRef]
- Jose, D.A.; Kumar, D.K.; Ganguly, B.; Das, A. Rugby-Ball-Shaped Sulfate–Water–Sulfate Adduct Encapsulated in a Neutral Molecular Receptor Capsule. Inorg. Chem. 2007, 46, 5817–5819. [Google Scholar] [CrossRef]
- Beer, P.D.; Gale, P.A. Anion Recognition and Sensing: The State of the Art and Future Perspectives. Angew. Chem. Int. Ed. 2001, 40, 486–516. [Google Scholar] [CrossRef]
- Rahmati, N.; Hoebeek, F.E.; Peter, S.; De Zeeuw, C.I. Chloride Homeostasis in Neurons With Special Emphasis on the Olivocerebellar System: Differential Roles for Transporters and Channels. Front. Cell. Neurosci. 2018, 12, 101. [Google Scholar] [CrossRef]
- Şan; Dey, A.K.; Giri, B. Fluoride Fact on Human Health and Health Problems: A Review. Med. Clin. Rev. 2016, 2, 1–6. [Google Scholar]
- Hendry-Hofer, T.B.; Ng, P.C.; Witeof, A.E.; Mahon, S.B.; Brenner, M.; Boss, G.R.; Bebarta, V.S. A Review on Ingested Cyanide: Risks, Clinical Presentation, Diagnostics, and Treatment Challenges. J. Med. Toxicol. 2019, 15, 128–133. [Google Scholar] [CrossRef]
- Moss, B. Water pollution by agriculture. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2008, 363, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Evans, N.H.; Beer, P.D. Advances in Anion Supramolecular Chemistry: From Recognition to Chemical Applications. Angew. Chem. Int. Ed. 2014, 53, 11716–11754. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.G.; Littlejohn, D.; Hu, K.Y. Disulfate Ion as an Intermediate to Sulfuric Acid in Acid Rain Formation. Science 1987, 237, 756–758. [Google Scholar] [CrossRef] [PubMed]
- Busschaert, N.; Caltagirone, C.; Van Rossom, W.; Gale, P.A. Applications of Supramolecular Anion Recognition. Chem. Rev. 2015, 115, 8038–8155. [Google Scholar] [CrossRef]
- Panja, A.; Ghosh, K. Pyridylazo Derivatives with Dicyanovinyl Appendage in Selective Sensing of CN− in Sol-Gel Medium. ChemistrySelect 2018, 3, 1809–1814. [Google Scholar] [CrossRef]
- Desiraju, G.R. Supramolecular Synthons in Crystal Engineering—A New Organic Synthesis. Angew. Chem. Int. Ed. Engl. 1995, 34, 2311–2327. [Google Scholar] [CrossRef]
- Sudhakaran Jayabhavan, S.; Kuppadakkath, G.; Damodaran, K.K. The Role of Functional Groups in Tuning the Self-Assembly Modes and Physical Properties of Multicomponent Gels. ChemPlusChem 2023, 88, e202300302. [Google Scholar] [CrossRef]
- Bondy, C.R.; Loeb, S.J. Amide based receptors for anions. Coord. Chem. Rev. 2003, 240, 77–99. [Google Scholar] [CrossRef]
- Molina, P.; Zapata, F.; Caballero, A. Anion Recognition Strategies Based on Combined Noncovalent Interactions. Chem. Rev. 2017, 117, 9907–9972. [Google Scholar] [CrossRef]
- Ishioka, Y.; Minakuchi, N.; Mizuhata, M.; Maruyama, T. Supramolecular gelators based on benzenetricarboxamides for ionic liquids. Soft Matter 2014, 10, 965–971. [Google Scholar] [CrossRef]
- Panja, S.; Panja, A.; Ghosh, K. Supramolecular gels in cyanide sensing: A review. Mater. Chem. Front. 2021, 5, 584–602. [Google Scholar] [CrossRef]
- Aletti, A.B.; Blasco, S.; Aramballi, S.J.; Kruger, P.E.; Gunnlaugsson, T. Sulfate-Templated 2D Anion-Layered Supramolecular Self-Assemblies. Chem 2019, 5, 2617–2629. [Google Scholar] [CrossRef]
- Ghosh, A.; Das, P.; Kaushik, R.; Damodaran, K.K.; Jose, D.A. Anion responsive and morphology tunable tripodal gelators. RSC Adv. 2016, 6, 83303–83311. [Google Scholar] [CrossRef]
- Malviya, N.; Das, M.; Mandal, P.; Mukhopadhyay, S. A smart organic gel template as metal cation and inorganic anion sensor. Soft Matter 2017, 13, 6243–6249. [Google Scholar] [CrossRef]
- Liu, J.; Yang, H.-L.; Sun, X.-W.; Zhang, Y.-M.; Yao, H.; Wei, T.-B.; Lin, Q. A simple pillar[5]arene assembled multi-functional material with ultrasensitive sensing, self-healing, conductivity and host–guest stimuli-responsive properties. Soft Matter 2021, 17, 8308–8313. [Google Scholar] [CrossRef]
- Zhao, Q.; Dai, X.-Y.; Yao, H.; Zhang, Y.-M.; Qu, W.-J.; Lin, Q.; Wei, T.-B. Stimuli-responsive supramolecular hydrogel with white AIE effect for ultrasensitive detection of Fe3+ and as rewritable fluorescent materials. Dye. Pigments 2021, 184, 108875. [Google Scholar] [CrossRef]
- de Windt, L.N.J.; Fernández, Z.; Fernández-Míguez, M.; Freire, F.; Palmans, A.R.A. Elucidating the Supramolecular Copolymerization of N- and C-Centered Benzene-1,3,5-Tricarboxamides: The Role of Parallel and Antiparallel Packing of Amide Groups in the Copolymer Microstructure. Chem. Eur. J. 2022, 28, e202103691. [Google Scholar] [CrossRef]
- Gudmundsson, T.A.; Kuppadakkath, G.; Ghosh, D.; Ruether, M.; Seddon, A.; Ginesi, R.E.; Doutch, J.; Adams, D.J.; Gunnlaugsson, T.; Damodaran, K.K. Nanoscale assembly of enantiomeric supramolecular gels driven by the nature of solvents. Nanoscale 2024, 16, 8922–8930. [Google Scholar] [CrossRef]
- Prasad, K. Rheology for Chemists—An Introduction. Appl. Rheol. 2019, 16, 69. [Google Scholar] [CrossRef]
- Guenet, J.-M. Organogels: Thermodynamics, Structure, Solvent Role, and Properties; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Adams, D.J. Does Drying Affect Gel Networks? Gels 2018, 4, 32. [Google Scholar] [CrossRef]
- Srinivasulu, G.; Sridhar, B.; Ravi Kumar, K.; Sreedhar, B.; Ramesh, V.; Srinivas, R.; Kunwar, A.C. Molecular self assembly of benzene-1,3,5-tricarbonyl phenylalanine. J. Mol. Struct. 2011, 1006, 180–184. [Google Scholar] [CrossRef]
- Jana, P.; Paikar, A.; Bera, S.; Maity, S.K.; Haldar, D. Porous Organic Material from Discotic Tricarboxyamide: Side Chain–Core interactions. Org. Lett. 2014, 16, 38–41. [Google Scholar] [CrossRef] [PubMed]
- Fornaro, T.; Burini, D.; Biczysko, M.; Barone, V. Hydrogen-Bonding Effects on Infrared Spectra from Anharmonic Computations: Uracil–Water Complexes and Uracil Dimers. J. Phys. Chem. A 2015, 119, 4224–4236. [Google Scholar] [CrossRef] [PubMed]
- Abul-Haija, Y.M.; Roy, S.; Frederix, P.W.J.M.; Javid, N.; Jayawarna, V.; Ulijn, R.V. Biocatalytically Triggered Co-Assembly of Two-Component Core/Shell Nanofibers. Small 2014, 10, 973–979. [Google Scholar] [CrossRef]
- Chevigny, R.; Sitsanidis, E.D.; Schirmer, J.; Hulkko, E.; Myllyperkiö, P.; Nissinen, M.; Pettersson, M. Nanoscale Probing of the Supramolecular Assembly in a Two-Component Gel by Near-Field Infrared Spectroscopy. Chem. Eur. J. 2023, 29, e202300155. [Google Scholar] [CrossRef]
- Nebot, V.J.; Armengol, J.; Smets, J.; Prieto, S.F.; Escuder, B.; Miravet, J.F. Molecular Hydrogels from Bolaform Amino Acid Derivatives: A Structure–Properties Study Based on the Thermodynamics of Gel Solubilization. Chem. Eur. J. 2012, 18, 4063–4072. [Google Scholar] [CrossRef]
- Morishita, Y.; Kaino, T.; Okamoto, R.; Izumi, M.; Kajihara, Y. Synthesis of D,L-amino acid derivatives bearing a thiol at the β-position and their enzymatic optical resolution. Tetrahedron Lett. 2015, 56, 6565–6568. [Google Scholar] [CrossRef]
| Solvent | MPBTA | MTBTA | ||
|---|---|---|---|---|
| MGC | Tgel | MGC | Tgel | |
| DMF/H2O (1:1, v/v) | 1.4 | 93.3 | 4.5 | 69.2 |
| DMSO/H2O (1:1, v/v) | 0.7 | 119.4 | Crystals * | - |
| p-xylene | 1.0 | 121.6 | Insoluble | - |
| ethanol | 4.0 | 67.8 | Solution ** | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuppadakkath, G.; Volkova, I.; Damodaran, K.K. Designing Stimuli-Responsive Supramolecular Gels by Tuning the Non-Covalent Interactions of the Functional Groups. Gels 2024, 10, 584. https://doi.org/10.3390/gels10090584
Kuppadakkath G, Volkova I, Damodaran KK. Designing Stimuli-Responsive Supramolecular Gels by Tuning the Non-Covalent Interactions of the Functional Groups. Gels. 2024; 10(9):584. https://doi.org/10.3390/gels10090584
Chicago/Turabian StyleKuppadakkath, Geethanjali, Ira Volkova, and Krishna K. Damodaran. 2024. "Designing Stimuli-Responsive Supramolecular Gels by Tuning the Non-Covalent Interactions of the Functional Groups" Gels 10, no. 9: 584. https://doi.org/10.3390/gels10090584
APA StyleKuppadakkath, G., Volkova, I., & Damodaran, K. K. (2024). Designing Stimuli-Responsive Supramolecular Gels by Tuning the Non-Covalent Interactions of the Functional Groups. Gels, 10(9), 584. https://doi.org/10.3390/gels10090584







