Abstract
Dermatophytes are keratinophilic fungi that cause a wide range of superficial infections in humans and animals. The Trichophyton mentagrophytes species complex is one of the most clinically important groups due to its broad host range, widespread distribution, and increasing involvement in antifungal-resistant infections. Here, we described the epidemiology of T. mentagrophytes over a period of 4 years detected in the northeastern part of Italy and provided the genomic characterization of clinical isolates. ITS sequence analysis revealed that among the 13 strains studied, 11 belonged to the T. mentagrophytes complex. In detail, nine were classified as genotype I/II and two as genotype VII. Analysis of the SQLE gene revealed that nine strains harbored a wild-type gene, while two carried a Lys276Asn mutation. Genomic analysis was performed on three clinical T. mentagrophytes strains that belonged to genotype I/II, revealing the presence of different virulence factors including MEP-1, MEP-2, MEP-3, and MEP-5. Phylogenetic analysis based on core-genome SNPs demonstrated that the two genomes included in this study were clonally related to a T. mentagrophytes strain isolated in China in 2024. In conclusion, our study highlights the importance of genomic characterization in order to trace the epidemiology of dermatophytes worldwide and to characterize emerging strains.
1. Introduction
In the last few decades, antifungal resistance has become an expanding and increasingly significant issue [1]. Pathogenic fungi, responsible for both invasive and superficial infections, are progressively developing resistance to various classes of antifungal drugs [2].
Dermatophytes, a group of keratinophilic fungi, are among the most common causative agents of superficial fungal infections [3,4]. Among different species, the most common etiological agents of dermatophytosis in human are Trichophyton rubrum, Trichophyton interdigitale and Trichophyton mentagrophytes, which are able to colonize keratinized tissues such as skin, hair, and nails [5,6].
Recently, emergence cases of T. mentagrophytes resistant to antifungal molecules have been reported in different countries [2,7]. In particular, different cases of dermatophytosis infections due to terbinafine-resistant T. mentagrophytes were reported in India. In this context, genotypic analysis demonstrated that clinical isolates belonged to genotype VIII, which was further named as T. indotineae by Kano et al. [2,7,8].
In the last few years, T. indotineae has become a leading cause of widespread and difficult-to-treat skin infections especially in India, where it has driven an epidemic of extensive dermatophytosis [1,9]. In particular, epidemiological and molecular studies have shown that T. indotineae initially spread in Asia, showing a gradually replacing Trichophyton rubrum as the predominant endemic dermatophyte. Currently, it has become the most widespread dermatophyte in different countries [1,2,3,4,5,6,7,8,9]. Due to its microscopic and macroscopic similarity to other species of the T. mentagrophytes complex, accurately identifying T. indotineae requires advanced molecular tests, such as the sequencing of ITS regions. T. indotineae exhibits commonly resistance to terbinafine, which is a first-line topical and systemic antifungal agent used to treat dermatophytic infections. It acts by inhibiting the enzyme squalene epoxidase, disrupting ergosterol biosynthesis, a vital component of fungal cell membranes, and leading to the toxic intracellular accumulation of squalene [9,10]. Although different studies reported the enlarging widespread of T. indotinae in different continents, few epidemiological data are available in Italy [1,2,3,4,5,6,7,8,9].
Based on these considerations, the aim of the present study was to characterize the T. mentagrophytes strains collected in a hospital located in the northeastern part of Italy over a period of 4 years and to evaluate the susceptibility to terbinafine and the related genetic mechanism of resistance. At the same time, we characterized the genomes of different clinical strains, the object of this study, in order to enlarge the knowledge of the T. mentagrophytes epidemiology.
2. Material and Methods
2.1. Clinical Isolates
Between 1 January 2020 and 31 December 2024, we collected all fungal strains from clinical samples of nail scales, skin scales, and hair from patients with superficial mycosis who were admitted to a large tertiary hospital located in the northeastern part of Italy. Species identification was initially performed by fungal culture and based on morphological examination of the colonies and following microscopic examination morphological characteristics of the fungal colonies following the routine workflow.
2.2. Evaluation of Terbinafine Sensitivity
To evaluate the sensitivity of the studied strains to terbinafine, the TCAM (Terbinafine Containing Agar Method) was used, which involves Sabouraud Dextrose Agar (SDA) enriched with terbinafine at a concentration of 0.2 mg/L [5,11]. The medium was prepared following these steps: 500 mL of distilled water was measured using a cylinder, and 15 g of Sabouraud Dextrose Liquid Medium (Thermo Scientific™ Oxoid™ Sabouraud Dextrose Broth, Waltham, MA, USA) was added to the water along with 7.5 g of Bacteriological Agar Type E (Microbiol Diagnostici, Macchiareddu, Italy). The mixture was sterilized in an autoclave at 121 °C for 15 min. After sterilization, the medium was allowed to cool to 50–60 °C, and 15 mL was poured into each Petri dish. Half of the plates were enriched with terbinafine to achieve a final concentration of 0.2 mg/L, while the other half were left as growth controls. Each strain was inoculated on both a TCAM plate and an SDA control plate without terbinafine and incubated at room temperature. Growth was evaluated after 7 and 14 days [5,11].
2.3. Molecular Identification of ITS Region and SQLE Gene of Clinical Isolates
DNA from the fungal colonies was extracted according to the manufacturer’s protocol using the InGenius system (Elitech, Signes, France). The identification of isolates from the T. mentagrophytes complex was performed at both the species and genotype level by PCR sequencing of the ribosomal DNA (rDNA) internal transcribed spacer (ITS), as previously described [12,13]. Terbinafine resistance was investigated by amplification of the SQLE gene by using TrSQLE-F1 (5′-ATGGTTGTAGAGGCTCCTCCC-3′) and TrSQLE-R1 (5′-CTAGCTTTGAAGTTCGGCAAA-3′) primers and related amplification settings as previously described [12]. The amplification products of the ITS regions and SQLE gene were sequenced using the Sanger method, and multiple sequence alignment was performed with ClustalOmega v1.2.4 and visualized with Unipro UGENE v49.1. Phylogenetic analysis was performed with MEGA v11.0.11 (available at: https://www.megasoftware.net; accessed on 10 January 2025) using the iterative Neighbor-Joining (NJ) algorithm based on the distance matrix. The list of T. mentagrophytes strains used for phylogenetic analysis based on the ITS sequence is shown in Table 1.

Table 1.
List of T. mentagrophytes strains used for the phylogenic analysis based on the ITS sequences.
2.4. Genomic Characterization of Clinical Isolates
Genomic DNA extraction, purification, quantification and library preparation were performed as previously described [13]. Briefly, DNA sequencing was conducted on the Illumina MiSeq system (Illumina, San Diego, CA, USA). The obtained paired-end reads underwent quality control with FastQC v0.12.1 (available at: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/; accessed on 10 January 2025), and adapters were trimmed with Trimmomatic v0.39 (available at: https://github.com/usadellab/Trimmomatic; accessed on 10 January 2025). Prior to proceeding with the genome assembly, reads were filtered to remove any contaminant sequences, as previously described [14]. Briefly, Illumina reads were mapped against the reference genome of the T. mentagrophytes ATCC18748 (Acc. no. BPUJ00000000.1) using Burrows–Wheeler Aligner (BWA) v.0.7.18 software. Mapped reads were extracted with Samtools 1.6 and used for the de novo genome assembly with SPAdes v4.0.0 using careful settings. Assemblies were annotated by using AUGUSTUS (available at: https://bioinf.uni-greifswald.de/augustus/; accessed on 10 January 2025), and gene prediction was manually investigated by BLAST analysis. Genes involved in virulence were detected using the PHI-base (Pathogen-Host Interaction) database by BLAST analysis.
The T. mentagrophytes genomes available in GenBank and used for the molecular epidemiological analysis are shown in Table 2. A phylogenetic tree based on core genome single nucleotide polymorphisms (SNPs) was performed using ParSNP software (available at: https://harvest.readthedocs.io/en/latest/content/parsnp.html; accessed on 10 January 2025) using the genome of T. mentagrophytes ATCC 18748 as a reference. In addition, ParSNP analysis was performed using the settings “-c”, “-a 13” and “-x” for all of the genomes, higher resolution mapping and the exclusion of SNPs located in regions of recombination. A maximum likelihood tree was constructed from the final alignment of core-genome SNPs using FastTree with generalized time-reversible mode.

Table 2.
List of the T. mentagrophytes genomes used for the phylogenomic analysis.
3. Results
3.1. Phenotypic and Genotypic Characterization of Dermatophytes Clinical Strains
During the study period, we isolated a total of 13 dermatophytes collected from different types of skin samples by culturing and macro and microscopic examination. The list of strains included in this study is shown in Table 3. In order to characterize genotypically the dermatophytes isolates included in this study and due to the impossibility of distinguishing between genotypes belonging to the T. mentagrophytes complex, the ITS rDNA were sequenced and compared to ITS regions derived from T. mentagrophytes strains belonging to different genotypes present in GenBank (Table 1). ITS analysis demonstrated that 1 out of 13 dermatophytes belonged to the T. verrucosum species, while 11 out of 13 clinical strains included in this study belonged to the T. mentagrophytes complex. Beauveria bassiana was excluded from the study by not belonging to the dermatophytes. Phylogenetic analysis based on the ITS region of the 11 isolates genotypically characterized as T. mentagrophytes showed that 9 (81.8%) strains belonged to genotype I/II, while 2 (18.2%) belonged to genotype VII (Figure 1).

Table 3.
Characteristics of the T. mentagrophytes clinical strains included in this study.
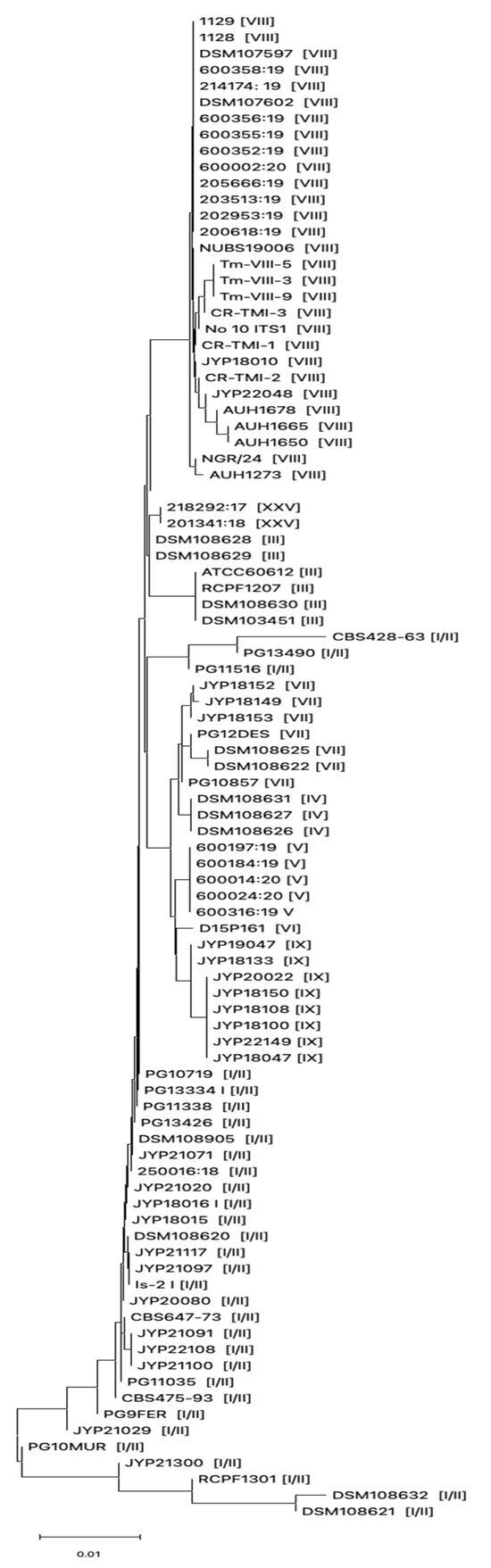
Figure 1.
Evolutionary history inferred using Neighbor-Joining method based on ITS sequences derived from T. mentagrophytes strains collected in this study and from different countries. Genotype for each strain is shown within the brackets.
Antifungal susceptibility testing results showed that all T. mentagrophytes strains included in this study were susceptible to terbinafine.
Genotypic characterization revealed that 9 out of 11 T. mentagrophytes clinical strains harbored the wild-type SQLE gene, while two strains harbored a Lysin for Asparagine substitution at position 276 (Table 3). Correlation with ITS genotypic results revealed that strains harboring the Lys276Asn substitution belonged to the T. mentagrophytes genotype I/II.
3.2. Genomic Characterization of T. mentagrophytes Genotype I/II
In order to characterize the T. mentagrophytes isolates included in this study, we performed the whole-genome sequencing (WGS) of selected strains included in this study (PG9FER, PG10MUR and PG11338).
WGS produced a total of 18,518,774, 13,421,006 and 13,838,658 Illumina reads 300 bp in length, respectively, for PG9FER, PG10MUR and PG11338 clinical strains.
Genome assemblies of PG9FER, PG10MUR and PG11338 generated, respectively, a total of 615, 679 and 712 contigs with a G + C content of 47.98, 48 and 47.52. The characteristics of the T. mentagrophytes genomes included in this study are shown in Table 4. Assemblies gave a total of 7.1924 (PG9FER), 7.140 (PG10MUR) and 7.140 (PG11338) of protein-coding DNA sequences (CDSs), thus showing a similar number of CDSs observed in the available T. mentagrophytes genomes previously annotated (Table 2).

Table 4.
Genomic characteristics of the T. mentagrophytes genomes included in this study.
A genomic analysis of T. mentagrophytes genomes obtained in this study showed that different virulence factors (i.e., metalloproteinase, MEP genes) were present (Figure S1 in the Supplementary Materials). In detail, the following virulence factors (MEP-1, MEP-2, MEP-3 and MEP-5) were present in the genomes of PG9FER, PG10MUR and PG11338, while MEP-4 was present in the complete form only in the PG9FER strain, and no isolates harbored complete ZAF-A (Table 5).

Table 5.
Aminoacid substitutions on virulence factors within T. mentagrophytes genomes included in this study.
To investigate the clonal relatedness of T. mentagrophytes collected in our study with the clinical available genomes isolated worldwide, phylogenetic analysis based on core genome SNPs was performed. A list of the selected genomes collected in this study and the T. mentagrophytes genomes available in GenBank and used for the genomic comparison is shown in Table 2. A phylogenetic tree demonstrated that all T. mentagrophytes genomes included in this study grouped into a monophyletic clade formed by isolates belonging to different genotypes, while T. mentagrophytes genomes belonging to genotype VIII (defined as T. indotinae) clustered separately (Figure 2). In detail, PG9FER and PG10MUR clustered closely to the LL-2024a clinical isolate, which was a strain isolated in China during 2024 from a patient with skin lesions. At the same time, PG11338 clustered separately to the genomes of PG9FER and PG10MUR strains, thus demonstrating a divergent evolution of strains belonging to this genotype. Of note, our phylogenetic analysis based on core-genome SNPs (Figure 2) revealed that the PG12DES strain, which belongs to the emergent genotype VII, was closely related to the D15P156 (NCBI GenBank GCA_003664385.1), which is a T. mentagrophytes strain collected in Moldova in 2017 from a patient suffering from tinea capitis who had contact with a rabbit.

Figure 2.
Maximum likelihood phylogeny based on SNPs in the core genomes of T. mentagrophytes. The T. mentagrophytes clinical isolates included in this study are highlighted in red, while T. mentagrophytes belonging to genotype VII recently isolated in Italy were highlighted in blue. The genotype for each strain is shown within the brackets. Abbreviations: NA, Not applicable.
A deep genome comparison with ATCC18748 and clonally related genomes (i.e., D15P152, LL-2024a) showed that PG9FER, used as a reference for comparison, exhibited OrthoANIu values of 98.2% (PG9FER vs. ATCC18748), 99.6% (PG9FER vs. D15P152), 99.94% (PG9FER vs. LL-2024a), 99.92% (PG9FER vs. PG10MUR) and 98.99% (PG9FER vs. PG11338) with an average aligned length (bp) of 16,812,337 bp. At the same time, genome comparison analysis showed that PG12DES differed by 4141 SNPs in comparison, respectively, to the D15P156 strain and showed an OrthoANIu value of 99.83% with an average aligned length (bp) of 17,009,500 bp.
4. Discussion
In this study, we described the epidemiology of T. mentagrophytes strains collected in a hospital located in the northeastern part of Italy over a period of 4 years. Our results demonstrated that the T. mentagrophytes included in this study belonged to genotypes I/II and VII, thus showing the endemic circulation of few genotypes among patients in the northeastern part of Italy. Although the number of patients included in this study was small, our results are in agreement with a previous study showing that genotype I/II was the most prevalent genotypes distributed worldwide [15], while T. mentagrophytes type VII (TMVII) was a recently emerged dermatophyte causing different outbreaks in different countries in Europe (i.e., Germany and France) and more recently in the USA [16,17,18,19]. This emerging genotype is closely related to T. indotineae, and it has been hypothesized to be transmitted from human to human through sexual contact with an increasing risk of infections among groups engaging in high-risk sexual behaviors, such as men who have sex with men (MSM) [16,17,18,19]. However, the PG12DES strain belonging to genotype VII was isolated from a clinical sample of skin scale located around the eyes of a young child in 2022, thus suggesting a larger spreading of genotype VII worldwide. Based on these findings, our data reinforced the importance of monitoring the spread of clinical strains in order to provide a complete picture T. mentagrophytes epidemiology and to characterize emerging genotypes worldwide. In particular, further genomic and/or genotypic studies need to be perform to better define the epidemiological diffusion of different genotypes among T. mentagrophytes in Italy.
Antifungal susceptibility analysis demonstrated that all strains were susceptible to terbinafine, and no mutations within the SQLE gene were observed in strains included in this study. In this context, the resistance to terbinafine diminishes the efficacy of available treatments, complicating clinical management and posing significant challenges for healthcare systems. Indeed, resistant fungal infections often lead to prolonged treatment durations, higher healthcare costs, and an increased risk of complications, underscoring their impact on public health [2].
The spread of antifungal resistance represents an escalating global health concern, thus limiting the therapeutic options available and made the management of fungal infections increasingly complex [1]. Indeed, the resistance has significant implications, requiring extended and more complex treatment regimens, which impact healthcare systems and the quality of life of affected patients.
Lastly, here, we provided the complete genome analysis of three T. mentagrophytes strains belonging to type I/II. Our results demonstrated that different virulence factors were conserved among our isolates (MEP-1, MEP-2, MEP-3 and MEP5), while ZafA was truncated in all strains. Genomic phylogenetic analysis revealed that the genomes of T. mentagrophytes included in this study were closely related to a clinical strain recently isolated in China, thus suggesting a high conservation degree of this genotype among dermatophytes. The high nucleotide homology of our strains with isolates collected from different countries indicated a high circulation of clonally related strains worldwide, requiring further genomic investigations
In conclusion, our study aims to provide a picture of the epidemiology of dermatophytosis in the northern part of Italy by providing a deep genome analysis of the common genotypes circulating in our region. Our study highlights the importance of monitoring the spread of dermatophytes in order to provide a complete view of the T. mentagrophytes epidemiology and to rapidly identify new genotypes or novel traits of resistance.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jof11080566/s1, Figure S1: Aminoacid aligment of virulence factors (MEP-1, panel A; MEP-2, panel B; MEP-3, panel C; MEP-4, panel D; MEP-5, panel E; ZafA, panel F) in the genomes of T. mentagrophytes genomes included in this study compared to reference alleles. Aminoacid variations were highlighted.
Author Contributions
Conceptualization, L.R., A.S. and P.G.; methodology, L.R. and A.S.; formal analysis, P.G.; investigation, A.S. and C.S.; data curation, L.R. and P.G.; writing—original draft preparation, L.R. and P.G.; writing—review and editing, C.S. and P.G. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Italian Ministry of Health (Ricerca Finalizzata, Giovani Ricercatori, GR-2019-12371428) and FUR2024 to Paolo Gaibani.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki, and ethical clearance is not required by Italian law for this type of study.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The draft genome assemblies of T. mentagrophytes strains have been deposited in the NCBI under the following accession numbers: JBLIVW000000000 (Biosample: SAMN46524735; PG9FER), JBLIVX000000000 (Biosample: SAMN46524736, PG10MUR), JBLIVY000000000 (Biosample: SAMN46524737, PG11338), and JBKIZU000000000 (Biosample: SAMN45682025, PG12DES).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lockhart, S.R.; Chowdhary, A.; Gold, J.A.W. The rapid emergence of antifungal-resistant human-pathogenic fungi. Nat. Rev. Microbiol. 2023, 21, 818–832. [Google Scholar] [CrossRef] [PubMed]
- Jabet, A.; Normand, A.C.; Brun, S.; Dannaoui, E.; Bachmeyer, C.; Piarroux, R.; Hennequin, C.; Moreno-Sabater, A. Trichophyton indotineae, from epidemiology to therapeutic. J. Mycol. Med. 2023, 33, 101383. [Google Scholar] [CrossRef] [PubMed]
- Deng, R.; Wang, X.; Li, R. Dermatophyte infection: From fungal pathogenicity to host immune responses. Front. Immunol. 2023, 14, 1285887. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; Long, X.; Hu, W.; Zhu, J.; Jiang, Y.; Ahmed, S.; de Hoog, G.S.; Liu, W.; Jiang, Y. The epidemic of the multiresistant dermatophyte Trichophyton indotineae has reached China. Front. Immunol. 2023, 13, 1113065. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Sabater, A.; Normand, A.C.; Bidaud, A.L.; Cremer, G.; Foulet, F.; Brun, S.; Bonnal, C.; Aït-Ammar, N.; Jabet, A.; Ayachi, A.; et al. Terbinafine Resistance in Dermatophytes: A French Multicenter Prospective Study. J. Fungi 2022, 8, 220. [Google Scholar] [CrossRef] [PubMed]
- Havlickova, B.; Czaika, V.A.; Friedrich, M. Epidemiological trends in skin mycoses worldwide. Mycose 2008, 51, 2–15. [Google Scholar] [CrossRef] [PubMed]
- Uhrlaß, S.; Verma, S.B.; Gräser, Y.; Rezaei-Matehkolaei, A.; Hatami, M.; Schaller, M.; Nenoff, P. Trichophyton indotineae-An Emerging Pathogen Causing Recalcitrant Dermatophytoses in India and Worldwide-A Multidimensional Perspective. J. Fungi 2022, 8, 757. [Google Scholar] [CrossRef] [PubMed]
- Kano, R.; Kimura, U.; Kakurai, M.; Hiruma, J.; Kamata, H.; Suga, Y.; Harada, K. Trichophyton indotineae sp. nov.: A new highly terbinafine-resistant anthropophilic dermatophyte species. Mycopathologia 2020, 185, 947–958. [Google Scholar] [CrossRef] [PubMed]
- Sonego, B.; Corio, A.; Mazzoletti, V.; Zerbato, V.; Benini, A.; di Meo, N.; Zalaudek, I.; Stinco, G.; Errichetti, E.; Zelin, E. Trichophyton indotineae, an Emerging Drug-Resistant Dermatophyte: A Review of the Treatment Options. J. Clin. Med. 2024, 13, 3558. [Google Scholar] [CrossRef] [PubMed]
- Kruithoff, C.; Gamal, A.; McCormick, T.S.; Ghannoum, M.A. Dermatophyte Infections Worldwide: Increase in Incidence and Associated Antifungal Resistance. Life 2023, 14, 1. [Google Scholar] [CrossRef] [PubMed]
- Bidaud, A.L.; Normand, A.C.; Jabet, A.; Brun, S.; Delliere, S.; Cremer, G.; Foulet, F.; Ayachi, A.; Imbert, S.; Hennequin, C.; et al. Reliability of a terbinafine agar containing method for the screening of dermatophyte resistance. Med. Mycol. 2023, 61, myad043. [Google Scholar] [CrossRef] [PubMed]
- Taghipour, S.; Shamsizadeh, F.; Pchelin, I.M.; Rezaei-Matehhkolaei, A.; Zarei Mahmoudabadi, A.; Valadan, R.; Ansari, S.; Katiraee, F.; Pakshir, K.; Zomorodian, K.; et al. Emergence of Terbinafine Resistant Trichophyton mentagrophytes in Iran, Harboring Mutations in the Squalene Epoxidase (SQLE) Gene. Infect. Drug Resist. 2020, 13, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Amadesi, S.; Amedeo, A.; Rinaldi, M.; Palombo, M.; Giannella, M.; Gaibani, P. In vivo emergence of cefiderocol and ceftazidime/avibactam cross-resistance in KPC- producing Klebsiella pneumoniae following ceftazidime/avibactam -based therapies. Diagn. Microbiol. Infect. Dis. 2024, 110, 116372. [Google Scholar] [CrossRef] [PubMed]
- Marangoni, A.; Amadesi, S.; Djusse, M.E.; Foschi, C.; Gaspari, V.; Lazzarotto, T.; Gaibani, P. Whole Genome Sequencing of a Chlamydia trachomatis Strain Responsible for a Case of Rectal Lymphogranuloma Venereum in Italy. Curr. Issues Mol. Biol. 2023, 45, 1852–1859. [Google Scholar] [CrossRef] [PubMed]
- Taghipour, S.; Pchelin, I.M.; Zarei Mahmoudabadi, A.; Ansari, S.; Katiraee, F.; Rafiei, A.; Shokohi, T.; Abastabar, M.; Taraskina, A.E.; Kermani, F.; et al. Trichophyton mentagrophytes and T interdigitale genotypes are associated with particular geographic areas and clinical manifestations. Mycoses 2019, 62, 1084–1091. [Google Scholar] [CrossRef] [PubMed]
- Kapranou, R.; Kotsafti, O.; Vrioni, G.; Giannoukos, A.; Papanikou, S.; Stratigos, A.; Nicolaidou, E. Trichophyton Mentagrophytes type VII (TMVII): An emerging sexually transmitted pathogen. QJM 2025, hcaf049. [Google Scholar] [CrossRef] [PubMed]
- Jabet, A.; Bérot, V.; Chiarabini, T.; Dellière, S.; Bosshard, P.P.; Siguier, M.; Tubiana, R.; Favier, M.; Canestri, A.; Makhloufi, S.; et al. Trichophyton mentagrophytes ITS genotype VII infections among men who have sex with men in France: An ongoing phenomenon. J. Eur. Acad. Dermatol. Venereol. 2025, 39, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Kupsch, C.; Czaika, V.A.; Deutsch, C.; Gräser, Y. Trichophyton mentagrophytes—A new genotype of zoophilic dermatophyte causes sexually transmitted infections. J. Dtsch. Dermatol. Ges. 2019, 17, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.S.; Sikora, M.; Strome, A.; Akoh, C.C.; Otto, C.; Chaturvedi, S.; Zampella, J.G. Potential Sexual Transmission of Tinea Pubogenitalis From TMVII. JAMA Dermatol. 2024, 160, 783–785. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).