Abstract
Leptosphaeriaceae is a widely distributed fungal family with diverse lifestyles. The family includes several genera that can be distinguished by morphology and molecular phylogenetic analysis. During our investigation of saprobic fungi on grasslands in Yunnan Province, China, four fungal taxa belonging to Leptosphaeriaceae associated with grasses were collected. Morphological observations and phylogenetic analyses of the combined SSU, LSU, ITS, tub2, and rpb2 loci based on maximum likelihood and Bayesian inference were used to reveal the taxonomic placement of these fungal taxa. This study introduces four new taxa, viz. Leptosphaeria yunnanensis, L. zhaotongensis, Paraleptosphaeria kunmingensis, and Plenodomus zhaotongensis. Colour photo plates, full descriptions, and a phylogenetic tree to show the placement of the new taxa are provided.
1. Introduction
Leptosphaeriaceae, introduced by Barr [1], are widely distributed and exhibit diverse lifestyles, including fungicolous, epiphytic, parasitic, saprobic, and hemibiotrophic on herbaceous and woody plants [2,3,4,5,6]. For example, Leptosphaeria species are saprobes, plant pathogens, or hemibiotrophs on cultivated, wild herbaceous, and woody plants [7,8,9,10,11]. In the order Pleosporales, the family Leptosphaeriaceae contains economically significant plant pathogens [2]: i.e., Zhang et al. [8] reported Leptosphaeria species cause a serious disease of oilseed rape (Brassica napus, canola) in China.
The sexual morphs of Leptosphaeriaceae species are characterised by immersed to superficial and conical or globose ascomata, a thick peridium with scleroplectenchymatous or plectenchymatous cells, cylindrical to oblong asci, and hyaline to brown ascospores that are transversely septate [2,3,6,12,13,14,15]. In addition, asexual morphs are coelomycetous or hyphomycetous [13,16,17]. Furthermore, the most recently evolved Leptosphaeria species produce paler, longer, fusiform, and narrower ascospores with three or multi-septa, compared with the primitive dark brown ascospores [2,5].
Leptosphaeria and Paraleptosphaeria are morphologically similar, but phylogenetic analysis can clearly distinguish them [9,17,18,19,20]. The same applies to Plenodomus, and the other six genera, viz. Alloleptosphaeria, Alternariaster, Neoleptosphaeria, Pseudoleptosphaeria, Sphaerellopsis, and Subplenodomus, that were re-circumscribed based on molecular phylogeny [2]. Based on the multilocus phylogeny of the combined LSU, SSU, and ITS datasets, seven other genera, viz. Angularia, Chaetoplea [21], Ochraceocephala [22], Praeclarispora [23], Heterosporicola, Querciphoma, and Sclerenchymomyces [6], have been described in Leptosphaeriaceae. The Index Fungorum [24] listed 1682 Leptosphaeria species (accessed on 29 March 2023) epithets, and many of them have been synonymised under other genera. The members of Paraleptosphaeria have been reported as saprobic, fungicolous, or pathogenic [2,4,20], and nine species epithets are listed in the Index Fungorum [24] (accessed on 29 March 2023). In addition, Plenodomus has often been reported as saprobic or pathogenic on Asteraceae, Fabaceae, Lamiaceae, Liliaceae, and Poaceae [2,6,13,25,26] and 101 records are listed in the Index Fungorum [24] (accessed on 29 March 2023).
Grasslands comprise a biome subjected to alternating drought, where grass and grass-like species dominate, and arboreous trees are uncommon [27]. In the grassland biome, several living organisms, such as herbivorous mammals, insects, and fungi (pathogenic, saprobic, and symbiotic), play essential roles in maintaining biomass and biodiversity [28]. Regarding fungi, a checklist of Ascomycetes on grasses was provided by Karunarathna et al. [29], which lists 3,165 fungal species. Studies of fungi on grasses include those of Thambugala et al. [30], Goonasekara et al. [31], and Brahmanage et al. [32].
This study describes four new Leptosphaeriaceae species that were collected from herbaceous plants in Kunming and Zhaotong, Yunnan Province, China. Phylogenetic analyses results based on SSU, LSU, ITS, tub2, and rpb2 loci, colour photo plates, complete descriptions of the four new species, and a summary of the morphological characteristics of Leptosphaeria, Paraleptosphaeria, and Plenodomus are provided.
2. Materials and Methods
2.1. Sample Collection, Isolation, and Identification
Herbaceous plants with fungal fruiting bodies were collected from Kunming and Zhaotong in Yunnan Province, China, stored in plastic bags, and returned to the mycology laboratory at the Kunming Institute of Botany. The samples were examined under an Olympus SZ-61 dissecting microscope (Tokyo, Japan). Fungal fruiting bodies were manually sectioned and mounted in double distilled water (ddH2O). Micro-morphological characteristics were captured using an OLYMPUS SZ2-ILST compound microscope connected to an Industrial Digital Camera 16NP USB3.0 (Panasonic, Osaka, Japan) microscope imaging system. Photo plates were processed using Adobe Photoshop CS6 Extended version 13.0.1 (Adobe Systems, San Jose, CA, USA). As described by Senanayake et al. [33], cultures were obtained via single-spore isolation and incubated under normal light at room temperature (25 °C). Germinating ascospores or conidia were observed under a stereo microscope and transferred to new potato dextrose agar (PDA) plates. Herbarium specimens were deposited in the herbarium at the Kunming Institute of Botany, Chinese Academy of Sciences (HKAS), and the Herbarium Mycologicum Academiae Sinicae, Beijing, China (HMAS), and living cultures were deposited in the China General Microbiological Culture Collection Center (CGMCC). The Index Fungorum [24] and Faces of Fungi (FoF) [34] numbers were registered for the new species.
2.2. DNA Extraction, PCR Amplification, and DNA Sequencing
Genomic DNA was extracted from fresh mycelia grown on PDA at 28 °C for two weeks using the Biospin Fungus Genomic DNA Extraction Ki-BSC14S1 (BioFlux®, Hangzhou, China) according to the manufacturer’s protocol. The E.Z.N.A. Forensic DNA Kit-D3591 (Omega Biotek, Inc., Norcross, Georgia) was used to extract DNA directly from fruiting bodies. Polymerase chain reactions (PCRs) were carried out for five genetic markers: internal transcribed spacer region (ITS) [35], partial 28S large subunit nuclear ribosomal DNA (LSU) [36], partial small subunit ribosomal RNA (SSU) [35], β-tubulin (tub2) [37], and partial RNA polymerase second largest subunit (rpb2) [38]. The primers and amplification conditions used are listed in Table 1. The total volume of PCR mixtures for amplification was 25 μL, containing 8.5 μL ddH2O, 12.5 μL 2xF8FastLong PCR MasterMix (Beijing Aidlab Biotechnologies Co. Ltd., Beijing, China), 2 μL of the DNA template, and 1 μL each of reverse and forward primer (10 pM). The PCR products were sequenced by Shanghai Sangon Biological Engineering Technology and Service Co., Ltd., Shanghai, China.

Table 1.
Details of genes/loci with PCR primers and thermal cycling program for PCR amplification.
2.3. Phylogenetic Analyses
The newly obtained sequences (SSU, LSU, ITS, tub2, and rpb2) were subjected to BLASTn searches against the GenBank database (https://blast.ncbi.nlm.nih.gov/Blast.cgi) (accessed on 29 March 2023) to identify closely related taxa. Reference isolates and accessions were obtained from recent studies [6,17,23,39,40,41] and downloaded from GenBank. Single-locus sequence datasets were aligned using MAFFT v. 7.505 [42], trimmed using TrimAl v. 1.3 [43] via the web server Phylemon2 (http://phylemon.bioinfo.cipf.es/utilities.html) (accessed on 29 March 2023), and concatenated using BioEdit v. 7.0.5.2 [44]. Phylogenetic reconstructions of individual and combined datasets were performed using maximum likelihood (ML) and Bayesian inference (BI) analyses.
Maximum likelihood trees were inferred using RAxML-HPC2 in the XSEDE v. 8.2.12 [45] in CIPRES Science Gateway v. 3.3 online platform [46] under the GTRGAMMA nucleotide substitution model with 1000 bootstrap replicates. Bayesian inference analysis was conducted using MrBayes on XSEDE v. 3.2.7a [47], under the substitution model GTR + I + G for all loci, estimated by MrModeltest v. 2.3 [48] using PAUP v. 4.0b10 [49]. Six simultaneous Markov chains were run for 10,000,000 generations, with trees sampled every 1000th generation. The run was configured to stop when the standard deviation of split frequencies dropped below 0.01, and the first 25% of the trees were discarded as burn-in.
Tree topologies were visualised and exported using FigTree v. 1.4.0 [50]. The phylogram was edited and annotated using Microsoft Office PowerPoint 2016 (Microsoft Inc., Redmond, WA, USA) and Adobe Photoshop CS6 Extended version 13.0.1 (Adobe Systems, San Jose, CA, USA). Finally, the newly generated sequences were deposited in the GenBank database (Table 2).

Table 2.
Names, Index Fungorum strain numbers, and corresponding GenBank accession numbers of the taxa used for phylogenetic analyses in this study.
The decision as to whether the new species should be introduced followed the polyphasic guidelines of Chethana et al. [51] and Maharachchikumbura et al. [52].
3. Results
3.1. Phylogenetic Analyses
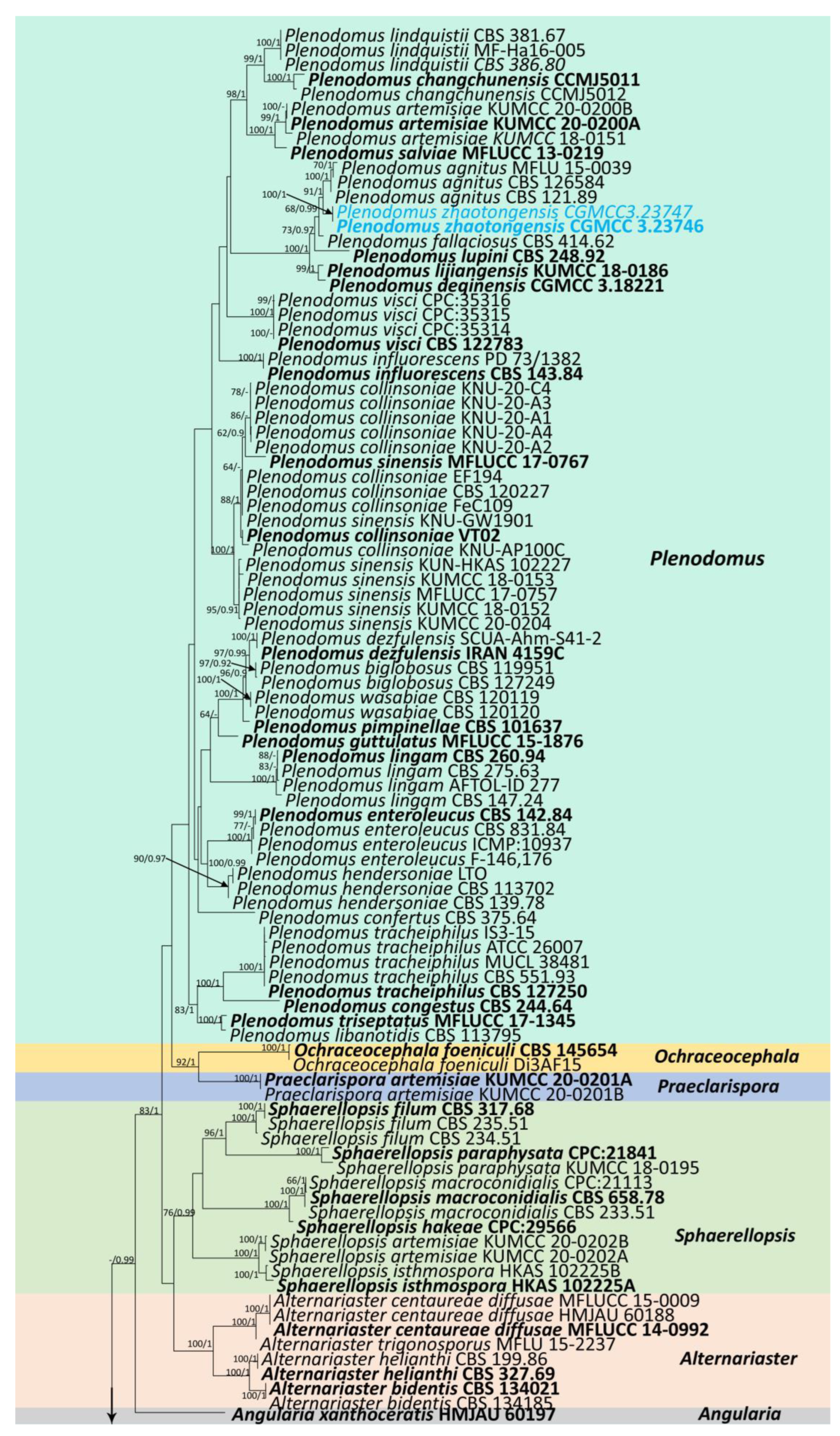
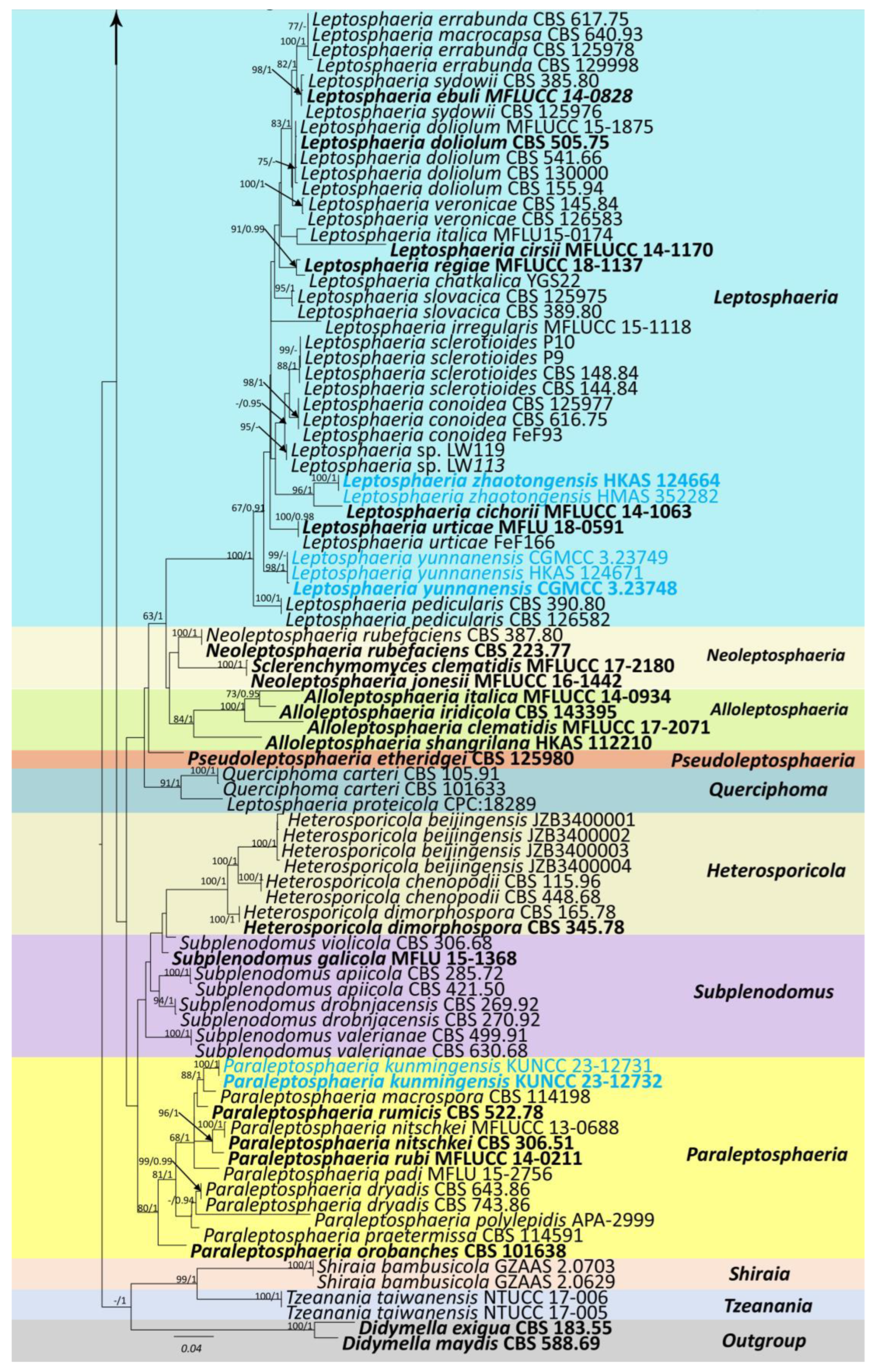
The combined sequence data of SSU, LSU, ITS, tub2, and rpb2 consisted of 177 strains of Leptosphaeriaceae, plus Didymella exigua (CBS 183.55) and D. maydis (CBS 588.69) as outgroup taxa (Figure 1). After trimming, the dataset consisted of 3981 sites, including gaps (SSU = 1–894 bp, LSU = 895–2224 bp, ITS = 2225–2780 bp, tub2 = 2781–3108 bp, and rpb2 = 3109–3981 bp). The phylogenetic tree topologies of the single and combined matrices were similar. The phylogenetic topologies obtained using the ML and BI methods also shared the same topology. RAxML analysis of the combined dataset yielded a best-scoring tree with a final ML optimization likelihood value of −31,560.945159. The matrix had 1315 distinct alignment patterns, with 45.47% undetermined characters or gaps. Estimated base frequencies were as follows: A = 0.243300, C = 0.230842, G = 0.272923, T = 0.252935; substitution rates were as follows: AC = 1.583725, AG = 4.540133, AT = 1.817809, CG = 0.949937, CT = 7.364395, GT = 1.00, alpha: 0.147156.
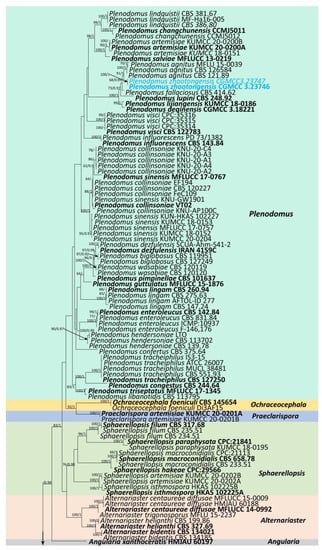
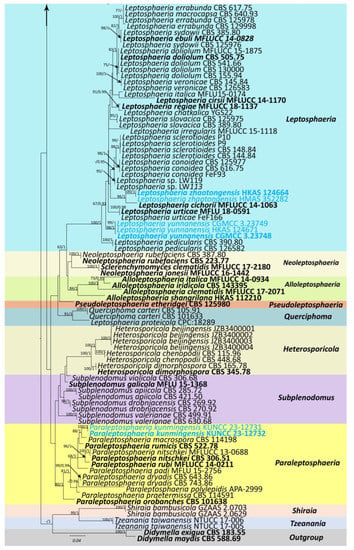
Figure 1.
Phylogenetic tree obtained from combined SSU, LSU, ITS, tub2, and rpb2 sequence data. Numerical values at the nodes indicate bootstrap support, and maximum likelihood bootstrap support ≥65% and Bayesian posterior probabilities ≥0.90 are displayed at the node. Ex-type strains are in bold, and the newly generated sequences are shown in red.
Our phylogenetic analysis of Leptosphaeriaceae is analogous to the analysis by Xu et al. [6]. Our isolate, Leptosphaeria zhaotongensis (HKAS 124664, HMAS 352282), is closely related to L. cichorii (MFLUCC 14-1063) with 96% ML and 1.00 BYPP bootstrap support (Figure 1). Leptosphaeria yunnanensis (CGMCC 3.23748, CGMCC 3.23749, HKAS 124671) formed a well-separated lineage from other Leptosphaeria species with 67% ML and 0.91 BYPP bootstrap support (Figure 1). Plenodomus zhaotongensis (CGMCC 3.23746, CGMCC 3.23747) is closely related to the Pl. agnitus strains with 91% ML and 1.00 BYPP bootstrap support (Figure 1). Paraleptosphaeria kunmingensis (KUNCC 23-12732, KUNCC 23-12731) is closely related to Pa. macrospora (CBS 114198) with 88% ML and 1.00 BYPP bootstrap support (Figure 1).
3.2. Taxonomy
3.2.1. Leptosphaeria yunnanensis Y. Gao and H. Gui, sp. nov.
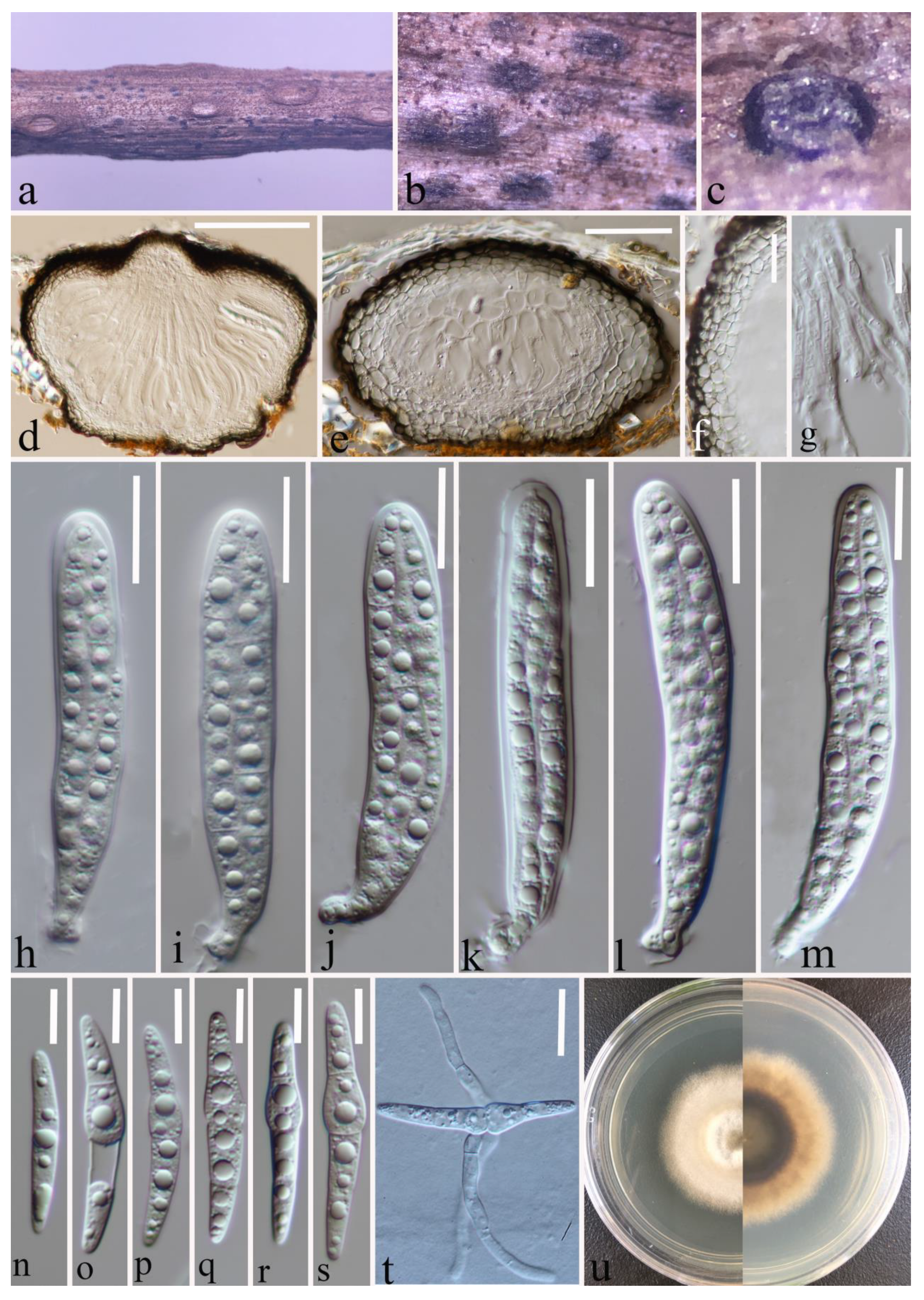
Index Fungorum number: IF 556122; Faces of Fungi number: FoF 12905; Figure 2.

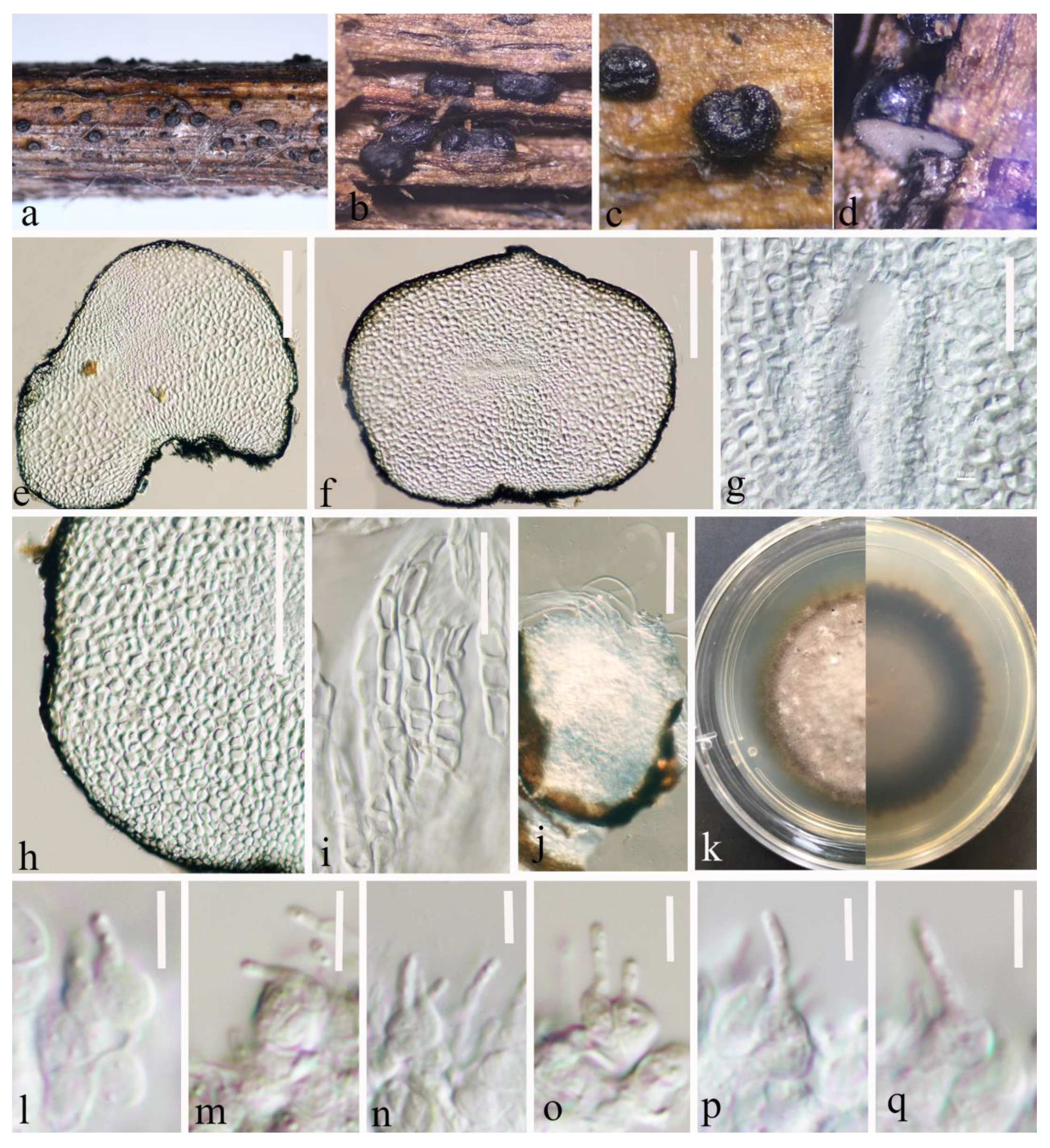
Figure 2.
Leptosphaeria yunnanensis (HKAS 124670 holotype). (a–d) Black conidiomata on the host surface. (e,f) Vertical sections of conidiomata. (g) Conidia location in conidiomata. (h,i) Peridium. (j) Germinating conidiomata. (k) Front and reverse of colony on PDA. (l–q) Conidiogenous cells and developing conidia in conidiomata. Scale bars: (e,f) = 150 μm. (g) = 50 μm. (h) = 100 μm. (i) = 50 μm. (j) = 100 μm. (l–q) = 5 μm.
Etymology: The specific epithet “yunnanensis” refers to Yunnan Province, where the holotype was collected.
Holotype: HKAS 124670
Saprobic on a decaying stalk of herbaceous plant. Sexual morph: Undetermined. Asexual morph: Conidiomata 280–515 μm diam × 170–370 μm high ( = 385 × 253 μm, n = 20), in small groups or scattered, solitary, erumpent to superficial, subglobose to globose, smooth-walled, easily removed from the host substrate, black, coriaceous, without ostiolate. Conidiomatal wall (132–)144–189(–202) μm thick, ( = 166 μm, n = 30), thick, almost fills the entire conidiomata, each cell-layer (9–)10.6–15.6(–18) μm wide, ( = 13 μm, n = 40), composed of flattened cells of textura angularis, highly pigmented on the outside and hyaline on the inside, with conidiogenous cells. Conidiogenous cells (2.8–)3.6–5.2(–6.4) μm long × (3.8–)4.4–5.8(–6.7) μm wide ( = 4.4 × 5 μm, n = 40), ampulliform or globose to subglobose, smooth-walled, hyaline. Conidia (2.5–)3.3–5.6(–6.6) μm long × (1–)1.2–1.5(–1.8) μm wide ( = 4.5 × 1.4 μm, n = 30), ellipsoidal to sub-cylindrical with obtuse ends, hyaline, guttulate, aseptate, smooth-walled.
Culture characteristics: Conidia germinated on PDA within 20 h. Colonies on PDA reaching 20 mm at four weeks at room temperature (25–27 °C), hairy or cottony, raised, white to grey, mycelium superficial, dark brown at the margin, white to light grey at the centre from above, grey in the centre, gradually black towards the edges from below.
Material examined: China, Yunnan Province, Zhaotong City, Daguan County, grassland (27°44′23″ N, 103°47′59″ E), on a decaying stalk of herbaceous plant, 21 August 2021, Ying Gao, ZG25A (HKAS 124670, holotype), ex-type, CGMCC 3.23748; ibid., ZG25C (HKAS 127125, paratype), ex-paratype CGMCC 3.23749; ibid., ZG25 (HKAS 124671, paratype).
Notes: Based on multilocus phylogenetic analyses, our isolates of L. yunnanensis (CGMCC 3.23748, CGMCC 3.23749, and HKAS 124671) showed a well-separated lineage within Leptosphaeria with moderate statistical support (67% ML, 0.91PP (Figure 1)). It clustered between L. urticae (MFLU 18-0591) and L. pedicularis (CBS 390.80) (Figure 1). The pairwise nucleotide comparison showed that our strain (CGMCC 3.23748) differs from L. urticae (MFLU 18-0591) in 44/487 bp of ITS (9.03%, with 5 gaps) and L. pedicularis (CBS 390.80) in 37/513 bp of ITS (7.23%, with 6 gaps) and 24/334 bp of tub2 (7.19%, with 2 gaps). Significant morphological differences were observed when compared with the literature for the genus (for example, [2,5,13,41,53]). Leptosphaeria yunnanensis differs from related species by having a unique conidiomatal wall that occupies almost the entire interior of the conidiomata and sporulation indistinctly in the centre. In addition, Leptosphaeria yunnanensis was reported as an asexual form, and although it is similar to L. cichorii in conidiomata and conidiogenous cells, the sizes are different (conidiomata 280–515 × 170–370 μm vs. 189–200 × 196–220 μm) (conidiogenous cells 2.8–6.5 × 3.5–6.5 μm vs. 2–5 × 2–4 μm) [2]. Furthermore, these two species formed a well-separated lineage (Figure 1). Therefore, based on the polyphasic approach recommended for species-boundary delimitation [51,52], we introduce L. yunnanensis as a novel taxon.
3.2.2. Leptosphaeria zhaotongensis Y. Gao and H. Gui, sp. nov.
Index Fungorum number: IF 556123; Faces of Fungi number: FoF 12904; Figure 3.
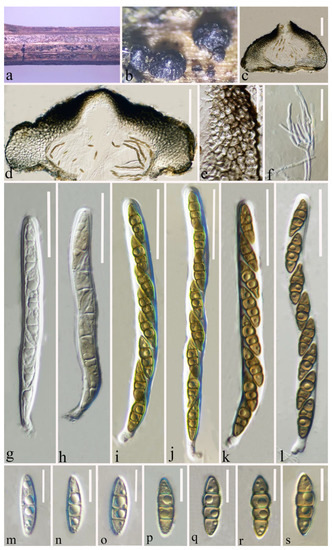
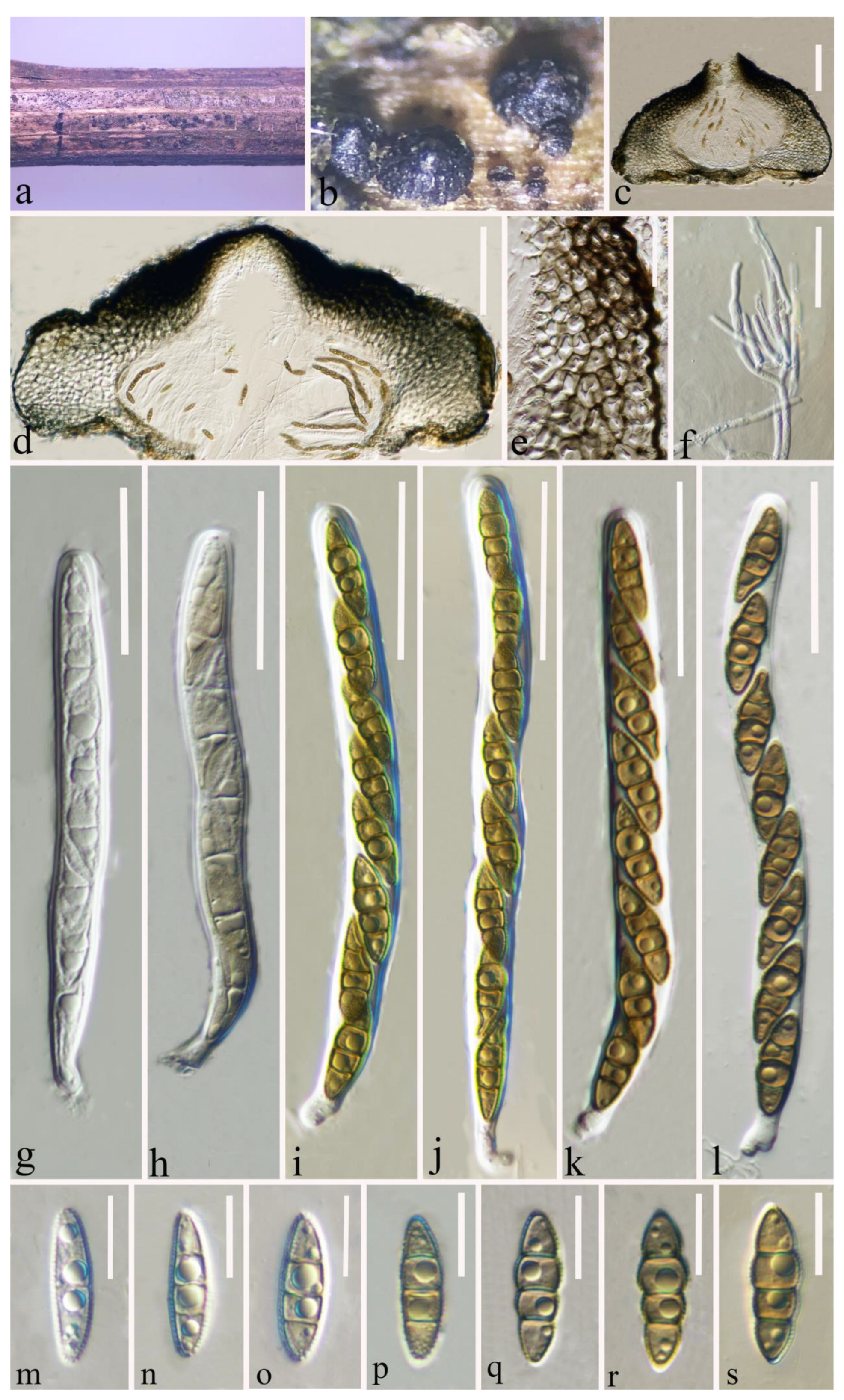
Figure 3.
Leptosphaeria zhaotongensis (HKAS 124664 holotype). (a,b) Ascomata on decaying stalk of herbaceous plant. (c,d) Vertical section of the ascoma. (e) Peridium. (f) Hamathecium. (g–l) Asci. (m–s) Ascospores. Scale bars: (c–d) = 100 μm. (e) = 30 μm. (f) = 20 μm. (g–l) = 30 μm. (m–s) = 10 μm.
Etymology: The specific epithet “zhaotongensis” refers to Zhaotong City, where the holotype was collected.
Holotype: HKAS 124664
Saprobic on a decaying stalk of a herb. Sexual morph: Ascomata 380–550 μm diam × 185–300 μm high ( = 461 × 234 μm, n = 15), scattered or in small groups, solitary, erumpent to superficial, globose to ampulliform, smooth-walled, easily removed from the host substrate, with a flattened base, black, coriaceous, uni-loculate, glabrous, ostiolate. Ostiole apex conical and with papilla. Peridium (35–)66–116.5(–127) μm wide, ( = 91 μm, n = 40), thick-walled, composed of 4–8 layers of flattened, light brown to dark brown cells of textura angularis. Hamathecium (1.5–)2–2.6(–3) μm wide, ( = 2.3 μm, n = 50), straight, septate, hyaline, branched, cellular pseudoparaphyses, partially embedded in a gelatinous matrix. Asci (84–)92–113(–118) × (7.7–)8.2–10(–11) μm ( = 102 × 9 μm, n = 30), eight-spored, arising from the base, cylindrical to cylindric-clavate, bitunicate, fissitunicate, apically rounded, short pedicellate with foot-like pedicel, with ocular chamber, hyaline. Ascospores (16.2–)17.6–20(–21.3) × (4.5–)4.8–5.7(–6.5) μm ( = 18.7 × 5.2 μm, n = 40), uniseriate, fusiform, partially overlapping, narrow to acute at both ends, guttulate, initially hyaline with one septum, becoming yellowish to brown, 3-septate at maturity, broader cells above central septum, often slightly constricted at septum, sometimes rough-walled, without mucilaginous sheath. Asexual morph: Undetermined.
Material examined: China, Yunnan Province, Zhaotong City, Daguan County, grassland (27°44′23″ N, 103°47′59″ E), on a decaying stalk of herbaceous plant, 21 August 2021, Ying Gao, GG (HKAS 124664, holotype); ibid., (HMAS 352282, paratype).
Notes: Based on our phylogenetic analysis of the combined SSU, LSU, ITS, tub2, and rpb2 sequence data, our novel species L. zhaotongensis (HKAS 124664, HMAS 352282) is closely related to L. cichorii (MFLUCC 14-1063) with 96% ML and 1.00 PP statistical support (Figure 1). Leptosphaeria zhaotongensis differs from L. cichorii in its larger ascomata (384–551 × 186–292 μm vs. 206–240 × 251–363 μm) and ascospores (16–21 × 5–7 μm, hyaline, yellowish to brown, guttulate vs. 11–20 × 3–6 μm, reddish to yellowish brown, without guttulate). The similarity of the ITS sequence data of L. cichorii (MFLUCC 14-1063) was 62/521 bp (11.9%, with 6 gaps) compared to L. cichorii (MFLUCC 14-1063). Therefore, based on the polyphasic approach recommended for species boundaries delimitation [51,52], we introduce L. zhaotongensis as a novel taxon.
3.2.3. Paraleptosphaeria kunmingensis Y. Gao and H. Gui, sp. nov.
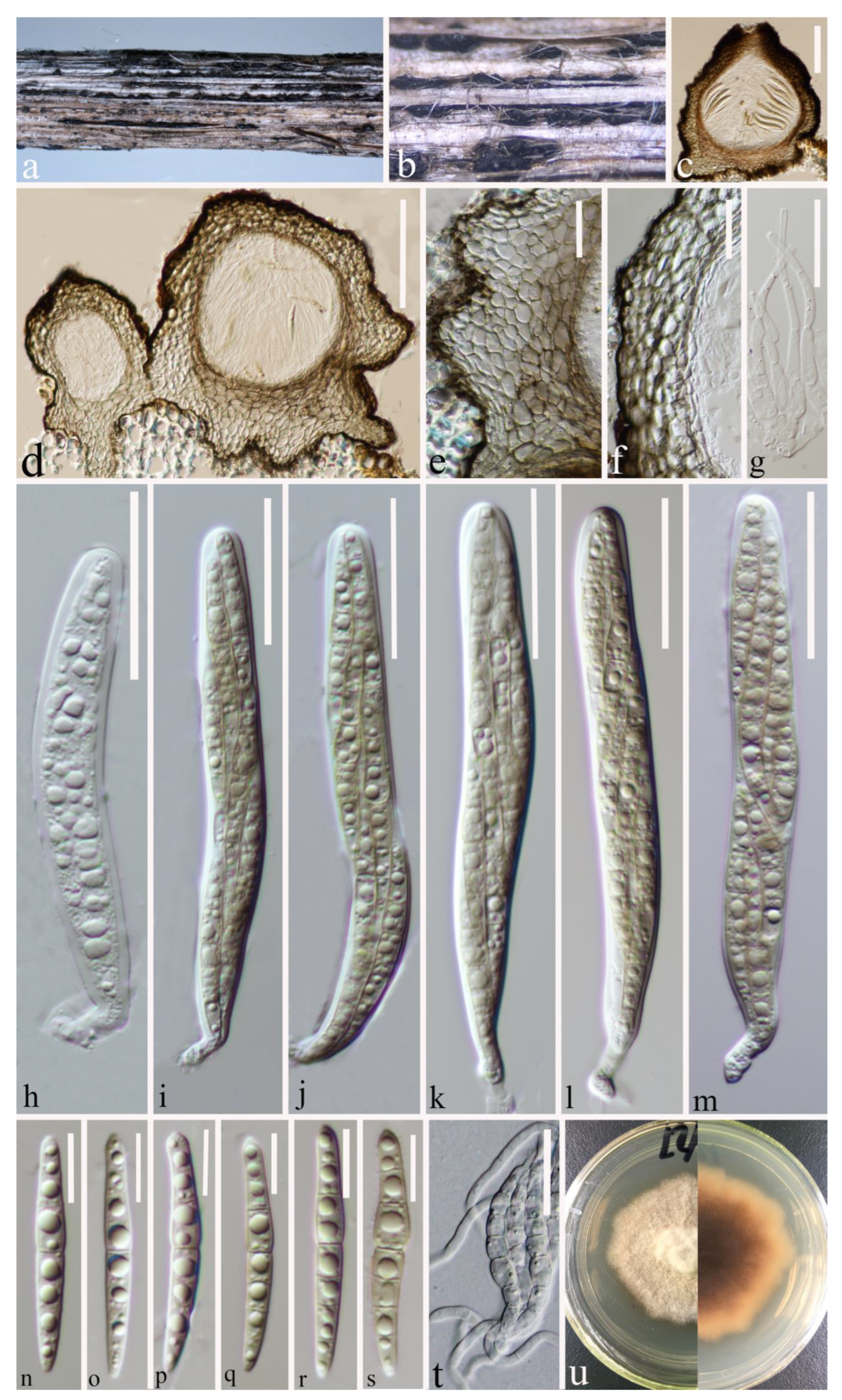
Index Fungorum number: IF 556124; Faces of Fungi number: FoF 12902; Figure 4.
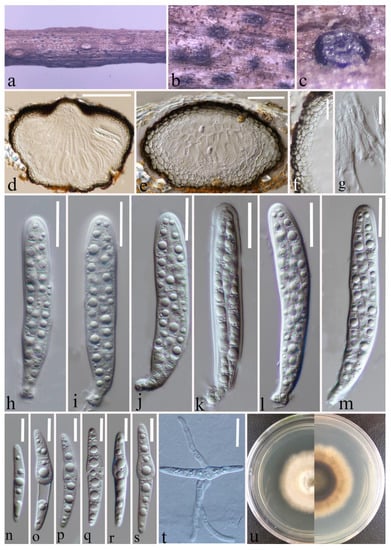
Figure 4.
Paraleptosphaeria kunmingensis (HKAS 124662 holotype). (a–c) Ascomata on decaying stalk of herbaceous plant. (d,e) Vertical section of the ascoma. (f) Peridium. (g) Hamathecium. (h–m) Asci. (n–s) Ascospores. (t) Germinating ascospore. (u) Front and reverse colony on PDA. Scale bars: (d) = 100 μm, (e) = 50 μm, (f) = 30 μm, (g–m) = 10 μm, (n–s) = 10 μm, (t) = 20 μm.
Etymology: The specific epithet “kunmingensis” refers to Kunming City, where the holotype was collected.
Holotype: HKAS 124662
Saprobic on a decaying stalk of herbaceous plant. Sexual morph: Ascomata 215–300 μm × 145–220 μm ( = 253 × 176 μm, n = 15), scattered, gregarious, immersed in the epidermis of the host, globose or subglobose and flat-globose, dark brown to black, uni-loculate, glabrous, shiny, papillate, with ostiole. Peridium (21–)24–34(–43) μm wide, ( = 29 μm, n = 35), composed of 2–4 layers of flattened, light brown to dark brown cells of textura angularis. Hamathecium (1.6–)2–3(–3.7) μm wide, ( = 2.5 μm, n = 40), straight, septate, hyaline, unbranched, cellular pseudoparaphyses, embedded in a gelatinous matrix. Asci (70–)73–92(–104) × (12–)13–15.7(–16.4) μm ( = 83 × 14 μm, n = 25), eight-spored, arising from base, fissitunicate, bitunicate, cylindrical to cylindric-clavate, short pedicellate with club-like pedicel, thick-walled at the apex, hyaline, with ocular chamber. Ascospores (33.5–)37.6–47(–50.5) × (5–)5.2–6.2(–7.2) μm ( = 42.3 × 5.7 μm, n = 30), overlapping, 2–3-seriate, hyaline, guttulate, lunate to long fusiform or inequilateral, straight or slightly curved, with 1–3 transverse septa, often slightly constricted at septum, swollen at the second cell, rounded to slightly pointed at both ends, guttulate, without a mucilaginous sheath. Asexual morph: Undetermined.
Culture characteristics: Ascospores germinated on PDA within 20 h, and a germ tube was initially produced from the middle. Colonies on PDA reaching 15 mm at two weeks at room temperature, circular, slightly raised, curled, floccose, pale yellow from above, dark brown in the centre, gradually pale yellow towards the edges from below, grows towards the filamentous edge.
Material examined: China, Yunnan Province, Kunming City, (25°8′19″ N, 102°44′25″ E), on a decaying stalk of herbaceous plant, 20 June 2021, Ying Gao, CCSG18A (HKAS 124662, holotype), ex-type KUNCC 23-12732. ibid., CCSG18 (HKAS 127126, paratype), ex-paratype KUNCC 23-12731.
Notes: Paraleptosphaeria kunmingensis is introduced as a new species based on its distinct morphology and phylogenetic analysis of combined SSU, LSU, ITS, tub2, and rpb2 datasets. Paraleptosphaeria kunmingensis is closely related to Pa. macrospora (CBS 114198) with 88% ML and 1.00 BYPP bootstrap support (Figure 1). The species differs from Pa. macrospora (Basionym: Metasphaeria macrospora) in its smaller asci (83 × 14 μm vs. 105 × 18 μm), smaller ascospores (42.3 × 5.7 μm vs. 44 × 8 μm), and the number of septa of ascospores (1–3 septa vs. three septa) [13,54]. In addition, the ITS pairwise nucleotide comparison of these species showed 18/523 bp differences (3.44%, without gaps). Our isolate differs from Pa. nitschkei in 7.51% (KT389833) and Pa. dryadis (GU371733) in 10.05% in the tub2 and rpb2 regions, respectively. Therefore, based on the polyphasic approach recommended for species boundaries delimitation [51,52], we introduce Pa. kunmingensis as a novel taxon.
3.2.4. Plenodomus zhaotongensis Y. Gao and H. Gui, sp. nov.
Index Fungorum number: IF 556124; Faces of Fungi number: FoF 12903; Figure 5.
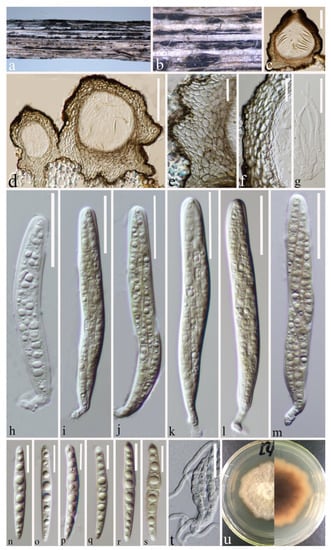
Figure 5.
Plenodomus zhaotongensis (HKAS 124668 holotype). (a,b) Ascomata on decaying stalk of herbaceous plant. (c,d) Vertical section of the ascoma. (e) Vertical section of the base of peridium. (f) Peridium. (g) Hamathecium. (h–m) Asci. (n–s) Ascospores. (t) Germinating ascospores. (u) Front and reverse of the colony on PDA. Scale bars: (c,d) = 100 μm, (e–m) = 30 μm, (n–s) = 10 μm, (t) = 20 μm.
Etymology: The specific epithet “zhaotongensis” refers to Zhaotong City, where the holotype was collected.
Holotype: HKAS 124668
Saprobic on a decaying stalk of herbaceous plant. Sexual morph: Ascomata 200–300 μm × 210–320 μm ( = 240 × 298 μm, n = 15), scattered, most are gregarious, raised, superficial with base seated in the substrate, globose to subglobose or irregular, apically conical, dark brown to black, uni-loculate, glabrous, coriaceous, ostiolate, papillate ostiole, wider and flattened at the base, connected by very thin stromatal tissue at the base. Peridium (22–)29–54(–79) μm wide, ( = 42 μm, n = 50), composed of two types of scleroplectenchymatous cells layers, thick-walled of unequal thickness, thickened at base, thinner toward sides and apex, inner layers composed of hyaline to pale brown cells of textura angularis to textura globulosa, outer layer of amorphous black cells. Hamathecium (1.4–)2–3.7(–6) μm wide, ( = 3 μm, n = 45), septate, hyaline, unbranched, broad at base, tapering upwards, pseudoparaphyses. Asci (80–)97–120(–132) × (10–)11–13(–14) μm ( = 109 × 12 μm, n = 20), eight-spored, arising from base, fissitunicate, bitunicate, cylindric-clavate, initially hyaline, short pedicellate with foot-like pedicel, with ocular chamber, thick-walled at the apex. Ascospores (34–)35–39(–42) × (3.5–)4–5(–6) μm ( = 37 × 4.5 μm, n = 30), overlapping, 2–3-seriate, initially hyaline, becoming pale yellowish at maturity, guttulate, lunate to long fusiform, straight or slightly curved, with six transverse septa at maturity, often constricted at medium septum, widest at the middle, rounded or slightly pointed at both ends, without a mucilaginous sheath. Asexual morph: Undetermined.
Culture characteristics: Ascospores germinated on PDA within 20 h, and germ tube initially produced from both ends of the ascospores. Colonies on PDA reaching 20 mm at four weeks at room temperature, irregular, flat, centre is slightly raised, panniform, mycelium grows on the surface of PDA, white from above, brown in the centre gradually pale yellow towards the edges from below. Asexual spores and sexual spores were not formed on PDA within 60 days.
Material examined: China, Yunnan Province, Zhaotong City, Daguan County, grassland (27°44′23″ N, 103°47′59″ E), on a decaying stalk of herbaceous plant, 21 August 2021, Ying Gao, ZG17A (HKAS 124668, holotype), ex-type, CGMCC 3.23746. ibid., ZG17 (HKAS 127124, paratype), ex-paratype, CGMCC 3.23747.
Notes: Plenodomus zhaotongensis is introduced as a new species based on its distinct morphology and phylogenetic analysis of combined SSU, LSU, ITS, tub2, and rpb2 sequence data. Plenodomus zhaotongensis is closely related to Pl. agnitus strains with 91% ML and 1.00 BYPP statistical support (Figure 1). A pairwise nucleotide comparison showed that Pl. zhaotongensis differs from Pl. agnitus (CBS 121.89) in 12/529 bp of ITS (2.27%, without gaps), 12/341 bp of tub2 (3.52%, without gaps), and 18/766 bp of rpb2 (2.35%, without gaps). Plenodomus zhaotongensis differs from Pl. agnitus (sexual morph: Leptosphaeria agnita (Desm.) Ces. & De Not., Comm. Soc. Crittog. Ital. 1: 236. 1863.) in its larger ascospores (34–42 × 3.5–6 μm, lunate to long fusiform vs. 31–35 × 4–5 μm, narrowly subcylindrical) [13,55]. Therefore, based on the guidelines for new species boundaries delimitation [51,52], we introduce Pl. zhaotongensis as a novel taxon.
4. Discussion
In this study, we introduce four new species, viz. L. yunnanensis, L. zhaotongensis, Pa. kunmingensis, and Pl. zhaotongensis, associated with grasses from Zhaotong and Kunming in Yunnan Province, southwestern China, based on polyphasic approaches [56,57] through multilocus analyses of five gene loci (SSU, LSU, ITS, tub2, and rpb2) and their morphological characteristics. Although Leptosphaeria is a speciose genus with 1682 species epithets, many are likely to belong to other genera [2] and need recollecting and sequencing. However, in speciose genera, many novel taxa can still be found [58], as in this study.
Paraleptosphaeria and Leptosphaeria have similar morphologies. In our study, Pa. kunmingensis fits within the generic concept of Paraleptosphaeria and is phylogenetically closely related to Pa. macrospora (Figure 1), whereas L. yunnanensis and L. zhaotongensis clustered distinctly in Leptosphaeria (Figure 1). All species differ in morphology (Figure 2, Figure 3 and Figure 5, Supplementary Table S2). Using molecular data, Piątek et al. [20] also showed that Paraleptosphaeria and Leptosphaeria are phylogenetically distinct. We also described Pl. zhaotongensis based on the morphological characteristics (Figure 5) and molecular phylogeny (Figure 1). Nevertheless, in Plenodomus, it is challenging to have well-resolved species delimitation because many species lack molecular data and detailed morphological descriptions [5,21]. Therefore, in future studies, precise morphological characteristics, molecular data, and phylogenetic analyses should be provided for all newly introduced and existing Plenodomus species.
Except for L. maculans, leptosphaeria-like taxa are diverse and widespread. However, they are mostly found in temperate regions (Supplementary Table S1, [59,60]), with 30 species reported on grasses [29]. In addition, Leptosphaeria species seem not to be host-specific, as they have been discovered on various plant families (i.e., Adoxaceae, Apiaceae, Asteraceae, Euphorbiaceae, Fabaceae, Gentianaceae, Juglandaceae, Lamiaceae, Orobanchaceae, Plantaginaceae, Rhamnaceae, and Urticaceae) [5]. Similarly, Plenodomus is widely distributed worldwide, mainly in temperate countries such as China, Greece, France, Japan, the Netherlands, and Spain (Supplementary Table S3, [6,61]), with four species, viz. Pl. acutus, Pl. changchunensis, Pl. enteroleucus, and Pl. sorghi, are associated with grasses [6,13,62,63]. This indicates that investigations of new host plants, especially those inhabiting decomposing litter [64,65], and unstudied environments will result in undescribed taxa in this and other families, contributing to the descriptive fungal curve [66,67].
The four described species in this study were collected from climate-contrasting grasslands in Yunnan Province. In Zhaotong, subtropical and warm temperate zones coexist, with an annual average temperature of 12.6 °C [68,69], while Kunming has distinct wet and dry seasons [70,71]. In terms of fungi, many new fungal species have been reported in Yunnan Province in the last two decades. More than 1300 new fungal species have been described, accounting for nearly 25% of the total fungal species described in China [72]. This scenario is consistent with Hyde et al. [66,67], who stated that continued exploration of new environments would result in undescribed taxa.
In Yunnan’s grasslands, many grass species are the primary source of carbohydrates and feed for livestock, and fungi play a pivotal role in maintaining and shaping grass communities. Each grass-associated fungal community is responsible for specific ecological properties of the environment [29]. Our study fills some gaps in the research on Leptosphaeriaceae species in grasslands by providing detailed information on four new species from China and insights into the number of grass-associated Leptosphaeria. In addition, fungi have never been reported in Zhaotong City; thus, future studies are needed to reveal the actual fungal diversity, especially those associated with grasses.
In addition, we compared our Leptosphaeria zhaotongensis with known species that have no molecular data in Shoemaker [73]. Leptosphaeria zhaotongensis is similar to L. galii, L. raphani, L. russellii, L. stellariae, and L. byssincola in ascomata, asci and ascospores. However, they differ from Leptosphaeria galii in ascospores (guttulate vs. without guttulate) and asci (84–118 × 7.7–11 μm, vs. 45–65 × 6–8 μm); Leptosphaeria zhaotongensis differs from Leptosphaeria raphani in ascospores (guttulate vs. without guttulate), asci (84–118 μm, vs. 60–80 μm), and ascomata (380–550 × 185–300 μm, vs. 200–280 × 200–280 μm); Leptosphaeria zhaotongensis differs from Leptosphaeria russellii in asci (84–118 μm, vs. 70–85 μm) and ascomata (380–550 × 185–300 μm, vs. 200–250 × 100–150 μm); Leptosphaeria zhaotongensis differs from Leptosphaeria stellariae in ascospores (16–21 μm × 4.5–6.5 μm, guttulate, rough, vs. 26–30 × 6–7 μm, without guttulate, smooth), asci (84–118 μm, vs. 80–180 μm), and ascomata (380–550 × 185–300 μm, vs. 140–190 × 90–110 μm); and Leptosphaeria zhaotongensis differs from Leptosphaeria byssincola in asci (84–118 × 8–11 μm, vs. 80–90 × 11–13 μm). There are also obvious differences in morphology between Leptosphaeria zhaotongensis and unsequenced Leptosphaeria species.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jof9060612/s1, Table S1: Synopsis of sexual and asexual morphological characteristics of Leptosphaeria species with molecular data; Table S2: Synopsis of sexual and asexual morphological characteristics of Paraleptosphaeria species with molecular data; Table S3: Synopsis of sexual and asexual morphological characteristics of Plenodomus species with molecular data. Refs. [74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91] are cited in the supplementary.
Author Contributions
Conceptualisation, Y.G.; methodology, Y.G. and S.T.; software, Y.G.; resources, H.G. and J.-C.X.; writing—original draft preparation, Y.G.; writing—review and editing, A.R.G.d.F., H.-B.J., S.C.K., S.T., and H.G.; visualisation, S.T.; supervision, H.G. and A.R.G.d.F.; funding acquisition, H.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant number 32001296, 32260004; the Youth Innovation Promotion Association of CAS, China, grant number 2022396, and the Strategic Priority Research Program of Chinese Academy of Sciences, grant number XDA26020203.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Beinn Purvis at World Agroforestry (ICRAF), Kunming Institute of Botany, China, is thanked for English editing. Shaun Pennycook is thanked for nomenclatural advice. We gratefully thank the Biology Experimental Center, Germplasm Bank of Wild Species, Kunming Institute of Botany, and the Chinese Academy of Sciences for providing molecular laboratory facilities. Xinyu Zhu is thanked for assisting with herbaria specimen deposition in the Herbarium Mycologicum Academiae Sinicae, Beijing, China (HMAS).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Barr, M.E. New taxa and combinations in the Loculoascomycetes. Mycotaxon 1987, 29, 501–505. [Google Scholar]
- Ariyawansa, H.A.; Phukhamsakda, C.; Thambugala, K.M.; Bulgakov, T.S.; Wanasinghe, D.N.; Perera, R.H.; Mapook, A.; Camporesi, E.; Kang, J.C.; Gareth Jones, E.B.G.; et al. Revision and phylogeny of Leptosphaeriaceae. Fungal Divers. 2015, 74, 19–51. [Google Scholar] [CrossRef]
- Phookamsak, R.; Hyde, K.D.; Jeewon, R.; Bhat, D.J.; Jones, E.B.G.; Maharachchikumbura, S.S.N.; Raspé, O.; Karunarathna, S.C.; Wanasinghe, D.N.; Hongsanan, S.; et al. Fungal diversity notes 929–1035: Taxonomic and phylogenetic contributions on genera and species of fungi. Fungal Divers. 2019, 95, 1–273. [Google Scholar] [CrossRef]
- Sun, J.Z.; Liu, X.Z.; McKenzie, E.H.C.; Jeewon, R.; Liu, J.K.; Zhang, X.L.; Zhao, Q.; Hyde, K.D. Fungicolous fungi: Terminology, diversity, distribution, evolution and species checklist. Fungal Divers. 2019, 95, 1–94. [Google Scholar] [CrossRef]
- Lestari, A.S.; Wanasinghe, D.N.; Gafforov, Y.; Tennakoon, D.S.; Chethana, K.W.T.; Aburazakov, A.; Promputtha, I.; Hyde, K.D. Taxonomy and phylogenetic appraisal of Leptosphaeria chatkalica sp. nov. (Leptosphaeriaceae, Pleosporales) from Uzbekistan. Phytotaxa 2021, 520, 155–168. [Google Scholar] [CrossRef]
- Xu, R.; Su, W.X.; Tian, S.Q.; Bhunjun, C.S.; Tibpromma, S.; Hyde, K.D.; Li, Y.; Phukhamsakda, C. Synopsis of Leptosphaeriaceae and introduction of three new taxa and one new record from China. J. Fungi 2022, 8, 416. [Google Scholar] [CrossRef]
- Howlett, B.J.; Idnurm, A.; Soledade, M.; Pedras, C. Leptosphaeria maculans, the causal agent of blackleg disease of Brassicas. Fungal Genet. Biol. 2001, 33, 1–14. [Google Scholar] [CrossRef]
- Zhang, X.; White, R.P.; Demir, E.; Jedryczka, M.; Lange, R.M.; Islam, M.; Li, Z.Q.; Huang, Y.J.; Hall, A.M.; Zhou, G.; et al. Leptosphaeria spp., phoma stem canker and potential spread of L. maculans on oilseed rape crops in China. Plant Pathol. 2014, 63, 598–612. [Google Scholar] [CrossRef]
- Wanasinghe, D.N.; Camporesi, E.; Hu, D.M. Neoleptosphaeria jonesii sp. nov., a novel saprobic sexual species, in Leptosphaeriaceae. Mycosphere 2016, 7, 1368–1377. [Google Scholar] [CrossRef]
- Hyde, K.D.; De Silva, N.; Jeewon, R.; Bhat, D.J.; Phookamsak, R.; Doilom, M.; Boonmee, S.; Jayawardena, R.S.; Maharachchikumbura, S.S.N.; Senanayake, I.C.; et al. AJOM new records and collections of fungi: 1–100. Asian J. Mycol. 2020, 3, 22–294. [Google Scholar] [CrossRef]
- Pem, D.; Hongsanan, S.; Doilom, M.; Tibpromma, S.; Wanasinghe, D.N.; Dong, W.; Liu, N.G.; Phookamsak, R.; Phillips, A.J.L.; Jeewon, R.; et al. https://www.dothideomycetes.org: An online taxonomic resource for the classification, identification, and nomenclature of Dothideomycetes. Asian J. Mycol. 2020, 3, 287–297. [Google Scholar] [CrossRef]
- Hyde, K.D.; Jones, E.B.G.; Liu, J.K.; Ariyawansa, H.; Boehm, E.; Boonmee, S.; Braun, U.; Chomnunti, P.; Crous, P.W.; Dai, D.Q.; et al. Families of Dothideomycetes. Fungal Divers. 2013, 63, 1–313. [Google Scholar] [CrossRef]
- Gruyter, J.de.; Woudenberg, J.H.C.; Aveskamp, M.M.; Verkley, G.J.M.; Groenewald, J.Z.; Crous, P.W. Redisposition of Phoma-like anamorphs in Pleosporales. Stud. Mycol. 2013, 75, 1–36. [Google Scholar] [CrossRef] [PubMed]
- Phukhamsakda, C.; McKenzie, E.H.C.; Phillips, A.J.L.; Gareth Jones, E.B.; Jayarama Bhat, D.; Stadler, M.; Bhunjun, C.S.; Wanasinghe, D.N.; Thongbai, B.; Camporesi, E.; et al. Microfungi associated with Clematis (Ranunculaceae) with an integrated approach to delimiting species boundaries. Fungal Divers. 2020, 102, 1–203. [Google Scholar] [CrossRef]
- Thiyagaraja, V.; Wanasinghe, D.N.; Karunarathna, S.C.; Tennakoon, D.S.; Hyde, K.D.; To-Anun, C.; Cheewangkoon, R. Alloleptosphaeria shangrilana sp. nov. and first report of the genus (Leptosphaeriaceae, Dothideomycetes) from China. Phytotaxa 2021, 491, 12–22. [Google Scholar] [CrossRef]
- Alves, J.L.; Woudenberg, J.H.C.; Duarte, L.L.; Crous, P.W.; Barreto, R.W. Reappraisal of the genus Alternariaster (Dothideomycetes). Persoonia 2013, 31, 77–85. [Google Scholar] [CrossRef]
- Aiello, D.; Vitale, A.; Polizzi, G.; Voglmayr, H. Ochraceocephala foeniculi gen. et sp. nov., a new pathogen causing crown rot of fennel in Italy. MycoKeys 2020, 66, 1–22. [Google Scholar] [CrossRef]
- Liu, J.K.; Hyde, K.D.; Jones, E.B.G.; Ariyawansa, H.A.; Bhat, D.J.; Boonmee, S.; Maharachchikumbura, S.S.N.; McKenzie, E.H.C.; Phookamsak, R.; Phukhamsakda, C.; et al. Fungal Diversity Notes 1-100: Taxonomic and phylogenetic contributions to fungal species. Fungal Divers. 2015, 72, 1–197. [Google Scholar] [CrossRef]
- Tibpromma, S.; Hyde, K.D.; Jeewon, R.; Maharachchikumbura, S.S.N.; Liu, J.K.; Bhat, D.J.; Jones, E.B.G.; McKenzie, E.H.C.; Camporesi, E.; Bulgakov, T.S. Fungal diversity notes 491–602: Taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers. 2017, 83, 1–261. [Google Scholar] [CrossRef]
- Piątek, M.; Rodriguez-Flakus, P.; Domic, A.; Palabral-Aguilera, A.N.; Gómez, M.I.; Flakus, A. Phylogenetic placement of Leptosphaeria polylepidis, a pathogen of Andean endemic Polylepis tarapacana, and its newly discovered mycoparasite Sajamaea mycophila gen. et sp. nov. Mycol. Prog. 2020, 19, 1–14. [Google Scholar] [CrossRef]
- Hongsanan, S.; Hyde, K.D.; Phookamsak, R.; Wanasinghe, D.N.; Mckenzie, E.H.C.; Sarma, V.V.; Boonmee, S.; Lücking, R.; Bhat, D.J.; Liu, N.G.; et al. Refined families of Dothideomycetes: Dothideomycetidae and Pleosporomycetidae. Mycosphere 2020, 11, 1553–2107. [Google Scholar] [CrossRef]
- Wijayawardene, N.N.; Hyde, K.D.; Dai, D.Q.; Sánchez-García, M.; Goto, B.T.; Saxena, R.K.; Erdoğdu, M.; Selçuk, F.; Rajeshkumar, K.C.; Aptroot, A.; et al. Outline of Fungi and fungus-like taxa—2021. Mycosphere 2022, 13, 53–453. [Google Scholar] [CrossRef]
- Doilom, M.; Hyde, K.D.; Dong, W.; Liao, C.F.; Suwannarach, N.; Lumyong, S. The plant family Asteraceae is a cache for novel fungal diversity: Novel species and genera with remarkable ascospores in Leptosphaeriaceae. Front. Microbiol. 2021, 12, 660261. [Google Scholar] [CrossRef] [PubMed]
- Index Fungorum. 2023. Available online: http://www.indexfungorum.org/Names/Names.asp (accessed on 29 March 2023).
- Marin-Felix, Y.; Groenewald, J.Z.; Cai, L.; Chen, Q.; Marincowitz, S.; Barnes, I.; Bensch, K.; Braun, U.; Camporesi, E.; Damm, U.; et al. Genera of phytopathogenic fungi: GOPHY 1. Stud. Mycol. 2017, 86, 99–216. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Crous, P.W.; Hou, L.W.; Duan, W.J.; Cai, L.; Ma, Z.Y.; Liu, F. Fungi of quarantine concern for China I: Dothideomycetes. Pers. Mol. Phylogeny Evol. Fungi 2021, 47, 45–105. [Google Scholar] [CrossRef]
- Risser, P.G. Diversity in and Among Grasslands. In Biodiversity; Wilson, E., Peter, F., Eds.; National Academics Press: Washington, DC, USA, 1988; pp. 176–180. [Google Scholar]
- Karunarathna, A.; Tibpromma, S.; Jayawardena, R.S.; Nanayakkara, C.; Asad, S.; Xu, J.-C.; Hyde, K.D.; Karunarathna, S.C.; Stephenson, S.L.; Lumyong, S.; et al. Fungal pathogens in Grasslands. Front. Cell. Infect. Microbiol 2021, 11, 695087. [Google Scholar] [CrossRef]
- Karunarathna, A.; Withee, P.; Pakdeeniti, P.; Haituk, S.; Tanakaew, N.; Senwanna, C.; Działak, P.; Karunarathna, S.C.; Tibpromma, S.; Promthep, T.; et al. Worldwide checklist on Grass Fungi: What do we know so far in Ascomycota. Chiang Mai J. Sci. 2022, 49, 742–984. [Google Scholar] [CrossRef]
- Thambugala, K.M.; Wanasinghe, D.N.; Phillips, A.J.L.; Camporesi, E.; Bulgakov, T.S.; Phukhamsakda, C.; Ariyawansa, H.A.; Goonasekara, I.D.; Phookamsak, R.; Dissanayake, A.; et al. Mycosphere notes 1-50: Grass (Poaceae) inhabiting Dothideomycetes. Mycosphere 2017, 8, 697–796. [Google Scholar] [CrossRef]
- Goonasekara, I.D.; Jayawardene, R.S.; Saichana, N.; Hyde, K.D. Checklist of microfungi on grasses in Thailand (excluding bambusicolous fungi). Asian J. Mycol. 2018, 1, 88–105. [Google Scholar] [CrossRef]
- Brahmanage, R.S.; Dayarathne, M.C.; Wanasinghe, D.N.; Thambugala, K.M.; Jeewon, R.; Chethana, K.W.T.; Samarakoon, M.C.; Tennakoon, D.S.; De Silva, N.I.; Camporesi, E.; et al. Taxonomic novelties of saprobic Pleosporales from selected dicotyledons and grasses. Mycosphere 2020, 11, 2481–2541. [Google Scholar] [CrossRef]
- Senanayake, I.C.; Rathnayake, A.R.; Marasinghe, D.S.; Calabon, M.S.; Gentekaki, E.; Lee, H.B. Morphological approaches in studying fungi: Collection, examination, isolation, sporulation and preservation. Mycosphere 2020, 11, 2678–2754. [Google Scholar] [CrossRef]
- Jayasiri, S.C.; Hyde, K.D.; Ariyawansa, H.A.; Bhat, J.; Buyck, B.; Cai, L.; Dai, Y.C.; Abd-Elsalam, K.A.; Ertz, D.; Hidayat, I.; et al. The Faces of Fungi database: Fungal names linked with morphology, phylogeny and human impacts. Fungal Divers. 2015, 74, 3–18. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. 38 - Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar] [CrossRef]
- Vilgalys, R.; Hester, M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 1990, 172, 4238–4246. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, K.; Cigelnik, E. Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungusfusariumare nonorthologous. Mol. Phylogenet. Evol. 1997, 7, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; Whelen, S.; Hall, B.D. Phylogenetic relationships amongascomycetes evidence from an RNA polymerse II subunit. Mol. Biol. Evol 1999, 16, 1799–1808. [Google Scholar] [CrossRef]
- Crous, P.W.; Wingfield, M.J.; Schumacher, R.K.; Akulov, A.; Bulgakov, T.S.; Carnegie, A.J.; Jurjević, Ž.; Decock, C.; Denman, S.; Lombard, L.; et al. New and Interesting Fungi. 3. Fungal Syst. Evol. 2020, 6, 157–231. [Google Scholar] [CrossRef]
- Hyde, K.D.; Dong, Y.; Phookamsak, R.; Jeewon, R.; Bhat, D.J.; Jones, E.B.G.; Liu, N.G.; Abeywickrama, P.D.; Mapook, A.; Wei, D.P.; et al. Fungal diversity notes 1151–1276: Taxonomic and phylogenetic contributions on genera and species of fungal taxa. Fungal Divers. 2020, 100, 5–277. [Google Scholar] [CrossRef]
- Pem, D.; Jeewon, R.; Selcuk, F.; Ulukapi, M.; Bhat, J.; Doilom, M.; Lumyong, S.; Hyde, K.D. Ribosomal and protein gene phylogeny reveals novel saprobic fungal species from Juglans regia and Urtica dioica. Front. Microbiol. 2020, 11, 1303. [Google Scholar] [CrossRef]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 2019, 20, 1160–1166. [Google Scholar] [CrossRef]
- Capella-Gutiérrez, S.; Silla-Martínez, J.M.; Gabaldón, T. trimAl: A tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 2009, 25, 1972–1973. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In Proceedings of the 2010 Gateway Computing Environments Workshop (GCE), New Orleans, LA, USA, 14 November 2010; pp. 1–8. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Nylander, J.A.A.; Wilgenbusch, J.C.; Warren, D.L.; Swofford, D.L. AWTY: A system for graphical exploration of MCMC convergence in Bayesian phylogenetics. Bioinformatics 2008, 24, 581–583. [Google Scholar] [CrossRef]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef]
- Rambaut, A. FigTree Version 1.4.0. 2012. Available online: http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 18 February 2023).
- Chethana, K.T.; Manawasinghe, I.S.; Hurdeal, V.G.; Bhunjun, C.S.; Appadoo, M.A.; Gentekaki, E.; Raspé, O.; Promputtha, I.; Hyde, K.D. What are fungal species and how to delineate them? Fungal Divers. 2021, 109, 1–25. [Google Scholar] [CrossRef]
- Maharachchikumbura, S.S.; Chen, Y.; Ariyawansa, H.A.; Hyde, K.D.; Haelewaters, D.; Perera, R.H.; Samarakoon, M.C.; Wanasinghe, D.N.; Bustamante, D.E.; Liu, J.K.; et al. Integrative approaches for species delimitation in Ascomycota. Fungal Divers. 2021, 109, 155–179. [Google Scholar] [CrossRef]
- Niranjan, M.; Sarma, V.V. Twelve new species of ascomycetous from Andaman Islands, India. Kavaka 2018, 50, 84–97. [Google Scholar]
- Saccardo, P.A. Sylloge Pyrenomycetum, In Sylloge Fungorum; Sumptibus Auctoris: Padua, Italy, 1883; Volume 2, pp. 1–813. [Google Scholar]
- Boerema, G.H.; de Gruyter, J.; van Kesteren, H.A. Contributions towards a monograph of Phoma (Coelomycetes)–III-1. Section Plenodomus: Taxa often with a Leptosphaeria teleomorph. Pers. Mol. Phylogeny Evol. Fungi 1994, 15, 431–487. [Google Scholar]
- Jayawardena, R.S.; Hyde, K.D.; de Farias, A.R.; Bhunjun, C.S.; Ferdinandez, H.S.; Manamgoda, D.S.; Udayanga, D.; Herath, I.S.; Thambugala, K.M.; Manawasinghe, I.S.; et al. What is a species in fungal plant pathogens? Fungal Divers. 2021, 109, 239–266. [Google Scholar] [CrossRef]
- Manawasinghe, I.S.; Phillips, A.J.; Xu, J.; Balasuriya, A.; Hyde, K.D.; Stępień, Ł.; Harischandra, D.L.; Karunarathna, A.; Yan, J.; Weerasinghe, J.; et al. Defining a species in fungal plant pathology: Beyond the species level. Fungal Divers. 2021, 109, 267–282. [Google Scholar] [CrossRef]
- Bhunjun, C.S.; Niskanen, T.; Suwannarach, N.; Wannathes, N.; Chen, Y.J.; McKenzie, E.H.C.; Maharachchikumbura, S.S.N.; Buyck, B.; Zhao, C.L.; Fan, Y.G.; et al. The numbers of fungi: Are the most speciose genera truly diverse? Fungal Divers. 2022, 114, 387–462. [Google Scholar] [CrossRef]
- Kirk, P.M.; Cannon, P.F.; Minter, D.W.; Stalpers, J.A. Dictionary of the Fungi, 10th ed.; CABI Bioscience: Wallingford, UK, 2008. [Google Scholar]
- CABI. Leptosphaeria maculans (stem canker). Distribution Maps of Plant Diseases; CAB International: Wallingford, UK, 2019; Available online: https://www.cabi.org/isc/datasheet/31468 (accessed on 20 February 2023).
- Farr, D.F.; Rossman, A.Y.; Fungal Databases. U.S. National Fungus Collections, ARS, USDA. 2021. Available online: https://nt.ars-grin.gov/fungaldatabases (accessed on 15 February 2023).
- Petrak, F. Mykologische Notizen. III. Annales Mycologici 1921, 19, 176–223. [Google Scholar]
- Petrak, F. List of New Species and Varieties of Fungi, New Combinations and New Names Published 1932-1935; Commonwealth Mycological Institute: Egham, UK, 1944; Volume 7, p. 983. [Google Scholar]
- Tennakoon, D.S.; Gentekaki, E.; Jeewon, R.; Kuo, C.H.; Promputtha, I.; Hyde, K.D. Life in leaf litter: Fungal community succession during decomposition. Mycosphere 2021, 12, 406–429. [Google Scholar] [CrossRef]
- Tennakoon, D.S.; Kuo, C.H.; Maharachchikumbura, S.S.; Thambugala, K.M.; Gentekaki, E.; Phillips, A.J.; Bhat, D.J.; Wanasinghe, D.N.; de Silva, N.I.; Promputtha, I.; et al. Taxonomic and phylogenetic contributions to Celtis formosana, Ficus ampelas, F. septica, Macaranga tanarius and Morus australis leaf litter inhabiting microfungi. Fungal Divers. 2021, 108, 1–215. [Google Scholar] [CrossRef]
- Hyde, K.D.; Norphanphoun, C.; Chen, J.; Dissanayake, A.J.; Doilom, M.; Hongsanan, S.; Jayawardena, R.S.; Jeewon, R.; Perera, R.H.; Thongbai, B.; et al. Thailand’s amazing diversity: Up to 96% of fungi in northern Thailand may be novel. Fungal Divers. 2018, 93, 215–239. [Google Scholar] [CrossRef]
- Hyde, K.D.; Jeewon, R.; Chen, Y.J.; Bhunjun, C.S.; Calabon, M.S.; Jiang, H.B.; Lin, C.G.; Norphanphoun, C.; Sysouphanthong, P.; Pem, D.; et al. The numbers of fungi: Is the descriptive curve flattening? Fungal Divers. 2020, 103, 219–271. [Google Scholar] [CrossRef]
- Liu, S.Q. Preliminary study on the development path of Zhaotong literature. Yunnan People’s Publ. House China 2015, 12, 178. [Google Scholar]
- Pei, Y. Analysis of temperature variation characteristics in Zhaotong City in recent 50 years. J. Agric. Catastrophol. 2022, 12, 3. [Google Scholar]
- Wang, L.; Shi, Z.; Ye, L.; Su, B. Analysis on the characteristics of extreme weather events in Kunming City during recent 20 Years. IOP Conf. Ser. Earth Environ. Sci. 2019, 252, 042124. [Google Scholar] [CrossRef]
- Wanasinghe, D.N.; Wijayawardene, N.N.; Xu, J.; Cheewangkoon, R.; Mortimer, P.E. Taxonomic novelties in Magnolia-associated pleosporalean fungi in the Kunming Botanical Gardens (Yunnan, China). PLoS ONE 2020, 15, e0235855. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Chen, S.L.; Dai, Y.C.; Jia, Z.F.; Li, T.H.; Liu, T.Z.; Phurbu, D.; Mamut, R.; Sun, G.Y.; Bau, T.; et al. Overview of China’s nomenclature novelties of fungi in the new century (2000–2020). Mycosystema 2021, 40, 822–833. [Google Scholar]
- Shoemaker, R.A. Canadian and some extralimital Leptosphaeria species. Can. J. Bot. 1984, 62, 2688–2729. [Google Scholar] [CrossRef]
- Hyde, K.D.; Hongsanan, S.; Jeewon, R.; Bhat, J.D.; McKenzie, E.H.C.; Jones, E.B.G.; Phookamsak, R.; Ariyawansa, H.A.; Boonmee, S.; Zhao, Q.; et al. Fungal diversity notes 367–490: Taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers. 2016, 80, 1–270. [Google Scholar] [CrossRef]
- Dayarathne, M.C.; Phookamsak, R.; Ariyawansa, H.A.; Jones, E.B.G.; Camporesi, E.; Hyde, K.D. Phylogenetic and morphological appraisal of Leptosphaeria italica sp. nov. (Leptosphaeriaceae, Pleosporales) from Italy. Mycosphere 2015, 6, 634–642. [Google Scholar] [CrossRef]
- Huhndorf, S.M. Systematics of Leptosphaeria species found on the Rosaceae. Ill. Nat. Hist. Surv. Bull. 1992, 34, 479–533. [Google Scholar] [CrossRef]
- Müller, E. Die schweizerischen arten der gattung Leptosphaeria und ihrer verwandten. Sydowia Ann. Mycol. Ed. Notitiam Sci. Mycol. Univers. 1950, 4, 185–319. [Google Scholar]
- Macia, M.J.; Palm, M.E.; Martin, M.P. A new species of Leptosphaeria (Ascomycotina, Pleosporales) on Rosaceae from Bolivia. Mycotaxon 2005, 93, 401–406. [Google Scholar]
- Boerema, G.H.; de Gruyter, J. Contributions towards a monograph of Phoma (Coelomycetes)–III-Supplement: Additional species of section Plenodomus. Pers. Mol. Phylogeny Evol. Fungi 1999, 17, 273–280. [Google Scholar]
- Quaedvlieg, W.; Verkley, G.J.; Shin, H.D.; Barreto, R.W.; Alfenas, A.C.; Swart, W.J.; Groenewald, J.Z.; Crous, P.W. Sizing up Septoria. Stud. Mycol. 2013, 75, 307–390. [Google Scholar] [CrossRef]
- Shoemaker, R.A.; Brun, H. The teleomorph of the weakly aggressive segregate of Leptosphaeria maculans. Can. J. Bot. 2001, 79, 412–419. [Google Scholar] [CrossRef]
- Dearness, J.; House, H.D. New or noteworthy species of fungi. II. Bull. New York State Mus. 1921, 233–234, 32–43. [Google Scholar]
- Lucas, M.T. Culture studies on Portuguese species of Leptosphaeria I. Trans. Br. Mycol. Soc. 1963, 46, 361–367. [Google Scholar] [CrossRef]
- Saccardo, P.A. Fungi nonnulli extra-Italici novi ex herbariis C.C. Gillet, P. Morthier, et G. Winter. Michelia 1878, 1, 357–360. [Google Scholar]
- Berlese, A.N. Excursion mycologique dans le Frioul. Bull. De La Société Mycol. De Fr. 1889, 5, 36–59. [Google Scholar]
- Fuckel, L. Symbolae mycologicae. Beiträge zur Kenntniss der Rheinischen Pilze. Jahrbücher des Nassauischen Vereins für Naturkunde 1870, 23–24, 1–459. [Google Scholar]
- Fuckel, L. Symbolae mycologicae. Beiträge zur Kenntniss der rheinischen Pilze. Zweiter Nachtrag; Julius Neidner: Wiesbaden, Germany, 1873; pp. 1–99. [Google Scholar]
- Lowen, R.; Sivanesan, A. Leptosphaeria pimpinellae and its Phoma anamorph. Mycotaxon 1989, 35, 205–210. [Google Scholar]
- Boerema, G.H.; Loerakker, W.M. Notes on Phoma. 2. Trans. Br. Mycol. Soc. 1985, 84, 289–302. [Google Scholar] [CrossRef]
- Ellis, J.B.; Everhart, B.M. New species of fungi from Washington Territory. Collected by W.N. Suksdorf during the summer and fall of 1883. Bull. Washburn Lab. Nat. Hist. 1884, 1, 3–6. [Google Scholar]
- Punithalingam, E.; Holliday, P. Deuterophoma tracheiphila. In Descriptions of Pathogenic Fungi and Bacteria; CABI International: Wallingford, UK, 1973; p. 399. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).