Abstract
The fungus Monilinia fructicola is responsible for brown rot on stone and pome fruit and causes heavy yield losses both pre- and post-harvest. Several mycoviruses are known to infect fungal plant pathogens. In this study, a metagenomic approach was applied to obtain a comprehensive characterization of the mycovirome in a worldwide collection of 58 M. fructicola strains. Deep sequencing of double-stranded (ds)RNA extracts revealed a great abundance and variety of mycoviruses. A total of 32 phylogenetically distinct positive-sense (+) single-stranded (ss)RNA viruses were identified. They included twelve mitoviruses, one in the proposed family Splipalmiviridae, and twelve botourmiaviruses (phylum Lenarviricota), eleven of which were novel viral species; two hypoviruses, three in the proposed family Fusariviridae, and one barnavirus (phylum Pisuviricota); as well as one novel beny-like virus (phylum Kitrinoviricota), the first one identified in Ascomycetes. A partial sequence of a new putative ssDNA mycovirus related to viruses within the Parvoviridae family was detected in a M. fructicola isolate from Serbia. The availability of genomic sequences of mycoviruses will serve as a solid basis for further research aimed at deepening the knowledge on virus–host and virus–virus interactions and to explore their potential as biocontrol agents against brown rot disease.
Keywords:
mycovirus; stone fruit; +ssRNA virus; mitovirus; splipalmivirus; botourmiavirus; benyvirus; hypovirus; fusarivirus; barnavirus; parvovirus 1. Introduction
Mycoviruses are viruses infecting a broad range of hosts in the major taxa of fungi and oomycetes, including many important pathogens of plants, insects, and humans [1,2,3,4], mycorrhizal fungi [5], yeasts [6], and mushrooms [7,8].
Recent studies have greatly enhanced our knowledge of the diversity and evolution of fungal viruses [9]. Mycoviruses are characterized by either single- or multi-segmented genomes that can be naked or differently packaged in virus-like particles (VLPs) with an isometric, spherical, bacilliform, or filamentous shape [10]. The majority of known mycoviruses possess linear double-stranded RNA (dsRNA) or positive-sense (+) single-stranded RNA (ssRNA) genomes [2,11], although mycoviruses with linear negative-sense (-) ssRNA [12] as well as single-stranded DNA (ssDNA) genomes [13] have been reported. Thanks to the advent of high-throughput next-generation sequencing (NGS), the number of mycoviral sequences available in the GenBank database has drastically increased to >770. According to the International Committee on Taxonomy of Viruses (ICTV; https://talk.ictvonline.org/taxonomy, accessed on 20 January 2022), mycoviruses are currently classified into 23 viral families and one unclassified viral genus [6]. However, taxonomic changes and the establishment of new families are very frequent due to the fast discovery of novel species that remain unclassified or are classified into taxa not yet formally approved [14,15].
Most mycoviruses lack an extracellular phase in their replication cycle and can be transmitted, with various efficiencies, horizontally to other fungal individuals via hyphal anastomosis, and vertically to asexual or sexual spores [1,16]. It is known that horizontal transmission may be hindered by vegetative incompatibility, a type of non-self-recognition that often induces programmed cell death and restricts the spread of molecular parasites [17]. This can pose limitations to the use of mycoviruses as biocontrol agents against fungal diseases. However, the strength of the barrier to mycovirus spreading due to vegetative incompatibility can vary considerably [18]. For instance, free virions of ssDNA viruses within the family Genomoviridae can infect fungal hosts on artificial media or in plant tissues [19,20], as well as mycophagous insects [21]. Moreover, some viruses, such as Sclerotinia sclerotiorum mycoreovirus 4 (SsMYRV4), can suppress vegetative incompatibility in a fungal host [22].
Recent studies on mycoviruses revealed fundamental aspects of the complex virus–host interactions that can be influenced by environmental conditions, host strain, temperature, and possible co-infecting viruses, resulting in various effects on their hosts [23]. Multiple viral infections are common in fungi and different viruses may co-exist and replicate in a single host [7,24,25]. Complex virus–virus interplays in co-infected hosts can be synergistic, antagonistic, or neutral interactions and have practical outcomes for the potential use of mycoviruses as biocontrol agents (e.g., [26]).
Mycovirus infections are mostly cryptic or responsible for mild symptoms with no apparent effects on fungal biology [27]. Nevertheless, some mycoviruses are associated with a range of altered phenotypic traits, beneficial or detrimental to their hosts [1,2]. Killer phenotypes in yeasts represent a classical example of a beneficial viral–host interaction [28]. Mycoviruses can increase the virulence (hypervirulence) of plant- and human-pathogenic fungi [29,30]. On the opposite, mycoviruses associated to host debilitation may cause reduced virulence (hypovirulence), impaired growth and sporulation, abnormal morphology and pigmentation, as well as altered metabolic regulation [1,2,31,32,33]. In mushroom cultivations, viruses are associated with economically important diseases causing heavy yield losses in several fungal species, such as Agaricus bisporus, Lentinula edodes, and Pleurotus spp. [7,34,35]. In pathogenic fungi, mycoviruses can cause hypovirulence and might be exploited as potential biocontrol agents against fungal diseases [18]. Moreover, mycoviruses might play a role in the adaptation of fungi to certain ecological niches. For instance, Pestalotiopsis theae chrysovirus-1 (PtCV1) modulates endophytic and phytopathogenic traits of Pestalotiopsis theae [36]; Curvularia thermal tolerance virus (CThTV), in a three-way symbiosis, confers heat tolerance to both the endophyte Curvularia protuberata and its host plant, a tropical panic grass, and allows both organisms to grow at high soil temperatures [37].
The most well-known example of successful biocontrol mediated by a mycovirus is the application of Cryphonectria hypovirus 1 (CHV-1) against the chestnut blight fungus Cryphonectria parasitica [38]. The widespread success of CHV-1 led to extensive studies of mycovirus-mediated hypovirulence, resulting in the discovery of several mycoviruses with biocontrol potentials in important phytopathogenic fungi, such as the white mould fungus Sclerotinia sclerotiorum [13], Ophiostoma novo-ulmi [39] causing the Dutch elm disease, the grey mould fungus Botrytis cinerea, the rice blast fungus Pyricularia oryzae [40], Plasmopara halstedii causing downy mildew on sunflower [41], and soil-borne pathogens, such as Rhizoctonia solani [42] and Rosellinia necatrix [43].
NGS technologies have changed the approach of investigations on fungal-associated viruses, allowing the detection of single or very few viral sequences in libraries from total RNA [44,45] or small RNA (sRNA) [46,47], although the first approach usually provides a more complete characterization of the fungal virome [48].
Monilinia fructicola (G. Winter) Honey is among the most destructive fungal pathogens of rosaceous fruit crops, mainly stone fruits. It induces American brown rot, causing blossom and twig blight and fruit decay, both in the field and postharvest, resulting in heavy yield losses. Following its introduction into Europe [49], the pathogen spread widely and rapidly, and for its greater fitness often prevails over the endogenous species Monilinia laxa and Monilinia fructigena [50,51].
There is only limited information available on mycoviruses infecting species of the genus Monilinia. Earlier studies have revealed the presence of viral dsRNAs and VLPs in M. fructicola isolates from peaches and nectarines in New Zealand [52], with no apparent effects on virulence, grow rate, and sporulation. Tran et al. [53] identified a hypovirus (Sclerotinia sclerotiorum hypovirus 2), a betapartitivirus (Fusarium poae virus 1), and a mycoflexivirus (Botrytis virus F) in M. fructicola and M. laxa isolates from Western Australia. Coinfection of the three mycoviruses in M. fructicola increases its grow rate in vitro by 10% but does not affect its virulence on fruits. The complete genome of a novel virus, Monilinia umbra-like virus 1 (MULV1), infecting M. fructigena was recently obtained by total RNA sequencing of mummified peach fruits [54]. Recently, Jo et al. [55] reported 20 partial and uncharacterized genome sequences of mycoviruses obtained by in silico shotgun assembly of Illumina RNA-Seq reads from 18 libraries of M. fructicola, M. fructigena, and M. laxa [56]. Most of them were from two Italian M. fructigena isolates.
The aim of the present work was to investigate the mycovirome of M. fructicola in a worldwide collection of isolates of the fungus from different hosts using an NGS-based metagenomic approach.
2. Materials and Methods
2.1. Fungal Isolates and Growth Conditions
A collection of fifty-eight isolates of M. fructicola from various host plants and locations worldwide was used (Table 1). All isolates, stored at −80 °C in glycerol (10% v/v) until use, were revitalised and grown on potato dextrose agar (PDA; infusion from 200 g peeled and sliced potatoes kept at 60 °C for 1 h, 20 g dextrose, adjusted at pH 6.5, 20 g agar Oxoid No. 3, per litre of distilled water), at 21 ± 1 °C in the dark for 7 days to obtain conidia. Aliquots (10 mL) of conidial suspension (0.7–1.3 × 106 conidia mL−1), obtained by scraping the plate surface with water with a few drops of Tween 20 added, were added to 190 mL of complete medium (CMV; 10 mL solution A (10 g KH2PO4, 100 mL−1 distilled water), 10 mL solution B (20 g NaNO3, 5 g MgSO4·7H2O, 0.1 g FeSO4, 100 mL−1 distilled water), 1 mL micro-nutritive solution [57], 10 g yeast extract Difco, 10 g casein acid-hydrolyzed Difco, 10 g bacto peptone Difco, 20 g glucose, and 1 mL vitamins stock solution [58] filtered and added to media after sterilization at 121 °C for 20 min) and maintained 60 h in an orbital shaker (150 rpm) at 25 ± 1 °C in the dark. Mycelium was collected on Miracloth (Calbiochem, San Diego, CA, USA) under a slight vacuum and washed twice with sterile distilled water.

Table 1.
Pools of M. fructicola strains used in the mycovirome characterization.
2.2. dsRNA Extraction and Purification
M. fructicola isolates were grouped in twelve pools, each of 4–5 isolates, basically according to their origin (Table 1). dsRNAs were isolated from each pool according to [59], with some modifications. In detail, a mix of an equal amount (2.5 g) of mycelium from each isolate was freeze dried and ground to a fine powder with a mortar and pestle in liquid nitrogen. To each pooled sample, 20 mL of GPS buffer (0.2 M glycine, 0.1 M Na2HPO4, 0.6 M NaCl, pH 9.5) were added and the mixture was stirred on ice for 30 min. Then, 2 mL of 10% SDS (sodium dodecyl-sulfate), 0.2 mL of β-mercaptoethanol, 20 mL of phenol pH 8.0, and 20 mL of chloroform-pentanol (25:1) were added and the mixture gently shaken for 30 min and then centrifuged at 9000 rpm for 20 min at 4 °C. The aqueous phase was recovered and extracted again with 20 mL of phenol and 20 mL of chloroform-pentanol (25:1). Afterwards, it was added with ethanol to a final concentration of 15% v:v and 1 g of CF11-cellulose (Whatman, Kent, UK), and stirred for 15 min. Nucleic acids adsorbed on cellulose were precipitated by centrifugation at 4500 rpm for 15 min at 4 °C. The pellet was resuspended with 10 mL of 15% ethanol STE (0.1 M NaCl, 0.05 M tris-HCl, 0.001 M Na2EDTA, pH 8.0) and used for the chromatographic column preparation by using a vacuum manifold (Supelco VisiprepTM, Bellefonte, PA, USA). After washing with 100 mL of STE added with 15% ethanol, nucleic acids were finally eluted with 10 mL STE and overnight precipitated with ethanol (1:2, v:v) at −20 °C. The pellet obtained after centrifugation at 14,000 rpm for 30 min at 4 °C was finally suspended in 200 μL of TE (0.01 M Tris-HCl, 0.001 M Na2EDTA, pH 8.0).
Nucleic acids were digested with 0.02 U mL−1 DNAase I (Life Technologies Corporation, Carlsbad, CA, USA) and then with 500 ng mL−1 ribonuclease A from bovine pancreas (Sigma-Aldrich, St. Louis, MO, USA), following the manufacturer’s instruction. Proteins were removed sequentially with equal volumes of phenol:chloroform-isoamyl alcohol (1:1, 24-1) and chloroform, and then dsRNA was pelleted at 14,000 rpm for 20 min after addition of 0.1 vol. 3 M sodium acetate (pH 5.5) and 2.5 vol. ethanol at −80 °C for 30 min. The pellet, washed with cold 70% ethanol, was vacuum-dried and re-dissolved in 15 μL of nuclease-free water.
The presence and quality of dsRNA were checked by electrophoresis on 1% (w/v) agarose (CertifiedTM Molecular Biology Agarose, Bio-Rad Laboratories, Hercules, CA, USA) gel run in TAE buffer (0.04 M tris-base, 0.02 M CH3COOH, 0.001 M Na2EDTA, pH 8.0) at 90 V for 110 min. The quality and quantity of dsRNA were assessed by absorption spectra using a NanoDrop 2000 Spectrophotometer (Thermo Scientific Inc., Wilmington, DE, USA). Prior to usage, dsRNA samples were also quantified using the Qubit RNA BR Assay (Invitrogen, Paisley, UK).
2.3. dsRNA-Seq Library Preparation and High-Throughput Sequencing
cDNA libraries were prepared from 0.8 µg dsRNA extracts from each pool of M. fructicola isolates using the TruSeqTM RNA Sample Preparation Kit v2 (Illumina, San Diego, CA, USA) in accordance with the manufacturer’s instructions, with some modifications. After validation by spectrophotometric (NanoDrop 2000) and fluorometric (Qubit® 2.0) assays and agarose gel electrophoresis, normalized cDNA libraries were multiplexed (12 libraries per lane) and custom-sequenced at the Genewiz Inc. NGS Service (South Plainfield, NJ, USA), using an Illumina HiSeq2500 platform with a 2 × 150 bp configuration and an average insert size of ~200 bp.
2.4. Bioinformatics Analysis
After a quality check using FastX-tools (http://hannonlab.cshl.edu/fastx_toolkit; accessed on 27 September 2020), the reads were trimmed and assembled de novo using CLC Genomics Workbench 7.0.3 (CLC Bio, Qiagen, Hilden, Germany) with the default settings. SeqMan Pro (Lasergene v.15.0.1; DNASTAR, Inc., Madison, WI, USA) was used for merging the smaller contigs into larger scaffolds. The consensus sequences were then subjected to BLASTN and BLASTX analysis against viral nucleotide and protein sequences in the NCBI database (www.ncbi.nlm.nih.gov; accessed on: 17 November 2020), with the default parameters and the E-value cut-off of 10−5. These sequences were filtered for redundancy at a 90% nucleotide identity over 90% of the length, and representative mycoviral sequences were selected from each pool. Selected sequences were classified according to the hit with the highest bit score and used in the subsequent analyses. To determine and correct assembly errors, row reads were mapped back to the de-novo assembled viral contigs, using SeqMan NGen and reads alignments visualized in SeqMan Pro. Frequencies of detection for each reconstructed mycovirus in each pool of isolates, expressed in RPKM (Reads Per Kilobase per Million mapped reads), were quantified using CLC Genomics Workbench.
Prediction of the open reading frames (ORFs) into the assembled viral genomes was performed using SeqBuilder (Lasergene v.15.0.1; DNASTAR). Protein molecular weight was calculated using the tool available at https://www.bioinformatics.org/sms/prot_mw.html (accessed on: 11 December 2021). Conserved protein domains were identified using MOTIF search (GenomeNet, Japan; https://www.genome.jp/tools/motif) in PFAM, NCBI-CDD, PROSITE pattern and profile databases (accessed on: 14 December 2021).
2.5. Phylogenetic Analysis
The amino acid sequences of the replication-associated proteins in M. fructicola viruses, the three closest homologues identified by BLASTP analysis, as well as the reference viruses for the family were used for multiple alignments with MUSCLE implemented in MEGA-X [60]. Phylogenetic trees were computed by MEGA-X using the maximum likelihood (ML) method set to the default parameters. Statistical support for the branches was evaluated using a bootstrap analysis [61] with 1000 replicates. iTOL v6 [62] was used to display, annotate, and manage the phylogenetic trees.
2.6. PCR Validation of Assembled ssDNA Viral Contigs
To confirm the presence of a putative ssDNA virus detected from pool No. 4, total DNA was extracted from each isolate in the pool using Gentra Puregene tissue kits (Qiagen, Milan, Italy), according to the manufacturer’s instructions. A PCR primer pair (p111Fw 5′-AGTGCCCAAATAGACGTCTCT-3′/ p2573Rv 5′-GCTGCATCGTGTTCTTTTGC-3′) was designed to generate a 1.67 kb product covering the genomic region included between the two available contigs. PCR products were assessed using gel electrophoresis and Sanger sequencing by external service (Genewiz, Takeley, UK).
3. Results
3.1. Extraction of dsRNA and Analysis of Electrophoretic Profiles
The total amount of dsRNA ranged from 2.2 μg (pool 8) to 20 μg (pool 11). The dsRNAs yielded on agarose gel 1–4 bands having sizes ranging from 1.6 to 3.0 kb for most dsRNA samples, except for the pool No. 5 that showed an additional band of about 11 kb (Supplementary Figure S1).
3.2. High-Throughput Sequencing and Reconstruction of Viral Genomes
A total of 340,001,023 paired-end (PE) reads were generated by Illumina NGS resulting in ~98 Gb of raw sequencing data with a mean quality score (QS) of 36.7. The number of non-redundant reads in the dsRNA samples ranged from 12,863,610 (pool No. 3) to 26,697,938 (pool No. 4) (Table 2).

Table 2.
Summary statistics of the sequencing data.
The assembly of the high-quality reads (QS ≥ 30) yielded an average of about 43,000 contigs per pool, corresponding to 34.1 Mb and 81.9–98.6% of the mapping reads (Table 3). The assembled contigs included 61–91% of small sequences with length < 1 kb (data not shown). An average number of 1339 contigs per pool showed similarity to viral proteins in the BLASTX search and were selected as putative mycoviral sequences. After filtering for redundancy, the number of identified sequences was reduced to an average of 193 per pool, ranging from 67 (pool No. 9) to 588 (pool No. 6).

Table 3.
Summary of the de novo assembly and BLASTP search of contigs.
Viruses from different pools showing identity at the amino acid level >95% were considered variants of the same virus; after manual inspection of alignment, the sequence of each virus with the highest read coverage and sequence length was selected as the representative one.
A total number of 33 viral genomes were identified from the assembled sequence reads (Table 4). Based on sequence analysis, mycoviruses were classified in different taxonomical groups, including 32 viruses that were predicted to represent +ssRNA viruses belonging to seven different viral families within the realm Riboviria, phyla Lenarviricota (Mitoviridae, the proposed family Splipalmiviridae, Botourmiaviridae), Pisuviricota (Hypoviridae, the proposed family Fusariviridae, Barnaviridae), Kitrinoviricota (Benyviridae), and only one ssDNA virus with similarity to viruses of the Parvoviridae family (phylum Cossaviricota, realm Monodnaviria).

Table 4.
Putative mycoviruses identified in Monilinia fructicola.
3.2.1. Mitoviridae and Splipalmiviridae
Twelve sequences showed similarity to viruses in the family Mitoviridae (Table 4). The size of the reconstructed sequences ranged from 2298 to 2816 nt, likely representing near-complete genomes, and contained a single ORF encoding an RdRp protein with a conserved domain (PF05919) and length ranging between 683 and 794 aa (77.47–80.84 kDa), except Monilinia fructicola mitovirus 11 (MfrcMV11) which showed a larger protein of 866 aa (99.99 kDa). The highest sequence identity of the RdRp sequence (89.7%) was found between MfrcMV7 and MfrcMV8, while the remaining viruses showed an identity at the amino acid level of ≤47%. Then, they included 11 distinct species, according to the demarcation criteria established by ICTV for mitoviruses (https://talk.ictvonline.org; accessed on: 10 January 2022). Most shared amino acid sequence identities ranging between 63.3% and 99.1%, with mitoviruses of B. cinerea (MfrcMV1, MfrcMV9 and MfrcMV12) or S. sclerotiorum (MfrcMV2 to 4, MfrcMV6 to 8, and MfrcMV10 and 11), while Monilinia fructicola mitovirus 5 (MfrcMV5) showed low identity (42.3%) with Ophiostoma mitovirus 6.
A putative new narna-like virus with two genomic segments (RNA1 and RNA2) was found and named Monilinia fructicola splipalmivirus 1 (MfrcSPV1). RNA1 was 2466 nt in length and coded for a single protein 785 aa in size and 89.2 kDa, while the partial sequence of RNA2 was 2123 nt in length and included an incomplete ORF coding for a protein 698 aa in size. RNA1 showed 77.2% aa sequence identity to Erisiphe necator associated narnavirus 13 and about 50% to Plasmopara viticola lesion associated narnavirus 4 and Alternaria tenuissima narnavirus 1; both segments showed aa sequence identity of 53.7% (RNA1) and 47.9% (RNA2) with Botrytis cinerea binarnavirus 2 and other ‘binarnaviruses’ found in B. cinerea [14], now included in a group of viruses for which alternative names of polynarnaviruses and splipalmiviruses have been proposed [5,63]. Like other viruses in this group, e.g., [63], MfrcSPV1 showed the RdRp palm subdomains split into the RNA1 (motifs F, A, and B) and RNA2 (motifs C and D) segments (data not shown).
The phylogenetic relationships of the M. fructicola mycoviruses and their relatives in the Mitoviridae and the proposed family Splipalmiviridae, within the phylum Lenarviricota, are shown in Figure 1. The phylogenetic analysis grouped mitoviruses into two major clades and 5 subclades and the M. fructicola viruses were distributed among them. MfrcSPV1 was grouped with Erysiphe necator associated narnavirus 13, Botrytis cinerea binarnavirus 2, and Plasmopara viticola lesion associated narnavirus 4 in a strongly supported clade much closer to Mitoviridae than to Narnaviridae.
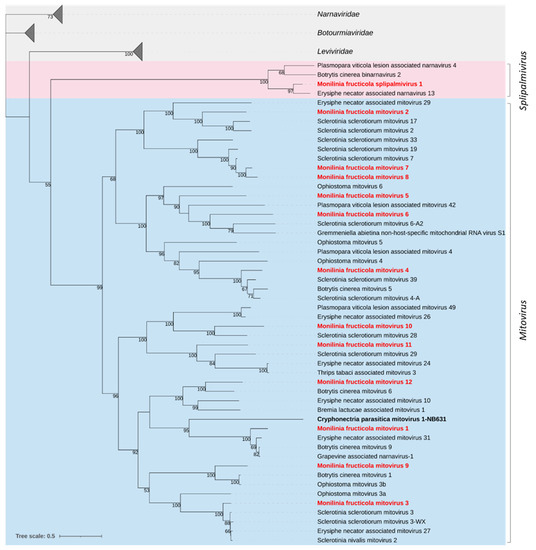
Figure 1.
Maximum likelihood phylogenetic tree based on multiple amino acid sequence alignment of the RdRp proteins of viruses in the family Mitoviridae (cyan) and the proposed family Splipalmiviridae (pink; [14]), and their relationships with the closest families Leviviridae, Narnaviridae, and Botourmiaviridae in the phylum Lenarviricota. Mycoviruses found in this work are in bold red. The numbers on nodes, shown for values greater than 50%, are the results of 1000 bootstrap analyses. The scale bar represents a genetic distance of 0.5 amino acid substitutions per site. The list of accession numbers of the analysed sequences is in Table S1.
3.2.2. Botourmiaviridae
Twelve viral genomes showed similarity to members of the family Botourmiaviridae. The genus Ourmiavirus includes plant viruses with a tripartite genome, while the other genera include viruses infecting fungi (Botoulivirus, Magoulivirus, Penoulivirus and Rhizoulivirus) or both plants and fungi (Scleroulivirus), with a monopartite +ssRNA genome and a single ORF encoding an RNA-directed RNA polymerase (RdRp).
The sequences from M. fructicola ranged from 2382 to 3004 nt in length and contained complete ORFs (645–780 aa; 70.88–89.14 kDa) with an RdRp conserved domain (PF05919). Three RdRp sequences showed high similarity with botourmiaviruses already known in Monilinia species. In details, Monilinia fructicola botourmiavirus 5 (MfrcBOV5) showed 99.7% aa sequence identity with Monilinia ourmiavirus B from M. fructicola; Monilinia fructicola botourmiavirus 9 and 10 (MfrcBOV9 and 10) showed 86.6% and 85.9% aa sequence identity, respectively, with two viruses identified in M. fructigena, Monilinia ourmiavirus I, and Monilinia ourmiavirus A/Mfrg. Four other sequences of Monilinia fructicola botourmiaviruses showed significant levels of similarity (70.3%-86.6% identity) with the RdRp proteins to botourmiaviruses identified in S. sclerotiorum (MfrcBOV1 and MfrcBOV3) and B. cinerea (MfrcBOV2 and MfrcBOV4).
According to the ICTV species demarcation criterium (RdRp identity ≤ 90%) within the family Botourmiaviridae, MfrcBOV1 to 4, 9, and 10 should represent new species of botourmiaviruses. The remaining viral genomes, Monilinia fructicola botourmiavirus 6, 7, 8, 11, and 12 (MfrcBOV6, 7, 8, 11 and 12), shared sequence identities lower than 55% with botourmiaviruses identified in other fungi and oomycetes. According to the genera demarcation criteria (RdRp identity ≤70%) these should therefore represent members of five new genera in the family Botourmiaviridae [64].
The phylogenetic analysis showed that the family Botourmiaviridae was subdivided into two major clades and six subclades, each representing a genus. Five of the identified Monilinia fructicola botourmiaviruses were clustered in the genus Scleroulivirus (MfrcBOV1, 6, 7, 8, and 12), four in the genus Penoulivirus (MfrcBOV4, 9,10, and 11), two in the genus Botoulivirus (MfrcBOV2 and 3), and one in the genus Magoulivirus (MfrcBOV5) (Figure 2).
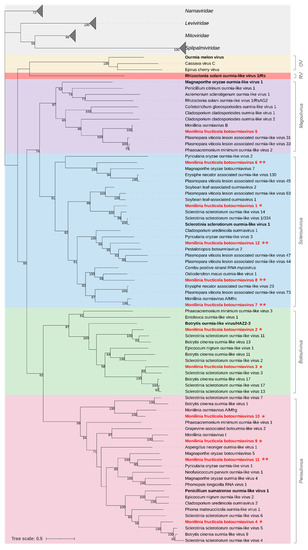
Figure 2.
Maximum likelihood phylogenetic tree based on multiple amino acid sequence alignment of the RdRp proteins of viruses in the family Botourmiaviridae and its relationships with the closest families Narnaviridae, Leviviridae, Splipalmiviridae, and Mitoviridae in the phylum Lenarviricota. The genera in the Botourmiaviridae family are represented with different coloured boxes: Ourmiavirus (OV, yellow), Rhizoulivirus (RV, red), Magoulivirus (purple), Scleroulivirus (cyan), Botoulivirus (green), and Penoulivirus (pink). The type species is in bold black. Mycoviruses found in this work are in bold red. Single or double asterisks indicate new proposed species and genera, respectively. The numbers on nodes, shown for values greater than 50%, are the results of 1000 bootstrap analyses. The scale bar represents a genetic distance of 0.5 amino acid substitutions per site. The list of accession numbers of the analysed sequences is in the Table S1.
3.2.3. Hypoviridae
Two large genomic sequences showing high homology with viruses of the family Hypoviridae were identified and tentatively named Monilinia fructicola hypovirus 1 and 2 (MfrcHV1 and 2) (Table 4). The family includes just one officially recognized genus, Hypovirus, comprising non-encapsidated viruses with +ssRNA genomes of 9.1–12.7 kb that possess one or two ORFs [65].
MfrcHV1, with a genome length of 9338 nt and a single ORF of 8754 nt coding for a polyprotein of 2918 aa and 331.49 kDa, showed high aa sequence identity with Sclerotinia sclerotiorum hypovirus 7 (97.7%) and Botrytis cinerea hypovirus 1 (96.9%). The polyprotein showed three conserved domains, an RdRp (PF00680), a PPPDE putative peptidase (PF05903), and a C-terminal helicase (PF00271).
MfrcHV2, with a genome length of 15,037 nt and a single ORF of 14,355 nt coding for a polyprotein of 4784 aa and 539.32 kDa, showed high aa sequence similarity with Monilinia hypovirus D (93.2%) and Sclerotinia sclerotiorum hypovirus 2 (85.3%). Two conserved domains were detected in the polyprotein, superfamilies 1 and 2 ATP-binding type-1 (PS51192), and C-terminal (PS51194) helicases.
Recently, it has been proposed to replace the genus Hypovirus in the family Hypoviridae with the two genera Alphahypovirus and Betahypovirus (e.g., [66]). Hu et al. [67] proposed a third genus, Gammahypovirus. Phylogenetic analysis based on the full-length aa sequence of the polyproteins (Figure 3), clustered MfrcHV1 with the type species Cryphonectria hypovirus 3 and Cryphonectria hypovirus 4, Sclerotinia sclerotiorum hypovirus 7, and Botrytis cinerea hypovirus 1 in the proposed genus Betahypovirus; MfrcHV2 was clustered with Sclerotinia sclerotiorum hypovirus 2 in the proposed genus Gammahypovirus including also Botrytis cinerea hypovirus 5, Sclerotium rolfsii hypovirus 1, and Monilinia hypovirus D.
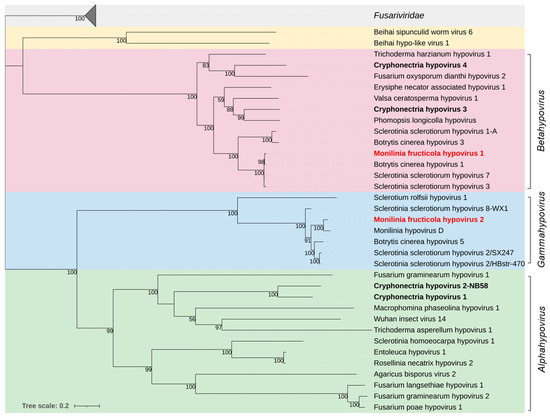
Figure 3.
Maximum likelihood phylogenetic tree based on multiple amino acid sequence alignment of the RdRp proteins of viruses in the family Hypoviridae and its relationship with the closely related proposed family Fusariviridae. The three genera in the Hypoviridae family are represented with different coloured boxes: Alphahypovirus (green), Betahypovirus (red), and the proposed genus Gammahypovirus (cyan). The Beihai hypo-like virus and Beihei sipunculid worm virus 6 (yellow) are proposed as members of an additional genus [65]. The type species are in bold black. Mycoviruses found in this work are in bold red. The numbers on nodes, shown for values greater than 50%, are the results of 1000 bootstrap analyses. The scale bar represents a genetic distance of 0.2 amino acid substitutions per site. The list of accession numbers of the analysed sequences is in Table S1.
3.2.4. Fusariviridae
Two near-complete viral genomes, provisionally named Monilinia fructicola fusarivirus 1 and 2 (MfrcFV1 and 2), and a partial genome of Monilinia fructicola fusarivirus 3 (MfrcFV3), all related to members of the proposed family Fusariviridae, were reconstructed. These consisted of +ssRNA genomes of 7425 nt (MfrcFV1), 7330 nt (MfrcFV2), and 5550 nt (MfrcFV3), each containing two ORFs, the largest ORF1 encoding a polyprotein containing an RdRp conserved domain (PF00680) and superfamilies 1 and 2 ATP-binding type-1 (PS51192) and C-terminal (PS51194) helicases, and the ORF2 encoding a hypothetical protein.
The MfrcFV1 polyprotein (1661 aa, 191.55 kDa) revealed an aa sequence identity of 81.2% to Monilinia fusarivirus G from M. fructigena and ~40% with other fusariviruses. The ORF2-encoded protein (504 aa, 57.74 kDa) revealed an aa identity of 71.5% to Monilinia fusarivirus G. It contained a conserved DUF1322 domain (PF07032).
The MfrcFV2 polyprotein (1667 aa, 192.23 kDa) revealed aa sequence identity (97.4%) to Monilinia fusarivirus B from M. fructigena and ≤ 52.9% to other fusariviruses. The ORF2-encoded protein (509 aa, 56.73 kDa) revealed aa identity of 95.5% identity to Monilinia fusarivirus B, with no conserved domains.
The partial MfcFV3 polyprotein (1399 aa) revealed an aa sequence identity (96.2%) to Monilinia barnavirus J from M. fructigena and ~88% to Botrytis cinerea fusarivirus 6 and Sclerotinia sclerotiorum fusarivirus 2. The ORF2-encoded protein (434 aa) revealed aa identity of 93.8% to Monilinia barnavirus J and 70% identity to Sclerotinia sclerotiorum fusarivirus 2. It showed SMC (COG1196) and Spc7 (PF08317) domains.
Phylogenetic analysis yielded two groups of fusariviruses, in agreement with Gilbert et al. [44]. MfrcFV1 and MfrcFV2 were included in Group 2, including other fusariviruses previously detected in M. fructigena as well as in other ascomycetes. MfrcFV3 was clustered in a clade of Group 1, including fusariviruses detected in Ascomycetes, and well distinguished by the other clade of the same Group, including fusariviruses from Basidiomycetes (Figure 4).
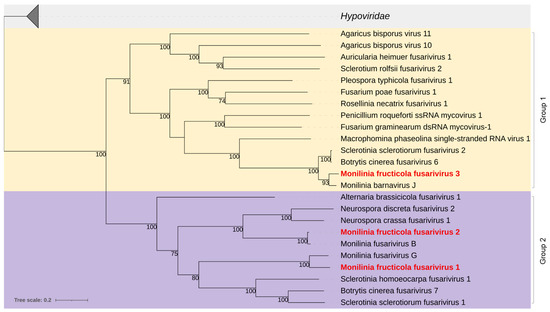
Figure 4.
Maximum likelihood phylogenetic tree based on multiple amino acid sequence alignment of the RdRp proteins of viruses in the proposed Fusariviridae family and its relationship with the closely related family Hypoviridae. Coloured boxes indicate Group 1 (yellow) and Group 2 (purple) of the fusariviruses [44]. Mycoviruses found in this work are in bold red. The numbers on nodes, shown for values greater than 50%, are the results of 1000 bootstrap analyses. The scale bar represents a genetic distance of 0.2 amino acid substitutions per site. The list of accession numbers of the analysed sequences is in Table S1.
3.2.5. Barnaviridae
A 4993 nt sequence showed similarity to barnaviruses and was provisionally named Monilinia fructicola barnavirus 1 (MfrcBV1). It included three ORFs, ORF1 codes for a peptidase (861 aa), ORF2 for a RdRp (691 aa), and ORF3 for a protein of unknown function (238 aa), with an overlapping of 991 bp and a -1 frameshifting between ORF1 and ORF2. ORF1 contained two conserved domains, a trypsin-like peptidase (PF13365) and a codanin-1 C-terminus (PF15296); ORF2 contained RdRp domains (PF02123 and PF00680), whereas no domains or motifs were detected in the ORF3. The three ORF-encoded proteins showed sequence identities from 94.5% to 99.6% to Monilinia barnavirus A identified in the transcriptome of M. fructigena [55] (Table 4).
Phylogenetic analysis of the RdRp clustered MfrcBV1 in a clade with Monilinia barnavirus A, Sclerotinia sclerotiorum barnavirus 1, and Riboviria spp. from soil, different from the clade including barnaviruses from basidiomycetes, the type-species Mushroom bacilliform virus LF-1, Rhizoctonia solani barnavirus 1, and Tulasnella barnavirus 1 (Figure 5).
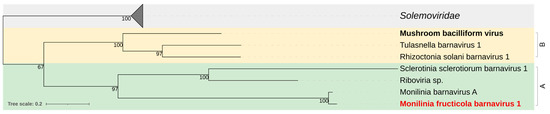
Figure 5.
Maximum likelihood phylogenetic tree based on multiple amino acid sequence alignment of the replicase protein of Monilinia fructicola barnavirus 1 and viruses of the genus Barnavirus (Barnaviridae family) reported in Basidiomycetes (B; yellow) and Ascomycetes (A; green), and the closely related members of the Solemoviridae family (grey). The type species is in bold black. The mycovirus found in this work is in bold red. The number on nodes, shown for values greater than 50%, are the results of 1000 bootstrap analyses. The scale bar represents a genetic distance of 0.2 amino acid substitutions per site. The list of accession numbers of the analysed sequences is in Table S1.
3.2.6. Benyviridae
A 5680 nt sequence showed significant similarity to members of the family Benyviridae and was provisionally named Monilinia fructicola beny-like virus 1 (MfrcBeLV1). MfrcBeLV1 contained two ORFs (Table 4). The ORF1 encoded a replication-associated protein (1623 aa, 182.96 kDa), with three conserved domains, a Superfamily 1 RNA helicase (PF01443), an RdRp (PF00978), and an Alphavirus-like methyltransferase PROSITE profile (PS51743). The protein showed 47.6% and 40.2% aa sequence identities to Lentinula edodes beny-like virus 1 and Agaricus bisporus virus 13, respectively [7,68]. The ORF2 codes for a hypothetical protein (214 aa; 23.55 kDa) and no conserved domains were detected.
Phylogenetic analysis of the ORF1-encoded protein clustered MfrcBeLV1 in a clade including other mycoviruses and well discriminated by the other two clades, one including viruses from insects and the other from plants (Figure 6).
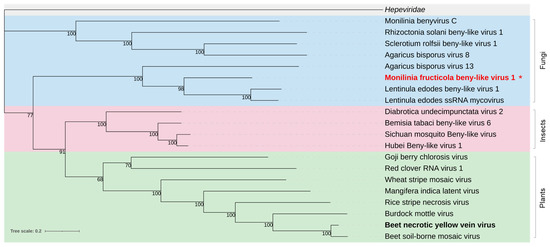
Figure 6.
Maximum likelihood phylogenetic tree based on multiple amino acid sequence alignment of the nonstructural replication-associated protein of Monilinia fructicola beny-like virus 1 and beny-like viruses (Benyviridae family) from various hosts, including fungi (blue), insects (pink), and plants (green). The Hepeviridae family (grey) was used as outgroup. The type species is in bold black. The mycovirus found in this work is in bold red. The asterisk indicates the newly proposed species. The number on the nodes, shown for values greater than 50%, are the results of 1000 bootstrap analyses. The scale bar represents a genetic distance of 0.2 amino acid substitutions per site. The list of accession numbers of the analysed sequences is in Table S1.
3.2.7. Parvoviridae
Two short sequences of 398 and 329 nt showing similarity to members of the family Parvoviridae were identified. Viruses in this family have linear, ssDNA genomes of 4–6 kb and typically infect vertebrates, including humans, and invertebrates. Due to the unexpected association of a parvo-like virus with a fungal host, we confirmed the finding through PCR. Total DNA from single isolates of the pool 4 was analysed and the Serbian Mfrc407 isolate (Table 1) showed to carry the virus. Hence, the region between the two contigs was reconstructed through Sanger sequencing. The new virus was provisionally named Monilinia fructicola parvo-like virus 1 (MfrcPV1). The partial genomic sequence (1617 nt; GenBank accession number ON038373) included incomplete ORFs coding for a nonstructural (NS) protein and a capsid virus protein (VP), which represent the two major gene cassettes in Parvoviridae members [69]. The NS protein showed identity of 67.7% to Human CSF-associated densovirus; the VP protein showed identity of 97.5% to a parvo-like virus from the wild bird Ciconia boyciana (Table 4). MfrcPV1 is the first member of the Parviviridae family detected in a fungal host.
3.2.8. Frequencies of Mycoviral Sequences in M. fructicola dsRNAs
The distribution and prevalence of mycoviruses of each family were examined in the pools of M. fructicola isolates (Figure 7), each including basically isolates of the same geographical origin (Table 1).
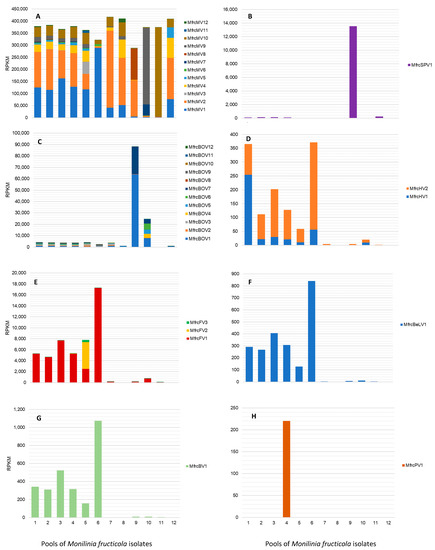
Figure 7.
Detection rate of viral families and single viruses in 12 pools of Monilinia fructicola isolates in RPKM (reads normalized per kilobases of viral genome length and millions of total reads mapped on the virome). (A) Mitoviriridae; (B) Splipalmiviridae; (C) Botourmiaviridae; (D) Hypoviridae; (E) Fusariviridae; (F) Benyviridae; (G) Barnaviridae; (H) Parvoviridae.
Mitoviruses were largely prevalent with high and quite uniform frequencies in all the pools, representing on average 95.5% of the total row reads, ranging from 73.9% (pool No. 9) to more than 99% (pools No 7, 8, 11, and 12). In detail, MfrcMV2 and MfrcMV1 were abundant in all the pools, followed by MfrcMV10, MfrcMV9, and MfrcMV4, while others (e.g., MfrcMV11 and MfrcMV3) were less represented and their genomes assembled from reads of few pools, such as pool No. 5. The genome sequence of the new putative splipalmivirus MfrcSPV1, mostly reconstructed from reads of the pool No. 9 (South of Italy, Basilicata), was also detected in other pools (i.e., No. 1–4 and 11). Viruses in the family Botourmiaviridae were detected at lower frequencies than mitoviruses in all the pools, corresponding to less than 1.1% of the row reads, except for pools No. 9 (22.6%) and No. 10 (6.2%). Overall, MfrcBOV1 and MfrcBOV7 were more frequent than MfrcBOV10, MfrcBOV11, MfrcBOV12, and MfrcBOV8. The latter was present with a low number of row reads only in pool No. 7, including isolates from China. The sequences of the two Monilinia hypoviruses were represented by a relatively low number of reads, up to 254 RPKM for MfrcHV1 (pool No. 1) and 315 RPKM for MfrcHV2 (pool No. 6). Similarly, the partial sequence of the putative fusarivirus MfrcFV3 was assembled from reads of pool No. 5 (397 RPKM), whereas the other two fusariviruses were more prevalent in pools No. 6 (17.3 × 103 RPKM) and No. 1–5 (2.5–7.7 × 103 RPKM) for MfrcFV1, and in pool No. 5 (4.9 × 103 RPKM) for MfrcFV2. The new beny-like virus MfrcBLV1 and the barnavirus MfrcBV1 were both more represented in pool No. 6, with RPKM values of 8.4 × 102 and 1.1 × 103, respectively, and at lower rate in the pools No. 1–5. A relatively low number of reads (2.2 × 102 RPKM) mapping on the partially assembled new parvo-like virus MfrcPV1 was exclusively found in pool No. 4, including isolates from Serbia and, in particular, the isolate Mfrc407 hosting the virus.
4. Discussion and Conclusions
In this study, a metagenomic-based strategy was used to explore the mycovirome associated with the plant-pathogenic fungus M. fructicola, inducing brown rot on stone and pome fruit. The approach combined deep sequencing analysis with Illumina technology, which is highly sensitive in viral detection [7,70,71], and dsRNA-enrichment in samples, to maximize the number of short reads derived from viruses [72]. dsRNA analysis indeed permits the detection of viruses with dsRNA as well as ssRNA or DNA genomes due to generation of transient dsRNA [73,74].
A worldwide collection of 58 M. fructicola isolates from several stone fruit (cherry, sour cherry, peach, flat peach, nectarine, and plum) and nine countries, encompassing four continents, were analysed aiming at obtaining the most comprehensive overview on viruses associated with the pathogen. The isolates were grouped in 12 pools basically on their geographical origin, as successfully performed by Ruiz-Padilla et al. [14].
Preliminarily, dsRNA extracts from single pools were analysed through gel electrophoresis and diverse patterns with variable numbers of bands were observed, as previously reported for M. fructicola [52] and the related species B. cinerea and S. sclerotiorum [14,45,75].
Illumina sequencing allowed to get a total of 100 Gb, which included 14 Gb (70 million of PE 2 × 150 bp short reads) representing the virome of the analysed isolates. Bioinformatic analysis allowed to reconstruct nearly full-length genomic sequences of 33 viruses. On the ground of their genomic structures, conserved domains, sequence homologies with known viruses, and phylogenetic analysis based on hallmarks in replication associated proteins (RdRp or polyprotein), these could be tentatively classified into three families (Mitoviridae, the proposed family Splipalmiviridae, and Botourmiaviridae) in the phylum Lenarviricota, three (Hypoviridae, the proposed family Fusariviridae, and Barnaviridae) in the phylum Pisuviricota, and one (Benyviridae) in the phylum Kitrinoviricota, which were the three phyla characterised by +ssRNA genomes (kingdom Orthornavirae) in the realm Riboviria. Just one virus had an ssDNA genome and was tentatively classified in the family Parvoviridae, phylum Cossaviricota of the realm Monodnaviria. To our knowledge, this is the first comprehensive analysis of viral diversity in M. fructicola.
Hence, most of the viruses identified in M. fructicola had a +ssRNA genome, thus belonging to Riboviria, the realm that encloses the largest and most diverse group of eukaryote-infecting viruses, including most plant viruses and many important human and animal pathogens [76]. The prevalence of +ssRNA in M. fructicola agrees with previous reports on the diversity and predominance of +ssRNA viruses over -ssRNA and dsRNA viruses in several fungal species [7,14,15,45,68,75]. This might be due to greater infection, replication, and transmission efficiency of these types of viruses in their hosts [77]. Nevertheless, it should be considered that this could be a result of the experimental approaches adopted in fungal growth, nucleic acid extraction, and sequencing procedures that might have favoured the selection of +ssRNA viruses. These may indeed have secondary and tertiary structures, increasing the cellulose binding and protection from nuclease activity [7].
Mycoviruses in the phylum Lenarviricota were prevalent in M. fructicola isolates, in agreement with previous findings in other fungal plant pathogens [14,15]. These included 12 viruses belonging to the family Mitoviridae and 12 viruses belonging to the family Botourmiaviridae. Members of the two families are naked +ssRNA viruses with very simple genomes of 2.2 to 4.4 kb encoding a single RdRp [5,78].
Viruses of the family Mitoviridae occur in fungi and some lead to latent infections while others, such as Botrytis cinerea mitovirus 1 and Sclerotinia sclerotiorum mitovirus 2/KL-1, have obvious phenotypic effects in the fungal hosts, causing swollen and malformed mitochondria and reduced fungal growth and virulence [79,80]. Although mitoviruses have never been reported in Monilinia species, we found that these are prevalent components of the virome in M. fructicola since they were detected at high frequency in all the analysed samples. This finding agrees with reports for other fungi [14,45,75,81]. Mitoviruses are presumably unaffected by antiviral RNA silencing in the host due to their mitochondrial localization and this might contribute to their widespread occurrence [82]. The 12 mitoviruses detected in M. fructicola fall into five distinct clades in the family Mitoviridae and this shows their broad genetic diversity.
Mitoviruses might play an important role in pathosystems other than inducing fungal hypovirulence. Indeed, there are reports of their horizontal transfer from fungi to plants as, for instance, in the case of Botrytis cinerea mitovirus 10 (BcMV10) transmitted from Botrytis cinerea to cucumber plants [83]. Moreover, integration events of cDNA of fragments or complete genomes of fungal mitoviruses, likely through a transposon-encoded reverse transcriptase, seems to occur in the mitochondrial DNA of vascular plants (nonretroviral endogenized RNA virus elements; NERVEs) [84]. The outcomes of these processes remain to be investigated. The issue is still debated since bioinformatic analysis revealed the existence of mitoviruses in plants so that a subdivision of the genus Mitovirus has been proposed to group viruses from both plants and fungi [85].
Monilinia fructicola splipalmivirus 1 (MfrcSPV1) along with the three more strictly related Erysiphe necator associated narnavirus 13, Botrytis cinerea binarnavirus 2, and Plasmopara viticola lesion associated narnavirus 4 were grouped phylogenetically in a single cluster much closer to Mitoviridae than Narnaviridae. This finding supports the proposed institution of the new family Splipalmiviridae, including viruses with segmented genomes coding for RdRp conserved motifs on separated segments [63], and highlights the uncertainty in the discrimination of these taxa.
Mycoviruses in Botourmiaviridae are closely related to plant ourmiaviruses and have a wide range of hosts, revealing rapid evolution and high diversity [86]. We found a great diversity in the 12 botourmiaviruses detected in M. fructicola that were indeed distributed among the genera Botoulivirus, Magoulivirus, Scleroulivirus [64], and the recently proposed genus Penoulivirus [14]. Viruses in this family do not appear to affect fungal hosts although there are few exceptions, such as Fusarium oxysporium ourmia-like virus 1 causing hypovirulence in Fusarium oxysporum f.sp. mormordicae [87]. ICTV established criteria for demarcation of species and genera in the Botourmiaviridae based on RdRp sequence identity lower than 90% and 70%, respectively [64]. Therefore, most detected viruses, except for Monilinia fructicola botourmiaviruses 5, should be new species, and the five Monilinia fructicola botourmiaviruses 6, 7, 8, 11, and 12, should be members of five new distinct genera. These findings suggest the need of a taxonomic revision of the family Botourmiaviridae, also taking into consideration that many new viruses have been recently added (e.g., [87,88,89]).
We found two hypoviruses in M. fructicola, Monilinia fructicola hypovirus 1 and 2 (MfrcHV1 and MfrcHV2). The phylogenetic analysis divided the family Hypoviridae into three major clades, corresponding to the genera Alphahypovirus and Betahypovirus, with the type species Cryphonectria hypovirus 1 and 2 and Cryphonectria hypovirus 3 and 4, respectively, and the proposed new genus Gammahypovirus [80,90]. MrfcHV1 showed 97% sequence identity to Botrytis cinerea hypovirus 1, a member of the genus Betahypovirus, inhibiting formation of infection cushions and causing hypovirulence in B. cinerea [67,91]. MfrcHV2 showed 85% sequence identity to Sclerotinia sclerotiorum hypovirus 2, inducing morphological and structural alterations and hypovirulence in S. sclerotiorum, and should be a member of the genus Gammahypovirus [78,90], along with Sclerotium rolfsii hypovirus 1, associated to hypovirulence [24], and Botrytis cinerea hypovirus 5 [14]. Therefore, the results here presented support the proposal of establishing the third genus Gammahypovirus in the family.
The three viruses Monilinia fructicola fusarivirus 1, 2, and 3 (MfrcFV1, 2 and 3) were related to the family Fusariviridae, proposed but not yet officially approved by ICTV, close to Hypoviridae. Phylogenetic analysis divided the family into two major clades, Group 1 and Group 2. This agrees with Gilbert et al. [44] and Picarelli et al. [81], who recognized the two groups due to different genome organization, with Group 1 having the functional domains SMC and Spc7 in the ORF2, as opposed to Group 2 that does not contain any predicted domains. MfrcFV3 should be a member of Group 1, having Fusarium graminearium dsRNA mycovirus-1 as a reference, while MfrcFV1 and MfrcFV2 should be members of Group 2.
Barnaviridae typically includes mycoviruses from the Basidiomycetes and has the Mushroom bacilliform virus LF-1/AUS as the type species. Monilinia fructicola barnavirus 1 (MfrcBV1) along with the almost identical Monilinia barnavirus A found in a M. fructigena transcriptome [55], and Sclerotinia sclerotiorum barnavirus 1 [92], are so far the only members associated with the Ascomycetes.
There is only the genus Benyvirus in the family Benyviridae, having four to five +ssRNAs of 6.7, 4.6, 1.8, 1.4, and 1.3 kb [93] and including viruses from plants, insects, and fungi. These were separated in three well-defined clades by phylogenetic analysis. In M. fructicola, we could detect, like in Rhizoctonia solani beny-like virus 1 [45], only the RNA1 segment encoding the RdRp protein of Monilinia fructicola benyvirus 1 (MfrcBenV1). This is the first Bernyvirus identified in the Ascomycetes and should be a new viral species, showing an RdRp sequence identity lower than 48% with the closest Lentinula edodes ssRNA mycovirus.
There are only few reports of ssDNA viruses associated with fungi. The few examples are the viruses belonging to the family Genomoviridae detected in S. sclerotiorum [13], Fusarium graminearum [94] and B. cinerea [14,20]. In contrast, we found two partial sequences of 329 and 398 nt of ssDNAs showing similarity to members of the Parvoviridae family. This family includes ssDNA viruses with a genome sized 4–6 kb and is divided into three subfamilies: Parvovirinae, Densovirinae, and the recently approved Hamaparvovirinae [95]. The host spectrum of these viruses is very broad and includes humans, mammals, fishes, birds, tunicates, arthropods, and flatworms. Some viruses are pathogenic for insects and are used as biocontrol agents [96]. This was a very unexpected result; therefore, the M. fructicola isolate hosting the virus Mfrc407 from Nectarine in Serbia was individuated among the pooled isolates. The sequence between the two original fragments was reconstructed by PCR and Sanger sequencing, obtaining a still partial sequence of 1617 nt. It contained two partial ORFs: ORF1 encoding a non-structural replication associated protein and ORF2 encoding the coat protein. ORF1 showed a sequence identity as high as 67% with the Human CFS-associated densovirus and ORF2 a sequence identity of 96.8% to the Ciconia boyciana parvovirus. Then, the virus was tentatively named Monilinia fructicola parvo-like virus 1 (MfrcPLV1), which is the first virus in the Parvoviridae family detected in a fungal host. The genome of the virus is incomplete and its characterization and possible role in fungal biology are worthwhile of further research.
In conclusion, this study provided new insights into the mycovirome diversity of M. fructicola, an important pathogen inducing brown rot of stone and pome fruit, thus enhancing the already broad collection of mycoviruses in plant pathogenic fungi. Although many mycoviruses are cryptic and do not alter the phenotypic traits of their fungal hosts, these may be a source of diversity and play important roles in their population biology. The availability of near full-length viral sequences associated with M. fructicola will provide crucial tools for investigations into the complex virus–host and virus–virus interactions and improve the background knowledge to explore the potential use of mycoviruses as biocontrol agents, which is certainly worthwhile of further research to obtain to sustainable and environmentally friendly control strategies against this economically important pathogen.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jof8050481/s1, Table S1: List of accession numbers of the sequences used for phylogenetic analyses; Figure S1: Example of electrophoretic patterns of dsRNA extracted from pools of Monilinia fructicola isolates on a 1.5% agarose gel.
Author Contributions
C.R. (Caterina Rotolo), R.M.D.M.A. and S.P. contributed to the acquisition of data; C.R. (Caterina Rotolo), C.R. (Celeste Raguseo), D.G. and R.M.D.M.A. contributed to data analysis; C.R. (Caterina Rotolo) and C.R. (Celeste Raguseo) contributed to the original draft preparation and R.M.D.M.A. substantively revised it. S.P. supervised in the data analysis and writing of the manuscript. F.F. planned the project, designed the experiments, supervised, and aided in the writing, and coordinated the collaboration of the authors. All authors participated to the study conception and design, to the interpretation of data. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the University of Bari Aldo Moro for the project “Epidemiology, genetics of plant pathogens and development of molecular diagnostic methods”, Code Disspa16Ricat15. The research working contract of Donato Gerin was financially supported by Regione Puglia under the Programme: Research for Innovation (REFIN)—POR Puglia FESR-FSE 2014/2020—Asse X—Azione 10.4, project: "Applications of new technologies for developing IPM strategies for table and wine grapes, stone fruit and pomegranate against pathogens of new introduction or mycotoxin producers to improve the sustainability of production processes and the quality and security of produce"—Code 119061D5.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All raw sequencing reads were stored in the Sequence Read Archive (SRA) database: BioProject accession number PRJNA818196, BioSample accession numbers from SAMN26849489 to SAMN26849500, and SRA runs from SRX14530422 to SRX14530433. Genome sequences of the mycoviruses identified have been deposited in GenBank under accession numbers ON038373 and ON038375—ON038406 (detailed in Supplementary Table S1).
Acknowledgments
The bioinformatic analysis was partially carried out using the facilities of the ReCaS Data Center of the University of Bari (https://www.recas-bari.it). The authors thank Cecile Guinet (Agence nationale de sécurité sanitaire de l’alimentation, de l’environnement et du travail—ANSES, France), George Karaoglanidis (Aristotle University of Thessaloniki, Greece), Bevan Weir (Landcare Research Manaaki Whenua, New Zealand), Jovana Hrustic (Istitute za Pesticide, Serbia), Guido Schnabel (Clemson University, USA), Antonieta De Cal (Instituto Nacional de Investigación y Tecnología Agraria y Alimentaria, Spain), Li-Yun Guo (China Agricultural University, China), Gerard C. M. Van Leeuwen (National Reference Centre—NPPO, The Netherlands), Themis Michailides (University of California, Berkeley, USA). Franco Nigro (DiSSPA—University of Bari, Italy) that provided isolates from their collections for this study. The authors also thank Massimo Turina, Luisa Rubino and Angelantonio Minafra (IPSP-CNR, Bari), for their useful comments and suggestions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pearson, M.N.; Beever, R.E.; Boine, B.; Arthur, K. Mycoviruses of filamentous fungi and their relevance to plant pathology. Mol. Plant Pathol. 2009, 10, 115–128. [Google Scholar] [CrossRef]
- Ghabrial, S.A.; Castón, J.R.; Jiang, D.; Nibert, M.L.; Suzuki, N. 50-plus years of fungal viruses. Virology 2015, 479, 356–368. [Google Scholar] [CrossRef] [Green Version]
- Hillman, B.I.; Annisa, A.; Suzuki, N. Viruses of plant-interacting fungi. Adv. Virus Res. 2018, 100, 99–116. [Google Scholar] [CrossRef]
- Uchida, K.; Sakuta, K.; Ito, A.; Takahashi, Y.; Katayama, Y.; Omatsu, T.; Mizutani, T.; Arie, T.; Komatsu, K.; Fukuhara, T.; et al. Two novel endornaviruses co-infecting a Phytophthora pathogen of Asparagus officinalis modulate the developmental stages and fungicide sensitivities of the host oomycete. Front. Microbiol. 2021, 12, 122. [Google Scholar] [CrossRef]
- Sutela, S.; Forgia, M.; Vainio, E.J.; Chiapello, M.; Daghino, S.; Vallino, M.; Martino, E.; Girlanda, M.; Perotto, S.; Turina, M. The virome from a collection of endomycorrhizal fungi reveals new viral taxa with unprecedented genome organization. Virus Evol. 2020, 6, veaa076. [Google Scholar] [CrossRef]
- Crucitti, D.; Chiapello, M.; Oliva, D.; Forgia, M.; Turina, M.; Carimi, F.; La Bella, F.; Pacifico, D. Identification and molecular characterization of novel mycoviruses in Saccharomyces and non-Saccharomyces yeasts of oenological interest. Viruses 2022, 14, 52. [Google Scholar] [CrossRef]
- Deakin, G.; Dobbs, E.; Bennett, J.M.; Jones, I.M.; Grogan, H.M.; Burton, K.S. Multiple viral infections in Agaricus bisporus-characterisation of 18 unique RNA viruses and 8 ORFans identified by deep sequencing. Sci. Rep. 2017, 7, 2469. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.H.; Fujita, M.; Chiba, S.; Hyodo, K.; Andika, I.B.; Suzuki, N.; Kondo, H. Two novel fungal negative-strand RNA viruses related to mymonaviruses and phenuiviruses in the shiitake mushroom (Lentinula edodes). Virology 2019, 533, 125–136. [Google Scholar] [CrossRef]
- Myers, J.M.; Bonds, A.E.; Clemons, R.A.; Thapa, N.A.; Simmons, D.R.; Carter-House, D.; Ortanez, J.; Liu, P.; Miralles-Durán, A.; Desirò, A.; et al. Survey of early-diverging lineages of fungi reveals abundant and diverse mycoviruses. mBio 2020, 11, e02027-20. [Google Scholar] [CrossRef]
- Sato, Y.; Castón, J.R.; Suzuki, N. The biological attributes, genome architecture and packaging of diverse multi-component fungal viruses. Curr. Opin. Virol. 2018, 33, 55–65. [Google Scholar] [CrossRef]
- Son, M.; Yu, J.; Kim, K.H. Five questions about mycoviruses. PLoS Pathog. 2015, 11, e1005172. [Google Scholar] [CrossRef]
- Kondo, H.; Chiba, S.; Toyoda, K.; Suzuki, N. Evidence for negative-strand RNA virus infection in fungi. Virology 2013, 435, 201–209. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.; Li, B.; Fu, Y.; Jiang, D.; Ghabrial, S.A.; Li, G.; Peng, Y.; Xie, J.; Cheng, J.; Huang, J.; et al. A geminivirus-related DNA mycovirus that confers hypovirulence to a plant pathogenic fungus. Proc. Natl. Acad. Sci. USA 2010, 107, 8387–8392. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Padilla, A.; Rodríguez-Romero, J.; Gómez-Cid, I.; Pacifico, D.; Ayllón, M.A. Novel mycoviruses discovered in the mycovirome of a necrotrophic fungus. mBio 2021, 12, e03705-20. [Google Scholar] [CrossRef]
- Chiapello, M.; Rodríguez-Romero, J.; Ayllón, M.A.; Turina, M. Analysis of the virome associated to grapevine downy mildew lesions reveals new mycovirus lineages. Virus Evol. 2020, 6, veaa058. [Google Scholar] [CrossRef]
- Ghabrial, S.A.; Suzuki, N. Viruses of plant pathogenic fungi. Annu. Rev. Phytopathol. 2009, 47, 353–384. [Google Scholar] [CrossRef]
- Choi, G.H.; Dawe, A.L.; Churbanov, A.; Smith, M.L.; Milgroom, M.G.; Nuss, D.L. Molecular characterization of vegetative incompatibility genes that restrict hypovirus transmission in the chestnut blight fungus Cryphonectria parasitica. Genetics 2012, 190, 113–127. [Google Scholar] [CrossRef] [Green Version]
- García-Pedrajas, M.D.; Cañizares, M.C.; Sarmiento-Villamil, J.L.; Jacquat, A.G.; Dambolena, J.S. Mycoviruses in biological control: From basic research to field implementation. Phytopathology 2019, 109, 1828–1839. [Google Scholar] [CrossRef]
- Yu, X.; Li, B.; Fu, Y.; Xie, J.; Cheng, J.; Ghabrial, S.A.; Li, G.; Yi, X.; Jiang, D. Extracellular transmission of a DNA mycovirus and its use as a natural fungicide. Proc. Natl. Acad. Sci. USA 2013, 110, 1452–1457. [Google Scholar] [CrossRef] [Green Version]
- Khalifa, M.E.; MacDiarmid, R.M. A mechanically transmitted DNA mycovirus is targeted by the defence machinery of its host, Botrytis cinerea. Viruses 2021, 13, 1315. [Google Scholar] [CrossRef]
- Liu, S.; Xie, J.; Cheng, J.; Li, B.; Chen, T.; Fu, Y.; Li, G.; Wang, M.; Jin, H.; Wan, H.; et al. Fungal DNA virus infects a mycophagous insect and utilizes it as a transmission vector. Proc. Natl. Acad. Sci. USA 2016, 113, 12803–12808. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Cheng, J.; Fu, Y.; Chen, T.; Jiang, D.; Ghabrial, S.A.; Xie, J. Virus-mediated suppression of host non-self recognition facilitates horizontal transmission of heterologous viruses. PLoS Pathog. 2017, 13, e1006234. [Google Scholar] [CrossRef] [Green Version]
- Hyder, R.; Pennanen, T.; Hamberg, L.; Vainio, E.J.; Piri, T.; Hantula, J. Two viruses of Heterobasidion confer beneficial, cryptic or detrimental effects to their hosts in different situations. Fungal Ecol. 2013, 6, 387–396. [Google Scholar] [CrossRef]
- Zhu, J.Z.; Zhu, H.J.; Da Gao, B.; Zhou, Q.; Zhong, J. Diverse, novel mycoviruses from the virome of a hypovirulent Sclerotium rolfsii strain. Front. Plant Sci. 2018, 9, 1738. [Google Scholar] [CrossRef] [Green Version]
- Osaki, H.; Sasaki, A.; Nomiyama, K.; Tomioka, K. Multiple virus infection in a single strain of Fusarium poae shown by deep sequencing. Virus Genes 2016, 52, 835–847. [Google Scholar] [CrossRef]
- Dobbs, E.; Deakin, G.; Bennett, J.; Fleming-Archibald, C.; Jones, I.; Grogan, H.; Burton, K. Viral interactions and pathogenesis during multiple viral infections in Agaricus bisporus. mBio 2021, 12, e03470-20. [Google Scholar] [CrossRef]
- Buck, K. Molecular variability of viruses of fungi. In Molecular Variability of Fungal Pathogens; Bridge, P.D., Couteaudier, Y., Clackson, J.M., Eds.; CAB International: Wallingford, UK, 1998; pp. 53–72. [Google Scholar]
- Schmitt, M.; Breinig, F. Yeast viral killer toxins: Lethality and self-protection. Nat. Rev. Microbiol. 2006, 4, 212–221. [Google Scholar] [CrossRef]
- Ahn, I.P.; Lee, Y.H. A viral double-stranded RNA up regulates the fungal virulence of Nectria radicicola. Mol. Plant-Microbe Interact. 2001, 14, 496–507. [Google Scholar] [CrossRef] [Green Version]
- Lau, S.K.P.; Lo, G.C.S.; Chow, F.W.N.; Fan, R.Y.Y.; Cai, J.J.; Yuen, K.Y.; Woo, P.C.Y. Novel Partitivirus enhances virulence of and causes aberrant gene expression in Talaromyces marneffei. mBio 2018, 9, e00947-18. [Google Scholar] [CrossRef] [Green Version]
- Nuss, D.L. Hypovirulence: Mycoviruses at the fungal-plant interface. Nat. Rev. Microbiol. 2005, 3, 632–642. [Google Scholar] [CrossRef]
- Fuke, K.; Takeshita, K.; Aoki, N.; Fukuhara, T.; Egusa, M.; Kodama, M.; Moriyama, H. The presence of double-stranded RNAs in Alternaria alternata Japanese pear pathotype is associated with morphological changes. J. Gen. Plant Pathol. 2011, 77, 248–252. [Google Scholar] [CrossRef]
- Kotta-Loizou, I. Mycoviruses and their role in fungal pathogenesis. Curr. Opin. Microbiol. 2021, 63, 10–18. [Google Scholar] [CrossRef]
- van der Lende, T.R.; Harmsen, M.C.; Go, S.J.; Wessels, J.G.; Wessel, J.G. Double-stranded RNA mycoviruses in mycelium of Pleurotus ostreatus. FEMS Microbiol. Lett. 1995, 125, 51–56. [Google Scholar] [CrossRef]
- Magae, Y. Molecular characterization of a novel mycovirus in the cultivated mushroom, Lentinula edodes. Virol. J. 2012, 9, 60. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Li, X.; Kotta-Loizou, I.; Dong, K.; Li, S.; Ni, D.; Hong, N.; Wang, G.; Xu, W. A mycovirus modulates the endophytic and pathogenic traits of a plant associated fungus. ISME J. 2021, 15, 1893–1906. [Google Scholar] [CrossRef]
- Márquez, L.M.; Redman, R.S.; Rodriguez, R.J.; Roossinck, M.J. A virus in a fungus in a plant: Three-way symbiosis required for thermal tolerance. Science 2007, 315, 513–515. [Google Scholar] [CrossRef] [Green Version]
- Feau, N.; Dutech, C.; Brusini, J.; Rigling, D.; Robin, C. Multiple introductions and recombination in Cryphonectria hypovirus 1: Perspective for a sustainable biological control of chestnut blight. Evol. Appl. 2014, 7, 580–596. [Google Scholar] [CrossRef]
- Brasier, C.M. Viruses as biological control agents of the dutch elm disease fungus Ophiostoma novo-ulmi. In The Elms; Dunn, C.P., Ed.; Springer: Boston, MA, USA, 2000; pp. 201–212. [Google Scholar] [CrossRef]
- Aihara, M.; Urayama, S.; Le, M.T.; Katoh, Y.; Higashiura, T.; Fukuhara, T.; Arie, T.; Teraoka, T.; Komatsu, K.; Moriyama, H. Infection by Magnaporthe oryzae chrysovirus 1 strain A triggers reduced virulence and pathogenic race conversion of its host fungus, Magnaporthe oryzae. J. Gen. Plant Pathol. 2018, 84, 92–103. [Google Scholar] [CrossRef]
- Grasse, W.; Zipper, R.; Totska, M.; Spring, O. Plasmopara halstedii virus causes hypovirulence in Plasmopara halstedii, the downy mildew pathogen of the sunflower. Fungal Genet. Biol. 2013, 57, 42–47. [Google Scholar] [CrossRef]
- Zheng, L.; Zhang, M.; Chen, Q.; Zhu, M.; Zhou, E. A novel mycovirus closely related to viruses in the genus Alphapartitivirus confers hypovirulence in the phytopathogenic fungus Rhizoctonia solani. Virology 2014, 456–457, 220–226. [Google Scholar] [CrossRef] [Green Version]
- Chiba, S.; Salaipeth, L.; Lin, Y.H.; Sasaki, A.; Kanematsu, S.; Suzuki, N. A novel bipartite double-stranded RNA mycovirus from the white root rot fungus Rosellinia necatrix: Molecular and biological characterization, taxonomic considerations, and potential for biological control. J. Virol. 2009, 83, 12801–12812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilbert, K.B.; Holcomb, E.E.; Allscheid, R.L.; Carrington, J.C. Hiding in plain sight: New virus genomes discovered via a systematic analysis of fungal public transcriptomes. PLoS ONE 2019, 14, e0219207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marzano, S.Y.; Nelson, B.D.; Ajayi-Oyetunde, O.; Bradley, C.A.; Hughes, T.J.; Hartman, G.L.; Eastburn, D.M.; Domier, L.L. Identification of diverse mycoviruses through metatranscriptomics characterization of the viromes of five major fungal plant pathogens. J. Virol. 2016, 90, 6846–6863. [Google Scholar] [CrossRef] [Green Version]
- Donaire, L.; Ayllon, M.A. Deep sequencing of mycovirus-derived small RNAs from Botrytis species. Mol. Plant Pathol. 2017, 18, 1127–1137. [Google Scholar] [CrossRef] [PubMed]
- Vainio, E.J.; Jurvansuu, J.; Streng, J.; Rajamäki, M.L.; Hantula, J.; Valkonen, J.P. Diagnosis and discovery of fungal viruses using deep sequencing of small RNAs. J. Gen. Virol. 2015, 96, 714–725. [Google Scholar] [CrossRef] [Green Version]
- Nerva, L.; Ciuffo, M.; Vallino, M.; Margaria, P.; Varese, G.C.; Gnavi, G.; Turina, M. Multiple approaches for the detection and characterization of viral and plasmid symbionts from a collection of marine fungi. Virus Res. 2016, 219, 22–38. [Google Scholar] [CrossRef] [PubMed]
- Lichou, J.; Myrin, J.F.; Breniaux, D. First report of brown rot caused by Monilinia fructicola in peach orchards in France. Phytoma 2002, 47, 22. [Google Scholar]
- Villarino, M.; Egüen, B.; Lamarca, N.; Segarra, J.; Usall, J.; Melgarejo, P.; De Cal, A. Occurrence of Monilinia laxa and M. fructigena after introduction of M. fructicola in peach orchards in Spain. Eur. J. Plant Pathol. 2013, 137, 835–845. [Google Scholar] [CrossRef]
- Abate, D.; Pastore, C.; Gerin, D.; De Miccolis Angelini, R.M.; Rotolo, C.; Pollastro, S.; Faretra, F. Characterization of Monilinia spp. populations on stone fruit in South Italy. Plant Dis. 2018, 102, 1708–1717. [Google Scholar] [CrossRef] [Green Version]
- Tsai, P.; Pearson, M.; Beever, R. Mycoviruses in Monilinia fructicola. Mycol. Res. 2004, 108, 907–912. [Google Scholar] [CrossRef]
- Tran, T.T.; Li, H.; Nguyen, D.Q.; Jones, M.G.; Wylie, S.J. Co-infection with three mycoviruses stimulates growth of a Monilinia fructicola isolate on nutrient medium but does not induce hypervirulence in a natural host. Viruses 2019, 11, 89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jo, Y.; Back, C.G.; Choi, H.; Cho, W.K. Comparative microbiome study of mummified peach fruits by metagenomics and metatranscriptomics. Plants 2020, 9, 1052. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.; Choi, H.; Chu, H.; Cho, W.K. Identification of viruses from fungal transcriptomes. BioRxiv, 2020; preprint. [Google Scholar] [CrossRef]
- De Miccolis Angelini, R.M.; Abate, D.; Rotolo, C.; Gerin, D.; Pollastro, S.; Faretra, F. De novo assembly and comparative transcriptome analysis of Monilinia fructicola, Monilinia laxa and Monilinia fructigena, the causal agents of brown rot on stone fruits. BMC Genom. 2018, 19, 436. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, K.E.; Srb, A.M. Heterokaryosis and parasexuality in the fungus Ascochyta imperfecta. Am. J. Bot. 1965, 52, 72–81. [Google Scholar] [CrossRef]
- Beadle, G.W.; Tatum, E.L. Neurospora. II. Methods of producing and detecting mutations concerned with nutritional requirements. Am. J. Bot. 1945, 32, 678–686. [Google Scholar] [CrossRef]
- Morris, T.J.; Dodds, J.A. Isolation and analysis of double-stranded RNA from virus-infected plant and fungal tissue. Phytopathology 1979, 69, 854–858. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547. [Google Scholar] [CrossRef]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef]
- Sato, Y.; Shahi, S.; Telengech, P.; Hisano, S.; Cornejo, C.; Rigling, D.; Kondo, H.; Suzuki, N. A new tetra-segmented splipalmivirus with divided RdRP domains from Cryphonectria naterciae, a fungus found on chestnut and cork oak trees in Europe. Virus Res. 2022, 307, 198606. [Google Scholar] [CrossRef] [PubMed]
- Ayllón, M.A.; Turina, M.; Xie, J.; Nerva, L.; Marzano, S.L.; Donaire, L.; Jiang, D. ICTV Virus taxonomy profile: Botourmiaviridae. J. Gen. Virol. 2020, 101, 454–455. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Ghabrial, S.A.; Kim, K.H.; Pearson, M.; Marzano, S.Y.L.; Yaegashi, H.; Xie, J.; Guo, L.; Kondo, H.; Koloniuk, I.; et al. ICTV virus taxonomy profile: Hypoviridae. J. Gen. Virol. 2018, 99, 615–616. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, M.E.; Pearson, M.N. Characterisation of a novel hypovirus from Sclerotinia sclerotiorum potentially representing a new genus within the Hypoviridae. Virology 2014, 464–465, 441–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Z.; Wu, S.; Cheng, J.; Fu, Y.; Jiang, D.; Xie, J. Molecular characterization of two positive-strand RNA viruses co-infecting a hypovirulent strain of Sclerotinia sclerotiorum. Virology 2014, 464–465, 450–459. [Google Scholar] [CrossRef] [Green Version]
- Guo, M.; Shen, G.; Wang, J.; Liu, M.; Bian, Y.; Xu, Z. Mycoviral diversity and characteristics of a negative-stranded RNA virus LeNSRV1 in the edible mushroom Lentinula edodes. Virology 2021, 555, 89–101. [Google Scholar] [CrossRef]
- Cotmore, S.F.; Agbandje-McKenna, M.; Canuti, M.; Chiorini, J.A.; Eis-Hubinger, A.M.; Hughes, J.; Mietzsch, M.; Modha, S.; Ogliastro, M.; Pénzes, J.J.; et al. Ictv Report Consortium. ICTV Virus Taxonomy Profile: Parvoviridae. J. Gen. Virol. 2019, 100, 367–368. [Google Scholar] [CrossRef]
- Roossinck, M.J.; Martin, D.P.; Roumagnac, P. Plant virus metagenomics: Advances in virus discovery. Phytopathology 2015, 105, 716–727. [Google Scholar] [CrossRef] [Green Version]
- Marzano, S.Y.L.; Domier, L.L. Novel mycoviruses discovered from metatranscriptomics survey of soybean phyllosphere phytobiomes. Virus Res. 2016, 213, 332–342. [Google Scholar] [CrossRef] [Green Version]
- Bartholomäus, A.; Wibberg, D.; Winkler, A.; Pühler, A.; Schlüter, A.; Varrelmann, M. Deep sequencing analysis reveals the mycoviral diversity of the virome of an avirulent isolate of Rhizoctonia solani AG-2-2 IV. PLoS ONE 2016, 11, e0165965. [Google Scholar] [CrossRef] [Green Version]
- Weber, F.; Wagner, V.; Rasmussen, S.B.; Hartmann, R.; Paludan, S.R. Double-stranded RNA is produced by positive-strand RNA viruses and DNA viruses but not in detectable amounts by negative-strand RNA viruses. J. Virol. 2006, 80, 5059–5064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Son, K.N.; Liang, Z.; Lipton, H.L. Double-stranded RNA is detected by immunofluorescence analysis in RNA and DNA virus infections, including those by negative-stranded RNA ciruses. J. Virol. 2015, 89, 9383–9392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mu, F.; Xie, J.; Cheng, S.; You, M.P.; Barbetti, M.J.; Jia, J.; Wang, Q.; Cheng, J.; Fu, Y.; Chen, T.; et al. Virome characterization of a collection of S. sclerotiorum from Australia. Front. Microbiol. 2018, 8, 2540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dolja, V.V.; Koonin, E.V. Common origins and host-dependent diversity of plant and animal viromes. Curr. Opin. Virol. 2011, 1, 322–331. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Mu, F.; Xie, J.; Cheng, J.; Fu, Y.; Jiang, D. A single ssRNA segment encoding RdRp is sufficient for replication, infection, and transmission of ourmia-like virus in fungi. Front. Microbiol. 2020, 11, 379. [Google Scholar] [CrossRef] [Green Version]
- Hillman, B.I.; Cai, G. The family Narnaviridae: Simplest of RNA viruses. Adv. Virus Res. 2013, 86, 149–176. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, L.; Li, G.; Jiang, D.; Ghabrial, S.A. Genome characterization of a debilitation-associated mitovirus infecting the phytopathogenic fungus Botrytis cinerea. Virology 2010, 406, 117–126. [Google Scholar] [CrossRef] [Green Version]
- Khalifa, M.E.; Pearson, M.N. Molecular characterization of three mitoviruses co-infecting a hypovirulent isolate of Sclerotinia sclerotiorum fungus. Virology 2013, 441, 22–30. [Google Scholar] [CrossRef] [Green Version]
- Picarelli, M.A.S.; Forgia, M.; Rivas, E.B.; Nerva, L.; Chiapello, M.; Turina, M.; Colariccio, A. Extreme diversity of mycoviruses present in isolates of Rhizoctonia solani AG2-2 LP from Zoysia japonica from Brazil. Front. Cell. Infect. Microbiol. 2019, 9, 244. [Google Scholar] [CrossRef] [Green Version]
- Shahi, S.; Eusebio-Cope, A.; Kondo, H.; Hillman, B.I.; Suzuki, N. Investigation of host range of and host defense against a mitochondrially replicating mitovirus. J. Virol. 2019, 93, e01503-18. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Zou, Q.; Dai, Z.; Hong, N.; Wang, G.; Wang, L. Four novel mycoviruses from the hypovirulent Botrytis cinerea SZ-2-3y isolate from Paris polyphylla: Molecular characterisation and mitoviral sequence transboundary entry into plants. Viruses 2022, 14, 151. [Google Scholar] [CrossRef] [PubMed]
- Bruenn, J.A.; Warner, B.E.; Yerramsetty, P. Widespread mitovirus sequences in plant genomes. PeerJ 2015, 3, e876. [Google Scholar] [CrossRef] [Green Version]
- Nibert, M.L.; Vong, M.; Fugate, K.K.; Debat, H.J. Evidence for contemporary plant mitoviruses. Virology 2018, 518, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Li, C.X.; Zhu, J.Z.; Gao, B.D.; Zhu, H.J.; Zhou, Q.; Zhong, J. Characterization of a novel ourmia-like mycovirus infecting Magnaporthe oryzae and implications for viral diversity and evolution. Viruses 2019, 11, 223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Zhang, Y.; Wan, X.; She, Y.; Li, M.; Xi, H.; Xie, J.; Wen, C. A novel ourmia-like mycovirus confers hypovirulence-associated traits on Fusarium oxysporum. Front. Microbiol. 2020, 11, 569869. [Google Scholar] [CrossRef] [PubMed]
- Ohkita, S.; Lee, Y.; Nguyen, Q.; Ikeda, K.; Suzuki, N.; Nakayashiki, H. Three ourmia-like viruses and their associated RNAs in Pyricularia oryzae. Virology 2019, 534, 25–35. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, L.; Esmael, A.; Duan, J.; Bian, X.; Jia, J.; Xie, J.; Cheng, J.; Fu, Y.; Jiang, D.; et al. Four novel botourmiaviruses co-infecting an isolate of the rice blast fungus Magnaporthe oryzae. Viruses 2020, 12, 1383. [Google Scholar] [CrossRef]
- Marzano, S.Y.L.; Hobbs, H.A.; Nelson, B.D.; Hartman, G.L.; Eastburn, D.M.; McCoppin, N.K.; Domier, L.L. Transfection of Sclerotinia sclerotiorum with in vitro transcripts of a naturally occurring interspecific recombinant of Sclerotinia sclerotiorum hypovirus 2 significantly reduces virulence of the fungus. J. Virol. 2015, 89, 5060–5071. [Google Scholar] [CrossRef] [Green Version]
- Hao, F.; Ding, T.; Wu, M.; Zhang, J.; Yang, L.; Chen, W.; Li, G. Two novel hypovirulence-associated mycoviruses in the phytopathogenic fungus Botrytis cinerea: Molecular characterization and suppression of infection cushion formation. Viruses 2018, 10, 254. [Google Scholar] [CrossRef] [Green Version]
- Jia, J.; Fu, Y.; Jiang, D.; Mu, F.; Cheng, J.; Lin, Y.; Li, B.; Marzano, S.-Y.L.; Xie, J. Interannual dynamics, diversity and evolution of the virome in Sclerotinia sclerotiorum from a single crop field. Virus Evol. 2021, 7, veab032. [Google Scholar] [CrossRef]
- Gilmer, D.; Ratti, C.; Consortium, I.R. ICTV virus taxonomy profile: Benyviridae. J. Gen. Virol. 2017, 98, 1571–1572. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wang, S.; Zhang, L.; Qiu, D.; Zhou, X.; Guo, L. A tripartite ssDNA mycovirus from a plant pathogenic fungus is infectious as cloned DNA and purified virions. Sci. Adv. 2020, 6, eaay9634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pénzes, J.J.; Söderlund-Venermo, M.; Canuti, M.; Eis-Hübinger, A.M.; Hughes, J.; Cotmore, S.F.; Harrach, B. Reorganizing the family Parvoviridae: A revised taxonomy independent of the canonical approach based on host association. Arch. Virol. 2020, 165, 2133–2146. [Google Scholar] [CrossRef] [PubMed]
- Kolliopoulou, A.; Taning, C.N.T.; Smagghe, G.; Swevers, L. Viral delivery of dsRNA for control of insect agricultural pests and vectors of human disease: Prospects and challenges. Front. Physiol. 2017, 8, 399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).