Abstract
This study delineated the characteristics of 24 (11.2%) culture-positive, influenza-associated pulmonary aspergillosis (IAPA) patients out of 215 patients with severe influenza during 2016–2019 in a medical center in southern Taiwan. Twenty (83.3%) patients did not have EORTC/MSG-defined host factors. The mean time from influenza diagnosis to Aspergillus growth was 4.4 days, and 20 (83.3%) developed IAPA within seven days after influenza diagnosis. All patients were treated in intensive care units and all but one (95.8%) received mechanical ventilation. Aspergillus tracheobronchitis was evident in 6 (31.6%) of 19 patients undergoing bronchoscopy. Positive galactomannan testing of either serum or bronchoalveolar lavage was noted in all patients. On computed tomography imaging, IAPA was characterized by peribronchial infiltrates, multiple nodules, and cavities superimposed on ground-glass opacities. Pure Aspergillus growth without bacterial co-isolation in culture was found in 17 (70.8%) patients. A. fumigatus (15, 62.5%), A. flavus (6, 25.0%), and A. terreus (4, 16.7%) were the major causative species. Three patients had mixed Aspergillus infections due to two species, and two had mixed azole-susceptible and azole-resistant A. fumigatus infection. All patients received voriconazole with an all-cause mortality of 41.6%. Of 14 survivors, the mean duration of antifungal use was 40.5 days. In conclusion, IAPA is an early and rapidly deteriorating complication following influenza that necessitates clinical vigilance and prompt diagnostic workup.
1. Introduction
While being sporadically reported decades ago, influenza-associated pulmonary aspergillosis (IAPA) has been recognized as one of the major complications following influenza to date [1]. It was reported in 16–23% of patients with severe influenza in Belgium, the Netherlands, and Taiwan [1,2,3,4]. Notably, only 27–32% of IAPA patients had classical host factors for invasive pulmonary aspergillosis (IPA) defined by the European Organization for Research and Treatment of Cancer and the Mycoses Study Group (EORTC/MSG), and 25–30% were previously healthy [1,2]. After careful assessment in a Dutch–Belgian multicenter study in 2018, influenza has been identified as an independent risk factor for IPA, and the incorporation of influenza as one of the host factors in defining IPA is thus considered appropriate [1]. Nevertheless, according to the EORTC/MSG definition, the classification of probable IPA requires the presence of at least one host factor, clinical feature, and piece of mycological evidence, whereas influenza is not included as a host factor [5]. Moreover, based on the AspICU algorithm, which was proposed for diagnosing IPA in critically ill patients without EORTC/MSG host factors, the classification of putative IPA requires a positive culture for Aspergillus in bronchoalveolar lavage fluid (BALF) without simultaneous bacterial growth, and therefore, patients suspected to have IPA but with bacterial co-isolation or not receiving bronchoscopic studies would remain unclassified [6]. Facing an unmet diagnostic need and to facilitate clinical studies, Paul E. Verweij et al. recently proposed a case definition of IAPA (referred to as the Amsterdam IAPA criteria herein) in 2020, which includes two disease categories, i.e., invasive Aspergillus tracheobronchitis (ATB) and IAPA without ATB, based on expert consensus [7].
Clinical features of IAPA have been described in many studies [1,4,8]. In general, it usually presented as an early and critical complication requiring intensive care following influenza infection with a high mortality rate (49–61%) [1,2,8]. However, the microbiological characteristics of Aspergillus from IAPA were addressed to a lesser extent, and the optimal treatment duration for IAPA remains to be determined as the current recommendation of a 6–12 weeks’ treatment duration for IPA is mainly based on the data from immunocompromised patients [9].
In Taiwan, a single-center study reported a high incidence (16.9%) and mortality rate (66.7%) of IPA among severe influenza patients during 2015–2016, and subsequently called physicians’ attention towards this life-threatening complication herein [4]. With the issue of a new case definition and increased vigilance towards IAPA, we aimed to describe the clinical and laboratory characteristics and treatment outcome of IAPA and identify clinical and laboratory clues for the detection of IAPA and its optimal treatment duration.
2. Materials and Methods
This study enrolled adults aged ≥20 years with influenza infection and temporally related isolation of Aspergillus species from respiratory samples, including sputum, endotracheal aspirate (ETA), or BALF at National Cheng Kung University Hospital (NCKUH), a tertiary medical center in southern Taiwan, during 2016–2019. Clinical, laboratory, bronchoscopic, and radiological data of eligible patients obtained from medical chart review were analyzed. The diagnosis and classification of IAPA were made according to the Amsterdam IAPA criteria as well as those of EORTC/MSG and AspICU [5,6,7]. Early onset IAPA was considered when Aspergillus isolates were recovered within seven days after detection of influenza.
In this study, influenza virus infection was impressed based on a positive result of the reverse transcriptase polymerase chain reaction (RT-PCR) test according to the World Health Organization protocol or a rapid antigen test for influenza A and B (BD Veritor™ System) for nasopharyngeal or throat swab, sputum, or BALF [10]. For severe influenza patients with pulmonary infiltrates, fungal cultures of sputum and/or ETA were performed and the serum galactomannan (GM) index was determined by the Platelia Aspergillus Ag assay (Bio-Rad, Marnes-la-Coquette, France). Bronchoscopic examination, which allows visualization of the large airways and obtaining BALF for fungal culture and GM testing, was recommended for critically ill influenza patients with suspected IPA due to either clinical deterioration, suspicious radiographic lesions, mold isolated from sputum and/or ETA, or positive serum GM testing, and the attending physicians in charge of patient care and pulmonologists who performed bronchoscopy made the final decision. According to the Amsterdam’s IAPA criteria, the sample was considered positive at a cut-off index >0.5 for serum and ≥1.0 for BALF. Consecutive Aspergillus isolates from each patient were collected and stored at −80 °C until use. They were identified based on morphology, sequence analysis of the internal transcribed spacer region and calmodulin gene along with additional phylogenetic analysis for cryptic species, and subjected to antifungal susceptibility testing for minimum inhibitory concentration (MIC) determination following the CLSI M38-A2 method [11,12]. For azole-resistant Aspergillus fumigatus (ARAF) isolates, the cyp51A gene was analyzed [13]. The inter- and intra-patient genetic relatedness of A. fumigatus, Aspergillus flavus, and Aspergillus terreus isolates were determined by microsatellite genotyping as previously described [14,15,16].
Using the SPSS statistics (version 17.0. Chicago: SPSS Inc.), univariate and multivariate analyses were performed to identify the independent factors associated with all-cause in-hospital mortality among IAPA patients. In univariate analyses, categorical variables were compared by the Fisher’s exact test or chi-squared test, and continuous variables by t-test; in multivariate analysis, binary logistic regression model was used. A p value of less than 0.05 was considered statistically significant, and all tests were two-tailed.
3. Results
During 2016–2019, 31 patients with laboratory-confirmed influenza infection and isolation of Aspergillus species from respiratory sample(s) with a temporal relationship were identified. Based on the Amsterdam IAPA criteria, 24 patients were classified as proven (case 1) or probable (n = 23, case 2–24) cases of IAPA, with 3, 6, 6, and 9 patients occurring in 2016, 2017, 2018, and 2019, respectively. Seven patients who did not meet the IAPA definition were not included for further analyses. During the study period, a total of 215 cases of severe influenza requiring intensive care were identified, and thus the prevalence of culture-positive IAPA was 11.2% (24/215) in those with severe influenza. Clinical and laboratory characteristics of 24 IAPA patients are presented in Table 1, Table 2, and Table S1. Sixteen (66.7%) of 24 cases of IAPA had more than one respiratory sample with Aspergillus growth, and thus a total of 52 Aspergillus isolates were recovered from 24 patients. Their antifungal susceptibility profile is provided in Table 3.

Table 1.
Characteristics of 24 patients with influenza-associated pulmonary aspergillosis (IAPA).

Table 2.
Clinical and laboratory data of 24 patients with proven or probable influenza-associated pulmonary aspergillosis based on the Amsterdam IAPA criteria.

Table 3.
Antifungal susceptibility profiles of 52 Aspergillus isolates determined by CLSI M38-A2.
Of 24 IAPA patients, four (16.7%) had EORTC/MSG host factors, and five (20.8%) were classified as proven or probable IPA based on the EORTC/MSG definition, while ten (41.7%) were classified as proven or putative IPA based on the AspICU definition. Eighteen (75%) patients were infected by influenza A (subtype H1: 11, 45.8% and H3: 7, 29.2%) and six (25%) by influenza B, confirmed by PCR in all but one case, in whom a positive rapid antigen test was noted. Of 19 patients in whom Aspergillus were recovered after influenza diagnosis, the mean time from influenza diagnosis to Aspergillus growth was 4.4 days (interquartile range (IQR) 1–6 days), whereas in the remaining five patients, influenza testing was performed after the notification of Aspergillus growth. All patients were treated in intensive care units (ICUs), and all but one (23, 95.8%) needed mechanical ventilation, among whom 15 (65.2%) had their first Aspergillus-positive culture either shortly prior to endotracheal intubation (3 patients: 1–2 days earlier) or immediately within one day after intubation (12 patients).
Twenty (83.3%) patients had a serum GM index >0.5. Of 19 patients undergoing bronchoscopy, 15 (78.9%) patients had a serum GM index >0.5 and 14 (73.7%) had a BALF GM index ≥1.0, which included all four patients with a serum GM index <0.5. Therefore, all 24 patients with IAPA had either a serum GM index >0.5 or a BALF GM index ≥1.0. Via bronchoscopy, ATB presenting as tracheobronchial ulceration, pseudomembrane, or whitish plaque was noted in six patients (31.6%) (Figure 1), and in whom their BALF GM index was ≥1.0. The BALF GM index in the six patients with ATB was higher than that in the thirteen patients without ATB, though the difference was not significant (4.3 vs. 3.0, p = 0.17).
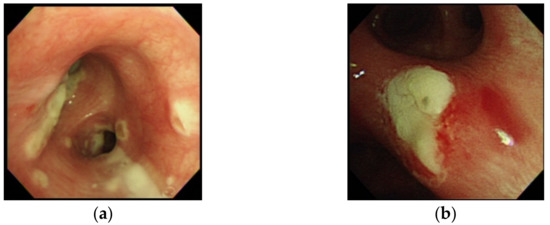
Figure 1.
Bronchoscopic examination in case 17 with influenza-associated pulmonary aspergillosis (IAPA) due to A. fumigatus, which demonstrated vesicles and whitish patches over bilateral bronchial tress (a), and in case 23 with IAPA due to A. flavus, which demonstrated an area of whitish plague over left secondary carina that was difficult to remove (b), but resolved under voriconazole in a follow-up bronchoscopic examination eight days later.
Of 19 patients with both ETA and BALF available for fungal cultures, nine (47.4%) had Aspergillus growth in BALF. All 19 patients had Aspergillus growth in ETA, which was obtained at a mean time of 2.4 (IQR 1–4) days earlier than the collection of BALF. Prior antifungal use did not decrease the fungal culture yield of BALF, as two of ten patients with BALF obtained before and seven of nine with BALF obtained after antifungal therapy had Aspergillus growth (20.0% vs. 77.8%, p = 0.04). Most (21, 87.5%) patients had a single Aspergillus species recovered from respiratory samples (13 A. fumigatus, 5 A. flavus, and 3 A. terreus), while three patients had concurrent isolation of two Aspergillus species (A. fumigatus plus A. pseudonomius, A. fumigatus plus A. flavus, and A. terreus plus A. allahabadii) (Table 4). Overall, A. fumigatus (15, 62.5%) was the most common cause of IAPA, followed by A. flavus (6, 25.0%), and A. terreus (4, 16.7%). Microsatellite genotypes of the isolates of three major species differed among patients, indicating no clonal spread (Table 4). Of fifteen patients with multiple isolates of the same species recovered, six (46.7%) harbored multiple microsatellite genotypes of one Aspergillus species, including A. fumigatus isolates belonging to 2–4 genotypes in four patients and A. flavus isolates belonging to two genotypes in two patients. Notably, a pure growth of Aspergillus without bacterial growth in the culture plate was found in seventeen (70.8%) patients, while co-isolation of pathogenic bacteria was found in five (20.8%) patients and co-isolation of commensal flora in two (8.3%) patients. Ten (41.7%) patients had bacterial pulmonary or bloodstream co-infections 0–7 days prior to the recovery of Aspergillus, and Klebsiella pneumoniae in four cases was the most common bacterial co-pathogen (Table S1).

Table 4.
Aspergillus isolates recovered from 24 patients with influenza-associated pulmonary aspergillosis and microsatellite genotypes of A. fumigatus, A. flavus, and A. terreus isolates.
Thirteen patients underwent chest computed tomography (CT) scanning (Figure 2), in which peribronchial infiltrate (13, 100%), multiple nodules (10, 76.9%), wedge-shaped consolidation (8, 61.5%), and cavities (4, 30.8%) were noted. Ground-glass opacity (GGO) was present in seven (53.8%) patients, among whom five of six patients tested for Pneumocystis jirovecii PCR had a negative result in a respiratory sample, suggesting a low possibility of Pneumocystis colonization or infection. Of eleven patients with CXR only, patchiness and/or consolidation were noted in all patients and nodules in three patients.
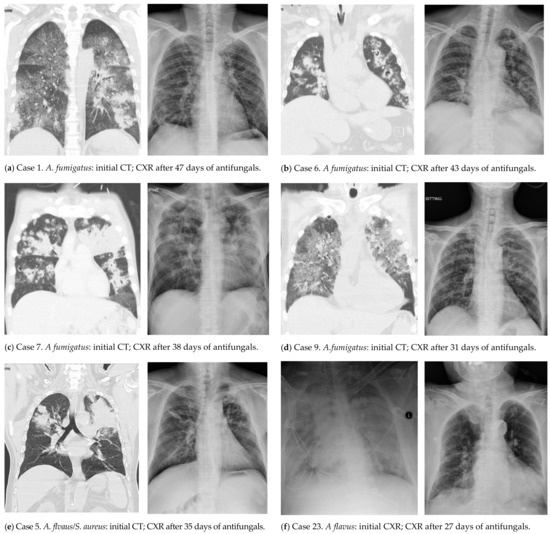
Figure 2.
Initial (left) and follow-up (right) radiological images of six patients with influenza-associated aspergillosis who survived.
All patients received anti-influenza agents (oseltamivir or preramivir) and voriconazole. The mean interval from the growth of Aspergillus (the day when the respiratory samples were collected for fungal cultures) to the initiation of antifungal therapy was short (4.0 days) and did not significantly differ between survivors and non-survivors (3.4 days vs. 4.9 days, p = 0.52). Azole-resistant A. fumigatus isolates were identified in two cases (no. 17 and 21). Case 17 started voriconazole treatment two days before the growth of both azole-susceptible and azole-resistant A. fumigatus isolates (voriconazole MIC: 2 μg/mL, isavuconazole MIC: 16 μg/mL; wild-type cyp51A) from ETA, but eventually died of IAPA after 18 days’ voriconazole and subsequent 12 days’ liposomal-amphotericin B treatment. Case 21 started voriconazole treatment two days after the growth of azole-susceptible A. fumigatus from ETA, but an azole-resistant A. fumigatus isolate (voriconazole MIC: 4 μg/mL; TR34/L98H mutation in cyp51A) was detected from ETA five days later. The patient eventually recovered with voriconazole therapy for 25 days without relapse.
The all-cause in-hospital and IAPA-attributable mortality rate was 41.6% (10/24) and 37.5% (9/24), respectively, among 24 IAPA patients. Univariate and multivariate analyses were not able to identify factors independently related to a fatal outcome. Among 14 survivors (13 without EORTC/MSG host factors), antifungal treatment was continued until there was no more growth of Aspergillus spp. in respiratory samples and a resolution or stabilization of pulmonary lesions in chest radiographs (Figure 2). The mean duration of antifungal use in 14 survivors was 40.5 (IQR 32–51) days and nine (64.3%) received voriconazole for ≤six weeks. Of 14 survivors, one patient died of lymphoma three months later, and ten patients receiving ≥six-month follow-up (nine receiving ≥nine-month follow-up) in the study hospital remained free from aspergillosis, among whom the mean duration of antifungal use was 42.1 (IQR 34–51) days and six received antifungal for ≤six weeks.
4. Discussion
This study delineated the clinical and laboratory characteristics of 24 patients with culture-positive IAPA at a medical center based on the Amsterdam IAPA criteria, which was composed of a combination of bronchoscopic, mycological, and radiological findings. Overall, we found that IAPA might occur in the absence of classical host factors, be caused by a mixture of Aspergillus isolates, and manifest as an early onset, rapidly deteriorating but treatable complication following influenza infection.
By analyzing culture-positive cases, the prevalence of IAPA in severe influenza patients was noted to be at least 11.2% here, which was within the range of those reported in the Dutch–Belgian study (19.2%), Switzerland (11.1%), Spain (7.2%), Canada (7.2%), China (5.4%), and a recent report from Taiwan (19.9%) [1,17,18,19,20,21]. As reported earlier, IAPA occurred not only following influenza A (both subtypes H1 and H3), but also influenza B [1]. The short interval from the influenza diagnosis to Aspergillus growth (4.4 days) was similar to that observed in Canada (5 days) and the U.S. (6 days) [19,22]. Notably, the first Aspergillus-positive cultures in about two-thirds (65.2%) of patients were noted shortly before or immediately after intubation, suggestive of the acquisition of Aspergillus pathogens before intubation. These findings also indicated that IAPA developed shortly following influenza when both mucosal and systemic immune defenses against inhaled airborne Aspergillus conidia were compromised by the influenza virus [7,22]. The vast majority of IAPA patients required ICU care and mechanical ventilation, as noted in earlier studies (89–100%) [1,3,8,17]. Taken together, IAPA was an early and rapidly deteriorating complication following influenza.
As delayed antifungal treatment is associated with an unfavorable outcome, early diagnosis and early antifungal therapy are crucial for IAPA management [2]. Clinical presentations of IAPA are non-specific, and thus the diagnosis of IAPA is usually dependent on a series of diagnostic tests, including, in order of invasiveness, serum GM testing, fungal cultures of sputum and/or ETA, chest CT, and bronchoscopy, which are all incorporated in the Amsterdam IAPA criteria [7]. This proposed definition seems more clinically feasible, as only 20.8% and 41.7% of IAPA patients herein were classified as IPA based on the EORTC/MSG and AspICU definitions, respectively. The merits of the Amsterdam IAPA criteria include that the classical EORTC/MSG host factors are not required, and those without bronchoscopic study or with bacterial co-isolation from BALF could be properly defined.
Previous studies reported a higher sensitivity of GM testing in BALF (88–94%) than in serum (65–78%) for detecting IAPA [1,2,22]. Though the sensitivity of GM testing in BALF (73.7%) was found to be slightly lower than in serum (83.3%) here, BALF GM testing remained important and was complementary to serum GM testing, as the former identified all four IAPA patients with negative serum GM. Moreover, our study revealed four microbiological features that were rarely addressed before. First, for IAPA, ETA samples had a higher culture yield than BALF samples. Together with a high sensitivity of serum GM testing, routine serum GM testing and fungal culture from ETA for patients with severe influenza upon ICU admission might aid the early detection of IAPA. A positive test result should promptly trigger diagnostic workup for invasive aspergillosis. Second, about 70% of IAPA patients showed pure growth of Aspergillus on culture plates without bacterial co-isolation, indicating that a dominant growth of Aspergillus in respiratory samples is associated with invasive fungal disease. The pure growth of Aspergillus also echoes the mycological criterion for putative IPA in the AspICU algorithm, i.e., semiquantitative Aspergillus growth in BALF without bacterial growth. Nevertheless, bacterial co-isolation was not uncommon in influenza patients, as seen in 29.2% of IAPA patients, and the drawback of the AspICU algorithm in such a scenario could be overcome by the Amsterdam IAPA criteria. Third, A. fumigatus was almost exclusively reported as the cause of IAPA in western countries [1,2,8,19,22], but A. flavus or A. terreus could be etiologic agents of IAPA here. A. flavus has been recognized to cause human aspergillosis particularly in Asia, the Middle East, and Africa, and was reported as the cause in 3 out of 10 culture-positive IAPA patients in China [23,24]. Though less common, IPA due to A. terreus occurred in certain geographical regions, such as Austria [25]. This study also identified less-common Aspergillus species, including A. pseudonomius (belonging to Aspergillus section Flavi) and A. allahabadii (Aspergillus section Terrei). Albeit rare, A. pseudonomius has been reported to cause human disease [26], while human infections due to A. allahabadii have not been identified yet. The pathogenic role of these uncommon species remained to be elucidated, since they co-existed with other well-recognized pathogenic Aspergillus species (A. fumigatus or A. terreus) here. Finally, mixed Aspergillus infections due to different species, microsatellite genotypes, or azole susceptibility profiles were not uncommon in IAPA, suggesting exposure to diverse Aspergillus conidia in the environment. Such a finding echoed the international guideline recommending antifungal susceptibility testing of multiple colonies (up to five) from a single culture [27]. Moreover, the discovery of ARAF due to the Aspergillus isolate with an environmental resistance mechanism (TR34/L98H mutation) following the initial azole-susceptible isolate in case 21 underlined the necessity of repeated susceptibility testing in the consecutive Aspergillus isolates, because the subsequent acquisition of environmental resistant isolates remains possible.
Our study and a French study shared a similar prevalence rate of ATB (31.6% and 28.6%, respectively) among IAPA patients, and both revealed that immunocompromised as well as immunocompetent patients were at risk for ATB [8]. We additionally found that A. flavus and A. terreus could be the causes of ATB, in addition to A. fumigatus reported in France. However, the association between ATB and a higher BALF GM level or mortality revealed in France was not found here, probably because of our limited number of cases.
The common CT images in patients with IAPA due to A. fumigatus herein included peribronchial infiltrates, multiple nodular consolidation, and cavitary lesions superimposed on areas of GGO. The appearance of GGO might be explained by the underlying viral pneumonia, as was seen in 45% of patients with H1N1 infection on CT imaging [28]. The features of peribronchial infiltrates reflect the pathogenic process of IAPA, where Aspergillus spreads along the tracheobronchial trees and eventually leads to ATB and multiple nodules and cavities within the lung parenchyma. Multiple nodules and cavities have been recognized as radiological characteristics of IAPA, and occurred less frequently in influenza patients without IPA [24]. These CT features in combination are regarded as radiological clues for IAPA.
The all-cause mortality rate of 24 IAPA patients with voriconazole therapy was 41.6%. Of note, case 21 survived with voriconazole treatment despite a high voriconazole MIC (4 μg/mL) of the causative A. fumigatus isolate. This favorable outcome might be explained by high voriconazole concentrations in pulmonary epithelial lining fluid (EFL), based on an average ELF-to-plasma ratio of 11 [29]. A relapse of IPA was not observed among ten survivors with available follow-up information who received a mean of six-week antifungal treatment and among six who received antifungal for ≤six weeks, and the treatment duration was shorter than that of 6–12 weeks recommended for IPA in patients with EORTC/MSG host factors. Furthermore, the resolution or stabilization of pulmonary lesions on chest radiographs could also be achieved with ≤six weeks antifungal treatment here. Therefore, our data suggested that antifungal therapy for six weeks might be sufficient for some IAPA patients without classical immunocompromised conditions.
Our study has several limitations. First, only culture-positive patients were enrolled, and thus true IAPA patients were considered for analysis. Culture-negative patients with positive GM results in blood or BAL were not included. With this restrictive criterion adopted herein, the incidence of IAPA is likely to be underestimated. Second, steroid use was previously found to be independently associated with IPA [3], but our study design cannot allow us to elucidate the role of steroid use in IAPA development. However, about two-thirds of IAPA patients did not have prior steroid exposure, indicating that steroid use might not be the only predisposing factor contributing to IAPA. Third, CT imaging was not performed in patients with monomicrobial IAPA due to A. flavus and A. terreus, so it is not clear whether both species presented similar CT findings as A. fumigatus. Finally, the case number of IAPA was limited. Further studies enrolling more cases are warranted to elucidate unanswered questions, such as the role of less-common Aspergillus species in IAPA and the prognostic impact of lymphopenia and steroid use during IAPA treatment, and to confirm the appropriateness of six-week antifungal therapy for IAPA patients without EORTC/MSG host factors.
5. Conclusions
Our study revealed that IAPA is an early and rapidly deteriorating complication of influenza that might occur in the absence of underlying disease and be caused by a mixture of Aspergillus isolates. Routine serum GM testing and fungal culture from ETA for patients with severe influenza upon ICU admission could be considered for early recognition of IAPA. A positive test result should promptly trigger diagnostic workup for invasive aspergillosis. Growth of pathogenic Aspergillus species without bacterial co-isolation, tracheobronchitis in a bronchoscopic study, and typical CT findings are useful clues for diagnosing IAPA. A high level of clinical vigilance, prompt diagnosis, and early treatment can ensure a better outcome of this dangerous but treatable complication following influenza.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jof8010049/s1, Table S1: Supplementary clinical and laboratory data of 24 patients with influenza-associated pulmonary aspergillosis.
Author Contributions
Conceptualization, C.-J.W. and W.-C.K.; Methodology, C.-J.W., H.-C.W., M.-I.H., P.-C.C. and W.-C.K.; Validation, C.-J.W.; Formal analysis, C.-J.W.; Investigation, C.-T.C., C.-W.C., W.-C.L., J.-C.L., P.-S.C., C.-C.H., W.-T.L., P.-L.S. and X.-M.L.; Resources, C.-T.C., C.-W.C., W.-C.L., J.-C.L., P.-S.C., C.-C.H., W.-T.L., P.-L.S. and X.-M.L.; Draft preparation, C.-J.W.; Manuscript review and editing, C.-J.W. and W.-C.K.; Funding acquisition, C.-J.W. All authors have read and agreed to the present version of the manuscript.
Funding
This work was funded by the National Health Research Institutes of Taiwan, Grant numbers IV-107-PP-08, IV-108-PP-08, and IV-109-PP-08.
Institutional Review Board Statement
This study was conducted according to the guidelines of the Declaration of Helsinki, which was approved by the Institutional Review Board NCKUH (IRB numbers B-ER-101-342 and B-ER-105-138).
Informed Consent Statement
Informed consent was obtained from the subjects participating in a study of invasive aspergillosis (IRB number B-ER-105-138) and was waived in the subjects participating in a retrospective chart review and isolate analysis (IRB number B-ER-101-342).
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.
Acknowledgments
We thank the medical staff at National Chen-Kung University Hospital for their contribution to patient care in this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Schauwvlieghe, A.; Rijnders, B.J.A.; Philips, N.; Verwijs, R.; Vanderbeke, L.; Van Tienen, C.; Lagrou, K.; Verweij, P.E.; Van de Veerdonk, F.L.; Gommers, D.; et al. Invasive aspergillosis in patients admitted to the intensive care unit with severe influenza: A retrospective cohort study. Lancet Respir. Med. 2018, 6, 782–792. [Google Scholar] [CrossRef]
- Van de Veerdonk, F.L.; Kolwijck, E.; Lestrade, P.P.; Hodiamont, C.J.; Rijnders, B.J.; van Paassen, J.; Haas, P.J.; Oliveira Dos Santos, C.; Kampinga, G.A.; Bergmans, D.C.; et al. Influenza-associated aspergillosis in critically ill patients. Am. J. Respir. Crit. Care Med. 2017, 196, 524–527. [Google Scholar] [CrossRef]
- Wauters, J.; Baar, I.; Meersseman, P.; Meersseman, W.; Dams, K.; De Paep, R.; Lagrou, K.; Wilmer, A.; Jorens, P.; Hermans, G. Invasive pulmonary aspergillosis is a frequent complication of critically ill H1N1 patients: A retrospective study. Intensive Care Med. 2012, 38, 1761–1768. [Google Scholar] [CrossRef]
- Ku, Y.H.; Chan, K.S.; Yang, C.C.; Tan, C.K.; Chuang, Y.C.; Yu, W.L. Higher mortality of severe influenza patients with probable aspergillosis than those with and without other coinfections. J. Formos. Med. Assoc. 2017, 116, 660–670. [Google Scholar] [CrossRef]
- Donnelly, J.P.; Chen, S.C.; Kauffman, C.A.; Steinbach, W.J.; Baddley, J.W.; Verweij, P.E.; Clancy, C.J.; Wingard, J.R.; Lockhart, S.R.; Groll, A.H.; et al. Revision and update of the consensus definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin. Infect. Dis. 2020, 71, 1367–1376. [Google Scholar] [CrossRef]
- Blot, S.I.; Taccone, F.S.; Van den Abeele, A.M.; Bulpa, P.; Meersseman, W.; Brusselaers, N.; Dimopoulos, G.; Paiva, J.A.; Misset, B.; Rello, J.; et al. A clinical algorithm to diagnose invasive pulmonary aspergillosis in critically ill patients. Am. J. Respir. Crit. Care Med. 2012, 186, 56–64. [Google Scholar] [CrossRef]
- Verweij, P.E.; Rijnders, B.J.; Brüggemann, R.J.; Azoulay, E.; Bassetti, M.; Blot, S.; Calandra, T.; Clancy, C.J.; Cornely, O.A.; Chiller, T. Review of influenza-associated pulmonary aspergillosis in ICU patients and proposal for a case definition: An expert opinion. Intensive Care Med. 2020, 46, 1524–1535. [Google Scholar] [CrossRef]
- Nyga, R.; Maizel, J.; Nseir, S.; Chouaki, T.; Milic, I.; Roger, P.A.; Van Grunderbeeck, N.; Lemyze, M.; Totet, A.; Castelain, S.; et al. Invasive tracheobronchial aspergillosis in critically ill patients with severe influenza. A clinical trial. Am. J. Respir. Crit. Care Med. 2020, 202, 708–716. [Google Scholar] [CrossRef]
- Patterson, T.F.; Thompson, G.R., 3rd; Denning, D.W.; Fishman, J.A.; Hadley, S.; Herbrecht, R.; Kontoyiannis, D.P.; Marr, K.A.; Morrison, V.A.; Nguyen, M.H.; et al. Practice Guidelines for the Diagnosis and Management of Aspergillosis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 63, e1–e60. [Google Scholar] [CrossRef]
- World Health Organization (WHO) Information for Molecular Diagnosis of Influenza Virus. Available online: https://www.who.int/influenza/gisrs_laboratory/WHO_information_for_the_molecular_detection_of_influenza_viruses_20171023_Final.pdf (accessed on 1 December 2021).
- Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi. Aproved Standard-Second Edition; CLSI document M38-A2; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008. [Google Scholar]
- Balajee, S.A.; Houbraken, J.; Verweij, P.E.; Hong, S.B.; Yaghuchi, T.; Varga, J.; Samson, R.A. Aspergillus species identification in the clinical setting. Stud. Mycol. 2007, 59, 39–46. [Google Scholar] [CrossRef]
- Snelders, E.; Karawajczyk, A.; Schaftenaar, G.; Verweij, P.E.; Melchers, W.J. Azole resistance profile of amino acid changes in Aspergillus fumigatus CYP51A based on protein homology modeling. Antimicrob. Agents Chemother. 2010, 54, 2425–2430. [Google Scholar] [CrossRef]
- De Valk, H.A.; Meis, J.F.; Curfs, I.M.; Muehlethaler, K.; Mouton, J.W.; Klaassen, C.H. Use of a novel panel of nine short tandem repeats for exact and high-resolution fingerprinting of Aspergillus fumigatus isolates. J. Clin. Microbiol. 2005, 43, 4112–4120. [Google Scholar] [CrossRef]
- Rudramurthy, S.M.; de Valk, H.A.; Chakrabarti, A.; Meis, J.F.; Klaassen, C.H. High resolution genotyping of clinical Aspergillus flavus isolates from India using microsatellites. PLoS ONE 2011, 6, e16086. [Google Scholar] [CrossRef]
- Rougeron, A.; Giraud, S.; Razafimandimby, B.; Meis, J.F.; Bouchara, J.P.; Klaassen, C.H. Different colonization patterns of Aspergillus terreus in patients with cystic fibrosis. Clin. Microbiol. Infect. 2014, 20, 327–333. [Google Scholar] [CrossRef][Green Version]
- Waldeck, F.; Boroli, F.; Suh, N.; Wendel Garcia, P.D.; Flury, D.; Notter, J.; Iten, A.; Kaiser, L.; Schrenzel, J.; Boggian, K.; et al. Influenza-associated aspergillosis in critically-ill patients-a retrospective bicentric cohort study. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 1915–1923. [Google Scholar] [CrossRef]
- Martin-Loeches, I.; Schultz, M.J.; Vincent, J.-L.; Alvarez-Lerma, F.; Bos, L.D.; Solé-Violán, J.; Torres, A.; Rodriguez, A. Increased incidence of co-infection in critically ill patients with influenza. Intensive Care Med. 2017, 43, 48–58. [Google Scholar] [CrossRef]
- Schwartz, I.S.; Friedman, D.Z.; Zapernick, L.; Dingle, T.C.; Lee, N.; Sligl, W.; Zelyas, N.; Smith, S.W. High rates of influenza-associated invasive pulmonary aspergillosis may not be universal: A retrospective cohort study from Alberta, Canada. Clin. Infect. Dis. 2020, 71, 1760–1763. [Google Scholar] [CrossRef]
- Zou, P.; Wang, C.; Zheng, S.; Guo, F.; Yang, L.; Zhang, Y.; Liu, P.; Shen, Y.; Wang, Y.; Zhang, X.; et al. Invasive pulmonary aspergillosis in adults with avian influenza a (H7N9) pneumonia in China: A retrospective study. J. Infect. Dis. 2020, 221, S193–S197. [Google Scholar] [CrossRef]
- Chao, C.M.; Lai, C.C.; Ou, H.F.; Ho, C.H.; Chan, K.S.; Yang, C.C.; Chen, C.M.; Yu, W.L. The Impacts of aspergillosis on outcome, burden and risks for mortality in influenza patients with critical illness. J. Fungi 2021, 7, 922. [Google Scholar] [CrossRef]
- Crum-Cianflone, N.F. Invasive aspergillosis associated with severe influenza infections. Open Forum Infect. Dis. 2016, 3, ofw171. [Google Scholar] [CrossRef]
- Rudramurthy, S.M.; Paul, R.A.; Chakrabarti, A.; Mouton, J.W.; Meis, J.F. Invasive aspergillosis by Aspergillus flavus: Epidemiology, diagnosis, antifungal resistance, and management. J. Fungi 2019, 5, 55. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, N.; Huang, X.; Xiong, S.; Feng, Y.; Zhang, Y.; Li, M.; Zhan, Q. Invasive pulmonary aspergillosis in patients with influenza infection: A retrospective study and review of the literature. Clin. Respir. J. 2019, 13, 202–211. [Google Scholar] [CrossRef]
- Risslegger, B.; Zoran, T.; Lackner, M.; Aigner, M.; Sanchez-Reus, F.; Rezusta, A.; Chowdhary, A.; Taj-Aldeen, S.J.; Arendrup, M.C.; Oliveri, S.; et al. A prospective international Aspergillus terreus survey: An EFISG, ISHAM and ECMM joint study. Clin. Microbiol. Infect. 2017, 23, 776-e1. [Google Scholar] [CrossRef]
- Salah, H.; Lackner, M.; Houbraken, J.; Theelen, B.; Lass-Florl, C.; Boekhout, T.; Almaslamani, M.; Taj-Aldeen, S.J. The emergence of rare clinical Aspergillus species in Qatar: Molecular characterization and antifungal susceptibility profiles. Front. Microbiol. 2019, 10, 1677. [Google Scholar] [CrossRef]
- Ullmann, A.J.; Aguado, J.M.; Arikan-Akdagli, S.; Denning, D.W.; Groll, A.H.; Lagrou, K.; Lass-Florl, C.; Lewis, R.E.; Munoz, P.; Verweij, P.E.; et al. Diagnosis and management of Aspergillus diseases: Executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin. Microbiol. Infect. 2018, 24 (Suppl. 1), e1–e38. [Google Scholar] [CrossRef]
- Schoen, K.; Horvat, N.; Guerreiro, N.F.C.; de Castro, I.; de Giassi, K.S. Spectrum of clinical and radiographic findings in patients with diagnosis of H1N1 and correlation with clinical severity. BMC Infect. Dis. 2019, 19, 964. [Google Scholar] [CrossRef]
- Capitano, B.; Potoski, B.A.; Husain, S.; Zhang, S.; Paterson, D.L.; Studer, S.M.; McCurry, K.R.; Venkataramanan, R. Intrapulmonary penetration of voriconazole in patients receiving an oral prophylactic regimen. Antimicrob. Agents Chemother. 2006, 50, 1878–1880. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
