Abstract
The dichloromethane extraction was applied to extracted volatile compounds of the six developmental stages of caps and stipes of an Amanita rubescens mushroom and the relative contents were measured with the gas chromatography-mass spectrometry. The number of identified compounds ranged between 53 and 52, respectively, with a high ratio of alkane volatiles. The significant differences between the aroma compounds were determined in caps to identify their stages of development. The fully mature stage caps were characterized by 4,6-dimethyl-dodecane (7.69 ± 1.15%), 2-hexyl-1-decanol (11.8 ± 1.61%), 1,3-di-tert-butylbenzene (11.4 ± 1.25%), heptadecyl pentadecafluorooctanoate (2.16 ± 0.31%), and 2-hexyl-1-dodecanol (13.5 ± 1.33%). Niacinamide (3.90 ± 0.07%) and glycerol (3.62 ± 1.27%) was present in the caps in the early-stage of the rotting mushroom, which represented the 10th–12th day of fructification. The caps and stipes from the 12th–15th day of fructification were characterized by 2,3-butanediol (11.7 ± 0.13% and 8.00 ± 0.10%, respectively). Moreover, the caps from this developmental stage were characterized by 2-methyl- and 3-methyl butanoic acids (0.18 ± 0.03% and 0.33 ± 0.02%, respectively) which are typical for the rotting stage. In this study, we confirmed the effect of A. rubescens developmental stages on the aroma profile.
1. Introduction
Aroma, formed by a combination of volatile and non-volatile compounds, is a key characteristic of food which significantly affects consumer preferences. Edible mushrooms are consumed as a delicacy not only because of their high nutritional value, but also because of their specific aroma [1]. Volatile organic compounds are synthesized as a protection of the organism or as by-products of metabolism [2]. They are released into the air and often have a characteristic odor [3]. There are many publications on fungal aroma. Altogether, 150 compounds have been identified, mainly from the categories of higher alcohols, ketones, esters, aldehydes, hydrocarbons, acids, heterocyclic and aromatic compounds [4,5,6,7,8]. In 1938, 1-octen-3-ol was first identified as a “mushroom–like flavor” and a “raw mushroom” with a characteristic earthy and sweet taste [5]. Other compounds have also been identified, specifically 1-octen-3-one, (E)-2-octen-1-ol, 1-octanol, 3-octanol, and (E)-2-octenal [9,10,11]. On the other hand, there is a lack of information around aroma profiles of different fruiting bodies and developmental stages of wild edible mushrooms, or Amanita rubescens.
European Blusher (Amanita rubescens Pers.) belongs to the Amanitaceae family. Many species of the family are inedible or toxic. On the other hand, A. rubescens, as well as some other species (A. fulva, A. baccata, A. caesarea), are edible [12,13,14,15]. The A. rubescens is a commonly collected and popular wild edible mushroom that is characterized by great sensory properties. Fruiting bodies of A. rubescens have a relatively high bioaccumulation capacity which can affect the spectrum of aromatic compounds and ultimately the increased risk of intoxication by heavy metals of the pickers [12].
According to SK Regulation 132/2014 [16] only wild edible mushrooms listed in the annex can be placed on the market. A. rubescens is absent from the annex, which contains 53 different wild edible mushroom species. In 2019, the consumption of “other vegetables and mushrooms” reached 13.3 kg per capita, according to Statistical Office of the Slovak Republic [17]. The consumption of exclusively wild edible mushrooms is not recorded in Slovakia. On the other hand, the Statistical Office of the Czech Republic recorded the consumption of 3.1 kg of “mushrooms” per capita in 2019 [18]. The aim of the work was to evaluate the effect of the various developmental stages of the Amanita rubescens Pers fruiting bodies from Slovakia on the volatile compounds, determined in a dichloromethane extract by the gas chromatography-mass spectrometry.
2. Materials and Methods
2.1. Sampling Area and Sampling
The six developmental stages of A. rubescens fruiting bodies (morphologically characterized by the expert mycologist prof. Kunca, and in compliance with the taxonomic keys [19]) were collected in Žakýlske Pleso area (Štavnické Vrchy, Slovakia) (GPS: 48°31′12.8″ N; 18°55′23.5″ E) on 3 July 2020. The area of the Štiavnické Vrchy is characterized by volcanic origins. The sampling area is predominantly forested with beech and hornbeam vegetation. The altitude of the sampling point is 757 m a.s.l., with an average annual temperature of 6–7 °C, and rainfall of 700–800 mm [20]. The region is characterized as very cold and humid, with a difference between potential evaporation and rainfall <50 mm (climate indicator for the months of June to August). The soil is characterized as loamy medium-heavy soil containing organic matter >20% [21].
The samples of individual developmental stages of A. rubescens fruiting bodies were taken in the morning from one 2 × 2 m area (Figure 1a). The advantage of such a collection is a high probability of homogeneous mycelium which creates a presumption of identical dynamics of nutrient uptake by the mycelium. Thus, identical or similar conditions for the formation of aromatic compounds were observed. After sampling, the fruiting bodies were removed from larger impurities and temporarily stored in ventilated polyethylene boxes (Figure 1b). Upon arrival at the laboratory, the samples were rinsed thoroughly in deionized water and divided into caps and stipes. The samples were dried at a temperature of 30 °C for ~22 h in a hot air dryer (Memmert UF 110m, Memmert GmbH & Co. KG, Schwabach, Germany). After thorough drying, the samples were homogenized on a rotary mill (IKA A10, IKA-Werke GmbH & Co. KG, Staufen, Germany) and stored in gas-tight 20 mL headspace vials.

Figure 1.
Amanita rubescens Pers: (a) Sampling place with different fruiting body developmental stages; (b) collected samples of six developmental stages in PE boxes.
The specific shape and size of the six developmental stages of the mushroom Amanita rubescens Pers are shown in Figure 2. The six developmental stages were sorted, according to Falandysz et al. [22], by an expert mycologist (prof. Kunca). The first developmental stage (6 individuals) with the smallest fruiting bodies was estimated to be the 2nd–3rd day of fructification, with a size of 7 cm (Figure 2a). The 3rd to 5th day of fructification represented the second developmental stage (2 individuals), when the fruiting body reached 9 cm (Figure 2b). Figure 2c shows the third developmental stage (3 individuals), which was estimated to be the 5th–8th day of fructification, at a height of 12 cm of the mushroom body. The 8th–10th day stage (3 individuals), which is 15 cm high, is characterized as the fully mature stage of fructification and thus the fourth developmental stage (Figure 2d). Figure 2e shows the 5th developmental stage (the rotting stage) which was estimated to be the 10th–12th day of fructification, at a size of 14 cm (2 individuals). The sixth developmental stage (2 individuals) was estimated to be the 12th–15th day of fructification when the size of the fruiting body remained the same as the previous stage.
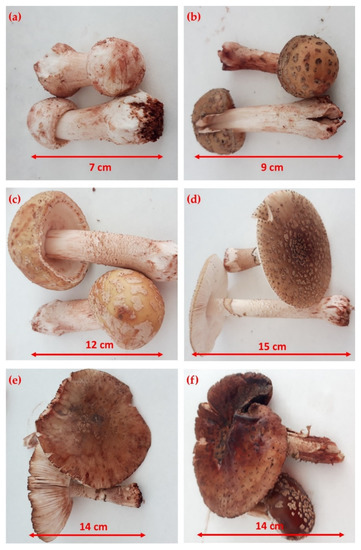
Figure 2.
Different developmental stages of Amanita rubescens Pers: (a) 2nd–3rd day of fructification; (b) 3rd–5th day of fructification; (c) 5th–8th day of fructification; (d) 8th–10th day of fructification; (e) 10th–12th day of fructification; (f) 12th–15th day of fructification.
2.2. Extraction Technique
An amount of 0.3 g of the sample was extracted with dichloromethane for HPLC ≥99.8% (2.5 mL; Sigma-Aldrich Merck KGaA, Darmstadt, Germany) by a shaker (Unimax 2010, Heidolph, Schwabach, Germany) at laboratory temperature (18 °C ± 0.1 °C) for 4 h and then filtered through a syringe PVDF filter (0.22 µm × 13 mm; Chromservis, Bratislava, Slovakia). Afterward, 150 µL of the sample extracts were stored in 2 mL vials with the microvolume insert (Agilent Technologies Inc., Santa Clara, CA, USA).
2.3. Volatile Compounds Analysis
The analysis was carried out using gas chromatography-mass spectrometry (GC-MS) (GC 7890B coupled by MSD 5977A; Agilent Technologies Inc.) equipped with CombiPal autosampler CTC120 (CTC Analytics AG, Zwingen, Switzerland). A column HP-5ms (30 m × 0.25 mm × 0.25 µm; Agilent Technologies Inc.) was used. One microliter of the sample extract was injected in the inlet, operated in a split mode 10:1 at 250 °C. The oven temperature program started at 40 °C. The temperature was held for 3 min, then increased to 250 °C at 3 °C/min and then held again for 10 min. Helium was used as carrier gas at the constant flow (1.2 mL/min). The mass detector parameters were as follows: ionization energy of filament: 70 eV, transfer line temperature: 250 °C, MS source temperature: 230 °C, quadrupole temperature: 150 °C. The mass spectrometer was programmed under electron impact (EI) in a full-scan mode at m/z 40–450 with a frequency of 1.8 scans/s. Each sample was measured in triplicate.
2.4. Volatile Compounds Determination
The compound identification was carried out by comparing mass spectra (over 80% match) with a commercial database NIST library 2017 (National Institute of Standards and Technology, Gaithersburg, MD, USA) and Wiley library, retention times of the reference mixture standard of n-alkanes (11 components, Restek Corporation, Bellefonte, PA, USA), and a comparison of data on the occurrence of edible fungi with the literature [23,24]. The relative percentage (%) of the determined volatile compounds was calculated by dividing the individual peak area by the total area of all peaks. Peaks under 0.1% were not counted.
2.5. Statistical Analysis
All the data obtained were analyzed by descriptive statistics arithmetic average and standard deviation. Then, all the variables were tested for normality. According to the Shapiro-Wilk test and the Kolmogorov-Smirnov test, all the tested variables did not follow the Gaussian distribution. The Kruskal-Wallis test was performed to compare significant differences between the developmental stages. The ten most numerous volatile compounds, characteristic of each stage, were used for PCA analysis. Principal Component Analysis (Spearman type) was used to find a pattern of similarity of the observations and the variables by displaying them as points on a map. Descriptive statistics, normality tests, Kruskal-Wallis test, and the PCA analysis were performed using the MS Excel and XLSTAT package program [25].
3. Results
In total, the sixty-two volatile compounds were identified by GC-MS analysis in dichloromethane extracts of the mushroom A. rubescens Pers. including 24 alkanes, 13 alcohols, 11 alkenes, 5 acids, 3 esters, 2 aldehydes, 2 amides, 1 imide, and 1 ketone. The values shown in Table 1 and Table 2 for each volatile compound are means of triplicate determinations with standard deviation. The developmental stages of A. rubescens were sorted into six developmental stages and the evaluation of the aroma composition in caps and stipes of the fruiting bodies were determined independently.

Table 1.
Relative percentage (%) of volatile compounds identified in the dichloromethane extract of the Amanita rubescens caps (1–6C) 1 in various developmental stages by the gas chromatography-mass spectrometry.

Table 2.
Relative percentage (%) of volatile compounds identified in the dichloromethane extract of the Amanita rubescens stipes (1–6S) 1 at various developmental stages by the gas chromatography-mass spectrometry.
The 2-hexyl-1-decanol (9.26–15.0% and 10.3–16.8%), 2-hexyl-1-dodecanol (13.2–16.2% and 6.00–15.3%), 2-butyl-1-octanol (3.95–6.58% and 4.91–6.19%), 2,4-di-tert-butylphenol (8.31–14.2% and 5.85–11.3%), 1,3-di-tert-butylbenzene (8.96–13.0% and 9.97–12.4%), 4,6-dimethyl-dodecane (6.40–8.72% and 6.35–8.88%), and 2,6-dimethyl-nonane (2.32–4.21% and 3.09–3.92%) in caps and stipes of A. rubescens showed high relative percentages. The caps contained more volatile compounds than stipes with different relative content. The relative percentage of 2,4-dimethyl-1-heptene in caps was 3.65–5.81% for developmental stages 1–5 (it was one of the higher contents) and 1.46% for developmental stage 6, while the relative percentage for stipes were 1.95–4.96%, and 3.75%, respectively.
Several unique volatiles that were present in one developmental stage and not in others can be marked as markers. The cap in stage 1 had the unique volatile succinimide. The cap in the next developmental stage 2 had the unique volatiles 7-methyl-6-tridecene and 5-ethyl-5-methyl-decane. The cap in stage 3 had no specific marker. The fully mature stage had the unique volatile 2-octyl-1-dodecanol. The early-stage of the rotting mushroom had specific volatiles N-methyliminopropylbenzene and nonanoic acid. The cap in developmental stage 6 had several unique acids: 2-methyl-butanoic acid and 3-methyl-butanoic acid.
The differences were also recorded in stipes. There were no markers determined in developmental stages 1, 3, and 4. The stipes in stage 2 were characterized by 11-methyldodecanol, 3-methoxy-1-butanol, 1,2-diethyl-cyclooctane, 1-iodo-eicosane, and tetradecyl-pentadecafluorooctanoate. The stipe of the early-stage of the rotting was characterized by 1-iodo-dodecane and the latest developmental stage included 3-methoxy-1-butanol acetate, α-bulnesene, 2,3-butanediol, dodecanoic acid, and tetradecanoic acid.
Moreover, different fructification stages (unripe and overripe) can be distinguished based on the presence of volatiles in the caps. The first two stages (1st and 2nd), which can be marked as the unripe stage, contained 4-methyl-dodecan-1-ol, 2-hydroxy-2,N-dimethyl-butanamide, 1,3,5-trimethyl-cyclohexane, 4-methylene-decane, heptadecane, 5-methyl-tridecane, 4,6-dimethyl-undecane, and 5-methyl-undecane. For the overripe or rotting mushroom stages (5th and 6th stage) compounds 2,3-butanediol and glycerol were identified.
There were significant differences (Kruskal-Wallis test) observed in the caps between the developmental stages in all identified compounds (p < 0.05) except for 7-methyl-6-tridecene (p = 0.1176), and 5-ethyl-5-methyl-decane (p = 0.1176). At the same time, there were no significant differences in stipes between the developmental stages in 1-tetradecene (p = 0.1215), 2,5-dimethyl-2,5-hexanediol (p = 0.0568), 2,6,10-trimethyltridecane (p = 0.6627), 3,7,11,15-tetramethyl-2-hexadecene (p = 0.0639), 1-iodo-dodecane (p = 0.1176), tetradecyl pentadecafluorooctanoate (p = 0.1176), pentadecane (p = 0.1489), 3,8-dimethyl-undecane (p = 0.0793), 4,6-dimethyl-undecane (p = 0.2261), and 4,8-dimethyl-undecane (p = 0.0759). The PCA for caps (Figure 3) revealed that 74.43% of the total variation embodied in 14 variables could be effectively condensed and explained by the first two principal components (PCs), with eigenvalues of 8.4 and 2.01, respectively. The PCA for stipes (Figure 4) explains 66.47% of total variability with the eigenvalues of the first two principal components (PCs) 6.01 and 2.63, respectively. The results show that stage 6 in both PCAs (caps, stipes) is characterized by 2,3-butanediol. In the case of caps, the early-stage of the rotting mushroom (stage 5) can be characterized by glycerol and niacinamide contents. In the case of stipes, it is possible to strictly characterize stage 3 by 2,4-di-tert-butylphenol. Other stages cannot be clearly distinguished.
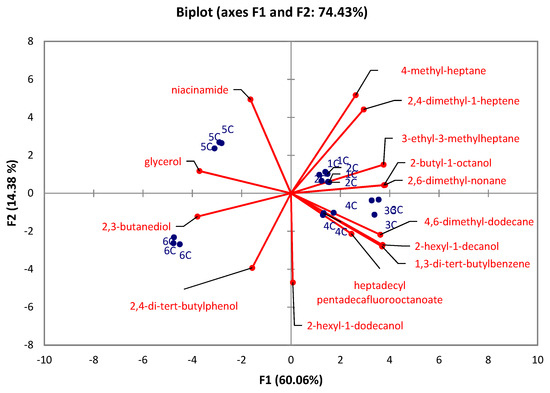
Figure 3.
PCA evaluation of six developmental stages (1C–6C) of A. rubescens Pers caps.
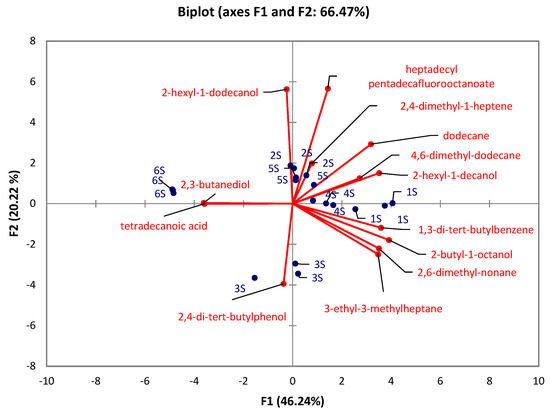
Figure 4.
PCA evaluation of six developmental stages (1S–6S) of A. rubescens Pers stipes.
4. Discussion
In this study, the fruit bodies of A. rubescens were divided into six developmental stages. According to Kalač [30], the lifetime of the fruiting body is estimated to be only 10–14 days. In this study, the sixth developmental stage was estimated to be the 12th–15th day of fructification, characterized by a dark brown color. The results showed that the caps contained higher quantities of volatile compounds than the stipes. On the other hand, the rotting mushroom cap stages 5 and 6 were statistically (PCA and Kruskal-Wallis test) characterized by unique aroma markers (2,3-butanediol (p < 0.0001), glycerol (p < 0.0001), and niacinamide (p = 0.0068)). The 2-methyl and 3-methyl butanoic acids (p = 0.0074) were characterized only for the 6th developmental stage and according to the literature, it is known to be sweaty [28]. In general, the acid compounds (i.e., nonanoic acid, and 2-methyl- and 3-methyl- butanoic acids) are responsible for the sweaty attribute, which is unpleasant and had a negative effect on the aroma profile of the mushrooms [31,32,33]. Most publications have focused on the sensory aroma quality of mushrooms from a technological aspect [11,34,35,36,37], while very few publications examined the sensory properties of mushroom developmental stages [38]. Lu et al. [38] characterized the aroma profile of two truffle species (T. indicum and T. pseudohimalayense) and the influence of the maturation stage on volatile organic compounds. The unripe stage was characterized by volatile compounds from groups: alcohols and phenols (7), esters (9), aldehydes and ketones (6), hydrocarbons (3), and 1 N-containing compound. In the mature stage, volatile compounds such as esters (2), alcohol (1), aldehydes and ketones (4), benzenes and methoxy compounds (5), hydrocarbons (2), and N-containing compounds (2) were identified. The same compound groups were also identified in the dichloromethane extracts of Amanita rubescens in our study.
According to the literature, the 1-octen-3-one is characteristic of a metallic mushroom-like odor with a low-odor threshold, and it makes a bigger contribution to the odor impressions of mushrooms than 1-octen-3-ol, which is known to have a higher content [8]. In this study, a series of eight-carbon aliphatic components were not detected in dichloromethane extracts of A. rubescens except 2-butyl-octanol (3.95–6.58%). Murray et al. [39] determined the key odorants responsible for the unique aroma of the diethyl ether extract of fragrant bolete, Suillus punctipes. The compounds 1-octen-3-one, 1-octen-3-ol, (2E)-oct-2-enal, linalool, δ-dodecalactone, and a mixture of octanal, nonanal, and decanal were essential for the unique aroma profile of S. punctipes. The n-hexadecanoic acid; 9,12-octadecadienoic acid (Z,Z)-, and 2(3H)-furanone were the most frequently occurring compounds in the methanolic extract of the Lentinus squarrosulus, Auricularia auricula-judae, Mycetinis copelandii, Baeospora myosura, Pleurotus ostreatus, and Volvariella volvacea [40]. De Pinho et al. [41] used the headspace solid-phase microextraction technique (HS-SPME) in the headspace of a wild edible mushroom powder and in the headspace of a mushroom dissolved in 10% ethanol. Their study included A. rubescens aromatic profile and it was characterized by 3-octanone, 3-octanol, benzoic acid, undecanal, and α-pinene. According to Portalo-Calero et al. [23] the 3-methyl-butanal, 2-methyl-butanal, hexanal, styrene, 3-octenol, 3-octanone, 3-octanol, and 2-ethyl-hexanol were identified by HS-SPME GC-MS in A. rubescens. This study is a first step in aiming to advance an understanding of the differentiation of developmental stages of Amanita rubescens through dichloromethane extracts.
5. Conclusions
This study provided a thorough comparison of the volatile compound profiles of Amanita rubescens at various developmental stages of fruiting bodies. Our results for the volatile compounds in A. rubescens show a minor change for caps and stipes during the fruit body maturation (from the smallest fruit body to full maturity). On the other hand, the eight compounds (4-methyl-dodecan-1-ol, 2-hydroxy-2,N-dimethyl-butanamide, 1,3,5-trimethy-cyclohexane, 4-methylene-decane, heptadecane, 5-methyl-tridecane, 4,6-dimethyl-undecane, and 5-methyl-undecane) were identified in the earlier stages of the fruit body while they were absent at the full-maturity stage. Statistical analysis showed that several compounds displayed different relationship patterns in the cap the rotting mushroom stage (2,3-butanediol, glycerol, and niacinamide) from that of the full-maturity stage (4,6-dimethyl-dodecane, 2-hexyl-1-decanol, 1,3-di-tert-butylbenzene, heptadecyl pentadecafluorooctanoate, and 2-hexyl-1-dodecanol). We confirmed that, based on aromatic profiles, it is possible to distinguish the initial developmental stage of a fruiting body from the fully mature and the decomposition stage. At the same time, it is better to determine the aromatic profiles of mushrooms in caps than stipes. For the first time, this study specifies the individual developmental stages of the wild edible mushroom Amanita rubescens. In conclusion, there is still a need for further confirmation of volatile organic compounds on more individual fruiting bodies. We recommend studying developmental stages of A. rubescens from different localities in Slovakia (possible effect of chemical-physical, and geochemical parameters of soil) or comparing the results with different developmental stages of other wild edible mushrooms.
Author Contributions
Conceptualization, J.Š. and J.Á.; methodology, J.Š. and J.Á; validation, J.Š.; formal analysis, J.Š., P.M., M.Š., V.K. and J.Á.; investigation, J.Š.; resources, J.Š., P.M., M.Š. and J.Á.; writing—original draft preparation, J.Š., P.M., M.Š., V.K. and J.Á.; writing—review and editing, J.Š., P.M., M.Š., V.K. and J.Á.; project administration, J.Á. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Education, Science, Research and Sport of the Slovak Republic, Slovakia, Project No. VEGA 1/0591/18 (1).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
We thank the Operational Program Research and Innovation: “Support of research activities in the ABT RC” 313011T465, co-financed by the European Regional Development Fund (0.1), the Operational Program Integrated Infrastructure within the project: Demand-driven research for the sustainable and innovative food, Drive4SIFood 313011V336, co-financed by the European Regional Development Fund (0.2), and the Grant Agency of The Slovak University of Agriculture in Nitra, the project number 17-GASPU-2021: Possibilities of reducing the level of contamination of edible wild-growing mushrooms from environmentally polluted areas of Slovakia while preserving their valuable biologically active substances (0.7). We thank Martin Hauptvogl for English proofreading.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mleczek, M.; Rzymski, P.; Budka, A.; Siwulski, M.; Jasińska, A.; Kalač, P.; Poniedziałek, B.; Gąsecka, M.; Niedzielski, P. Elemental characteristics of mushroom species cultivated in China and Poland. J. Food Compos. Anal. 2018, 66, 168–178. [Google Scholar] [CrossRef]
- Burgos, N.; Mellinas, A.C.; García-Serna, E.; Jiménez, A. 17-nanoencapsulation of flavor and aromas in food packaging. In Food Packaging, 1st ed.; Grumezescu, A.M., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 567–601. [Google Scholar] [CrossRef]
- Zhang, X.; Cheng, Z.; Ma, L.; Li, J. A study on accumulation of volatile organic compounds during ochratoxin a biosynthesis and characterization of the correlation in Aspergillus carbonarius isolated from grape and dried vine fruit. Food Chem. 2017, 227, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Combet, E.; Eastwood, D.C.; Burton, K.S.; Combet, E.; Henderson, J.; Henderson, J.; Combet, E. Eight-carbon volatiles in mushrooms and fungi: Properties, analysis, and biosynthesis. Mycoscience 2006, 47, 317–326. [Google Scholar] [CrossRef]
- Wang, X.M.; Zhang, J.; Wu, L.H.; Zhao, Y.L.; Li, T.; Li, J.Q.; Wang, Y.Z.; Liu, H.G. A mini-review of chemical composition and nutritional value of edible wild-grown mushroom from China. Food Chem. 2014, 151, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Pei, F.; Yang, W.; Ma, N.; Fang, Y.; Zhao, L.; An, X.; Xin, Z.; Hu, Q. Effect of the two drying approaches on the volatile profiles of button mushroom (Agaricus bisporus) by headspace GC-MS and electronic nose. LWT Food Sci. Technol. 2016, 72, 343–350. [Google Scholar] [CrossRef]
- Sun, L.; He, W.; Xin, G.; Cai, P.; Zhang, Y.; Zhang, Z.; Wei, Y.; Sun, B.; Wen, X. Volatile components total phenolic compounds. and antioxidant capacities of worm-infected Gomphidius rutilus. Food Sci. Hum. Wellness 2018, 7, 148–155. [Google Scholar] [CrossRef]
- Aisala, H.; Sola, J.; Hopia, A.; Linderborg, K.M.; Sandell, M. Odor-contributing volatile compounds of wild edible Nordic mushrooms analyzed with HS–SPME–GC–MS and HS–SPME–GC–O/FID. Food Chem. 2019, 283, 566–578. [Google Scholar] [CrossRef]
- Zawirska-Wojtasiak, R. Optical purity of (R)-(−)-1-octen-3-ol in the aroma of various species of edible mushrooms. Food Chem. 2004, 86, 113–118. [Google Scholar] [CrossRef]
- Bozok, F.; Zarifikhosroshahi, M.; Kafkas, E.; Taşkin, H.; Buyukalaca, S. Comparison of volatile compounds of fresh Boletus edulis and B. pinophilus in Marmara Region of Turkey. Not. Bot. Horti Agrobot. Cluj Napoca 2015, 43, 192–195. [Google Scholar] [CrossRef] [Green Version]
- Du, X.; Sissons, J.; Shanks, M.; Plotto, A. Aroma and flavor profile of raw and roasted Agaricus bisporus mushrooms using a panel trained with aroma chemicals. LWT Food Sci. Technol. 2021, 138, 110596. [Google Scholar] [CrossRef]
- Drewnowska, M.; Jarzyńska, G.; Kojta, A.K.; Falandysz, J. Mercury in European Blusher. Amanita rubescence, mushrooms and topsoils: Bioconcentration potential and intake assessment. J. Environ. Sci. Health B 2012, 47, 466–474. [Google Scholar] [CrossRef]
- Falandysz, J.; Drewnowska, M. Distribution of mercury in Amanita fulva (Schaeff.) Secr. Mushrooms: Accumulation, loss in cooking and dietary intake. Ecotox. Environ. Saf. 2015, 115, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Li, X.; Lüli, Y.; Li, X.; Chen, Z.H.; Yuan, P.; Yang, Z.L.; Li, G.; Luo, H. Novel Cyclic Peptides from Lethal Amanita Mushrooms through a Genome-Guided Approach. J. Fungi 2021, 7, 204. [Google Scholar] [CrossRef]
- Zhang, Y.; Mo, M.; Yang, L.; Mi, F.; Cao, Y.; Liu, C.; Tang, X.; Wang, P.; Xu, J. Exploring the Species Diversity of Edible Mushrooms in Yunnan. Southwestern China by DNA Barcoding. J. Fungi 2021, 7, 310. [Google Scholar] [CrossRef] [PubMed]
- Decree no. 132/2014 of Ministry of Agriculture and Rural Development of the Slovak Republic of 15 May 2014 on Processed Fruit and Vegetables, Edible Mushrooms, Oilseeds, Dried Nuts, Potatoes and Products Thereof. Available online: https://www.slov-lex.sk/pravne-predpisy/SK/ZZ/2014/132/20140601 (accessed on 20 July 2021).
- Statistical Office of the Slovak Republic. Food Consumption in the SR in 2019. Available online: www.statistics.sk (accessed on 20 July 2021).
- Czech Statistical Office. Food Consumption–2019. Available online: www.czso.cz (accessed on 20 July 2021).
- Hagara, L.; Antonín, V.; Baier, J. Vel’ký Atlas Húb; Ottovo Nakladatel’stvo: Bratislava, Slovakia, 2005; ISBN 8073603330. [Google Scholar]
- Bučinová, K.; Janík, R.; Jamnická, G.; Kuklová, M. Accumulation and bioconcentration factors of mineral macronutrients in representative species of macrofungi prevailing in beech-dominated forests affected by air pollution. Czech Mycol. 2014, 66, 193–207. [Google Scholar] [CrossRef]
- Džatko, M.; Sobocká, J.; Granec, M.; Bezák, P. Príručka Pre Používanie Máp Pôdnoekologických Jednotiek. Inovovaná Príručka Pre Bonitáciu a Hodnotenie Poľnohospodárskych Pôd Slovenska; Výskumný Ústav Pôdoznalectva a Ochrany Pôdy: Bratislava, Slovakia, 2009; ISBN 978-80-89128-55-6. [Google Scholar]
- Falandysz, J.; Hanć, A.; Barałkiewicz, D.; Zhang, J.; Treu, R. Metallic and metalloid elements in various developmental stages of Amanita muscaria (L.) Lam. Fungal Biol. 2020, 124, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Portalo-Calero, F.; Arroyo, P.; Suárez, J.I.; Lozano, J. Triangular Test of Amanita Mushrooms by Using electronic Nose and Sensory Panel. Foods 2019, 8, 414. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Pu, D.; Sun, B.; Ren, F.; Zhang, Y.; Chen, H. Characterization and comparison of key aroma compounds in raw porcini mushroom (Boletus edulis) by aroma extract dilution analysis, quantitation and aroma recombination experiments. Food Chem. 2018, 258, 260–268. [Google Scholar] [CrossRef]
- Addinsoft, XLSTAT. Analyse de Données et Statistique Avec MS Excel; Addinsoft: New York, NY, USA, 2014. [Google Scholar]
- The Good Scents Company Information System [WWW Document], n.d. Available online: http://www.thegoodscentscompany.com/ (accessed on 14 April 2020).
- Selli, S.; Guclu, G.; Sevindik, O.; Kelebek, H. Variations in the key aroma and phenolic compounds of champignon (Agaricus bisporus) and oyster (Pleurotus ostreatus) mushrooms after two cooking treatments as elucidated by GC–MS-O and LC-DAD-ESI-MS/MS. Food Chem. 2021, 354, 129576. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, D.; Pu, D.; Zhang, Y.; Chen, H.; Sun, B.; Ren, F. Multivariate relationships among sensory attributes and volatile components in commercial dry porcini mushrooms (Boletus edulis). Food Res. Int. 2020, 133, 109112. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, J.; Xiao, Q.; Feng, T.; Huang, Q.; Ho, C.-T.; Song, S. Comparative flavor profile analysis of four different varieties of Boletus mushrooms by instrumental and sensory techniques. Food Res. Int. 2020, 136, 109485. [Google Scholar] [CrossRef] [PubMed]
- Kalač, P. Trace element contents in European species of wild growing edible mushrooms: A review for the period 2000–2009. Food Chem. 2010, 122, 2–15. [Google Scholar] [CrossRef]
- Fukami, K.; Ishiyama, S.; Yaguramaki, H.; Masuzawa, T.; Nabeta, Y.; Endo, K.; Shimoda, M. Identification of Distinctive Volatile Compounds in Fish Sauce. J. Agric. Food Chem. 2002, 50, 5412–5416. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xue, J.L.; Feng, C.P. Effects of different drying methods on volatile flavor compounds in Lentinus edodes. Sci. Technol. Food Ind. 2018, 39, 224–229. [Google Scholar] [CrossRef]
- Hou, H.; Liu, L.; Lu, C.; Fang, D.; Hu, Q.; Zhang, Y.; Zhao, L. Characterization of flavor frame in shiitake mushrooms (Lentinula edodes) detected by HS-GC-IMS coupled with electronic tongue and sensory analysis: Influence of drying techniques. LWT 2021, 146, 111402. [Google Scholar] [CrossRef]
- Cho, H.; Kim, S.Y.; Choi, H.-K.; Kim, Y.-S. Characterization of Volatile Compounds in Raw and Cooked Pine-Mushrooms (Tricholoma matsutake Sing.). J. Agric. Food Chem. 2006, 54, 6332–6335. [Google Scholar] [CrossRef]
- Politowicz, J.; Lech, K.; Sánchez-Rodríguez, L.; Szumny, A.; Carbonell-Barrachina, Á.A. Volatile composition and sensory profile of Cantharellus cibarius Fr. as affected by drying method. J. Sci. Food Agric. 2017, 97, 5223–5232. [Google Scholar] [CrossRef]
- Aisala, H.; Laaksonen, O.; Manninen, H.; Raittola, A.; Hopia, A.; Sandell, M. Sensory properties of Nordic edible mushrooms. Food Res. Int. 2018, 109, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Aisala, H.; Manninen, H.; Laaksonen, T.; Linderborg, K.M.; Myoda, T.; Hopia, A.; Sandell, M. Linking volatile and non-volatile compounds to sensory profiles and consumer liking of wild edible Nordic mushrooms. Food Chem. 2020, 304, 125403. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Perez-Moreno, J.; Zhang, F.; Rinaldi, A.C.; Yu, F. Aroma profile of two commercial truffle species from Yunnan and Sichuan, China: Inter-and intraspecific variability and shared key compounds. Food Sci. Hum. Wellness 2021, 10, 163–173. [Google Scholar] [CrossRef]
- Murray, A.F.; Wickramasinghe, P.C.K.; Munafo, J.P. Key odorants from the fragrant bolete, Suillus punctipes. J. Agric. Food Chem. 2020, 68, 8621–8628. [Google Scholar] [CrossRef] [PubMed]
- Oni, J.O.; Akomaye, F.A.; Arkson, A.-A.A.; Egwu, A.C. GC-MS analysis of bioactive compounds in some wild-edible mushrooms from Calabar, Southern Nigeria. Eur. J. Biol. Biotech. 2020, 1, 1–9. [Google Scholar] [CrossRef]
- De Pinho, P.G.; Ribeiro, B.; Gonçalves, R.F.; Baptista, P.; Valentão, P.; Seabra, R.M.; Andrade, P.B. Correlation between the Pattern Volatiles and the Overall Aroma of Wild Edible Mushrooms. J. Agric. Food Chem. 2008, 56, 1704–1712. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).