Abstract
Mycotoxin-producing Aspergilli (Circumdati, Flavi, and Nigri), usually associated with contaminated food, may also cause respiratory disorders and are insufficiently studied in water-damaged indoor environments. Airborne (N = 71) and dust borne (N = 76) Aspergilli collected at post-flood and control locations in Croatia resulted in eleven different species based on their calmodulin marker: A. ochraceus, A. ostianus, A. pallidofulvus, A. sclerotiorum, and A. westerdijkiae (Circumdati); A. flavus (Flavi); and A. tubingensis, A. welwitschiae, A. niger, A. piperis, and A. uvarum (Nigri). Most of the airborne (73%) and dust borne (54%) isolates were found at post-flood locations, and the highest concentrations measured in indoor air (5720 colony-forming units (CFU)/m3) and dust (2.5 × 105 CFU/g) were up to twenty times higher than in the control locations. A. flavus dominated among airborne isolates (25%) at the unrepaired locations, while 56% of the dust borne Aspergilli were identified as A. tubingensis and A. welwitschiae. The ability of identified isolates to produce mycotoxins aflatoxin B1 (AFB1), fumonisin B2 (FB2), and ochratoxin A were assessed by LC-MS analysis. All ochratoxin A (OTA)-producing Circumdati belonged to A. westerdijkiae (13.7 ± 15.81 µg/mL); in the section, Flavi A. flavus produced AFB1 (2.51 ± 5.31 µg/mL), while A. welwitschiae and A. niger (section Nigri) produced FB2 (6.76 ± 13.51 µg/mL and 11.24 ± 18.30 µg/mL, respectively). Water damage dominantly supported the occurrence of aflatoxigenic A. flavus in indoor environments. Yet unresolved, the causal relationship of exposure to indoor Aspergilli and adverse health effects may support the significance of this research.
1. Introduction
As a result of climate change, we are witnessing an increasing number of storms, hurricanes, and other events resulting in flooding. Among the numerous consequences associated with these disastrous events, fungal proliferation complicates and increases the costs of renovations, and it represents a health threat to those exposed to it [1,2,3,4,5]. Building dampness or moisture problems are a typical cause of “building decay”, primarily because water promotes fungal growth, and fungi degrade the substrate on which they grow. With an ability to tolerate water activities (aw) 0.75–0.90, Aspergilli are the most common indoor fungi [6], while at the same time, their concentration in outdoor air is low. Aspergilli from the section Nidulantes series Versicolores are the most prevalent indoor Aspergilli [7,8,9,10,11,12,13,14,15], while only a limited number of studies reported Aspergilli from the sections Circumdati, Flavi, and Nigri in air or dust samples from indoor environments [16,17,18,19,20,21,22,23,24,25]. The Aspergillus species belonging to the sections Circumdati, Flavi, and Nigri are primary colonizers that can grow at a lower water activity than secondary (e.g., Cladosporium spp.) and tertiary colonizers (e.g., Trichoderma spp., Stachybotrys spp., and Chaetomium spp.). In addition, they are among the microbes most resistant to various disinfecting compounds [26]. Thus, they may remain in the indoor environment following water damage even after certain remediation activities are applied e.g., removing excess water, drying, ventilation, disinfection, etc. Unless thorough sporicidal fumigation is performed, their spores may remain indoors and proliferate when favorable conditions occur. Therefore, they could be a good indicator of successful or non-successful indoor post-flood remediation.
There are many reasons why monitoring Aspergilli from the sections Circumdati, Flavi, and Nigri matters. They may cause a variety of clinical pictures in humans, from simple allergies to severe asthma with fungal sensitization, especially in children [27]. They are also implicated in severe illnesses such as chronic pulmonary aspergillosis [28,29], and there are reports of its association with exposure to moulds at home [30]. A special concern arises from the ability of these species to produce mycotoxins such as ochratoxin A (OTA), aflatoxin B1 (AFB1), and fumonisin B2 (FB2), which can be deposited in various substrates, including dust in an indoor environment. In addition to their toxic, mutagenic, genotoxic, and cancerogenic properties [31,32,33,34,35], all of these mycotoxins may modulate immune responses and, as such, they may contribute to impaired response to infection or to chronic inflammatory disorders [36].
In 2014, catastrophic floods occurred in the areas of east Croatia, northwest Bosnia, and central Serbia [37]. The municipality of Gunja, in eastern Croatia, suffered heavy damage. The water levels measured in housing ranged from a few cm to 4 m, and the majority of inhabitants were evacuated. Two years after this catastrophic flood, most of these houses have undergone repair. While fungal indoor colonisation was still visible in some of the repaired homes, unrepaired houses were heavily contaminated by fungal growth. In order to evaluate post-flood fungal damage related to the presence of Aspergilli, air samples and dust were collected at repaired and unrepaired locations in Gunja during the winter and summer of 2016 and 2017. Houses as well as the school in Gornji Stupnik were chosen as the control locations as they are settled in a similar riverside environment in western Croatia. Airborne and dust borne AFB1, OTA, and FB2 producing and non-producing Aspergilli from the sections Circumdati, Flavi, and Nigri were studied by cultivation on specific agar plates and identified to species level based on partial calmodulin (CaM) sequences. To assess mycotoxin-producing abilities, a micro-extraction procedure [38,39] was applied for an each isolate following liquid chromatography-tandem mass spectrometry (LC/MS) analysis of mycotoxins AFB1, OTA, and FB2 in corresponding mould extracts.
2. Materials and Methods
2.1. Solvents, Reagents, and Mycotoxin Standards
Czapek Yeast Extract Agar (CYA) was prepared from Czapek Dox Broth (Difco; Becton, Dickinson and Company, Franklin Lakes, NJ, USA), Yeast Extract (Difco; Becton, Dickinson and Company, Franklin Lakes, NJ, USA), trace elements salts ZnSO4 × 7 H2O and CuSO4 × 5 H2O, and agar–agar (Kemig, Zagreb, Croatia) following the manual [40]. Malt Extract Agar (MEA), Dichloran 18%-glycerol agar (DG-18), and Dichloran Rose-Bengal Cloramphenicol Agar (DRBC) were purchased from Oxoid Limited, Thermo Fisher Scientific, Basingstoke, UK. Distilled water was prepared with Niro-VV-Atlantic 3 (Nirosta d. o. o., Zagreb, Croatia). Molecular biology grade water was purchased from Lonza (Basel, Switzerland). The solvents acetonitrile (MeCN) and methanol (MeOH) were of LC-MS grade and purchased from Kemig (Zagreb, Croatia). Dichloromethane, ethyl acetate (EtAc), formic acid (HCOOH), and acetic acid (AcOH) were of pro analysi grade and purchased from Lach-Ner d.o.o. Zagreb, Croatia. Aflatoxin B1 (AFB1) and ochratoxin A (OTA) were purchased from Sigma-Aldrich (Deisenhofen, Germany) and fumonisin B2 (FB2) was purchased from Cayman Chemical Company (Ann Arbor, MI, USA).
2.2. Sampling: Source, Locations, and Periods
According to the information obtained through interviews with the residents of Gunja, the sampling locations were selected based on renovation activities following the flood. When the water receded after the flood, excess water was pumped out of the buildings, and the entire area was treated with disinfectant fluids. Since then, the majority of the homes in the village have undergone thorough renovation, including drying, reconstruction works, wall painting and repeated application of chlorine-based disinfectant (Izosan® G). Among these thoroughly repaired locations, we chose five houses and the elementary school as post-flood repaired locations, and five houses were selected from those that had not undergone thorough remediation (unrepaired locations). In addition, six control locations were included in this study, which were comprised of five houses and one elementary school from a similar riverside area that was not affected by flooding. Sampling of air and dust was performed in four sampling periods, late winter (February) and late summer (September) of 2016 and 2017. Altogether, 60 indoor and 12 outdoor air samples, along with 12 samples of dust (control and repaired locations), and 40 indoor and 10 outdoor air samples, along with 10 samples of dust from unrepaired locations, were taken in each sampling period.
Airborne moulds were sampled 1 m above ground using a Mas 100 Eco air sampler (Merck, Darmstadt, Germany) with 400 holes (hole to agar impactor) and DG-18 and MEA plates [41]. A volume of 50 L was sampled with the impaction velocity of the sampler at approximately 10.8 m/s and an airflow rate of 100 L/min. The dust samples were collected by occupants in a nylon sampling sock by vacuuming an area of 1–3 m2 in home/school locations. After field sampling, a mycological analysis of dust samples was performed using the plate count method; 1 g of dust was suspended in peptone broth (9 mL) and dilutions from 10−1 to 10−5 were plated (0.1 mL) on DG-18 and DRBC [41]. The plates with prepared air and dust samples were incubated for five days in the dark at 25 ± 2 °C. After the mould colonies were developed, in order to separate Aspergilli from the sections Circumdati, Flavi, and Nigri, Aspergilli were re-isolated on CYA and incubated for seven days in the dark at 25 ± 2 °C.
Statistical Analysis
The number of colony-forming units (CFU) of total Aspergilli and each Aspergillus section, per m3 of air or per gram of dust, was analysed by mixed modelling. As the fixed factors group was nested within the location (levels Gunja: Unrepaired locations, Repaired locations, Outdoor air–air only, and Gornji Stupnik: Control locations, Outdoor air–air only) crossed with Season (levels Winter and Summer). The object identifier was included as a random intercept to account for multiple measures taken from the same object. Due to skewed distribution, CFU/m3 or CFU/g were transformed by inverse transformation of ranks to the standard normal distribution prior to the analysis. Based on the fitted model, t-test post-hoc was applied for Winter and Summer periods separately, comparing the difference in CFU/m3 or CFU/g between Unrepaired and Repaired locations, between Repaired locations and Control locations, and between Outdoor air in Gunja and Gornji Stupnik (air models only). Due to multiple testing, the false discovery rate was controlled using the Bonferroni method. Statistical significance was set at a level of 0.05. All statistical analyses were performed in R.
2.3. Identification of Aspergilli to the Species Level
The extraction of template DNA for PCR amplification was achieved using a NucleoSpin® Plant II kit (Macherey-Nagel GmbH & Co. KG, Düren, Germany), which was optimised and modified for the extraction of genomic DNA from fungi. The mycelia of three-day-old Aspergilli isolates cultured on CYA in darkness at 25 °C were scrapped by a sterile loop and transferred to 1 mL of SDS-based buffer in a sterile falcone tube containing approximately 1 g of sterile glass beads and vortexed at maximum speed for 3–5 min. Protein-precipitation reagent (500 µL) was added to the mixture, vortexed for 30 s, and incubated for 10 min at −20 °C. After this, the protocol provided by the manufacturer was followed. The isolated genomic DNA was used as the template DNA for PCR reactions. Part of the calmodulin gene (CaM) was amplified with 0.2 μM cmd5 and cmd6 primers (Hong et al., 2006). The reaction mixture, set to 20 μL volume, contained Hot Start polymerase Master Mix 2x and water (Takara Bio Inc, Kusatsu, Japan). Amplifications (T-100 Thermal Cycler, Bio Rad, Hercules, USA) in 35 cycles: 95 °C (5 min 1st, then 20 s), 56 °C (20 s), 72 °C (40 s; 5 min final). The successful amplifications were confirmed by electrophoresis (Sub-Cell® GT Agarose Gel Electrophoresis Systems, BioRad, Hercules, USA) on a 1.5% agarose gel by mixing the PCR product with fluorescent dye GelStar (Basel, Switzerland) against the reference ladder (100 bp, Lonza, Basel, Switzerland) under UV light (UV transilluminator Uvitec, Cambridge, UK). NucleoSpin® Gel and PCR Clean-up (Macherey-Nagel GmbH & Co., Düren, Germany) was used for purification of the PCR products following the procedure provided by manufacturer. The sequencing was done using the same pair of primers at Macrogen Inc., Amsterdam, The Netherlands.
Phylogeny
Sequences of the ex-type strains of all the species in section Circumdati, Flavi, and Nigri were obtained from the most recent phylogeny study of Aspergillus [42], downloaded from GenBank, and added to the analyses. An alignment of partial CaM sequences was made using Prank v 0.150803 [43] with default settings. The datasets were partitioned to exons and introns, and the best-fitting model was estimated by using ModelTest-NG v. 0.1.4 [44], allowing only gamma rate heterogeneity. Model selection was based on the corrected Akaike Information Criterion [45,46]. The proposed substitution models for intron and exon partitions, respectively, were HKY+G4 and TrN+G4 (section Circumdati), GTR+G4 and TIM1+G4 (section Flavi), and HKY+G4 and TIM2+G4 (section Nigri). Phylogenetic reconstruction was conducted using Maximum Likelihood (ML) analyses using RAxML-NG v0.9.0 [47]. Branch support was estimated by 500 bootstrap replicates. All identified isolates were stored under the appropriate number in the working microbial culture collection of the Department of Microbiology, Faculty of Pharmacy and Biochemistry, University of Zagreb.
2.4. Mycotoxin-Producing Abilities
Each of the Aspergillus isolates was extracted as described previously [19] following the procedure by Smedsgaard [38] for isolates from the sections Flavi and Circumdati, i.e., detection of AFB1 and OTA, and the procedure by Frisvad et al. [39] for the section Nigri, i.e., detection of FB2. The extracts were ultrasonicated, and the organic phases were separated, filtered through polypropylene (PP) or polytetrafluoroethylene (PTFE) syringe filters, evaporated to dryness in rota-vapour concentrator (Concentrator Plus, Eppendorf, Germany), weighed, dissolved in MeOH:H2O 0.7:0.3, v/v, and stored at −20 °C until the LC/MS analysis. All experiments were performed using the Agilent 1100 Series LC-MSD Trap system (Agilent Technologies, Waldbronn, Germany). Instrument control, data acquisition, and evaluation were done using ChemStation for LC 3D and LC/MSD Trap v. 5.2 software. The details about LC/MS parameters are included in Appendix A.
3. Results
3.1. The Airborne Aspergilli
The frequency and the concentrations of total Aspergilli in indoor and outdoor air samples from the post-flood locations (repaired and unrepaired) and control locations, including the proportion of Aspergilli from the sections Circumdati, Flavi, and Nigri, are presented in Figure 1, while the details regarding the concentrations of Aspergilli assigned to the sections Circumdati, Flavi, and Nigri are provided in the supplementary materials (Tables S1–S3). Aspergilli were dominantly isolated from indoor air collected at the repaired and the unrepaired locations in winter (92 and 85%, respectively), while they were less frequent in indoor air samples collected at the control locations (75%). In both sampling periods, the concentrations of Aspergilli in indoor air were higher than at the control locations, while the difference was less pronounced in the summer than in winter (Figure 1). The highest concentrations of Aspergilli in outdoor air were measured at the unrepaired locations, and they were twenty-five times higher than in outdoor air from the repaired locations and almost fifty times higher than in outdoor air from control locations (Figure 1). The opposite seasonal pattern was observed at the unrepaired locations compared to the control and repaired post-flood locations as their concentrations in outdoor air were ten times lower in the summer than in the winter sampling period.
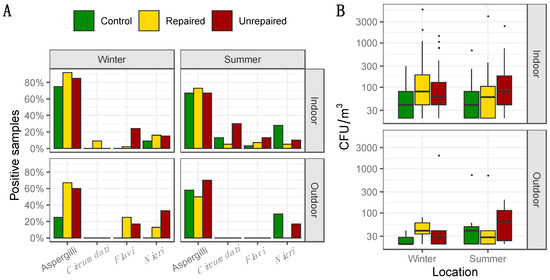
Figure 1.
Percentage of positive samples where total airborne Aspergilli, as well as Aspergilli from the sections Circumdati, Flavi, and Nigri, were detected based on analysis of 60 indoor air and 12 outdoor air samples for control and repaired locations and 40 indoor and 10 outdoor air samples for unrepaired locations in each sampling period i.e., winter and summer (panel A); Concentration of total Aspergilli (colony-forming units (CFU)/m3) detected in indoor and outdoor air. Each location is represented by a box–whisker plot in a different colour in each sampling period (winter and summer) as specified (panel B).
When compared to other sections, Aspergilli from the section Circumdati were rare, isolated only from indoor air samples, and dominant at the post-flood locations (Figure 1). Statistically, there were significantly more Aspergilli from the section Circumdati in the indoor air of post-flood unrepaired locations compared to the repaired locations in Gunja in the summer sampling period (Supplementary Materials, Table S1), while in the winter period, there were significantly more Aspergilli from the section Flavi in the indoor air of unrepaired locations compared to the repaired locations in Gunja (Supplementary Materials, Table S2). In addition, they were dominant in outdoor air collected at the post-flood locations in winter, comprising 25% and 17% of total airborne Aspergilli in the samples from the repaired and unrepaired locations, respectively (Figure 1). Aspergilli from the section Nigri were more abundant at repaired locations in winter (Max 1100 CFU/m3). In the summer, this was inversed, as the black Aspergilli were up to five times more common in the samples from the control locations compared to the post-flood locations (Figure 1 and Supplementary Materials Table S3). Exceptionally high concentrations of black Aspergilli were observed in the outdoor air samples collected at the unrepaired locations (Max 1980 CFU/m3) in winter. In the summer, at both post-flood locations, the concentrations of black Aspergilli in indoor and outdoor air decreased (Supplementary materials, Table S3).
3.2. The Dust Borne Aspergilli
The frequency and concentrations of total Aspergilli in dust collected at the post-flood locations (repaired and unrepaired) and control locations, including the proportion of Aspergilli from the sections Circumdati, Flavi, and Nigri, are presented in Figure 2. Details regarding the concentrations of Aspergilli assigned to the sections Circumdati, Flavi, and Nigri are provided in the Supplementary Materials (Tables S4–S6). The dust borne Aspergilli were present in every dust sample collected at the control locations and repaired post-flood locations. The highest concentrations of total dust borne Aspergilli were measured in the dust from the repaired locations in the winter sampling period (Max 2.5 × 105 CFU/g). They were up to four times higher than in the dust from the control locations and up to ten times higher than in the dust from the unrepaired locations (Figure 2). At both locations, concentrations of the dust borne Aspergilli declined in the summer sampling period (Figure 2). At the unrepaired locations, the dust borne Aspergilli were less frequent and presented in lower concentrations compared to other locations. In addition, the opposite seasonal pattern was observed as the concentrations of the dust borne Aspergilli measured in the summer doubled compared to the winter sampling period (Figure 2).
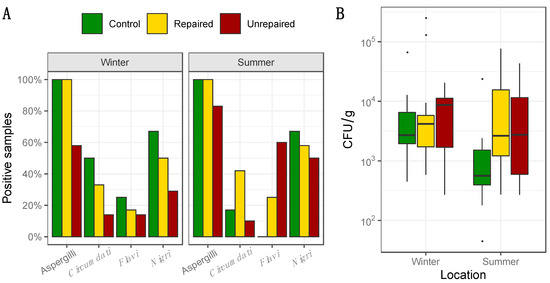
Figure 2.
Percentage of positive samples where total dust borne Aspergilli, as well as Aspergilli from the sections Circumdati, Flavi, and Nigri, were detected based on analysis of 12 samples of dust collected at control and repaired locations, and 10 samples of dust collected at unrepaired locations in each sampling period, i.e., winter and summer (panel A); Concentration of total Aspergilli (CFU/g) detected in dust. Each location is represented by a box–whisker plot in a different shade of gray in each sampling period (winter and summer) as specified (panel B).
While Aspergilli from the section Circumdati were not present in indoor or outdoor air at the control locations in the winter sampling period, their maximum concentration in dust samples (42,727 CFU/g) were up to seventy times higher compared to other locations (Supplementary Materials, Table S4). However, their concentrations drastically dropped in the summer (max 50 CFU/g). At post-flood locations, the dust borne Circumdati were more frequent at repaired compared to unrepaired locations, where they were detected in only one sample (Figure 2, Supplementary Materials, Table S4). Aspergilli from the section Flavi dominated in dust collected at the post-flood locations, while at the control locations, they were obtained only from winter samples (Figure 2). Their maximum concentrations in the dust collected at the repaired locations in winter (545 CFU/g) were more than three times higher compared to the control locations. The highest concentrations of Flavi were measured in dust from unrepaired locations in the summer (17,568 CFU/g), and they were eighty-seven times higher than at the same location in winter and thirty-five times higher than in dust from repaired post-flood locations in the same sampling period (Supplementary Materials, Table S5). Aspergilli from the section Nigri comprised 50–67% of the dust borne Aspergilli at control and post-flood repaired locations. The highest concentrations of dust borne Nigri were measured in the samples collected in the winter sampling period at the control locations (4545 CFU/g). Their median concentration (1024 CFU/g) was 1.5 times higher than in the samples from the repaired locations. Summer concentrations of black Aspergilli in dust from the control locations were about ten times lower than in the winter period, while at repaired locations, they were similar in both winter and summer (Supplementary Materials, Table S6). Black Aspergilli followed the previously mentioned opposite seasonal pattern at the unrepaired locations, as they prevailed in summer compared to winter (Supplementary Materials, Table S6). However, their median concentration in dust from unrepaired locations (143 CFU/g) was 3.6 times lower compared to the repaired locations (Supplementary Materials, Table S6).
3.3. Identification of the Species from the Sections Circumdati, Flavi, and Nigri and Mycotoxin-Producing Capacities
Airborne (N = 71) and dust borne (N = 76) Aspergillus isolates were identified as eleven different species: A. ochraceus, A. ostianus, A. pallidofulvus, A. sclerotiorum, and A. westerdijkiae (section Circumdati), A. flavus (section Flavi), A. niger, A. piperis, A. tubingensis, A. uvarum, and A. welwitschiae (section Nigri). The identification was based on the comparison of the DNA sequence of a PCR-amplified CaM fragment from each isolate to the CaM sequences for reference strains and supported by phylogenetic analysis (Supplementary materials, Figures S1–S3). The extracts prepared from each of the isolates were tested for the presence of the mycotoxin relevant for each section, i.e., ochratoxin A (OTA) in the section Circumdati, aflatoxin B1 (AFB1) in the section Flavi, and fumonisin B2 (FB2) in the section Nigri (Table 1, Supplementary Materials, Tables S7–S9). Additionally, the extracts prepared from the isolates of A. niger and A. welwitschiae from the section Nigri were tested on OTA, but it was not detected. The HPLC-ESI-MS chromatograms are shown for a representative isolate producing OTA, AFB1, and FB2, along with the corresponding standards (OTA, AFB1, and FB2) in the Supplementary Materials, Figures S4–S6.

Table 1.
Mycotoxin-producing abilities of the species assigned to the sections Circumdati, Flavi, and Nigri, and the distribution of mycotoxin-producing isolates over control and post-flood locations.
All OTA-producing and AFB1-producing isolates belonged to A. westerdijkiae and A. flavus, respectively, while FB2-producing isolates belonged to A. niger and A. welwitschiae (Table 1, Supplementary Materials, Tables S7–S9).
The OTA-producing airborne isolates were from indoor air collected at the repaired locations in winter and at the control locations in the summer sampling period. The only dust borne isolate was from the dust collected at the repaired locations in the summer (Table 1, Supplementary Materials, Table S7).
The AFB1-producing A. flavus isolates were only detected among the isolates from the post-flood locations (Table 1, Supplementary Materials, Table S8). Only one AFB1-producing airborne isolate originated from the repaired locations, and it was isolated from outdoor air collected in winter. All other isolates of AFB1-producing A. flavus were winter airborne isolates and summer dust borne isolates from the unrepaired locations (Table 1, Supplementary Materials, Table S8).
FB2-producing A. niger isolates were recovered from an indoor and an outdoor air sample, respectively, collected at the control locations in the summer. A single dust borne isolate of FB2-producing A. niger was isolated at a repaired location in winter (Table 1, Supplementary Materials, Table S9). Both airborne isolates of FB2-producing A. welwitschiae were recovered from indoor air collected at the repaired locations in winter and summer, respectively. The dust borne isolates of FB2-producing A. welwitschiae were collected at the control locations in both sampling periods, while in dust from both post-flood locations, they were isolated only in summer (Table 1, Supplementary Materials, Table S9).
3.4. Seasonal Distribution of the Species from the Sections Circumdati, Flavi, and Nigri at Post-Flood and Control Locations
The airborne and dust borne Aspergilli followed the different seasonal patterns of species distribution at the post-flood and the control locations as shown on the corresponding area plot diagrams (Figure 3 and Figure 4, respectively).
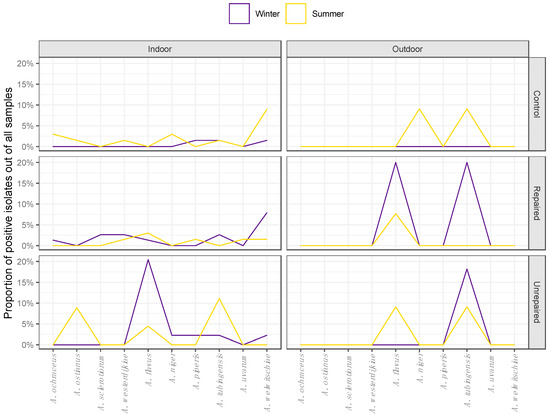
Figure 3.
Seasonal distribution patterns of Aspergilli from the sections Circumdati (A. ochraceus, A. ostianus, A. sclerotiorum, and A. westerdijkiae), Flavi (A. flavus) and Nigri (A. niger, A. piperis, A. tubingensis, A. uvarum, and A. welwitschiae) in indoor and outdoor air during winter and summer sampling periods in repaired houses, unrepaired houses, and control houses.
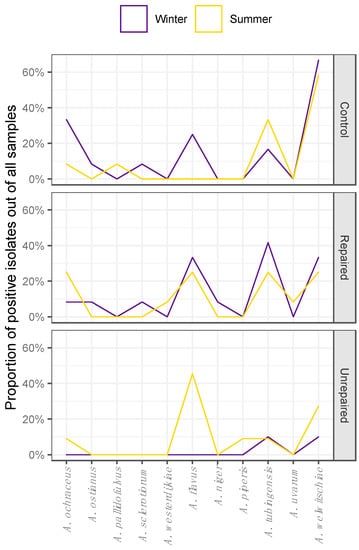
Figure 4.
Seasonal distribution patterns of Aspergilli from the sections Circumdati (A. ochraceus, A. ostianus, A. pallidofulvus, A. sclerotiorum, and A. westerdijkiae), Flavi (A. flavus) and Nigri (A. niger, A. piperis, A. tubingensis, A. uvarum, and A. welwitschiae) in dust collected during winter and summer sampling periods in repaired houses, unrepaired houses, and control houses.
The least diverse were the airborne isolates collected at the control locations in the winter sampling period, all of which belonged to the section Nigri (Figure 3). At the same location in the summer, airborne Aspergilli were represented by species from all three sections, predominantly by black Aspergilli, which comprised 15.5% of all airborne Aspergilli. While A. welwitschiae and A. tubingensis were detected in both seasons, A. piperis was identified only in winter and A. niger was identified only in summer (Figure 3). The airborne Aspergilli from the section Circumdati were represented by A. ochraceus, A. ostianus, and A. westerdijkiae, comprising 5.6% of all airborne Aspergilli (Figure 3).
The airborne Aspergilli were the most diverse at the repaired locations, and different seasonal patterns were observed compared to the control locations (Figure 3). Black Aspergilli dominated at this location, while winter isolates of A. tubingensis and A. welwitschiae comprised 5.6% and 8.5% of airborne Aspergilli. Aspergilli from the section Circumdati comprised 7% of airborne Aspergilli and included the species A. sclerotiorum, which was not detected at other locations. The proportion of isolates assigned to A. flavus (section Flavi) remained unchanged from winter to summer at this location (Figure 3), while the species compositions in other sections changed. Although less abundant, black Aspergilli were more diverse in the summer compared to the winter, and there were no isolates of A. tubingensis. The section Circumdati was less diverse in the summer compared to the winter, and A. westerdijkiae was the only species detected.
Airborne isolates from unrepaired locations were more diverse in winter. Most of the isolates belonged to A. flavus, which is a particularly dominant species at this location, comprising 12.7% of all airborne Aspergilli. The section Nigri was represented by four different species: A. tubingensis, A. welwitschiae, A. niger, and A. piperis (Figure 3). While they were not detected in the winter sampling period, in the summer, the section Circumdati comprised 5.6% of airborne Aspergilli, all belonging to A. ostianus. The proportion of isolates assigned to A. flavus declined compared to winter, while among black Aspergilli, only A. tubingensis was detected (Figure 3).
The Aspergilli isolated from dust collected at the control locations in winter were highly diverse, and the isolates from the sections Circumdati, Flavi, and Nigri comprised 7.9%, 4%, and 13%, respectively, of all dust borne Aspergilli. In the summer, the species diversity followed the decline in proportion of dust borne Aspergilli at this location. The only species detected in the section Circumdati were A. ochraceus and A. pallidofulvus, and there were no isolates of A. flavus. However, there were more isolates of A. tubingensis in the summer than in the winter at this location.
The Aspergilli in dust collected at repaired locations were more diverse compared to control locations, and different seasonal patterns in species distribution were observed compared to control locations (Figure 4). There were about two times less Aspergilli from the section Circumdati in winter compared to the control locations, and there was no difference in the species composition. While A. ochraceus was present at both locations in both sampling periods, A. ostianus and A. sclerotiorum were detected only in the winter. In the summer, A. pallidofulvus was detected at the control location and A. westerdijkiae at the repaired post-flood location. Isolates assigned to A. flavus were detected at both locations but prevailed at the repaired post-flood location, namely in winter, and comprised 5.3% of the total dust borne Aspergilli. A. welwitschiae dominated among all dust borne Aspergilli (35%) being 2.5 times more frequent than A. tubingensis at control locations. At repaired locations, A. tubingensis was slightly more frequent than A. welwitschiae, comprising 10.5% and 9.21%, respectively, of total dust borne Aspergilli collected at repaired post-flood locations. In addition, black Aspergilli were more diverse at repaired post-flood locations compared to the control, as A. niger and A. uvarum were detected at these locations in winter and summer, respectively.
In the dust collected in the winter, only isolates from the section Nigri were detected at the unrepaired locations and belonged to A. tubingensis and A. welwitschiae. Although the dust borne Aspergilli from the section Flavi were detected in the samples from unrepaired locations in the winter sampling period (Figure 2; Supplementary Materials, Table S8), they could not be isolated due to overgrowth with Trichoderma spp. In the summer, the dust borne Aspergilli were more diverse at this location, namely section Nigri (A. piperis, A. tubingensis, and A. welwitschie), while most of the dust borne isolates (6.6%) belonged to A. flavus. In addition, the section Circumdati was represented by A. ochraceus (Figure 4).
4. Discussion
The purpose of this study was to explore the occurrence and species distribution of airborne and dust borne Aspergilli from the sections Circumdati, Flavi, and Nigri at post-flood locations, repaired and unrepaired, compared to control locations. In addition, the role of seasonality on species distribution was inspected at each of the sampling locations. This is the first research of its kind conducted in Croatia, and Europe, regarding the presence of these medically and economically important groups of Aspergilli.
While assessing and evaluating fungal contamination in an indoor environment, it is important to take a sample where contamination is suspected along with another sample from a similar but unsuspected location for comparison [48]. Equally important is the sampling method and the sample itself, i.e., air and dust. Among the many available methods of collecting airborne fungi [49], direct impact on nutrient agar is by far the most widely used procedure to assess the concentration and composition of fungi in an indoor environment [50]. Dust samples may be an even more representative indoor sample in the assessment of human exposure to indoor fungi, as they contain indoor fungi, as well as those brought indoors from various outdoor sources [51]. In the presented research, houses and schools were categorised into the same group, i.e., post-flood repaired locations and control locations, as they represent similar indoor environments in regard to the expected fungal contamination [52].
Since water is a major promoter of fungal proliferation [6], the expected aftermath of flooding was more indoor fungi in the affected environment. The concentrations of airborne and dust borne Aspergilli at the post-flood locations were up to twenty times higher compared to the control locations. This observation is in agreement with the previously documented proliferation of Aspergilli after flooding [1,2,3,4,5]. Generally, airborne and dust borne Aspergilli dominate in post-flood indoor environments in the winter, while their concentrations in outdoor air increase in the summer. Decreased concentrations of dust borne fungi during summer months are already known in the literature, and they are explained by an increased ventilation rate [50]. Aspergilli grow better in darkness, and these conditions also support mycotoxin production [53], so an additional explanation may be found in shorter daylight periods and fewer sunny days in the winter compared to the summer. However, in this study, it has been observed that Aspergilli at the unrepaired post-flood locations followed the opposite seasonal pattern from that observed at the repaired or control locations. These houses are vacant, and as such, the air ventilation remains the same throughout the year. In addition, there is no heating, air-conditioning, activities such as cooking and washing, or any other human influence. Thus, this may account for observed differences in seasonal pattern, as these variables contribute to differences in the established equilibrium between indoor and outdoor environments.
The adverse health effects due to exposure to indoor airborne fungi are not fully clarified, but it is generally accepted that fungal rhinitis, hypersensitivity pneumonia, and/or asthma are more prevalent among occupants of water damaged, mouldy environments [54]. For instance, a study conducted following the hurricanes Irene and Sandy on the east coast of the USA demonstrated that the post-hurricane population had become more sensitised and reactive to moulds [55]. Specifically, the portion of Aspergillus-sensitised subjects increased from 15% to 45% in the post-hurricane population [55].
Aspergilli are ubiquitous fungi, and their presence in indoor and outdoor environments is not abnormal per se. Presented results have shown elevated concentrations of Aspergilli at control locations in certain sampling periods, e.g., dust borne Circumdati in the winter sampling period, or airborne Nigri in the summer period of sampling, while Aspergilli from the section Flavi always prevailed at post-flood locations.
In the case of the section Circumdati, the important observation was that their concentrations dropped in the summer, and that they were not detected in indoor air at the same location in the same season. Although A. ochraceus was most frequently identified among the Circumdati isolates, and its capacities to produce OTA were previously reported [56], the only OTA-producing species was A. westerdijkiae, which is most frequently isolated at post-flood locations. The amount of OTA detected in corresponding fungal extracts was 13.7 ± 15.81 µg/mL (Table 1, Supplementary Materials, Table S7). The isolates of A. sclerotiorum detected in air and dust samples of post-flood locations and control locations did not produce OTA, while in a previous study conducted in Croatia, the concentrations of OTA detected in spore extracts of A. sclerotiorum were 0.3–28 µg/mL [57]. Other species detected among the airborne and dust borne isolates included A. ostianus and A. pallidofulvus, which did not produce OTA, although according to the literature, some isolates of A. ostianus may produce OTA in trace amounts [56]. To the best of our knowledge, this is the first report of A. pallidofulvus in the indoor environment. It was described as a separate species from A. ochraceus in 2014, and its origins were green coffee beans from India as well as two unrecorded sources [56]. More recently, it was isolated from Pu-erh tea [58]. Previously, A. westerdijkiae was reported in high amounts in house dust from South Africa and air samples from The Netherlands [56], and A. ochraceus was reported in floor-related material and the wet-concrete of water-damaged buildings [11], while A. ostianus was detected in indoor air in Denmark [56]. Severe health hazards linked to the inhalation of OTA have been described in occupational settings, i.e., in a granary where workers exposed to OTA-producing Aspergilli suffered from acute renal failure [59]. Although studies about the exposure to mycotoxins in indoor environment are rare, OTA was detected in the urine of occupants in water-damaged buildings and associated with focal segmental glomerulosclerosis [60]. Furthermore, all of the identified species may be associated with infections, including skin and nail infections [61] but also allergic bronchopulmonary aspergillosis and otomycosis [56].
In the presented study, A. flavus was identified almost exclusively at the post-flood locations, where it comprised 17–25% of total airborne Aspergilli in the winter and 7–13% in the summer (Figure 1, Figure 3). All isolates assigned to the section Flavi were identified as A. flavus, and 7/33 isolates produced AFB1 in concentrations of 2.51 ± 5.31 µg/mL (Table 1, Supplementary Materials, Table S8). None of the AFB1-producing A. flavus were detected at the control locations. Interestingly, a single AFB1-producing isolate from a repaired post-flood location was recovered from an outdoor air sample. In previous research on indoor Aspergilli in living and occupational environments in Croatia, A. parasiticus was the only other species, besides A. flavus, detected among airborne isolates [19]. In other studies, some of the isolates from house dust were assigned to other species from this section, including A. tamarii, A. nomius, and A. pseudonomius [16]. The dominant association of aflatoxigenic A. flavus with water-damaged environments highlights a need for immediate action, i.e., the remediation of unrepaired locations, as they may serve as a sink for their proliferation and spread through the surrounding area.
Exposure to AFB1 via inhalation was investigated in industrial settings, where it was associated with pulmonary interstitial fibrosis in agricultural and textile workers [62]. Its role in primary liver cancer was supported by detectable levels of AFB1 in the serum of about 60% of the exposed workers in poultry farms and none of the control subjects [63]. This species is the second leading cause of invasive aspergillosis and is associated with approximately 10% of bronchopulmonary aspergillosis [64]. It may cause various diseases, including keratomycosis [65] and endophthalmitis [66].
Interesting results were obtained for the Aspergilli from the section Nigri. They were the most frequent among the three sections of interest and prevailed at post-flood locations, where they comprised 33% of airborne and 28% of dust borne Aspergilli. Most of the isolates were identified as A. tubingensis and A. welwitschiae, in agreement with previously conducted research on black Aspergilli in indoor air [17,18]. Mycotoxin FB2 was produced by A. niger and A. welwitschiae, while the concentrations of FB2 detected in corresponding fungal extracts were 11.24 ± 18.30 µg/mL and 6.76 ±13.51 µg/mL, respectively (Table 1, Supplementary Materials, Table S9). Despite a relatively small sample, our results are in agreement with the conclusions of previous studies, according to which a majority of A. niger and a minority of A. welwitschiae isolates produce FB2 [67]. The concentrations of black Aspergilli in indoor air samples at control locations were several times higher than those measured at the post-flood locations, and they were also elevated compared to outdoor air samples. This observation suggests an inner source of contamination with black Aspergilli at some of the control locations. The most common species among airborne and dust borne black Aspergilli is A. welwitschiae, and at the control locations, it prevailed over A. tubingensis, which was much more frequent at the post-flood locations. It was shown that a decline in water activities negatively affects the growth of A. tubingensis [68], and it may explain why this species prevailed at the post-flood locations over control locations, especially in the winter sampling period. The inability of A. tubingensis to produce mycotoxins could have implied a lower risk for the occupants compared to mycotoxin-producing A. welwitschiae. However, A. tubingensis is capable of producing other biologically active metabolites, including naphto-y-pyrones asperazine and malformins [69], which may contribute to deleterious effects in affected tissue. Furthermore, some studies in the clinical settings, where both A. welwitschiae and A. tubingensis are frequently isolated, [70,71,72] have shown that A. tubingensis is associated with the chronic pulmonary aspergillosis, while A. welwitschiae is suspected to be more related to fungal colonisation than infection [73]. Regarding other species from the section Nigri, this is the first report of A. uvarum in an indoor environment in Croatia. It was isolated from indoor air and dust samples from post-flood repaired locations in the summer sampling season. In 2008, it was introduced in the section Nigri and isolated from grape berries from multiple Southern European countries [74]. Later, it was reported in indoor and outdoor air samples in the United States [17], as well as indoor air samples from Turkey [18]. In this study, A. niger was isolated from indoor and outdoor air samples at post-flood locations in the winter and control locations in the summer, in addition to a single dust borne isolate collected at a repaired location in the winter. Airborne and dust borne isolates of A. piperis were collected at post-flood and control locations in both seasons. Both A. niger and A. piperis were previously reported as indoor air isolates from Croatia, while A. niger was isolated from indoor air in Hungary, The Netherlands, and Thailand [18].
5. Conclusions
The results of the presented study indicate that houses and schools, especially after water damage, may be important sources of various Aspergilli that are recognised human pathogens. In addition, this research provides valuable information regarding the composition of important mycotoxin-producing and non-producing Aspergilli at water-damaged locations and control locations, which is a study that is the first of its kind in Croatia and Europe. The exclusive presence of aflatoxigenic species A. flavus at the post-flood locations is the most worrying finding and infers the need for a thorough remediation. New genotyping strategies that would link environmental and clinical isolates could be of great importance for a better understanding of the significance of Aspergillus exposure after flooding events. The role of mycotoxins in adverse health effects upon inhalation remains a subject of debate. More information regarding their detection in clinically relevant samples supported by their presence in living and occupational settings might bring us closer to understanding their role in disease.
Supplementary Materials
The following are available online at https://www.mdpi.com/2309-608X/6/4/282/s1, Tables S1–S3: Concentrations of airborne Aspergilli from the section Circumdati (Table S1), Flavi (Table S2) and Nigri (Table S3) (CFU/m3) isolated from indoor air samples during winter and summer sampling periods at the locations specified; Tables S4–S6: Concentrations of Aspergilli from the section Circumdati (Table S4), Flavi (Table S5) and Nigri (Table S6) in dust (CFU/g) collected during winter and summer sampling periods at the locations specified. Table S7: Ochratoxin A (OTA)-producing abilities of airborne and dust-borne Aspergilli from the section Circumdati; Table S8. Aflatoxin B1 (AFB1)-producing abilities of airborne and dust-borne Aspergilli from the section Flavi: Table S9: Fumonisin B2 (FB2)-producing abilities of airborne and dust-borne Aspergilli from the section Nigri; Figure S1–S3: Phylogenetic trees based on the datasets of CaM sequences showing the relationship between isolates assigned to Aspergillus section Circumdati (Figure S1), Flavi (Figure S2), and Nigri (Figure S3). The bootstrap percentages of the maximum likelihood (ML) analysis are presented at the nodes; The bar indicates the number of substitutions per site. The phylogram is rooted with Aspergillus tanneri NRRL 62425 (Figure S1), Aspergillus flavipes NRRL 302T (Figure S2), and A. nidulans NRRL 187T (Figure S3). Figure S4: HPLC-ESI-MS total ion current chromatogram of selected extract of an OTA-producing isolate of A. westerdijkiae MFBF AC12375, RT (OTA) = 8.8 min. (a); corresponding HPLC-ESI-MS spectrum (b) and MS2 spectra (c); Concentration of OTA in the extract was 31.50 µg/mL and extrapolated from OTA standard calibration line constructed by plotting the peak area of m/z 260.9 obtained from HPLC-ESI-MS/MS extracted ion chromatogram vs. OTA concentration; HPLC-ESI-MS spectrum of OTA standard (d) and HPLC-ESI-MS/MS fragmentation pattern of a major ion m/z 426.2 [M+Na]+(e). Figure S5: HPLC-ESI-MS total ion current chromatogram of selected extract of AFB1-producing A. flavus isolate MFBF AF12404 (a); corresponding HPLC-ESI-MS extracted ion chromatogram (EIC) of major ions m/z 313.0 [MH]+ (black, higher peak) and m/z 335.0 [MNa]+ (light gray, shorter peak) at RT 5.2 min. Concentration of AFB1 in the extract was 14.52 µg/mL and extrapolated from AFB1 standard calibration line constructed by plotting the peak area of m/z 313 obtained from extracted ion chromatogram vs. AFB1 concentration (b); HPLC-ESI-MS spectrum of AFB1 standard (c). Figure S6: HPLC-ESI-MS total ion current chromatogram of selected extract of a FB2-producing isolate of A. niger MFBF AN12137 (light gray) overlaid with extracted ion m/z 706 chromatogram at RT 8.4 min (black) (a) and corresponding MS2 spectra (b). Concentration of FB2 in the extract was 32.383 µg/mL and extrapolated from FB2 standard calibration line constructed by plotting the peak area of m/z 336.2 obtained from HPLC-ESI-MS/MS extracted ion chromatogram vs FB2 concentration; HPLC-ESI-MS spectrum of FB2 standard (c) and HPLC-ESI-MS/MS fragmentation pattern of a major molecular ion [M+H]+ m/z 706 (d).
Author Contributions
Conceptualisation, M.Š.K.; Methodology, D.J., S.K., M.S., A.M., B.N., M.Š.K; Software, S.K., D.K. and M.S.; Validation, M.S., A.M. and B.N.; Formal analysis, M.S., I.K., D.J. and S.K.; Data curation, M.S., D.K., S.K., D.J.; Writing—original draft preparation, D.J. Writing—review and editing, D.J., M.S., S.K., I.K., D.K., A.M., B.N. and M.Š.K.; Supervision, M.Š.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Croatian Science Foundation under project no IP-09-2014-5982 (MycotoxA).
Acknowledgments
The authors want to thank Marijana Markoljević, Senka Klišanić, Matilda Šokčević, Jasminka Hot and Stipica Mišura, from Elementary School Antun and Stjepan Radić in Gunja as well as Štefica Babić, our technician, and teachers at Elementary School Gornji Stupnik for their hospitality and invaluable help in collecting the samples. The authors would like to thank to Heather Wynn for English language editing.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
Parameters for LC/MS Analysis
Prior to use, the mobile phases were filtered through a cellulose nitrate filter, diameter 47 mm, pore size 0.45 µm (Sartorius, Goettingen, Germany). Degassing of the mobile phase was carried out continuously with an on-line degasser. The samples and the standards were kept in glass (OTA, AFB1) and polypropylene (FB2) HPLC vials at 4 °C in the autosampler temperature control system, and in each analysis, 10 µL were injected. Symmetry C18, 4.6 × 150 mm (3.5 µm) column (Agilent Technologies, Waldbronn, Germany) was used as the stationary phase in all performed chromatographic separations, incubated at 35.0 ± 0.1 °C (OTA and FB2) and 40.0 ± 0.1 °C (AFB1). A separation of OTA was performed in isocratic mode at flow rate of 0.6 mL/min, while the mobile phase consisted of MeOH:H2O:AcOH = 70:30:0.1% (v/v). Separation of AFB1 and FB2 followed the gradient protocol. The mobile phase set at the flow rate of 0.5 mL/min (for AFB1) and 0.7 mL/min (FB2) consisted of MeCN: H2O:HCOOH = 50:50:0.1% (v/v) as eluent A (for AFB1 and FB2), while eluent B was MeCN for AFB1 and H2O:HCOOH = 100:0.1% (v/v) for FB2. The time program to elute AFB1 was 100% A (0–6 min), 100→90% A (6–8 min), 90% A (8–10 min), 90→100% A (10–12 min), and 100% A (12–14 min). In the analysis of FB2, the time program was 75% B (0–1 min), 75 → 60% B (1–5 min), 60% B (5–10 min), 75 → 60% B (10–12 min), and 75% B (12–17 min).
Mass spectrometry analysis were carried out with electrospray ionisation (ESI) source in the positive ion mode. The entire effluent was delivered to the mass spectrometer. Nitrogen was used both as drying gas at a flow rate of 6.0 L/min (OTA, AFB1), 10.0 L/min (FB2), and as nebulizer gas at a pressure of 10.0 psi (OTA, AFB1) and 15.0 psi (FB2). To maximize method sensitivity, the electrospray source temperature was optimized at 325 °C, and the capillary voltage was set at 3.5 kV. The full scan mass spectra were acquired over a range m/z 100–800 with max accumulation time of 200 ms. Quantitative analysis was performed in Selected reaction monitoring (SRM) mode by monitoring the transition m/z 426.0 → 260.9 (OTA), m/z 313 (AFB1) and 706.0 → 336.0 (FB2).
References
- Dumon, H.; Palot, A.; Charpin-Kadouch, C.; Quéralt, J.; Lehtihet, K.; Garans, M.; Charpin, D. Mold species identified in flooded dwellings. Aerobiologia 2009, 25, 341–344. [Google Scholar] [CrossRef]
- Solomon, G.M.; Hjelmroos-Koski, M.; Rotkin-Ellman, M.; Hammond, S.K. Airborne Mold and Endotoxin Concentrations in New Orleans, Louisiana, after Flooding, October through November 2005. Environ. Health Perspect. 2006, 114, 1381–1386. [Google Scholar] [CrossRef]
- Bloom, E.; Grimsley, L.F.; Pehrson, C.; Lewis, J.; Larsson, L. Molds and mycotoxins in dust from water-damaged homes in New Orleans after hurricane Katrina. Indoor Air 2009, 19, 153–158. [Google Scholar] [CrossRef]
- Barbeau, D.N.; Grimsley, L.F.; White, L.E.; El-Dahr, J.M.; Lichtveld, M. Mold Exposure and Health Effects Following Hurricanes Katrina and Rita. Annu. Rev. Public Heal. 2010, 31, 165–178. [Google Scholar] [CrossRef]
- Hsu, N.-Y.; Chen, P.-Y.; Chang, H.-W.; Su, H.-J. Changes in profiles of airborne fungi in flooded homes in southern Taiwan after Typhoon Morakot. Sci. Total Environ. 2011, 409, 1677–1682. [Google Scholar] [CrossRef]
- Adan, O.C.G.; Huinink, H.P.; Bekker, M. Water relations of fungi in indoor environments. In Fundamentals of Mold Growth in Indoor Environments and Strategies for Healthy Living; Wageningen Academic Publishers: Wageningen, The Netherlands, 2011; pp. 41–65. [Google Scholar]
- Jurjevic, Z.; Peterson, S.W.; Horn, B.W. Aspergillus section Versicolores: Nine new species and multilocus DNA sequence based phylogeny. IMA Fungus 2012, 3, 59–79. [Google Scholar] [CrossRef]
- Piecková, E. Domestic Environment: Indoor Mycobiota As a Public Health Risk Factor. Environ. Mycol. Public Heal. 2016, 129–146. [Google Scholar] [CrossRef]
- Jakšić Despot, D.; Kocsubé, S.; Bencsik, O.; Kecskeméti, A.; Szekeres, A.; Vágvölgyi, C.; Varga, J.; Klarić, M.Š. Species diversity and cytotoxic potency of airborne sterigmatocystin-producing Aspergilli from the section Versicolores. Sci. Total Environ. 2016, 562, 296–304. [Google Scholar] [CrossRef]
- Gravesen, S.; Nielsen, P.A.; Iversen, R.; Nielsen, K.F. Microfungal contamination of damp buildings--examples of risk constructions and risk materials. Environ. Health Perspect. 1999, 107, 505–508. [Google Scholar] [CrossRef]
- Andersen, B.; Frisvad, J.C.; Sondergaard, I.; Rasmussen, I.S.I.S.; Larsen, L.S.; Søndergaard, I.; Rasmussen, I.S.I.S.; Larsen, L.S. Associations between fungal species and water-damaged building materials. Appl. Environ. Microbiol. 2011, 77, 4180–4188. [Google Scholar] [CrossRef]
- Jussila, J.; Komulainen, H.; Kosma, V.-M.; Nevalainen, A.; Pelkonen, J.; Hirvonen, M.-R. Spores of Aspergillus versicolor isolated from indoor air of a moisture-damaged building provoke acute inflammation in mouse lungs. Inhal. Toxicol. 2002, 14, 1261–1277. [Google Scholar] [CrossRef]
- Veršilovskis, A.; Bartkevičs, V.; Miķelsone, V. Sterigmatocystin presence in typical Latvian grains. Food Chem. 2008, 109, 243–248. [Google Scholar] [CrossRef]
- Piontek, M.; Łuszczyńska, K.; Lechów, H. Occurrence of the Toxin-Producing Aspergillus versicolor Tiraboschi in Residential Buildings. Int. J. Environ. Res. Public Health 2016, 13, 862. [Google Scholar] [CrossRef]
- Engelhart, S.; Loock, A.; Skutlarek, D.; Sagunski, H.; Lommel, A.; Färber, H.; Exner, M. Occurrence of toxigenic Aspergillus versicolor isolates and sterigmatocystin in carpet dust from damp indoor environments. Appl. Environ. Microbiol. 2002, 68, 3886–3890. [Google Scholar] [CrossRef]
- Visagie, C.M.; Hirooka, Y.; Tanney, J.B.; Whitfield, E.; Mwange, K.; Meijer, M.; Amend, A.S.; Seifert, K.A.; Samson, R.A. Aspergillus, Penicillium and Talaromyces isolated from house dust samples collected around the world. Stud. Mycol. 2014, 78, 63–139. [Google Scholar] [CrossRef]
- Jurjević, Ž.; Peterson, S.W.; Stea, G.; Solfrizzo, M.; Varga, J.; Hubka, V.; Perrone, G. Two novel species of Aspergillus section Nigri from indoor air. IMA Fungus 2012, 3, 159–173. [Google Scholar] [CrossRef]
- Varga, J.; Kocsubé, S.; Szigeti, G.; Baranyi, N.; Vágvölgyi, C.; Jakšić Despot, D.; Magyar, D.; Meijer, M.; Samson, R.A.; Šegvić Klarić, M.; et al. Occurrence of black Aspergilli in indoor environments of six countries. Arh. Ind. Hyg. Toxicol. 2014, 65, 219–223. [Google Scholar] [CrossRef]
- Jakšić, D.; Kocsubé, S.; Bencsik, O.; Kecskeméti, A.; Szekeres, A.; Jelić, D.; Kopjar, N.; Vágvölgyi, C.; Varga, J.; Šegvić Klarić, M. Aflatoxin production and in vitro toxicity of Aspergilli section Flavi isolated from air samples collected from different environments. Mycotoxin Res. 2019, 35, 217–230. [Google Scholar] [CrossRef]
- Jakšić, D.; Kocsubé, S.; Bencsik, O.; Kecskeméti, A.; Szekeres, A.; Jelić, D.; Kopjar, N.; Vágvölgyi, C.; Varga, J.; Šegvić Klarić, M. Fumonisin production and toxic capacity in airborne black Aspergilli. Toxicol. Vitr. 2018, 53, 160–171. [Google Scholar] [CrossRef]
- Pyrri, I.; Tripyla, E.; Zalachori, A.; Chrysopoulou, M.; Parmakelis, A.; Kapsanaki-Gotsi, E. Fungal contaminants of indoor air in the National Library of Greece. Aerobiologia 2020, 36, 387–400. [Google Scholar] [CrossRef]
- Chegini, F.M.; Baghani, A.N.; Hassanvand, M.S.; Sorooshian, A.; Golbaz, S.; Bakhtiari, R.; Ashouri, A.; Joubani, M.N.; Alimohammadi, M. Indoor and outdoor airborne bacterial and fungal air quality in kindergartens: Seasonal distribution, genera, levels, and factors influencing their concentration. Build. Environ. 2020, 175, 106690. [Google Scholar] [CrossRef]
- Dannemiller, K.C.; Weschler, C.J.; Peccia, J. Fungal and bacterial growth in floor dust at elevated relative humidity levels. Indoor Air 2017, 27, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, M. Characteristics of biological and non-biological aerosol particles in indoor environment and their inhalable fractions in the human lung. Arab J. Nucl. Sci. Appl. 2020, 53, 46–55. [Google Scholar] [CrossRef]
- Crawford, J.A.; Rosenbaum, P.F.; Anagnost, S.E.; Hunt, A.; Abraham, J.L. Indicators of airborne fungal concentrations in urban homes: Understanding the conditions that affect indoor fungal exposures. Sci. Total Environ. 2015, 517, 113–124. [Google Scholar] [CrossRef]
- Mattei, A.S.; Madrid, I.M.; Santin, R.; Schuch, L.F.D.; Meireles, M.C.A. In vitro activity of disinfectants against aspergillus spp. Braz. J. Microbiol. 2013, 44, 481–484. [Google Scholar] [CrossRef][Green Version]
- Bush, A. Kids, Difficult Asthma and Fungus. J. Fungi 2020, 6, 55. [Google Scholar] [CrossRef]
- Kousha, M.; Tadi, R.; Soubani, A.O. Pulmonary aspergillosis: A clinical review. Eur. Respir. Rev. 2011, 20, 156–174. [Google Scholar] [CrossRef]
- Bongomin, F.; Asio, L.G.; Baluku, J.B.; Kwizera, R.; Denning, D.W. Chronic Pulmonary Aspergillosis: Notes for a Clinician in a Resource-Limited Setting Where There Is No Mycologist. J. Fungi 2020, 6, 75. [Google Scholar] [CrossRef]
- Schweer, K.E.; Jakob, B.; Liss, B.; Christ, H.; Fischer, G.; Vehreschild, M.J.G.T.; Cornely, O.A.; Vehreschild, J.J. Domestic mould exposure and invasive aspergillosis—Air sampling of aspergillus spp. Spores in homes of hematological patients, a pilot study. Med. Mycol. 2016, 54, 576–583. [Google Scholar] [CrossRef]
- Magnussen, A.; Parsi, M.A. Aflatoxins, hepatocellular carcinoma and public health. World J. Gastroenterol. 2013, 19, 1508–1512. [Google Scholar] [CrossRef]
- Šegvić Klarić, M.; Pepeljnjak, S.; Rozgaj, R. Genotoxicity of fumonisin B1, beauvericin and ochratoxin A in porcine kidney PK15 cells: Effects of individual and combined treatment. Croat. Chem. Acta 2008, 81, 139–146. [Google Scholar]
- Doi, K.; Uetsuka, K. Mechanisms of Mycotoxin-induced Dermal Toxicity and Tumorigenesis Through Oxidative Stress-related Pathways. J. Toxicol. Pathol. 2014, 27, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Malir, F.; Ostry, V.; Pfohl-Leszkowicz, A.; Malir, J.; Toman, J. Ochratoxin A: 50 Years of Research. Toxins 2016, 8, 191. [Google Scholar] [CrossRef] [PubMed]
- Joint FAO/WHO Expert Committee on Food Additives. Safety Evaluation of Certain Mycotoxins in Food; FAO: Rome, Italy; WHO: Geneva, Switzerland, 2001; ISBN 9251046646r9241660473r0254-4725. [Google Scholar]
- Bondy, G.S.; Pestka, J.J. Immunomodulation by fungal toxins. J. Toxicol. Environ. Health. B. Crit. Rev. 2000, 3, 109–143. [Google Scholar] [PubMed]
- Strelec Mahović, N.; Renko, T.; Tutiš, V.; Trošić, T. Synoptic analysis of the Catastrophic Floods in SE Europe. Eur. Forecast. 2014. Available online: http://www.euroforecaster.org/newsletter20/dhmz.pdf (accessed on 1 September 2020).
- Smedsgaard, J. Micro-scale extraction procedure for standardized screening of fungal metabolite production in cultures. J. Chromatogr. A 1997, 760, 264–270. [Google Scholar] [CrossRef]
- Frisvad, J.C.; Smedsgaard, J.; Samson, R.A.; Larsen, T.O.; Thrane, U. Fumonisin B2 Production by Aspergillus niger. J. Agric. Food Chem. 2007, 55, 9727–9732. [Google Scholar] [CrossRef]
- Samson, R.A.; Houbraken, J.; Thrane, U.; Frisvad, J.C.; Andersen, B. Food and Indoor Fungi; Springer: Utrecht, The Netherlands, 2010; ISBN 978-90-70351-82-3. [Google Scholar]
- Pitt, J.I.; Hocking, A.D. Fungi and Food Spoilage; Springer US: Boston, MA, USA, 2009; ISBN 978-0-387-92206-5. [Google Scholar]
- Houbraken, J.; Kocsubé, S.; Visagie, C.M.; Yilmaz, N.; Wang, X.-C.; Meijer, M.; Kraak, B.; Hubka, V.; Samson, R.A.; Frisvad, J.C. Classification of Aspergillus, Penicillium, Talaromyces and related genera (Eurotiales): An overview of families, genera, subgenera, sections, series and species. Stud. Mycol. 2020, 95, 5–169. [Google Scholar] [CrossRef]
- Löytynoja, A. Phylogeny-aware alignment with PRANK. Methods Mol. Biol. 2014, 1079, 155–170. [Google Scholar]
- Darriba, D.; Posada, D.; Kozlov, A.M.; Stamatakis, A.; Morel, B.; Flouri, T. ModelTest-NG: A New and Scalable Tool for the Selection of DNA and Protein Evolutionary Models. Mol. Biol. Evol. 2020, 37, 291–294. [Google Scholar] [CrossRef]
- Sugiura, N. Further Analysis of the Data by Anaike’s Information Criterion and the Finite Corrections. Commun. Stat. Theory Methods 1978, 7, 13–26. [Google Scholar] [CrossRef]
- Hurvich, C.M.; Tsai, C.L. Regression and time series model selection in small samples. Biometrika 1989, 76, 297–307. [Google Scholar] [CrossRef]
- Kozlov, A.M.; Darriba, D.; Flouri, T.; Morel, B.; Stamatakis, A.; Wren, J. RAxML-NG: A fast, scalable and user-friendly tool for maximum likelihood phylogenetic inference. Bioinformatics 2019, 35, 4453–4455. [Google Scholar] [CrossRef] [PubMed]
- Nevalainen, A.; Hyvärinen, A. Fungi in Low-contamination Occupational Environments. Environ. Mycol. Public Heal. 2016, 107–125. [Google Scholar] [CrossRef]
- Méheust, D.; Le Cann, P.; Reboux, G.; Millon, L.; Gangneux, J.-P. Indoor fungal contamination: Health risks and measurement methods in hospitals, homes and workplaces. Crit. Rev. Microbiol. 2014, 40, 248–260. [Google Scholar] [CrossRef]
- Oppliger, A.; Duquenne, P. Highly Contaminated Workplaces. Environ. Mycol. Public Heal. 2016, 79–105. [Google Scholar] [CrossRef]
- Täubel, M.; Hyvärinen, A. Occurrence of Mycotoxins in Indoor Environments. Environ. Mycol. Public Heal. 2016, 299–323. [Google Scholar] [CrossRef]
- Mandal, J.; Brandl, H. Bioaerosols in Indoor Environment—A Review with Special Reference to Residential and Occupational Locations. Open Environ. Biol. Monit. J. 2011, 4, 83–96. [Google Scholar]
- Samson, R.A. Ecology and general characteristics of indoor fungi. In Fundamentals of Mold Growth in Indoor Environments and Strategies for Healthy Living; Wageningen Academic Publishers: Wageningen, The Netherlands, 2011; pp. 101–116. [Google Scholar]
- WHO. Guidelines for Indoor Air Quality and Dampness; World Health Organization: Geneva, Switzerland, 2010; ISBN 9789289002134. [Google Scholar]
- Saporta, D.; Hurst, D. Increased sensitization to mold allergens measured by intradermal skin testing following hurricanes. J. Environ. Public Health 2017, 2017. [Google Scholar] [CrossRef]
- Visagie, C.M.; Varga, J.; Houbraken, J.; Meijer, M.; Kocsubé, S.; Yilmaz, N.; Fotedar, R.; Seifert, K.A.; Frisvad, J.C.; Samson, R.A. Ochratoxin production and taxonomy of the yellow aspergilli (Aspergillus section Circumdati). Stud. Mycol. 2014, 78, 1–61. [Google Scholar] [CrossRef]
- Šegvić Klarić, M.; Jakšić Despot, D.; Kopjar, N.; Rašić, D.; Kocsubé, S.; Varga, J.; Peraica, M. Cytotoxic and genotoxic potencies of single and combined spore extracts of airborne OTA-producing and OTA-non-producing Aspergilli in Human lung A549 cells. Ecotoxicol. Environ. Saf. 2015, 120, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Ma, C.; Ren, X.; Xia, T.; Zheng, C.; Liu, X. Correlation analysis between filamentous fungi and chemical compositions in a pu-erh type tea after a long-term storage. Food Sci. Nutr. 2020, 8, 2501–2511. [Google Scholar] [CrossRef] [PubMed]
- Di Paolo, N.; Guarnieri, A.; Garosi, G.; Sacchi, G.; Mangiarotti, A.M.; Di Paolo, M. Inhaled mycotoxins lead to acute renal failure. Nephrol. Dial. Transplant 1994, 9 (Suppl. 4), 116–120. [Google Scholar] [PubMed]
- Hope, J.H.; Hope, B.E. A Review of the Diagnosis and Treatment of Ochratoxin A Inhalational Exposure Associated with Human Illness and Kidney Disease including Focal Segmental Glomerulosclerosis. J. Environ. Public Health 2012, 2012, 1–10. [Google Scholar] [CrossRef]
- Bongomin, F.; Batac, C.R.; Richardson, M.D.; Denning, D.W. A Review of Onychomycosis Due to Aspergillus Species. Mycopathologia 2018, 183, 485–493. [Google Scholar] [CrossRef]
- Dvorácková, I.; Píchová, V. Pulmonary interstitial fibrosis with evidence of aflatoxin B1 in lung tissue. J. Toxicol. Environ. Health 1986, 18, 153–157. [Google Scholar] [CrossRef]
- Viegas, S.; Veiga, L.; Malta-Vacas, J.; Sabino, R.; Figueredo, P.; Almeida, A.; Viegas, C.; Carolino, E. Occupational exposure to aflatoxin (AFB1) in poultry production. J. Toxicol. Environ. Health Part A Curr. Issues 2012, 75, 1330–1340. [Google Scholar] [CrossRef]
- Rudramurthy, S.M.; Paul, R.A.; Chakrabarti, A.; Mouton, J.W.; Meis, J.F. Invasive Aspergillosis by Aspergillus flavus: Epidemiology, Diagnosis, Antifungal Resistance, and Management. J. Fungi 2019, 5, 55. [Google Scholar] [CrossRef]
- Manikandan, P.; Varga, J.; Kocsubé, S.; Revathi, R.; Anita, R.; Dóczi, I.; Németh, T.T.M.; Narendran, V.; Vágvölgyi, C.; Bhaskar, M.; et al. Keratitis caused by the recently described new species Aspergillus brasiliensis: Two case reports. J. Med. Case Rep. 2010, 4, 1–4. [Google Scholar] [CrossRef]
- Gupta, N.; Kodan, P.; Mittal, A.; Singh, G.; Netto, G.; Ramteke, P.; Malla, S.; Kumar, R.; Kumar, T.P.; Singh, K.; et al. Role of Voriconazole in the Management of Invasive Central Nervous System Aspergillosis: A Case Series from a Tertiary Care Centre in India. J. Fungi 2020, 6, 139. [Google Scholar] [CrossRef]
- Susca, A.; Proctor, R.H.; Morelli, M.; Haidukowski, M.; Gallo, A.; Logrieco, A.F.; Moretti, A. Variation in fumonisin and ochratoxin production associated with differences in biosynthetic gene content in Aspergillus niger and A. welwitschiae isolates from multiple crop and geographic origins. Front. Microbiol. 2016, 7, 1412. [Google Scholar] [CrossRef] [PubMed]
- Chiotta, M.L.; Sosa, D.M.; Ponsone, M.L.; Chulze, S.N. Effect of water activity and temperature on growth of Aspergillus carbonarius and Aspergillus tubingensis and their interactions on ochratoxin A production. World Mycotoxin J. 2015, 8, 99–105. [Google Scholar] [CrossRef]
- Bugni, T.S.; Ireland, C.M. Marine-derived fungi: A chemically and biologically diverse group of microorganisms. Nat. Prod. Rep. 2004, 21, 143–163. [Google Scholar] [CrossRef] [PubMed]
- D’hooge, E.; Becker, P.; Stubbe, D.; Normand, A.-C.; Piarroux, R.; Hendrickx, M. Black aspergilli: A remaining challenge in fungal taxonomy? Med. Mycol. 2019, 57, 773–780. [Google Scholar] [CrossRef]
- Bathoorn, E.; Escobar Salazar, N.; Sepehrkhouy, S.; Meijer, M.; de Cock, H.; Haas, P.-J. Involvement of the opportunistic pathogen Aspergillus tubingensis in osteomyelitis of the maxillary bone: A case report. BMC Infect. Dis. 2013, 13, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Toyotome, T.; Saito, S.; Koshizaki, Y.; Komatsu, R.; Matsuzawa, T.; Yaguchi, T. Prospective survey of Aspergillus species isolated from clinical specimens and their antifungal susceptibility: A five-year single-center study in Japan. J. Infect. Chemother. 2020, 26, 321–323. [Google Scholar] [CrossRef]
- Takeda, K.; Suzuki, J.; Watanabe, A.; Matsuki, M.; Higa, K.; Inoue, E.; Akashi, S.; Shimada, M.; Kawashima, M.; Ohshima, N.; et al. Species identification, antifungal susceptibility, and clinical feature association of Aspergillus section Nigri isolates from the lower respiratory tract. Med. Mycol. 2019, 58, 310–314. [Google Scholar] [CrossRef]
- Perrone, G.; Varga, J.; Susca, A.; Frisvad, J.C.; Stea, G.; Kocsubé, S.; Tóth, B.; Kozakiewicz, Z.; Samson, R.A. Aspergillus uvarum sp. nov., an uniseriate black Aspergillus species isolated from grapes in Europe. Int. J. Syst. Evol. Microbiol. 2008, 58, 1032–1039. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).