Enhancing the Yield of Pleurotus ostreatus Through the Addition of Nucleotides and Nucleosides
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Materials
2.2. Medium and Culture Conditions
2.3. Analysis Methods
2.3.1. Mycelial Growth Rate and Biomass
2.3.2. Yield and Biological Efficiency of Mushroom
2.3.3. Components of Lignocellulose
2.3.4. Enzymatic Activity
2.3.5. Nutritional Evaluation and Heavy Metals
2.4. Statistical Analysis
3. Results
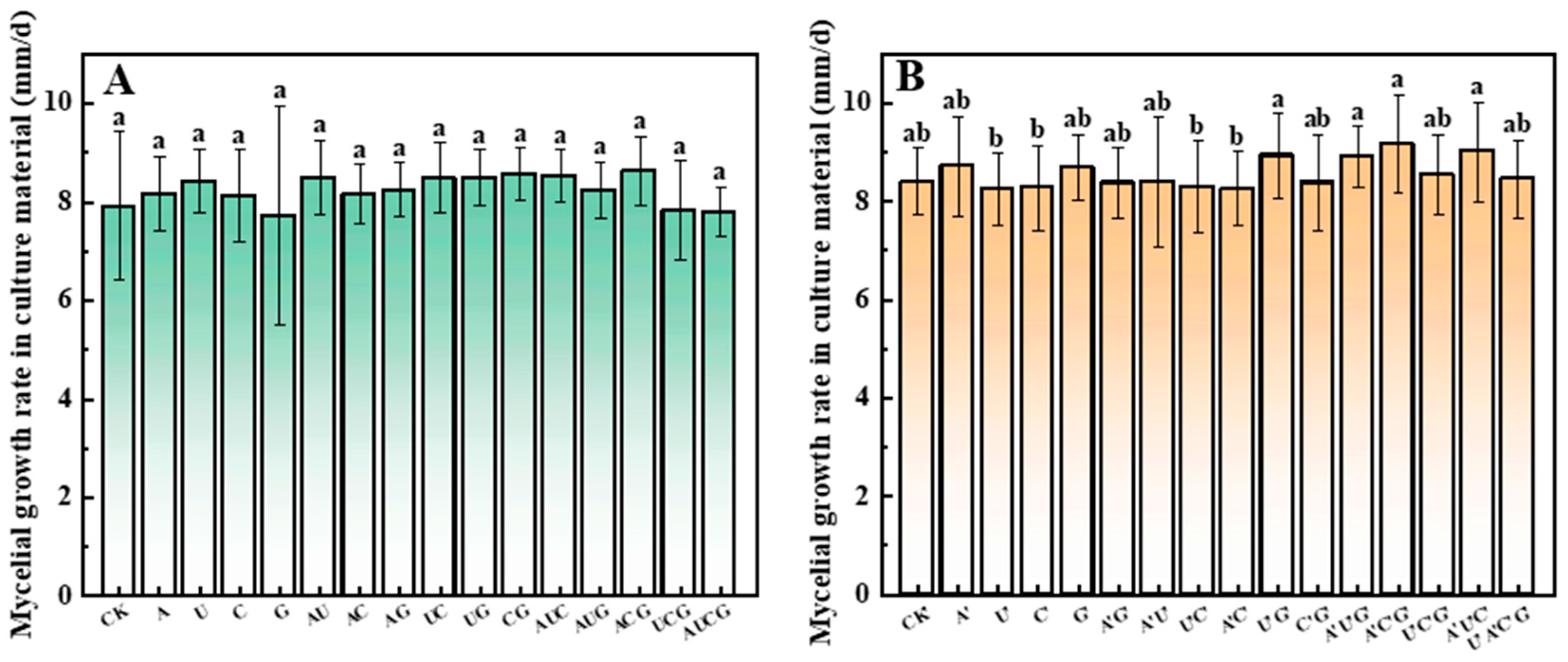
3.1. The Mycelium Growth Rate
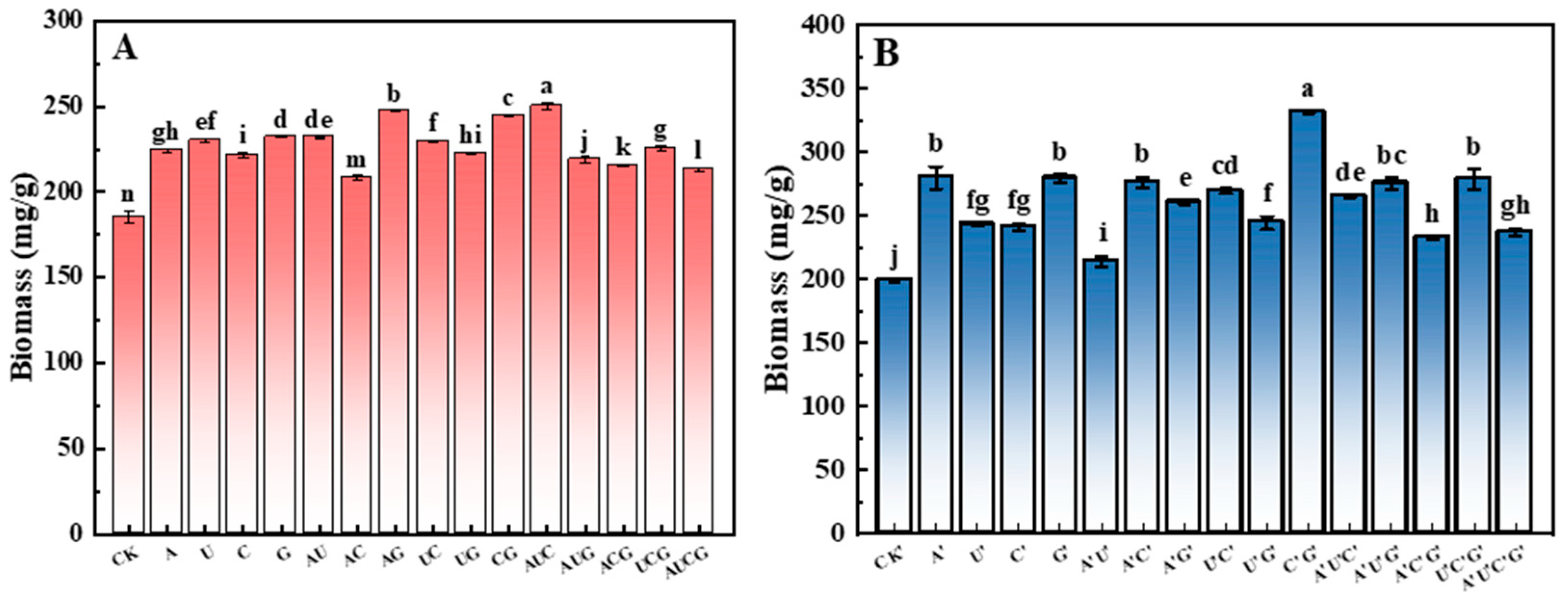
3.2. Analysis of Biomass
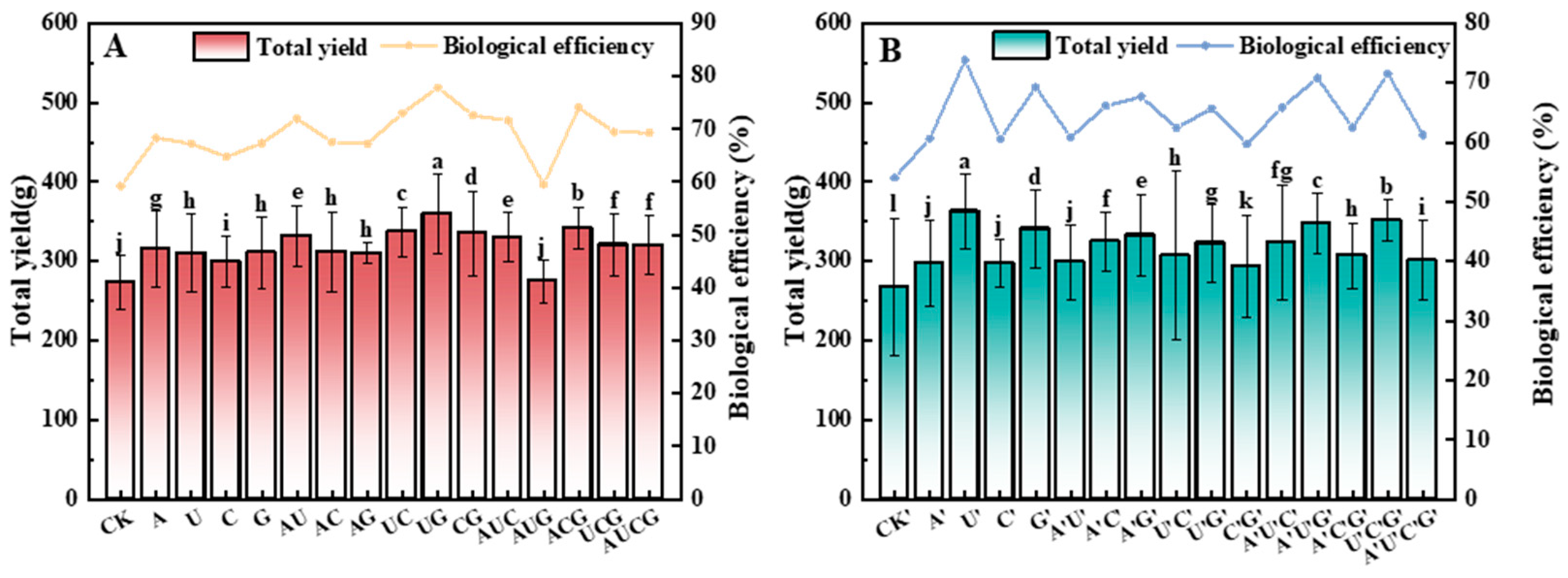
3.3. The Total Yield and Biological Efficiency of P. ostreatus
3.4. The Morphology of the Fruiting Body
3.5. The Nutrients in the Fruiting Bodies of P. ostreatus
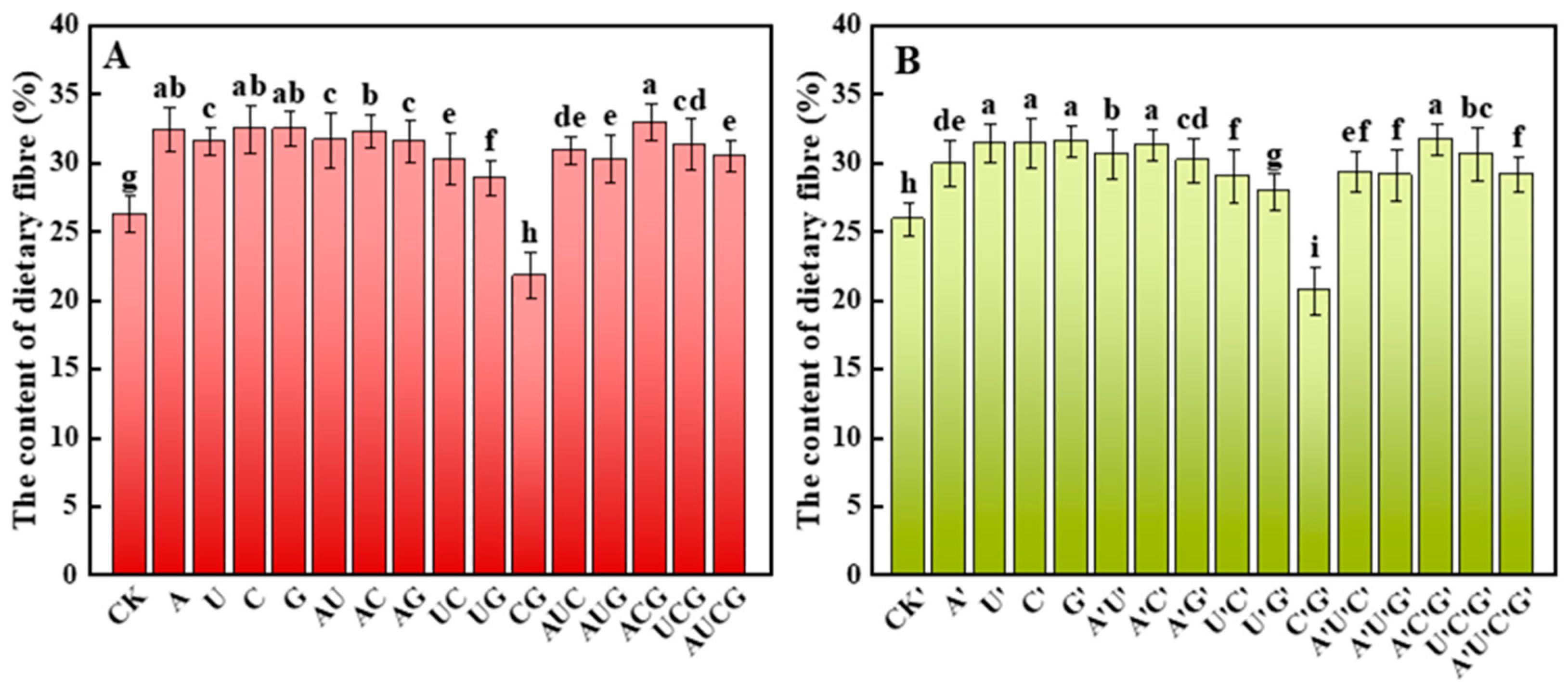
3.5.1. Dietary Fiber
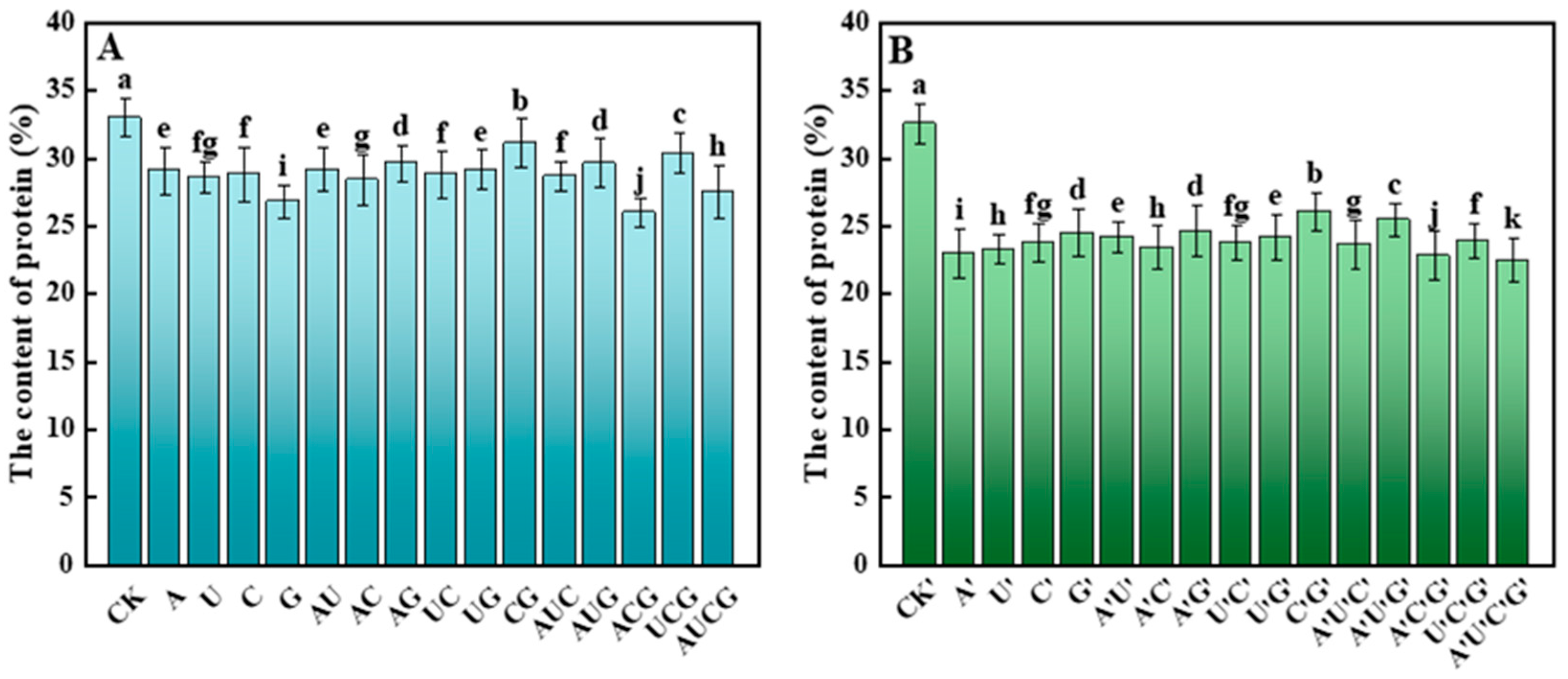
3.5.2. Protein
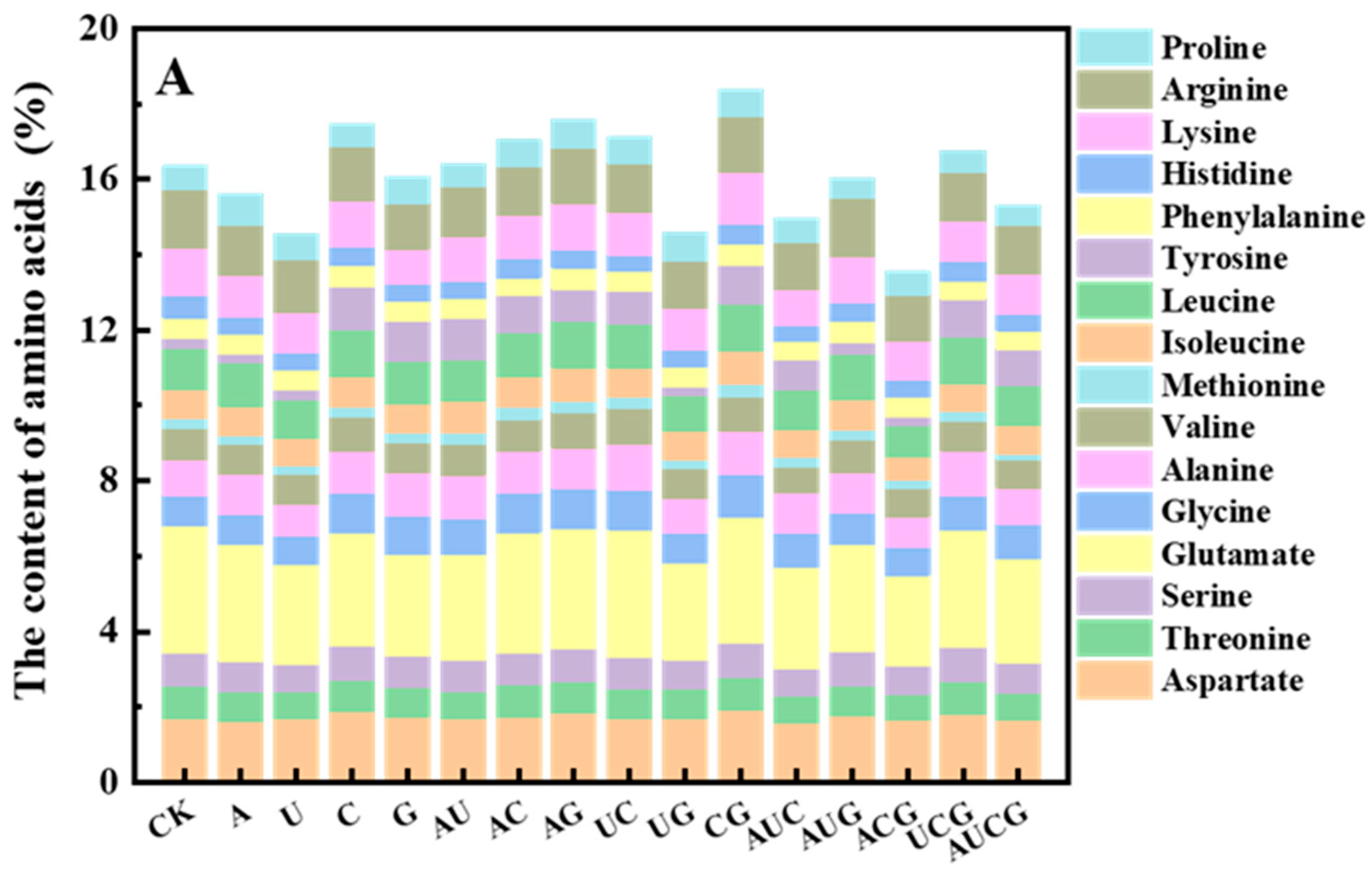
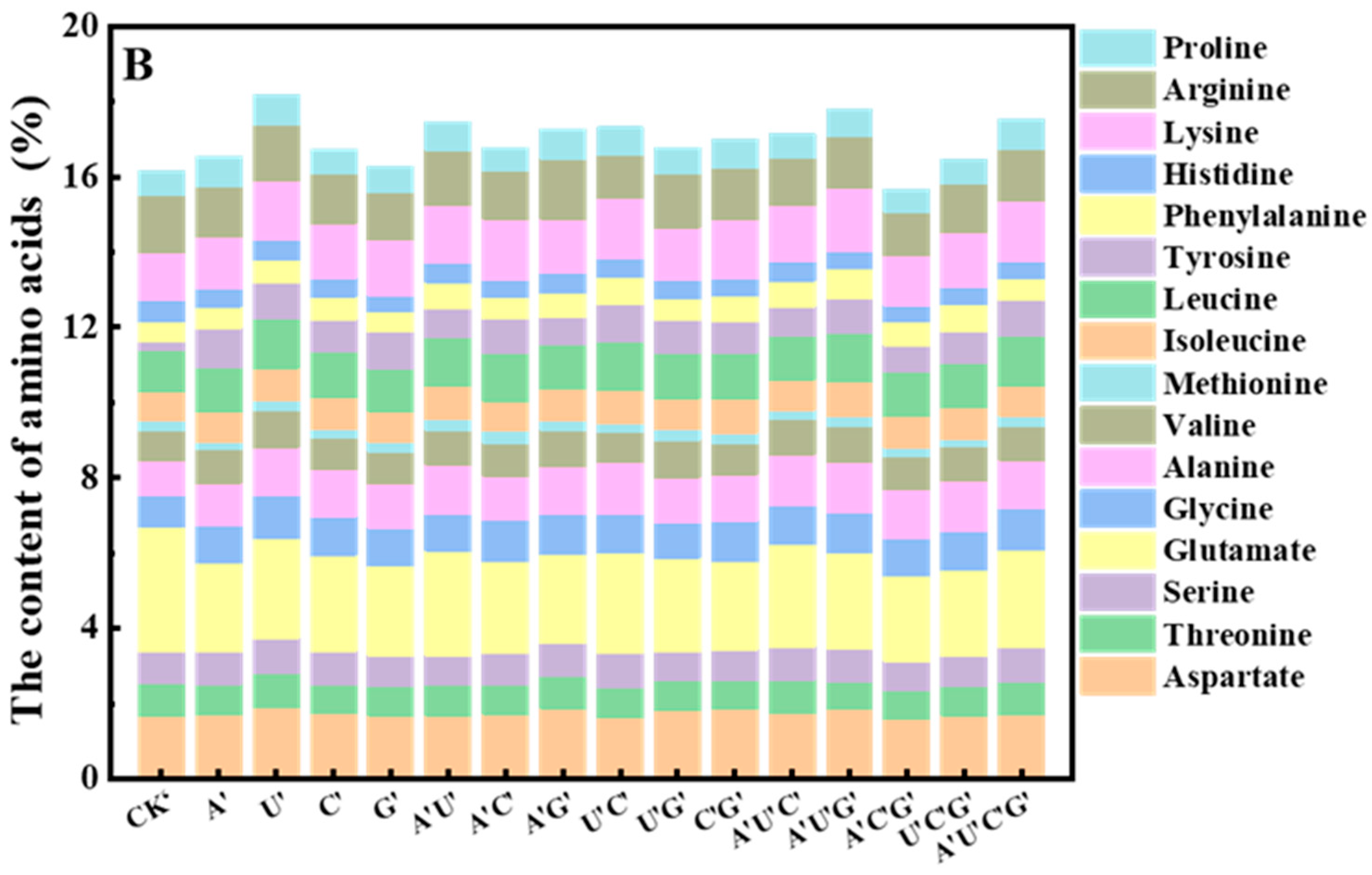
3.5.3. Amino Acid
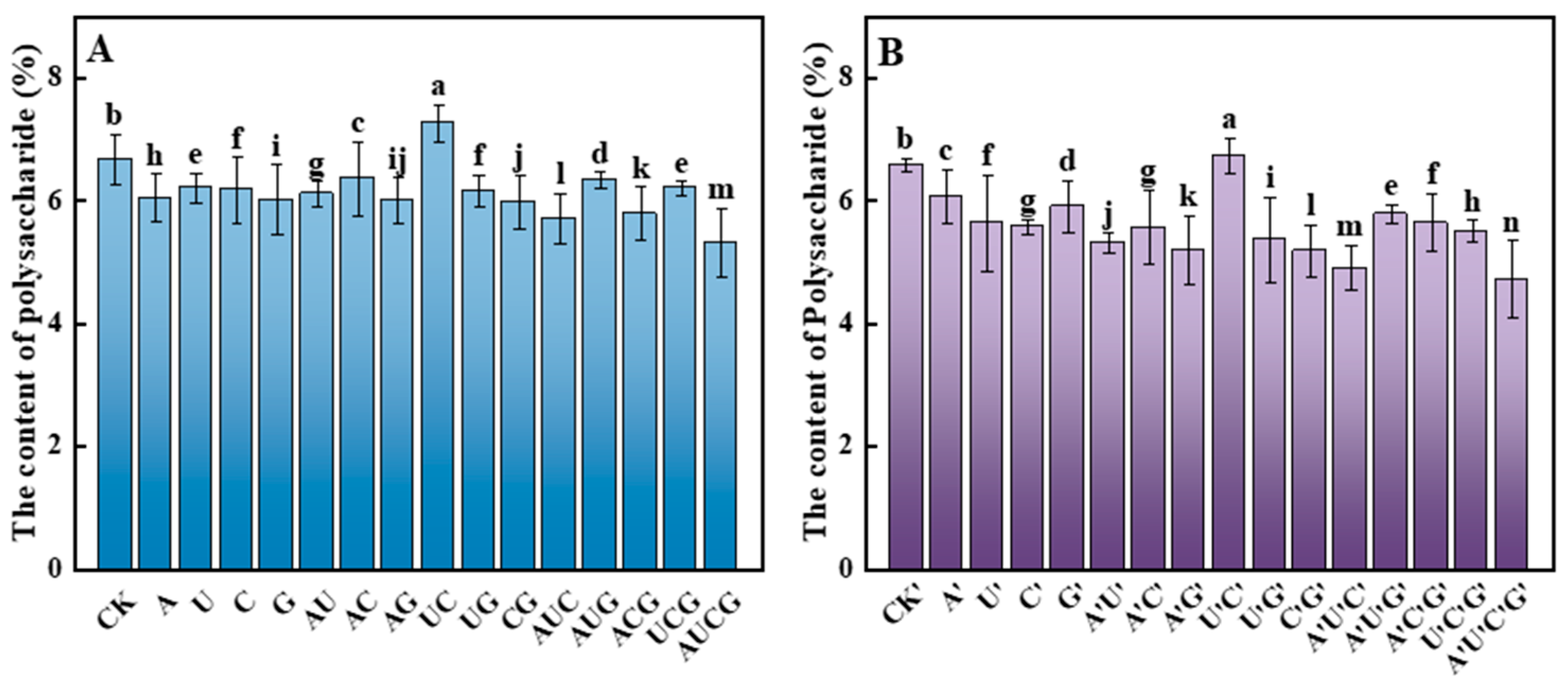
3.5.4. Polysaccharides
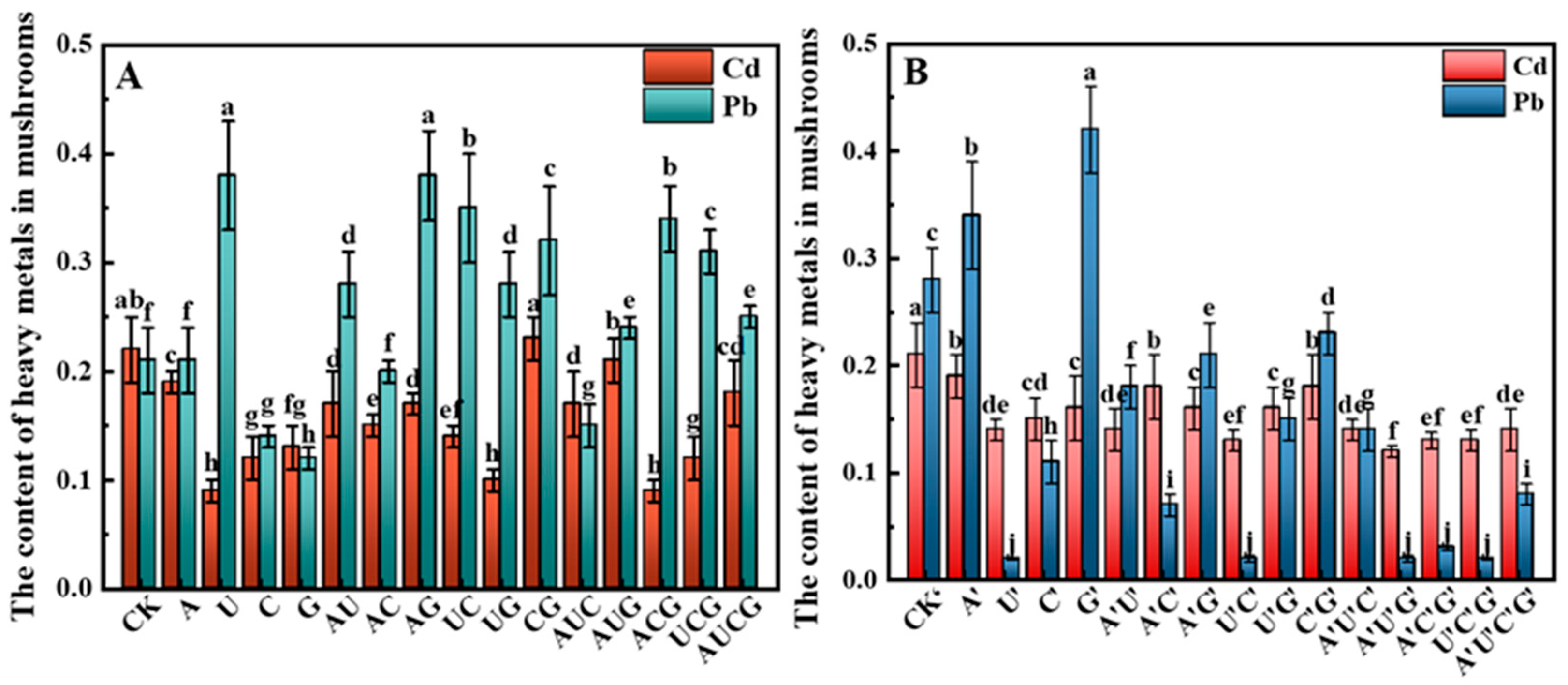
3.5.5. Heavy Metal Ions
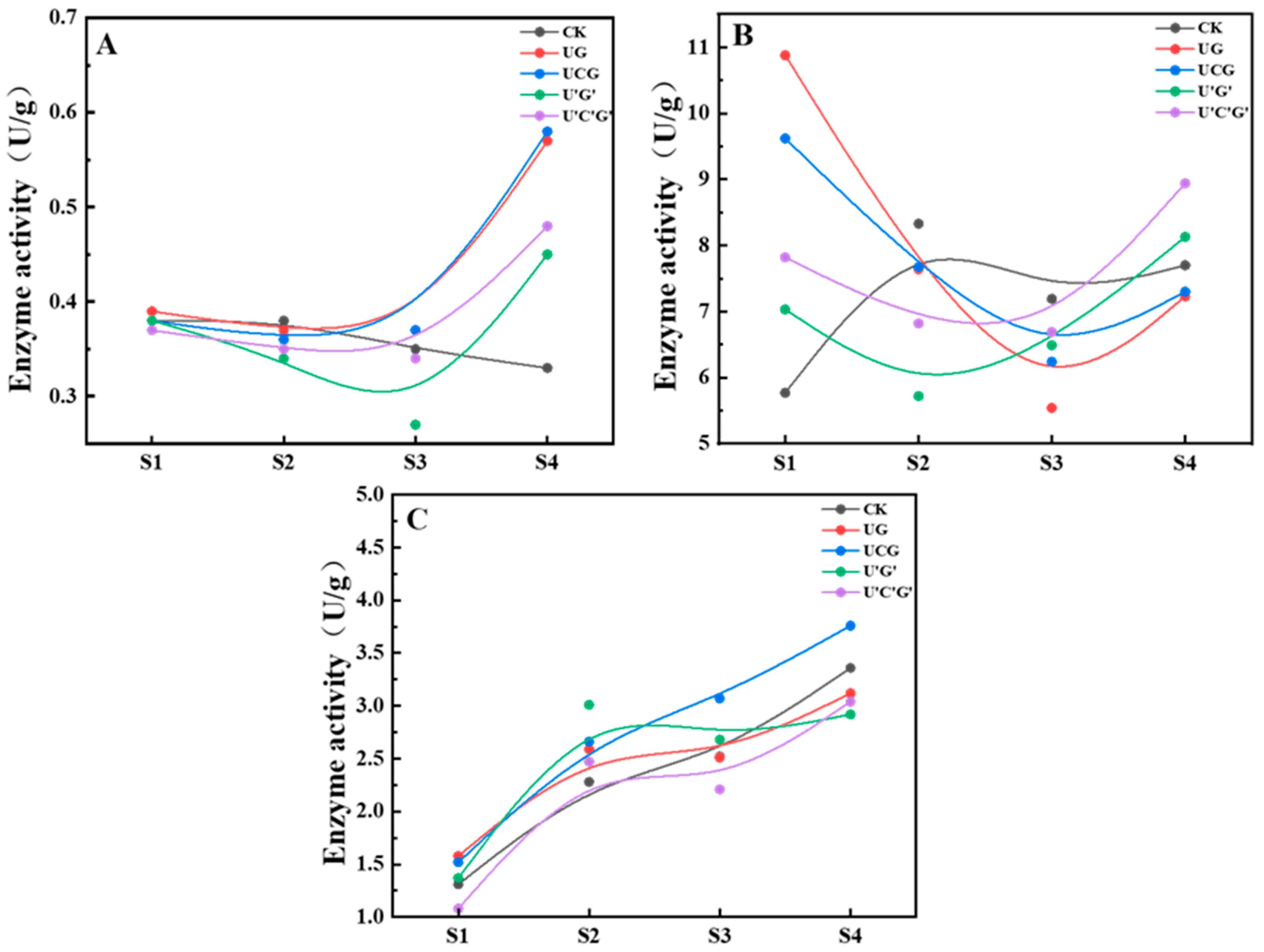
3.6. Analysis of Enzyme Activity at Various Growth Phases of P. ostreatus
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, Y.; Chu, M.H.; Ahmadi, F.; Agar, O.T.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A.R. A Comprehensive Review on Phytochemical Profiling in Mushrooms: Occurrence, Biological Activities, Applications and Future Prospective. Food Rev. Int. 2024, 40, 924–951. [Google Scholar] [CrossRef]
- Reis, F.S.; Barros, L.; Martins, A.; Ferreira, I. Chemical composition and nutritional value of the most widely appreciated cultivated mushrooms: An inter-species comparative study. Food Chem. Toxicol. 2012, 50, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Kong, W.L.; Cui, X.; Hu, S.J.; Shi, Z.W.; Wu, J.; Zhang, Y.T.; Qiu, L.Y. Dynamic succession of microbial compost communities and functions during Pleurotus ostreatus mushroom cropping on a short composting substrate. Front. Microbiol. 2022, 13, 946777. [Google Scholar] [CrossRef] [PubMed]
- Jafarpour, M.; Zand, A.J.; Dehdashtizadeh, B.; Eghbalsaied, S. Evaluation of agricultural wastes and food supplements usage on growth characteristics of Pleurotus ostreatus. Afr. J. Agric. Res. 2010, 5, 3291–3296. [Google Scholar]
- Bhattacharjya, D.K.; Paul, R.K.; Miah, M.N.A.; Ahmed, K.U. Comparative Study on Nutritional Composition of Oyster Mushroom (Pleurotus ostreatus Fr.) Cultivated on Different Sawdust Substrates. Biores. Commun. 2015, 1, 93–98. [Google Scholar]
- Pardo-Giménez, A.; Catalán, L.; Carrasco, J.; lvarez-Ortí, M.; Pardo, J.E. Effect of supplementing crop substrate with defatted pistachio meal on Agaricus bisporus and Pleurotus ostreatus production. J. Sci. Food Agric. 2015, 96, 3838–3845. [Google Scholar] [CrossRef] [PubMed]
- Coles, P.S.; Mcgiffen, M.E.; Xu, H.; Frutos, M. Compost Filling Methods Affect Green Mold Disease Incidence in Commercial Mushrooms. Plant Dis. 2024, 108, 666–670. [Google Scholar] [CrossRef] [PubMed]
- Kertesz, M.A.; Thai, M. Compost bacteria and fungi that influence growth and development of Agaricus bisporus and other commercial mushrooms. Appl. Microbiol. Biotechnol. 2018, 102, 1639–1650. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, J.; Zied, D.C.; Pardo, J.E.; Preston, G.M.; Pardo-Giménez, A. Supplementation in mushroom crops and its impact on yield and quality. AMB Express 2018, 8, 146. [Google Scholar] [CrossRef] [PubMed]
- Ding, T.; Song, G.; Liu, X.; Xu, M.; Li, Y. Nucleotides as optimal candidates for essential nutrients in living organisms: A review. J. Funct. Foods 2021, 82, 104498. [Google Scholar] [CrossRef]
- Claus-Peter, W.; Marco, H. Nucleotides and nucleotide derivatives as signal molecules in plants. J. Exp. Bot. 2024, 75, 6918–6938. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Klebansky, B.; Fine, R.M.; Xu, H.; Pronin, A.; Liu, H.; Tachdjian, C.; Li, X. Molecular mechanism for the umami taste synergism. Proc. Natl. Acad. Sci. USA 2008, 105, 20930–20934. [Google Scholar] [CrossRef] [PubMed]
- Brock, M. Fungal metabolism in host niches. Curr. Opin. Microbiol. 2009, 12, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Fox, E.M.; Howlett, B.J. Secondary metabolism: Regulation and role in fungal biology. Curr. Opin. Microbiol. 2008, 11, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Economou, C.N.; Diamantopoulou, P.A.; Philippoussis, A.N. Valorization of spent oyster mushroom substrate and laccase recovery through successive solid state cultivation of Pleurotus, Ganoderma, and Lentinula strains. Appl. Microbiol. Biotechnol. 2017, 101, 5213–5222. [Google Scholar] [CrossRef] [PubMed]
- Morais, A.R.C.; Zhang, J.; Dong, H.; Otto, W.G.; Mokomele, T.; Hodge, D.; Balan, V.; Dale, B.E.; Lukasik, R.M.; Sousa, L.D. Development of an ammonia pretreatment that creates synergies between biorefineries and advanced biomass logistics models. Green Chem. 2022, 24, 4443–4462. [Google Scholar] [CrossRef]
- Sandhya, C.; Sumantha, A.; Szakacs, G.; Pandey, A. Comparative evaluation of neutral protease production by Aspergillus oryzae in submerged and solid-state fermentation. Process Biochem. 2005, 40, 2689–2694. [Google Scholar] [CrossRef]
- Masuko, T.; Minami, A.; Iwasaki, N.; Majima, T.; Nishimura, S.I.; Lee, Y.C. Carbohydrate analysis by a phenol-sulfuric acid method in microplate format. Anal. Biochem. 2005, 339, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zheng, X.; Du, R.; Shao, Y.; Wen, Q.; Shen, X.; Wang, F.; Qi, Y.; Shen, J.; Hu, Y. Enrichment characteristics of Cd and Hg and regulation of heavy metal transporter signaling in Pleurotus ostreatus. Sci. Total Environ. 2024, 955, 176909. [Google Scholar] [CrossRef] [PubMed]
- AOAC. AOAC Official Method 991.43. Total, Soluble, and Insoluble Dietary Fiber in Foods; Association of Official Analytical Chemists: Washington, DC, USA, 1996. [Google Scholar]
- Zhang, Y.F.; Zhu, J.; Zou, Y.; Ye, Z.; Guo, L.; Zheng, Q. Insoluble dietary fiber from five commercially cultivated edible mushrooms: Structural, physiochemical and functional properties. Food Biosci. 2024, 57, 103514. [Google Scholar] [CrossRef]
- Guan, A.; Wang, M.; Gong, Y.; Huang, T.; Du, Y.; Zong, S. Optimization of selenium biofortification by liquid fermentation based on 2,4-dichlorophenoxyacetic acid and its effect on nutritional value of Pleurotus ostreatus. J. Food Compos. Anal. 2025, 137, 106850. [Google Scholar] [CrossRef]
- Song, F.; Su, D.; Shen, L.; Qiu, J.; Keyhani, N.O.; Wang, C. Influence of selenium on the mycelia of the shaggy bracket fungus, Inonotus hispidus. J. Sci. Food Agric. 2022, 102, 3762–3770. [Google Scholar] [CrossRef] [PubMed]
- Singh, U.; Sharma, S. Impact of bioaccumulated selenium on nutraceutical properties and volatile compounds in submerged fermented Pleurotus eryngii mycelia. J. Food Process. Preserv. 2022, 46, e17024. [Google Scholar] [CrossRef]
- Feng, P.; Gao, M.; Burgher, A.; Zhou, T.H.; Pramuk, K. A nine-country study of the protein content and amino acid composition of mature human milk. Food Nutr. Res. 2016, 60, 31042. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.D.; Chavez, J.L.; Mechref, Y. Sugar nucleotide quantification using multiple reaction monitoring liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2013, 27, 1794–1800. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.X.; Dong, Q.; Chen, M.J.; Zhao, R.H.; Zha, L.; Zhao, Y.; Zhang, M.K.; Zhang, B.S.; Ma, A.M. The Effect of Mushroom Dietary Fiber on the Gut Microbiota and Related Health Benefits: A Review. J. Fungi 2023, 9, 1028. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Dong, S.J.; Luo, J.H.; Ma, F.F.; Jiang, W.M.; Han, C.C. Research Progress on the Function and Application of Proteins of Edible and Medicinal Mushrooms: A Review. Int. J. Med. Mushrooms 2022, 24, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Leong, Y.K.; Yang, F.C.; Chang, J.S. Extraction of polysaccharides from edible mushrooms: Emerging technologies and recent advances. Carbohydr. Polym. 2021, 251, 117006. [Google Scholar] [CrossRef] [PubMed]
- Širić, I.; Humar, M.; Kasap, A.; Kos, I.; Mioč, B.; Pohleven, F. Heavy metal bioaccumulation by wild edible saprophytic and ectomycorrhizal mushrooms. Environ. Sci. Pollut. Res. Int. 2016, 23, 18239–18252. [Google Scholar] [CrossRef] [PubMed]
- Tschowri, N. Cyclic Dinucleotide-Controlled Regulatory Pathways in Streptomyces Species. J. Bacteriol. 2016, 198, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Dikec, J.; Olivier, A.; Bobée, C.; D’Angelo, Y.; Catellier, R.; David, P.; Filaine, F.; Herbert, S.; Lalanne, C.; Lalucque, H.; et al. Hyphal network whole field imaging allows for accurate estimation of anastomosis rates and branching dynamics of the filamentous fungus Podospora anserina. Sci. Rep. 2020, 10, 3131. [Google Scholar] [CrossRef] [PubMed]
- Wiemer, A.J.; Wiemer, D.F. Prodrugs of Phosphonates and Phosphates: Crossing the Membrane Barrier. In Phosphorus Chemistry I: Asymmetric Synthesis and Bioactive Compounds; Montchamp, J.L., Ed.; Topics in Current Chemistry-Series; Springer: Berlin/Heidelberg, Germany, 2015; Volume 360, pp. 115–160. [Google Scholar]
- Atila, F. Lignocellulosic and proximate based compositional changes in substrates during cultivation of Hericium erinaceus mushroom. Sci. Hortic. 2019, 258, 108779. [Google Scholar] [CrossRef]
- Mikiashvili, N.; Wasser, S.P.; Nevo, E.; Elisashvili, V. Effects of carbon and nitrogen sources on Pleurotus ostreatus ligninolytic enzyme activity. World J. Microbiol. Biotechnol. 2006, 22, 999–1002. [Google Scholar] [CrossRef]
- Levin, D.E. Regulation of cell wall biogenesis in Saccharomyces cerevisiae: The cell wall integrity signaling pathway. Genetics 2011, 189, 1145–1175. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Feng, Y.; Shang, Y.; Xu, H.; Xia, R.; Hou, Z.; Pan, S.; Li, L.; Bian, Y.; Zhu, J.; et al. Sexual spores in edible mushroom: Bioactive components, discharge mechanisms and effects on fruiting bodies quality. Food Sci. Hum. Wellness 2023, 12, 2111–2123. [Google Scholar] [CrossRef]
- Liu, L.W.; Fang, J.G.; Liang, X.F.; He, S. Nucleotide promotes feed intake and protein utilization via regulating the gene expression of feeding and nitrogen metabolism in juvenile Chinese perch (Siniperca chuatsi). Aquac. Nutr. 2020, 26, 1702–1712. [Google Scholar] [CrossRef]
- Gameiro, P.A.; Laviolette, L.A.; Kelleher, J.K.; Iliopoulos, O.; Stephanopoulos, G. Cofactor Balance by Nicotinamide Nucleotide Transhydrogenase (NNT) Coordinates Reductive Carboxylation and Glucose Catabolism in the Tricarboxylic Acid (TCA) Cycle. J. Biol. Chem. 2013, 288, 12967–12977. [Google Scholar] [CrossRef] [PubMed]
- Salar-García, M.J.; Bernal, V.; Pastor, J.M.; Salvador, M.; Argandoña, M.; Nieto, J.J.; Vargas, C.; Cánovas, M. Understanding the interplay of carbon and nitrogen supply for ectoines production and metabolic overflow in high density cultures of Chromohalobacter salexigens. Microb. Cell Fact. 2017, 16, 23. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Zhang, J.; Ma, F.; Tang, C.; Tang, Q.; Zhang, X. Investigation of lignocellulolytic enzymes during different growth phases of Ganoderma lucidum strain G0119 using genomic, transcriptomic and secretomic analyses. PLoS ONE 2018, 13, e0198404. [Google Scholar] [CrossRef] [PubMed]
- Elisashvili, V.; Kachlishvili, E.; Penninckx, M.J. Lignocellulolytic enzymes profile during growth and fruiting of Pleurotus ostreatus on wheat straw and tree leaves. Acta Microbiol. Immunol. Hung. 2008, 55, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Zhuo, F.; Fan, R.; Liu, F.; Zhang, H. Enhancing the Laccase Production and Laccase Gene Expression in the White-Rot Fungus Trametes velutina 5930 with Great Potential for Biotechnological Applications by Different Metal Ions and Aromatic Compounds. PLoS ONE 2013, 8, e79307. [Google Scholar]
- Masi, A.; Mach, R.L.; Mach-Aigner, A.R. The pentose phosphate pathway in industrially relevant fungi: Crucial insights for bioprocessing. Appl. Microbiol. Biotechnol. 2021, 105, 4017–4031. [Google Scholar] [CrossRef] [PubMed]
- Rauscher, R.; Würleitner, E.; Wacenovsky, C.; Aro, N.; Stricker, A.R.; Zeilinger, S.; Kubicek, C.P.; Penttilä, M.; Mach, R.L. Transcriptional regulation of xyn1, encoding xylanase I, in Hypocrea jecorina. Eukaryot. Cell 2006, 5, 447–456. [Google Scholar] [CrossRef] [PubMed]

| Nucleotide Groups | A (%) | U (%) | C (%) | G (%) |
| CK | 0.000 | 0.000 | 0.000 | 0.000 |
| A | 0.040 | 0.000 | 0.000 | 0.000 |
| U | 0.000 | 0.040 | 0.000 | 0.000 |
| C | 0.000 | 0.000 | 0.040 | 0.000 |
| G | 0.000 | 0.000 | 0.000 | 0.040 |
| AU | 0.020 | 0.020 | 0.000 | 0.000 |
| AC | 0.020 | 0.000 | 0.020 | 0.000 |
| AG | 0.020 | 0.000 | 0.000 | 0.020 |
| UC | 0.000 | 0.020 | 0.020 | 0.000 |
| UG | 0.000 | 0.020 | 0.000 | 0.020 |
| CG | 0.000 | 0.000 | 0.020 | 0.020 |
| AUC | 0.013 | 0.013 | 0.013 | 0.00 |
| AUG | 0.013 | 0.013 | 0.000 | 0.013 |
| ACG | 0.013 | 0.000 | 0.013 | 0.013 |
| UCG | 0.000 | 0.013 | 0.013 | 0.013 |
| AUCG | 0.010 | 0.010 | 0.010 | 0.010 |
| Nucleoside Group | A’ (%) | U’ (%) | C’ (%) | G’ (%) |
| CK | 0.000 | 0.000 | 0.000 | 0.000 |
| A’ | 0.040 | 0.000 | 0.000 | 0.000 |
| U’ | 0.000 | 0.040 | 0.000 | 0.000 |
| C’ | 0.000 | 0.000 | 0.040 | 0.000 |
| G’ | 0.000 | 0.000 | 0.000 | 0.040 |
| A’U’ | 0.020 | 0.020 | 0.000 | 0.000 |
| A’C’ | 0.020 | 0.000 | 0.020 | 0.000 |
| A’G’ | 0.020 | 0.000 | 0.000 | 0.020 |
| U’C’ | 0.000 | 0.020 | 0.020 | 0.000 |
| U’G’ | 0.000 | 0.020 | 0.000 | 0.020 |
| C’G’ | 0.000 | 0.000 | 0.020 | 0.020 |
| A’U’C’ | 0.013 | 0.013 | 0.013 | 0.000 |
| A’U’G’ | 0.013 | 0.013 | 0.000 | 0.013 |
| A’C’G’ | 0.013 | 0.000 | 0.013 | 0.013 |
| U’C’G’ | 0.000 | 0.013 | 0.013 | 0.013 |
| A’U’C’G’ | 0.010 | 0.010 | 0.010 | 0.010 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, C.; Gao, Y.; An, Z.; Sajid, A.Q.; Ying, H.; Wang, Z.; Liu, D. Enhancing the Yield of Pleurotus ostreatus Through the Addition of Nucleotides and Nucleosides. J. Fungi 2025, 11, 537. https://doi.org/10.3390/jof11070537
Tang C, Gao Y, An Z, Sajid AQ, Ying H, Wang Z, Liu D. Enhancing the Yield of Pleurotus ostreatus Through the Addition of Nucleotides and Nucleosides. Journal of Fungi. 2025; 11(7):537. https://doi.org/10.3390/jof11070537
Chicago/Turabian StyleTang, Chenmin, Yixuan Gao, Zhiguo An, Abdul Qadeer Sajid, Hanjie Ying, Zhenyu Wang, and Dong Liu. 2025. "Enhancing the Yield of Pleurotus ostreatus Through the Addition of Nucleotides and Nucleosides" Journal of Fungi 11, no. 7: 537. https://doi.org/10.3390/jof11070537
APA StyleTang, C., Gao, Y., An, Z., Sajid, A. Q., Ying, H., Wang, Z., & Liu, D. (2025). Enhancing the Yield of Pleurotus ostreatus Through the Addition of Nucleotides and Nucleosides. Journal of Fungi, 11(7), 537. https://doi.org/10.3390/jof11070537







