Abstract
Background: We aim to investigate the characteristics of invasive pulmonary aspergillosis (IPA) in patients with HBV-related acute on chronic liver failure (HBV-ACLF). Methods: A total of 44 patients with probable IPA were selected as the case group, and another 88 patients without lung infections were chosen as the control group. Results: HBV-ACLF patients with probable IPA had more significant 90-day mortality (38.6% vs. 15.9%, p = 0.0022) than those without. The white blood cell (WBC) count was the independent factor attributed to the IPA development [odds ratio (OR) 1.468, p = 0.027]. Respiratory failure was associated with the mortality of HBV-ACLF patients with IPA [OR 26, p = 0.000]. Twenty-seven patients received voriconazole or voriconazole plus as an antifungal treatment. Plasma voriconazole concentration measurements were performed as therapeutic drug monitoring in 55.6% (15/27) of the patients. The drug concentrations exceeded the safe range with a reduced dosage. Conclusions: The WBC count might be used to monitor patients’ progress with HBV-ACLF and IPA. The presence of IPA increases the 90-day mortality of HBV-ACLF patients mainly due to respiratory failure. An optimal voriconazole regimen is needed for such critical patients, and voriconazole should be assessed by closely monitoring blood levels.
1. Introduction
Invasive pulmonary aspergillosis (IPA) is a rapidly progressive, frequently fatal disease. It mainly occurs in immunocompromised patients, such as patients with neutropenia, patients with hematological malignancies, recipients of hematopoietic stem cell transplantation or solid organ transplantation, or patients undergoing corticosteroid treatment and other immunosuppressive therapies [1]. In recent years, studies have shown that IPA occurs in patients with decompensated cirrhosis and liver failure [2,3].
Liver failure is a life-threatening condition. Due to the rare survival of patients with liver failure, Aspergillus detection was limited; none of the genus Aspergillus was identified in patients with liver failure before 2006 [4]. Recently, with the advancement of diagnosis and treatment, the survival rate of patients with liver failure has been improved, and the detection rate of various fungal infections has also been significantly increased. Owing to multiple immunologic defects, patients with liver failure are prone to various infections, including bacterial, viral, and fungal infections such as IPA [4,5,6]. IPA lacks specific clinical manifestations in the early stage, and symptoms such as fever, cough, and dyspnea are easily overlooked by symptoms of liver failure or its complications.
Acute on chronic liver failure (ACLF) is a prevalent kind of liver failure as a result of chronic liver disease or an acute decompensation of an end-stage liver disease [7]. Hepatitis B virus (HBV) infection remains Asia’s leading cause of ACLF [7]. The studies for IPA in patients with HBV-related acute on chronic liver failure (HBV-ACLF) remain limited. In our retrospective study, we aim to investigate the prevalence, clinical manifestations, risk factors, outcomes, and antifungal treatment of IPA in patients with HBV-ACLF and to improve the prognosis of patients with HBV-ACLF complicated by IPA.
2. Materials and Methods
2.1. Study Subjects
This retrospective study included patients with probable IPA diagnosis admitted with ACLF to the West China Hospital of Sichuan University from February 2008 to September 2023. The data of patients without pulmonary infection were collected from the same databases. Clinical data from all selected patients were reviewed, including demographic features, predisposing factors, clinical manifestations, results of laboratory tests, chest computed tomography (CT) images, treatments, and prognosis. Informed consent was not obtained since it was a retrospective analysis of the collected data, and the participants’ identities were kept confidential. The study protocol was approved by the Ethics Committee of the West China Hospital of Sichuan University (Chengdu, Sichuan, China) following the ethical guidelines of the 1975 Declaration of Helsinki (approval number: 2023-1884). Subjects with other clinical liver diseases, such as autoimmune liver diseases, alcoholic liver disease, drug-induced liver injury, hepatocellular carcinoma, hematologic malignancy, bile duct obstruction, Wilson’s disease, chronic hepatitis C infection, or human immunodeficiency virus coinfection, were excluded.
2.2. Enrolment Criteria
ACLF was defined according to the following criteria specified by the Asian Pacific Association for the Study of the Liver [7] and the Guideline for Diagnosis and Treatment of Liver Failure in China [8]: (1) extreme fatigue with severe digestive symptoms such as apparent anorexia, abdominal distension, or nausea and vomiting; (2) the acute deterioration of pre-existing chronic liver disease/cirrhosis; (3) serum total bilirubin ≥ 178 μmol/L; and (4) coagulopathy (INR ≥ 1.5).
The diagnosis of IPA was classified as proven, probable, possible IPA, or Aspergillus colonization according to the definitions of the EORTC/MSG consensus [9]. Due to the severe coagulopathy and the concern of bleeding complications, none of the ACLF patients underwent bronchoscopy for needle aspiration biopsy, and only two patients underwent bronchoalveolar lavage. Considering ACLF as a host risk factor for IPA, patients with possible IPA were defined as having probable IPA if they met both of the following criteria: (1) the presence of abnormal radiologic findings compatible with pulmonary infection, including classical signs of IPA (dense, well-circumscribed lesions with or without a halo sign, air crescent sign, or cavity) or non-classical findings such as masses and (2) the presence of Aspergillus species indicated by direct test (direct microscopy or culture) or galactomannan (GM) antigen detected in plasma with a cut-off point value of 0.5 (Figure 1).
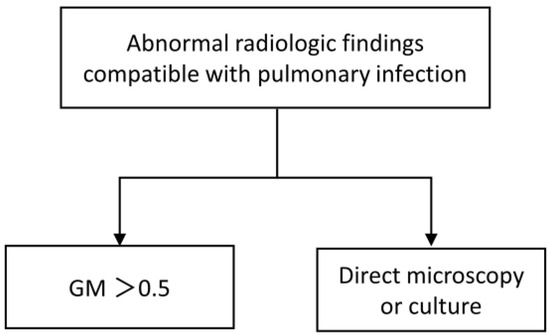
Figure 1.
IPA criteria.
Patients with HBV-ACLF whose CT scans did not show infiltrates in the lung were included as controls.
2.3. Patient Outcomes
The outcomes noted were the 90-day mortality.
2.4. Statistical Analyses
All statistical analyses were performed using SPSS version 22.0 (SPSS, Inc., Chicago, IL, USA). Continuous variables without a normal distribution are expressed as the median and inter-quartile range (IQR). Categorical variables are expressed as frequencies and percentages. The χ2 test or Fisher’s exact probability test was used to examine categorical variables such as sex and age. The Mann–Whitney U test was used to analyze continuous variables. Predictors for the development of IPA and mortality were determined by the odds ratio (OR) and 95% confidence interval (CI), which were calculated using multivariable binomial logistic regression analysis. Survival analysis was presented using the Kaplan–Meier method and compared by the log-rank test. A two-sided p-value < 0.05 was considered statistically significant.
3. Results
3.1. Study Populations and the Incidence of IPA
A diagram of the population selection in the study is shown in Figure 2. In this study, 4534 patients with liver failure were screened. Of these, the patients with HBV-ACLF were 2380, and with IPA were 44; the overall incidence of IPA in patients with HBV-ACLF was 44/2380 (1.84%). We enrolled 88 HBV-ACLF patients diagnosed with no lung infection by random sampling and compared their characteristics with those of 44 IPA patients.
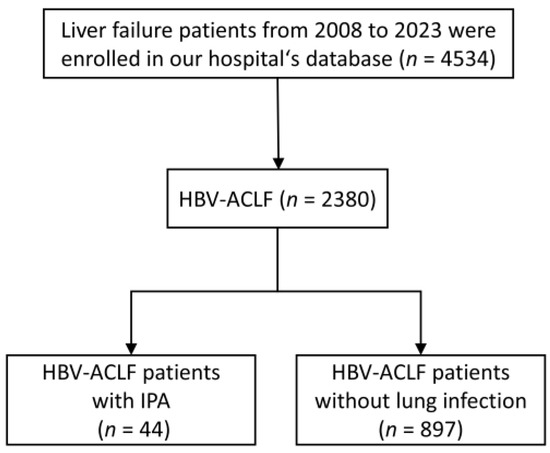
Figure 2.
Flow chart of patient selection.
Table 1 presents the differences in baseline characteristics, complications, laboratory findings, and outcome parameters between the IPA and non-IPA patients. The two groups did not differ in terms of age, sex, and hospital days. The percentage of underlying diseases of cardiovascular disease and diabetes mellitus type 2 was higher in the IPA group than in the non-IPA group. The incidence of upper gastrointestinal bleeding and septic shock in the IPA group was significantly higher than those in the non-IPA group. Compared to the non-IPA group, patients with IPA had lower ALT, AST, GLB, and Hb, a higher WBC count and neutrophil ratio, a lower platelet count, and higher procalcitonin. IPA developments were not attributed to the severity of liver failures, as indicated by MELD and MELD-Na score between these two groups. Antibiotic usage and steroid or immunosuppressant exposure were higher in the IPA group than in the non-IPA group. The 90-day mortality of patients with IPA was 38.6%, which was higher than that of patients without IPA (15.9%) (Figure 3).

Table 1.
Characteristics of patients with HBV-ACLF in the IPA and control groups.
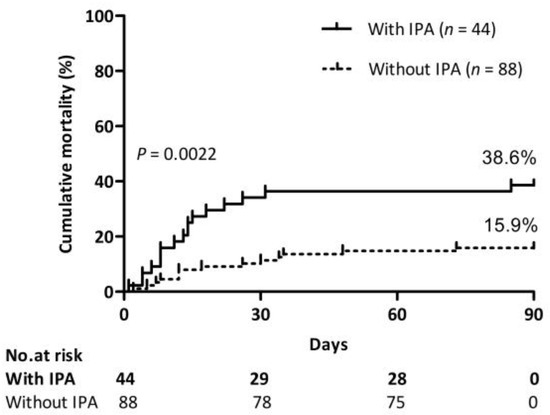
Figure 3.
Impact of IPA on mortality. Cumulative 90-day mortality of patients with or without IPA. Log-rank test (38.6% vs. 15.9%, p = 0.0022).
3.2. Factors Associated with the Development of IPA
On multivariate logistic regression analysis, WBC count [odds ratio (OR) 1.468, p = 0.027] was the significant predictor for the development of IPA in patients with HBV-ACLF (Table 2). The parameter might have been involved in the development of IPA in patients with HBV-ACLF; therefore, measuring it might help monitor the occurrence of IPA.

Table 2.
Logistic regression analysis of risk factors associated with IPA among patients with HBV-ACLF.
3.3. Clinical Characteristics of Patients with IPA
Table 3 and Table 4 present the clinical characteristics of patients with IPA. Five patients were admitted to the intensive care unit. Mechanical ventilation (thirteen patients), artificial liver support system (thirty-one patients), liver transplantation (four patients), vasoactive agents (seven patients), and continuous renal replacement therapy (six patients) were applied. In addition, ten patients received steroid or immunosuppressant treatment. All patients with IPA used one or more antibiotics.

Table 3.
The characteristics of HBV-ACLF patients with IPA.

Table 4.
Clinical manifestations, imaging findings, and laboratory parameters of HBV-ACLF patients with IPA.
Patients with IPA had fever and respiratory symptoms, including cough, hemoptysis, and dyspnea. These were not specific symptoms but may have been useful indicators of the early stage of IPA. Imaging findings were significant for IPA diagnosis. Halo signs or air-crescent signs help diagnose IPA and could guide antifungal therapy at an early stage. In the study, the imaging results from the chest CT scan were not specific, and nodules were the most common change rather than the halo sign or air crescent sign. Two (2/44, 4.5%) and 26 (26/44, 59.1%) patients had a positive GM measurement in bronchoalveolar lavage fluid and serum, respectively. About cultures, 56.8% (25/44) of cases were identified as positive for Aspergillus spp. (nineteen with A. fumigatus, two with A. flavus and four with unclassified Aspergillus).
3.4. Risk Factors of Mortality in IPA Patients
Among the IPA patients, 17 died within 90 days. There were differences in the outcomes among patients with the complications of respiratory failure, upper gastrointestinal bleeding, and septic shock; the treatments of the ventilator, vasoactive agents and continuous renal replacement therapy, and voriconazole; the symptoms of dyspnea; and the mycological findings of the serum level of GM test (Table 3 and Table 4). The above nine variables were considered for this logistic regression model. Multivariate logistic regression analysis showed that respiratory failure was an independent risk factor for predicting mortality in IPA patients with HBV-ACLF [OR 26, p = 0.000] (Table 5).

Table 5.
Logistic regression analysis of risk factors associated with mortality in HBV-ACLF patients with IPA.
3.5. Treatment with Antifungals
All but four patients (4/44, 9.1%) received antifungal therapy. Twenty-seven (27/44, 61.4%) patients received voriconazole or voriconazole plus as antifungal treatment, eight (18.2%) received caspofungin, and five (11.4%) received micafungin (Table 3).
Voriconazole is a first-line option for the primary treatment of IPA, and therapeutic regimens consisting of voriconazole therapy were the most common. Patients treated with a voriconazole-based regimen had a higher 90-day survival compared with those treated with a non-voriconazole regimen (77.8% vs. 30.8%, p = 0.0022; Figure 4a). However, experience of its use in ACLF patients is limited due to concerns of potential liver injuries. The loading dose ranged from 100 to 400 mg twice daily (Figure 4b). Patients began with the dose of the standard regimen (loading dose, 400 mg twice daily) or reduced regimen (loading dose ranged from 100 to 300 mg twice daily).
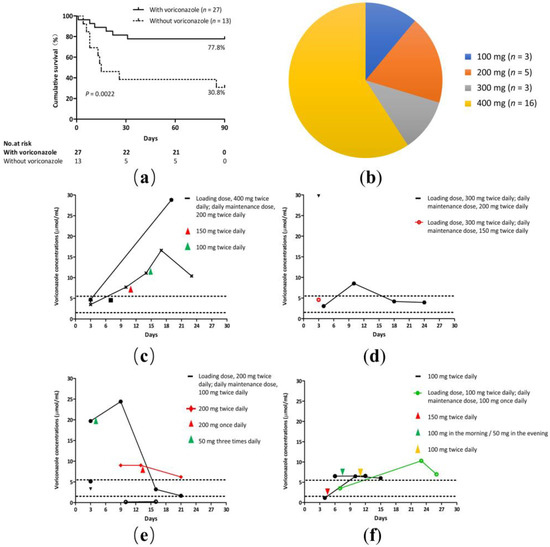
Figure 4.
Voriconazole regimen for IPA. (a) A cumulative 90-day survival of patients with or without voriconazole regimen. Log-rank test (77.8% vs. 30.8%, p = 0.0022). (b) Loading doses of voriconazole in HBV-ACLF patients. The loading dose ranged from 100 to 400 mg twice daily. (c–f) Trough voriconazole concentrations comparing patients treated with different regimens. Nine patients underwent plasma voriconazole concentration monitoring more than twice; the maintenance dose was based on the plasma voriconazole concentration. (c) A standard voriconazole regimen. (d–f) A reduced voriconazole regimen.
Plasma voriconazole concentration measurements were performed as therapeutic drug monitoring in 55.6% (15/27) of the patients. Our hospital’s normal range of plasma voriconazole concentration levels were 1.5 μg/mL to 5.5 μg/mL. Nine patients underwent plasma voriconazole concentration monitoring more than twice; the maintenance dose was based on the plasma voriconazole concentration. The drug concentrations exceeded the safe range in 60% (9/15) of the patients (Figure 4c–f). Out of four patients treated with a standard dose regimen (loading dose, 400 mg twice daily; daily maintenance dose, 200 mg twice daily), in two cases the plasma concentrations exceeded the safe range (Figure 4c). The remaining 11 patients were treated with a reduced dosage regimen. Out of three patients treated with a reduced dose regimen (loading dose, 300 mg twice daily; daily maintenance dose, 150/200 mg twice daily), in two cases the plasma concentrations exceeded the safe range (Figure 4d). Out of four patients treated with a reduced dose regimen (loading dose, 200 mg twice daily; daily maintenance dose, 100 mg twice daily) and one with a reduced dose regimen (200 mg twice daily), in two cases the plasma concentrations exceeded the safe range and in one case the plasma concentration was less than 1.5 μg/mL (Figure 4e). Out of one patient treated with a reduced dose regimen (loading dose, 100 mg twice daily; daily maintenance dose, 100 mg once daily) and two with a reduced dose regimen (100 mg twice daily), all three cases had plasma concentrations that exceeded the safe range (Figure 4f).
4. Discussion
This study investigated the prevalence, clinical manifestations, risk factors, outcomes, and antifungal treatment in HBV-ACLF patients with IPA. First, we found the prevalence of IPA in HBV-ACLF patients is 1.84%. Second, we described the detailed clinical features of IPA in HBV-ACLF patients and found that WBC count was the independent factor attributed to the IPA development. Third, we demonstrated that respiratory failure was associated with the mortality of HBV-ACLF patients with IPA. Finally, we suggested that voriconazole should be assessed by closely monitoring blood levels.
A study by W. Wang & C et al. reported that the prevalence of IPA in HBV-ACLF patients is 66/798 (8.3%) [4]. Meanwhile, in the study by Jiajia Chen et al., 37/787 (4.7%) of the patients with HBV-ACLF developed IPA [10]. The short-term mortality observed in these patients ranged from 95% to 100%. In our study, the incidence was 44/2380 (1.84%), and the mortality was 38.6%, which was lower than previous reports. The incidence of IPA is underestimated, and this discrepancy may be attributed to a population selection bias, as our study enrolled patients from only Sichuan Province, a southwest region of China. The IPA rate in patients with HBV-ACLF is not high, but once it occurs, the mortality is very high. W. Wang & C et al. reported that in the context of HBV-ACLF, patients with IPA died mainly due to respiratory failure within a very short time of IPA diagnosis, regardless of various antifungal therapies [4]. Our study’s multivariate logistic regression analysis showed that respiratory failure was a risk factor for predicting mortality in patients with IPA, consistent with previous reports. Therefore, we suggested the importance of a prompt IPA diagnosis to improve HBV-ACLF patients’ outcomes.
Accordingly, a large number of studies have reported lots of IPA risk factors for liver failure to provide methods for early clinical diagnosis and treatment. The previous studies showed age, sex, antibiotics use, steroid exposure, encephalopathy, diabetes, hepatorenal syndrome, frequent invasive procedures, and plasma exchange were independent risk factors associated with the occurrence of IPA in patients with liver failure [4,10,11,12]. In our study, multivariate logistic regression analysis found that WBC count was an independent predictor for IPA. IPA has been traditionally regarded as an infection mainly occurring in patients with well-established risk factors, such as neutropenia. However, an increasing number of reports underline the susceptibility of some categories of nonneutropenic patients to invasive fungal infections [13,14,15]. A study enrolled 43 nonneutropenic patients, and the underlying diseases in these patients included twelve cases of bronchiectasis, eleven cases of old pulmonary tuberculosis, eleven cases of nasosinusitis, nine cases of diabetes, seven cases of chronic obstructive pulmonary disease, three cases of bronchial asthma, two cases of lung cancer, and one case of pulmonary fibrosis [14]. It found that a WBC count > 20.0 × 109/L may aid in the early diagnosis of IPA [14]. Moreover, a case report showed that a diabetes patient with an elevated WBC count (WBC count 33 × 109/L) was diagnosed as having IPA [15]. IPA patients with combined underlying lung disease, diabetes, liver cirrhosis, and so on usually present with nonspecific symptoms and signs as well as nonspecific CT findings, and the diagnosis of IPA in nonneutropenic patients is more challenging. In this study, we, for the first time, confirmed that the high WBC count was a risk factor for IPA in HBV-ACLF patients. This means that patients with HBV-ACLF and a high WBC count will have an increased risk for IPA. Therefore, different WBC counts for diagnosing IPA should be considered based on the underlying condition. The conventional laboratory parameter of WBC count might be used to monitor patients’ progress with HBV-ACLF and IPA.
The effects of antifungal agents and the duration of treatment for IPA in patients with HBV-ACLF remain inadequate. Voriconazole is recommended as the first-line treatment for IPA according to the guidelines of the Infectious Diseases Society of America in 2016 [16]. It is associated with potential liver damage. Voriconazole drug instructions indicate that for patients with mild to moderate liver cirrhosis (Child–Pugh A and B), the loading dose remains unchanged and the maintenance dose is halved. However, no recommendation has been given for patients with severe liver cirrhosis (Child–Pugh C) and liver failure [17]. Jie Gao et al. showed that a voriconazole-based regimen achieved a comparable 90-day survival to ACLF patients with IPA [18]. In agreement with this finding, our analysis showed that patients treated with a voriconazole-based regimen had a higher 90-day survival than those treated with a non-voriconazole regimen (77.8% vs. 30.8%, p = 0.0022). How much of the dosage of voriconazole needs to be used in patients with liver failure, given concerns of the hepatotoxicity of voriconazole? To date, there are few studies on the use of voriconazole in ACLF patients, and some researchers have analyzed its individualized regime, but its safety is still unknown. Jie Gao et al. reported that ALCF patients (n = 8) treated with an optimal voriconazole regimen (loading doses, 200 mg twice daily; maintenance doses, 100 mg once daily) resulted in rational trough plasma drug concentrations (1–5 μg/mL), 90-day survival rate, and no observed adverse events [18]. Danli Chen et al. enrolled 102 patients treated with a voriconazole regimen; the loading dose of voriconazole ranged from 100 to 800 mg/day, and the maintenance dose ranged from 0 to 800 mg/day [19]. They confirmed that the voriconazole regimen (loading dose, 200 mg twice daily; daily maintenance dose, 100 mg once daily) achieved comparable 28-day survival and optimal trough drug concentrations (1–5 μg/mL) [19]. However, we observed that the drug concentrations exceeded the safe range with a reduced dosage (loading dose ranged from 100 to 300 mg twice daily; maintenance doses, 100 to 200 mg twice daily). This discrepancy may be attributed to factors such as age, liver function, drug interactions, and polymorphisms of human cytochrome P450 enzymes [20,21]. We suggested that voriconazole should be assessed by closely monitoring blood levels and adverse effects; however, as the current investigation included a small number of patients for therapeutic drug monitoring, a large sample, prospective study is warranted to validate the optimal voriconazole regimen for IPA in HBV-ACLF patients.
A. fumigatus is the primary causative agent of IPA. In our study, the A. fumigatus species remained the most commonly identified species, with a proportion of 43.2% of tested clinical isolates. However, studies have also observed that isolates of cryptic Aspergillus species and other sections can cause IPA [22]. Cryptic Aspergillus species are morphologically indistinguishable from others but differentiated by molecular methods. They often show intrinsic resistance to several classes of antifungals, making some treatments less effective. Furthermore, the diagnostic challenges of accurately identifying cryptic Aspergillus species, frequently misidentified as A. fumigatus, can lead to the inappropriate use of antifungals. Therefore, we should improve the awareness of the importance of cryptic Aspergillus species in the clinical setting. Recently, voriconazole resistance has been an increasing problem in invasive aspergillosis. A multicenter retrospective cohort study by Pieter P Lestrade et al. found that 37/196 (19%) cases of invasive aspergillosis in patients with A. fumigatus positive cultures harbored a voriconazole-resistant infection [23]. We should also be alert to the possible occurrence of voriconazole resistance.
Our study had some limitations. First, the study was a retrospective design. Second, this was a single-center study, and the generalizability of the results may be limited. Third, lung biopsies could not be performed in patients due to their coagulation function and platelet status, which will not lead to a precise diagnosis.
5. Conclusions
In summary, IPA is more common in HBV-ACLF patients with high WBC counts. Once IPA occurs in patients with HBV-ACLF, the fatality rate is very high. Respiratory failure was associated with the mortality of HBV-ACLF patients with IPA. A voriconazole regimen is needed for such critical patients, and therapeutic drug monitoring should be performed. Clinicians should increase the awareness of the risk factors of IPA in patients with HBV-ACLF and make early diagnoses and treatments to improve patients’ prognoses.
Author Contributions
Data curation, M.Y., N.H., D.L., W.H. and M.Z.; writing—original draft preparation, M.Y.; writing—review and editing, L.Y. and H.T.; funding acquisition, L.Y. and H.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key Research and Development Program of China (No. 2022YFC2304800), the 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University (No. ZYGD23030), and the Science and Technology project of the Health Commission of Sichuan Province (No. 23009).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of the West China Hospital of Sichuan University (Chengdu, Sichuan, China) (protocol code: 2023-1884; date of approval: October 2023).
Informed Consent Statement
Informed consent was not obtained since it was a retrospective analysis of the collected data, and the participants’ identities were kept confidential.
Data Availability Statement
Data associated with this study has not been deposited into a publicly available repository and will be made available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Latgé, J.P.; Chamilos, G. Aspergillus fumigatus and Aspergillosis in 2019. Clin. Microbiol. Rev. 2019, 33, e00140-18. [Google Scholar] [CrossRef] [PubMed]
- Levesque, E.; Ait-Ammar, N.; Dudau, D.; Clavieras, N.; Feray, C.; Foulet, F.; Botterel, F. Invasive pulmonary aspergillosis in cirrhotic patients: Analysis of a 10-year clinical experience. Ann. Intensive Care 2019, 9, 31. [Google Scholar] [CrossRef] [PubMed]
- Lahmer, T.; Peçanha-Pietrobom, P.M.; Schmid, R.M.; Colombo, A.L. Invasive fungal infections in acute and chronic liver impairment: A systematic review. Mycoses 2022, 65, 140–151. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, C.Y.; Zhou, J.Y.; Wang, Y.D.; Shen, C.; Zhou, D.F.; Yin, H.Z. Invasive pulmonary aspergillosis in patients with HBV-related liver failure. Eur. J. Clin. Microbiol. Infect. Dis. 2011, 30, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Fernández, J.; Acevedo, J.; Wiest, R.; Gustot, T.; Amoros, A.; Deulofeu, C.; Reverter, E.; Martínez, J.; Saliba, F.; Jalan, R.; et al. Bacterial and fungal infections in acute-on-chronic liver failure: Prevalence, characteristics and impact on prognosis. Gut 2018, 67, 1870–1880. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, M.C.; Hayes, P.C.; Simpson, K.J. Role of inflammation and infection in the pathogenesis of human acute liver failure: Clinical implications for monitoring and therapy. World J. Gastroenterol. 2016, 22, 5958–5970. [Google Scholar] [CrossRef]
- Sarin, S.K.; Choudhury, A.; Sharma, M.K.; Maiwall, R.; Al Mahtab, M.; Rahman, S.; Saigal, S.; Saraf, N.; Soin, A.S.; Devarbhavi, H.; et al. Acute-on-chronic liver failure: Consensus recommendations of the Asian Pacific association for the study of the liver (APASL): An update. Hepatol. Int. 2019, 13, 353–390. [Google Scholar] [CrossRef]
- Liver Failure and Artificial Liver Group, Chinese Society of Infectious Diseases, Chinese Medical Association; Severe Liver Disease and Artificial Liver Group, Chinese Society of Hepatology, Chinese Medical Association. Guideline for diagnosis and treatment of liver failure. Zhonghua Gan Zang Bing. Za Zhi 2019, 27, 18–26. [Google Scholar]
- De Pauw, B.; Walsh, T.J.; Donnelly, J.P.; Stevens, D.A.; Edwards, J.E.; Calandra, T.; Pappas, P.G.; Maertens, J.; Lortholary, O.; Kauffman, C.A.; et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin. Infect. Dis. 2008, 46, 1813–1821. [Google Scholar]
- Chen, J.; Yang, Q.; Huang, J.; Li, L. Risk factors for invasive pulmonary aspergillosis and hospital mortality in acute-on-chronic liver failure patients: A retrospective-cohort study. Int. J. Med. Sci. 2013, 10, 1625–1631. [Google Scholar] [CrossRef]
- Zhang, X.; Shen, S.; Dai, X.; Bi, Y.; Zhang, J.; Wu, Y.; Shi, Y.; Wei, R.; Gao, H. Clinical Risk Score for Invasive Pulmonary Aspergillosis in Patients with Liver Failure: A Retrospecwith Study in Zhejiang. Front. Med. 2021, 8, 762504. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, M.; Hu, J.; Zhao, H.; Li, L. Epidemiology of invasive pulmonary aspergillosis in patients with liver failure: Clinical presentation, risk factors, and outcomes. J. Int. Med. Res. 2018, 46, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Falcone, M.; Vena, A.; Venditti, C.; Mancini, C.; Morelli, A.; Venditti, M. Invasive pulmonary aspergillosis in non-neutropenic patients: Analysis of a 14-month prospective clinical experience. J. Chemother. 2011, 23, 290–294. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Che, C. Clinical manifestations and outcome analysis of invasive pulmonary aspergillosis infection: A retrospective study in 43 nonneutropenic patients. J. Int. Med. Res. 2019, 47, 5680–5688. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.L.; Zhang, Q.; Wang, M.H.; Li, L.; Fu, A.; Liu, C.; Zhang, H.; Li, W.; Chen, Y.; Zhang, S.; et al. Negative (1,3)-β-D-glucan and Elevated White Blood Cells Combined Procalcitonin Masquerading as Severe Pneumonia Eventually Diagnosed as Invasive Pulmonary Aspergillosis Proven by Bronchoalveolar Lavage Fluid Culture in a Diabetes Patient: A Case Report and Literature Review. Clin. Lab. 2019, 65, 1551–1554. [Google Scholar]
- Patterson, T.F.; Thompson, G.R., 3rd; Denning, D.W.; Fishman, J.A.; Hadley, S.; Herbrecht, R.; Kontoyiannis, D.P.; Marr, K.A.; Morisson, V.A.; Nguyen, M.H.; et al. Practice Guidelines for the Diagnosis and Management of Aspergillosis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 63, e1–e60. [Google Scholar] [CrossRef]
- Yu, X.; Xu, L.; Zheng, J.; Lei, Z.; Pang, Y.; Li, X.; Zhu, J.; Liu, J. Efficacy and safety of voriconazole in the treatment of invasive pulmonary aspergillosis in patients with liver failure: Study protocol for a randomized controlled clinical trial. Trials 2023, 24, 811. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zhang, Q.; Wu, Y.; Li, Y.; Qi, T.; Zhu, C.; Liu, S.; Yu, R.; He, Q.; Wem, W.; et al. Improving survival of acute-on-chronic liver failure patients complicated with invasive pulmonary aspergillosis. Sci. Rep. 2018, 8, 876. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Qian, Z.; Su, H.; Meng, Z.; Lv, J.; Huang, Y.; Gao, Y.; Liu, J.; Zhao, C.; Gao, H.; et al. Invasive Pulmonary Aspergillosis in Acute-on-Chronic Liver Failure Patients: Short-Term Outcomes and Antifungal Options. Infect. Dis. Ther. 2021, 10, 2525–2538. [Google Scholar] [CrossRef] [PubMed]
- Elewa, H.; El-Mekaty, E.; El-Bardissy, A.; Ensom, M.H.; Wilby, K.J. Therapeutic Drug Monitoring of Voriconazole in the Management of Invasive Fungal Infections: A Critical Review. Clin. Pharmacokinet. 2015, 54, 1223–1235. [Google Scholar] [CrossRef]
- Seyedmousavi, S.; Mouton, J.W.; Verweij, P.E.; Brüggemann, R.J. Therapeutic drug monitoring of voriconazole and posaconazole for invasive aspergillosis. Expert. Rev. Anti Infect. Ther. 2013, 11, 931–941. [Google Scholar] [CrossRef] [PubMed]
- Geremia, N.; Giovagnorio, F.; Colpani, A.; De Vito, A.; Caruna, G.; Meloni, M.C.; Madeddu, G.; Panese, S.; Parisi, S.G. What do We Know about Cryptic Aspergillosis? Microorganisms 2024, 12, 886. [Google Scholar] [CrossRef] [PubMed]
- Lestrade, P.P.; Bentvelsen, R.G.; Schauwvlieghe, A.F.A.D.; Schalekamp, S.; van der Velden, W.J.F.M.; Kuiper, E.J.; van Paassen, J.; van der Hoven, B.; van der Lee, H.A.; Melchers, W.J.G.; et al. Voriconazole Resistance and Mortality in Invasive Aspergillosis: A Multicenter Retrospective Cohort Study. Clin. Infect. Dis. 2019, 68, 1463–1471. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).