Abstract
Underexpansion of a self-expanding transcatheter aortic valve (TAVI) is a critical issue that can negatively impact long-term outcomes, including paravalvular leak, valve thrombosis, and increased mortality. This paper provides a comprehensive review of the pathophysiology and consequences of such complications, including three primary mechanisms: (1) infolding, (2) incorrect site of crossing and (3) true underexpansion. It also discusses strategies to address these challenges, including pre-procedural planning and procedural techniques to ensure proper valve deployment and expansion. Mitigating these issues is essential to improving both immediate and long-term outcomes in TAVI patients.
1. Introduction
Transcatheter aortic valve implantation (TAVI) for the management of severe aortic stenosis offers an effective and less invasive alternative to traditional surgical valve replacement. However, despite the substantial advances in technique and prosthesis design, complications still remain a significant challenge [].
Self-expanding valves (SEVs) represent an evolving field with different devices available and showing unique features. This includes models with both supra-annular leaflets like the Evolut (Medtronic, Minneapolis, MN, USA) or the Acurate Neo (Boston Scientific, Marlborough, MA, USA) or intra-annular devices like the Navitor (Abbott, North Chicago, IL, USA). Most SEVs can be either repositioned and/or retrieved, offering a larger effective orifice area, reduced transvalvular gradient and decreased incidence of severe prosthesis–patient mismatch compared to intra-annular valves. Some limitations in maneuverability, especially in horizontal aortas, or coronary re-access with some devices along with a lower radial force are important factors to consider while choosing the correct transcatheter heart valve (THV) for each patient.
TAVI underexpansion refers to the failure of the transcatheter valve to fully expand during deployment, potentially leading to suboptimal hemodynamic results, clinical complications and impaired long-term durability. In this review, we explore the mechanisms underlying THV underexpansion, discuss its clinical implications and examine current strategies for prevention, detection and management. Understanding the underlying pathophysiology is essential for optimizing procedural outcomes and improving long-term prognosis.
2. Definition and Clinical Implications
While there is no universally accepted definition in the medical literature, underexpansion in TAVI procedures generally refers to the incomplete expansion of the THV stent frame, which fails to reach its intended nominal diameter during deployment.
With balloon-expandable THVs, circularity is relatively preserved, often achieving over 90% of expansion [], but due to their lower radial force, SEVs frequently develop deformation and eccentric underexpansion at the inflow level []. This has been shown to impact valve functionality and durability by increasing leaflet stress and strain. It has been suggested that 5–10% of underexpansion can be clinically tolerable [], but when this deformation extends into the functional portion, it can result in impaired valve motion or increased risk of leaflet thrombosis. Fuchs et al. [] showed that moderate-to-severe regional THV underexpansion was associated with a significantly higher incidence of leaflet thickening than in cases of full regional THV expansion (24% vs. 3%, p < 0.01). They hypothesize that incomplete stent frame and leaflet expansion may result in the “wrinkling” of the leaflet as found in in vitro tests, making it more prone to the development of a thin thrombus layer on its surface [].
Underexpansion also is associated with the presence of residual paravalvular leak (PVL), which has been consistently related to adverse events [].The five-year follow up from the PARTNER-1 trial showed that moderate-to-severe aortic regurgitation after TAVI was associated with lower survival (HR 1.46, CI: 1.03–2.07, p = 0.04) []. In a prospective registry by Okuno et al., even mild paravalvular regurgitation after TAVI had a higher mortality risk at five years than those with none/mild leak (54.6% vs. 43.8%; HR adjusted 1.26, 95% CI: 1.06–1.50) [].
Special consideration must be given when using SEVs with lower radial force, as pre-dilatation is highly encouraged by the manufacturer and post-dilatation is often needed to optimize the hemodynamic results. Recently, the ACURATE IDE trial [] missed its non-inferiority primary endpoint when comparing the ACURATE neo2 (Boston Scientific) vs. the Evolut (Medtronic) and Sapien 3 (Edwards Lifesciences) platforms. At 1 year, the combined rate of all-cause mortality, stroke, or rehospitalization was 16.16% in patients treated with ACURATE neo2 and 9.53% among those treated with the other transcatheter valves. A post hoc analysis showed that underexpansion of the Acurate Neo 2 platform was present in nearly 20% of those devices, and when comparing underexpanded versus fully expanded valves, the first group showed numerically higher 1-year rates of hospitalization (18.8% vs. 12.4%; p = 0.050), death (7.4% vs. 3.7%; p = 0.054) and stroke (11.0% vs. 3.5%; p < 0.001). Interestingly, the fully expanded Acurate Neo 2 showed similar rates of adverse events when paired against the other two platforms.
3. Mechanisms of TAVI Underexpansion and Management
The mechanisms underlying underexpansion are complex and multifactorial, involving both patient-specific anatomical features and technical aspects of the procedure.
Understanding these factors is crucial for both prevention and management. In this section, we will review the key elements contributing to this complication and discuss strategies for optimizing the procedure, including pre- and post-deployment measures, aimed at mitigating its clinical impact.
3.1. Valve Infolding
Although it is now a rare complication affecting nearly 0,7% of implants with a second-generation Evolut platform [,], infolding can lead to severe consequences such as an increased risk of PVL, hemodynamic collapse, peri-procedural stroke and death [,]. This term describes the inward folding of the stent frame of an SEV along the vertical axis starting from the valve inflow.
The first preventive measure to avoid infolding is to inspect the valve before implantation to ensure proper loading [].
Once deployment has started, the two main fluoroscopic clues to identify it are (1) the appearance of a dense vertical line on the valve, reflecting the overlapping layers of the infolded stent cage, and (2) visual signs of underexpansion with a narrower-than-expected width [] (Figure 1A).
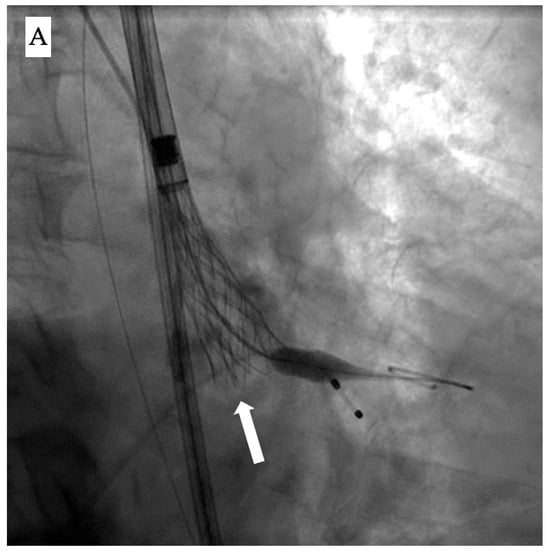
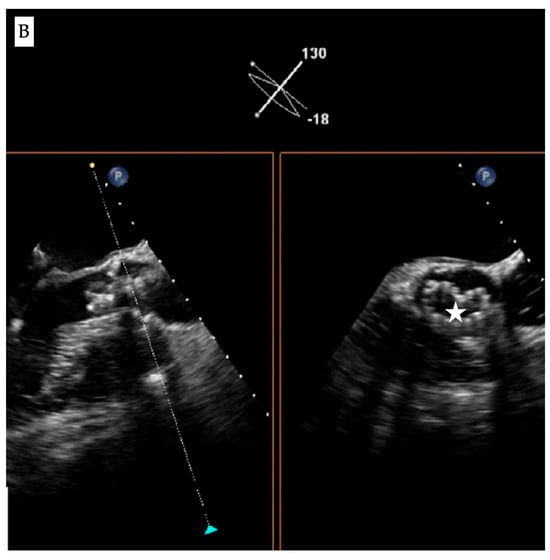
Figure 1.
Fluoroscopic and echocardiographic appearance of TAVI infolding. (A) Visual clues for recognizing this complication include a dense vertical line across the valve frame (white arrow) and the inability to reach the intended valve expansion. (B) Short axis window in echocardiography revealing the “Pac man” sign (white star).
Accordingly, evaluation of the bio-prosthesis in two orthogonal views or performing a c-arm fluoroscopic rotation is recommended to identify such complications. Infolding can also be confirmed by echocardiography through visualization of the “Pac-man” sign in the short axis view [] (Figure 1B).
Several factors have been described as increasing the risk of TAVI infolding, as shown in Table 1.

Table 1.
Risk factors for valve infolding *.
Depending on the timing of the infolding diagnosis, there are different correction measures:
- Before full release: The recommendation is to recapture, retrieve and reload the valve onto a new delivery catheter.If a high risk of infolding persists after several re-sheathing maneuvers, a more aggressive valve predilatation may be effective. Finally, switching to a BEV could be considered as a bail-out option.
- After full release: Failure to identify this complication usually leads to hemodynamic instability due to severe aortic regurgitation. In this situation, urgent balloon post-dilatation is necessary to resolve the acute prosthetic dysfunction []. Careful manipulation of the guidewire is essential to avoid losing wire position and the risk of rewiring through the valve’s stent frame.A valve-in-valve implantation using a BEV or emergency cardiac surgery have been suggested as bail-out measures, if post-dilatation is insufficient to improve the patient’s clinical condition [].
3.2. Guidewire Crossing Through an Inadequate Pathway
Stenotic disease of the aortic valve encompasses a wide range of anatomopathological variations, including the presence of fibrotic or calcific bridges between the cusps (Figure 2) that could allow passage of a guidewire through a lateral orifice.
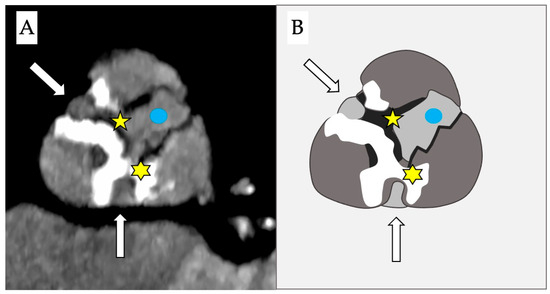
Figure 2.
Computed tomography (A) and schematic figure (B) of a stenotic aortic valve that depicts the presence of 2 simultaneous bridges: one fibrotic between the right and the non-coronary cusps (yellow star) and a second calcific bridge between the left and the non-coronary cusps (yellow asterisk). This creates two anomalous new orifices (white arrows) where the guidewire can pass through and which are distinct from the true anatomical orifice (blue dot).
Other possibilities are the presence of cusp perforations as a complication following endocarditis, or iatrogenic as sequalae from previous surgical interventions (e.g., aortic valvuloplasty) or due to percutaneous procedures (e.g., advancement of high-tip-load guidewires through the native cusp, complications from coronary atherectomy [], etc.).
In this scenario, pre-dilating the native aortic valve without a clear understanding of the wire position can lead to severe aortic regurgitation and sudden onset of cardiac arrest [].
The “commissural drop” wiring technique may allow for a safer crossing during the procedure [,]. It describes the movement of the pre-shaped super stiff wire (e.g., Safari, Boston Scientific) from the center of the valve into the inter-commissural space between the non-coronary and right cusps, thus leaning towards the outer curvature of the aorta (Figure 3A,B). This is usually a sign of correct crossing through the central valvular orifice and provides a visual clue to confirm a safe position before proceeding.
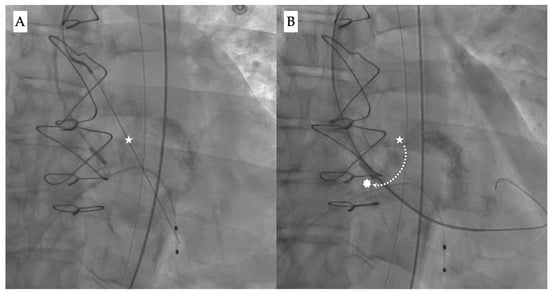
Figure 3.
Fluoroscopy images showing (A) the initial position of the wire during valve crossing (white star), and (B) guidewire drop down (dotted line) from the center position into the inter-commissural space between the right and non-coronary cusp (white asterisk).
If crossing the aortic valve is difficult, and in order to avoid losing wire position, another indirect technique could be attempting to advance an uninflated peripheral balloon through the guidewire in the left ventricle and checking for any resistance during advancement.
Finally, imaging techniques like transesophageal echocardiography could also help the operator assess the correct position of the guidewire.
3.3. True Underexpansion
This refers to an unsuccessful deployment due to mechanical factors that obstruct the expansion of the valve frame.
The most common one is severe calcification, which has been identified as one of the most important contributors, not only for underexpansion, but for complications such as annular rupture, need for pacemaker or PVL []. Contrary to non-contrast scans in which a fixed threshold of 130 Hounsfield units (HUs) is suggested, the threshold for calcium detection on contrast-enhanced CT scans has yet to be standardized in published reports. This lack of standardized acquisition parameters explains some of the difficulty in developing a uniform classification, although the range of 300–850 HU has been recommended []. CT is a fundamental part of the pre-planning phase and can help predict the risk of this complication, not only by estimating the amount of calcium, but also its distribution and characteristics (e.g., LVOT calcification, calcific nodules, etc.). This critical step can influence many decisions of the procedure such as THV selection, planning for upfront pre- and post-dilatation, depth of implant, need for coronary protection, pacemaker placement, etc.
Considering these issues, pre-dilatation is usually recommended in most bicuspid cases as it is for tri-leaflet valves with severe calcification measured by computed tomography (CT) scans (e.g., Agatston > 5000) or echocardiography. Operators must also be aware, especially in bicuspid anatomy, of the recoil phenomenon due to the fibro-calcific raphe, which enhances the difficulty of modifying the original “fish-mouth” shape.
During aortic valve-in-valve (ViV) procedures, there is also a risk of underexpansion.
One of the most critical points is to identify, whether with CT images or medical history, the type and size of the surgical prosthesis. Stentless surgical valves are similar to native aortic stenosis in terms of sizing at the virtual basal ring due to the absence of a rigid scaffold, but they are usually implanted in small root anatomies, which increases the risk for coronary occlusion []. In cases of stented prostheses, the direct measurement of the surgical internal diameter is a key component of proper sizing and avoiding mismatch. Also, the THV is anchored at the sewing ring but its expansion is also determined by the degree of calcification or pannus of the leaflets and the surgical posts. The depth of the implant is also relevant because a deeper deployment creates an overlap between the functional part of the surgical and transcatheter valves, thus increasing the risk of underexpansion [].Taking into account the potential obstruction caused by the surgical posts, a low implantation would also be more likely to hinder proper expansion at the functional portion.
To mitigate this risk during ViV, careful pre-planning should be performed, including the implant depth and the possible need for post-dilatation or fracture of the surgical prosthesis []. This should also consider the potential risks involved as higher implantation can cause coronary obstruction or hinder coronary access and post-dilatation/fracture could lead to leaflet damage or increased risk of stroke [].
Figure 4 summarizes the main recommendations to avoid and to manage TAVI underexpansion.
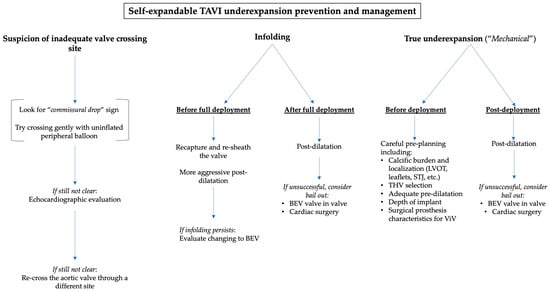
Figure 4.
A practical algorithm to manage TAVI underexpansion depending on the suspected cause.
4. Technical Considerations on Postdilatation
The decision to perform post-dilatation remains primarily based on functional assessments, such as the presence of paravalvular leak (PVL) and transvalvular gradients, which are frequently assessed by echocardiography. On the other hand, the use of angiographic features to guide post-dilatation remains less well-established, and further data are required to define clear criteria based solely on the fluoroscopic appearance of the prosthesis. Interestingly, some authors have proposed methods of evaluating underexpansion on BEVs [] and SEVs [], usually involving direct measurements of the valve frame and the use of different fluoroscopic views.
Proper balloon sizing is therefore critical as undersizing may fail to improve hemodynamics, while oversizing can lead to complications including leaflet damage, annular rupture, stroke or coronary artery occlusion []. To minimize these risks, safe post-dilatation should consider several key anatomical factors, including the presence of calcification at the left coronary cusp and the left ventricle outflow tract (LVOT). The bottom of the left cusp represents a point of less resistance and a higher risk of rupture compared to the right cusp, which is better “anatomically protected” by the presence of a muscular portion of the LVOT and is externally surrounded by the right ventricular outflow tract. Below the non-coronary cusp, there are the membranous septum and conduction bundles, particularly the atrioventricular node and the left bundle branch. Another important element is the size of the sinotubular junction, which is also a weak point and whose damage can lead to intramural hematoma or aortic dissection with the potential involvement of the coronary ostia []. In a recent study, LVOT calcium and STJ oversizing were the two strongest anatomical predictors of annular rupture when using BEV [].
Additionally, a “patient-tailored” approach is required to guide balloon sizing and ensure that the prosthesis is adequately expanded without exceeding the tolerance of the surrounding structures. This involves understanding the relationship between the perimeter and the area of the landing zone: two geometrical figures can have the same perimeter but different area, with the circle being the shape with the largest possible area (Figure 5). By choosing the balloon size based on the “area-derived diameter” instead of “perimeter-derived diameter”, just as is done with BEVs, we avoid the risk of oversizing when circularizing the virtual basal ring with the postdilatation [].
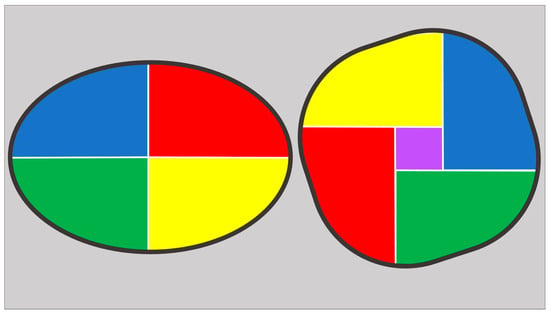
Figure 5.
Ellipse (on the left) and circular figures (on the right) with the same perimeter. Despite having the same perimeter, the circular one has a larger area, as demonstrated by the presence of the purple square inside it. This increase in area when transitioning to a circle (while maintaining the same perimeter) is the effect achieved with the implantation of a BEV or with post-dilatation of an SEV.
5. Conclusions
In conclusion, TAVI underexpansion remains a significant concern, with various mechanisms contributing to its occurrence. Early detection and correction of these issues, coupled with careful procedural planning and execution, are essential for minimizing the associated risks. Future advancements in imaging and device technology may further reduce its incidence and help to improve long-term outcomes.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| TAVI | Transcatheter aortic valve implantation |
| THV | Transcatheter heart valve |
| SEV | Self-expanding transcatheter valve |
| BEV | Balloon-expandable transcatheter valve |
| PVL | Paravalvular leak |
| HU | Hounsfield unit |
| ViV | Valve-in-valve |
| CT | Computed tomography |
| LVOT | Left ventricle outflow tract |
| CT | Computed tomography |
References
- Zou, Q.; Wei, Z.; Sun, S. Complications in transcatheter aortic valve replacement: A comprehensive analysis and management strategies. Curr. Probl. Cardiol. 2024, 49, 102478. [Google Scholar] [CrossRef] [PubMed]
- Kazuno, Y.; Maeno, Y.; Kawamori, H.; Takahashi, N.; Abramowitz, Y.; Babak, H.; Kashif, M.; Chakravarty, T.; Nakamura, M.; Cheng, W.; et al. Comparison SAPIEN 3 SAPIEN XT transcatheter heart valve stent-frame expan-sion: Evaluation using multi-slice computed tomography. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Schultz, C.J.; Piazza, N.; Mollet, N.; Krestin, G. Geometry degree apposition CoreValve ReValving system multislice computed to-mography after implantation patients aortic stenosis. J. Am. Coll. Cardiol. 2009, 54, 911–918. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.; Sun, W. Transcatheter valve underexpansion limits leaflet durability: Im-plications valve-in-valve procedures. Ann. Biomed. Eng. 2017, 45, 394–404. [Google Scholar] [CrossRef]
- Fuchs, A.; De Backer, O.; Brooks, M.; de Knegt, M.; Bieliauskas, G.; Yamamoto, M.; Yanagisawa, R.; Hayashida, K.; Søndergaard, L.; Kofoed, K. Subclinical leaflet thickening and stent frame geometry in self-expanding transcatheter heart valves. EuroIntervention 2017, 13, e1067–e1075. [Google Scholar] [CrossRef]
- Gunning, P.S.; Saikrishnan, N.; Yoganathan, A.P.; McNamara, L.M. Total ellipse of the heart valve: The impact of eccentric stent distortion on the regional dynamic deformation of pericardial tissue leaflets of a transcatheter aortic valve replacement. J. R. Soc. Interface 2015, 12, 20150737. [Google Scholar] [CrossRef]
- Chau, K.C.; Chen, S.; Crowley, A.; Redfors, B.; Li, D.; Hahn, R.H.; Douglas, P.D.; Alu, M.A.; Finn, M.F.; Kodali, S.; et al. Paravalvular regurgitation after transcatheter aortic valve replacement in intermediate-risk patients: A pooled PARTNER 2 study. EuroIntervention 2022, 17, 1053–1060. [Google Scholar] [CrossRef]
- Mack, M.J.; Leon, M.B.; Smith, C.R.; Miller, D.C.; Moses, J.W.; Tuzcu, E.M.; Webb, J.G.; Douglas, P.S.; Anderson, W.N.; Blackstone, E.H.; et al. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): A randomised controlled trial. Lancet 2015, 385, 2477–2484. [Google Scholar] [CrossRef]
- Reardon, M. One-Year Outcomes of Acurate Neo2 vs Approved TAVR Devices in All-Risk Patients with Severe AS: The ACURATE IDE Trial; TCT: Washington, DC, USA, 2024. [Google Scholar]
- Musallam, A.; Rogers, T.; Ben-Dor, I.; Torguson, R.; Khan, J.M.; Satler, L.F.; Waksman, R. Self-expanding transcatheter aortic valve-frame infolding: A case series with a warning message. JACC Cardiovasc. Interv. 2020, 13, 789–790. [Google Scholar] [CrossRef]
- Veulemans, V.; Piuhola, J.; Niemelä, M.; Maier, O.; Piayda, K.; Polzin, A.; Jung, C.; Westenfeld, R.; Kelm, M.; Zeus, T. Incidence Risk Assessment Infolding Using Self-Expandable De-vices TAVR. Struct. Heart 2022, 6, 100008. [Google Scholar] [CrossRef]
- Ben-Dor, I.; Rogers, T.; Satler, L.F.; Waksman, R. A word of caution using self-expanding transcatheter aortic valve-frame infolding. Catheter. Cardiovasc. Interv. 2019, 93, 555–558. [Google Scholar] [CrossRef] [PubMed]
- Abdelghani, M.; Ghalban, A.E.; Landt, M.; Richardt, G. Mohamed Abdel-Wahab, Vivo Stent Frame Infolding Self-Expanding Transcatheter Aortic Valve After Resheathing. JACC Cardiovasc. Interv. 2018, 11, 1204–1206. [Google Scholar] [CrossRef] [PubMed]
- Ancona, M.B.; Romano, V. Self-expanding transcatheter aortic valve infolding: Current evidence, diagnosis, management. Catheter. Cardiovasc. Interv. 2020, 98, E299–E305. [Google Scholar] [CrossRef]
- Pozzi, M.; Rioufol, G.; Delannoy, B. “PAC-MAN” sign: Insidious complication after transcatheter aortic valve deployment. Int. J. Cardiol. 2013, 167, e192–e194. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, F.; Rodés-Cabau, J.; Tirado-Conte, G.; Santos, I.A.; Plachtzik, C.; Cura, F.; Sztejfman, M.; Mangione, F.; Tumeleiro, R.; Esteves, V.; et al. Incidence, predictor, and clinical outcomes of multiple resheathing with self-expanding valves during transcatheter aortic valve replacement. J. Am. Heart Assoc. 2021, 10, e020682. [Google Scholar] [CrossRef]
- Karrowni, W.; Fakih, S.; Nassar, P. Infolding of self-expandable transcatheter heart valve: Case report and review of literature. Cureus. Cureus 2020, 12, e10093. [Google Scholar] [CrossRef]
- Armijo, G.; Castro-Mejía, A.F.; Jimenez-Quevedo, P.; Vera-Urquiza, R.; de Agustín, J.A.; Nombela-Franco, L. Stent frame infolding of a 34-mm evolut-R in a patient with a mechanical mitral valve. JACC Cardiovasc. Interv. 2020, 13, e25–e27. [Google Scholar] [CrossRef]
- Willens, H.J.; Wolpowitz, A.; Nitzberg, W.D.; Kessler, K.M. Transesophageal echocardiography to diagnose and assess repair of aortic cusp perforation caused by directional coronary atherectomy. Am. Heart J. 1995, 130, 612–613. [Google Scholar] [CrossRef]
- Ancona, M.B.; Vella, C.; Beneduce, A.; Moroni, F.; Ancona, F.; Russo, F.; Ferri, L.A.; Bellini, B.; Verzini, A.; Monaco, F.; et al. Impella implantation as a rescue strategy in balloon aortic valvuloplasty complicated by acute aortic regurgitation. J. Cardiovasc. Med. 2022, 23, e1–e2. [Google Scholar] [CrossRef]
- Ghizzoni, G.; Ancona, M.B.; Romano, V.; Bellini, B.; Ferri, L.; Russo, F.; Vella, C.; Gentile, D.; Chionchio, G.; Macelletti, V.; et al. Mind the “commissural drop” when crossing the aortic valve! Catheter. Cardiovasc. Interv. 2023, 102, 538–541. [Google Scholar] [CrossRef]
- Du, Y.; Sorajja, P.; Fukui, M.; Hashimoto, G.; Ahmed, A.; Gössl, M. “Commissural drop” wiring technique facilitates catheter crossing of severely stenotic aortic valve. Chin. Med. J. 2020, 134, 245–246. [Google Scholar] [CrossRef] [PubMed]
- Pio, M.; Bax, S.; Delgado, J. How valvular calcification can affect outcomes transcatheter aortic valve implantation. Expet. Rev. Med. Dev. 2020, 17, 773–784. [Google Scholar]
- Flores-Umanzor, E.; Keshvara, R.; Reza, S.; Asghar, A.; Anwar, M.R.; Cepas-Guillen, P.L.; Osten, M.; Halankar, J.; Abrahamyan, L.; Horlick, E. A systematic review of contrast-enhanced computed tomography calcium scoring methodologies and impact of aortic valve calcium burden on TAVI clinical outcomes. J. Cardiovasc. Comput. Tomogr. 2023, 17, 373–383. [Google Scholar] [CrossRef]
- Blanke, P.; Weir-McCall, J.R.; Achenbach, S.; Delgado, V.; Hausleiter, J.; Jilaihawi, H.; Marwan, M.; Nørgaard, B.L.; Piazza, N.; Schoenhagen, P.; et al. Computed Tomography Imaging in theContext of Transcatheter Aortic ValveImplantation (TAVI)/TranscatheterAortic Valve Replacement (TAVR). JACC Cardiovasc. Imaging 2019, 12, 1–24. [Google Scholar] [CrossRef]
- Fukui, M.; Cavalcante, J.L.; Bapat, V.N. Deformation in transcatheter heart valves: Clinical implications and considerations. J. Cardiol. 2024, 83, 351–358. [Google Scholar] [CrossRef]
- Allen, K.B.; Chhatriwalla, A.K.; Cohen, D.J.; Saxon, J.T.; Aggarwal, S.; Hart, A.; Baron, S.; Davis, J.R.; Pak, A.F.; Dvir, D.; et al. Bioprosthetic valve fracture to facilitate transcatheter valve-in-valve implantation. Ann. Thorac. Surg. 2017, 104, 1501–1508. [Google Scholar] [CrossRef] [PubMed]
- Beaupré, F.; Garot, P. 3 ways to mitigate the risk of transcatheter heart valve underexpansion and dysfunction in valve-in-valve TAVR. JACC Cardiovasc. Interv. 2023, 16, 527–529. [Google Scholar] [CrossRef]
- Husain, A.; Tzimas, G.; Akodad, M.; Jelisejevas, J.; Offen, S.; Millar, K.; Leipsic, J.A.; Blanke, P.; Wood, D.A.; Sellers, S.L.; et al. Novel real-time fluoroscopic assessment method of transcatheter heart valve expansion following balloon-expandable TAVR. Circ. Cardiovasc. Interv. 2025, 18, e014617. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, U.; Hachinohe, D.; Kobayashi, K.; Shitan, H.; Horita, R.; Ootake, R.; Fujita, T. Steep right anterior oblique view of self-expandable transcatheter aortic valve to timely detect stent under-expansion or non-uniform expansion before final release: A case series. Eur. Heart J.-Case Rep. 2024, 8, ytae405. [Google Scholar] [CrossRef]
- Tarantini, G.; Basso, C.; Fovino, L.N.; Fraccaro, C.; Thiene, G.; Rizzo, S. Left ventricular outflow tract rupture during transcatheter aortic valve implantation: Anatomic evidence of the vulnerable area. Cardiovasc. Pathol. 2017, 29, 7–10. [Google Scholar] [CrossRef]
- Kim, W.-K.; Charitos, E.; Renker, M.; Hamm, C.; Seidler, T.; Hamm, C.W.; Choi, Y.-H.; Sossalla, S. Contemporary risk assessment of aortic root injury for new-generation balloon-expandable transcatheter heart valves. Can. J. Cardiol. 2024, 40, 1266–1269. [Google Scholar] [CrossRef] [PubMed]
- Ancona, M.B.; Cianfanelli, L.; Romano, V.; Montorfano, M. Rottura dell’anello aortico durante impianto transcatetere di valvola aortica: Prevenzione e gestione della complicanza. G. Ital. Cardiol. 2020, 21, 13–16. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).