Abstract
Pulmonary hypertension (PH) refers to a pathologic elevation of the mean pulmonary artery pressure (mPAP) and is associated with increased morbidity and mortality in a wide range of medical conditions. These conditions are classified according to similarities in pathophysiology and management in addition to their invasive hemodynamic profiles. The 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension present the newest clinical classification system and includes significant updates to the hemodynamic definitions. Pulmonary hypertension is now hemodynamically defined as an mPAP > 20 mmHg, reduced from the previous threshold of ≥25 mmHg, due to important insights from both normative and prognostic data. Pulmonary vascular resistance has been extended into the definition of pre-capillary pulmonary hypertension, with an updated threshold of >2 Wood Units (WU), to help differentiate pulmonary vascular disease from other causes of increased mPAP. Exercise pulmonary hypertension has been reintroduced into the hemodynamic definitions and is defined by an mPAP/cardiac output slope of >3 mmHg/L/min between rest and exercise. While these new hemodynamic thresholds will have a significant impact on the diagnosis of pulmonary hypertension, no evidence-based treatments are available for patients with mPAP between 21–24 mmHg and/or PVR between 2–3 WU or with exercise PH. This review highlights the evidence underlying these major changes and their implications on the diagnosis and management of patients with pulmonary hypertension.
1. Introduction
Pulmonary hypertension (PH) refers to a group of diseases characterized by a pathologic elevation in the mean pulmonary artery pressure (mPAP). The physiology causing the elevated pulmonary pressures varies significantly based on the underlying etiology including intrinsic pulmonary vasculopathy, changes involving the left heart or pulmonary parenchyma, and chronic thromboembolic disease, among others. Given the large number of potential causes, these diseases are classified using the WHO Clinical Classification which divides the diseases based on their similar underlying mechanisms, presentations, hemodynamics, as well as treatment strategies (Table 1) [1]. Since the publication of the first clinical classification of PH, several advances have been made in understanding the pathophysiologic mechanisms underpinning certain forms of PH. To obtain a diagnosis of PH, regardless of etiology, direct hemodynamic confirmation by right heart catheterization is required. The diagnosis of PH is established based on mPAP but additional hemodynamic findings, including the pulmonary vascular resistance (PVR) and pulmonary arterial wedge pressure (PAWP), are used to further classify the diagnosis (Table 2). Over the past 50 years, as our understanding of the pathologies underlying PH has evolved, the thresholds for these hemodynamic categories have changed, as reflected in the published international guidelines for the diagnosis and management of PH [1,2,3,4,5,6,7,8,9,10] (Figure 1). As the hemodynamic criteria serve as the foundation to the diagnosis of PH, these updates can drastically impact both the diagnosis and the subsequent interventions which patients are offered [1]. This review focuses primarily on the updated hemodynamic definitions in the recent 2022 ESC/ERS Guidelines for the Diagnosis and Treatment of Pulmonary Hypertension and their clinical implications.

Table 1.
Current clinical classification of pulmonary hypertension, adapted from [1].

Table 2.
Current hemodynamic definitions of pulmonary hypertension, adapted from [1].
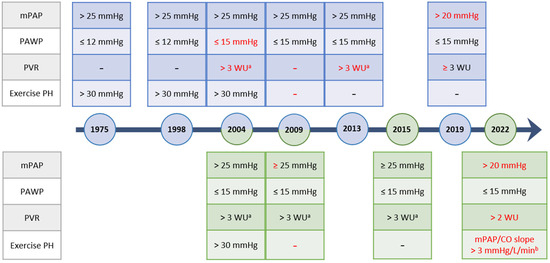
Figure 1.
Evolution of the World Symposium (Blue) and ESC/ERS (Green) Hemodynamic Definitions Over Time. Dates are listed based on the year of publication. Updates are highlighted in red. mPAP, mean pulmonary artery pressure; PAWP, pulmonary artery wedge pressure; PVR, pulmonary vascular resistance; PH, pulmonary hypertension; WU, Wood Units. a: applies only to Group 1 PAH, b: mean pulmonary artery pressure to cardiac output slope (mPAP/CO slope) measured between rest and exercise.
2. Clinical Classification of Pulmonary Hypertension
The updated clinical classification of PH is based around five categories of diseases organized around similar pathophysiology, hemodynamics, and/or therapeutic management strategies (Table 1). Group 1 pulmonary arterial hypertension (PAH) represents a heterogenous group of conditions, all characterized by progressive pathologic remodeling of the small-calibre pulmonary arteries leading to progressive right ventricular dysfunction and death. The group encompasses idiopathic PAH, heritable PAH, drug and toxin associated PAH, and PAH associated with systemic diseases. Group 2 PH includes diseases of the left heart, such as heart failure with reduced ejection fraction, heart failure with preserved ejection fraction and valvular heart disease. Group 3 PH includes PH due to lung diseases and/or hypoxia. Group 4 pulmonary hypertension is associated with pulmonary artery obstruction such as chronic thromboembolic pulmonary hypertension. Group 5 pulmonary hypertension is due to conditions leading to elevated pressures for unclear and/or multifactorial mechanisms. These groups were retained from prior iterations of the clinical classification, but three main updates and some minor changes were included.
Firstly, idiopathic PAH (within Group 1) was divided based on response to acute vasoreactivity testing results at right heart catheterization. During right heart catheterization, patients are exposed to an agent, typically inhaled nitric oxide, to assess for acute changes in pulmonary hemodynamics. The criteria for a positive response remain unchanged from previous iterations, and is defined as a reduction in the mPAP by ≥10 mmHg to an absolute value of ≤40 mmHg with an unchanged or increased cardiac output [11]. A positive response identifies patients, termed “acute responders”, who may benefit from high dose calcium channel blocker therapy and predicts a favourable long-term outcome [12]. However, while approximately 12% of patients are found to have an acute response at vasoreactivity testing, only around 7% have a persistent clinical and hemodynamic response after at least one year on high dose calcium channel blocker therapy [11]. These longer-term responders are the ones with the more favourable outcome and should be included within this classification. While response to vasoreactivity testing has been included as a subcategory of idiopathic PAH, patients with heritable and drug- or toxin-associated PH may also be acute responders and may benefit from calcium channel blocker therapy, thus testing is also indicated in those subgroups.
The second change is the recategorization of PAH with features of venous/capillary (pulmonary veno-occlusive disease/pulmonary capillary haemangiomatosis (PVOD/PCH) involvement) and persistent PH of the newborn into group 1 PAH. In the prior guidelines, they were provided with their own special subcategorization of 1′ and 1″, respectively.
The third main change is the terminology change for group 3.4 from sleep disordered breathing to the new term hypoventilation syndrome. This change comes from the accumulation of data showing that PH with obstructive sleep apnea is rare when not associated with conditions leading to hypoxemia [13].
Some other minor changes include the movement of PH associated with lymphangioleiomyomatosis (LAM) into group 3 PH associated with chronic lung disease. Recent studies revealed that PH in patients with LAM was correlated to the degree of pulmonary function impairment and hypoxemia, making it more appropriately classified into group 3 PH. Additionally, splenectomy and thyroid disorders have been removed from the classification schema as these conditions are not felt to cause PH, but are rather associated comorbidities. These minor changes are in line with the proceedings of the 6th World Symposium of Pulmonary Hypertension (WSPH) [14].
Overall, while the importance of the clinical classification in defining further management cannot be understated, the changes included in this 2022 ESC/ERS classification update are unlikely to significantly impact clinical practice in contrast to the changes in the hemodynamic definitions.
3. Hemodynamic Definition of Pulmonary Hypertension
PH has been defined as mPAP of ≥25 mmHg since the proceedings of the first WSPH in 1975 [2]. However, the mPAP alone is insufficient for adequate discrimination between patients with pulmonary vascular disease from causes such as an increased cardiac output (CO) or increased left ventricular filling pressures [2,9]. As a result, subsequent hemodynamic definitions included thresholds for the PAWP and, more recently, the PVR to help distinguish different causes of the increased mPAP. In the 2015 ESC/ERS Guidelines, pre-capillary PH was defined as also requiring a PAWP of ≤15 mmHg to distinguish it from PH due to left heart disease. While a PVR threshold was not included in the diagnosis of pre-capillary PH, the diagnosis of Group 1 PAH required a threshold of ≥3 Wood units (WU) in the absence of other causes of PH, such as severe parenchymal lung disease [9]. In the 2022 update, major updates from the 2015 iteration were made to the hemodynamic definition reducing the thresholds of PH to an mPAP > 20 mmHg and the PVR threshold to >2 WU to define pre-capillary PH, while the PAWP cut-off of ≤15 mmHg was maintained to distinguish pre-capillary from post-capillary PH. Additionally, exercise induced PH was reintroduced into the hemodynamic classification. Other hemodynamic variables that have been shown to provide important prognostic data, such as pulmonary artery compliance, were not included in the updated definitions [15].
3.1. Mean Pulmonary Artery Pressure
The mPAP threshold was established relatively arbitrarily during the first WSPH as ≥25 mmHg, despite evidence at that time that the normal resting mPAP typically does not exceed 15 mmHg at rest with little impact by age [2]. Over the subsequent decades, this arbitrary choice continued to be controversial but remained essentially unchanged due to the absence of normative data and ethical concerns regarding invasive testing without a clinical indication prevented the collection of such data [16].
The first major breakthrough was a large meta-analysis which reviewed the hemodynamic data of 1187 healthy individuals [17]. In this study, the mPAP in this cohort was found to be 14.0 ± 3.3 mmHg with the upper limit of normal defined as 2 standard deviations above the mean or 20.6 mmHg. This threshold for the upper limit of normal was now established by a scientific approach and was not arbitrarily set in contrast to the original definition. Despite this knowledge, the 2015 ESC/ERS guidelines retained the definition of PH as ≥25 mmHg as the clinical significance of “borderline” elevations in mPAP (20–25 mmHg) had not been adequately studied [9,18]. Appropriately, an update to the hemodynamic definition of PH required data suggesting prognostic implications of the decision [14].
However, data suggesting poorer outcomes in patients with lower levels of mPAP had started to accumulate within a wide range of diseases. This included findings of increased mortality in patients with idiopathic pulmonary fibrosis (IPF), chronic obstructive pulmonary disease (COPD), sickle cell disease, systemic sclerosis, and in a relatively unselected population with concern for PH [19,20,21,22,23]. Further confirmation was provided by two large retrospective database reviews totalling more than 25,000 patients, many whom had left heart disease, revealing an increased hazard ratio for death with mPAP between 19–24 mmHg [24,25]. Further confirmation was provided by pathological data revealing adverse remodeling occurring in patients who had milder elevations in mPAP with systemic sclerosis [26].
This accumulation of normative physiologic data coupled with prognostic data from various conditions led to the adoption of a reduced diagnostic threshold of the mPAP for PH at the 6th WSPH and more recently in the newest ESC/ERS guidelines [1,14].
3.2. Pulmonary Arterial Wedge Pressure
By wedging the balloon on the tip of the Swan-Ganz catheter in a mid-sized pulmonary artery a “stop flow” phenomenon is created in the occluded pulmonary artery and the downstream pulmonary capillary bed and pulmonary veins. In the absence of flow, pressure equilibrates across the pulmonary capillary bed so that the pressure in a same-sized pulmonary vein can be estimated by the so called PAWP. A PAWP ≤ 15 mmHg is recommended to distinguish pre-capillary from post-capillary PH [1]. However, it should be noted that a PAWP value of 12 mmHg is generally regarded as the upper limit of normal in healthy individuals [2,27,28]. Recent data have suggested that a PAWP threshold of 12 mmHg provides the highest sensitivity and specificity for distinguishing between pre-capillary and post-capillary PH [29].
However, available data on the best PAWP threshold are contradictory and a higher threshold is recommended for the invasive diagnosis of heart failure by the ESC Heart Failure Association [1,30]. Additionally, almost all randomized controlled trials (RCT) in PAH have utilized an PAWP ≤ 15 mmHg as an inclusion criterion [1]. Consequently, the current ESC/ERS guidelines recommend a PAWP threshold ≤ 15 mmHg is recommended by for the differentiation between pre-capillary and post-capillary PH, while acknowledging the presence of a grey area between 13 and 15 mmHg [1]. This highlights the crucial role of accurately phenotyping patients during the diagnostic evaluation.
3.3. Pulmonary Vascular Resistance
PVR has been variably included in the diagnosis of PH over the last several iterations of international guidelines for the management PH [31]. The PVR criterion was first introduced in the proceedings of the 3rd World Symposium in 2003 with a threshold of greater than 3 WU applied only to the definition of PAH but with little discussion on the rationale for inclusion nor the source of this threshold [4]. However, this definition was excluded in the first two iterations of the ESC/ERS guidelines in 2004 and 2009 but was subsequently introduced into the ESC/ERS guidelines in 2015, similarly only applying to the diagnosis of PAH [9,18]. While not specifically addressed in the 2015 ESC/ERS document, the prior World Symposium proceedings outline the rationale for choosing 3 WU instead of 2 WU as patients with a PVR of less than 3 WU are unlikely to have PAH [18].
Similar to the discussion above on changes to the mPAP, the first observations to challenge the prevailing definition were the publication of normative data on PVR among healthy individuals [32]. This systematic review identified that the upper limit of normal for PVR was 2 WU over a large range of ages. However, as the PVR threshold of ≥3 WU was deemed clinically relevant due to its use in other clinical scenarios, such as congenital heart disease and heart transplantation, it was not adopted during the 6th World Symposium [9,10].
Prognostic data were subsequently released to further justify re-examination of the PVR threshold. In a population of systemic sclerosis patients with mildly elevated mPAP (21–24 mmHg), a PVR of ≥2 WU was associated with physiological limitations with reduced walk distances and pulmonary arterial compliance as well as reduced long-term survival [33]. This was shortly followed by a large retrospective review of two large databases where increased all-cause mortality hazard for PVR increased progressively starting around 2 WU, with a clinical significant mortality HR identified at 2.2 WU [34]. This finding was independent of the PAWP, with increased mortality identified in patients with both pre- and post-capillary PH. Pathologic data again confirmed the findings of adverse vascular remodeling in patients with lower PVR beginning approximately at 1.8–2 WU [35].
This evolution in our understanding was thus accepted in the new ESC/ERS guidelines establishing the upper limit of normal and the lowest prognostically relevant PVR threshold of 2 WU within the new definition of pre-capillary PH [1].
3.4. Exercise PH
Similar to PVR, exercise PH has been intermittently included in published guidelines since the proceedings of the first world symposium in 1975 [2]. In that document, the authors conclude that the mPAP does not normally exceed 30 mmHg during exercise, but acknowledged that in athletes with high cardiac outputs, pressures have been demonstrated to exceed this value. Despite this, PH on exercise continued to be defined as a mPAP greater than 30 mmHg until the publication of a systematic review of normative data revealed that both age and the level of exercise significantly impacted the mPAP on exercise leading to readings greater than 30 mmHg in otherwise healthy patients [17]. As a result, the ESC/ERS guidelines acknowledged that a definition for PH on exercise, as assessed by RHC, was not supported by the data leading to its removal in 2009 [7].
The definition of exercise PH continued to evolve with a focus on alternative hemodynamic parameters, as it was clear that a pressure threshold alone was not a suitable [36]. The next major development was the publication of a systematic review discussing the flow-dependant changes in exercise hemodynamics [32]. By reviewing 250 patients with exercise hemodynamics, the authors were able to identify linear relationship between the mPAP and CO. Two important observations were that the mPAP/CO slope was positive and that there was a significant increase with age [37]. Specifically, the mean values were 0.8 ± 0.4 mmHg/L/min in patients around age 30, 1.6 ± 0.2 mmHg/L/min around age 50, and 2.4 ± 0.5 mmHg/L/min around age 70 with upper limits of normal of 1.6, 2.1, and 3.3 mmHg/L/min respectively. These data along with other observations established the upper limit of normal for the mPAP/CO relationship around 3 mmHg/L/min [38].
The mPAP/CO slope was subsequently investigated for prognostic relevance similarly to the mPAP and PVR thresholds [39,40,41,42]. The largest study was performed in a group of patients evaluated for unexplained dyspnea [41]. The authors found that an mPAP/CO threshold of 3 mmHg/L/min for exercise PH was associated with a worse cardiovascular (CV) event-free survival regardless of whether there was resting PH. Further, both pre- and post-capillary contributions to the abnormal mPAP/CO slope were independently associated with increased hazard of CV hospitalization or death. In systemic sclerosis patients without manifest PH, exercise PH is a known predictor of disease progression and poor outcomes but further investigation found that an mPAP/CO slope > 3.5 mmHg/L/min identifies those with increased mortality at 10 years despite normal resting hemodynamics [42,43].
Based on these emerging normative and prognostic data, exercise PH was re-introduced into the hemodynamic definitions of PH in the 2022 ESC/ERS updated guidelines [1]. However, while included in the hemodynamic definitions, exercise hemodynamic testing is a technically challenging procedure that may not be available to all PH centres [36].
4. Management Strategy
The overall management strategy for patients with pulmonary hypertension remains unchanged compared to the prior guidelines. The guidelines outline general measures, baseline and follow-up risk stratification, and PAH specific therapy use in patients diagnosed with PH. While this article is not focused on the updated treatment paradigm in PAH, one area that warrants discussion are cardiopulmonary comorbidities and their impact on treatment selection.
Over the last few decades, there has been a shift in the demographic of patients diagnosed with idiopathic PAH, specifically patients are being diagnosed older with more comorbidities [44]. Further analysis revealed that, in addition to the classic PAH phenotype (generally young, mostly female patients without comorbidities), two other phenotypes have emerged [45]. One of the phenotypes include elderly, mostly female patients who have risk factors for left heart disease however hemodynamically meet criteria for pre-capillary pulmonary hypertension. The other phenotype is composed primarily of elderly males with comorbidities, a history of smoking, and a low diffusing capacity for carbon monoxide (DLCO). These two phenotypes encompass the majority of patients in registries with idiopathic PAH and have similar recommendations for initial therapy and are referred to in the guidelines as patients with cardiopulmonary comorbidities.
The guidelines differentiate initial treatment selection for those patients without comorbidities (i.e., the classical phenotype) and those with comorbidities [1]. Patients without comorbidities have their initial treatment selection based on risk stratification. Those patients at low- or intermediate-risk are recommended to start with dual upfront combination therapy compared to those at high-risk starting treatment with triple upfront combination therapy including a parenteral prostanoid. Patients who remain intermediate- or high-risk at follow-up are recommended for escalation of therapy.
In contrast, treatment recommendations for patients with cardiopulmonary comorbidities are less clear given the lack of published data as these patients have typically been excluded in modern landmark studies [46]. Data suggest that these patients tend to experience more side effects from PAH therapies leading to discontinuation and have less clinical improvement compared to the classical phenotype [47]. Patients with cardiopulmonary comorbidities are recommended to start treatment with initial monotherapy, regardless of baseline risk stratification. At follow-up, escalation of therapy may be considered on an individualized basis.
5. Clinical Implications of the Updated Hemodynamic Definitions
As the hemodynamic definition of PH is the foundation for the diagnosis and subsequent management of patients, changing these definitions has wide reaching implications for both patients and clinicians. A particular tension arises as, currently, no guideline recommended medical therapies exist for patients who meet the new criteria for resting (between mPAP 21–24 mmHg and/or PVR 2–3 WU) or exercise PH [1].
In the previous era of the 2015 ESC/ERS guidelines, patients who met the hemodynamic definition were given a diagnosis of PH. Along with the label of PH, there were serious implications to the patient impacting future quality and quantity of life which were, to some extent, mitigated by the provision of interventions. These included recommendations for general measures such as physical activity, vaccination, psychosocial supports, and management of contributory risk factors in conjunction with the initiation of PAH-specific therapies to directly treat the vasculopathy for those with Group 1 PAH [9].
With the recent update in 2022, there are now a significant number of patients who will receive a life altering diagnosis of PH, resting or with exercise, who would not previously have met criteria [48]. All these patients will benefit from access to multidisciplinary care teams available at PH centres, including specialized nursing, social workers, dieticians, pharmacists, and other allied healthcare workers who serve a critical role in the holistic management of these complex patients. However, the question of PAH therapies in this population has yet to be answered [1]. While early data appear promising, treatment with PAH therapies is reserved only for those patients who were included in prior randomized control trials for these therapeutics, namely those who met the prior hemodynamic definition of an mPAP ≥ 25 mmHg, PAWP of ≤15 mmHg, and PVR of >3 WU [1,49,50,51].
Over the last several years, convincing arguments have been raised against lowering the hemodynamic definitions for PH. The main objection is the risk of overdiagnosis and overtreatment leading to exposure to potentially harmful therapeutics [52]. As the upper limit of normal is defined by two standard deviations, approximately 2.5% of patients will have hemodynamics above these thresholds but be free from disease [31]. This highlights the pivotal role of the PH centre and PH practitioner in identifying patients appropriate for potent, and costly, medical therapies. Even more emphasis will need to be placed on adequate exclusion of alternative causes of PH and ensuring that hemodynamic assessments are performed correctly given the risk of misclassification if performed inadequately [53]. Previously, there existed a gray area between normal hemodynamics and a diagnosis of PAH, specifically those with an mPAP 21–24 mmHg and/or PVR 2–3 WU, which was proposed to help limit overdiagnosis and overtreatment. Now patients with, for instance, Group 2 PH who are misclassified as PAH, perhaps if provocative testing is not performed in a patient with a borderline PAWP, may be at risk of worsening clinical outcomes following exposures to PAH therapies [54]. Indeed, retrospective data already reveals a concerning trend of prescribing PAH therapies off-label and the expected increase in the population of patients diagnosed with PH, particularly with milder disease, may further worsen this trend [55,56]. Special attention is required to carefully evaluate patients at these margins, and they are likely to benefit greatly from specialized evaluation at expert centres, especially given the higher prevalence of cardiorespiratory comorbidities in this group of patients [48]. However, despite increasing awareness of PH among practitioners and the increased availability of screening modalities, namely echocardiography, over the last several decades, patients with a new diagnosis of PAH are still presenting late with hemodynamic abnormalities greatly exceeding these new thresholds [57]. A greater impact of these changes may be felt by patients who are part of screening cohorts, such as those with systemic sclerosis, who are diagnosed earlier with less severe hemodynamic findings [1,58].
Patients meeting the new hemodynamic criteria, but not thresholds for treatment, should be followed regularly given the risk of progression to manifest PH warranting therapy, particularly in groups at high risk of developing PAH [33,42]. The question remains how best to monitor patients for progression as even in systemic sclerosis patients, who are at high risk of developing PAH, the conversion from an mPAP 21–24 mmHg to ≥25 mmHg at 3 years of follow-up occurred in around 25% of patients with only the minority (7%) being diagnosed with PAH [59].
There is a paucity of research in this hemodynamic group of patients and specific attention should be made by the PH community to enrol this group of patients into PH registries and, when available, interventional clinical trials to establish the role of PAH therapies in patients with milder hemodynamic abnormalities.
6. Conclusions
The classification and hemodynamic definitions of PH have evolved greatly since the first WHO meeting on chronic cor pulmonale held in Geneva in 1960, in what was only then emerging as a new field of medicine. The 2022 ESC/ERS guidelines for the diagnosis and management of PH outlines the newest and most evidence based hemodynamic definitions for PH that significantly impacts clinical practice. While now supported by firm normative and prognostic data, these changes raise many new questions and identifies exciting new directions for inquiry. Over the years, the hard work and dedication of generations of clinicians and researchers has led, and will continue to lead, to significant advances in our shared understanding of this collection of diseases and improve the lives of patients around the world.
Author Contributions
Writing-original draft preparation, M.K., C.G. and D.M.; writing-review and editing, M.J. and M.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
M.K. declares honorarium from AstraZeneca and advisory board compensation from Merck. D.M. declares grants from Acceleron, Janssen, Merck MSD; consulting fees from Acceleron, Merck MSD, Janssen, Ferrer; honoraria from Bayer, Janssen, Boerhinger, Chiesi, GSK, Ferrer, Merck MSD. M.H. declares grants from Acceleron, AOP Orphan, Janssen, Merck, Shou Ti; consulting fees from 35 Pharma, Aerovate, AOP Orphan, Bayer, Chiesi, Ferrer, Janssen, Keros, Merck, MorphogenIX, Shou Ti, United Therapeutics; honoraria from Janssen, Merck; monitoring or advisory board compensation from Acceleron, Altavant, Janssen, Merck, United Therapeutics. CG has received compensation for scientific symposia from AstraZeneca, AOPHealth, Cordis, Janssen and MSD, speaker fees from AOPHealth, AstraZeneca, Janssen and Ferrer, and an educational grant from OrphaCare. M.J. declares no conflicts of interest.
References
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.; Brida, M.; Carlsen, J.; Coats, A.J.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Respir. J. 2023, 61, 2200879. [Google Scholar] [CrossRef] [PubMed]
- Hatano, S.; Strasser, T.; World Health Organization. Primary Pulmonary Hypertension: Report on a WHO Meeting, Geneva, 15–17 October 1973/Edited by Shuichi Hatano and Toma Strasser; World Health Organization: Geneva, Switzerland, 1975. [Google Scholar]
- Rich, S. Primary Pulmonary Hypertension: Exective Summary from the World Symposium. Primary Pulmonary Hypertension; World Health Organization: Geneva, Switzerland, 1998. [Google Scholar]
- Galiè, N.; Rubin, L.J. Introduction: New insights into a challenging disease: A review of the third world symposium on pulmonary arterial hypertension. J. Am. Coll. Cardiol. 2004, 43 (Suppl. S12), 1s–90s. [Google Scholar] [CrossRef]
- Galie, N.; Torbicki, A.; Barst, R.; Dartevelle, P.; Haworth, S.; Higenbottam, T.; Olschewski, H.; Peacock, A.; Pietra, G.; Rubin, L.J.; et al. Guidelines on diagnosis and treatment of pulmonary arterial hypertension. The Task Force on Diagnosis and Treatment of Pulmonary Arterial Hypertension of the European Society of Cardiology. Eur. Heart J. 2004, 25, 2243–2278. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Humbert, M.; McLaughlin, V.V. The 4th World Symposium on Pulmonary Hypertension. J. Am. Coll. Cardiol. 2009, 54 (Suppl. S1), S1–S2. [Google Scholar] [CrossRef]
- Galie, N.; Hoeper, M.M.; Humbert, M.; Torbicki, A.; Vachiery, J.L.; Barbera, J.A.; Beghetti, M.; Corris, P.; Gaine, S.; Gibbs, J.S.; et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: The Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur. Heart J. 2009, 30, 2493–2537. [Google Scholar] [CrossRef]
- Galiè, N.; Simonneau, G. The Fifth World Symposium on Pulmonary Hypertension. J. Am. Coll. Cardiol. 2013, 62 (Suppl. S25), D1–D3. [Google Scholar] [CrossRef]
- Galiè, N.; Humbert, M.; Vachiery, J.-L.; Gibbs, S.; Lang, I.; Torbicki, A.; Simonneau, G.; Peacock, A.; Vonk Noordegraaf, A.; Beghetti, M.; et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Heart J. 2016, 37, 67–119. [Google Scholar] [CrossRef]
- Galie, N.; McLaughlin, V.V.; Rubin, L.J.; Simonneau, G. An overview of the 6th World Symposium on Pulmonary Hypertension. Eur. Respir. J. 2019, 53, 1802148. [Google Scholar] [CrossRef] [PubMed]
- Sitbon, O.; Humbert, M.; Jaïs, X.; Ioos, V.; Hamid, A.M.; Provencher, S.; Garcia, G.; Parent, F.; Hervé, P.; Simonneau, G. Long-term response to calcium channel blockers in idiopathic pulmonary arterial hypertension. Circulation 2005, 111, 3105–3111. [Google Scholar] [CrossRef]
- Rich, S.; Kaufmann, E.; Levy, P.S. The effect of high doses of calcium-channel blockers on survival in primary pulmonary hypertension. N. Engl. J. Med. 1992, 327, 76–81. [Google Scholar] [CrossRef]
- Thurnheer, R.; Ulrich, S.; Bloch, K.E. Precapillary Pulmonary Hypertension and Sleep-Disordered Breathing: Is There a Link? Respiration 2017, 93, 65–77. [Google Scholar] [CrossRef]
- Simonneau, G.; Montani, D.; Celermajer, D.S.; Denton, C.P.; Gatzoulis, M.A.; Krowka, M.; Williams, P.G.; Souza, R. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur. Respir. J. 2019, 53, 1801913. [Google Scholar] [CrossRef]
- Weatherald, J.; Zanini, U.; Humbert, M. Illuminating the Importance of Pulmonary Arterial Compliance in Pulmonary Hypertension. Am. J. Respir. Crit. Care Med. 2023, 208, 231–233. [Google Scholar] [CrossRef]
- Maron, B.A.; Brittain, E.L.; Choudhary, G.; Gladwin, M.T. Redefining pulmonary hypertension. Lancet Respir. Med. 2018, 6, 168–170. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, G.; Berghold, A.; Scheidl, S.; Olschewski, H. Pulmonary arterial pressure during rest and exercise in healthy subjects: A systematic review. Eur. Respir. J. 2009, 34, 888–894. [Google Scholar] [CrossRef] [PubMed]
- Hoeper, M.M.; Bogaard, H.J.; Condliffe, R.; Frantz, R.; Khanna, D.; Kurzyna, M.; Langleben, D.; Manes, A.; Satoh, T.; Torres, F.; et al. Definitions and diagnosis of pulmonary hypertension. J. Am. Coll. Cardiol. 2013, 62 (Suppl. S25), D42–D50. [Google Scholar] [CrossRef] [PubMed]
- Hamada, K.; Nagai, S.; Tanaka, S.; Handa, T.; Shigematsu, M.; Nagao, T.; Mishima, M.; Kitaichi, M.; Izumi, T. Significance of pulmonary arterial pressure and diffusion capacity of the lung as prognosticator in patients with idiopathic pulmonary fibrosis. Chest 2007, 131, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Weitzenblum, E.; Hirth, C.; Ducolone, A.; Mirhom, R.; Rasaholinjanahary, J.; Ehrhart, M. Prognostic value of pulmonary artery pressure in chronic obstructive pulmonary disease. Thorax 1981, 36, 752–758. [Google Scholar] [CrossRef]
- Gladwin, M.T.; Sachdev, V.; Jison, M.L.; Shizukuda, Y.; Plehn, J.F.; Minter, K.; Brown, B.; Coles, W.A.; Nichols, J.S.; Ernst, I.; et al. Pulmonary hypertension as a risk factor for death in patients with sickle cell disease. N. Engl. J. Med. 2004, 350, 886–895. [Google Scholar] [CrossRef]
- Bae, S.; Saggar, R.; Bolster, M.B.; Chung, L.; Csuka, M.E.; Derk, C.; Domsic, R.; Fischer, A.; Frech, T.; Goldberg, A.; et al. Baseline characteristics and follow-up in patients with normal haemodynamics versus borderline mean pulmonary arterial pressure in systemic sclerosis: Results from the PHAROS registry. Ann. Rheum. Dis. 2012, 71, 1335–1342. [Google Scholar] [CrossRef]
- Douschan, P.; Kovacs, G.; Avian, A.; Foris, V.; Gruber, F.; Olschewski, A.; Olschewski, H. Mild Elevation of Pulmonary Arterial Pressure as a Predictor of Mortality. Am. J. Respir. Crit. Care Med. 2018, 197, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Maron, B.A.; Hess, E.; Maddox, T.M.; Opotowsky, A.R.; Tedford, R.J.; Lahm, T.; Joynt, K.E.; Kass, D.J.; Stephens, T.; Stanislawski, M.A.; et al. Association of Borderline Pulmonary Hypertension with Mortality and Hospitalization in a Large Patient Cohort: Insights from the Veterans Affairs Clinical Assessment, Reporting, and Tracking Program. Circulation 2016, 133, 1240–1248. [Google Scholar] [CrossRef] [PubMed]
- Assad, T.R.; Maron, B.A.; Robbins, I.M.; Xu, M.; Huang, S.; Harrell, F.E.; Farber-Eger, E.H.; Wells, Q.S.; Choudhary, G.; Hemnes, A.R.; et al. Prognostic Effect and Longitudinal Hemodynamic Assessment of Borderline Pulmonary Hypertension. JAMA Cardiol. 2017, 2, 1361–1368. [Google Scholar] [CrossRef]
- Hsu, S.; Kokkonen-Simon, K.M.; Kirk, J.A.; Kolb, T.M.; Damico, R.L.; Mathai, S.C.; Mukherjee, M.; Shah, A.A.; Wigley, F.M.; Margulies, K.B.; et al. Right Ventricular Myofilament Functional Differences in Humans with Systemic Sclerosis-Associated Versus Idiopathic Pulmonary Arterial Hypertension. Circulation 2018, 137, 2360–2370. [Google Scholar] [CrossRef] [PubMed]
- Hellems, H.K.; Haynes, F.W.; Dexter, L. Pulmonary capillary pressure in man. J. Appl. Physiol. 1949, 2, 24–29. [Google Scholar] [CrossRef]
- Paulus, W.J.; Tschope, C.; Sanderson, J.E.; Rusconi, C.; Flachskampf, F.A.; Rademakers, F.E.; Marino, P.; Smiseth, O.A.; De Keulenaer, G.; Leite-Moreira, A.F.; et al. How to diagnose diastolic heart failure: A consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur. Heart J. 2007, 28, 2539–2550. [Google Scholar] [CrossRef]
- Gerges, C.; Gerges, M.; Skoro-Sajer, N.; Zhou, Y.; Zhang, L.; Sadushi-Kolici, R.; Jakowitsch, J.; Lang, M.B.; Lang, I.M. Hemodynamic Thresholds for Precapillary Pulmonary Hypertension. Chest 2016, 149, 1061–1073. [Google Scholar] [CrossRef]
- Pieske, B.; Tschope, C.; de Boer, R.A.; Fraser, A.G.; Anker, S.D.; Donal, E.; Edelmann, F.; Fu, M.; Guazzi, M.; Lam, C.S.P.; et al. How to diagnose heart failure with preserved ejection fraction: The HFA-PEFF diagnostic algorithm: A consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur. Heart J. 2019, 40, 3297–3317. [Google Scholar] [CrossRef]
- Brusca, S.B.; Zou, Y.; Elinoff, J.M. How low should we go? Potential benefits and ramifications of the pulmonary hypertension hemodynamic definitions proposed by the 6th World Symposium. Curr. Opin. Pulm. Med. 2020, 26, 384–390. [Google Scholar] [CrossRef]
- Kovacs, G.; Olschewski, A.; Berghold, A.; Olschewski, H. Pulmonary vascular resistances during exercise in normal subjects: A systematic review. Eur. Respir. J. 2012, 39, 319–328. [Google Scholar] [CrossRef]
- Xanthouli, P.; Jordan, S.; Milde, N.; Marra, A.; Blank, N.; Egenlauf, B.; Gorenflo, M.; Harutyunova, S.; Lorenz, H.M.; Nagel, C.; et al. Haemodynamic phenotypes and survival in patients with systemic sclerosis: The impact of the new definition of pulmonary arterial hypertension. Ann. Rheum. Dis. 2020, 79, 370–378. [Google Scholar] [CrossRef]
- Maron, B.A.; Brittain, E.L.; Hess, E.; Waldo, S.W.; Barón, A.E.; Huang, S.; Goldstein, R.H.; Assad, T.; Wertheim, B.M.; Alba, G.A.; et al. Pulmonary vascular resistance and clinical outcomes in patients with pulmonary hypertension: A retrospective cohort study. Lancet Respir. Med. 2020, 8, 873–884. [Google Scholar] [CrossRef]
- Maron, B.A.; Kleiner, D.E.; Arons, E.; Wertheim, B.M.; Sharma, N.S.; Haley, K.J.; Samokhin, A.O.; Rowin, E.J.; Maron, M.S.; Rosing, D.R.; et al. Evidence of Advanced Pulmonary Vascular Remodeling in Obstructive Hypertrophic Cardiomyopathy with Pulmonary Hypertension. Chest 2023, 163, 678–686. [Google Scholar] [CrossRef]
- Kovacs, G.; Herve, P.; Barbera, J.A.; Chaouat, A.; Chemla, D.; Condliffe, R.; Garcia, G.; Grunig, E.; Howard, L.; Humbert, M.; et al. An official European Respiratory Society statement: Pulmonary haemodynamics during exercise. Eur. Respir. J. 2017, 50, 1700578. [Google Scholar] [CrossRef] [PubMed]
- Zeder, K.; Olschewski, H.; Kovacs, G. Updated definition of exercise pulmonary hypertension. Breathe 2022, 18, 220232. [Google Scholar] [CrossRef]
- Naeije, R.; Vanderpool, R.; Dhakal, B.P.; Saggar, R.; Saggar, R.; Vachiery, J.L.; Lewis, G.D. Exercise-induced pulmonary hypertension: Physiological basis and methodological concerns. Am. J. Respir. Crit. Care Med. 2013, 187, 576–583. [Google Scholar] [CrossRef]
- Douschan, P.; Avian, A.; Foris, V.; Sassmann, T.; Bachmaier, G.; Rosenstock, P.; Zeder, K.; Olschewski, H.; Kovacs, G. Prognostic Value of Exercise as Compared to Resting Pulmonary Hypertension in Patients with Normal or Mildly Elevated Pulmonary Arterial Pressure. Am. J. Respir. Crit. Care Med. 2022, 206, 1418–1423. [Google Scholar] [CrossRef] [PubMed]
- Hasler, E.D.; Muller-Mottet, S.; Furian, M.; Saxer, S.; Huber, L.C.; Maggiorini, M.; Speich, R.; Bloch, K.E.; Ulrich, S. Pressure-Flow During Exercise Catheterization Predicts Survival in Pulmonary Hypertension. Chest 2016, 150, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.E.; Zern, E.K.; Lau, E.S.; Wooster, L.; Bailey, C.S.; Cunningham, T.; Eisman, A.S.; Hardin, K.M.; Farrell, R.; Sbarbaro, J.A.; et al. Exercise Pulmonary Hypertension Predicts Clinical Outcomes in Patients with Dyspnea on Effort. J. Am. Coll. Cardiol. 2020, 75, 17–26. [Google Scholar] [CrossRef]
- Zeder, K.; Avian, A.; Bachmaier, G.; Douschan, P.; Foris, V.; Sassmann, T.; Moazedi-Fuerst, F.C.; Graninger, W.B.; Hafner, F.; Brodmann, M.; et al. Exercise Pulmonary Resistances Predict Long-Term Survival in Systemic Sclerosis. Chest 2021, 159, 781–790. [Google Scholar] [CrossRef]
- Condliffe, R.; Kiely, D.G.; Peacock, A.J.; Corris, P.A.; Gibbs, J.S.; Vrapi, F.; Das, C.; Elliot, C.A.; Johnson, M.; DeSoyza, J.; et al. Connective tissue disease-associated pulmonary arterial hypertension in the modern treatment era. Am. J. Respir. Crit. Care Med. 2009, 179, 151–157. [Google Scholar] [CrossRef]
- Hoeper, M.M.; Huscher, D.; Ghofrani, H.A.; Delcroix, M.; Distler, O.; Schweiger, C.; Grunig, E.; Staehler, G.; Rosenkranz, S.; Halank, M.; et al. Elderly patients diagnosed with idiopathic pulmonary arterial hypertension: Results from the COMPERA registry. Int. J. Cardiol. 2013, 168, 871–880. [Google Scholar] [CrossRef]
- Hoeper, M.M.; Pausch, C.; Grunig, E.; Klose, H.; Staehler, G.; Huscher, D.; Pittrow, D.; Olsson, K.M.; Vizza, C.D.; Gall, H.; et al. Idiopathic pulmonary arterial hypertension phenotypes determined by cluster analysis from the COMPERA registry. J. Heart Lung Transplant. 2020, 39, 1435–1444. [Google Scholar] [CrossRef]
- Galie, N.; Barbera, J.A.; Frost, A.E.; Ghofrani, H.A.; Hoeper, M.M.; McLaughlin, V.V.; Peacock, A.J.; Simonneau, G.; Vachiery, J.L.; Grunig, E.; et al. Initial Use of Ambrisentan plus Tadalafil in Pulmonary Arterial Hypertension. N. Engl. J. Med. 2015, 373, 834–844. [Google Scholar] [CrossRef]
- Rosenkranz, S.; Pausch, C.; Coghlan, J.G.; Huscher, D.; Pittrow, D.; Grunig, E.; Staehler, G.; Vizza, C.D.; Gall, H.; Distler, O.; et al. Risk stratification and response to therapy in patients with pulmonary arterial hypertension and comorbidities: A COMPERA analysis. J. Heart Lung Transplant. 2023, 42, 102–114. [Google Scholar] [CrossRef]
- Karia, N.; Howard, L.; Johnson, M.; Kiely, D.G.; Lordan, J.; McCabe, C.; Pepke-Zaba, J.; Ong, R.; Preiss, M.; Knight, D.; et al. Predictors of outcomes in mild pulmonary hypertension according to 2022 ESC/ERS Guidelines: The EVIDENCE-PAH UK study. Eur. Heart J. 2023, 44, 4678–4691. [Google Scholar] [CrossRef]
- Hadinnapola, C.; Bleda, M.; Haimel, M.; Screaton, N.; Swift, A.; Dorfmuller, P.; Preston, S.D.; Southwood, M.; Hernandez-Sanchez, J.; Martin, J.; et al. Phenotypic Characterization of EIF2AK4 Mutation Carriers in a Large Cohort of Patients Diagnosed Clinically with Pulmonary Arterial Hypertension. Circulation 2017, 136, 2022–2033. [Google Scholar] [CrossRef]
- Ratwatte, S.; Anderson, J.; Strange, G.; Corrigan, C.; Collins, N.; Celermajer, D.S.; Dwyer, N.; Feenstra, J.; Keating, D.; Kotlyar, E.; et al. Pulmonary arterial hypertension with below threshold pulmonary vascular resistance. Eur. Respir. J. 2020, 56, 1901654. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Marra, A.M.; Benjamin, N.; Eichstaedt, C.A.; Blank, N.; Bossone, E.; Cittadini, A.; Coghlan, G.; Denton, C.P.; Distler, O.; et al. Early treatment with ambrisentan of mildly elevated mean pulmonary arterial pressure associated with systemic sclerosis: A randomized, controlled, double-blind, parallel group study (EDITA study). Arthritis Res. Ther. 2019, 21, 217. [Google Scholar] [CrossRef] [PubMed]
- Provencher, S.; Boucherat, O.; Potus, F.; Bonnet, S. Pulmonary hypertension thresholds: Time to lower further? Lancet Respir. Med. 2020, 8, 834–836. [Google Scholar] [CrossRef] [PubMed]
- Viray, M.C.; Bonno, E.L.; Gabrielle, N.D.; Maron, B.A.; Atkins, J.; Amoroso, N.S.; Fernandes, V.L.C.; Maran, A.; Nielsen, C.D.; Powers, E.R.; et al. Role of Pulmonary Artery Wedge Pressure Saturation during Right Heart Catheterization: A Prospective Study. Circ. Heart Fail. 2020, 13, e007981. [Google Scholar] [CrossRef]
- Bermejo, J.; Yotti, R.; Garcia-Orta, R.; Sanchez-Fernandez, P.L.; Castano, M.; Segovia-Cubero, J.; Escribano-Subias, P.; San Roman, J.A.; Borras, X.; Alonso-Gomez, A.; et al. Sildenafil for improving outcomes in patients with corrected valvular heart disease and persistent pulmonary hypertension: A multicenter, double-blind, randomized clinical trial. Eur. Heart J. 2018, 39, 1255–1264. [Google Scholar] [CrossRef]
- Maron, B.A.; Ryan, J.J. A Concerning Trend for Patients with Pulmonary Hypertension in the Era of Evidence-Based Medicine. Circulation 2019, 139, 1861–1864. [Google Scholar] [CrossRef] [PubMed]
- Wijeratne, D.T.; Lajkosz, K.; Brogly, S.B.; Lougheed, M.D.; Jiang, L.; Housin, A.; Barber, D.; Johnson, A.; Doliszny, K.M.; Archer, S.L. Increasing Incidence and Prevalence of World Health Organization Groups 1 to 4 Pulmonary Hypertension: A Population-Based Cohort Study in Ontario, Canada. Circ. Cardiovasc. Qual. Outcomes 2018, 11, e003973. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.M.; Sitbon, O.; Doelberg, M.; Feldman, J.; Gibbs, J.S.R.; Grunig, E.; Hoeper, M.M.; Martin, N.; Mathai, S.C.; McLaughlin, V.V.; et al. Three- Versus Two-Drug Therapy for Patients with Newly Diagnosed Pulmonary Arterial Hypertension. J. Am. Coll. Cardiol. 2021, 78, 1393–1403. [Google Scholar] [CrossRef] [PubMed]
- Humbert, M.; Yaici, A.; de Groote, P.; Montani, D.; Sitbon, O.; Launay, D.; Gressin, V.; Guillevin, L.; Clerson, P.; Simonneau, G.; et al. Screening for pulmonary arterial hypertension in patients with systemic sclerosis: Clinical characteristics at diagnosis and long-term survival. Arthritis Rheum. 2011, 63, 3522–3530. [Google Scholar] [CrossRef] [PubMed]
- Coghlan, J.G.; Wolf, M.; Distler, O.; Denton, C.P.; Doelberg, M.; Harutyunova, S.; Marra, A.M.; Benjamin, N.; Fischer, C.; Grunig, E. Incidence of pulmonary hypertension and determining factors in patients with systemic sclerosis. Eur. Respir. J. 2018, 51, 1701197. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).