Prophylactic Pulmonary Artery Banding in Pediatric Dilated Cardiomyopathy: An Additional Therapeutic Option
Abstract
1. Introduction
2. Materials and Methods
2.1. Cohort, Demographic, and Clinical Data
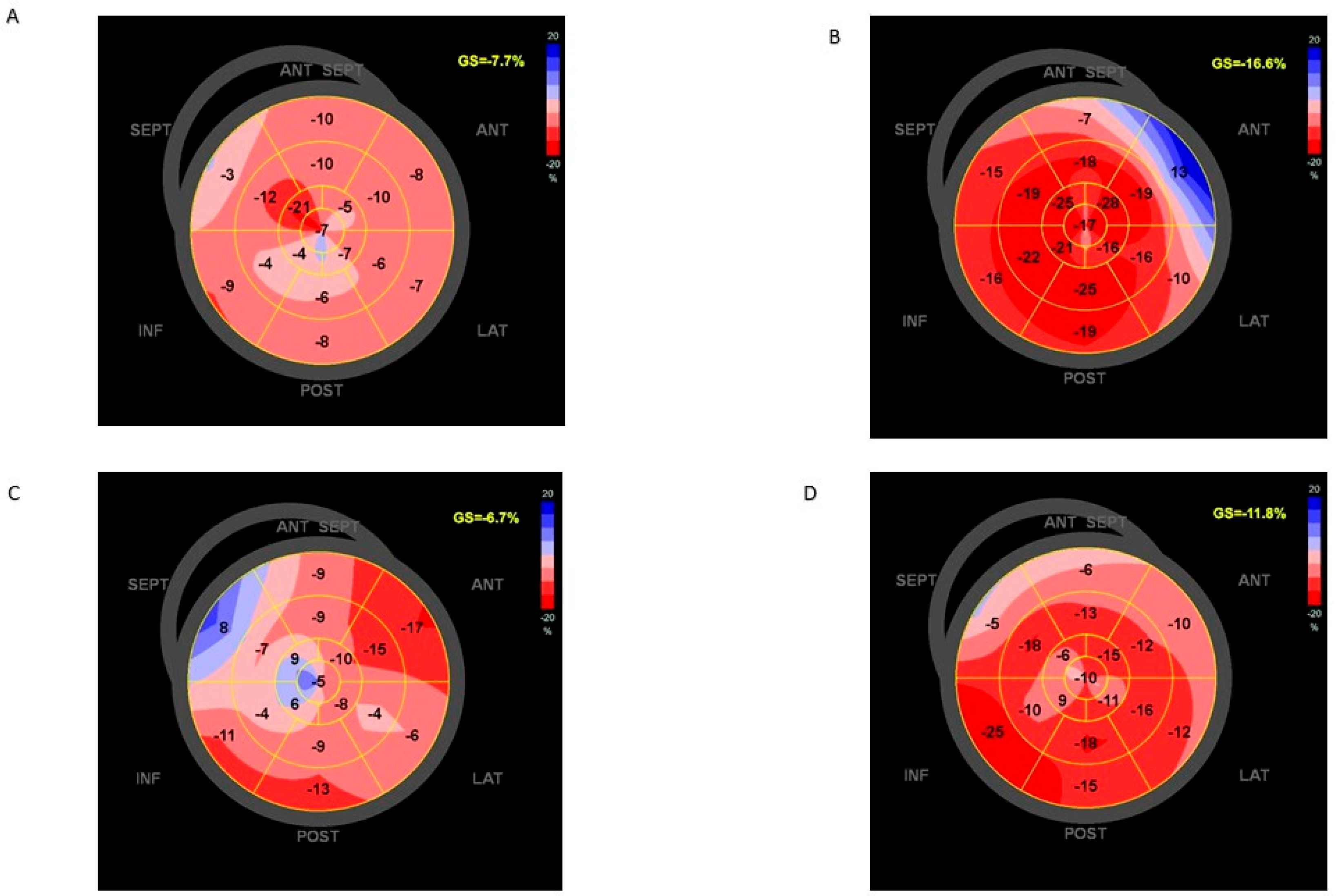
2.2. Cardiac Imaging
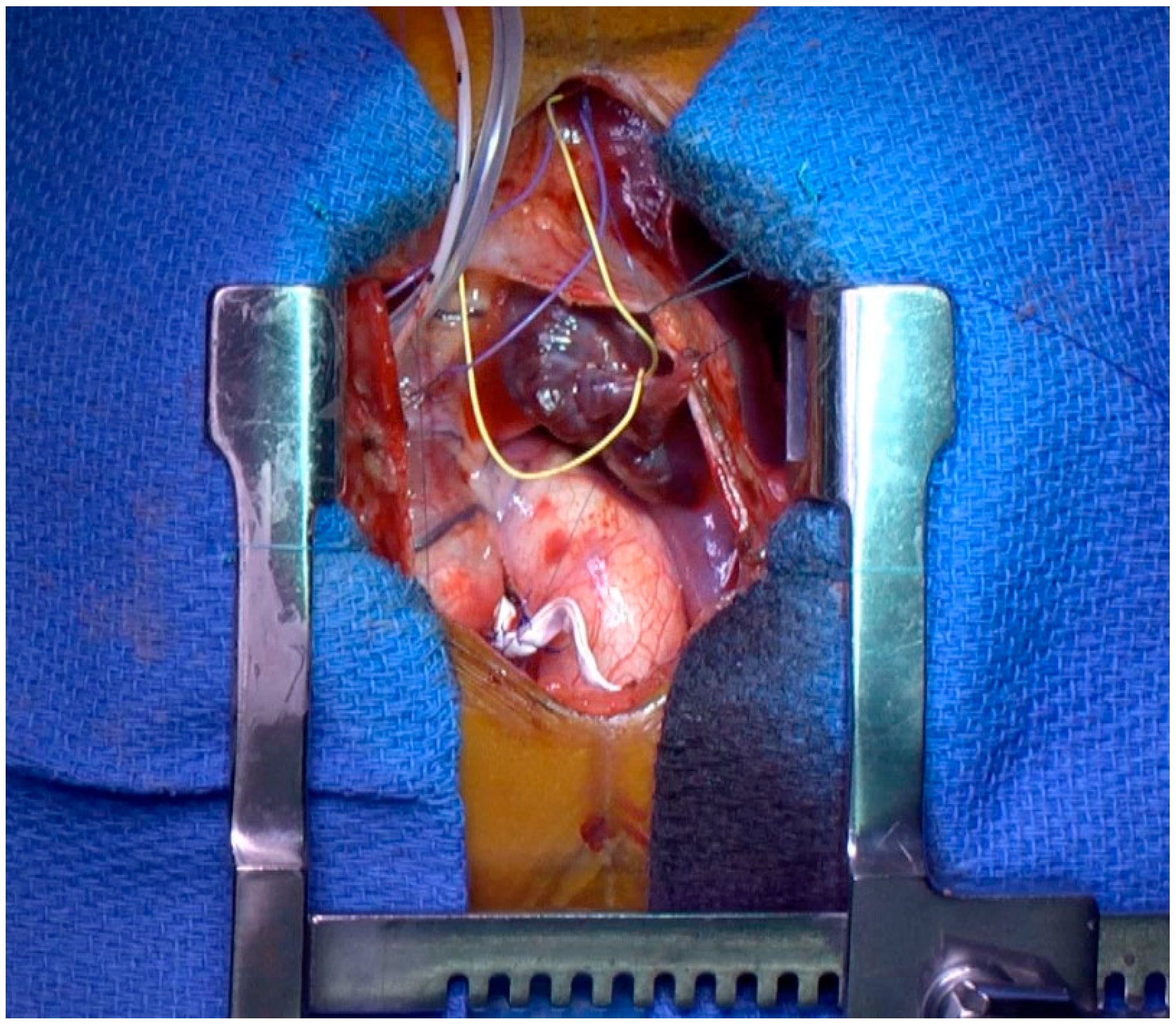
2.3. Procedural Data
2.4. Statistical Analysis
3. Results
3.1. Study Population at Diagnosis and Clinical Evolution before PAB
3.2. Procedural Data and Periprocedural Outcome
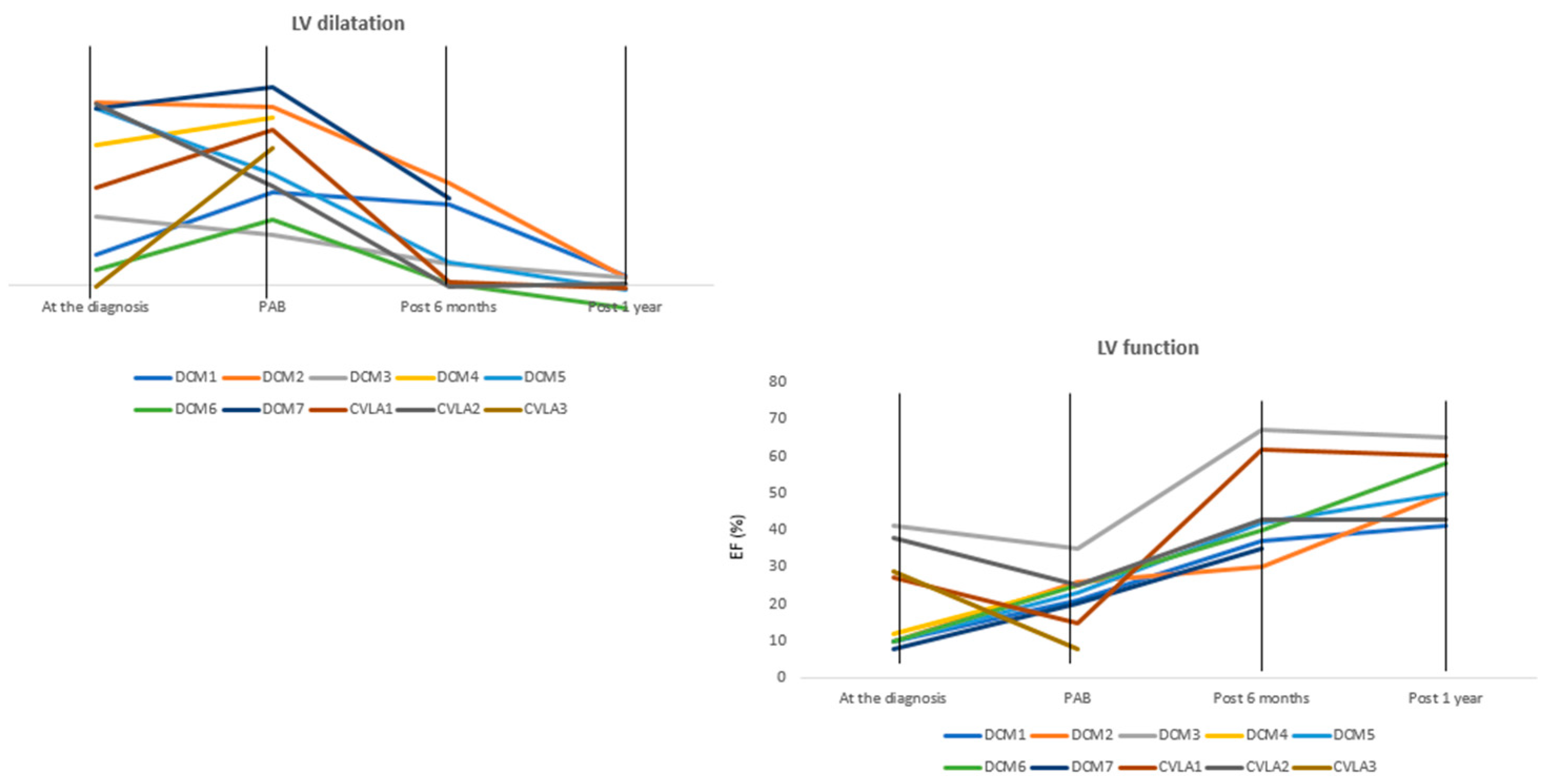
3.3. Clinical Status and Reversal Ventricular Remodeling from PAB to 1 Year of Follow-Up
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nugent, A.W.; Daubeney, P.E.; Chondros, P.; Carlin, J.B.; Cheung, M.; Wilkinson, L.C.; Davis, A.M.; Kahler, S.G.; Chow, C.W.; Wilkinson, J.L.; et al. The epidemiology of childhood cardiomyopathy in Australia. N. Engl. J. Med. 2003, 348, 1639–1646. [Google Scholar] [CrossRef]
- Lipshultz, S.E.; Sleeper, L.A.; Towbin, J.A.; Lowe, A.M.; Orav, E.J.; Cox, G.F.; Lurie, P.R.; McCoy, K.L.; McDonald, M.A.; Messere, J.E.; et al. The incidence of pediatric cardiomyopathy in two regions of the United States. N. Engl. J. Med. 2003, 348, 1647–1655. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, J.A.; Orav, E.J.; Wilkinson, J.D.; Fleming, L.E.; Lee, D.J.; Sleeper, L.A.; Rusconi, P.G.; Colan, S.D.; Hsu, D.T.; Canter, C.E.; et al. Pediatric Cardiomyopathy Registry Investigators. Competing risks for death and cardiac transplantation in children with dilated cardiomyopathy: Results from the pediatric cardiomyopathy registry. Circulation 2011, 124, 814–823. [Google Scholar] [CrossRef]
- Canter, C.E.; Shaddy, R.E.; Bernstein, D.; Hsu, D.T.; Chrisant, M.R.; Kirklin, J.K.; Kanter, K.R.; Higgins, R.S.D.; Blume, E.D.; Rosenthal, D.N.; et al. Indications for heart transplantation in pediatric heart disease: A scientific statement from the American Heart Association Council on Cardiovascular Disease in the Young; the Councils on Clinical Cardiology, Cardiovascular Nursing, and Cardiovascular Surgery and Anesthesia; and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation 2007, 115, 658–676. [Google Scholar] [PubMed]
- Muller, W.H., Jr.; Danimann, J.F., Jr. The treatment of certain congenital malformations of the heart by the creation of pulmonic stenosis to reduce pulmonary hypertension and excessive pulmonary blood flow; a preliminary report. Surg. Gynecol. Obstet. 1952, 95, 213–219. [Google Scholar] [PubMed]
- Murtuza, B.; Barron, D.J.; Stumper, O.; Stickley, J.; Eaton, D.; Jones, T.J.; Brawn, W.J. Anatomic repair for congenitally corrected transposition of the great arteries: A single-institution 19-year experience. J. Thorac. Cardiovasc. Surg. 2011, 142, 1348–1357.e1. [Google Scholar] [CrossRef] [PubMed]
- Metton, O.; Gaudin, R.; Ou, P.; Gerelli, S.; Mussa, S.; Sidi, D.; Vouhé, P.; Raisky, O. Early prophylactic pulmonary artery banding in isolated congenitally corrected transposition of the great arteries. Eur. J. Cardiothorac. Surg. 2010, 38, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Schranz, D.; Rupp, S.; Müller, M.; Schmidt, D.; Bauer, A.; Valeske, K.; Michel-Behnke, I.; Jux, C.; Apitz, C.; Thul, J.; et al. Pulmonary artery banding ininfants and young children with left ventricular dilated cardiomyopathy: A novel therapeutic strategy before heart transplantation. J. Heart Lung Transplant. 2013, 32, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Spigel, Z.A.; Razzouk, A.; Nigro, J.J.; Karamlou, T.B.; Kavarana, M.N.; Roeser, M.E.; Adachi, I. Pulmonary Artery Banding for Children With Dilated Cardiomyopathy: US Experience. Semin. Thorac. Cardiovasc. Surg. Pediatr. Card. Surg. Annu. 2020, 23, 69–76. [Google Scholar] [CrossRef]
- Marijon, E.; Ou, P.; Fermont, L.; Concordet, S.; Le Bidois, J.; Sidi, D.; Bonnet, D. Diagnosis and outcome in congenital ventricular diverticulum and aneurysm. J. Thorac. Cardiovasc. Surg. 2006, 131, 433–437. [Google Scholar] [CrossRef]
- Recla, S.; Schmidt, D.; Logeswaran, T.; Esmaeili, A.; Schranz, D. Pediatric heart failure therapy: Why β1-receptor blocker, tissue ACE-I and mineralocorticoid-receptor-blocker? Transl. Pediatr. 2019, 8, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.D. The Ross Classification for Heart Failure in Children After 25 Years: A Review and an Age-Stratified Revision. Pediatr. Cardiol. 2012, 33, 1295–1300. [Google Scholar] [CrossRef] [PubMed]
- Kiess, A.; Green, J.; Willenberg, A.; Ceglarek, U.; Dähnert, I.; Jurkutat, A.; Körner, A.; Hiemisch, A.; Kiess, W.; Vogel, M. Age-Dependent Reference Values for hs-Troponin T and NT-proBNP and Determining Factors in a Cohort of Healthy Children (The LIFE Child Study). Pediatr. Cardiol. 2022, 43, 1071–1083. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Bierig, M.; Devereux, R.B.; Flachskampf, F.A.; Foster, E.; Pellikka, P.A.; Picard, M.H.; Roman, M.J.; Seward, J.; Shanewise, J.S.; et al. Recommendations for chamber quantification: A report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J. Am. Soc. Echocardiogr. 2005, 18, 1440–1463. [Google Scholar]
- Lee, C.K.; Margossian, R.; Sleeper, L.A.; Canter, C.E.; Chen, S.; Tani, L.Y.; Shirali, G.; Szwast, A.; Tierney, E.S.S.; Campbell, M.J.; et al. Variability of M-mode versus two-dimensional echocardiography measurements in children with dilated cardiomyopathy. Pediatr. Cardiol. 2014, 35, 658–667. [Google Scholar] [CrossRef]
- Pettersen, M.D.; Du, W.; Skeens, M.E.; Humes, R.A. Regression equations for calculation of z scores of cardiac structures in a large cohort of healthy infants, children, and adolescents: An echocardiographic study. J. Am. Soc. Echocardiogr. 2008, 21, 922–934. [Google Scholar] [CrossRef]
- Quiñones, M.A.; Otto, C.M.; Stoddard, M.; Waggoner, A.; Zoghbi, W.A. Recommendations for quantification of Doppler echocardiography: A report from the Doppler Quantification Task Force of the Nomenclature and Standards Committee of the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2002, 15, 167–184. [Google Scholar] [CrossRef] [PubMed]
- Kapusta, L.; Thijssen, J.M.; Groot-Loonen, J.; Antonius, T.; Mulder, J.; Daniëls, O. Tissue Doppler imaging in detection of myocardial dysfunction in survivors of childhood cancer treated with anthracyclines. Ultrasound Med. Biol. 2000, 26, 1099–1108. [Google Scholar] [CrossRef]
- Balasubramanian, S.; Punn, R.; Smith, S.N.; Houle, H.; Tacy, T.A. Left Ventricular Systolic Myocardial Deformation: A Comparison of Two- and Three-Dimensional Echocardiography in Children. J. Am. Soc. Echocardiogr. 2017, 30, 974–983. [Google Scholar] [CrossRef]
- Di Candia, A.; Castaldi, B.; Bordin, G.; Cerutti, A.; Reffo, E.; Biffanti, R.; Di Salvo, G.; Vida, V.L.; Padalino, M.A. Pulmonary Artery Banding for Ventricular Rehabilitation in Infants With Dilated Cardiomyopathy: Early Results in a Single-Center Experience. Front. Pediatr. 2020, 8, 347. [Google Scholar] [CrossRef]
- Ponzoni, M.; Frigo, A.C.; Castaldi, B.; Cerutti, A.; Di Salvo, G.; Vida, V.L.; Padalino, M.A. Surgical strategies for the management of end-stage heart failure in infants and children: A 15-year experience with a patient- tailored approach. Artif. Organs 2021, 45, 1543–1553. [Google Scholar] [CrossRef]
- Schranz, D.; Akintuerk, H.; Bailey, L. Pulmonary Artery Banding for Functional Regeneration of End-Stage Dilated Cardiomyopathy in Young Children: World Network Report. Circulation 2018, 137, 1410–1412. [Google Scholar] [CrossRef]
- Beyar, R.; Dong, S.J.; Smith, E.R.; Belenkie, I.; Tyberg, J.V. Ventricular interaction and septal deformation: A model compared with experimental data. Am. J. Physiol. 1993, 265 Pt 2, H2044–H2056. [Google Scholar] [CrossRef]
- Acar, P.; Sidi, D.; Bonnet, D.; Aggoun, Y.; Bonhoeffer, P.; Kachaner, J. Maintaining tricuspid valve competence in double discordance: A challenge for the paediatric cardiologist. Heart 1998, 80, 479–483. [Google Scholar] [CrossRef]
- Schranz, D.; Recla, S.; Malcic, I.; Kerst, G.; Mini, N.; Akintuerk, H. Pulmonary artery banding in dilative cardiomyopathy of young children: Review and protocol based on the current knowledge. Transl. Pediatr. 2019, 8, 151–160. [Google Scholar] [CrossRef]
- Raimondi, F.; Iserin, F.; Raisky, O.; Laux, D.; Bajolle, F.; Boudjemline, Y.; Boddaert, N.; Bonnet, D. Myocardial inflammation on cardiovascular magnetic resonance predicts left ventricular function recovery in children with recent dilated cardiomyopathy. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Alexander, P.M.; Daubeney, P.E.; Nugent, A.W.; Lee, K.J.; Turner, C.; Colan, S.D.; Robertson, T.; Davis, A.M.; Ramsay, J.; Justo, R.; et al. Long-term outcomes of dilated cardiomyopathy diagnosed during childhood: Results from a national population-based study of childhood cardiomyopathy. Circulation 2013, 128, 2039–2046. [Google Scholar] [CrossRef] [PubMed]
- Al-Wakeel-Marquard, N.; Degener, F.; Herbst, C.; Kühnisch, J.; Dartsch, J.; Schmitt, B.; Kuehne, T.; Messroghli, D.; Berger, F.; Klaassen, S. RIKADA Study Reveals Risk Factors in Pediatric Primary Cardiomyopathy. J. Am. Heart Assoc. 2019, 8, e012531. [Google Scholar] [CrossRef]
- Traister, A.; Patel, R.; Huang, A.; Patel, S.; Plakhotnik, J.; Lee, J.E.; Medina, M.G.; Welsh, C.; Ruparel, P.; Zhang, L.; et al. Cardiac regenerative capacity is age- and disease-dependent in childhood heart disease. PLoS ONE 2018, 13, e0200342. [Google Scholar] [CrossRef] [PubMed]
- Latus, H.; Hachmann, P.; Gummel, K.; Recla, S.; Voges, I.; Mueller, M.; Bauer, J.; Yerebakan, C.; Akintuerk, H.; Apitz, C.; et al. Biventricular response to pulmonary artery banding in children with dilated cardiomyopathy. J. Heart Lung Transplant. 2016, 35, 934–938. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, J.F.; Vesel, T.P.; Jeewa, A.; Adachi, I. Pulmonary Artery Band Reduces Left Atrial Pressure in Dilated Cardiomyopathy. Ann. Thorac. Surg. 2015, 100, e35–e36. [Google Scholar] [CrossRef] [PubMed]
| Heart Failure Etiology | Clinical Status at Diagnosis | ||||||
|---|---|---|---|---|---|---|---|
| P/LP Variant 1 | P/LP Variant 2 | Genetic Testing of Parents | Age (Months) | Cardiogenic Shock/ICU | Modified Ross Class | Weight–Length (Z-Score) | |
| DCM1 | ACTC1 c664G>A (p.(Ala222Thr)); class 4 | None | None | 0.7 | Yes | 4 | −2.7 |
| DCM2 | VCL c.1225C>T(p.(Arg409)); class 4 | None | VCLc.1225C>T(p.(Arg409)) (mother) | 6.1 | No | 3 | −3.60 |
| DCM3 | FLNC c.3937C>T(p.(Arg1313)); class 4 | None | Not available | 2.3 | No | 1 | −1.4 |
| DCM4 | CSRP3 c140C>G (p.Thr47Arg); class 4 | MYH6 c4999G>A (p.Asp1667Asn); class 4 | MYH6 c4999G>A (p.Asp1667Asn) (mother); CSRP3 c140C>G (p.Thr47Arg) (father) | 1.9 | Yes | 4 | −1.4 |
| DCM5 | DTNA c1841G>A (p.(Arg614Lys); class 3 | None | Not available | 8.9 | Yes | 4 | −0.4 |
| DCM6 | ALMS1. c.2816T>A,pLeu939 *; class 5 | ALMS1. c.2816T>A,pLeu939 * | ALMS1; c.2816T>A,pLeu939 * (mother, father) | 0.8 | Yes | 4 | −1.87 |
| DCM7 | FLNC phe1529leufs10; class 5 | None | Not available | 4.96 | Yes | 3 | −0.11 |
| CLVA1 | 12.6 | No | 1 | +1.2 | |||
| CLVA2 | 1.9 | No | 2 | −0.5 | |||
| CLVA3 | 0.10 | No | 1 | −1.20 | |||
| Clinical Data at Time of PAB and During Follow-Up (6 Months, 1 Year) | Echocardiographic Data at Time of PAB and During Follow-Up (6 Months, 1 Year) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Weight–Length (Z-Score) | Modified Ross Class a | NT-proBNP (pg/mL) | Enteral Nutritio n | Loop Diuretic | Status at 6 m/1 y | LVEDD Z-Score | EF (%) | LAD Z-Score | RVFAC (%) | RVED Surface | |
| DCM1 | −1.3/−0.9/−0.73 | 3/2/2 | 2663/605/- | Yes/No/No | Yes/Yes/Yes | Alive | 6.5/5.8/0.73 | 21/37/41 | 4.5/2.7//1.1 | 63/55/41 | 6.5/9.3/9.1 |
| DCM2 | −0.51/−0.6/−0,56 | 3/3/2 | 2080/-/- | Yes/No | Yes/Yes/No | Alive | 12.6/7.3/0.6 | 26/30/50 | 4.5/1.4/1.3 | 49/45/- | 4/7.2/- |
| DCM3 | −1.8/−0.5/−0.9 | 2/2/2 | 422/43/183 | Yes/No/No | N/No/No | Alive | 3.6/1.5/0.6 | 35/67/65 | −0.2/-/−0.9/−1.6 | 52/52/27 | 7/11/17 |
| DCM4 | +1.3/-/- | 3-/- | 3980/-/- | Yes/-/- | Yes/-/- | Decease d (day 69 afterPAB) | 11.8/-/- | 25/-/- | 6/-/- | 44/-/- | 8.7/-/- |
| DCM5 | +0.3/+0.5/+0.2 | 2/2/2 | 576/202/324 | Yes/No/No | Yes/No/No | Alive | 7.9/1.7/−0.3 | 23/42/50 | 3.3/0.1/0.5 | 53/40/40 | 6.5/8.8/8.3 |
| DCM6 | +0.8/+2.3/2.02 | 3/2/2 | 101/-/- | Yes/No/No | Yes/No/No | Alive | 4.6/0.2 | 25/40 | 3.6/1/- | 56/44/- | 4/5/- |
| DCM7 | −2.5/+1.2 | 3/2/2 | 16,200/303/- | Yes/No/No | Yes/No/No | Alive | 14/6.2/5.4 | 20/35/32 | 2.4/1.4/2.5 | 30/45/- | 6.5/7.5/- |
| CLVA1 | +0.4/+1.3/+1.2 | 1/1/1 | 576/611,6 | No/No/No | No/No/No | Alive | 11/0.24/−0.2 | 15/43/60 | 1.1/−0.3/- | 60/51/- | 9/9.4/- |
| CLVA2 | −2.9/−0.4/+0.4 | 1/1/1 | 196/-/180 | No/No/No | No/No/No | Alive | 7.1/−0.1/0.2 | 25/43/43 | 0.3/−1.1/−3 | 47/43/42 | 5/7.6/8 |
| CLVA 3 | −0.04/-/- | 3-/- | 11,000/-/- | No/-/- | Yes/-/- | Deceased (day 77 after PAB) | 9.6/-/- | 8/-/- | 4.9/-/- | 48/-/- | 5.5/-/- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panaioli, E.; Khraiche, D.; Pontailler, M.; Ader, F.; Raisky, O.; Gaudin, R.; Bonnet, D. Prophylactic Pulmonary Artery Banding in Pediatric Dilated Cardiomyopathy: An Additional Therapeutic Option. J. Cardiovasc. Dev. Dis. 2024, 11, 79. https://doi.org/10.3390/jcdd11030079
Panaioli E, Khraiche D, Pontailler M, Ader F, Raisky O, Gaudin R, Bonnet D. Prophylactic Pulmonary Artery Banding in Pediatric Dilated Cardiomyopathy: An Additional Therapeutic Option. Journal of Cardiovascular Development and Disease. 2024; 11(3):79. https://doi.org/10.3390/jcdd11030079
Chicago/Turabian StylePanaioli, Elena, Diala Khraiche, Margaux Pontailler, Flavie Ader, Olivier Raisky, Regis Gaudin, and Damien Bonnet. 2024. "Prophylactic Pulmonary Artery Banding in Pediatric Dilated Cardiomyopathy: An Additional Therapeutic Option" Journal of Cardiovascular Development and Disease 11, no. 3: 79. https://doi.org/10.3390/jcdd11030079
APA StylePanaioli, E., Khraiche, D., Pontailler, M., Ader, F., Raisky, O., Gaudin, R., & Bonnet, D. (2024). Prophylactic Pulmonary Artery Banding in Pediatric Dilated Cardiomyopathy: An Additional Therapeutic Option. Journal of Cardiovascular Development and Disease, 11(3), 79. https://doi.org/10.3390/jcdd11030079







