Abstract
Surgical correction of severe mitral regurgitation (MR) can reverse left ventricular (LV) remodeling in patients with mitral valve prolapse (MVP). However, whether this process is similar to the case in Barlow’s Disease (BD) and Fibro-elastic Deficiency (FED) is currently unknown. The aim of this study is to evaluate post-operative LV reverse remodeling and function in patients with BD versus FED. In this study, 100 MVP patients (BD = 37 and FED = 63) with severe MR who underwent mitral valve surgery at three Belgian centers were retrospectively included. Transthoracic echocardiography was used to assess MR severity, LV volumes and function before surgery and 6 months thereafter. Baseline MR severity, LV ejection fraction (LVEF), indexed LV end-diastolic (LVEDVi) and end-systolic volumes (LVESVi) were not different between the groups. After a median follow-up of 278 days, there was a similar decrease in LVEDVi, but a trend towards a smaller decrease in LVESVi in BD compared to FED (−3.0 ± 11.2 mL/m2 vs. −5.3 ± 9.0 mL/m2; p = 0.154). This resulted in a significantly larger decrease in LVEF in BD (−8.3 ± 9.6%) versus FED (−3.9 ± 6.9%) after adjusting for baseline LVEF (p < 0.001) and type of surgical intervention (p = 0.01). These findings suggest that LV (reverse) remodeling in BD could be affected by other mechanisms beyond volume overload, potentially involving concomitant cardiomyopathy.
1. Introduction
Mitral valve prolapse (MVP) is the most common cause of primary mitral regurgitation (MR) and has a prevalence of 2–3% in the general population [1]. While the disease course of MVP appears to be benign in some patients, chronic MR can cause substantial volume and pressure overload, subsequently leading to severe remodeling with left ventricular (LV) dilatation and/or dysfunction [2,3]. MVP can be subcategorized as primarily related to Fibro-elastic Deficiency (FED) or to Barlow’s Disease (BD), the two ends of the MVP spectrum. Patients with FED typically present with isolated leaflet or segment prolapse due to degenerative processes, whereas BD is characterized by bileaflet prolapse with thick and myxomatous valvular infiltration [4,5]. Interestingly, LV dilation in FED is thought to result entirely from volume overload in the presence of severe MR. However, significant LV dilation has been observed in BD even in the absence of MR, potentially involving an underlying cardiomyopathy in certain patients with BD [6,7,8]. The evolution of LV dilatation after correction of MR could further underscore this hypothesis.
In the presence of severe MR and associated symptoms or markers of LV dilation and/or dysfunction, mitral valve surgery is indicated to eliminate volume overload and reverse the process of LV remodeling [9,10]. Nevertheless, it remains unknown whether LV reverse remodeling following mitral valve surgery is similar in both MVP subtypes, especially since other factors could affect the process of LV (reverse) remodeling beyond volume overload in patients with BD [3,7]. Therefore, this study aims to evaluate the impact of the MVP subtype on LV reverse remodeling after mitral valve surgery.
2. Materials and Methods
We retrospectively included all consecutive patients with mitral valve prolapse (MVP) who underwent isolated mitral valve surgery (+/− tricuspid valve annuloplasty) in 3 Belgian referral centers: University Hospital Antwerp (from January 2013 to October 2022), Hospital Oost-Limburg (from January 2017 to October 2022) and Sint-Jan Hospital Bruges (from January 2019 to December 2021). Approval of the central ethical committee (UZA) was obtained. All eligible patients had to (1) have (moderate-to) severe primary MR, (2) be age 18 years or older, and (3) have comprehensive echocardiography at baseline and after a minimum of 6 months post-operative follow-up. Subjects were excluded if they presented with any of the following: (1) history of cardiac surgery, (2) coronary artery disease, (3) significant concomitant left-sided valve disease (≥moderate aortic stenosis/insufficiency and/or ≥moderate mitral stenosis), (4) syndromic MVP (e.g., Marfan’s syndrome), (5) congenital heart disease, (6) inadequate quality of echocardiographic images;, and (7) follow-up at a referral center (Figure 1).
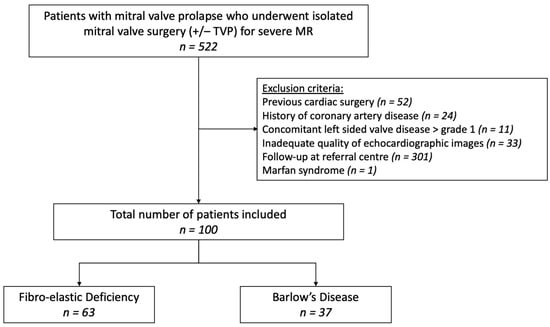
Figure 1.
Flowchart patient selection. MR, mitral regurgitation; TVP, tricuspid valve annuloplasty.
Clinical characteristics were collected from the electronic patient files. Patients were classified as BD or FED according to the intra-operative diagnosis of myxomatous mitral valve (=BD) or overall normal valvular tissue (=FED). Ventricular arrhythmias were defined as a reported history of ventricular tachycardia (minimum of 3 consecutive beats) or ventricular fibrillation; screening with Holter was not performed. The baseline preoperative echocardiography and first follow-up echocardiography after a minimum of 6 months were analyzed. LV end-diastolic and end-systolic volumes and ejection fractions (LVEFs) were calculated using the modified Simpson’s method. All ventricular and atrial volumes were indexed to body surface area (LVEDVi and LVESVi, respectively). The change in volumes and function was expressed as ΔLVEDVi (LVEDVifollow-up − LVEDVibaseline), ΔLVESVi (LVESVifollow-up − LVESVibaseline) and ΔLVEF (LVEFfollow-up − LVEFbaseline). In addition, the relative change in LVEF was calculated as ΔLVEF/LVEFbaseline.
A multi-integrative approach was used to quantify MR severity. Regurgitant volume (Rvol) was calculated using the proximal isovelocity surface area (PISA) method. MR severity was classified as no/trace (grade 0), mild (grade 1), moderate (grade 2), moderate to severe (grade 3) or severe (grade 4) [11]. The prolapse volume, defined as the end-systolic volume between the mitral annular plane and mitral valve leaflets, was calculated from the apical 4-chamber and 2-chamber views, as described previously [12]. Systolic pulmonary artery pressures were calculated from the maximal tricuspid regurgitant jet velocity and an estimate of the right atrial pressure based on inferior caval vein dimension and collapsibility. All echocardiographic images were analyzed by a single observer per center.
Continuous variables are expressed as mean ± standard deviation for normally distributed variables or median with interquartile range (IQR) for parameters with a non-normal distribution. Categorical variables are expressed as numbers and percentages and were compared using a Chi-square test or Fisher’s exact test. Baseline and follow-up LV volumes and LVEF (in the 2 subgroups separately) were compared with a paired Student’s t-test or nonparametric alternative (Wilcoxon signed-rank test). Comparisons between the 2 MVP subtypes were performed with a Student’s t-test or nonparametric alternative (Mann–Whitney U test). Follow-up echocardiographic variables were corrected for their respective baseline variables using a simple linear regression analysis. Univariable and multivariable linear regression analyses were conducted to identify the determinants of post-operative change in LVEFs. Only variables with a p-value of ≤0.05 on univariate analysis were included in the multivariable linear regression analysis. Additional multicollinearity statistics were gathered (variance inflation factors and tolerance) to exclude a significant correlation between the independent determinants in the multivariable regression analysis. Statistical analyses were performed using SPSS version 28.0 (SPSS Inc., Chicago, IL, USA). A p-value of ≤0.05 was considered statistically significant.
3. Results
3.1. Baseline Characteristics
A total of 100 patients were included, 37 with BD and 63 with FED (Figure 1). The baseline clinical characteristics are shown in Table 1. Patients with FED tended to be older compared to BD and had a marked male predominance and a higher body mass index; however, body surface area was not significantly different. The presence of symptoms, cardiovascular risk factors, family history of valvular heart disease and atrial fibrillation was similar in both groups. Furthermore, there was no significant difference regarding the prevalence of ventricular arrhythmias between BD and FED.

Table 1.
Baseline clinical characteristics.
3.2. Baseline Echocardiography
Baseline echocardiographic characteristics are presented in Table 2. At baseline, BSA-indexed LV end-diastolic (LVEDVi) and end-systolic volume (LVESVi) were similar between BD and FED. In addition, preoperative MR severity was not significantly different between both groups. Patients with BD presented with a larger prolapse volume and a larger mitral annular diameter compared to patients with FED, while chordal rupture was more prevalent in FED. Furthermore, the left atrial volume index was similar in both groups, and there was no significant difference in systolic pulmonary artery pressures between BD and FED.

Table 2.
Baseline echocardiography characteristics.
3.3. Surgical Intervention
Most patients underwent mitral valve repair (76%) using one or more of the following techniques: mitral valve annuloplasty, Neochord and/or triangular/quadrangular resection (Table 3). Mitral valve replacement (MVR) was performed in selected patients when valve repair was not feasible, mainly in BD (46% vs. 11%; p < 0.001). Most patients undergoing MVR received a mechanical mitral valve prosthesis (58%). Additional tricuspid valve annuloplasty was performed in 9% of patients, with no difference between BD and FED. In selected patients with a history of atrial fibrillation, surgical pulmonary vein isolation (6%) and/or left atrial appendage occlusion (21%) was additionally performed with no difference among the MVP groups. At discharge, significant residual MR (grade ≥ 2) was observed in four FED patients.

Table 3.
Surgical data.
3.4. Follow-Up Echocardiography
Follow-up echocardiographic characteristics are presented in Table 4. After a median follow-up period of 278 days in BD and 288 days in FED (p = 0.753), there was no difference between both groups regarding the presence of residual MR ≥ grade 2. We observed a significant decrease in LVEDVi from baseline to follow-up in both BD and FED (p < 0.001). While patients with FED experienced a significant post-operative decrease in LVESVi as well (p < 0.001), there was only a limited decrease in the less load-dependent LVESVi in patients with BD from baseline to follow-up (p = 0.188) (Figure 2). At follow-up, we observed a larger decrease in LVEF in BD as compared to FED after correction for baseline LVEF (p < 0.001) (Figure 3). A LVEF of < 50% was present in 28% of patients with BD compared to 12% in FED (p = 0.050); however, this difference did not remain statistically significant after correction for baseline LVEF (p = 0.060). Of note, we observed a limited decrease in LVESVi and significantly lower post-operative LVEF in patients with mitral valve replacement compared to mitral valve repair (respectively 0.2 ± 12.4 mL/m2 vs. −5.8 ± 8.6 mL/m2, p = 0.025 and −10.1 ± 9.2% vs. −4.2 ± 7.5%, p = 0.005). Additional subgroup analysis was performed excluding patients who underwent valve replacement without preservation of the subvalvular apparatus (n = 90), which showed no significant difference between LVEF decrease at follow-up between mitral valve replacement (−8.1 ± 10.1%) versus mitral valve repair (−4.2 ± 7.5%) (p = 0.091). Furthermore, this subgroup analysis presented with similar results regarding the evolution of post-operative LVEF in BD compared to FED (−6.8 ± 9.9% vs. −3.9 ± 6.9%; p = 0.006). Of note, subgroup analysis of only patients that underwent mitral valve repair (n = 76) showed a clear trend but not a statistically significant difference between LVEF decrease in BD versus FED (−5.7 ± 8.5% vs. −3.7 ± 7.1%, p = 0.059) (Supplementary Table S1).

Table 4.
Follow-up echocardiography characteristics.
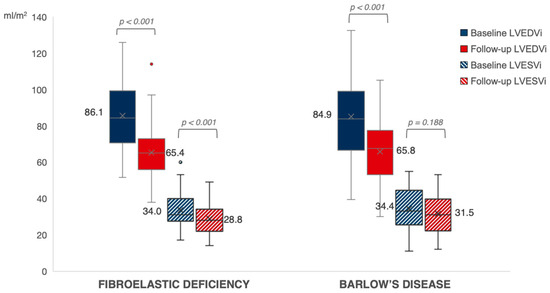
Figure 2.
Evolution of left ventricular volumes in FED vs. BD. The evolution of LV end-diastolic and end-systolic volumes at baseline (in blue) towards follow-up (in red) in patients with FED versus BD. Expressed values are mean (shown as “x”). LVEDVi, left ventricular end-diastolic volume index; LVESVi, left ventricular end-systolic volume index.
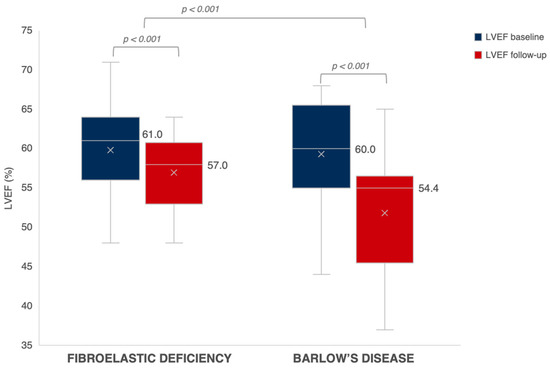
Figure 3.
Evolution of LVEF in FED vs. BD. The evolution of LVEF at baseline (in blue) towards follow-up (in red) in patients with FED versus BD. Expressed values are median. The mean is shown as “x”. LVEF, left ventricular ejection fraction.
Table 5 shows the univariable linear regression analyses used to identify the predictors of post-operative LVEF decrease (ΔLVEF). Based on these univariable regression analyses, baseline LVEF, baseline LVESVi, MVR and BD were significantly associated with a greater decrease in LVEF after follow-up. Multivariable linear regression analysis showed that only baseline LVEF and MVP subtype remained a significant determinant of post-op change in LVEF (Table 6). Additional multicollinearity statistics verified that the predictors of BD and MVR were not closely correlated with one another. Thus, the effect of the MVP subtype on LVEF cannot be explained by the higher rate of MVR in BD. Of note, ΔLVEF was independent of age, sex, comorbidities, medical therapy and symptoms.

Table 5.
Univariable linear regression analysis for change in LVEF (ΔLVEF).

Table 6.
Multivariable linear regression analysis for change in LVEF (ΔLVEF).
4. Discussion
The present study investigated reverse remodeling in MVP patients undergoing mitral valve surgery for severe MR. The key findings are that (1) the BD subtype is independently associated with a reduced LVEF recovery after intervention, even after correction for baseline LVEF and type of surgical intervention, and (2) while baseline LVESVi and MVR were associated with ΔLVEF in the univariable regression analysis, they were not independently associated with post-operative change in LVEF.
In 2019, Le Tourneau et al. described the phased process of LV reverse remodeling after mitral valve surgery in primary MR. In short, the post-operative reduction in MR regurgitant volume results in an abrupt decrease in LV preload. Since LVEDV is known to be largely load-dependent, the early reverse remodeling phase is characterized by a decline in LVEDV, while LVESV does not change yet, resulting in a decreased stroke volume (LVEDV − LVESV). Accordingly, LVEF (stroke volume/LVEDV) will decline as well. After several months, LV end-systolic volume usually decreases towards normal values with recovery of LVEF [13]. The observed LVESVi and LVEF at >6 month follow-up in FED patients in our cohort is in line with this late reverse remodeling phase. In contrast, the BD patients in our study showed a trend towards less favorable reverse remodeling with higher LVESVi and lower LVEF at follow-up.
Furthermore, our study confirms the recent findings of Althunayyan et al. that LV dysfunction after mitral valve surgery is not uncommon in patients with MVP [14]. In our study, clinically significant LV dysfunction with LVEF < 50% at follow-up [15] was more prevalent in patients with BD compared to FED, but this difference did not remain significant after correction for baseline LVEF.
Moreover, patients with BD had a higher rate of MVR compared to FED, which has previously been associated with a lower post-op LVEF compared to MV repair [16], yet MVP subtype remained a significant determinant for change in LVEF even after correction for surgical intervention. In fact, effective LV contraction is partially supported by the dynamic relationship between the mitral valve annulus (including papillary muscles and chorda tendineae) and the LV free wall. Therefore, disrupting this annulo-ventricular continuity due to the (partial) removal of the subvalvular apparatus during MV replacement is hypothesized to result in less favorable post-operative LV geometry and function compared to MV repair. However, MVR with the preservation of the papillary muscles and/or chordae tendineae is associated with similar post-operative LV reverse remodeling compared to MV repair, as was recently observed by Craven et al. [17]. Their findings are in line with our data and subgroup analysis excluding patients without MV subvalvular apparatus preservation, which confirmed that BD was associated with a significantly larger post-operative decrease in LVEF compared to FED.
Therefore, the question arises whether the previously observed effect of MVR on post-operative LVEF could be partially explained by the higher prevalence of BD together with regular resection of the subvalvular apparatus in prior surgical cohorts, but thus far, this remains only a hypothesis.
What explains the difference in post-operative LV remodeling in BD versus FED remains to be elucidated; however, a number of observations deserve attention. First, BD is generally associated with longstanding LV volume overload due to chronic MR compared to the more (sub)acute MR following chordal rupture in FED. Therefore, patients with BD may present with already more structural LV adaptation in the preoperative phase compared to FED. Yet, we need to recognize that a difference in the time course of MR cannot be the complete explanation since significant LV remodeling was observed by Malev et al. in a very young cohort of BD patients without longstanding volume overload. Second, several studies have demonstrated that BD can be associated with severe LV dilatation and dysfunction as well as myocardial fibrosis, even in the absence of significant MR [6,7,8,18]. Third, BD has been associated with an underlying genetic substrate [19,20] and ventricular arrhythmias, including frequent premature ventricular contractions [21], which could contribute to dilated cardiomyopathy. Finally, El-Tallawi et al. suggested that the disproportionate LV dilatation in BD could be explained by a larger total volume load, defined as the sum of the MR volume and prolapse volume [22,23]. Due to annular dilatation and bileaflet prolapse in BD, these patients can present with a significant prolapse volume, but this is a rather unlikely explanation of the limited post-op reverse remodeling in BD since the prolapse volume is also corrected by mitral valve surgery.
Consequently, we hypothesize that other factors beyond volume overload affect the process of LV (reverse) remodeling in patients with BD. The presence of an underlying genetic and/or ectopy-induced cardiomyopathy could potentially elucidate why the successful elimination of volume overload through mitral valve surgery cannot completely reverse LV remodeling in some patients with BD. Furthermore, we could even speculate that a theoretically non-severe MR volume may be hemodynamically significant in a patient with BD due to their underlying myocardial substrate, which potentially suggests a benefit for early intervention in this patient group. Yet, this is currently no more than a hypothesis that requires further investigation.
We tried to overcome the inherent limitations of the retrospective study design by screening consecutive cases in three centers. Despite the multicenter aspect of the study, the sample size is rather small. The main reason for drop out was if patients received their echocardiographic follow-up with the referring cardiologist. Therefore, we expect no significant effect of patient selection on the differences between MVP subtypes. In addition, the ratio of included FED to BD patients (2:1) is similar to what would be expected in the general population. In addition, the rate of mitral valve replacement in this study (especially for BD patients) was higher than in previously published cohorts. Therefore, outcomes might be different in centers with higher repair rates for BD. Importantly, we only had access to the available standard 2D echocardiographic images that were obtained in clinical routine, while 3D echocardiography and cardiac magnetic resonance imaging are known to be superior in the assessment of LV volumes [24].
5. Conclusions
While patients with BD and FED showed similar MR severity, LV volumes and LVEF at baseline, the post-operative recovery of LVEF was significantly smaller in patients with BD, even after correction for baseline values and surgical intervention. These findings suggest that LV (reverse) remodeling in BD could be affected by other mechanisms beyond volume overload, including concomitant cardiomyopathy.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcdd11030071/s1, Table S1: Subanalysis of LV remodelling parameters at follow-up in patients who underwent mitral valve repair.
Author Contributions
Conceptualization, L.L.P., E.M.V.C. and C.M.V.D.H.; methodology, L.L.P. and C.M.V.D.H.; formal analysis, L.L.P., P.B.B., P.D. and C.M.V.D.H.; investigation, L.L.P., P.B.B., P.D., S.D., B.H., B.P.P., D.D.B., H.H. and C.M.V.D.H.; data curation, L.L.P., P.D., S.D. and B.H.; writing—original draft preparation, L.L.P.; writing—review and editing, L.L.P., P.B.B., P.D., S.D., B.H., B.P.P., D.D.B., H.H., E.M.V.C. and C.M.V.D.H.; visualization, L.L.P. and C.M.V.D.H.; supervision, C.M.V.D.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of University Hospital Antwerp (protocol code 5337, date of approval: 24 April 2023).
Informed Consent Statement
Patient consent was waived due to the retrospective nature of this study.
Data Availability Statement
The data underlying this article will be shared upon reasonable request to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Freed, L.A.; Levy, D.; Levine, R.A.; Larson, M.G.; Evans, J.C.; Fuller, D.L.; Lehman, B.; Benjamin, E.J. Prevalence and clinical outcome of mitral-valve prolapse. N. Engl. J. Med. 1999, 341, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Delling, F.N.; Vasan, R.S. Epidemiology and pathophysiology of mitral valve prolapse: New insights into disease progression, genetics, and molecular basis. Circulation 2014, 129, 2158–2170. [Google Scholar] [CrossRef] [PubMed]
- Pype, L.L.; Bertrand, P.B.; Paelinck, B.P.; Heidbuchel, H.; Van Craenenbroeck, E.M.; Van De Heyning, C.M. Left Ventricular Remodeling in Non-syndromic Mitral Valve Prolapse: Volume Overload or Concomitant Cardiomyopathy? Front. Cardiovasc. Med. 2022, 9, 862044. [Google Scholar] [CrossRef] [PubMed]
- Anyanwu, A.C.; Adams, D.H. Etiologic classification of degenerative mitral valve disease: Barlow’s disease and fibroelastic deficiency. Semin. Thorac. Cardiovasc. Surg. 2007, 19, 90–96. [Google Scholar] [CrossRef]
- Adams, D.H.; Rosenhek, R.; Falk, V. Degenerative mitral valve regurgitation: Best practice revolution. Eur. Heart J. 2010, 31, 1958–1966. [Google Scholar] [CrossRef] [PubMed]
- Yiginer, O.; Keser, N.; Ozmen, N.; Tokatli, A.; Kardesoglu, E.; Isilak, Z.; Uz, O.; Uzun, M. Classic mitral valve prolapse causes enlargement in left ventricle even in the absence of significant mitral regurgitation. Echocardiography 2012, 29, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Malev, E.; Reeva, S.; Vasina, L.; Timofeev, E.; Pshepiy, A.; Korshunova, A.; Prokudina, M.; Zemtsovsky, E. Cardiomyopathy in young adults with classic mitral valve prolapse. Cardiol. Young 2014, 24, 694–701. [Google Scholar] [CrossRef]
- Yang, L.T.; Ahn, S.W.; Li, Z.; Benfari, G.; Mankad, R.; Takeuchi, M.; Levine, R.A.; Enriquez-Sarano, M.; Michelena, H.I. Mitral Valve Prolapse Patients with Less than Moderate Mitral Regurgitation Exhibit Early Cardiac Chamber Remodeling. J. Am. Soc. Echocardiogr. 2020, 33, 815–825.e2. [Google Scholar] [CrossRef]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., 3rd; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 143, e35–e71. [Google Scholar]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease: Developed by the Task Force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2021, 75, 524. [Google Scholar] [CrossRef]
- Lancellotti, P.; Pibarot, P.; Chambers, J.; La Canna, G.; Pepi, M.; Dulgheru, R.; Dweck, M.; Delgado, V.; Garbi, M.; Vannan, M.A.; et al. Multi-modality imaging assessment of native valvular regurgitation: An EACVI and ESC council of valvular heart disease position paper. Eur. Heart J. Cardiovasc. Imaging 2022, 23, e171–e232. [Google Scholar] [CrossRef] [PubMed]
- Luyten, P.; Heuts, S.; Cheriex, E.; Olsthoorn, J.R.; Crijns, H.J.G.M.; Winkens, B.; Roos-Hesselink, J.W.; Nia, P.S.; Schalla, S. Mitral prolapsing volume is associated with increased cardiac dimensions in patients with mitral annular disjunction. Neth. Heart J. 2021, 30, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Le Tourneau, T.; Topilsky, Y.; Inamo, J.; Mahoney, D.W.; Suri, R.; Schaff, H.V.; Sarano, M. Reverse Left Ventricular Remodeling after Surgery in Primary Mitral Regurgitation: A Volume-Related Phased Process. Struct. Heart 2019, 3, 383–390. [Google Scholar] [CrossRef]
- Althunayyan, A.M.; Alborikan, S.; Badiani, S.; Wong, K.; Uppal, R.; Patel, N.; Petersen, S.E.; Lloyd, G.; Bhattacharyya, S. Determinants of post-operative left ventricular dysfunction in degenerative mitral regurgitation. Eur. Heart J. Cardiovasc. Imaging 2023, 24, 1252–1257. [Google Scholar] [CrossRef]
- Enriquez-Sarano, M.; Tajik, A.; Schaff, H.V.; Orszulak, T.A.; McGoon, M.D.; Bailey, K.R.; Frye, R.L. Echocardiographic prediction of left ventricular function after correction of mitral regurgitation: Results and clinical implications. J. Am. Coll. Cardiol. 1994, 24, 1536–1543. [Google Scholar] [CrossRef] [PubMed]
- Enriquez-Sarano, M.; Schaff, H.V.; Orszulak, T.A.; Tajik, A.J.; Bailey, K.R.; Frye, R.L. Valve repair improves the outcome of surgery for mitral regurgitation. A multivariate analysis. Circulation 1995, 91, 1022–1028. [Google Scholar] [CrossRef]
- Craven, T.P.; Chew, P.G.; Dobson, L.E.; Gorecka, M.; Parent, M.; Brown, L.A.E.; Saunderson, C.E.D.; Das, A.; Chowdhary, A.; Jex, N.; et al. Cardiac reverse remodeling in primary mitral regurgitation: Mitral valve replacement vs. mitral valve repair. J. Cardiovasc. Magn. Reson. 2023, 25, 43. [Google Scholar] [CrossRef]
- Constant Dit Beaufils, A.L.; Huttin, O.; Jobbe-Duval, A.; Senage, T.; Filippetti, L.; Piriou, N.; Cueff, C.; Venner, C.; Mandry, D.; Sellal, J.M.; et al. Replacement Myocardial Fibrosis in Patients with Mitral Valve Prolapse: Relation to Mitral Regurgitation, Ventricular Remodeling and Arrhythmia. Circulation 2021, 18, 1763–1774. [Google Scholar] [CrossRef]
- Le Tourneau, T.; Mérot, J.; Rimbert, A.; Le Scouarnec, S.; Probst, V.; Le Marec, H.; A Levine, R.; Schott, J.-J. Genetics of syndromic and non-syndromic mitral valve prolapse. Heart 2018, 104, 978–984. [Google Scholar] [CrossRef]
- van Wijngaarden, A.L.; Hiemstra, Y.L.; Koopmann, T.T.; Ruivenkamp, C.A.L.; Aten, E.; Schalij, M.J.; Bax, J.J.; Delgado, V.; Barge-Schaapveld, D.Q.C.M.; Marsan, N.A. Identification of known and unknown genes associated with mitral valve prolapse using an exome slice methodology. J. Med. Genet. 2020, 57, 843–850. [Google Scholar] [CrossRef]
- Essayagh, B.; Sabbag, A.; Antoine, C.; Benfari, G.; Yang, L.-T.; Maalouf, J.; Asirvatham, S.; Michelena, H.; Enriquez-Sarano, M. Presentation and Outcome of Arrhythmic Mitral Valve Prolapse. J. Am. Coll. Cardiol. 2020, 76, 637–649. [Google Scholar] [CrossRef] [PubMed]
- El-Tallawi, K.C.; Kitkungvan, D.; Xu, J.; Cristini, V.; Yang, E.Y.; Quinones, M.A.; Lawrie, G.M.; Zoghbi, W.A.; Shah, D.J. Resolving the Disproportionate Left Ventricular Enlargement in Mitral Valve Prolapse Due to Barlow Disease: Insights From Cardiovascular Magnetic Resonance. JACC Cardiovasc. Imaging 2020, 14, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Levy, F.; Iacuzio, L.; Marechaux, S.; Civaia, F.; Dommerc, C.; Wautot, F.; Tribouilloy, C.; Eker, A. Influence of Prolapse Volume in Mitral Valve Prolapse. Am. J. Cardiol. 2021, 157, 64–70. [Google Scholar] [CrossRef]
- Van De Heyning, C.M.; Magne, J.; Piérard, L.A.; Bruyère, P.-J.; Davin, L.; De Maeyer, C.; Paelinck, B.P.; Vrints, C.J.; Lancellotti, P. Assessment of left ventricular volumes and primary mitral regurgitation severity by 2D echocardiography and cardiovascular magnetic resonance. Cardiovasc. Ultrasound 2013, 11, 46. [Google Scholar] [CrossRef] [PubMed][Green Version]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).