Simple Summary
The employment of stem cells in the treatment of diverse animals’ illnesses and the engineering of various body tissues continues to evolve quickly. Many animal models have been utilized to assess the safety and efficacy of cell-based therapies. They were exploited mainly at a preclinical scale to measure the potential of the application of such therapies in humans and/or animals. Significant research endeavors are conducted to expand their use to include clinical cases. Herein, the main objective of the present article is to discuss the research efforts conducted to use stem cell therapies in veterinary practice, either on experimental animals or clinical cases based on the currently available knowledge.
Abstract
The introduction of new regenerative therapeutic modalities in the veterinary practice has recently picked up a lot of interest. Stem cells are undifferentiated cells with a high capacity to self-renew and develop into tissue cells with specific roles. Hence, they are an effective therapeutic option to ameliorate the ability of the body to repair and engineer damaged tissues. Currently, based on their facile isolation and culture procedures and the absence of ethical concerns with their use, mesenchymal stem cells (MSCs) are the most promising stem cell type for therapeutic applications. They are becoming more and more well-known in veterinary medicine because of their exceptional immunomodulatory capabilities. However, their implementation on the clinical scale is still challenging. These limitations to their use in diverse affections in different animals drive the advancement of these therapies. In the present article, we discuss the ability of MSCs as a potent therapeutic modality for the engineering of different animals’ tissues including the heart, skin, digestive system (mouth, teeth, gastrointestinal tract, and liver), musculoskeletal system (tendons, ligaments, joints, muscles, and nerves), kidneys, respiratory system, and eyes based on the existing knowledge. Moreover, we highlighted the promises of the implementation of MSCs in clinical use in veterinary practice.
1. Introduction
The key purpose of tissue engineering is to renovate damaged tissues and replace them with new functional ones. Consequently, these tissues can restore their entire function. This process is multidisciplinary and needs the study of biochemistry, cell biology, developmental biology, biomaterials, and bioengineering [1]. Over the years, a wide range of therapeutic choices have been investigated in different animal models and at diverse scales of research to be used for animals’ and humans’ regenerative therapeutic purposes [2,3,4,5,6,7]. However, the subject of stem cells continues to pique the scientific community’s curiosity. They can be classified as cells with the capacity for self-renewal and cell differentiation in the most basic sense [8,9]. From the time the ovum is fertilized until the time of death, these cells are found in every living thing. Their existence enables the tissues to grow and keep the equilibrium of somatic cells. They also support organ and tissue regeneration by swapping out somatic cells that age or sustain injury [10]. The development of techniques for obtaining stem cells has brought us considerably closer to realizing humanity’s long-held goal of replacing diseased and worn-out cells and/or tissues with fresh ones that are produced in a lab. The Nobel Prize, given to John Gurdon and Shinya Yamanaka for producing what is known as induced pluripotent stem cells (iPSCs), served as a reminder of the significance of stem cells in medicine. They are modified somatic cells that develop stem cell characteristics [11]. It is now possible to employ stem cells in human and animal medicine as a result of countless studies on them in different scientific domains [12,13]. Numerous authors have cited the following characteristics of stem cells: their simple composition and deficient differentiation; self-renewal, which enables maintenance of a persistent population of cells over the life of an animal; asymmetric division, which produces a larger and a smaller stem cell that undergoes additional linear differentiation; the capacity to discriminate into cells of many tissues; and the expression of proteins [8,14,15,16]. The information that is currently known regarding stem cell usage in veterinary medicine is included in this publication. Based on chosen scientific papers, it covers different stem cell types, their immunomodulatory characteristics, and an assessment of the therapy’s efficacy in the treatment of the heart, skin, digestive system (mouth, teeth, gastrointestinal tract, and liver), musculoskeletal system (tendons, ligaments, joints, muscles, and nerves), kidneys, respiratory system, and eyes in the veterinary field.
2. Stem Cells Classification
Stem cells are distinguished by being undifferentiated cells present at diverse life stages from the embryonic to the adult stage, producing the adult cells of various body tissues and organs. They are classified based on variable criteria, including their differentiation potential, their origin, and their relationship to the recipient [17].
2.1. Differentiation Potential
Concerning the differentiation capacity of stem cells, they may be totipotent, pluripotent, multipotent, oligo-/bipotent, or unipotent [17]. Totipotent stem cells are those cells with an infinite capacity for division. Due to their ability to differentiate into any cell that contributes to the development of the embryo and extra-embryonic tissues (placenta, umbilical cord), these cells can give rise to the complete body. As shown by monozygotic twins made from several blastomeres, the totipotent cell is a fertilized ovum (zygote), and cells are acquired from the first germinal stage (morula) [18,19]. Pluripotent stem cells are the cells that have the potential to differentiate into cells that emerge from the germ layers: endoderm, ectoderm, and mesoderm. Hence, unlike the totipotent cells, they are unable to give rise to the full body tissues. The blastocyst germ cells, also known as the inner cell mass (ICM), are an example of pluripotent cells [20]. Multipotent cells are the cells with an outstanding ability to differentiate into various kinds of cells developed from a single germ layer (germ layer-derived cells) [21,22] (Figure 1). They are characterized by their unique proliferation potential, their potential to differentiate into diverse body cells of various origins, and their correlation to the receiver. They can be obtained from diverse tissues such as adipose tissue, bone marrow, umbilical cord blood, and bone Wharton’s jelly. Mesenchymal stem cells are the most investigated multipotent stem cells [17,23]. Oligo-/bipotent stem cells are those cells with the capacity to differentiate into two or more lineages inside a specific tissue. For instance, swine ocular cells have been proven to produce both conjunctival and corneal cells [24]. Unipotent stem cells, also known as precursor cells, exhibit a specialized differentiation pathway into a particular kind of adult body cell. The reproductive layer cells of the epidermis are one example of how they enable the maintenance of a consistent cell population in the tissues under normal circumstances [22].
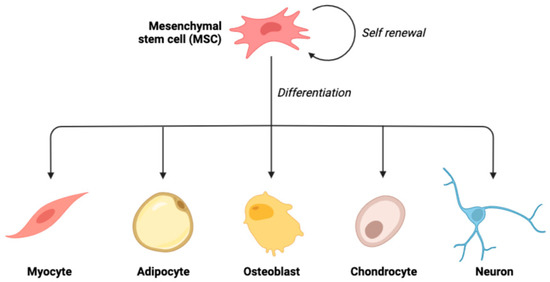
Figure 1.
Schematic illustration of the ability of mesenchymal stem cells to undergo multilineage differentiation.
2.2. Origin of Stem Cells
According to their origin, stem cells may be embryonic, adult, tissue-derived, or iPSCs [17]. Totipotent embryonic stem cells (ESCs) are those cells isolated from the embryo’s blastomeres. However, the most often employed pluripotent cells for experimentation are those from the blastocyst embryonic node. Fetal tissues, umbilical cord tissues (such as Wharton’s jelly), umbilical cord blood, and amniotic cells are the sources of fetal stem cells (FSCs) [25]. Embryonic stem cells have an unlimited capacity for self-renewal and differentiation into all cell types in vitro (Figure 2). However, it has not yet been feasible to establish exact methods for controlling their differentiation and division at the site of injection in vivo, which might, among other factors, result in the emergence of cancerous lesions realized in experimental animals [26]. These challenges, along with ethical concerns, are the major causes of the lack of ESC utilization in clinical stem cell treatment [27]. Somatic or mature cells are synonyms for adult stem cells (ASCs). They are multi- or unipotent, undifferentiated cells.
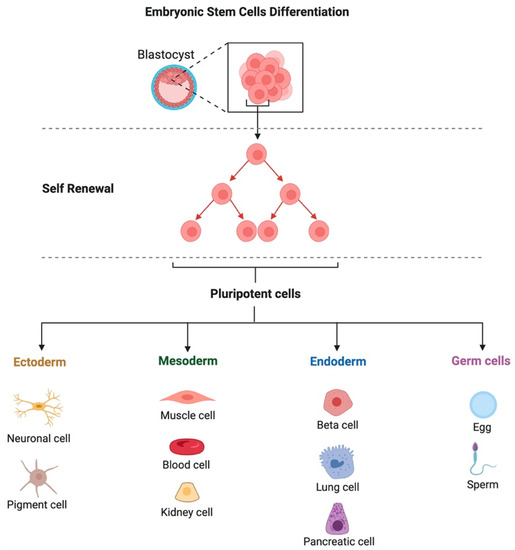
Figure 2.
Schematic illustration of the source and multilineage differentiation of embryonic stem cells.
The possibility of tissues and organs in which they exist is given by their occurrence in the body throughout the postnatal period [25,28]. As an alternative to pluripotent cells of embryonic origin, autologous stem cells of a pluripotent type can be produced by converting adult somatic cells, such as fibroblasts (cultured in vitro). They are produced by inserting the genes for the transcription factors (c-Myc, Klf 4, Oct 3/4, Sox 2) required for the formation of embryonic cells. Induced pluripotent stem cells are the resultant cells, which may develop similarly to embryonic cells [27]. Unfortunately, this process is not very successful, and when laboratory mice were given these cells, they developed teratomas such as ESC [10].
2.3. Relationship to the Recipient
Based on their relation to the recipient, stem cells can be autogenic, allogeneic, and xenogeneic [29]. Autogenic cells are the most currently investigated. These cells are separated from the recipient animal that is also the donor (for example, bone marrow, adipose tissue, or cord blood). Allogeneic stem cells are harvested from a different animal of the same species. They serve as the cornerstone of therapies that employ both adult and embryonic stem cells [28]. The utilization of allogenic stem cells provides ready-to-use products that enable suitable timing of the regenerative therapy implementation and skip the required time for the expansion of the cultured autogenic cells. However, the key restriction factor of this cell type is the need for a donor. Xenogeneic stem cells are alternatives to autogenic and allogenic stem cells [30]. Xenogenic stem cells can be harvested and obtained from various animals of different species. Numerous investigations using antlerogenic (xenogenic) cells revealed that some species, including rabbits and horses [31], had a regenerative response when their tissues were exposed to stem cells denoted by the macrophage inhibitory cytokine 1 (MIC-1) [32].
3. Sources of MSCs in Tissue
Mesenchymal stem cells may be effectively extracted from practically any tissue in a live organism. The finest outcomes are, however, attained when extracting cells from adult tissues such as adipose tissue (AD-MSCs), bone marrow (BM-MSCs), peripheral blood (PB-MSCs), or fetal tissue such as the placenta (P-MSCs), umbilical cord blood (UCB-MSCs), or umbilical cord (UC-MSCs) [33,34]. This is because of the proliferative and differentiation potential of these cells. It was discovered that the kind of cells, based on their origin, has a substantial influence on both their ability to differentiate in vivo and their physiologically important characteristics [26]. The usage of MSCs in a particular therapy should be a key consideration when choosing the source of those cells. Bone marrow is one of the most researched sources of MSC origin in veterinary treatment, similar to human medicine [35]. Sadly, collecting the samples is an intrusive operation done under the effect of general anesthetic in canines and sedatives with or without local anesthetic in equines, both of which have a danger of postoperative problems, including infection and/or bleeding [36]. Several reports of serious unintentional cardio-thoracic punctures [37] as well as nonfatal pneumopericardium in horses [38] have been published. However, in these animals, the BM-MSC collection process may be carried out on a standing animal with little to no danger of the previously stated problems. Today, it is acknowledged that sternal biopsy utilizing a Jamshidi needle at the 4th or 5th sternebra is the most appropriate location [39,40]. The quantity of BM-MSCs diminishes with aging and makes up a very minor portion of all bone marrow stromal mononuclear cells [41]. The aspirate that was previously taken from the donor is used for MSC culture and passage in vitro. The therapeutic number of MSCs strictly relies on the number of cultured MSCs, which can be passaged four times maximum. This culture includes various phases (establishing of the culture, cell growth, change of the medium, and completion of the culture with the determination of the cell’s phenotype).
Adipose tissue has grown in popularity and accessibility as a source of material for MSC extraction and separation in recent years [42]. The simplest method of collecting the material is a planned lipectomy or lipoaspiration carried out during preventive ovariohysterectomy in dogs and cats. Subcutaneous or visceral fat has been proposed as a source of AD-MSCs [43]. Immediately upon collection, a small number of nucleated cells with evidence of AD-MSCs may be extracted from the aspirate. Similar to BM-MSCs, it is essential to culture fat-derived cells in vitro to acquire a therapeutic dosage. This requires particular laboratory techniques, such as enzymatic digestion, followed by serial washing and centrifugation. Since AD-MSCs have been demonstrated to present a high ability for differentiation as well as proliferation, regenerative medicine routinely uses these cells as a source. These cells have been and are often employed in the therapy of musculoskeletal illnesses, and when compared to BM-MSCs, they have a higher bioavailability and are easier to get [44,45]. An alternate source of MSCs is thought to be PB-MSCs. The least contentious and least dangerous one that does not need pharmacologically sedating the animal is collecting a blood sample, which is superior to the other ways mentioned above. Attributed to the limited bioavailability of PB-MSCs in the peripheral blood of dogs, horses, and humans, the current studies must be continued. Additionally, these cells were effectively extracted from guinea pigs, mice, and rabbits [46]. According to the authors, only 3 out of 10 test horses had adequate blood cells for further cell culture. To boost the quantity of PB-MSCs in the peripheral circulation, these animals were additionally given particular treatments (hyperbaric chambers) [47,48]. Fetal tissues, including the placenta, fetal membranes, amniotic fluid, Wharton jelly (WJ) of the umbilical cord, and umbilical cord blood, are another potential and increasingly often employed source of stem cells in veterinary medicine [49,50]. It is well recognized that MSC performance decreases with donor aging. The ability of cells isolated from fetal tissues to differentiate into not just mesenchymal cells but also cells from the other three germ layers may be a sign of their pluripotency [51]. The process for gathering the material is categorized as a minimally invasive approach and takes place in the perinatal period as opposed to the collection of BM-MSCs or AD-MSCs. The reduced sterility of the method itself may be the noted drawback of the material packing approach [52].
4. Autogenous Allogeneous, and Xenogenic MSCs
Allogeneic and autologous stem cells are the two main kinds of stem cells employed in modern regenerative therapy. The difference in the safety and effectiveness of employing these cells in treatment is a topic of continuous discussion among experts worldwide. As previously indicated, MSCs can be categorized as being of allogeneic, autologous, or xenogeneic origin, depending on the donor-recipient relationship. The recipient of allogeneic cells is a member of the same species as the donor. Compared to autologous cells, successful in vitro cultivation of these cells drastically decreases the time needed to perform the therapy. The antlerogenic stem cells MIC-1 (obtained from a deer) were therapeutically administered to horses and experimentally injected into the tissues of injured rabbits [53]. They are the least often employed source of MSCs because the donor and recipient are from different species. Researchers have been attempting to establish whether allogeneic or autologous cells are superior throughout the heyday of regenerative medicine and at the height of the hunt for alternative therapies. One must weigh the benefits and drawbacks of each type of MSC before choosing the kind of therapy to be utilized. The safest source of MSCs, according to logic, should be cells extracted directly from the same animal. Hence, autologous cells are thought to be safer for therapy, but on the other hand, acquiring and preparing a therapeutic amount of these MSCs is a time-consuming process that frequently involves surgery during harvesting with high possibilities of infection and disease transmission [54]. This technique is only sometimes employed because of the aforementioned issues and the overall expense of the process. The animal’s age, sex, and kind of sickness are all strongly correlated with the quantity and quality of autologous cells in that animal [55,56,57]. On the other side, after donation, allogeneic cells can be cultivated and stored in a cell bank forever (Figure 3). This makes it possible to identify a single-cell batch with a stable MSC quantity, safety level, and potential for differentiation.
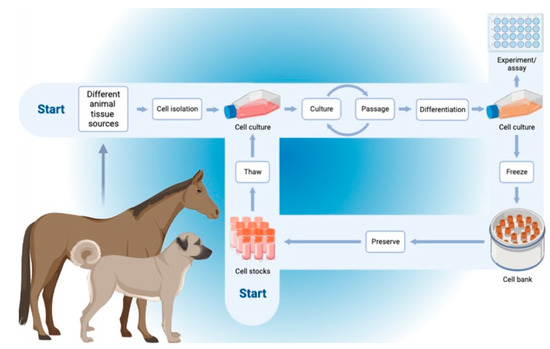
Figure 3.
Schematic representation of the workflow of the autogenic and allogenic mesenchymal stem cells from the harvesting, culturing, storage, and use.
Such a strategy would shorten the time required for material collection and cell growth, minimize usual variability, as in the autogenic cells, and allow for uniformity of the therapy and the anticipated outcomes [57,58]. The national legal guidelines about the utilization of medical preparations should also be considered when using allogeneic cells. Bertoni et al. [59] provided intriguing test findings after analyzing the impact of auto- and allogeneic stem cells on the fetlocks of healthy horses. Although there was no discernible change in the characteristics of the synovial fluid, it was declared that BM-MSCs considerably increased synovial effusion when compared to UCB-MSCs. Compared to the placebo, MSC injections caused slight to moderate topical inflammatory symptoms, whereas more cells showed reduced inflammation during clinical and ultrasonographic tests [59]. Two allogeneic cell treatment products for horses and one for dogs that have a marketing license from the European Medicines Agency, among others, are proof of the tolerance of allogeneic stem cells [60]. Many investigations have studied the use of xenogeneic stem cells. Various experimental animals, including mice, rats, rabbits, dogs, and baboons, were used to cultivate these cells both locally and systemically [61]. Studies on rats revealed that 12 weeks after being injected into the carotid artery, xenogeneic cells (murine) were present in the bone marrow. In addition to the bone marrow, their presence has been detected in the myocardial infarction tissue as immature myocytes [62]. Further effective trials to use xenogeneic cells have been reported. For instance, rat stem cells were used to regenerate bone tissue in rabbits [63], human bone marrow stem cells were used to treat rat spinal cord injuries [64], and the same cells were used to regenerate bone in mice [65]. A growing number of researchers believe that the use of xenogeneic stem cells can replace the use of allogeneic or autogenous stem cells in regenerative medicine after analyzing the already available data. The immunogenicity of xenogeneic stem cells is comparable to that of autologous and allogeneic stem cells, according to studies [25,28].
5. Immunomodulatory Potential of MSCs
The presence of the major histocompatibility complex (MHC) on the surfaces of the living cells plays a key role in the detection of the acceptance or rejection of the donated tissue by the recipient’s body. Hence, there is a possibility for the rejection of the allogenic cells or organs. Almost every nucleated cell in the body possesses a class I MHC (MHC I) molecule, whereas class II molecules (MHC II) are associated with immune cells such as macrophages, dendritic cells, and lymphocytes. As a result, recipient CD8+ T cells detect every allogeneic cell with MHC I surface molecules, resulting in the direct cytotoxicity of the foreign cells. Moreover, they can be identified by the recipient CD4+ T cells if they carry MHC II molecules, which can trigger a cytotoxic or humoral immune response. After being indirectly recognized by antigen-presenting cells, B cells may also produce alloantibodies [66]. Despite the absence of clear information on their immunogenicity, adult MSCs are thought to be low-immunogenic. This is attributed, in part, to the lack of MHC II antigens on their surface and the poor expression of the MHC I. They prevent NK cells, T lymphocytes, and B lymphocytes from proliferating or functioning normally. Additionally, they block antigen-presenting cells and promote the growth of suppressor T cells [67]. Only MHC I molecules are expressed on the surface of MSCs following their stimulation to differentiate along the adipogenic, osteogenic, and chondrogenic lineages [68]. The allogeneic variety of MSCs may be a widely available source for regenerative therapy in veterinary medicine since they appear to be immunologically privileged. However, it is important to consider the possibility that the receiver may contract the donor’s illness [69]. Hence, it is necessary to schedule a specialized systemic strategy for the management of possible viral, bacterial, and fungal contaminations for each species. The MSC secretome is rich in anti-inflammatory cytokines such as interleukin 18 (IL18), IL13, IL10, neurotrophin 3 (NT-3), and ciliary neurotrophic factor (CNTF). Thus, it presents an outstanding immunomodulatory ability [70]. Furthermore, it has been proven to be safer than MSCs despite being administered in high doses in some cases without major adverse effects. Additionally, its use exhibits a lower possibility of emboli formation post-intravenous administration or pathological or tumorigenic transformations compared to those caused by the hysterical cell differentiation. In addition, it allows the utilization of allogenic therapies without immune stimulation as it represents the structure of the parental cells [71].
6. Clinical Applications of Stem Cells in Regeneration and Bioengineering of Different Body Organs and Tissues in Veterinary Practice
Stem cells have so far been employed, primarily at experimental scales, to cure a wide range of illnesses in many animal species. The early focus of veterinary regenerative medicine was on orthopedic illnesses, but it is currently swiftly spreading to include additional conditions, such as those affecting the heart, digestive tract, liver, kidneys, lungs, nerves, and muscles. Stem cells have frequently been employed in dogs and horses with various tissue diseases. Moreover, in cats, renal and respiratory affections and several inflammatory disorders were treated with these cells [26]. In this section, the employment of stem cell therapy for engineering and treating of different body tissue affections in diverse animals is discussed, and some representative examples are presented in Table 1.
6.1. Heart
Cardiovascular diseases are the leading reason for death in humans worldwide. Likewise, they are very serious in animals as well, with the challenge that most cases passed go undiagnosed. However, the introduction of novel cardiac imaging tools in veterinary medicine has significantly helped in the early diagnosis or even prediction of cardiac dysfunctions [72,73,74,75,76,77,78,79,80,81]. That, in turn, will help to refer them to available therapeutic options.

Table 1.
Employment of diverse kinds of stem cells in the regeneration of various body tissues in different animals.
Table 1.
Employment of diverse kinds of stem cells in the regeneration of various body tissues in different animals.
| Body Systems and Tissues | The Type of Cells Used | Animal Model | Route of Administration | Outcomes | Refs. | |
|---|---|---|---|---|---|---|
| Heart | Adipose-derived mesenchymal stem cells (AD-MSCs) | Dobermann dogs with dilated cardiomyopathy | Retrograde coronary venous delivery | The stem cell therapy was safe. However, it did not prevent the progression of the disease | [82] | |
| Allogenic Cardiosphere-derived cells (CDCs) | Doberman pinscher dogs with spontaneous DCM | Intracoronary infusion | Safety was confirmed with an effective improvement of the heart functions | [83] | ||
| Allogenic puppy deciduous teeth stem cells (PDSCs) | Dogs with chronic valvular heart disease | Intravenous injection | Amelioration of the cardiac functions and improvement of the quality of the life scores | [84] | ||
| Skin | Caprine amniotic fluid and bone marrow-derived mesenchymal stem cells | New Zealand white rabbits | Subcutaneous injection | Amniotic fluid-derived cells were superior to bone marrow-derived cells for enhancement of skin wound healing | [85] | |
| Peripheral blood-derived MSCs | Sheep | Subcutaneous injection in the margins of the skin wound | Improvement of both superficial and deep wound healing | [86] | ||
| Umbilical cord-blood-derived equine MSCs | Horses | Subcutaneous injection in the margins of the skin wound | Stem cell therapy is a promising choice for the treatment of distal extremity wounds in horses | [87] | ||
| Digestive System | Mouth and teeth | Adipose-derived multi-lineage progenitor cells (ADMPC) | Micro-mini pig | Topical transplantation of autologous or allogeneic ADMPC-fibrin gel complex | ADMPC presented an immune-modulation action and enhanced periodontal tissue regeneration | [88] |
| Dental pulp stem cells (DPSCs) | Mongrel dogs | Direct pulp capping method | DPSCs have exhibited a promising capacity for regeneration of the damaged dentin. | [89] | ||
| Mobilized dental pulp stem cells (MDPSCs) isolated from the abdominal subcutaneous adipose tissue | Dogs | Topical transplantation in the pulpectomized dogs | Safety was confirmed with the enhancement of total pulp regeneration | [90] | ||
| Gastrointestinal tract | Adipose tissue-derived MSCs (AD-MSCs) | Dogs with inflammatory bowel disease (IBD) | Intravascular (IV) infusion | IV infusion of AD-MSCs was safe and effective in dogs with IBD | [91] | |
| Adipose-derived feline mesenchymal stem cells (fMSC) | Cats with chronic enteropathy | Intravenous injection | fMSCs were safe and effective for the treatment of cats with chronic enteropathy | [92] | ||
| Liver | Canine adipose-derived mesenchymal stem cells (cADSCs) | Dogs with induced acute liver injury | Intraperitoneal injection | cADSCs played an important role in the regeneration of canine liver | [93] | |
| Bone marrow-derived mesenchymal stem cells (BM-MSCs) | Dogs with induced liver fibrosis | Intravenous infusion | Improvement of liver functions with no adverse effects | [94] | ||
| Musculoskeletal system | Tendons and ligament | Bone marrow-derived mesenchymal stem cells (BM-MSCs) | Horses with tendon or ligament injuries | Topical intralesional injection | BM-MSCs were safe and successfully regenerated equine tendons and ligaments | [95] |
| Bone marrow-derived mesenchymal stem cells (BM-MSCs) | Polo with an injured superficial digital flexor tendon | Topical injection at the lesion site | Improvement of the regeneration capacity of the injured tendon | [96] | ||
| Joints | Bone marrow-derived mesenchymal stem cells (BM-MSCs) | Horses with a stifle injury | Intra-articular administration | Improvement of the stifle injury with enhanced ability of the animals to return to work | [97] | |
| Adipose-derived MSCs (AD-MSCs) | Horses with osteoarthritis | Intra-articular administration | AD-MSCs were efficient and safe | [98] | ||
| Muscles and nerves | Neurogenically-induced bone marrow-derived mesenchymal stem cells (NIBM-MSCs) | Dogs with paraplegia | Percutaneous transplantation | NIBM-MSC therapy was a promising option for the treatment of spinal cord injuries | [99] | |
| Bone marrow-derived mesenchymal stem cells (BM-MSCs) | Dogs with paraplegia | Intralesional injection | Enhanced regeneration of the injured spinal cord | [100] | ||
| Kidneys | Bone marrow-derived or adipose tissue-derived MSCs (BM-MSCs or AD-MSCs) | Cats with chronic kidney disease (CKD) | Ultrasound-guided intrarenal injection | Improved kidney regeneration and function | [101] | |
| Amniotic membrane-derived MSCs (AMSCs) | Cats with chronic kidney disease (CKD) | Ultrasound-guided intrarenal injection and intravenous infusion | AMSCs exhibited a renoprotective effect and enhanced kidney function | [102] | ||
| Respiratory system | Bone marrow-derived mononuclear cells (BMMCs) | Horses with recurrent airway obstruction (RAO) | Intratracheal instillation | BMMCs could reduce inflammatory reactions | [103] | |
| Human umbilical cord-derived mesenchymal stem cells (MSCs) | Dogs with radiation-induced lung injury | Intratracheal transplantation | Reduced lung injury and declined inflammation | [104] | ||
| Eye | Bone marrow-derived mesenchymal stem cells (BM-MSCs) | Horses with unilateral immune-mediated keratitis (IMMK) | Subconjunctival injection | Improved corneal clarity with improved regeneration of the corneal tissue | [105] | |
| Feline adipose-derived mesenchymal stromal cells (fAd-MSCs) | Cats with feline eosinophilic keratitis (FEK) | Subconjunctival injection | fAd-MSCs were safe and effective for the treatment of FEK | [106] | ||
Cardiac stem cell treatments were employed many years ago in human medicine to heal the damaged myocardium after an acute or chronic myocardial infarction [107]. Companion animals seldom get primary myocardial infarction [108]. However, dilated cardiomyopathy is a rather prevalent condition in large dog breeds. This condition will inevitably progress, which will result in refractory congestive heart failure and death. Retrograde coronary venous allogeneic AD-MSCs administration was used in Dobermans as an experimental therapy for this condition. Although safe, stem cell therapy did not present any positive outcomes [82]. Likewise, transplanting allogeneic cardiosphere-derived stem cells into coronary arteries did not improve the condition of animals with dilated cardiomyopathy [83]. The most prevalent cardiac condition in smaller dog breeds is degenerative valve disease, which is frequently complicated by ventricular enlargement and cardiac dysfunction [109]. Petchdee and Sompeewong [84] have investigated the utility of puppy deciduous teeth-derived stem cells administered intravenously in the treatment of degenerative valvular conditions. Even though this was a small trial, the left ventricular ejection fraction improved. Hence, further research will be required to confirm any possible beneficial findings.
6.2. Skin
The process of successful wound healing is usually disrupted by inadequate cellular and molecular pathways. This frequently results in a persistent, chronic wound that makes the animal uncomfortable. Owing to their anti-inflammatory and regenerative capabilities, MSCs may thus be a potentially effective therapy for chronic wounds with significant inflammation and a hyperplastic response [110]. The positive effects of MSC therapy on wound healing in caprines [85], ovines [86], horses [87], and canines have been demonstrated in several investigations using animal models [111]. The utilization of MSC-generated extracellular vesicles (ECVs) injected locally to treat dogs’ wounds led to noticeably better cutaneous wound healing [112]. In another work, peripheral blood stem cells (PBSCs) were infused topically and systemically into four horses with spontaneous infected wounds that had not responded to traditional treatments. Within four weeks of therapy, tissue expansion was seen in all four cases as a result of the formation of crusts and tiny scars in the middle of the incision [113]. In a further investigation, full healing of the non-healing skin lesion was also shown in a filly following repeated topical applications of heterologous WJ-generated MSCs with the utilization of carboxymethylcellulose gel. In 5 days, the wound had entirely healed [114]. On the other hand, MSCs were utilized to treat atopic dermatitis, one of the most prevalent skin conditions in dogs, in addition to wound healing. Two trials employing a comparable amount of IV-administered AD-MSCs in dogs with atopic dermatitis have found conflicting outcomes. At first, there was no discernible improvement in the clinical symptoms or pruritus [115]. Twenty-two dogs with atopic dermatitis (AD) who had not responded to conventional treatment were enrolled in the second study. After receiving allogeneic AD-MSCs intravenously for one month, cadesi-04 scores and pruritus were both drastically reduced. Clinical symptoms were in remission for at least 6 months with no side effects noticed [116]. Stem cells may be an intriguing potential therapeutic to support chronic wound healing, according to diverse research conducted on both laboratory and clinical veterinary animals. However, many issues must be resolved in further research before such medicines are used widely in clinical practice. Since there is a dearth of information on AD in dogs, it is hard to foresee, at this time, whether stem cell therapies will be effective.
6.3. Digestive System
6.3.1. Mouth and Teeth
The quality of the animal’s life might be significantly impacted by oral discomfort and mastication issues [117]. Recently, oral and dental disorders have become widely prevalent among dogs and cats worldwide and are associated with severe pain and potential topical and systemic infections [118,119]. Stem cells have gained attention for the repair of oro-dental tissues as regenerative cell treatment has advanced. Studies emphasize the ability of MSCs to modulate the immune responses to promote the regeneration of periodontal and dental tissues, as well as their differentiation capability to enhance the strength of the implant and bone tissue healing in alveolar defects. In the mini-pig periodontal defect model, allogeneic AD-MSCs alone could also stimulate the regeneration of periodontal tissue [88]. Autologous [89] and allogeneic [90] dental pulp stem cells, or autogenous BM-MSCs [120], were effective in regenerating canine dental pulp. Even while these studies suggest that oro-dental tissue regeneration is possible, other research suggests that stem cells have no positive effects on dental disorders such as problems with dental implants [121]. Animal model studies do provide a base and a point of reference for the utilization of stem cells and tissue engineering in boosting oro-dental tissue regeneration. The effectiveness and utility of stem cell therapies for the treatment of oro-dental issues in animals with natural disorders, however, still need significant investigation. Feline chronic gingivostomatitis (FCGS) is a painful and crippling oral illness in cats characterized by persistent gingival inflammation that extends to the buccal and caudal oral mucosa. Anorexia, oral discomfort, weight loss, ptyalism, halitosis, and a lack of grooming are the major symptoms of FCGS [122]. Currently available treatments include corticosteroids [123], cyclosporin [124], and tooth extraction surgery [125], and have varying response rates and many potential side effects [126]. The employment of MSC therapy in FCGS has shown extremely positive results. In most cats, full clinical and histological remission or a decrease in the severity of the clinical condition were the outcomes of IV therapy with autogenic AD-MSCs, according to research by Arzi et al. [127]. The stabilization of immune cell subsets, cytokine levels, and serum protein levels served as proof of the immunomodulation of MSCs. The study’s findings also indicated that the lack of cells with low expression of CD8 (CD8lo cells) could serve as a biomarker to gauge how well MSC treatment will work. It is interesting to note that allogeneic AD-MSCs appear to have poorer clinical effectiveness than autogenic MSCs in the treatment of FCGS [128]. About 70% of cats with FCGS treated with IV allogenous AD-MSC had a clinical, histopathological, and systemic response [29].
6.3.2. Gastrointestinal Tract
Inflammatory bowel disease (IBD) is an idiopathic disorder of the gastrointestinal tract distinguished by continual or repeated gastroenteric symptoms with inflammatory evidence with unknown underlying reasons [129,130]. Some dogs are resistant to the standard, ongoing therapies, including cyclosporine or steroids. Nine out of 11 dogs with severe IBD experienced clinical remission following a single IV infusion of allogenic AD-MSCs six weeks after the treatment, and blood levels of albumin, cobalamin, and folate significantly increased [91]. In cats, IBD is also rather typical, with frequent vomiting and diarrhea. Allogeneic AD-MSCs were used to treat cats with IBD in placebo-controlled, blinded research. Five out of seven cats’ owners reported considerable improvement or full remission of the clinical symptoms. In contrast, there was no improvement in the clinical signs or perhaps deterioration in cats who received the placebo [92]. Attributed to their immunomodulatory and anti-inflammatory properties, MSCs appeared to be a potential alternative therapy for canines and felines with IBD. The preliminary study findings are encouraging, but larger follow-up studies and more investigation are required to prove that MSC therapy is a secure and efficient means of treating IBD in animals.
6.3.3. Liver
Stem cell therapies for canine liver disease were the subject of several investigations. In an investigation, Yan et al. [93] explored the impact of IV injection of autogenic AD-MSCs for the treatment of experimentally induced canine acute hepatic damage. The homing of AD-MSCs to the liver decreased the blood-liver enzyme levels, and the restoration of hepatic tissue structure following the therapy points to the possibility of using MSCs to treat liver disorders in canines. Additionally, MSCs were used in a canine liver cirrhosis model. Without causing any negative side effects, IV administration of autogenic BM-MSCs dramatically reduced the amount of hepatic fibrosis and enhanced hepatic function in the cell received group [94]. In a canine model of liver fibrosis, IA administration of BM-MSCs was demonstrated to be safe, similar to IV administration, but intriguingly, IA application of MSC had a longer-lasting impact on lowering liver enzyme levels in the peripheral blood [131]. Additionally, 10 dogs with degenerative hepatopathy were repeatedly treated with autogenic AD-MSCs intravenously. When compared to the control group, all animals showed noticeably better liver function in terms of the drop in hepatic biomarkers following each treatment [132]. Additionally presented was a clinical instance of MSCs-treated hepato-cutaneous syndrome. Allogeneic AD-MSCs were repeatedly injected intravenously or into the hepatic parenchyma. For this condition, the dog’s survival with relapsed or limited clinical indications was longer than anticipated [133]. The IV route of delivery seems reasonable for treating hepatic disorders that are sensitive to the MSC treatment in animals because IV injection of MSCs causes the buildup of cells in the liver after they have been removed from the lungs [134]. The few trials that have been done on clinical cases make it difficult to draw firm conclusions on the optimum delivery method as well as the effectiveness and safety of allogenic MSCs in treating hepatic disorders. Therefore, further research is required to solve these difficulties.
6.4. Musculoskeletal System
6.4.1. Tendons and Ligaments
Tendon and ligament traumatic and stress injuries naturally recover with the growth of scar tissue, which is less functional than healthy tissue. While the initial damage reduces structural stiffness, fibrosis destroys the tendon or ligament’s physiological architecture and function [135]. This leads to impaired locomotor function that is vulnerable to further harm [135]. Therefore, the goal of the ideal treatment should be to restore to the tissue’s natural structure and function. Traditional treatments for tendon injuries in horses include chilling [136], bandaging, and a period of recovery during which regulated activity is performed. Corticosteroids or other anti-inflammatory medications can be used as pharmacological therapy; however, surgery is frequently necessary [137,138]. Reinjury is frequent with these conservative methods, and animals are frequently not able to function as well as they did before the injury [137]. Therefore, the ideal course of treatment should focus on tendon matrix regeneration. Due to its potential as a tool for improved tissue regeneration, the use of MSCs has been proposed as a substitute for the conventional strategy [95]. The goal of regenerative cell-based treatment is healing with correct collagen fiber production and effective restoration of normal tendon function with a reduced chance of recurrence. It is anticipated that the most suitable source of MSCs for stem cell therapy will be MSCs derived from the same tissue that needs to be treated. Therefore, tendon-derived stem cells would be the ideal source of stem cells for tendinopathies [139], but it is exceedingly difficult to isolate stem cells from tendonous tissues, and there is no accepted stimulation procedure for tendon genesis [140]. Therefore, for tendon regeneration, stem cells from several sources, primarily from adipose tissue and bone marrow were employed. First described in 2003, autogenic BM-MSC transplantation into the equine superficial digital flexor tendon [96]. Significant clinical healing was documented when cells were injected into 11 racehorses with superficial digital flexor tendon injuries [141]. Similarly, intralesional administration of autogenous BM-MSCs caused 28% or more reinjuries in all 141 racehorses included in the cohort research with naturally occurring superficial digital flexor tendon injury [35]. When horses were given intralesional injections of beta aminopropionitrile fumarate, hyaluronan, or polysulfated glycosaminoglycans for the same kind of damage and follow-up, the results demonstrated a considerable decrease in the rate of reinjury [142]. Smith et al. [143] revealed that autogenic BM-MSC therapy of natural tendinopathies causes the production of tissue matching a typical tendon matrix as opposed to the fibrous tissue that is generated throughout the normal healing procedures. Besides autogenous MSC therapy, allogeneic MSC therapy has shown promise in treating tendon and ligament problems such as desmitis of the suspensory and inferior check ligaments and tendinitis of the superficial and deep digital flexor tendons [144]. However, as compared to alternative therapies such as platelet-rich plasma (PRP), autogenic BM- or AD-MSC therapy showed little or extremely little enhancement in surgically produced lesions of the horse’s superficial digital flexor tendons [145,146]. Dogs were also given experimental MSC therapies, much the same as horses. A cranial cruciate ligament rupture in the stifle joint is a frequent injury in dogs [147]. Its rupture is the most characteristic cause of lameness in older dogs and is connected to stifle osteoarthritis [148]. Currently, surgical correction is the advised course of treatment [149]. The efficacy of MSC usage in this disease was underlined by positive treatment outcomes from multiple investigations. It has been shown that dogs needing tibial plateau leveling osteotomy (TPLO) may experience less post-operative pain and lameness following a single intra-articular injection of allogenic BM-MSCs than they would following a one-month course of oral non-steroidal anti-inflammatory drug (NSAID) administration [150]. According to research [151], autogenic BM-MSCs administered intraarticularly engraft in the region of damaged cranial essential ligaments and have an anti-inflammatory effect. In dogs with the same disease, post-operative intraarticular or IV injection of autogenic MSCs led to lower CD8+ T-cell counts, serum and synovial CRP, and synovial IFN- levels that lasted for more than 8 weeks after BM-MSC injection [152]. Promising findings were gathered from the retrospective study, where autogenous BM-MSC treatment combined with PRP avoided additional degeneration of the joint and canine contralateral ligament rupture in cases of incomplete tears without disruption of the stifle joint, where surgery is not the best treatment [153].
6.4.2. Joints
The ability of cartilage tissue to regenerate itself is somewhat constrained due to its relative hypocellularity and avascularization. It is further impacted in horses by the significant mechanical stress and loading forces applied to the articular surfaces throughout the performance [154]. Joint problems, with osteoarthritis being the most prevalent, are one of the most frequent causes of horse athletes’ careers ending and persistent lameness [155]. Horses’ athletic performance is frequently related to the traditional remedy for musculoskeletal affections that destroy the articular cartilages, ligaments, and menisci [156]. It has been documented that intra-articular MSC therapy for cartilage, bone, and meniscal diseases in horses was beneficial in vivo. Bone spavin, a degenerative joint condition where standard therapy is centered on the administration of corticosteroids for reducing pain and inflammation, is the most researched and characterized locomotive system ailment in horses. The study’s findings, which involved treating 16 horses with bone spavin with autogenous intraarticular AD-MSCs, point to the beneficial and durable effects of MSC treatment. At 180 days following treatment, there was no evidence of lameness in the treated horses compared to the control group. This was corroborated by scintigraphy, which contrasted the horses’ treated tarsal joints with the control group’s still-inflamed joints and showed no evidence of an inflammatory process in the horses’ treated tarsal joints [157]. In horses with meniscal injury, MSC therapy is also quite promising. Compared to horses treated only with arthroscopy, those treated with intraarticular injections of autogenic BM-MSC had a higher percentage of horses returning to work [97]. In one study, allogeneic AD-MSCs were used to treat 80 horses with osteoarthritis, and throughout the 90-day follow-up period, a substantial decrease in lameness was seen, indicating the positive impact of allogeneic cells [98]. Similar results were shown when horses with metacarpophalangeal/metatarsophalangeal osteoarthritis were treated with allogenic UC-MSCs. However, there were no clinically meaningful improvements after 6 months. However, several investigations have shown that administering allogeneic MSCs repeatedly to osteoarthritic horses resulted in negative clinical reactions [158]. Allogeneous MSCs have been observed to cause slight to moderate topical inflammatory symptoms even after a single injection [59]. According to several studies conducted on dogs, injecting MSC into arthritic joints reduces pain and improves function in animals. The much slower course of osteoarthritis in joints treated with autogenic AD-MSCs than in joints treated with placebo revealed a considerable reduction in lameness in dogs with stifle osteoarthritis [159]. Black et al. [160] and Vilar et al. [45] observed comparable outcomes in canines with hip osteoarthritis. According to research with up to a 4-year follow-up, the effects of intra-articular administration of autogenous AD-MSCs to treat canine osteoarthritis of various joints appear to be long-lasting [161]. Utilizing allogeneic AD-MSCs has also been demonstrated to significantly enhance MSC treatment for osteoarthritis. No side effects were found in 74 dogs treated with allogeneic AD-MSCs and placebo-controlled research, and effectiveness in lowering clinical symptoms was demonstrated in contrast to the control group [162]. Another large study that had 203 dogs with serious osteoarthritis that caused significant chronic pain and lameness revealed that, 10 weeks after therapy, 90% of young dogs exhibited great recovery and 60% of older dogs showed good improvement [163]. In contrast to the control group, cartilage repair was recorded in a dog model of osteoarthritis treated with allogeneic UC-MSCs. This cartilage repair was observed as cartilage neogenesis, a diminished amount of the joint fluid, a lowered inflammatory reaction, and enhanced healing of the adjacent tissues [164]. In contrast to research done on horses, recurrent allogeneic MSC treatment has been demonstrated to be safe with only moderate, self-limiting inflammatory responses and no negative consequences even two years following intraarticular MSC injection [165]. Both autogenic and allogeneic MSC treatments for canine osteoarthritis have been examined and shown to be effective when combined with PRP or hyaluronic acid [166]. When osteoarthritis in dogs was treated with AD-MSCs and PRP, MSC therapy exhibited more potent and advantageous results [44]. ECV treatment has already been used to treat suspensory ligament injuries in a stallion, with excellent outcomes demonstrated by enhanced angiogenesis, lesion filling, and tendon flexibility [167].
6.4.3. Muscles and Nerves
Spinal cord injuries (SCI), which can cause permanent disability, are among the most frequent neuromuscular injuries in both humans and animals [99]. Dogs who suffer from spinal cord damage may do so due to trauma or a herniated disc. Treatments using stem cells have been investigated and found to be effective for both illnesses. Through hemilaminectomy, autogenic BM-MSC treatment was investigated in dogs for spontaneous spinal cord damage brought on by spinal trauma. Some of the animals showed slight to moderate improvements in their locomotion, nociception, and proprioception [55,99]. In a different study, individuals with severe spinal cord injuries who received both allogeneic BM-MSCs and regular drug therapy had a statistically significant increase in their functional recovery compared to those who received only the usual medication [100]. Positive outcomes of MSC treatment were shown in dogs with acute disc herniation, much as they had been in dogs with traumatic spinal cord injuries. AD-MSCs were applied epidurally to dogs with acute paraplegia, and these dogs recovered their locomotion more quickly than dogs only receiving surgical decompression [168]. However, autogenic BM-MSC transplantation had no impact on clinical outcomes in dogs with naturally occurring degenerative intervertebral disc degeneration, and none of the three dogs presented any regenerative benefits [169]. The results of trials employing MSCs to treat traumatic spinal cord damage and disc herniation in canines did indicate some favorable outcomes, but further research is required to find a way to enhance the therapeutic advantages of MSC therapies that have already been identified. An option is a tissue engineering strategy. In one investigation, spinal cord transplantation of canine MSC-originated neural network tissue led to the progressive recovery of the motor functions of the paralyzed limb [170]. Therefore, more research and advancements are required to determine whether cell therapy and tissue bioengineering strategies are helpful for spinal cord damages, particularly in animals with spontaneous damages where the disease progression is frequently highly different from the pathologies induced in experiments.
6.5. Kidneys
Chronic tubulointerstitial nephritis, tubular atrophy, and interstitial fibrosis are the hallmarks of chronic kidney disease (CKD), a frequent medical problem encountered in aged cats. Currently, the only treatment that might restore renal function is kidney transplantation [171]. Therefore, stem cell-based treatments may offer less drastic therapeutic choices. Intravenous injection of stem cells is the recommended method of cell delivery since intrarenal stem cell inoculation has serious side effects and anesthesia-related dangers [101,102]. However, there were no negative effects described following IV delivery of allogeneic AD-MSCs to cats with kidney illness, and there were also no enhancements of the renal function in the short run [172,173]. However, in a work by Vidane et al. [102], cats with spontaneous CKD were regularly given IV injections of allogeneous MSCs produced from feline amniotic membranes, and a substantial improvement in kidney function was detected after the second transplantation of MSCs. Particularly, urine-specific gravity rose, and urine protein, and serum creatinine concentrations fell. The general clinical health of cats, including their eating habits and social interactions, has also significantly improved. Inconsistent findings from a few trials make it difficult to draw a firm judgment on the appropriateness of MSC treatment in cats with CKD. Thus, the employment of MSC therapy in (FCGS) has shown extremely positive results. Additional research is required to ascertain the potential impact of various variables, such as tissue source for MSCs, single or recurrent MSCs administrations, and timing of application concerning disease stage, on the outcomes of MSC treatment in cats with CKD. Further research is needed on the issue of allogeneic cell safety in cats because there has not been nearly enough studies done in this area.
6.6. Respiratory System
Respiratory illnesses are a common issue in veterinary medicine. Asthma, which includes several disorders including recurrent airway obstruction (RAO) and/or inflammatory airway illness, is a serious medical ailment for which there is no effective cure, particularly in horses. Moldy hay, pollen, and dusty straw are all conducive to the disease’s development. Horses frequently cough, require more effort to breathe when at rest, and are intolerant to activity. The use of corticosteroids, bronchodilators, or altering the environment can all be used to manage clinical symptoms. However, new therapeutic approaches are required to avoid the possible negative side effects of these medications. In horses with RAO, Barussi et al. [103] investigated the impact of intratracheal injections of bone marrow-derived mononuclear cells (BM-MNCs) on the progression of respiratory inflammation. In contrast to oral therapy with dexamethasone and single intratracheal delivery of autogenic cells, BM-MNCs reduced the clinical symptoms and the inflammatory reaction in horses with RAO. After cell therapy, IL-10 levels rose and were noticeably greater than in the dexamethasone-treated control group. The findings of this investigation are consistent with those of experimental studies in dogs [104] and cats that used induced respiratory circumstances [174].
6.7. Eye
Ophthalmology also conducts research on stem cell treatment. Some ocular conditions, such as corneal ulcers, can be treated with the present therapeutic strategies. Three cases of persistent corneal ulcers and one incidence of retinal detachment in horses that had not responded to conventional therapy were both treated with autogenous peripheral blood stem cells (PB-SCs). Cells were administered intravenously (IV), topically into the ophthalmic artery, subconjunctivally, or as an eye drop formulation. After therapy, all four animals showed considerable progress, with the epithelial surface being restored and the level of inflammation decreasing [175]. In another work, in three out of four horses, subconjunctival delivery of autogenic BM-MSC also improved immune-mediated keratitis, as evidenced by improved corneal clarity, less local neovascularization, and diminished surface abnormalities [105]. Equine recurrent uveitis (ERU) may be altered by the immunomodulatory actions of MSCs, as indicated by the reduced production of IFN- by CD4+ T cells from horses with ERU after being incubated with AD-MSCs in vitro. It has been demonstrated that PGE2 signaling and contact-dependent mechanisms reduce CD4+ T cell activation [176]. The outstanding outcomes exhibited by the subconjunctivally implanted allogenic AD-MSCs, including a significant reduction in the clinical manifestations of feline eosinophilic keratitis (FEK), rendered the use of MSC therapy for the treatment of these affections highly promising [106]. Moreover, keratoconjunctivitis sicca (KCS), often known as a dry eye condition, has been successfully treated in dogs with MSCs. The local allogeneic AD-MSC implantation around both lacrimal glands dramatically and sustainably decreased the clinical symptoms [177]. Likewise, the work of Sgrignoli et al. [178] showed that 6 months after recurrent local delivery of allogenic AD-MSCs into the dog conjunctival sac, exhibited a considerable reduction in the expression of KCS markers; IL-6, IL-1, TNF, and CD4. Based on the present knowledge, MSC therapy offers novel treatments for several animal eye affections, including corneal ulcers, recurrent uveitis, immune-mediated and eosinophilic keratitis, and dry keratoconjunctivitis. Though, further blinded prospective trials are required to gauge the in vivo impact of MSC treatment more precisely, particularly for horses with ERU. To completely comprehend the complexity and severity of certain illnesses as well as the regenerative effects of stem cell treatments used in the treatment of the eyes, ongoing scientific study is unquestionably required.
7. Conclusions and Future Perspectives
Research on regenerative medicine for animals is ongoing. In recent years, significant progress has been made toward producing safe and efficient stem cell treatments. Significant improvements in MSC therapy have been achieved in the treatment of various disorders, such as FCGS and IBD, as well as for wound healing. Notable effects of MSC treatments have been recorded, particularly for orthopedic problems in dogs and horses. Positive results from various studies indicate significant potential for stem cell therapy for various animal illnesses in the future. However, some obstacles still need to be resolved. The best source for MSC isolation is one of them. Most studies employed MSCs generated from adipose and bone marrow, mainly because they are accessible and simple to deal with. Therefore, it is possible that, in the future, stem cells from other organs will be shown to be more effective for treating definite disorders. Future research is also needed to determine how age and maybe even sex affect the therapeutic benefits of MSCs. There are significant differences between studies in terms of the illness progression time, cell dose, and MSC administration method. The ideal treatment methods for a certain condition are not suggested by any defined standards of the protocol. Despite claims of short viability and quick cell discharge, intravenous administration of MSCs has frequently been employed to treat a variety of animal ailments. Apoptosis-mediated immunomodulation via the immune cells is, according to some research, maybe the trigger for healing immunomodulatory mechanisms in the body, which might lengthen the effect of MSCs. Another significant problem in the realm of systemic stem cell treatments is the trapping of MSCs in the lungs following IV administration. The major substitute for IV administration of MSCs may be the systemic administration of ECVs, while different methods of administration have been taken into consideration to prevent lung entrapment. Early studies indicated that ECVs may be a promising cell-free treatment that might minimize lung entrapment and prevent potential pulmonary embolism induced by IV injection of MSCs. However, more research is required to determine the efficacy and safety of ECVs. The use of autogenic or allogeneic cells is an additional issue that has not been addressed. Although autogenic cells are safer, using them is more difficult and expensive for animal owners. Therefore, allogenic cells from healthy donors are a viable substitute, albeit there are still issues to be overcome about their immunogenicity and propensity to incite an immunological reaction in the receiver of the cells. Although veterinary regenerative medicine has made significant strides recently, this area is still in its infancy and requires a lot more effort to address many concerns before it is proven and standardized medicines can be provided to clinical cases. We are in an exciting period as more and more breakthrough regenerative medicines are developed. One may have faith that ongoing research in this field will eventually get us to a place where stem cell treatments could be a potent therapeutic option in both veterinary and human medicine for the curing of a variety of diseases that are presently incurable, rather than just a theoretical possibility.
Author Contributions
Conceptualization, H.M.E.-H. and R.T.; data curation, H.M.E.-H., E.A.M. and M.A.Y.H.; writing, original draft preparation, H.M.E.-H. and E.A.M.; writing and editing, H.M.E.-H., E.A.M. and M.A.Y.H.; supervision, H.M.E.-H. and R.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Caddeo, S.; Boffito, M.; Sartori, S. Tissue Engineering Approaches in the Design of Healthy and Pathological In Vitro Tissue Models. Front. Bioeng. Biotechnol. 2017, 5, 40. [Google Scholar] [CrossRef]
- El-Husseiny, H.M. Evaluation of Some Prosthetic Implants for Surgical Management of Different Varieties of Hernias in Domestic Animals. Master’s Thesis, Faculty of Veterinary Medicine, Benha University, Benha, Egypt, 2017; pp. 42–43. [Google Scholar]
- El-Husseiny, H.M.; El-Maghraby, H.M.; Alakraa, A.M.; Kandiel, M.M.M. Platelet Rich Fibrin Augmented Versus Non-Augmented Glycerolized Bovine Pericardium and Polypropylene Mesh for Repairing of Large Abdominal Wall Defects. Eur. J. Med. Nat. Sci. 2019, 3, 33–48. [Google Scholar] [CrossRef]
- El-Husseiny, H.M.; Mady, E.A.; El-Dakroury, W.A.; Doghish, A.S.; Tanaka, R. Stimuli-Responsive Hydrogels: Smart State of-the-Art Platforms for Cardiac Tissue Engineering. 2022. Available online: https://www.researchsquare.com/article/rs-2011475/v1 (accessed on 10 September 2022). [CrossRef]
- El-Husseiny, H.M.; Mady, E.A.; El-Dakroury, W.A.; Zewail, M.B.; Noshy, M.; Abdelfatah, A.M.; Doghish, A.S. Smart/stimuli-responsive hydrogels: State-of-the-art platforms for bone tissue engineering. Appl. Mater. Today 2022, 101560. [Google Scholar] [CrossRef]
- El-Husseiny, H.M.; Mady, E.A.; Hamabe, L.; Abugomaa, A.; Shimada, K.; Yoshida, T.; Tanaka, T.; Yokoi, A.; Elbadawy, M.; Tanaka, R. Smart/stimuli-responsive hydrogels: Cutting-edge platforms for tissue engineering and other biomedical applications. Mater. Today Bio 2022, 13, 100186. [Google Scholar] [CrossRef] [PubMed]
- Abd Elkodous, M.; El-Husseiny, H.M.; El-Sayyad, G.S.; Hashem, A.H.; Doghish, A.S.; Elfadil, D.; Radwan, Y.; El-Zeiny, H.M.; Bedair, H.; Ikhdair, O.A.; et al. Recent advances in waste-recycled nanomaterials for biomedical applications: Waste-to-wealth. Nanotechnol. Rev. 2021, 10, 1662–1739. [Google Scholar] [CrossRef]
- Dziubińska, P.; Jaskólska, M.; Przyborowska, P.; Adamiak, Z. Stem cells in dentistry—Review of literature. Pol. J. Vet. Sci. 2013, 16, 135–140. [Google Scholar] [CrossRef]
- Hendawy, H.; Uemura, A.; Ma, D.; Namiki, R.; Samir, H.; Ahmed, M.F.; Elfadadny, A.; El-Husseiny, H.M.; Chieh-Jen, C.; Tanaka, R. Tissue Harvesting Site Effect on the Canine Adipose Stromal Vascular Fraction Quantity and Quality. Animals 2021, 11, 460. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, M.; Zuba-Surma, E.; Ratajczak, J. Stem Cell Therapeutics—Hope and Concerns. Acta Haematol. Pol. 2009, 40, 289–303. [Google Scholar]
- Yamanaka, S. Induced Pluripotent Stem Cells: Past, Present, and Future. Cell Stem Cell 2012, 10, 678–684. [Google Scholar] [CrossRef]
- Jayasuriya, C.T.; Hu, N.; Li, J.; Lemme, N.; Terek, R.; Ehrlich, M.G.; Chen, Q. Molecular characterization of mesenchymal stem cells in human osteoarthritis cartilage reveals contribution to the OA phenotype. Sci. Rep. 2018, 8, 7044. [Google Scholar] [CrossRef]
- Frisbie, D.D.; Smith, R.K.W. Clinical update on the use of mesenchymal stem cells in equine orthopaedics. Equine Vet. J. 2010, 42, 86–89. [Google Scholar] [CrossRef]
- Chopra, H.; Kumar Hans, M.; Shetty, S. Stem cells-the hidden treasure: A strategic review. Dent. Res. J. 2013, 10, 421–427. [Google Scholar]
- Krampera, M.; Franchini, M.; Pizzolo, G.; Aprili, G. Mesenchymal stem cells: From biology to clinical use. Blood Transfus. 2007, 5, 120–129. [Google Scholar] [CrossRef]
- Emura, M. Stem cells of the respiratory epithelium and their in vitro cultivation. Vitr. Cell. Dev. Biol.-Anim. 1997, 33, 3–14. [Google Scholar] [CrossRef]
- Kolios, G.; Moodley, Y. Introduction to Stem Cells and Regenerative Medicine. Respiration 2013, 85, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.R. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc. Natl. Acad. Sci. USA 1981, 78, 7634–7638. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.J.; Kaufman, M.H. Establishment in culture of pluripotential cells from mouse embryos. Nature 1981, 292, 154–156. [Google Scholar] [CrossRef] [PubMed]
- De Miguel, M.P.; Fuentes-Julián, S.; Alcaina, Y. Pluripotent Stem Cells: Origin, Maintenance and Induction. Stem Cell Rev. Rep. 2010, 6, 633–649. [Google Scholar] [CrossRef]
- Guercio, A.; Di Marco, P.; Casella, S.; Cannella, V.; Russotto, L.; Purpari, G.; Di Bella, S.; Piccione, G. Production of canine mesenchymal stem cells from adipose tissue and their application in dogs with chronic osteoarthritis of the humeroradial joints. Cell Biol. Int. 2012, 36, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Nantavisai, S.; Egusa, H.; Osathanon, T.; Sawangmake, C. Mesenchymal stem cell-based bone tissue engineering for veterinary practice. Heliyon 2019, 5, e02808. [Google Scholar] [CrossRef]
- Augello, A.; Kurth, T.B.; De Bari, C. Mesenchymal stem cells: A perspective from in vitro cultures to in vivo migration and niches. Eur. Cells Mater. 2010, 20, e33. [Google Scholar] [CrossRef] [PubMed]
- Majo, F.; Rochat, A.; Nicolas, M.; Jaoudé, G.A.; Barrandon, Y. Oligopotent stem cells are distributed throughout the mammalian ocular surface. Nature 2008, 456, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Burdzinska, A.; Idziak, M. Stem Cells in Veterinary Medicine—Facts and Myths. Mag. Weter 2013, 7, 695–704. [Google Scholar]
- Voga, M.; Adamic, N.; Vengust, M.; Majdic, G. Stem Cells in Veterinary Medicine—Current State and Treatment Options. Front. Vet. Sci. 2020, 7, 278. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-S.; Lin, G.; Lue, T.F. Allogeneic and Xenogeneic Transplantation of Adipose-Derived Stem Cells in Immunocompetent Recipients Without Immunosuppressants. Stem Cells Dev. 2012, 21, 2770–2778. [Google Scholar] [CrossRef] [PubMed]
- Arzi, B.; Peralta, S.; Fiani, N.; Vapniarsky, N.; Taechangam, N.; Delatorre, U.; Clark, K.C.; Walker, N.J.; Loscar, M.R.; Lommer, M.J.; et al. A multicenter experience using adipose-derived mesenchymal stem cell therapy for cats with chronic, non-responsive gingivostomatitis. Stem Cell Res. Ther. 2020, 11, 115. [Google Scholar] [CrossRef]
- Kalisiak, O.; Cegielski, M. Stem cells in treatment of tendon disorders in horses. Życie Weter. 2013, 88, 112–114. [Google Scholar]
- Cegielski, M.; Kalisiak, O. Stem Cells Therapy—A Hope For the Treatment of Tendons in Horses. Życie Weter. 2008, 83, 754–756. [Google Scholar]
- Cegielski, M.; Izykowska, I.; Chmielewska, M.; Wojciech, D.; Bochnia, M.; Calkonski, I.; Dziegiel, P. Characteristics of MIC-1 Antlerogenic Stem Cells and Their Effect on Hair Growth in Rabbits. Vivo 2013, 27, 97–106. [Google Scholar]
- Ding, D.-C.; Shyu, W.-C.; Lin, S.-Z. Mesenchymal Stem Cells. Cell Transplant. 2011, 20, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Bearden, R.N.; Huggins, S.S.; Cummings, K.J.; Smith, R.; Gregory, C.A.; Saunders, W.B. In-vitro characterization of canine multipotent stromal cells isolated from synovium, bone marrow, and adipose tissue: A donor-matched comparative study. Stem Cell Res. Ther. 2017, 8, 218. [Google Scholar] [CrossRef] [PubMed]
- Godwin, E.E.; Young, N.J.; Dudhia, J.; Beamish, I.C.; Smith, R.K.W. Implantation of bone marrow-derived mesenchymal stem cells demonstrates improved outcome in horses with overstrain injury of the superficial digital flexor tendon. Equine Vet. J. 2012, 44, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Peters, A.E.; Watts, A.E. Biopsy Needle Advancement during Bone Marrow Aspiration Increases Mesenchymal Stem Cell Concentration. Front. Vet. Sci. 2016, 3, 23. [Google Scholar] [CrossRef]
- Jacobs, R.M.; Kociba, G.J.; Ruoff, W.W. Monoclonal Gammopathy in a Horse with Defective Hemostasis. Vet. Pathol. 1983, 20, 643–647. [Google Scholar] [CrossRef]
- Durando, M.M.; Zarucco, L.; Schaer, T.P.; Ross, M.; Reef, V.B. Pneumopericardium in a horse secondary to sternal bone marrow aspiration. Equine Vet. Educ. 2006, 18, 75–79. [Google Scholar] [CrossRef]
- Désévaux, C.; Laverty, S.; Doizé, B. Sternal bone biopsy in standing horses. Vet. Surg. 2000, 29, 303–308. [Google Scholar]
- Kasashima, Y.; Ueno, T.; Tomita, A.; Goodship, A.E.; Smith, R.K.W. Optimisation of bone marrow aspiration from the equine sternum for the safe recovery of mesenchymal stem cells. Equine Vet. J. 2011, 43, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Rebelatto, C.K.; Aguiar, A.M.; Moretão, M.P.; Senegaglia, A.C.; Hansen, P.; Barchiki, F.; Oliveira, J.; Martins, J.; Kuligovski, C.; Mansur, F.; et al. Dissimilar Differentiation of Mesenchymal Stem Cells from Bone Marrow, Umbilical Cord Blood, and Adipose Tissue. Exp. Biol. Med. 2008, 233, 901–913. [Google Scholar] [CrossRef]
- Orbay, H.; Tobita, M.; Mizuno, H. Mesenchymal Stem Cells Isolated from Adipose and Other Tissues: Basic Biological Properties and Clinical Applications. Stem Cells Int. 2012, 2012, 461718. [Google Scholar] [CrossRef]
- Webb, T.L.; Quimby, J.M.; Dow, S.W. In vitro comparison of feline bone marrow-derived and adipose tissue-derived mesenchymal stem cells. J. Feline Med. Surg. 2012, 14, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Cuervo, B.; Rubio, M.; Sopena, J.; Dominguez, J.M.; Vilar, J.; Morales, M.; Cugat, R.; Carrillo, J.M. Hip Osteoarthritis in Dogs: A Randomized Study Using Mesenchymal Stem Cells from Adipose Tissue and Plasma Rich in Growth Factors. Int. J. Mol. Sci. 2014, 15, 13437–13460. [Google Scholar] [CrossRef] [PubMed]
- Vilar, J.M.; Morales, M.; Santana, A.; Spinella, G.; Rubio, M.; Cuervo, B.; Cugat, R.; Carrillo, J.M. Controlled, blinded force platform analysis of the effect of intraarticular injection of autologous adipose-derived mesenchymal stem cells associated to PRGF-Endoret in osteoarthritic dogs. BMC Vet. Res. 2013, 9, 131. [Google Scholar] [CrossRef] [PubMed]
- Koerner, J.; Nesic, D.; Romero, J.D.; Brehm, W.; Mainil-Varlet, P.; Grogan, S.P. Equine Peripheral Blood-Derived Progenitors in Comparison to Bone Marrow-Derived Mesenchymal Stem Cells. Stem Cells 2006, 24, 1613–1619. [Google Scholar] [CrossRef] [PubMed]
- Dhar, M.; Neilsen, N.; Beatty, K.; Eaker, S.; Adair, H.; Geiser, D. Equine peripheral blood-derived mesenchymal stem cells: Isolation, identification, trilineage differentiation and effect of hyperbaric oxygen treatment. Equine Vet. J. 2012, 44, 600–605. [Google Scholar] [CrossRef] [PubMed]
- Spaas, J.H.; Schauwer, C.D.; Cornillie, P.; Meyer, E.; Soom, A.V.; Van de Walle, G.R. Culture and characterisation of equine peripheral blood mesenchymal stromal cells. Vet. J. 2013, 195, 107–113. [Google Scholar] [CrossRef]
- Chen, M.Y.; Lie, P.C.; Li, Z.L.; Wei, X. Endothelial differentiation of Wharton’s jelly-derived mesenchymal stem cells in comparison with bone marrow-derived mesenchymal stem cells. Exp. Hematol 2009, 37, 629–640. [Google Scholar] [CrossRef]
- In’t Anker, P.S.; Scherjon, S.A.; Kleijburg-van der Keur, C.; de Groot-Swings, G.M.J.S.; Claas, F.H.J.; Fibbe, W.E.; Kanhai, H.H.H. Isolation of Mesenchymal Stem Cells of Fetal or Maternal Origin from Human Placenta. Stem Cells 2004, 22, 1338–1345. [Google Scholar] [CrossRef]
- Horse Embryonic Stem Cell Lines from the Proliferation of Inner Cell Mass Cells. Stem Cells Dev. 2006, 15, 523–531. [CrossRef]
- Passeri, S.; Nocchi, F.; Lamanna, R.; Lapi, S.; Miragliotta, V.; Giannessi, E.; Abramo, F.; Stornelli, M.R.; Matarazzo, M.; Plenteda, D.; et al. Isolation and expansion of equine umbilical cord-derived matrix cells (EUCMCs). Cell Biol. Int. 2009, 33, 100–105. [Google Scholar] [CrossRef]
- Prządka, P.; Kiełbowicz, Z.; Osiński, B.; Dzimira, S.; Madej, J.A.; Nowacki, W.; Kubiak, K.; Reichert, P.; Cegielski, M. Reconstruction of cranial cruciate ligament in rabbits using polyester implants saturated with PRP, antlerogenic stem cells MIC-1 and their homogenate. Connect. Tissue Res. 2017, 58, 464–478. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhao, H.; Cheng, L.; Wang, B. Allogeneic vs. autologous mesenchymal stem/stromal cells in their medication practice. Cell Biosci. 2021, 11, 187. [Google Scholar] [CrossRef] [PubMed]
- Penha, E.M.; Meira, C.S.; Guimarães, E.T.; Mendonça, M.V.P.; Gravely, F.A.; Pinheiro, C.M.B.; Pinheiro, T.M.B.; Barrouin-Melo, S.M.; Ribeiro-dos-Santos, R.; Soares, M.B.P. Use of Autologous Mesenchymal Stem Cells Derived from Bone Marrow for the Treatment of Naturally Injured Spinal Cord in Dogs. Stem Cells Int. 2014, 2014, 437521. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Lu, A.; Gao, H.; Qian, C.; Zhang, J.; Lin, T.; Zhao, Y. Safety of Allogeneic Umbilical Cord Blood Stem Cells Therapy in Patients with Severe Cerebral Palsy: A Retrospective Study. Stem Cells Int. 2015, 2015, 325652. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, W.; Lim, C.; Chung, S.G. Treatment of Lateral Epicondylosis by Using Allogeneic Adipose-Derived Mesenchymal Stem Cells: A Pilot Study. Stem Cells 2015, 33, 2995–3005. [Google Scholar] [CrossRef]
- Sullivan, M.J. Banking on cord blood stem cells. Nat. Rev. Cancer 2008, 8, 555–563. [Google Scholar] [CrossRef]
- Bertoni, L.; Branly, T.; Jacquet, S.; Desancé, M.; Desquilbet, L.; Rivory, P.; Hartmann, D.-J.; Denoix, J.-M.; Audigié, F.; Galéra, P.; et al. Intra-Articular Injection of 2 Different Dosages of Autologous and Allogeneic Bone Marrow- and Umbilical Cord-Derived Mesenchymal Stem Cells Triggers a Variable Inflammatory Response of the Fetlock Joint on 12 Sound Experimental Horses. Stem Cells Int. 2019, 2019, 9431894. [Google Scholar] [CrossRef]
- Prządka, P.; Buczak, K.; Frejlich, E.; Gąsior, L.; Suliga, K.; Kiełbowicz, Z. The Role of Mesenchymal Stem Cells (MSCs) in Veterinary Medicine and Their Use in Musculoskeletal Disorders. Biomolecules 2021, 11, 1141. [Google Scholar] [CrossRef]
- Devine, S.M.; Bartholomew, A.M.; Mahmud, N.; Nelson, M.; Patil, S.; Hardy, W.; Sturgeon, C.; Hewett, T.; Chung, T.; Stock, W.; et al. Mesenchymal stem cells are capable of homing to the bone marrow of non-human primates following systemic infusion. Exp. Hematol. 2001, 29, 244–255. [Google Scholar] [CrossRef]
- Saito, T.; Kuang, J.-Q.; Bittira, B.; Al-Khaldi, A.; Chiu, R.C.J. Xenotransplant cardiac chimera: Immune tolerance of adult stem cells. Ann. Thorac. Surg. 2002, 74, 19–24. [Google Scholar] [CrossRef]
- Kim, H.-J.; Park, J.-B.; Lee, J.K.; Park, E.-Y.; Park, E.-A.; Riew, K.D.; Rhee, S.-K. Transplanted xenogenic bone marrow stem cells survive and generate new bone formation in the posterolateral lumbar spine of non-immunosuppressed rabbits. Eur. Spine J. 2008, 17, 1515–1521. [Google Scholar] [CrossRef] [PubMed]
- Pal, R.; Gopinath, C.; Rao, N.M.; Banerjee, P.; Krishnamoorthy, V.; Venkataramana, N.K.; Totey, S. Functional recovery after transplantation of bone marrow-derived human mesenchymal stromal cells in a rat model of spinal cord injury. Cytotherapy 2010, 12, 792–806. [Google Scholar] [CrossRef] [PubMed]
- Guan, M.; Yao, W.; Liu, R.; Lam, K.S.; Nolta, J.; Jia, J.; Panganiban, B.; Meng, L.; Zhou, P.; Shahnazari, M.; et al. Directing mesenchymal stem cells to bone to augment bone formation and increase bone mass. Nat. Med. 2012, 18, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek, M.; Abualrous, E.T.; Sticht, J.; Álvaro-Benito, M.; Stolzenberg, S.; Noé, F.; Freund, C. Major Histocompatibility Complex (MHC) Class I and MHC Class II Proteins: Conformational Plasticity in Antigen Presentation. Front. Immunol. 2017, 8, 292. [Google Scholar] [CrossRef]
- Opiela, J.; Samiec, M. Characterization of mesenchymal stem cells and their application in experimental embryology. Pol. J. Vet. Sci. 2013, 16, 593–599. [Google Scholar] [CrossRef]
- de Vasconcellos Machado, C.; da Silva Telles, P.D.; Nascimento, I.L.O. Immunological characteristics of mesenchymal stem cells. Rev. Bras. Hematol. E Hemoter. 2013, 35, 62. [Google Scholar] [CrossRef] [PubMed]
- Fortier, L.A. Stem Cells: Classifications, Controversies, and Clinical Applications. Vet. Surg. 2005, 34, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Vizoso, F.J.; Eiro, N.; Cid, S.; Schneider, J.; Perez-Fernandez, R. Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. Int. J. Mol. Sci. 2017, 18, 1852. [Google Scholar] [CrossRef]
- Bari, E.; Ferrarotti, I.; Torre, M.L.; Corsico, A.G.; Perteghella, S. Mesenchymal stem/stromal cell secretome for lung regeneration: The long way through “pharmaceuticalization” for the best formulation. J. Control. Release 2019, 309, 11–24. [Google Scholar] [CrossRef]
- El-Husseiny, H.; Mady, E.; Shimada, K.; Hamabe, L.; Yoshida, T.; Ma, D.; Mandour, A.; Hendawy, H.; Sasaki, K.; Fukuzumi, S.; et al. Intraventricular pressure gradient: A promising tool to predict the post-infarction chronic congestive heart failure in rats. Eur. Heart J.-Cardiovasc. Imaging 2022, 23, jeab289.390. [Google Scholar] [CrossRef]
- El-Husseiny, H.M.; Mady, E.A.; Ma, D.; Hamabe, L.; Takahashi, K.; Tanaka, R. Intraventricular pressure gradient: A novel tool to assess the post-infarction chronic congestive heart failure. Front. Cardiovasc. Med. 2022, 9, 944717. [Google Scholar] [CrossRef]
- Ma, D.; Mandour, A.S.; Elfadadny, A.; Hendawy, H.; Yoshida, T.; El-Husseiny, H.M.; Nishifuji, K.; Takahashi, K.; Zhao, Y.; Zhou, Z. Changes in cardiac function during the development of uremic cardiomyopathy and the effect of Salvianolic acid B administration in a rat model. Front. Vet. Sci. 2022, 734, 905759. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Mandour, A.S.; Hendawy, H.; Yoshida, T.; El-Husseiny, H.M.; Ozai, Y.; Takeuchi, A.; Takahashi, K.; Uemura, A.; Tanaka, R. Renovascular Hypertension-Induced Cardiac Changes in a Rat Model: Feasibility Of Conventional And Recent Echocardiography. J. Hypertens. 2021, 39, e403–e404. [Google Scholar] [CrossRef]
- Mandour, A.S.; Samir, H.; Yoshida, T.; Matsuura, K.; Abdelmageed, H.A.; Elbadawy, M.; Al-Rejaie, S.; El-Husseiny, H.M.; Elfadadny, A.; Ma, D.; et al. Assessment of the Cardiac Functions Using Full Conventional Echocardiography with Tissue Doppler Imaging before and after Xylazine Sedation in Male Shiba Goats. Animals 2020, 10, 2320. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Ma, D.; Mandour, A.S.; Ozai, Y.; Yoshida, T.; Matsuura, K.; Takeuchi, A.; Cheng, C.-J.; El-Husseiny, H.M.; Hendawy, H.; et al. Evaluation of Changes in the Cardiac Function before and after Transcatheter Edge-to-Edge Mitral Valve Repair in Healthy Dogs: Conventional and Novel Echocardiography. Animals 2022, 12, 56. [Google Scholar] [CrossRef]
- Yairo, A.; Mandour, A.S.; Matsuura, K.; Yoshida, T.; Ma, D.; Kitpipatkun, P.; Kato, K.; Cheng, C.-J.; El-Husseiny, H.M.; Tanaka, T.; et al. Effect of Loading Changes on the Intraventricular Pressure Measured by Color M-Mode Echocardiography in Rats. Diagnostics 2021, 11, 1403. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Mandour, A.S.; Matsuura, K.; Shimada, K.; El-Husseiny, H.M.; Hamabe, L.; Yilmaz, Z.; Uemura, A.; Tanaka, R. Changes in the Pulmonary Artery Wave Reflection in Dogs with Experimentally-Induced Acute Pulmonary Embolism and the Effect of Vasodilator. Animals 2021, 11, 1977. [Google Scholar] [CrossRef]
- Yoshida, T.; Matsuura, K.; Chieh-Jen, C.; Aboshi, Y.; Yamada, S.; Yotsuida, H.; Hasegawa, M.; Hendawy, H.A.; El-Husseiny, H.M.; Takahashi, Y.; et al. Surgical treatment for left atrial rupture due to myxomatous mitral valve disease in three dogs: A case report. Vet. Med. Sci. 2022, 1–7. [Google Scholar] [CrossRef]
- Hamabe, L.; Shimada, K.; Mandour, A.S.; Yoshida, T.; Hirose, M.; Hendawy, H.; El-Husseiny, H.M.; Tanaka, R. Evaluation of Left Ventricular Function in Healthy Retrievers Using Standard and 2D Speckle-Tracking Echocardiography. Vet. Sci. 2022, 9, 529. [Google Scholar] [CrossRef]
- Pogue, B.; Estrada, A.H.; Sosa-Samper, I.; Maisenbacher, H.W.; Lamb, K.E.; Mincey, B.D.; Erger, K.E.; Conlon, T.J. Stem-cell therapy for dilated cardiomyopathy: A pilot study evaluating retrograde coronary venous delivery. J. Small Anim. Pract. 2013, 54, 361–366. [Google Scholar] [CrossRef]
- Hensley, M.T.; Tang, J.; Woodruff, K.; Defrancesco, T.; Tou, S.; Williams, C.M.; Breen, M.; Meurs, K.; Keene, B.; Cheng, K. Intracoronary allogeneic cardiosphere-derived stem cells are safe for use in dogs with dilated cardiomyopathy. J. Cell. Mol. Med. 2017, 21, 1503–1512. [Google Scholar] [CrossRef] [PubMed]
- Petchdee, S.; Sompeewong, S. Intravenous administration of puppy deciduous teeth stem cells in degenerative valve disease. Vet. World 2016, 9, 1429. [Google Scholar] [CrossRef] [PubMed]
- Pratheesh, M.D.; Dubey, P.K.; Gade, N.E.; Nath, A.; Sivanarayanan, T.B.; Madhu, D.N.; Somal, A.; Baiju, I.; Sreekumar, T.R.; Gleeja, V.L.; et al. Comparative study on characterization and wound healing potential of goat (Capra hircus) mesenchymal stem cells derived from fetal origin amniotic fluid and adult bone marrow. Res. Vet. Sci. 2017, 112, 81–88. [Google Scholar] [CrossRef]
- Martinello, T.; Gomiero, C.; Perazzi, A.; Iacopetti, I.; Gemignani, F.; DeBenedictis, G.M.; Ferro, S.; Zuin, M.; Martines, E.; Brun, P.; et al. Allogeneic mesenchymal stem cells improve the wound healing process of sheep skin. BMC Vet. Res. 2018, 14, 202. [Google Scholar] [CrossRef] [PubMed]
- Textor, J.A.; Clark, K.C.; Walker, N.J.; Aristizobal, F.A.; Kol, A.; LeJeune, S.S.; Bledsoe, A.; Davidyan, A.; Gray, S.N.; Bohannon-Worsley, L.K.; et al. Allogeneic Stem Cells Alter Gene Expression and Improve Healing of Distal Limb Wounds in Horses. Stem Cells Transl. Med. 2017, 7, 98–108. [Google Scholar] [CrossRef]
- Venkataiah, V.S.; Handa, K.; Njuguna, M.M.; Hasegawa, T.; Maruyama, K.; Nemoto, E.; Yamada, S.; Sugawara, S.; Lu, L.; Takedachi, M.; et al. Periodontal Regeneration by Allogeneic Transplantation of Adipose Tissue Derived Multi-Lineage Progenitor Stem Cells in vivo. Sci. Rep. 2019, 9, 921. [Google Scholar] [CrossRef]
- Abdelaz, P.; ElZoghbi, A.; Shokry, M.; Ahmed, A.-Z.; Rasha, H. Reparative dentin formation using stem cell therapy versus calcium hydroxide in direct pulp capping: An animal study. Braz. Dent. J. 2019, 30, 542–549. [Google Scholar] [CrossRef]
- Iohara, K.; Utsunomiya, S.; Kohara, S.; Nakashima, M. Allogeneic transplantation of mobilized dental pulp stem cells with the mismatched dog leukocyte antigen type is safe and efficacious for total pulp regeneration. Stem Cell Res. Ther. 2018, 9, 116. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Merino, E.M.; Usón-Casaús, J.M.; Duque-Carrasco, J.; Zaragoza-Bayle, C.; Mariñas-Pardo, L.; Hermida-Prieto, M.; Vilafranca-Compte, M.; Barrera-Chacón, R.; Gualtieri, M. Safety and efficacy of allogeneic adipose tissue-derived mesenchymal stem cells for treatment of dogs with inflammatory bowel disease: Endoscopic and histological outcomes. Vet. J. 2015, 206, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Webb, T.L.; Webb, C.B. Stem cell therapy in cats with chronic enteropathy: A proof-of-concept study. J. Feline Med. Surg. 2015, 17, 901–908. [Google Scholar] [CrossRef]
- Yan, Y.; Fang, J.; Wen, X.; Teng, X.; Li, B.; Zhou, Z.; Peng, S.; Arisha, A.H.; Liu, W.; Hua, J. Therapeutic applications of adipose-derived mesenchymal stem cells on acute liver injury in canines. Res. Vet. Sci. 2019, 126, 233–239. [Google Scholar] [CrossRef]
- Matsuda, T.; Takami, T.; Sasaki, R.; Nishimura, T.; Aibe, Y.; Paredes, B.D.; Quintanilha, L.F.; Matsumoto, T.; Ishikawa, T.; Yamamoto, N.; et al. A canine liver fibrosis model to develop a therapy for liver cirrhosis using cultured bone marrow–derived cells. Hepatol. Commun. 2017, 1, 691–703. [Google Scholar] [CrossRef] [PubMed]
- Renzi, S.; Riccò, S.; Dotti, S.; Sesso, L.; Grolli, S.; Cornali, M.; Carlin, S.; Patruno, M.; Cinotti, S.; Ferrari, M. Autologous bone marrow mesenchymal stromal cells for regeneration of injured equine ligaments and tendons: A clinical report. Res. Vet. Sci. 2013, 95, 272–277. [Google Scholar] [CrossRef]
- Smith, R.K.W.; Korda, M.; Blunn, G.W.; Goodship, A.E. Isolation and implantation of autologous equine mesenchymal stem cells from bone marrow into the superficial digital flexor tendon as a potential novel treatment. Equine Vet. J. 2003, 35, 99–102. [Google Scholar] [CrossRef]
- Ferris, D.J.; Frisbie, D.D.; Kisiday, J.D.; McIlwraith, C.W.; Hague, B.A.; Major, M.D.; Schneider, R.K.; Zubrod, C.J.; Kawcak, C.E.; Goodrich, L.R. Clinical Outcome After Intra-Articular Administration of Bone Marrow Derived Mesenchymal Stem Cells in 33 Horses With Stifle Injury. Vet. Surg. 2014, 43, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Mariñas-Pardo, L.; Rodríguez-Hurtado, I.; Rodríguez-García, M.I.; Núñez-Naveira, L.; Hermida-Prieto, M. Allogeneic Adipose-Derived Mesenchymal Stem Cells (Horse Allo 20) for the Treatment of Osteoarthritis-Associated Lameness in Horses: Characterization, Safety, and Efficacy of Intra-Articular Treatment. Stem Cells Dev. 2018, 27, 1147–1160. [Google Scholar] [CrossRef] [PubMed]
- Besalti, O.; Aktas, Z.; Can, P.; Akpinar, E.; Elcin, A.E.; Elcin, Y.M. The use of autologous neurogenically-induced bone marrow-derived mesenchymal stem cells for the treatment of paraplegic dogs without nociception due to spinal trauma. J. Vet. Med. Sci. 2016, 78, 1465–1473. [Google Scholar] [CrossRef]
- Bhat, I.A.; Sivanarayanan, T.B.; Somal, A.; Pandey, S.; Bharti, M.K.; Panda, B.S.; Verma, M.; Sonwane, A.; Kumar, G.S.; Chandra, V.; et al. An allogenic therapeutic strategy for canine spinal cord injury using mesenchymal stem cells. J. Cell. Physiol. 2019, 234, 2705–2718. [Google Scholar] [CrossRef]
- Quimby, J.M.; Webb, T.L.; Gibbons, D.S.; Dow, S.W. Evaluation of intrarenal mesenchymal stem cell injection for treatment of chronic kidney disease in cats: A pilot study. J. Feline Med. Surg. 2011, 13, 418–426. [Google Scholar] [CrossRef]
- Vidane, A.S.; Pinheiro, A.O.; Casals, J.B.; Passarelli, D.; Hage, M.; Bueno, R.S.; Martins, D.S.; Ambrósio, C.E. Transplantation of amniotic membrane-derived multipotent cells ameliorates and delays the progression of chronic kidney disease in cats. Reprod. Domest. Anim. 2017, 52, 316–326. [Google Scholar] [CrossRef]
- Barussi, F.C.M.; Bastos, F.Z.; Leite, L.M.B.; Fragoso, F.Y.I.; Senegaglia, A.C.; Brofman, P.R.S.; Nishiyama, A.; Pimpão, C.T.; Michelotto, P.V. Intratracheal therapy with autologous bone marrow-derived mononuclear cells reduces airway inflammation in horses with recurrent airway obstruction. Respir. Physiol. Neurobiol. 2016, 232, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Ran, Y.; Lu, B.; Li, J.; Zhang, J.; Feng, C.; Fang, J.; Ma, R.; Qiao, Z.; Dai, X.; et al. Therapeutic Effects of Human Umbilical Cord–Derived Mesenchymal Stem Cells on Canine Radiation-Induced Lung Injury. Int. J. Radiat. Oncol. Biol. Phys. 2018, 102, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.B.; Schnabel, L.V.; Gilger, B.C. Subconjunctival bone marrow-derived mesenchymal stem cell therapy as a novel treatment alternative for equine immune-mediated keratitis: A case series. Vet. Ophthalmol. 2019, 22, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Villatoro, A.J.; Claros, S.; Fernández, V.; Alcoholado, C.; Fariñas, F.; Moreno, A.; Becerra, J.; Andrades, J.A. Safety and efficacy of the mesenchymal stem cell in feline eosinophilic keratitis treatment. BMC Vet. Res. 2018, 14, 116. [Google Scholar] [CrossRef]
- Wernly, B.; Mirna, M.; Rezar, R.; Prodinger, C.; Jung, C.; Podesser, B.K.; Kiss, A.; Hoppe, U.C.; Lichtenauer, M. Regenerative Cardiovascular Therapies: Stem Cells and Beyond. Int. J. Mol. Sci. 2019, 20, 1420. [Google Scholar] [CrossRef]
- Hoffman, A.M.; Dow, S.W. Concise Review: Stem Cell Trials Using Companion Animal Disease Models. Stem Cells 2016, 34, 1709–1729. [Google Scholar] [CrossRef]
- Fox, P.R. Pathology of myxomatous mitral valve disease in the dog. J. Vet. Cardiol. 2012, 14, 103–126. [Google Scholar] [CrossRef]
- Ojeh, N.; Pastar, I.; Tomic-Canic, M.; Stojadinovic, O. Stem Cells in Skin Regeneration, Wound Healing, and Their Clinical Applications. Int. J. Mol. Sci. 2015, 16, 25476–25501. [Google Scholar] [CrossRef]
- Johnson, V.; Webb, T.; Norman, A.; Coy, J.; Kurihara, J.; Regan, D.; Dow, S. Activated Mesenchymal Stem Cells Interact with Antibiotics and Host Innate Immune Responses to Control Chronic Bacterial Infections. Sci. Rep. 2017, 7, 9575. [Google Scholar] [CrossRef]
- El-Tookhy, O.S.; Shamaa, A.A.; Shehab, G.G.; Abdallah, A.N.; Azzam, O.M. Histological Evaluation of Experimentally Induced Critical Size Defect Skin Wounds Using Exosomal Solution of Mesenchymal Stem Cells Derived Microvesicles. Int. J. Stem Cells 2017, 10, 144–153. [Google Scholar] [CrossRef]
- Spaas, J.H.; Broeckx, S.; Van de Walle, G.R.; Polettini, M. The effects of equine peripheral blood stem cells on cutaneous wound healing: A clinical evaluation in four horses. Clin. Exp. Dermatol. 2013, 38, 280–284. [Google Scholar] [CrossRef]
- Lanci, A.; Merlo, B.; Mariella, J.; Castagnetti, C.; Iacono, E. Heterologous Wharton’s Jelly Derived Mesenchymal Stem Cells Application on a Large Chronic Skin Wound in a 6-Month-Old Filly. Front. Vet. Sci. 2019, 6, 9. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.; Rosenkrantz, W.S.; Hong, J.; Griffin, C.E.; Mendelsohn, C. Evaluation of the potential use of adipose-derived mesenchymal stromal cells in the treatment of canine atopic dermatitis: A pilot study. Vet. Ther. 2010, 11, E1–E14. [Google Scholar] [PubMed]
- Villatoro, A.J.; Hermida-Prieto, M.; Fernández, V.; Fariñas, F.; Alcoholado, C.; Rodríguez-García, M.I.; Mariñas-Pardo, L.; Becerra, J. Allogeneic adipose-derived mesenchymal stem cell therapy in dogs with refractory atopic dermatitis: Clinical efficacy and safety. Vet. Rec. 2018, 183, 654. [Google Scholar] [CrossRef] [PubMed]
- Babu, N.C.; Gomes, A.J. Systemic manifestations of oral diseases. J. Oral Maxillofac. Pathol. 2011, 15, 144. [Google Scholar] [CrossRef]
- Javdani, M.; Nikousefat, Z. Prevalence of dental problems in pet dogs in Shiraz, Iran Res. Opin. Anim. Vet. Sci 2011, 1, 666–668. [Google Scholar]
- Niemiec, B.; Gawor, J.; Nemec, A.; Clarke, D.; McLeod, K.; Tutt, C.; Gioso, M.; Steagall, P.V.; Chandler, M.; Morgenegg, G.; et al. World Small Animal Veterinary Association Global Dental Guidelines. J. Small Anim. Pract. 2020, 61, E36–E161. [Google Scholar] [CrossRef]
- El-Zekrid, M.H.; Mahmoud, S.H.; Ali, F.A.; Helal, M.E.; Grawish, M.E. Healing Capacity of Autologous Bone Marrow–derived Mesenchymal Stem Cells on Partially Pulpotomized Dogs’ Teeth. J. Endod. 2019, 45, 287–294. [Google Scholar] [CrossRef]
- Sánchez-Garcés, M.À.; Alvira-González, J.; Sánchez, C.M.; Cairó, J.R.B.; Del Pozo, M.R.; Gay-Escoda, C. Bone Regeneration Using Adipose-Derived Stem Cells with Fibronectin in Dehiscence-Type Defects Associated with Dental Implants: An Experimental Study in a Dog Model. Int. J. Oral Maxillofac. Implant. 2017, 32, e97–e106. [Google Scholar] [CrossRef]
- Bellei, E.; Dalla, F.; Masetti, L.; Pisoni, L.; Joechler, M. Surgical therapy in chronic feline gingivostomatitis (FCGS). Vet. Res. Commun. 2008, 32, 231–234. [Google Scholar] [CrossRef]
- Hennet, P.R.; Camy, G.A.L.; McGahie, D.M.; Albouy, M.V. Comparative efficacy of a recombinant feline interferon omega in refractory cases of calicivirus-positive cats with caudal stomatitis: A randomised, multi-centre, controlled, double-blind study in 39 cats. J. Feline Med. Surg. 2011, 13, 577–587. [Google Scholar] [CrossRef]
- Lommer, M.J. Efficacy of Cyclosporine for Chronic, Refractory Stomatitis in Cats: A Randomized, Placebo-Controlled, Double-Blinded Clinical Study. J. Vet. Dent. 2013, 30, 8–17. [Google Scholar] [CrossRef]
- Druet, I.; Hennet, P. Relationship between Feline calicivirus Load, Oral Lesions, and Outcome in Feline Chronic Gingivostomatitis (Caudal Stomatitis): Retrospective Study in 104 Cats. Front. Vet. Sci. 2017, 4, 209. [Google Scholar] [CrossRef] [PubMed]
- Winer, J.N.; Arzi, B.; Verstraete, F.J.M. Therapeutic Management of Feline Chronic Gingivostomatitis: A Systematic Review of the Literature. Front. Vet. Sci. 2016, 3, 54. [Google Scholar] [CrossRef] [PubMed]
- Arzi, B.; Mills-Ko, E.; Verstraete, F.J.M.; Kol, A.; Walker, N.J.; Badgley, M.R.; Fazel, N.; Murphy, W.J.; Vapniarsky, N.; Borjesson, D.L. Therapeutic Efficacy of Fresh, Autologous Mesenchymal Stem Cells for Severe Refractory Gingivostomatitis in Cats. Stem Cells Transl. Med. 2015, 5, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Arzi, B.; Clark, K.C.; Sundaram, A.; Spriet, M.; Verstraete, F.J.M.; Walker, N.J.; Loscar, M.R.; Fazel, N.; Murphy, W.J.; Vapniarsky, N.; et al. Therapeutic Efficacy of Fresh, Allogeneic Mesenchymal Stem Cells for Severe Refractory Feline Chronic Gingivostomatitis. Stem Cells Transl. Med. 2017, 6, 1710–1722. [Google Scholar] [CrossRef]
- Craven, M.; Simpson, J.W.; Ridyard, A.E.; Chandler, M.L. Canine inflammatory bowel disease: Retrospective analysis of diagnosis and outcome in 80 cases (1995–2002). J. Small Anim. Pract. 2004, 45, 336–342. [Google Scholar] [CrossRef]
- Hall, E.J. Inflammatory Bowel Disease in Dogs and Cats; Hill’s Pet Nutrition, Inc.: Topeka, KS, USA, 2009; Available online: https://protrain.hs.llnwd.net/e1/sitefiles/642/Documents/GI%20technical%20booklet.pdf (accessed on 10 September 2022).
- Nishimura, T.; Takami, T.; Sasaki, R.; Aibe, Y.; Matsuda, T.; Fujisawa, K.; Matsumoto, T.; Yamamoto, N.; Tani, K.; Taura, Y.; et al. Liver regeneration therapy through the hepatic artery-infusion of cultured bone marrow cells in a canine liver fibrosis model. PLoS ONE 2019, 14, e0210588. [Google Scholar] [CrossRef]
- Gardin, C.; Ferroni, L.; Bellin, G.; Rubini, G.; Barosio, S.; Zavan, B. Therapeutic Potential of Autologous Adipose-Derived Stem Cells for the Treatment of Liver Disease. Int. J. Mol. Sci. 2018, 19, 4064. [Google Scholar] [CrossRef]
- Nam, A.; Han, S.M.; Go, D.M.; Kim, D.Y.; Seo, K.W.; Youn, H.Y. Long-Term Management with Adipose Tissue-Derived Mesenchymal Stem Cells and Conventional Treatment in a Dog with Hepatocutaneous Syndrome. J. Vet. Intern. Med. 2017, 31, 1514–1519. [Google Scholar] [CrossRef]
- de Witte, S.F.H.; Luk, F.; Sierra Parraga, J.M.; Gargesha, M.; Merino, A.; Korevaar, S.S.; Shankar, A.S.; O’Flynn, L.; Elliman, S.J.; Roy, D.; et al. Immunomodulation By Therapeutic Mesenchymal Stromal Cells (MSC) Is Triggered Through Phagocytosis of MSC By Monocytic Cells. Stem Cells 2018, 36, 602–615. [Google Scholar] [CrossRef]
- Dakin, S.G.; Jespers, K.; Warner, S.; O’hara, L.K.; Dudhia, J.; Goodship, A.E.; Wilson, A.M.; Smith, R.K.W. The relationship between in vivo limb and in vitro tendon mechanics after injury: A potential novel clinical tool for monitoring tendon repair. Equine Vet. J. 2011, 43, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Petrov, R.; MacDonald, M.H.; Tesch, A.M.; Hoogmoed, L.M.V. Influence of topically applied cold treatment on core temperature and cell viability in equine superficial digital flexor tendons. Am. J. Vet. Res. 2003, 64, 835–844. [Google Scholar] [CrossRef]
- Chan, K.-M.; Fu, S.-C. Anti-inflammatory management for tendon injuries-friends or foes? BMC Sport. Sci. Med. Rehabil. 2009, 1, 23. [Google Scholar] [CrossRef] [PubMed]
- Eliashar, E.; Schramme, M.C.; Schumacher, J.; Smith, R.K.W.; Ikada, Y. Use of a bioabsorbable implant for the repair of severed digital flexor tendons in four horses. Vet. Rec. 2001, 148, 506–509. [Google Scholar] [CrossRef]
- Filomeno, P.; Dayan, V.; Touriño, C. Stem cell research and clinical development in tendon repair. Muscles Ligaments Tendons J. 2012, 2, 204. [Google Scholar]
- Costa-Almeida, R.; Calejo, I.; Gomes, M.E. Mesenchymal Stem Cells Empowering Tendon Regenerative Therapies. Int. J. Mol. Sci. 2019, 20, 3002. [Google Scholar] [CrossRef] [PubMed]
- Pacini, S.; Spinabella, S.; Trombi, L.; Fazzi, R.; Galimberti, S.; Dini, F.; Carlucci, F.; Petrini, M. Suspension of Bone Marrow–Derived Undifferentiated Mesenchymal Stromal Cells for Repair of Superficial Digital Flexor Tendon in Race Horses. Tissue Eng. 2007, 13, 2949–2955. [Google Scholar] [CrossRef] [PubMed]
- Dyson, S.J. Medical management of superficial digital flexor tendonitis: A comparative study in 219 horses (1992–2000). Equine Vet. J. 2004, 36, 415–419. [Google Scholar] [CrossRef]
- Smith, R.K.W.; Werling, N.J.; Dakin, S.G.; Alam, R.; Goodship, A.E.; Dudhia, J. Beneficial Effects of Autologous Bone Marrow-Derived Mesenchymal Stem Cells in Naturally Occurring Tendinopathy. PLoS ONE 2013, 8, e75697. [Google Scholar] [CrossRef]
- Van Loon, V.J.F.; Scheffer, C.J.W.; Genn, H.J.; Hoogendoorn, A.C.; Greve, J.W. Clinical follow-up of horses treated with allogeneic equine mesenchymal stem cells derived from umbilical cord blood for different tendon and ligament disorders. Vet. Q. 2014, 34, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Romero, A.; Barrachina, L.; Ranera, B.; Remacha, A.R.; Moreno, B.; de Blas, I.; Sanz, A.; Vázquez, F.J.; Vitoria, A.; Junquera, C.; et al. Comparison of autologous bone marrow and adipose tissue derived mesenchymal stem cells, and platelet rich plasma, for treating surgically induced lesions of the equine superficial digital flexor tendon. Vet. J. 2017, 224, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Geburek, F.; Roggel, F.; van Schie, H.T.M.; Beineke, A.; Estrada, R.; Weber, K.; Hellige, M.; Rohn, K.; Jagodzinski, M.; Welke, B.; et al. Effect of single intralesional treatment of surgically induced equine superficial digital flexor tendon core lesions with adipose-derived mesenchymal stromal cells: A controlled experimental trial. Stem Cell Res. Ther. 2017, 8, 129. [Google Scholar] [CrossRef] [PubMed]
- Harasen, G. Diagnosing rupture of the cranial cruciate ligament. Can. Vet. J. 2002, 43, 475. [Google Scholar]
- Chuang, C.; Ramaker, M.A.; Kaur, S.; Csomos, R.A.; Kroner, K.T.; Bleedorn, J.A.; Schaefer, S.L.; Muir, P. Radiographic Risk Factors for Contralateral Rupture in Dogs with Unilateral Cranial Cruciate Ligament Rupture. PLoS ONE 2014, 9, e106389. [Google Scholar] [CrossRef]
- Mölsä, S.H.; Hyytiäinen, H.K.; Hielm-Björkman, A.K.; Laitinen-Vapaavuori, O.M. Long-term functional outcome after surgical repair of cranial cruciate ligament disease in dogs. BMC Vet. Res. 2014, 10, 266. [Google Scholar] [CrossRef]
- Taroni, M.; Cabon, Q.; Fèbre, M.; Cachon, T.; Saulnier, N.; Carozzo, C.; Maddens, S.; Labadie, F.; Robert, C.; Viguier, E. Evaluation of the Effect of a Single Intra-articular Injection of Allogeneic Neonatal Mesenchymal Stromal Cells Compared to Oral Non-Steroidal Anti-inflammatory Treatment on the Postoperative Musculoskeletal Status and Gait of Dogs over a 6-Month Period after Tibial Plateau Leveling Osteotomy: A Pilot Study. Front. Vet. Sci. 2017, 4, 83. [Google Scholar] [CrossRef]
- Linon, E.; Spreng, D.; Rytz, U.; Forterre, S. Engraftment of autologous bone marrow cells into the injured cranial cruciate ligament in dogs. Vet. J. 2014, 202, 448–454. [Google Scholar] [CrossRef]
- Muir, P.; Hans, E.C.; Racette, M.; Volstad, N.; Sample, S.J.; Heaton, C.; Holzman, G.; Schaefer, S.L.; Bloom, D.D.; Bleedorn, J.A.; et al. Autologous Bone Marrow-Derived Mesenchymal Stem Cells Modulate Molecular Markers of Inflammation in Dogs with Cruciate Ligament Rupture. PLoS ONE 2016, 11, e0159095. [Google Scholar] [CrossRef]
- Canapp, S.O.; Leasure, C.S.; Cox, C.; Ibrahim, V.; Carr, B.J. Partial Cranial Cruciate Ligament Tears Treated with Stem Cell and Platelet-Rich Plasma Combination Therapy in 36 Dogs: A Retrospective Study. Front. Vet. Sci. 2016, 3, 112. [Google Scholar] [CrossRef]
- Frisbie, D.D.; Stewart, M.C. Cell-based Therapies for Equine Joint Disease. Vet. Clin. N. Am. Equine Pract. 2011, 27, 335–349. [Google Scholar] [CrossRef]
- Rossdale, P.; Hopes, R.; Digby, N. Epidemiological study of wastage among racehorses 1982 and 1983. Vet. Rec. 1985, 116, 66–69. [Google Scholar] [CrossRef]
- Walmsley, J.P.; Phillips, T.J.; Townsend, H.G.G. Meniscal tears in horses: An evaluation of clinical signs and arthroscopic treatment of 80 cases. Equine Vet. J. 2003, 35, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Nicpoń, J.; Marycz, K.; Grzesiak, J. Therapeutic effect of adipose-derived mesenchymal stem cell injection in horses suffering from bone spavin. Pol. J. Vet. Sci. 2013, 16, 753–754. [Google Scholar] [CrossRef] [PubMed]
- Joswig, A.-J.; Mitchell, A.; Cummings, K.J.; Levine, G.J.; Gregory, C.A.; Smith, R.; Watts, A.E. Repeated intra-articular injection of allogeneic mesenchymal stem cells causes an adverse response compared to autologous cells in the equine model. Stem Cell Res. Ther. 2017, 8, 42. [Google Scholar] [CrossRef] [PubMed]
- Mohoric, L.; Zorko, B.; Ceh, K.; Majdic, G. Blinded placebo study of bilateral osteoarthritis treatment using adipose derived mesenchymal stem cells. Slov. Vet. Res. 2016, 53, 167–174. [Google Scholar]
- Black, L.L.; Gaynor, J.; Gahring, D.; Adams, C.; Aron, D.; Harman, S.; Gingerich, D.A.; Harman, R. Effect of adipose-derived mesenchymal stem and regenerative cells on lameness in dogs with chronic osteoarthritis of the coxofemoral joints: A randomized, double-blinded, multicenter controlled trial. Vet. Ther. 2007, 8, 272. [Google Scholar]
- Srzentić Dražilov, S.; Mrkovački, J.; Spasovski, V.; Fazlagić, A.; Pavlović, S.; Nikčević, G. The use of canine mesenchymal stem cells for the autologous treatment of osteoarthritis. Acta Vet. Hung. 2018, 66, 376–389. [Google Scholar] [CrossRef]
- Harman, R.; Carlson, K.; Gaynor, J.; Gustafson, S.; Dhupa, S.; Clement, K.; Hoelzler, M.; McCarthy, T.; Schwartz, P.; Adams, C. A Prospective, Randomized, Masked, and Placebo-Controlled Efficacy Study of Intraarticular Allogeneic Adipose Stem Cells for the Treatment of Osteoarthritis in Dogs. Front. Vet. Sci. 2016, 3, 81. [Google Scholar] [CrossRef]
- Shah, K.; Drury, T.; Roic, I.; Hansen, P.; Malin, M.; Boyd, R.; Sumer, H.; Ferguson, R. Outcome of Allogeneic Adult Stem Cell Therapy in Dogs Suffering from Osteoarthritis and Other Joint Defects. Stem Cells Int. 2018, 2018, 7309201. [Google Scholar] [CrossRef]
- Zhang, B.-y.; Wang, B.-y.; Li, S.-c.; Luo, D.-z.; Zhan, X.; Chen, S.-f.; Chen, Z.-s.; Liu, C.-y.; Ji, H.-q.; Bai, Y.-s.; et al. Evaluation of the Curative Effect of Umbilical Cord Mesenchymal Stem Cell Therapy for Knee Arthritis in Dogs Using Imaging Technology. Stem Cells Int. 2018, 2018, 1983025. [Google Scholar] [CrossRef]
- Cabon, Q.; Febre, M.; Gomez, N.; Cachon, T.; Pillard, P.; Carozzo, C.; Saulnier, N.; Robert, C.; Livet, V.; Rakic, R.; et al. Long-Term Safety and Efficacy of Single or Repeated Intra-Articular Injection of Allogeneic Neonatal Mesenchymal Stromal Cells for Managing Pain and Lameness in Moderate to Severe Canine Osteoarthritis Without Anti-inflammatory Pharmacological Support: Pilot Clinical Study. Front. Vet. Sci. 2019, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Duan, X.; Fan, Z.; Chen, L.; Xing, F.; Xu, Z.; Chen, Q.; Xiang, Z. Mesenchymal Stem Cells in Combination with Hyaluronic Acid for Articular Cartilage Defects. Sci. Rep. 2018, 8, 9900. [Google Scholar] [CrossRef]
- Kornicka-Garbowska, K.; Pędziwiatr, R.; Woźniak, P.; Kucharczyk, K.; Marycz, K. Microvesicles isolated from 5-azacytidine-and-resveratrol-treated mesenchymal stem cells for the treatment of suspensory ligament injury in horse—A case report. Stem Cell Res. Ther. 2019, 10, 394. [Google Scholar] [CrossRef]
- Bach, F.S.; Rebelatto, C.L.K.; Fracaro, L.; Senegaglia, A.C.; Fragoso, F.Y.I.; Daga, D.R.; Brofman, P.R.S.; Pimpão, C.T.; Engracia Filho, J.R.; Montiani-Ferreira, F.; et al. Comparison of the Efficacy of Surgical Decompression Alone and Combined With Canine Adipose Tissue-Derived Stem Cell Transplantation in Dogs With Acute Thoracolumbar Disk Disease and Spinal Cord Injury. Front. Vet. Sci. 2019, 6, 383. [Google Scholar] [CrossRef] [PubMed]
- Bone Marrow-Derived Mesenchymal Stem Cells as Autologous Therapy in Dogs with Naturally Occurring Intervertebral Disc Disease: Feasibility, Safety, and Preliminary Results. Tissue Eng. Part C Methods 2017, 23, 643–651. [CrossRef] [PubMed]
- Wu, G.-H.; Shi, H.-J.; Che, M.-T.; Huang, M.-Y.; Wei, Q.-S.; Feng, B.; Ma, Y.-H.; Wang, L.-J.; Jiang, B.; Wang, Y.-Q.; et al. Recovery of paralyzed limb motor function in canine with complete spinal cord injury following implantation of MSC-derived neural network tissue. Biomaterials 2018, 181, 15–34. [Google Scholar] [CrossRef] [PubMed]
- Adin, C.A.; Gregory, C.R.; Kyles, A.E.; Cowgill, L. Diagnostic Predictors of Complications and Survival after Renal Transplantation in Cats. Vet. Surg. 2001, 30, 515–521. [Google Scholar] [CrossRef]
- Quimby, J.M.; Webb, T.L.; Habenicht, L.M.; Dow, S.W. Safety and efficacy of intravenous infusion of allogeneic cryopreserved mesenchymal stem cells for treatment of chronic kidney disease in cats: Results of three sequential pilot studies. Stem Cell Res. Ther. 2013, 4, 48. [Google Scholar] [CrossRef]
- Quimby, J.M.; Webb, T.L.; Randall, E.; Marolf, A.; Valdes-Martinez, A.; Dow, S.W. Assessment of intravenous adipose-derived allogeneic mesenchymal stem cells for the treatment of feline chronic kidney disease: A randomized, placebo-controlled clinical trial in eight cats. J. Feline Med. Surg. 2016, 18, 165–171. [Google Scholar] [CrossRef]
- Trzil, J.E.; Masseau, I.; Webb, T.L.; Chang, C.-H.; Dodam, J.R.; Liu, H.; Quimby, J.M.; Dow, S.W.; Reinero, C.R. Intravenous adipose-derived mesenchymal stem cell therapy for the treatment of feline asthma: A pilot study. J. Feline Med. Surg. 2016, 18, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Marfe, G.; Massaro-Giordano, M.; Ranalli, M.; Cozzoli, E.; Di Stefano, C.; Malafoglia, V.; Polettini, M.; Gambacurta, A. Blood derived stem cells: An ameliorative therapy in veterinary ophthalmology. J. Cell. Physiol. 2012, 227, 1250–1256. [Google Scholar] [CrossRef] [PubMed]
- Saldinger, L.K.; Nelson, S.G.; Bellone, R.R.; Lassaline, M.; Mack, M.; Walker, N.J.; Borjesson, D.L. Horses with equine recurrent uveitis have an activated CD4+ T-cell phenotype that can be modulated by mesenchymal stem cells in vitro. Vet. Ophthalmol. 2020, 23, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Villatoro, A.J.; Fernández, V.; Claros, S.; Rico-Llanos, G.A.; Becerra, J.; Andrades, J.A. Use of Adipose-Derived Mesenchymal Stem Cells in Keratoconjunctivitis Sicca in a Canine Model. BioMed Res. Int. 2015, 2015, 527926. [Google Scholar] [CrossRef] [PubMed]
- Sgrignoli, M.R.; Silva, D.A.; Nascimento, F.F.; Sgrignoli, D.A.M.; Nai, G.A.; da Silva, M.G.; de Barros, M.A.; Bittencourt, M.K.W.; de Morais, B.P.; Dinallo, H.R.; et al. Reduction in the inflammatory markers CD4, IL-1, IL-6 and TNFα in dogs with keratoconjunctivitis sicca treated topically with mesenchymal stem cells. Stem Cell Res. 2019, 39, 101525. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).