Simple Summary
The presented manuscript provides the first data about the important zoonotic disease, brucellosis, in the population of wild boars on the territory of Serbia. Brucellosis is an important disease of animals, both domestic and wild, and humans, and is of exceptional importance. Recently, the disease has re-emerged in some countries and is a threat to public health. The infection was never investigated before in the population of wild boars in Serbia, although the reported infections in domestic pigs indicate the possible pathogen transmission from wild to domestic pigs. Applied serology assays provided Brucella seroprevalences in wild boars, while a wealth of statistics delivered important data. The obtained results confirm the presence of the infection in the population of wild boars in Serbia and open new chapters for the future investigations of brucellosis in wild boars in Serbia.
Abstract
Brucellosis is one of the most important bacterial zoonotic diseases worldwide, characterized in domestic animals by long-term reproductive disorders. As known, wild boars (Sus scrofa) are natural hosts for Brucella suis biovar 2, in which the infection passes in inapparent form, increasing the pathogen transmission risk to domestic pigs, other domestic animals and humans. So far, no studies regarding brucellosis in wild boars in Serbia have been published. During the hunting season 2020/2021, 480 sera of wild boars living in Serbia were collected and tested for the presence of anti-Brucella antibodies. For the serological survey, the Rose Bengal Test (RBT) and competitive enzyme-linked immunosorbent assay (c-ELISA) were used. Of the 480 sera, 45 sera tested positive, indicating the acquired Brucella seroprevalence in wild boars of 9.4%. The greatest numbers of Brucella seropositive animals were detected in the eastern parts of the country and in one of the central districts, i.e., Pomoravski, Branicevski, Borski and Juznobanatski. This study provides the first data regarding brucellosis in the wild boar population in Serbia, revealing the seroprevalence of Brucella, thus indicating that wild boars as natural hosts and/or vectors of Brucella likely present a risk for the infection of other animals.
1. Introduction
Brucellosis is one of the most important contagious diseases of animals, including humans, as the disease has been registered on almost every continent in the past and is still one of the most significant bacterial zoonoses. Brucella is a group of small facultative, intracellular, Gram-negative, non-capsulated and non-motile coccobacilli. Within the genus Brucella, twelve species have been recognised so far. Beside the six well known species, i.e., the so-called classical Brucella species, B. abortus, B. melitensis, B. ovis, B. suis, B. canis and B. neotomae, novel classification involves B. ceti, B. pinnipedialis, B. microti, B. inopinata [1], B. vulpis [2], and last reported B. papionis [3]. The number of species illustrates the large variety of animals, also including wildlife, predisposed to infection with Brucella.
The disease in pigs is dominantly caused by B. suis, within which five different biovars are identified. B. suis biovars 1 and 3 circulate in the population of domestic pigs, biovar 2 in wild boars, biovar 4 in reindeer and biovar 5 in rodents.
Wild boars (Sus scrofa) are the natural hosts and reservoirs for numerous infectious diseases and zoonoses, including brucellosis [4]. The population of wild boars in Europe is constantly increasing, primarily due to the absence of natural predators combined with artificial feeding, high reproductive potential etc., [5] with estimated population density throughout Europe of up to 15 individuals/km2 [6]. As B. suis is considered practically eradicated on commercial pig farms across Europe, USA, Canada and Australia, wild boars represent a constant risk for the reintroduction of brucellosis into domestic pig population. Additionally, wild boars also represent a risk for the infection of cattle, being consequently hazardous for public health. Limited ways for decreasing the prevalence of brucellosis in natural reservoirs, as well as the high zoonotic potential of certain biovars, indicate that B. suis still owns an important place on the list of infectious agents. Although wild boars together with European hares (Lepus europaeus) are natural hosts for B. suis biovar 2, infections with other Brucella species (B. melitensis and B. abortus) were also registered [7]. B. microti was notably isolated from the submandibular lymph node of wild boar, although it is known that reservoirs for B. microti are voles [8], while the bacteria were also found in foxes [9] and soil [10].
The reported prevalence of brucellosis in wild boars is estimated as ranging from 8 to 32% throughout Europe [11]. On the other hand, using serological investigations, researchers reported Brucella seroprevalence in wild boars ranged from 0 to almost 60% [12,13,14,15,16].
Brucellosis in pigs is characterized by long-term reproductive disorders. However, the infection in wild boars is considered to be inapparent, without characteristic clinical symptoms or pathomorphological lesions, despite the successful isolation of the pathogen. The basis for this can lie in the low pathogenicity of B. suis biovar 2 for wild boars and/or in other as yet undefined reasons. The spread of infection is mostly via mating or by ingestion of contaminated feed or water.
Brucellosis can be diagnosed in two ways: directly via isolation of Brucella, or indirectly by detection of specific antibodies. Due to the required laboratory working conditions (biosafety level 3), the risk to laboratory staff’s health and frequent failures to isolate bacteria, classical microbiological methods are rarely performed. Serological investigations in wildlife are the most frequently carried out for screening purposes, using the Rose Bengal Test (RBT) together with the complement fixation test (CFT), both recommended as general-purpose diagnostic tests in all wildlife species. Nevertheless, enzyme-linked immunosorbent assay (ELISA), as a more reliable test, appeared to be useful for epidemiological serosurveys [7].
In Serbia, B. melitensis, B. canis and B. suis biovar 2 have been isolated so far [17]. To the best of our knowledge, there are no published data regarding brucellosis in wild boars in Serbia. Hence, the lack of data regarding brucellosis in wild boars hinders understanding of the potential spread of this infection to domestic pigs, other domestic animals and humans. Therefore, our study aimed to investigate the presence of anti-Brucella antibodies in wild boar sera and, thus, provide the first information about this significant infection in the population of wild boars in Serbia.
2. Materials and Methods
2.1. Sample Collection
The samples were collected during 2020 and 2021 from hunted wild boars living in six different administrative districts in Serbia: Branicevski, Borski, Zajecarski, Pomoravski, Juznobanatski and Sumadijski (Figure 1).
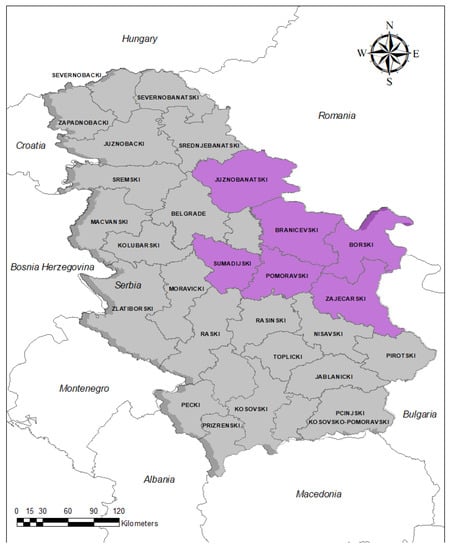
Figure 1.
Administrative districts in Serbia where the tested wild boar sera were collected (purple areas).
All of the wild boars were shot by registered hunters during the annual hunting season for wild boars. Age determination was conducted according to SCHEDA Ecological Associates, Inc. based on the number of permanent molars (one molar 6–18 months, two molars 1.5–2.5 years, and three molars over 2.5 years of age). Sampling was performed at the time of slaughter in the location where each animal was shot. Blood samples were collected from the thoracic cavity, into sterile 10 mL vacutainers containing clot activator, and sent to the Immunology Department, Scientific Institute of Veterinary Medicine of Serbia. After spontaneous coagulation and centrifugation (at 1500 g for 10 min), the sera were decanted and stored at −20 °C until further diagnostics. The sample size corresponded to the planned wild boar annual hunting bag that, with its range, exceeded a statistically representative sample as determined by statistical methods.
2.2. Serological Tests
Sera were analysed by two methods. A serum was considered positive when the two applied tests both showed positive reactions. The applied serology tests were RBT, used as a screening test, and c-ELISA, used as a confirmatory test. Antigen for RBT test was a suspension of inactivated Brucella abortus biovar 1 Weybridge strain No 99, obtained from ID.vet (Grabels, France). For this test, equal volumes (25 µL) of serum and antigen were mixed on a glass plate, forming a zone approximately 2 cm in diameter, gently stirred, and visualised for the presence of agglutination. The reaction was read after four minutes and any visible reaction was considered positive [7].
C-ELISA assay (SVANOVIR®, Brucella-Ab C-ELISA, Svanova, Uppsala, Sweden) was performed according to the manufacturer’s instructions. The optical density (OD) was measured using an ELISA reader (Tecan) at a single wave length of 450 nm. Sera were considered positive when the percent inhibition (PI value) was ≥30%. According to the manufacturer, diagnostic sensitivity and specificity values of the used ELISA kit were 99.5% and 99.6%, respectively.
2.3. Statistical Analysis
In the statistical analysis, we used descriptive statistical methods, Fisher’s exact test, risk ratio and prevalence estimation. We used Cohen’s Kappa test to assess the agreement between RBT and c-ELISA tests. Confidence intervals were calculated using the Wilson score method. Fisher’s exact test was used to determine the significance of the data. We also calculated the diagnostic parameters of the tests: negative and positive predictive value, expected number of true positive results, false negative and true negative results, the likelihood ratio for a positive result and likelihood ratio for a negative result, risk ratio and odds ratio (OR). In addition, we analysed the association of the age of wild boars with the seroprevalence of brucellosis. The strength of association between dependent and explanatory variables was estimated using ORs with 95% confidence intervals (95 CIs). Binary logistic regression (BLR) was used to model the relationship between predictors (age and sex) and dependent variables, i.e., seropositivity (for more information see Supplementary material, Table S1). The initial working hypothesis was that the age of wild boars was a risk factor for Brucella infection, so the hunted wild boars were stratified into four strata: up to 6 months of age, 6 to 18 months, 1.5 to 2.5 years, and boars older than 2.5 years. Within each stratum formed, we determined the seroprevalence. The statistical analyses were done using the statistical software packages IBM SPSS v. 26.0.0.0, XLSTAT Version 2014.5.03. and OpenEpi Version 3 (https://www.openepi.com/Menu/OE_Menu.htm (accessed on 17 June 2022).
3. Results
In total, 480 blood samples were collected from 304 (63.33%) male and 176 (36.66%) female individuals. The collected sera were examined for the presence of anti-Brucella antibodies, out of which 45 tested positive. The overall apparent seroprevalence of Brucella infection was 9.4% and the true prevalence was 9.06% bounded by 95 CIs of 6.74% and 12.02%. Seroprevalence data of Brucella in wild boars in Serbia are presented in Table 1. The greatest percentages of Brucella seropositive animals were detected mainly in the eastern parts of the country and in one of the central districts, i.e., Pomoravski, Branicevski, Borski and Juznobanatski.

Table 1.
Seroprevalence of Brucella in wild boars in Serbia.
Following the expected sensitivity and specificity of the tests as given by the manufacturers, RBT and c-ELISA should classify correctly 98.96% and 99.58% of samples, respectively. RBT should misclassify 1.04% of samples (two false-negative results and three false-positive results, while c-ELISA only 0.42% (two false-negative results and one false-positive result). Table 2 and Table 3 show the properties of the RBT and c-ELISA tests used in the study.

Table 2.
Properties of RBT.

Table 3.
Properties of c-ELISA test.
The two applied serology test methods showed some deviation, as 50 sera were positive according to RBT, while out of these, 45 sera with positive reactions were confirmed by the c-ELISA test. Sera were only considered as positive when both of the two applied tests gave positive reactions. As shown in Table 4 and Table 5, RBT and c-ELISA tests showed excellent, almost perfect mutual agreement (Cohen’s Kappa test 0.94).

Table 4.
c-ELISA vs. RBT cross tabulation.

Table 5.
Test agreement of RBT and c-ELISA (Cohen’s Kappa test).
The majority of tested animals were 6–18 months old (46.5%), while the categories 0–6 months, 1.5–2.5 years and older than 2.5 years contained 2.2%, 32.1% and 19.1% of the animals, respectively. Brucella seropositive wild boars belonged to the age categories 0–6 months (n = 3), 6–18 months (n = 14), 1.5–2.5 years (n = 23) and older than 2.5 years (n = 5). Table 6 shows the results of wild boar sera testing with the c-ELISA test, stratified by the age categories.

Table 6.
The results of c-ELISA stratified by age.
By comparing the seroprevalences in different age groups of wild boars, we found there were obvious differences between the age strata and that these differences were significant. The highest percentage of Brucella seropositive boars was found in the category of young animals, aged up to 6 months (27.12%), then in pigs aged 1.5 to 2.5 years (14.67%), then in pigs aged 6–18 months (5.93%), and the lowest in pigs older than 2.5 years (5.08%) (Table 7).

Table 7.
Seroprevalence of brucellosis in wild boars stratified by age.
Table 8 and Table 9 show the values of the relative risks and risk differences in different age groups of wild boars tested for the presence of specific antibodies against Brucella.

Table 8.
The relative risk of brucellosis in different age groups of wild boars.

Table 9.
Risk differences of brucellosis between different age groups of wild boars.
Brucella seroprevalence differences between different age groups as a whole were statistically significant (Fisher’s exact test two-tailed p value = 0.0038). Wild boars aged 6–18 months have a 1.07-fold higher risk of having Brucella antibodies than wild boars over 2.5 years old, which makes a negligible difference between these two age categories. However, wild boars aged 1.5–2.5 years have a 2.75-fold higher risk of harbouring Brucella antibodies than wild boars over 2.5 years of age, whereas wild boars aged 1.5–2.5 years have a 2.56-fold higher risk of harbouring Brucella antibodies than wild boars aged 6–18 months. Risk ratio analysis indicated that wild boars between 1.5 and 2.5 years of age are at the greatest risk of infection.
Figure 2 provides a comparative overview of the distribution of tested sera, the number of Brucella seropositive individuals and the established seroprevalence of brucellosis in different age groups of wild boars.
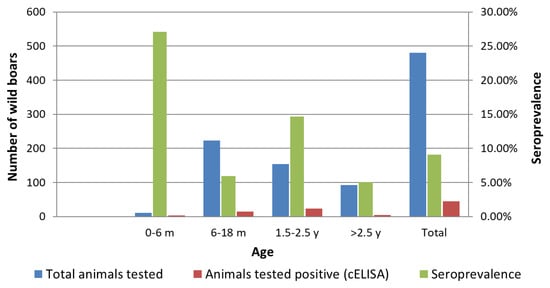
Figure 2.
Distribution of the total tested wild boars, the number of Brucella seropositive individuals and the determined seroprevalence of brucellosis in different age groups of wild boars.
BLR analysis proved a negative and positive association between the different age groups of wild boars and seropositivity, i.e., the registered number of Brucella seropositive wild boars compared to the youngest analysed category (age 6–18 months) as a reference category. The regression coefficient, B, for the model predictor variables was positive and significant for age group 1.5–2.5 years, indicating that this age group is more susceptible to infection than age group 6 to 18 months, which was not the case with the age group over 2.5 years where the regression coefficient, B, was negative and not significant. The regression coefficients for the different age groups of wild boars are given in Table 10. Regarding the OR, an exponential value of B of 2.613 for the age group 1.5–2.5 years indicates this age group is 2.613 times more likely to be infected with Brucella than the age group 6–18 months (Table 10; Supplementary material, Tables S1–S4; Supplementary material, Figures S1 and S2).

Table 10.
Results of the regression analysis (predicted variable: wild boar age; explanatory variable: Brucella seroprevalence).
Table 11 shows the results of mutual comparisons of the significance of the differences of different age groups and different sexes of wild boars. The sex of the boars was not a significant predictor of seropositivity (regression coefficient p = 0.358) (Table 11).

Table 11.
Results of mutual comparisons of the significance of the differences of different age groups of wild boars and different sexes.
4. Discussion
Brucellosis is a significant bacterial disease causing direct production losses in domestic animals, and thus, it is constantly highly ranked among the most economically important zoonoses worldwide. During the 1990s, due to armed conflict and uncontrolled movement of infected sheep, brucellosis spread to different parts of Serbia [19]. Recently, brucellosis in Serbia in domestic pigs has been sporadically confirmed, mostly in backyard farmed pigs after reproductive disorders were reported [20], while a serosurvey covering the period 2011–2015 detected 88 Brucella seropositive domestic pigs [21]. To date, no studies regarding Brucella infection in wild boars in Serbia have been published.
The prevalence of brucellosis has noticeably increased in many countries where pigs exist together with an overabundance of wildlife. In parallel with the growth of the wild boar population, in most countries, the prevalence of brucellosis has increased [15]. The population of wild boars in Serbia in recent years has remained reasonably constant, and in 2019, the population size was about 25,536 individuals [22]. To date, the presence in Serbia of B. melitensis, B. suis biovar 2 and B. canis has been reported [17]. B. suis biovar 2 was confirmed in two aborted foetuses from outdoor reared pigs in the northwestern part of the country, Srem, a mostly forested plains district, was reported by Zutic et al. [23]. The authors assumed that direct or indirect contact with wild boars led to the infection. Rogozarski et al. [24] isolated the same biovar from the epididymal puncture of a boar affected with brucellosis.
Our study revealed the true seroprevalence of Brucella infection in the wild boar population of 9.06%, and shows the first published data regarding Brucella seropositivity in the wild boar population in Serbia, to date. The presence of Brucella in the wild boar population in Serbia was not unexpected since this microorganism has been confirmed in neighbouring countries, where B. suis biovar 2 was predominantly isolated [25,26]. Moreover, Cvetnic et al. [14] confirmed the presence of B. suis biovar 3 in wild boars.
A global comprehensive literature review and meta-analysis of Brucella in pigs from 2000 to 2020 reported that the overall prevalence of brucellosis in wild boars between 2006 and 2010 was 22.3% [27], while after 2010, the prevalence gradually decreased after the World Organisation for Animal Health (WOAH) proposed control safety standards for animal production [28]. De facto, the Brucella seroprevalence in wild boars in Croatia varied through the years, ranging from 1.3% to 27.6% during different periods [13,14,25]. These data are in accordance with our obtained results, showing a Brucella seroprevalence of 9.06%. In our study, by comparing the Brucella seroprevalence in different age groups of wild boar, we found that these differences are statistically significant. Based on the BLR analysis, it was established that the age group of 1.5–2.5 years is the most susceptibile to infection, which was not the case with the age group over 2.5 years. Those findings are in line with the fact the greatest number and percentage of Brucella seropositive animals detected in the current study were in the age category 1.5–2.5 years. The findings are also consistent with the fact that the majority of wild boars reach puberty and start to mate within the first year of life, which favours the spread of infection. On the other hand, of 11 tested piglets aged 0–6 months, three were Brucella seropositive. This high seroprevalence in pigs aged 0–6 months cannot be reliably explained. However, taking into account biological plausibility and common modes of transmission and routes of infection, influencing factors could be maternal antibodies, piglets being vertically infected [29] and false-positive serological results due to Yersinia enterocolitica O:9 infection causing cross-reactivity. Analysis showed the sex of the boars is not a significant predictor of seropositivity.
A limitation of this study was the significantly smaller number of sera tested in the young age stratum compared to other age strata (total of 11 sera in the <6 month stratum). Due to this limitation in terms of sample size tested in this category of very young animals, and also due to the possible influence of unknown confounders, we concluded that it would be unjustifiable to include this stratum in the further statistical and epizootiological analyses. In that sense, no statistical comparisons were made of this stratum with other strata, i.e., other older categories of wild boars.
Both serological tests used in the study proved to be reliable for this type of study, especially when used in tandem.
Brucellosis can be a permanent risk even in the face of continuously applied monitoring programs, not only for domestic pigs, but for other domestic animals and humans. Since Serbia is not recognized as being officially free of brucellosis, legislation on brucellosis in Serbia prescribes mandatory serology testing for cattle older than 12 months, for sheep and goats older than 6 months, and for all breeding boars, rams and bulls kept either for natural breeding or artificial insemination [30]. Furthermore, every abortion in sows must be notified and examined for brucellosis [30]. Long-term brucellosis surveillance is well-regulated for domestic animals, but in contrast, brucellosis monitoring programs in wildlife are not conducted.
Our study indicates that wild boars are a very likely reservoir of Brucella for domestic pigs, considering pigs are frequently reared outdoors with low biosafety measures applied. These circumstances allow close contact of domestic and wild pigs, often resulting in them mating. In addition, the risk factors for transmission of the pathogen between wild boars and outdoor-reared pigs are linked to the presence and density of wild boar and domestic pig populations, disease prevalence, the features of the inhabited regions, fence characteristics etc. [31]. Wild boar meat has recently become more popular, and harvesting this product facilitates pathogen transmission [32]. Moreover, we hypothesise that the wild boar population is growing, and this has likely led to more frequent free movement of wild boars near commercial pig farms, perhaps increasing the risk of infection transmission to domestic pigs.
The greatest numbers of Brucella seropositive animals were detected in the eastern parts of the country and in one of the central districts, i.e., Pomoravski, Branicevski, Borski and Juznobanatski. The most likely reason for higher seropositivity in certain areas is the higher density of wild boars living in these localities. Additionally intriguing is the fact that Brucella seropositive wild boars were found in eastern parts of the country, i.e., border areas with neighbouring countries Hungary and Bulgaria, indicating the need for further, extended studies that will cover other administrative districts and include more animals. Therefore, cooperation between countries in the region is essential and should provide a better understanding of the epizootiological situation, since genetic similarity among circulating Brucella strains in the population of domestic and wild pigs originating from Hungary, Croatia and Germany was observed, highlighting the inability of state borders to control the spread of the pathogen [26,33].
The complexity of interactions among animals and humans is evident, and so the global picture of brucellosis remains incomplete, while in wild boars the disease is insufficiently clarified. Since the disease has recently re-emerged as a public health concern, brucellosis should be considered thoughtfully and disease control measures should be constantly maintained. Additional, comprehensive studies regarding Brucella biological characteristics, epizootiology and risk of transmission to domestic pigs and humans are in crucial need.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/vetsci9100575/s1, Table S1: Age structure, gender of investigated wild boar population and results of ELISA test (detection of Brucella specific antibodies); Table S2: Summery statistics; Table S3: Goodness of fit statistics (Variable ELISA); Table S4: Standardized coefficients (Variable ELISA); Figure S1: Standardized coefficients (Variable ELISA); Figure S2: ROC Curve (Variable ELISA) AUC- Area under the curve.
Author Contributions
S.R. and J.Z. designed and supervised this study. Z.Z.S. wrote the original draft of the manuscript. Z.Z.S. and N.S. carried out the laboratory diagnosis. S.S. performed statistical analysis. V.M. and M.R. together with S.R. and J.Z. reviewed the draft and prepared the final version of the manuscript. All authors contributed to the analysis and interpretation of data. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Ministry of Education, Science and Technological Development Republic of Serbia (Contract No 451-03-68/2022-14/200030 and 451-03-68/2022-14/200143).
Institutional Review Board Statement
Ethical review and approval were waived for this study, since animals were sampled during regular hunting activities regulated by national and regional laws, and no animals were killed for the research purposes.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Scholz, H.C.; Nöckler, K.; Göllner, C.; Bahn, P.; Vergnaud, G.; Tomaso, H.; Al Dahouk, S.; Kämpfer, P.; Cloeckaert, A.; Maquart, M.; et al. Brucella inopinata sp. nov., isolated from a breast implant infection. Int. J. Syst. Evol. Microbiol. 2010, 60, 801–808. [Google Scholar] [CrossRef] [PubMed]
- Scholz, H.C.; Revilla-Fernández, S.; Dahouk, S.A.; Hammerl, J.A.; Zygmunt, M.S.; Cloeckaert, A.; Koylass, M.; Whatmore, A.M.; Blom, J.; Vergnaud, G.; et al. Brucella vulpis sp. nov., isolated from mandibular lymph nodes of red foxes (Vulpes vulpes). Int. J. Syst. Evol. Microbiol. 2016, 66, 2090–2098. [Google Scholar] [CrossRef] [PubMed]
- Whatmore, A.M.; Davison, N.; Cloeckaert, A.; Al Dahouk, S.; Zygmunt, M.S.; Brew, S.D.; Perrett, L.L.; Koylass, M.S.; Vergnaud, G.; Quance, C.; et al. Brucella papionis sp. nov., isolated from baboons (Papio spp.). Int. J. Syst. Evol. Microbiol. 2014, 64, 4120–4128. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.J.; Lindsay, D.S.; Sriranganathan, N. Wild boars as sources for infectious diseases in livestock and humans. Philo. Trans. R. Soc. B 2009, 364, 2697–2707. [Google Scholar] [CrossRef] [PubMed]
- Bratton, S.P. The effect of Wild Boar, Sus scrofa on Gray Beech Forest in the Great Smokey Mountains. Ecology 1975, 56, 1356–1366. [Google Scholar] [CrossRef]
- ENETWILD-consortium; Acevedo, P.; Aleksovski, V.; Apollonio, M.; Berdión, O.; Blanco-Aguiar, J.; del Rio, L.; Ertürk, A.; Fajdiga, L.; Escribano, F.; et al. Wild boar density data generated by camera trapping in nineteen European areas. EFSA Support. Publ. 2022, 19, 7214E. [Google Scholar]
- WOAH. Chapter 3.1.4. Brucellosis (Infection with B. abortus, B. melitensis and B. suis): World Organisation for Animal Health (WOAH). 2022. Available online: https://www.woah.org/fileadmin/Home/eng/Health_standards/tahm/3.01.04_BRUCELLOSIS.pdf (accessed on 18 April 2022).
- Rónai, Z.; Kreizinger, Z.; Dán, Á.; Drees, K.; Foster, J.T.; Bányai, K.; Marton, S.; Szeredi, L.; Jánosi, S.; Gyuranecz, M. First isolation and characterization of Brucella microti from wild boar. BMC Vet. Res. 2015, 11, 147. [Google Scholar] [CrossRef]
- Scholz, H.C.; Hofer, E.; Vergnaud, G.; Le Fleche, P.; Whatmore, A.M.; Al Dahouk, S.A.; Pfeffer, M.; Krüger, M.; Cloeckaert, A.; Tomaso, H. Isolation of Brucella microti from mandibular lymph nodes of red foxes, Vulpes vulpes, in lower Austria. Vector-Borne Zoonotic Dis. 2009, 9, 153–156. [Google Scholar] [CrossRef]
- Scholz, H.C.; Hubalek, Z.; Nesvadbova, J.; Tomaso, H.; Vergnaud, G.; Le Flèche, P.; Whatmore, A.M.; Al Dahouk, S.; Krüger, M.; Lodri, C.; et al. Isolation of Brucella microti from Soil. Emerg. Infect. Dis. 2008, 14, 1316–1317. [Google Scholar] [CrossRef]
- Olsen, S.C.; Tatum, F.M. Swine brucellosis: Current perspectives. Vet. Med. 2016, 8, 1–12. [Google Scholar] [CrossRef]
- Hälli, O.; Ala-Kurikka, E.; Nokireki, T.; Skrzypczak, T.; Raunio-Saarnisto, M.; Peltoniemi, O.A.; Heinonen, M. Prevalence of and risk factors associated with viral and bacterial pathogens in farmed European wild boar. Vet. J. 2012, 194, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Cvetnic, Z.; Toncic, J.; Spicic, S.; Lojkic, M.; Terzic, S.; Jemersic, L.; Humski, A.; Curic, S.; Mitak, M.; Habrun, B.; et al. Brucellosis in wild boar (Sus scrofa) in the Republic of Croatia. Vet. Med. 2004, 49, 115–122. [Google Scholar] [CrossRef]
- Cvetnic, Z.; Spicic, S.; Toncic, J.; Majnaric, D.; Benic, M.; Albert, D.; Thiébaud, M.; Garin-Bastuji, B. Brucella suis infection in domestic pigs and wild boar in Croatia. Rev. Sci. Technol. 2009, 28, 1057–1067. [Google Scholar] [CrossRef]
- Grégoire, F.; Mousset, B.; Hanrez, D.; Michaux, C.; Walravens, K.; Linden, A. A serological and bacteriological survey of brucellosis in wild boar (Sus scrofa) in Belgium. BMC Vet. Res. 2012, 8, 80. [Google Scholar] [CrossRef] [PubMed]
- Al Dahouk, S.; Nöckler, K.; Tomaso, H.; Splettstoesser, W.D.; Jungersen, G.; Riber, U.; Petry, T.; Hoffmann, D.; Scholz, H.C.; Hensel, A.; et al. Seroprevalence of brucellosis, tularemia, and yersiniosis in wild boars (Sus scrofa) from north-eastern Germany. J. Vet. Med. B. Infect. Dis. Vet. Public Health 2005, 52, 444–455. [Google Scholar] [CrossRef]
- Radojicic, S. Brucellosis: Epizootiologic and diagnostic challenge. Vet. Glas 2005, 5, 79–87. [Google Scholar] [CrossRef]
- Etman, R.H.; Barsoum, S.A.; Ibrahim, I.G.A.; El-Ashmawy, W.R.; Abou-Gazia, K.A. Evaluation of efficacy of some serological tests used for diagnosis of brucellosis in cattle in Egypt using latent class analysis. Sokoto J. Vet. Sci. 2014, 12, 1–7. [Google Scholar] [CrossRef]
- Djuricic, B. Brucellosis in the Republic of Serbia—The epizootiological situation. Maced. J. Med. Sci. 2010, 3, 246–250. [Google Scholar] [CrossRef]
- Zutic, J.; Stanojevic, S.; Plavsic, B.; Vojinovic, D. Prevalence and significance of brucellosis in the Republic of Serbia. In Book of Abstract of the Symposium Brucellosis in Croatia and Neighbouring Countries; Zagreb, Croatia, 25 September 2013; Croatian Academy of Sciences and Arts and Croatian Veterinary Institute: Zagreb, Croatia, 2013; pp. 1–2. [Google Scholar]
- Zutic, J.; Vojinovic, D.; Stanojevic, S.; Cvetojevic, D.J.; Plavsic, B. Seroepizootiological situation of brucellosis in Republic of Serbia. In Proceedings of the 2nd International Symposium of Veterinary Medicine, Belgrade, Serbia, 22–24 June 2016; pp. 30–35. [Google Scholar]
- Statistical Office of the Republic of Serbia. Numbers of Wild Boars. 2019. Available online: https://data.stat.gov.rs/Home/Result/13040601?languageCode=sr-Latn (accessed on 5 April 2022).
- Zutic, J.; Cvetojevic, D.J.; Veljovic, L.J.; Radanovic, O.; Stanojevic, S.; Kureljusic, B. First report of Brucella suis biovar 2 in outdoor reared pigs (Sus Scrofa domesticus) in Serbia. Turk. J. Vet. Anim. Sci. 2017, 41, 700–704. [Google Scholar] [CrossRef]
- Rogozarski, D.; Potkonjak, A.; Gagrcin, M.; Lako, B.; Plavsic, B. Isolation and identification of Brucella suis biotype 2 from epididymal puncture performed on a boar affected with brucellosis. Vet. Glas 2009, 63, 153–161. [Google Scholar] [CrossRef]
- Cvetnic, Z.; Duvnjak, S.; Zdelar-Tuk, M.; Reil, I.; Mikulic, M.; Cvetnic, M.; Spicic, S. Swine Brucellosis caused by Brucella suis biovar 2 in Croatia. SVR 2017, 54, 149–154. [Google Scholar] [CrossRef]
- Kreizinger, Z.; Foster, J.T.; Rónai, Z.; Sulyok, K.M.; Wehmann, E.; Jánosi, S.; Gyuranecz, M. Genetic relatedness of Brucella suis biovar 2 isolates from hares, wild boars and domestic pigs. Vet. Microbiol. 2014, 172, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Gong, Q.-L.; Sun, Y.-H.; Yang, Y.; Zhao, B.; Wang, Q.; Li, J.-M.; Ge, G.-Y.; Chen, Z.-Y.; Shi, K.; Leng, X.; et al. Global comprehensive literature review and meta-analysis of Brucella spp. in swine based on publications from 2000 to 2020. Front. Vet. Sci. 2021, 8, 630960. [Google Scholar] [CrossRef] [PubMed]
- Knight-Jones, T.J.D.; Mylrea, G.E.; Kahn, S. Animal production food safety: Priority pathogens for standard setting by the World Organisation for Animal Health. Rev. Sci. Technol. 2010, 29, 523–535. [Google Scholar] [CrossRef]
- Cilia, G.; Fratini, F.; Turchi, B.; Angelini, M.; Cerri, D.; Bertelloni, F. Genital Brucella suis biovar 2 infection of wild boar (Sus scrofa) hunted in Tuscany (Italy). Microorganisms 2021, 9, 582. [Google Scholar] [CrossRef]
- Official Gazette of the Republic of Serbia No. 36/2021, Regulation on Animal Health Programme for 2021. Available online: https://www.vet.minpolj.gov.rs/srb/program-mera-2021 (accessed on 15 May 2021).
- Wu, N.; Abril, C.; Thomann, A.; Grosclaude, E.; Doherr, G.M.; Boujon, P.; Ryser-Degiorgis, M.R. Risk factors for contacts between wild boar and outdoor pigs in Switzerland and investigations on potential Brucella suis spill-over. BMC Vet. Res. 2012, 8, 116. [Google Scholar] [CrossRef]
- Petrovic, J.M.; Prodanov-Radulovic, J.Z.; Mirceta, J.D. Wild boar meat safety. IOP Conf. Ser. Earth Environ. Sci. 2019, 333, 012015. [Google Scholar] [CrossRef]
- Ferreira, A.C.; Corrêa de Sá, M.I.; Dias, R.; Tenreiro, R. MLVA-16 typing of Brucella suis biovar 2 strains circulating in Europe. Vet. Microbiol. 2017, 210, 77–82. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).