Shape of the Pulmonary Doppler Sonography Blood Flow Profile of the Congo Grey Parrot (Psittacus erithacus) and the Influence of Heart Disease
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Examined Congo Grey Parrots
2.2. Doppler Sonographic Examination
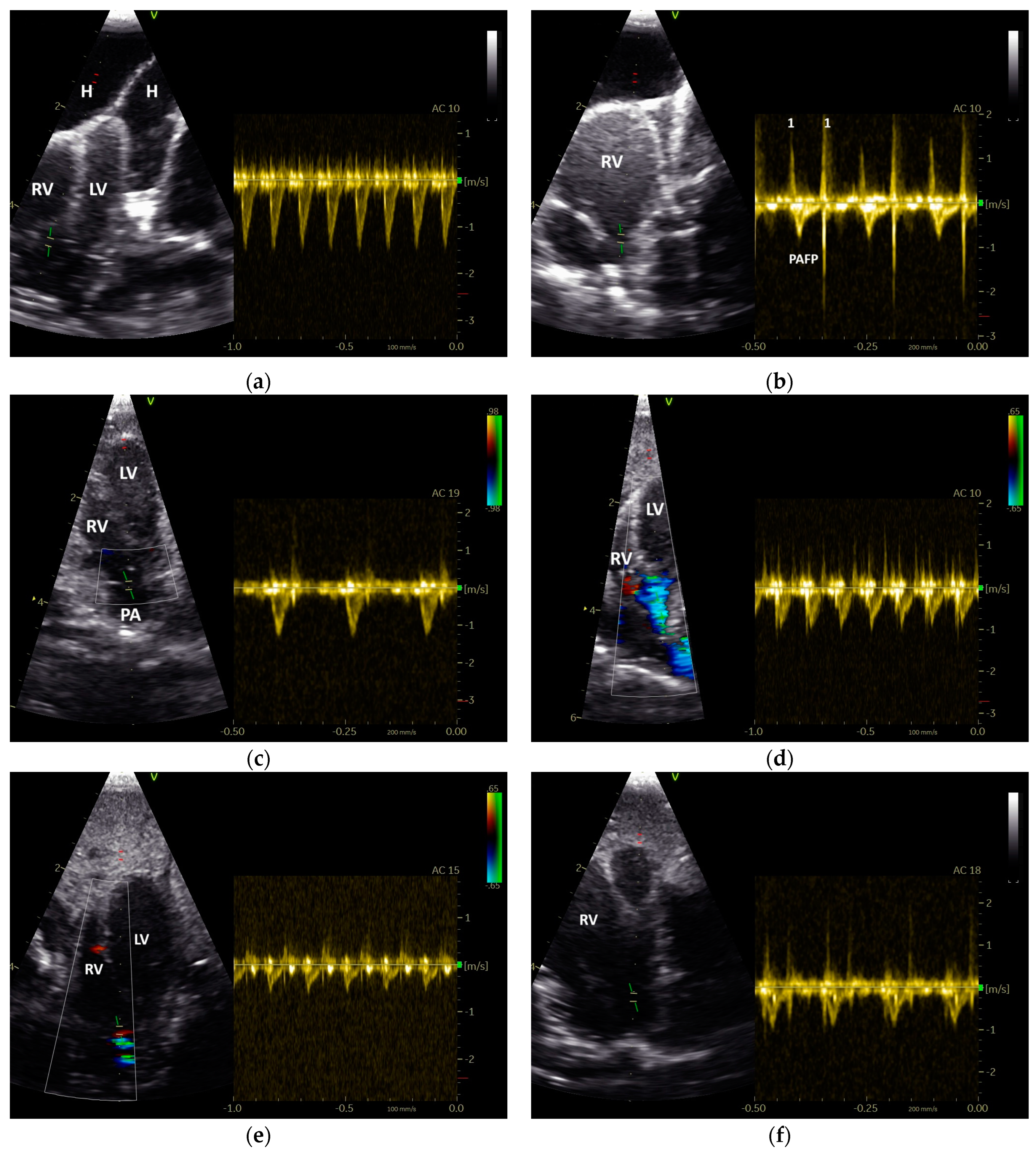
2.3. Measurments of the Heart Diameter and of Doppler Blood Flow Profiles
2.4. Statistical Analysis
3. Results
3.1. Shape of PAFP and AOFP of Grey Parrots
3.2. The Influence of Heart Failure on the Shape of the PAFP and AOFP
3.3. Influence of the Heart Rate on the Pulmonary and Aortic Blood Flow Profiles of the GPs
3.4. Correlation of Diastolic and Systolic Blood Flow Velocities with the Shape of the Pulmonary and Aortic Blood Flow Profiles in the Examined Diseased Grey Parrots
3.5. Correlation of the Pulmonary and Aortic Blood Flow Profiles with Different Heart Diameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pees, M.; Krautwald-Junghanns, M.-E.; Straub, J. Evaluating and treating the cardiovascular system. In Clinical Avian Medicine, 1st ed.; Harrison, G.J., Lightfoot, T., Eds.; Spix Publishing: Palm Beach, FL, USA, 2006; pp. 379–394. [Google Scholar]
- Fitzgerald, B.C.; Beaufrère, H. Cardiology. In Current Therapy in Avian Medicine and Surgery, 1st ed.; Speer, B.L., Ed.; Elsevier: St Louis, MO, USA, 2016; pp. 252–328. [Google Scholar]
- Oglesbee, B.L.; Oglesbee, M.J. Results of post-mortem examination of psittacine birds with cardiac disease: 26 cases (1991−1995). J. Am. Vet. Med. Assoc. 1998, 212, 1737–1742. [Google Scholar] [CrossRef] [PubMed]
- Beaufrère, H.; Nevarez, J.G.; Holder, K.; Pariaut, R.; Tully, T.N.; Wakamatsu, N. Characterization and classification of psittacine atherosclerotic lesions by histopathology, digital image analysis, transmission and scanning electron microscopy. Avian Pathol. 2011, 40, 531–544. [Google Scholar] [CrossRef] [PubMed]
- Beaufrère, H. Avian atherosclerosis: Parrots and beyond. J. Exot. Pet. Med. 2013, 22, 336–347. [Google Scholar] [CrossRef]
- Krautwald-Junghanns, M.-E.; Braun, S.; Pees, M.; Straub, J.; Valerius, H.P. Research on the anatomy and pathology of the psittacine heart. J. Avian Med. Surg. 2004, 18, 2–11. [Google Scholar] [CrossRef]
- Straub, J.; Pees, M.; Krautwald-Junghanns, M.-E. Measurement of the cardiac silhouette in psittacines. J. Am. Vet. Med. Assoc. 2002, 221, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Beaufrère, H.; Rodriguez, D.; Pariaut, R.; Gaschen, L.; Schnellbacher, R.; Nevarez, J.G.; Tully, T.N. Estimation of intrathoracic arterial diameter by means of computed tomographic angiography in Hispaniolan Amazon parrots. Am. J. Vet. Res. 2011, 72, 210–218. [Google Scholar] [CrossRef]
- Hein, R.F.; Kiefer, I.; Pees, M. A spectral computed tomography contrast study: Demonstration of the avian cardiovascular anatomy and function. Vet. Clin. Exot. Anim. Pract. 2022, 25, 435–451. [Google Scholar] [CrossRef]
- Megan, L.; Brust, K.; Spriet, M.; Ruivo, P.; Gómez-Ponce, M.; Beaufrère, H. 18F-sodium fluoride positron emission tomography is a sensitive imaging technique to detect atherosclerosis in Amazon parrots (Amazona spp.). Am. J. Vet. Res. 2025, 86, ajvr.24.10.0303. [Google Scholar] [CrossRef]
- Pees, M.; Straub, J.; Krautwald-Junghanns, M.-E. Echocardiographic examinations of 60 African grey parrots and 30 other psittacine birds. Vet. Rec. 2004, 155, 73–76. [Google Scholar] [CrossRef]
- Pees, M.; Straub, J.; Schumacher, J.; Gompf, R.; Krautwald-Junghanns, M.-E. Pilotstudie zu echokardiographischen Untersuchungen mittels Farb-und pulsed-wave-Spektraldoppler an Blaukronenamazonen (Amazona ventralis) und Blaustimamazonen (Amazona a. aestiva). Dtsch. Tierärztl. Wochenschr. 2005, 112, 39–43. [Google Scholar]
- Pees, M.; Krautwald-Junghanns, M.-E. Avian Echocardiography. Semin. Avian Exot. Pet Med. 2005, 14, 14–21. [Google Scholar] [CrossRef]
- Legler, M.; Koy, L.; Kummerfeld, N.; Fehr, M. Evaluation of blood flow velocity in the ascending aorta and pulmonary artery of clinically healthy racing pigeons (Columba livia f. dom.) by pulsed-wave Doppler echocardiography. Wiener Tierärztliche Mon. 2019, 106, 179–187. [Google Scholar]
- Legler, M.; Koy, L.; Kummerfeld, N. Differences between the filling velocities of the left and right heart ventricle in racing pigeons (Columba livia f. domestica) and the influence of anesthesia with isoflurane. Vet. Sci. 2019, 6, 79. [Google Scholar] [CrossRef]
- Sargo, R.; Alves, J.; Silva, F. Feasibility of pulmonary artery blood flow evaluation in 10 wild avian species, a retrospective study. In Proceedings of the International Conference on Avian, Herpetological, Exotic Mammal, Zoo and Wildlife Medicine, Budapest, Hungary, 27–30 March 2022. [Google Scholar]
- Masoudifard, M.; Bidgoli, V.R.; Madani, S.A.; Vahji, A.; Davoodipoor, S.; Vali, Y. Normal echocardiographic findings in healthy pigeons. Iran. J. Vet. Surg. 2016, 11, 7–13. [Google Scholar]
- Boon, J.A. Manual of Veterinary Echocardiography, 1st ed.; Blackwell Publishing: Oxford, UK, 2006. [Google Scholar]
- Krautwald-Junghanns, M.-E.; Schulz, U.; Konicek, C.; Pees, M. Evaluation of diagnostic criteria in grey parrots (Psittacus erithacus) with suspected atherosclerosis. Tierarztl. Prax. Ausg. K. Kleintiere/Heimtiere 2022, 50, 411–422. [Google Scholar] [CrossRef]
- Girard, C.; Koy, L.; Kummerfeld, N.; Pees, M.; Fehr, M.; Legler, M. Shape of the doppler sonographic systolic blood flow profile of the pulmonary artery of healthy racing pigeons and the influence of anesthesia. Vet. Sci. 2024, 11, 679. [Google Scholar] [CrossRef]
- Kirberger, R.M.; Bland-van den Berg, P.; Darazs, B. Doppler echocardiography in the normal dog: Part I Velocity findings and flow patterns. Vet. Radiol. Ultrasound 1992, 33, 370–379. [Google Scholar] [CrossRef]
- Kirberger, R.M.; Bland-van den Berg, P.; Grimbeek, R.J. Doppler echocardiography in the normal dog: Part II Factors influencing blood flow velocities and a comparison between left and right heart blood flow. Vet. Radiol. Ultrasound 1992, 33, 380–386. [Google Scholar] [CrossRef]
- Arkles, J.S.; Opotowsky, A.R.; Ojeda, J.; Rogers, F.; Liu, T.; Prassana, V.; Marzec, L.; Palevsky, H.I.; Ferrari, V.A.; Forfia, P.R. Shape of the right ventricular Doppler envelope predicts hemodynamics and right heart function in pulmonary hypertension. Am. J. Respir. Crit. Care Med. 2011, 183, 268–276. [Google Scholar] [CrossRef]
- Legler, M.; Koy, L.; Kummerfeld, N. The influence of anesthesia with isoflurane on the pulmonary and aortic blood flow of Racing Pigeons (Columba livia f. domestica) measured by pulsed wave Doppler echocardiography. Open Vet. J. 2019, 9, 18–26. [Google Scholar] [CrossRef]
- Joye, S.; Bhattacharya, S.; Kharrat, A.; Jasani, B.; Giesinger, R.E.; McNamara, P.J.; Jain, A. Shape of Pulmonary Artery Doppler Flow Profile and Right Ventricular Hemodynamics in Neonates. J. Pediatr. 2024, 266, 113864. [Google Scholar] [CrossRef] [PubMed]
- Temple, I.P.; Monfredi, O.; Quigley, G.; Schneider, H.; Zi, M.; Cartwright, E.J.; Boyett, M.R.; Mahadevan, V.S.; Hart, G. Macitentan treatment retards the progression of established pulmonary arterial hypertension in an animal model. Int. J. Cardiol. 2014, 177, 423–428. [Google Scholar] [CrossRef]
- Kitabatake, A.; Inoue, M.; Asao, M.; Masuyama, T.; Tanouchi, J.; Morita, T.; Mishima, M.; Uematsu, M.; Shimazu, T.; Hori, M.; et al. Noninvasive evaluation of pulmonary hypertension by a pulsed Doppler technique. Circulation 1983, 68, 302–309. [Google Scholar] [CrossRef]
- Kushwaha, S.P.; Zhao, Q.H.; Liu, Q.Q.; Wu, W.H.; Wang, L.; Yuan, P.; Zhang, R.; Jing, Z.C. Shape of the Pulmonary Artery Doppler-Flow Profile Predicts the Hemodynamics of Pulmonary Hypertension Caused by Left-Sided Heart Disease. Clin. Cardiol. 2016, 39, 150–156. [Google Scholar] [CrossRef]
- López-Candales, A.; Edelman, K. Shape of the right ventricular outflow Doppler envelope and severity of pulmonary hypertension. Eur. Heart J.—Cardiovasc. Imaging 2012, 13, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Ginghina, C.; Muraru, D.; Vladaia, A.; Jurcut, R.; Popescu, B.A.; Calin, A.; Giusca, S. Doppler Flow Patterns in the Evaluation of Pulmonary Hypertension. Rom. J. Intern. Med. 2009, 47, 109–121. [Google Scholar] [PubMed]
- Lumeij, J.T.; Stokhof, A.A. Electrocardiogram of the Racing Pigeon (Columba livia forma domestica). Res. Vet. Sci. 1985, 38, 275–278. [Google Scholar] [CrossRef]
- Riedel, U. Die Ultraschalluntersuchung des Vogels am Beispiel der Brieftaube (Columba livia forma domestica) mit Hilfe der Schnittbildechokardiographie. Ph.D. Thesis, Justus-Liebig-Universität Gießen, Gießen, Germany, 1995. [Google Scholar]
- Schulz, M. Morphologische und Funktionelle Messungen am Herzen von Brieftauben (Columba livia forma domestica) mit Hilfe der Schnittbildechokardiographie. Ph.D. Thesis, Justus-Liebig-Universität Gießen, Gießen, Germany, 1995. [Google Scholar]
- Dzialowski, E.M.; Crossley, D.A. The cardiovascular system. In Sturkie’s Avian Physiology, 6th ed; Scanes, C.G., Ed.; Academic Press: New York, NY, USA, 2015; pp. 193–283. [Google Scholar]
- West, J.B.; Watson, R.R.; Fu, Z. Major differences in the pulmonary circulation between birds and mammals. Respir. Physiol. Neurobiol. 2007, 157, 382–390. [Google Scholar] [CrossRef]
- Rosenkranz, S.; Gibbs, J.S.; Wachter, R.; De Marco, T.; Vonk-Noordegraaf, A.; Vachiéry, J.L. Left ventricular heart failure and pulmonary hypertension. Eur. Heart J. 2016, 37, 942–954. [Google Scholar] [CrossRef]
- Galiè, N.; Hoeper, M.M.; Humbert, M.; Torbicki, A.; Vachiery, J.-L.; Barbera, J.A.; Beghetti, M.; Corris, P.; Gaine, S.; Gibbs, J.S.; et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: The Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation. Eur. Heart J. 2009, 30, 2493–2537. [Google Scholar]
- Reinero, C.; Visser, L.C.; Kellihan, H.B.; Masseau, I.; Rozanski, E.; Clercx, C.; Williams, K.; Abbott, J.; Borgarelli, M.; Scansen, B.A. ACVIM consensus statement guidelines for the diagnosis, classification, treatment, and monitoring of pulmonary hypertension in dogs. J. Vet. Intern. Med. 2020, 34, 549–573. [Google Scholar] [CrossRef] [PubMed]
- Tossavainen, E.; Söderberg, S.; Grönlund, C.; Gonzalez, M.; Henein, M.Y.; Lindqvist, P. Pulmonary artery acceleration time in identifying pulmonary hypertension patients with raised pulmonary vascular resistance. Eur. Heart J. Cardiovasc. Imaging 2013, 14, 890–897. [Google Scholar] [CrossRef] [PubMed]
- Kingma, I.; Tyberg, J.V.; Smith, E.R. Effects of diastolic transseptal pressure gradient on ventricular septal position and motion. Circulation 1983, 68, 1304–1314. [Google Scholar] [CrossRef] [PubMed]
- Little, W.C.; Reeves, R.C.; Arciniegas, J.; Katholi, R.E.; Rogers, E.W. Mechanism of abnormal interventricular septal motion during delayed left ventricular activation. Circulation 1982, 65, 1486–1491. [Google Scholar] [CrossRef]
- Hoeper, M.M.; Krowka, M.J.; Strassburg, C.P. Portopulmonary hypertension and hepatopulmonary syndrome. Lancet 2004, 363, 1461–1468. [Google Scholar] [CrossRef]
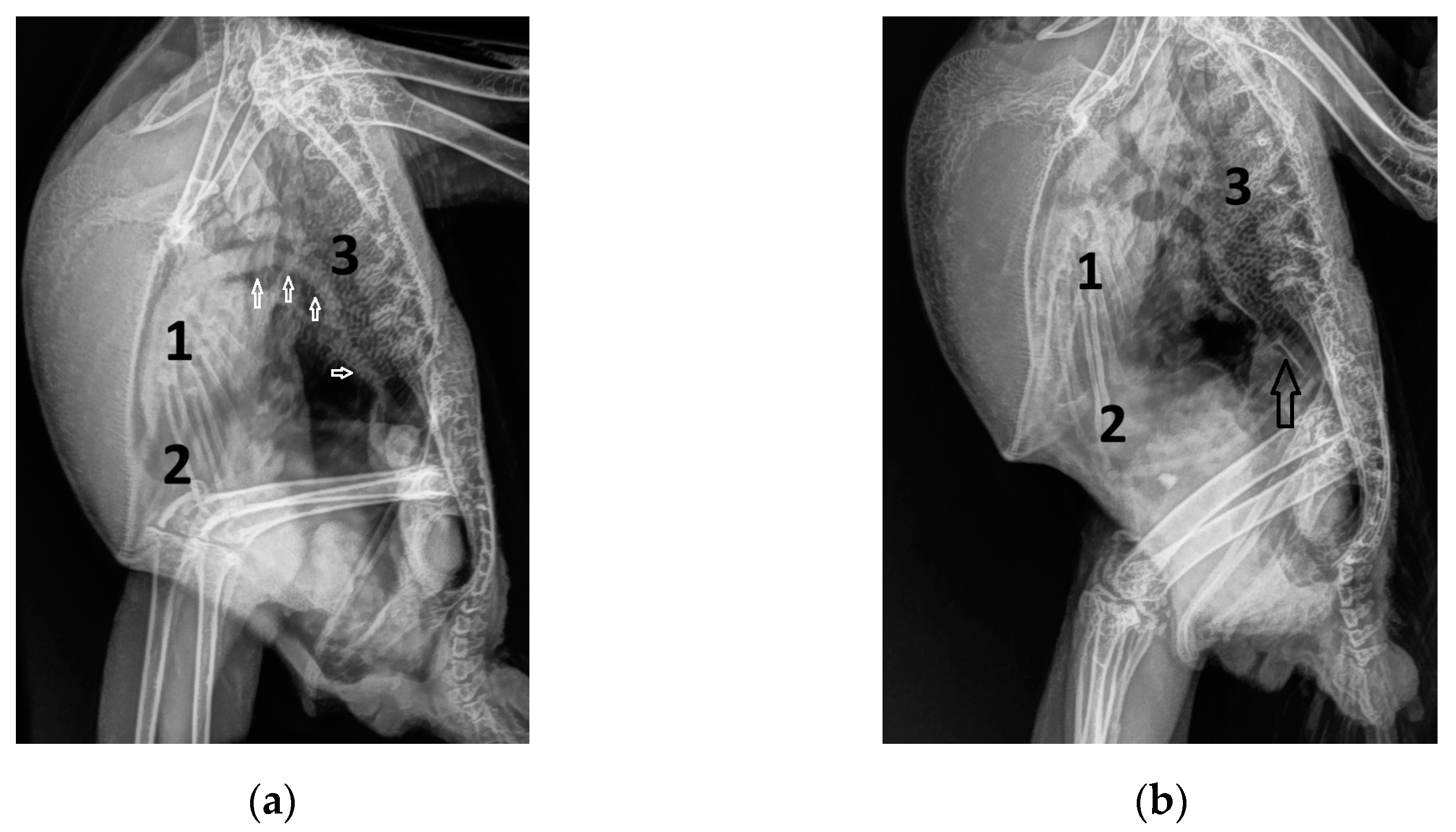
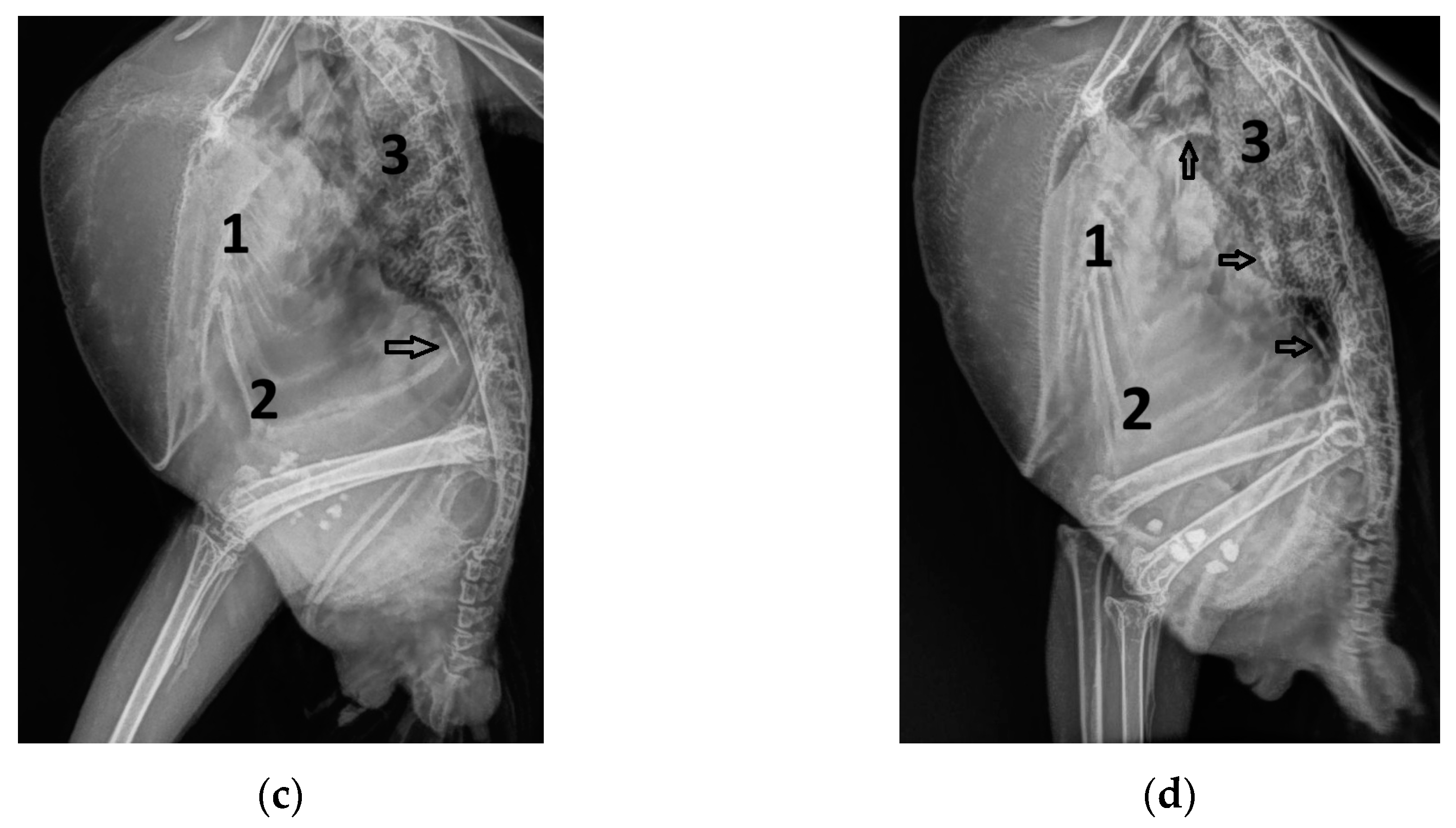
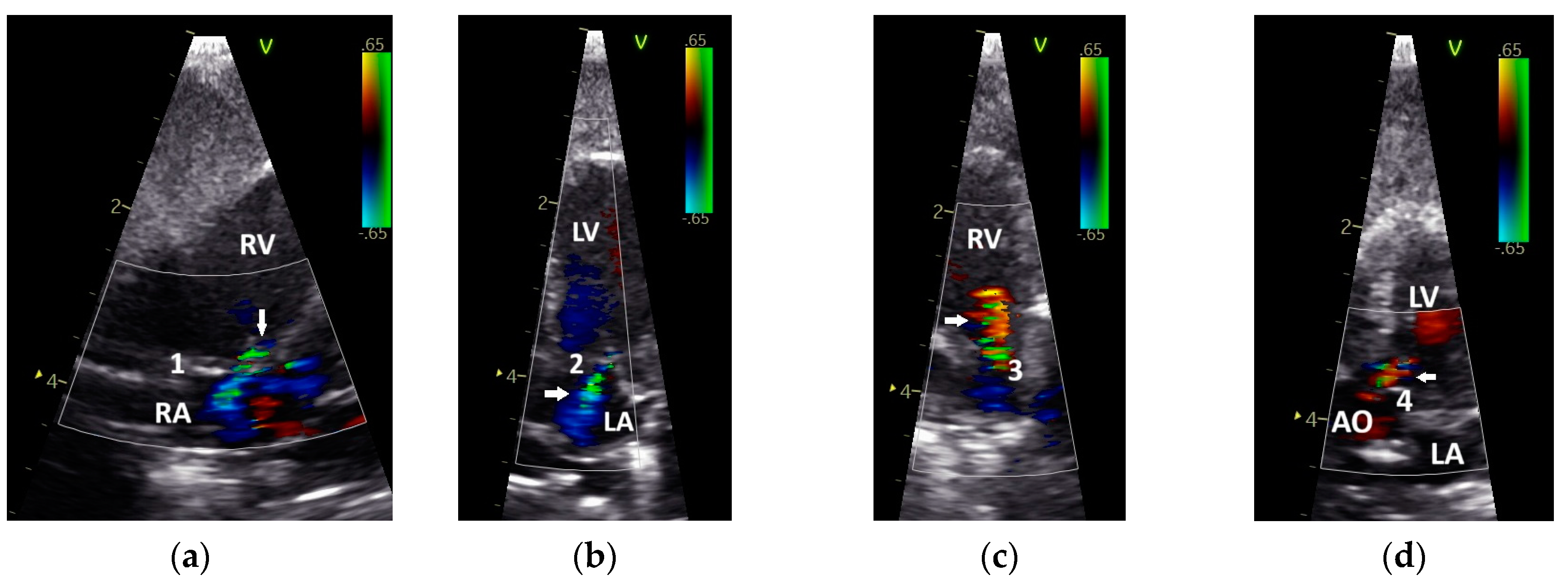
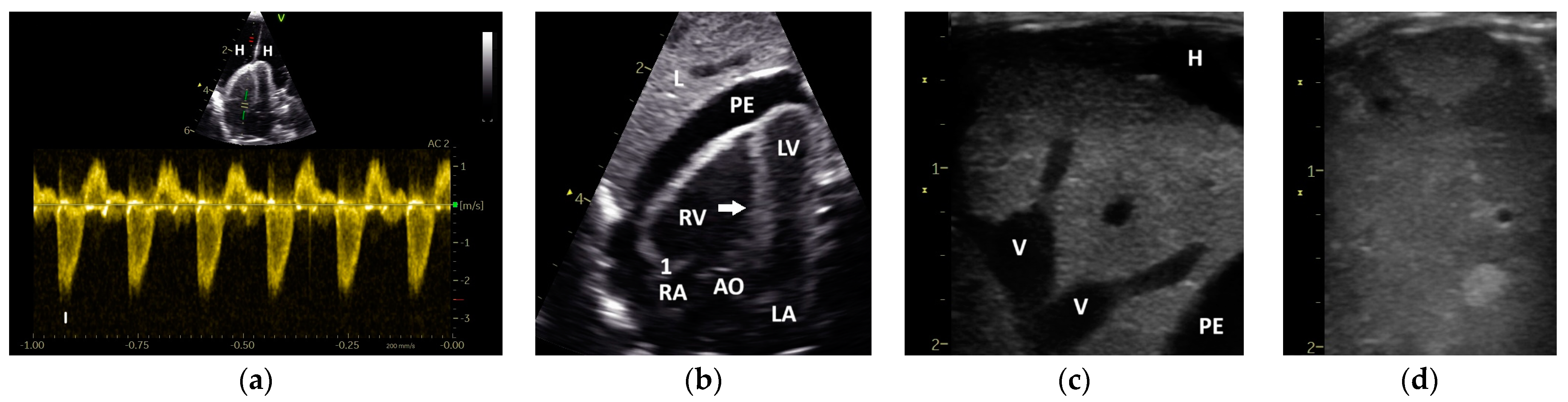



| Abbreviation | Unit | Doppler Sonographic Parameter |
|---|---|---|
| AO | - | Aorta |
| AOV | - | Aortic valve |
| PA | - | Pulmonary artery |
| PAV | - | Pulmonary valve |
| AV | - | Atrioventricular valve |
| AT | ms | Acceleration time |
| DT | ms | Deceleration time |
| ET | ms | Ejection time |
| AP | % | Percentage of AT on ET |
| DP | % | Percentage of DT on ET |
| PAAT | ms | AT of the blood flow of the PA |
| PADT | ms | DT of the blood flow of the PA |
| PAAP (%) | % | AP of the blood flow of the PA |
| PADP (%) | % | DP of the blood flow of the PA |
| PAET (ms) | ms | ET of the blood flow of the PA |
| PAVmax (m/s) | m/s | Peak flow velocities of the PA |
| ERVVmax (m/s) | m/s | Peak E wave velocities of the right heart |
| ARVVmax (m/s) | m/s | Peak A wave velocities of the right heart |
| E to A ratio right | - | E to A wave velocity ratio of the right heart |
| HRPA (beats/min) | beats/min | Heart rate during measurements of the PA |
| AOAT (ms) | ms | AT of the blood flow of the AO |
| AODT (ms) | ms | DT of the blood flow of the AO |
| AOAP (%) | % | AP of the blood flow of the AO |
| AODP (%) | % | DP of the blood flow of the AO |
| AOET (ms) | ms | ET of the blood flow of the AO |
| AOVmax (m/s) | m/s | Peak flow velocities of the AO |
| ELVVmax (m/s) | m/s | Peak E wave velocities of the left heart |
| ALVVmax (m/s) | m/s | Peak A wave velocities of the left heart |
| E to A ratio left | - | E to A wave velocity ratio of the left heart |
| HRAO (beats/min) | beats/min | Heart rate during measurements of the AO |
| LVd | cm | Left ventricle dimension end-diastolic |
| LVs | cm | Left ventricle dimension end-systolic |
| RVd | cm | Right ventricle dimension end-diastolic |
| RVs | cm | Right ventricle dimension end-systolic |
| LA | cm2 | Left atrium area (B-Mode) |
| RA | cm2 | Right atrium area (B-Mode) |
| FSLV | % | Fractional shortening LV |
| FSRV | % | Fractional shortening RV |
| Insufficiencies | SM | LD | H | PE | ||||
|---|---|---|---|---|---|---|---|---|
| AOV | PAV | AVleft | AVright | |||||
| Group 1 | 0/23 1 | 0/20 | 0/23 | 0/20 | 0/26 | 1/26 | 0/26 | 0/26 |
| Group 2 | 10/58 | 0/46 | 25/58 | 0/46 | 0/60 | 7/60 | 4/60 | 0/60 |
| Group 3 | 4/56 | 2/48 | 16/56 | 18/50 | 7/63 | 15/63 | 10/63 | 9/63 |
| LV | RV | LV and RV | |
|---|---|---|---|
| Group 1 | 0/23 1 | 0/20 | 0/20 |
| Group 2 | 34/58 | 0/46 | 0/46 |
| Group 3 | 4/61 | 27/50 | 22/50 |
| FS | Mean ± SD 1 | Xmin–Xmax | Median | 25% Percentile | 75% Percentile | |
|---|---|---|---|---|---|---|
| Group 1 | FSLV (%) | 30.1 ± 8.8 | 14.0–47.8 | 30.7 | 23.2 | 36.4 |
| FSRV (%) | 55.4 ± 12.3 | 32.9–76.7 | 55.3 | 46.0 | 64.5 | |
| Group 2 | FSLV (%) | 22.2 ± 9.1 | 2.6–48.1 | 22.2 | 16.3 | 27.3 |
| FSRV (%) | 55.8 ± 15.8 | 30.2–100.0 | 53.1 | 44.1 | 69.2 | |
| Group 3 | FSLV (%) | 22.4 ± 10.8 | 2.6–56.9 | 23.0 | 14.6 | 29.5 |
| FSRV (%) | 38.5 ± 18.2 | 10.1–79.4 | 36.1 | 24.2 | 52.2 |
| Parameter | Mean ± SD 1 | Xmin–Xmax | Median | 25% Percentile | 75% Percentile |
|---|---|---|---|---|---|
| PAAT (ms) | 23.6 ± 8.3 | 8.8–47.5 | 24.3 | 18.0 | 28.4 |
| PADT (ms) | 27.8 ± 7.0 | 19.3–48.2 | 27.7 | 21.4 | 31.8 |
| PAAP (%) | 45.3 ± 9.9 | 15.5–60.1 | 45.7 | 41.2 | 51.8 |
| PADP (%) | 54.7 ± 9.9 | 39.9–84.5 | 54.3 | 48.3 | 58.9 |
| PAET (ms) | 53.5 ± 10.9 | 37.0–85.3 | 52.6 | 46.3 | 59.7 |
| PAVmax (m/s) | 1.57 ± 0.80 | 0.65–3.69 | 1.31 | 1.10 | 1.82 |
| PAVmean max (m/s) | 1.12 ± 0.61 | 0.48–2.83 | 0.93 | 0.78 | 1.24 |
| HRPA (beats/min) | 395.5 ± 96.8 | 240.0–600.0 | 390.0 | 465.0 | 480.0 |
| EARVVmax (m/s) | 0.61 ± 0.13 | 0.41–0.84 | 0.60 | 0.51 | 0.74 |
| HREARV (beats/min) | 396.7 ± 101.6 | 240.0–600.0 | 360.0 | 322.5 | 480.0 |
| AOAT (ms) | 10.7 ± 2.6 | 6.0–18.0 | 10.5 | 9.0 | 12.6 |
| AODT (ms) | 41.7 ± 8.3 | 28.8–55.5 | 40.3 | 35.2 | 50.2 |
| AOAP (%) | 21.2 ± 5.5 | 9.8–33.8 | 21.3 | 18.2 | 24.5 |
| AODP (%) | 78.9 ± 5.5 | 66.2–90.2 | 78.7 | 75.5 | 81.8 |
| AOET (ms) | 56.6 ± 8.7 | 43.8–74.9 | 56.1 | 47.7 | 62.9 |
| AOVmax (m/s) | 1.32 ± 0.20 | 0.96–1.62 | 1.32 | 1.16 | 1.50 |
| AOVmean max (m/s) | 0.85 ± 0.13 | 0.63–1.09 | 0.85 | 0.73 | 0.95 |
| HRAO (beats/min) | 412.2 ± 85.4 | 240.0–600.0 | 420.0 | 360.0 | 480.0 |
| EALVVmax (m/s) | 0.93 ± 0.31 | 0.41–1.56 | 0.93 | 0.64 | 1.11 |
| HREALV (beats/min) | 410.5 ± 90.4 | 240.0–600.0 | 390.0 | 337.5 | 480.0 |
| Parameter | Mean ± SD 1 | Xmin–Xmax | Median | 25% Percentile | 75% Percentile |
|---|---|---|---|---|---|
| PAAT (ms) | 21.2 ± 5.7 | 12.3–43.7 | 20.8 | 17.78 | 25.0 |
| PADT (ms) | 31.4 ± 7.0 | 17.3–55.0 | 26.9 | 27.0 | 36.0 |
| PAAP (%) | 40.7 ± 8.4 | 22.8–56.2 | 41.8 | 28.8 | 46.7 |
| PADP (%) | 59.8 ± 9.0 | 43.8–77.9 | 58.2 | 53.4 | 65.5 |
| PAET (ms) | 54.5 ± 9.2 | 37.7–92.3 | 54.0 | 49.6 | 58.0 |
| PAVmax (m/s) | 1.85 ± 0.79 | 0.68–4.16 | 1.59 | 1.20 | 2.46 |
| PAVmean max (m/s) | 1.24 ± 0.53 | 0.50–2.45 | 1.07 | 0.82 | 1.53 |
| HRPA (beats/min) | 425.9 ± 72.0 | 300.0–720.0 | 420.0 | 360.0 | 480.0 |
| EARVVmax (m/s) | 0.76 ± 0.23 | 0.50–1.67 | 0.71 | 0.62 | 0.77 |
| HREARV (beats/min) | 422.7 ± 65.9 | 300.0–600.0 | 420.0 | 360.0 | 480.0 |
| AOAT (ms) | 9.77 ± 2.6 | 4.5–18.7 | 9.8 | 8.0 | 11.2 |
| AODT (ms) | 43.4 ± 9.6 | 25.7–63.2 | 42.8 | 35.9 | 50.2 |
| AOAP (%) | 18.7 ± 5.5 | 9.0–29.5 | 17.7 | 14.1 | 22.8 |
| AODP (%) | 81.1 ± 5.4 | 70.5–91.0 | 81.9 | 77.1 | 85.3 |
| AOET (ms) | 58.5 ± 10.7 | 39.2–99.3 | 57.7 | 50.8 | 66.5 |
| AOVmax (m/s) | 1.25 ± 0.31 | 0.71–2.57 | 1.21 | 1.08 | 1.38 |
| AOVmean max (m/s) | 0.76 ± 0.18 | 0.41–1.49 | 0.73 | 0.64 | 0.86 |
| HRAO (beats/min) | 419.1 ± 66.9 | 240.0–600.0 | 420.0 | 360.0 | 480.0 |
| EALVVmax (m/s) | 0.99 ± 0.28 | 0.47–1.70 | 0.95 | 0.80 | 1.19 |
| HREALV (beats/min) | 423.7 ± 55.7 | 300.0–600.0 | 420.0 | 400.0 | 480.0 |
| Parameter | Mean ± SD 1 | Xmin–Xmax | Median | 25% Percentile | 75% Percentile |
|---|---|---|---|---|---|
| PAAT (ms) | 20.7 ± 6.8 | 7.8–38.3 | 18.8 | 16.0 | 26.0 |
| PADT (ms) | 40.9 ± 12.8 | 19.3–75.2 | 43.0 | 29.9 | 50.3 |
| PAAP (%) | 34.5 ± 12.0 | 13.1–62.2 | 31.8 | 24.7 | 41.3 |
| PADP (%) | 65.5 ± 12.0 | 37.8–86.9 | 68.2 | 58.8 | 75.2 |
| PAET (ms) | 62.9 ± 11.5 | 44.2–105.4 | 62.8 | 55.5 | 70.8 |
| PAVmax (m/s) | 1.35 ± 0.82 | 0.57–3.89 | 1.07 | 0.87 | 1.54 |
| PAVmean max (m/s) | 0.90 ± 0.59 | 0.36–2.85 | 0.68 | 0.50 | 1.06 |
| HRPA (beats/min) | 388.5 ± 76.1 | 180.0–480.0 | 420.0 | 360.0 | 460.0 |
| EARVVmax (m/s) | 0.85 ± 0.30 | 0.28–2.14 | 0.80 | 0.65 | 1.01 |
| HREARV (beats/min) | 394.2 ± 82.9 | 150.0–540.0 | 420.0 | 360.0 | 480.0 |
| AOAT (ms) | 11.2 ± 3.2 | 5.0–19.8 | 10.9 | 9.3 | 12.9 |
| AODT (ms) | 46.3 ± 10.6 | 25.2–87.0 | 45.1 | 38.1 | 52.9 |
| AOAP (%) | 19.9 ± 5.5 | 7.9–32.6 | 19.4 | 16.3 | 23.3 |
| AODP (%) | 80.0 ± 5.4 | 67.4–92.1 | 80.3 | 76.5 | 82.9 |
| AOET (ms) | 62.8 ± 14.3 | 40.4–120.2 | 60.9 | 52.8 | 71.9 |
| AOVmax (m/s) | 1.25 ± 0.30 | 0.73–2.04 | 1.20 | 1.02 | 1.47 |
| AOVmean max (m/s) | 0.76 ± 0.18 | 0.47–1.18 | 0.72 | 0.64 | 0.88 |
| HRAO (beats/min) | 399.3 ± 82.3 | 180.0–540.0 | 420.0 | 360.0 | 480.0 |
| EALVVmax (m/s) | 1.00 ± 0.28 | 0.41–1.90 | 1.00 | 0.80 | 1.15 |
| HREALV (beats/min) | 402.0 ± 75.2 | 180.0–540.0 | 420.0 | 360.0 | 480.0 |
| Heart Score (%) | Mean ± SD 1 | Xmin–Xmax | Median | 25% Percentile | 75% Percentile |
|---|---|---|---|---|---|
| Group 1 | 49.2 ± 6.0 | 40.0–61.0 | 49.0 | 44.0 | 53.5 |
| Group 2 | 52.5 ± 5.2 | 40.0–63.0 | 53.0 | 48.8 | 56.0 |
| Group 3 | 56.8 ± 6.6 | 44.0–76.0 | 56.0 | 52.0 | 61.0 |
| FP Parameter | EAleft | EAright | PA | PAmean | AO | AOmean | HR |
|---|---|---|---|---|---|---|---|
| PAAT (ms) | p = 0.21 r = −0.08 | p = 0.09 r = −0.12 | p = 0.06 r = 0.12 | p = 0.01 r = 0.16 | p = 0.10 r = 0.11 | p = 0.01 r = 0.16 | p = 0.02 r = −0.21 |
| PADT (ms) | p = 0.13 r = 0.10 | p = 0.10 r = 0.11 | p = 0.09 r = −0.11 | p = 0.02 r = −0.15 | p = 0.41 r = −0.05 | p = 0.03 r = −0.14 | p = 0.21 r = −0.12 |
| PAET (ms) | p = 0.05 r = 0.13 | p = 0.15 r = 0.10 | p = 0.33 r = −0.06 | p = 0.15 r = −0.09 | p = 0.75 r = −0.02 | p = 0.16 r = −0.09 | p = 0.03 r = −0.20 |
| PAAP (%) | p = 0.08 r = −0.12 | p = 0.07 r = −0.12 | p = 0.02 r = 0.15 | p = 0.002 r = 0.20 | p = 0.21 r = 0.08 | p < 0.01 r = 0.18 | p = 0.52 r = −0.06 |
| PADP (%) | p = 0.09 r = 0.11 | p = 0.09 r = 0.12 | p = 0.02 r = −0.14 | p = 0.004 r = −0.19 | p = 0.23 r = −0.08 | p < 0.01 r = 0.18 | p = 0.46 r = −0.07 |
| AOAT (ms) | p < 0.001 r = −0.36 | p = 0.66 r = 0.04 | p = 0.001 r = −0.30 | p < 0.01 r = −0.32 | p = 0.58 r = −0.05 | p = 0.36 r = 0.08 | p = 0.02 r = −0.26 |
| AODT (ms) | p = 0.18 r = 0.12 | p = 0.76 r = 0.03 | p = 0.66 r = −0.04 | p = 0.76 r = 0.03 | p = 0.78 r = 0.03 | p = 0.03 r = −0.18 | p = < 0.001 r = −0.28 |
| AOET (ms) | p = 0.88 r = −0.01 | p = 0.55 r = −0.06 | p = 0.35 r = −0.09 | p = 0.46 r = −0.07 | p = 0.55 r = 0.05 | p = 0.15 r = −0.12 | p = < 0.001 r = −0.40 |
| AOAP (%) | p < 0.001 r = −0.32 | p = 0.91 r = 0.01 | p = 0.03 r = −0.21 | p = 0.02 r = −0.23 | p = 0.75 r = −0.03 | p = 0.03 r = −0.19 | p = 0.65 r = −0.04 |
| AODP (%) | p = 0.002 r = 0.27 | p = 0.78 r = −0.03 | p = 0.05 r = 0.19 | p = 0.04 r = 0.20 | p = 0.75 r = 0.03 | p = 0.03 r = 0.19 | p = 0.98 r = −0.002 |
| FP Parameter | LVd | LVs | RVd | RVs | LA | RA | FSLV | FSRV |
|---|---|---|---|---|---|---|---|---|
| PAAT (ms) | p = 0.54 r = 0.04 | p = 0.94 r = −0.01 | p = 0.05 r = −0.14 | p = 0.004 r = −0.20 | p = 0.32 r = −0.08 | p = 0.76 r = −0.05 | p = 0.34 r = 0.06 | p = 0.01 r = 0.17 |
| PADT (ms) | p = 0.77 r = 0.02 | p = 0.05 r = 0.13 | p = 0.003 r = 0.21 | p < 0.001 r = 0.23 | p = 0.02 r = 0.18 | p = 0.01 r = 0.39 | p = 0.02 r = −0.20 | p = 0.01 r = −0.19 |
| PAET (ms) | p = 0.60 r = 0.03 | p = 0.05 r = 0.13 | p = 0.02 r = 0.21 | p = 0.01 r = 0.18 | p = 0.03 r = −0.16 | p = 0.02 r = 0.38 | p = 0.003 r = −0.19 | p = 0.03 r = −0.14 |
| PAAP (%) | p = 0.95 r = −0.01 | p = 0.22 r = −0.08 | p = 0.02 r = −0.21 | p < 0.001 r = −0.27 | p = 0.05 r = −0.15 | p = 0.07 r = −0.29 | p = 0.02 r = 0.15 | p < 0.001 r = 0.23 |
| PADP (%) | p = 0.95 r = 0.01 | p = 0.21 r = −0.08 | p = 0.004 r = 0.20 | p < 0.001 r = 0.26 | p = 0.05 r = 0.15 | p = 0.18 r = 0.21 | p = 0.03 r = −0.15 | p = 0.002 r = −0.21 |
| AOAT (ms) | p = 0.35 r = −0.08 | p = 0.44 r = −0.07 | p = 0.43 r = 0.07 | p = 0.13 r = 0.14 | p = 0.92 r = 0.01 | p = 0.06 r = 0.33 | p = 0.001 r = 0.13 | p = 0.02 r = −0.22 |
| AODT (ms) | p = 0.17 r = 0.12 | p = 0.04 r = 0.18 | p = 0.20 r = 0.12 | p = 0.98 r = 0.002 | p < 0.001 r = 0.33 | p = 0.68 r = 0.08 | p = 0.09 r = −0.15 | p = 0.30 r = 0.10 |
| AOET (ms) | p = 0.19 r = 0.11 | p = 0.06 r = 0.16 | p = 0.09 r = 0.15 | p = 0.78 r = 0.03 | p = 0.004 r = 0.29 | p = 0.62 r = 0.09 | p = 0.09 r = −0.15 | p = 0.46 r = 0.07 |
| AOAP (%) | p = 0.07 r = −0.16 | p = 0.03 r = −0.20 | p = 0.78 r = −0.03 | p = 0.28 r = 0.10 | p = 0.07 r = −0.18 | p = 0.09 r = 0.31 | p = 0.16 r = 0.12 | p = 0.01 r = −0.23 |
| AODP (%) | p = 0.07 r = 0.16 | p = 0.01 r = 0.23 | p = 0.93 r = 0.01 | p = 0.27 r = −0.10 | p = 0.08 r = 0.18 | p = 0.06 r = −0.34 | p = 0.10 r = −0.15 | p = 0.03 r = 0.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Girard, C.; Kummerfeld, N.; Pees, M.; Fehr, M.; Legler, M. Shape of the Pulmonary Doppler Sonography Blood Flow Profile of the Congo Grey Parrot (Psittacus erithacus) and the Influence of Heart Disease. Vet. Sci. 2025, 12, 468. https://doi.org/10.3390/vetsci12050468
Girard C, Kummerfeld N, Pees M, Fehr M, Legler M. Shape of the Pulmonary Doppler Sonography Blood Flow Profile of the Congo Grey Parrot (Psittacus erithacus) and the Influence of Heart Disease. Veterinary Sciences. 2025; 12(5):468. https://doi.org/10.3390/vetsci12050468
Chicago/Turabian StyleGirard, Carolin, Norbert Kummerfeld, Michael Pees, Michael Fehr, and Marko Legler. 2025. "Shape of the Pulmonary Doppler Sonography Blood Flow Profile of the Congo Grey Parrot (Psittacus erithacus) and the Influence of Heart Disease" Veterinary Sciences 12, no. 5: 468. https://doi.org/10.3390/vetsci12050468
APA StyleGirard, C., Kummerfeld, N., Pees, M., Fehr, M., & Legler, M. (2025). Shape of the Pulmonary Doppler Sonography Blood Flow Profile of the Congo Grey Parrot (Psittacus erithacus) and the Influence of Heart Disease. Veterinary Sciences, 12(5), 468. https://doi.org/10.3390/vetsci12050468





