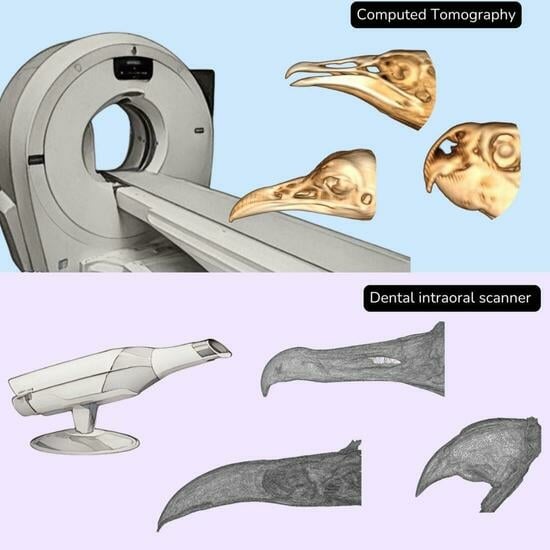
Computed Tomography and a Dental Intraoral Scanner to Generate Three-Dimensional Models of the Beaks of Three Bird Species
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Species Selection
2.2. Computed Tomography
2.3. Dental Intraoral Scanner
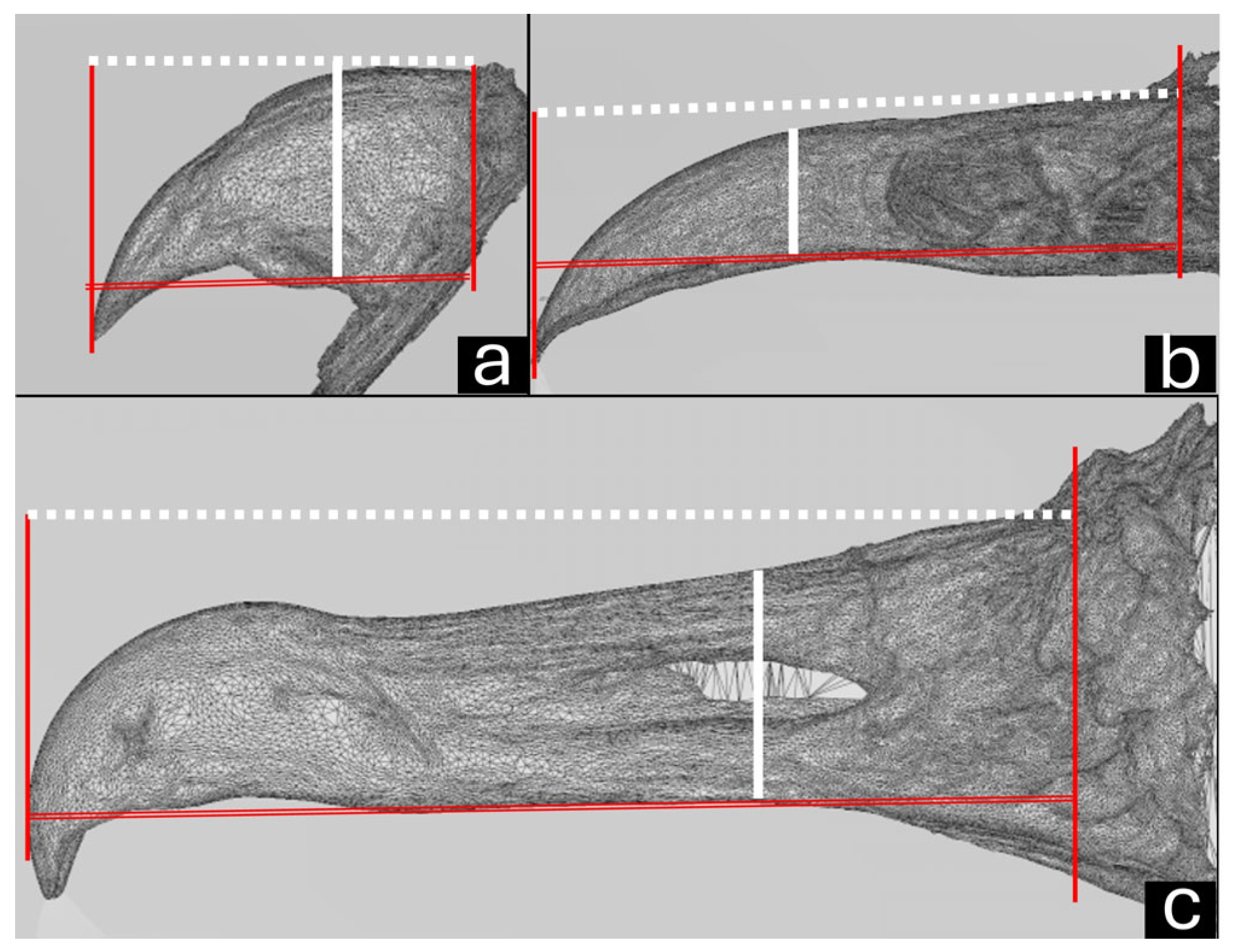
2.4. Macroscopic Measurements
2.5. Statistical Analysis
3. Results
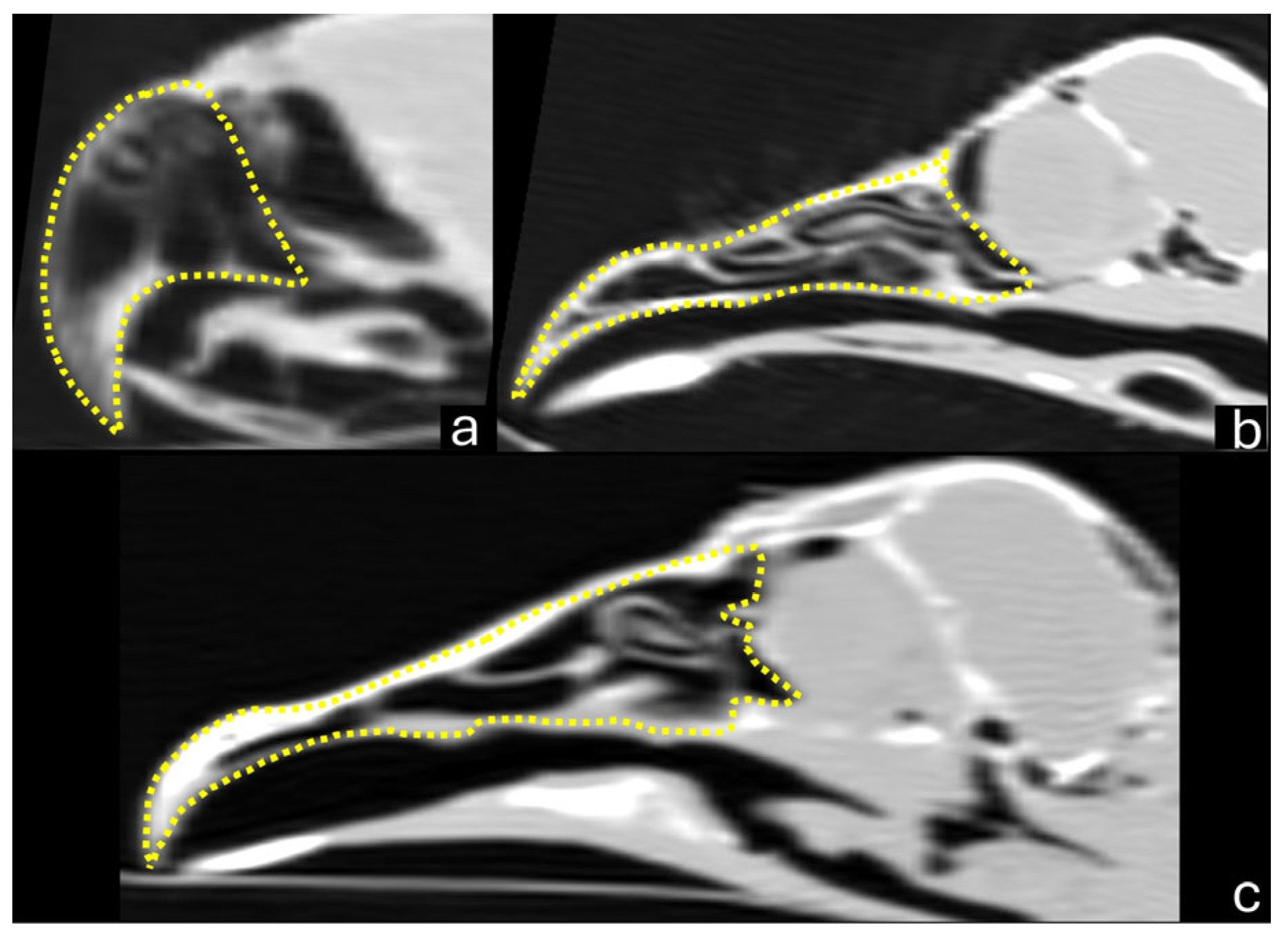
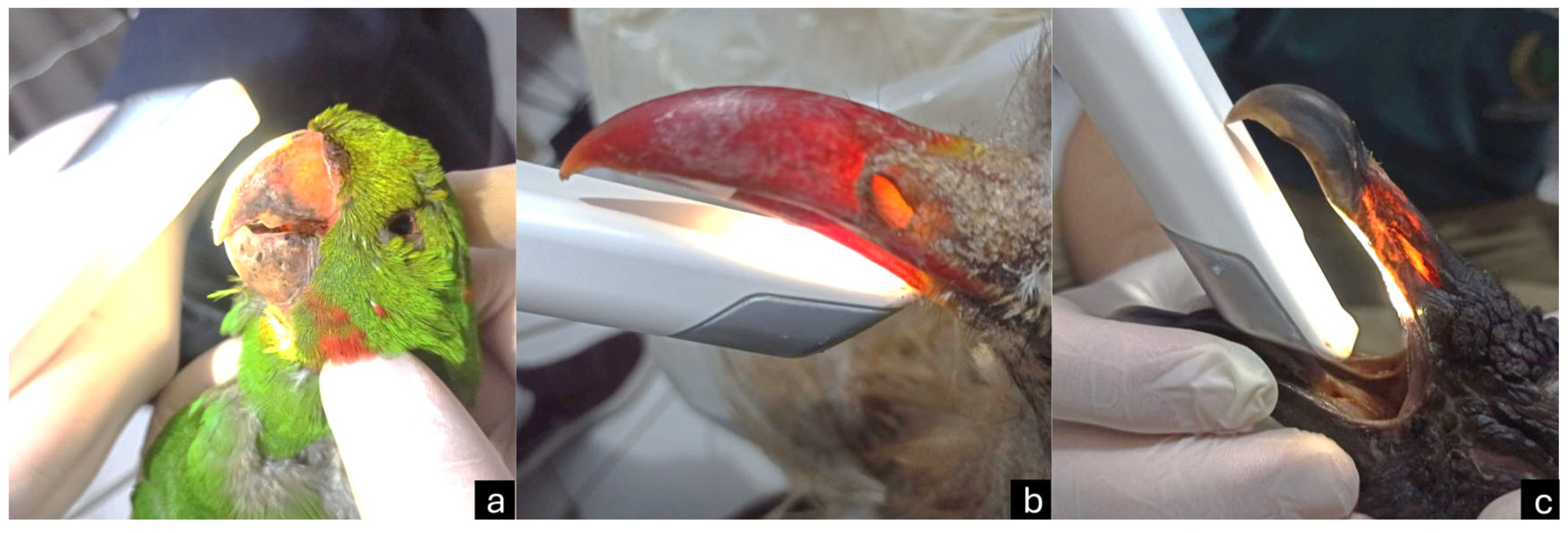
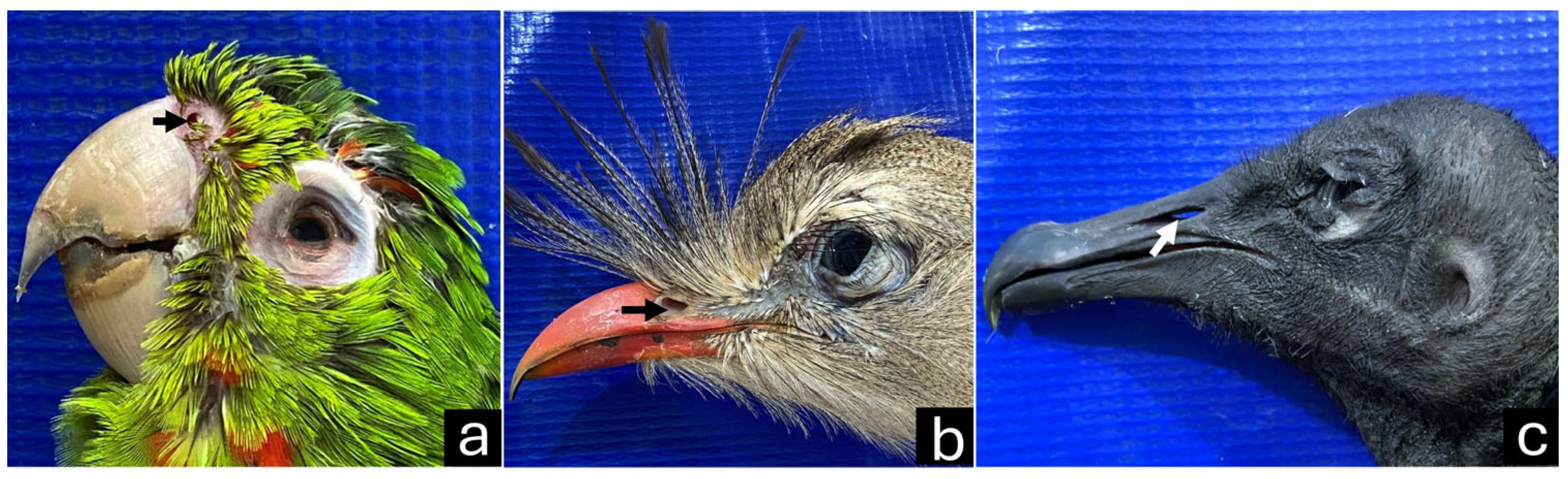
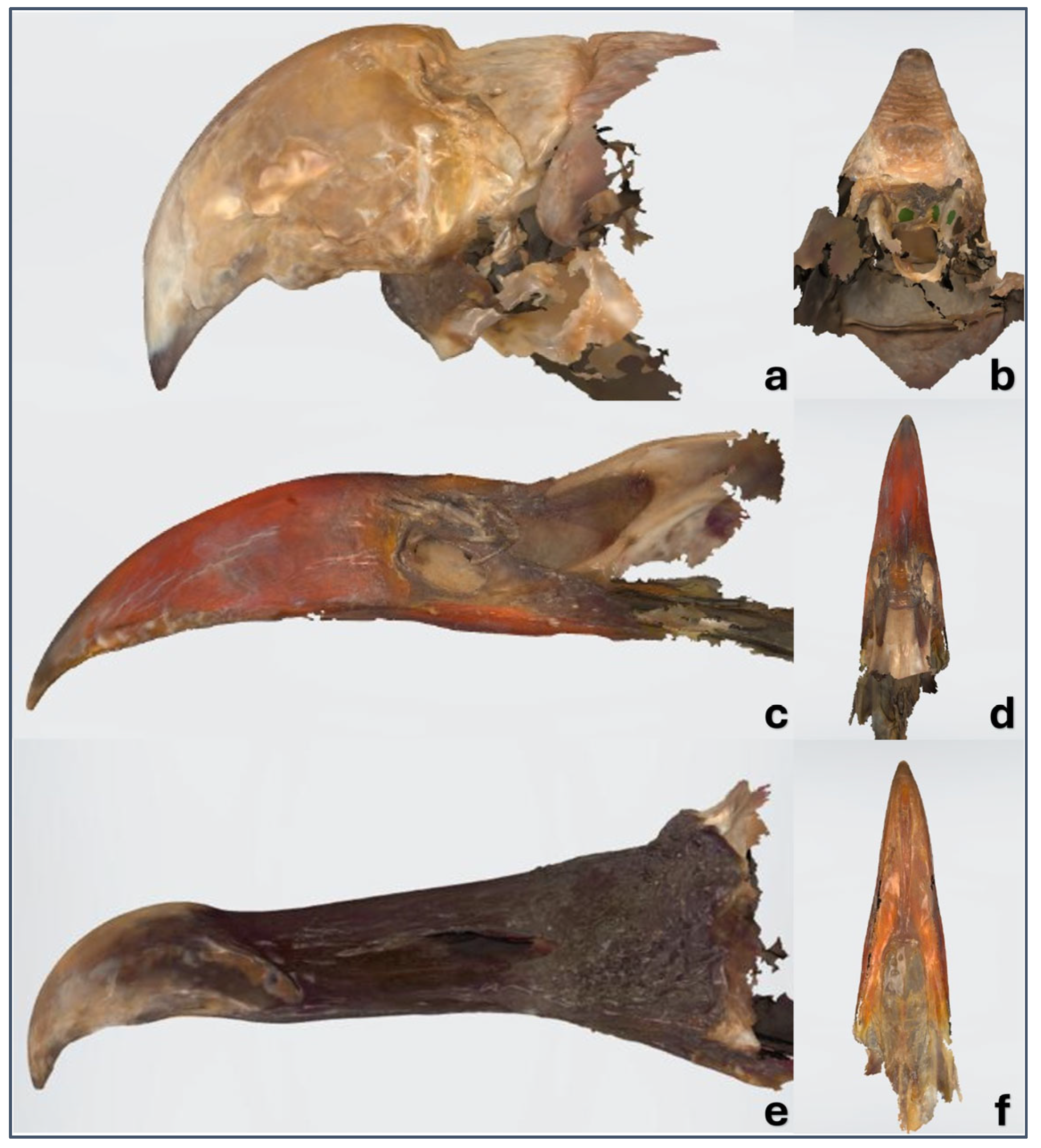
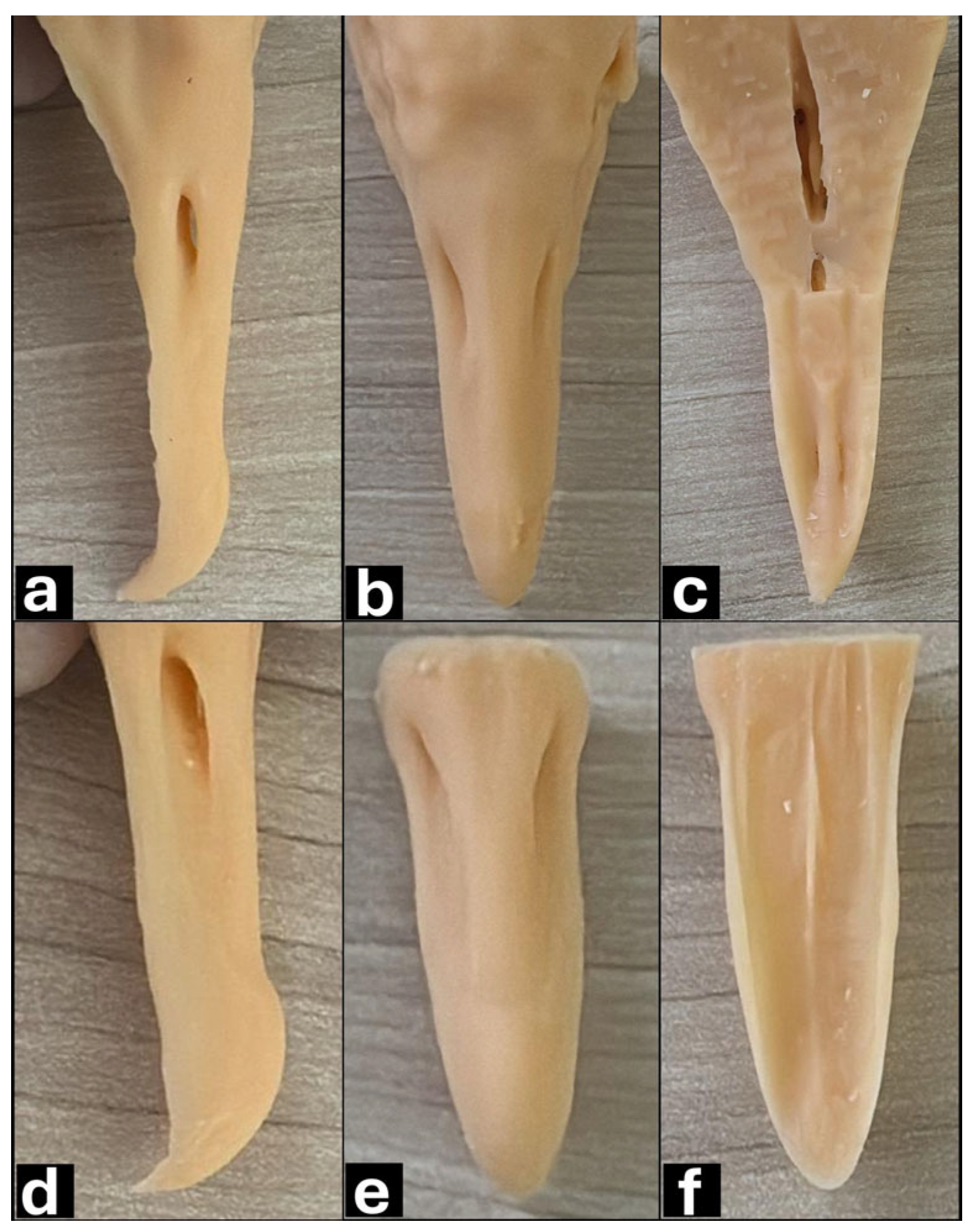
3.1. Macroscopy, CT, and Dental Intraoral Scanner
3.2. Statistical Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CT | Computed tomography |
| 3D | Three-dimensional |
References
- Ritchie, B.W.; Harrison, G.J.; Harisson, L.R. Avian Medicine: Principles and Application; Wingers Publishing: Lake Worth, FL, USA, 1994. [Google Scholar]
- Worell, A.B. Dermatological conditions affecting the beak, claws, and feet of captive avian species. Vet. Clin. N. Am. Exot. Anim. Pract. 2013, 16, 777–799. [Google Scholar] [CrossRef]
- Fecchio, R.S. Beakistry. In Zoo and Wild Animal; Emily, P.P., Eisner, E.R., Eds.; Wiley Blackwell: Hoboken, NJ, USA, 2021; pp. 99–117. [Google Scholar]
- Coles, B. Essentials of Avian Medicine and Surgery, 3rd ed.; Blackwell Publishing: Oxford, UK, 2008. [Google Scholar]
- Wheler, C.L. Orthopedic conditions of the avian head. Vet. Clin. N. Am. Exot. Anim. Pract. 2002, 5, 83–95. [Google Scholar] [CrossRef]
- Doneley, B. Avian Medicine and Surgery in Practice; Manson Publishing Ltd.: London, UK, 2010. [Google Scholar]
- Huynh, M.; González, M.S.; Beaufrére, H. Avian skull orthopedics. Vet. Clin. N. Am. Exot. Anim. Pract. 2019, 22, 253–283. [Google Scholar] [CrossRef] [PubMed]
- Speer, B.; Powers, L.V. Anatomy and disorders of the beak and oral cavity of birds. Vet. Clin. N. Am. Exot. Anim. Pract. 2016, 19, 707–736. [Google Scholar] [CrossRef] [PubMed]
- Morris, P.J.; Weigel, J.P. Methacrylate beak prosthesis in a marabou stork (Leptoptilos crumeniferus). J. Assoc. Avian Vet. 1990, 4, 103–106. [Google Scholar] [CrossRef]
- Rong, C.; Changlin, D.; Guodong, W.; Meirong, L.; Shujie, W.; Hongyi, L.; Menglin, Z.; Chengping, L. Comparison of 3d-printed titanium alloy and polyether ether ketone prosthetic beaks for an injured red-crowned crane (Grus japonensis). J. Avian Med. Surg. 2022, 35, 445–450. [Google Scholar] [CrossRef]
- Kim, H.J.; Cho, C.; Kim, K.T. Application of 3D-printed prosthetic lower beak in an Oriental stork (Ciconia boyciana). J. Vet. Med. Sci. 2023, 85, 1190–1194. [Google Scholar] [CrossRef] [PubMed]
- Krautwald-Junghanns, M.E.; Kostka, V.M.; Dörsch, B. Comparative studies on the diagnostic value of conventional radiography and computed tomography in evaluating the heads of psittacine and raptorial birds. J. Avian Med. Surg. 1998, 12, 149–157. [Google Scholar]
- Mangano, F.; Gandolfi, A.; Luongo, G.; Logozzo, S. Intraoral scanners in dentistry: A review of the current literature. BMC Oral. Health 2017, 17, 149. [Google Scholar] [CrossRef]
- Hwang, H.H.; Chou, C.; Chen, Y.; Yao, C.J. An overview of digital intraoral scanners: Past, present and future- from an orthodontic perspective. Taiwan. J. Orthod. 2018, 30, 148–162. [Google Scholar] [CrossRef]
- Nikoyan, L.; Patel, R. Intraoral scanner, three-dimensional imaging, and three-dimensional printing in the dental office. Dent. Clin. N. Am. 2020, 64, 365–378. [Google Scholar] [CrossRef] [PubMed]
- Gumpenberger, M.; Henninger, W. The use of computed tomography in avian and reptile medicine. Semin. Avian Exot. Pet. Med. 2001, 10, 174–180. [Google Scholar] [CrossRef]
- Greco, A.; Meomartino, L.; Gnudi, G.; Brunetti, A.; Di Giancamillo, M. Imaging techniques in veterinary medicine. Part II: Computed tomography, magnetic resonance imaging, nuclear medicine. Eur. J. Radiol. Open. 2022, 10, 100467. [Google Scholar] [CrossRef] [PubMed]
- Aswani, K.; Wankhade, S.; Khalikar, A.; Deogade, S. Accuracy of an intraoral digital impression: A review. J. Indian Prosthodont. Soc. 2020, 20, 27–37. [Google Scholar] [CrossRef]
- Shah, N.; Thakur, M.; Gill, S.; Shetty, O.; Alqahtani, N.M.; Al-Qarni, M.A.; Alqahtani, S.M.; Elagib, M.F.A.; Chaturvedi, S. Validation of digital impressions’ accuracy obtained using intraoral and extraoral scanners: A systematic review. J. Clin. Med. 2023, 12, 5833. [Google Scholar] [CrossRef] [PubMed]
- Alkadi, L.A. Comprehensive review of factors that influence the accuracy of intraoral scanners. Diagnostics 2023, 13, 3291. [Google Scholar] [CrossRef]
- Winkler, J.; Gkantidis, N. Trueness and precision of intraoral scanners in the maxillary dental arch: An in vivo analysis. Sci. Rep. 2020, 10, 1172. [Google Scholar] [CrossRef]
- Amornvit, P.; Rokaya, D.; Sanohkan, S. Comparison of accuracy of current ten intraoral scanners. Biomed. Res. Int. 2021, 2021, 2673040. [Google Scholar] [CrossRef]
- Grespan, A.; Raso, T.F. Psittaciformes (Araras, Papagaios, Periquitos, Calopsitas e Cacatuas). In Tratado de Animais Selvagens: Medicina Veterinária; Cubas, Z.S., Silva, J.C.R., Catão-Dias, J.L., Eds.; Roca: São Paulo, Brazil, 2014; pp. 550–589. [Google Scholar]
- Harcourt-Brown, N.H. Anatomy and physiology. In BSAVA Manual of Psittacine Birds; Harcourt-Brown, N.H., Chitty, J., Eds.; British Small Animal Veterinary Association: Glouster, UK, 2005; pp. 7–21. [Google Scholar]
- Stager, K.E. The role of olfaction in food location by the turkey vulture (Cathartes aura). Contrib. Sci. 1964, 81, 1–63. [Google Scholar]
- Bang, B.G. The nasal organs of the black and turkey vultures; A comparative study of the cathartid species Coragyps atratus atratus and Cathartes aura septentrionalis (with notes on Cathartes aura falklandica, pseudogyps bengalensis, and Neophron percnopterus). J. Morphol. 1964, 115, 153–183. [Google Scholar] [CrossRef]
- Jackson, J.A. American black vulture. In Handbook of North American Birds; Palmer, R., Ed.; Yale University Press: New Haven, CT, USA, 1998; pp. 11–24. [Google Scholar]
- Sick, H. Ornitologia Brasileira; Editora Universidade de Brasília: Brasília, Brazil, 1984. [Google Scholar]
- Fecchio, R.S.; Seki, Y.; Bodde, S.G.; Gomes, M.S.; Kolososki, J.; Rossi, J.L., Jr.; Gioso, M.A.; Meyers, M.A. Mechanical behavior of prosthesis in Toucan beak (Ramphastos toco). Mat. Sci. Eng. C 2010, 30, 460–464. [Google Scholar] [CrossRef]
- Song, C.; Wang, A.; Wu, Z.; Chen, Z.; Yang, Y.; Wang, D. The design and manufacturing of a titanium alloy beak for Grus japonensis using additive manufacturing. Mater. Des. 2017, 17, 410–416. [Google Scholar] [CrossRef]
- Xie, S.; Cai, B.; Rasidi, E.; Yen, C.C.; Hsu, C.D.; Chow, W.T.; De Busscher, V.; Hsu, L.C. The use of a 3D-printed prosthesis in a Great Hornbill (Buceros bicornis) with squamous cell carcinoma of the casque. PLoS ONE 2019, 14, e0220922. [Google Scholar] [CrossRef] [PubMed]
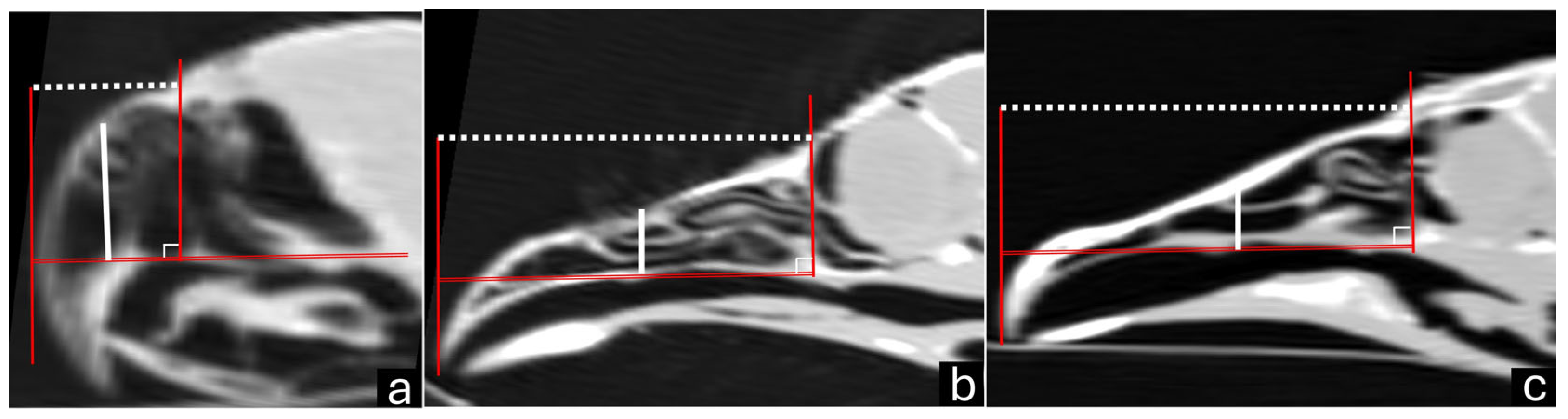
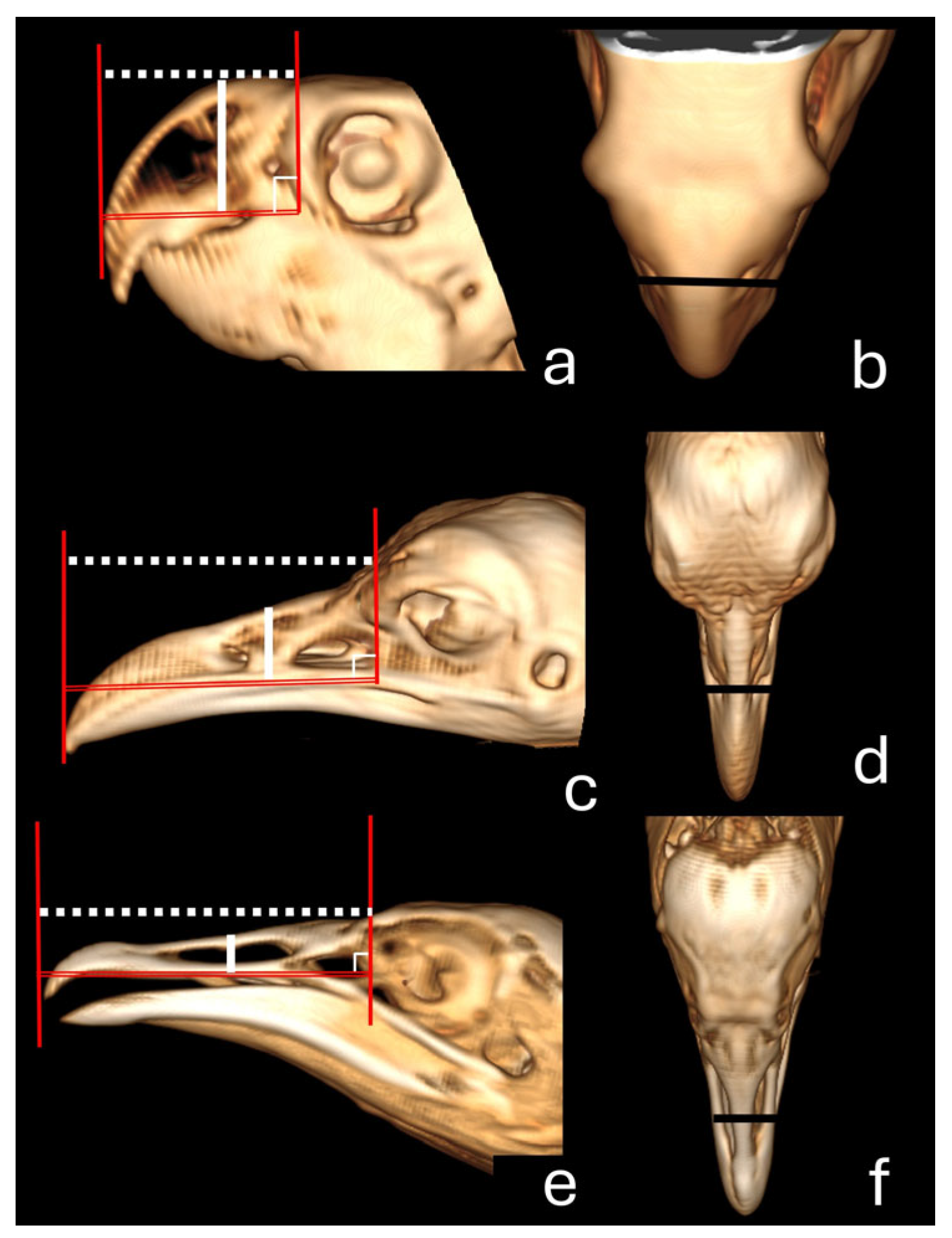

| Bird/Number | Sagittal Length | Sagittal Height | Sagittal Area | 3D naris Height | 3D naris Length |
|---|---|---|---|---|---|
| Parakeet 1 | 2.13 | 1.33 | 2.35 | 0.1 | 0.1 |
| Parakeet 2 | 2.18 | 1.35 | 2.5 | 0.12 | 0.1 |
| Parakeet 3 | 2.0 | 1.36 | 2.34 | 0.12 | 0.1 |
| Parakeet 4 | 2.32 | 1.28 | 2.5 | 0.1 | 0.11 |
| Parakeet 5 | 2.06 | 1.3 | 2.3 | 0.1 | 0.1 |
| Mean ± standard deviation | 2.13 ± 0.12 | 1.32 ± 0.03 | 2.39 ± 0.09 | 0.11 ± 0.0 | 0.11 ± 0.01 |
| Red-legged seriema 1 | 6.4 | 1.28 | 8.59 | 0.24 | 0.48 |
| Red-legged seriema 2 | 6.69 | 1.25 | 7.13 | 0.25 | 0.46 |
| Red-legged seriema 3 | 6.27 | 1.12 | 6.12 | 0.22 | 0.42 |
| Red-legged seriema 4 | 7.21 | 1.32 | 8.32 | 0.2 | 0.45 |
| Red-legged seriema 5 | 6.76 | 1.32 | 8.27 | 0.23 | 0.48 |
| Mean ± standard deviation | 6.66 ± 0.36 | 1.25 ± 0.08 | 7.68 ± 1.03 | 0.22 ± 0.01 | 0.45 ± 0.02 |
| Black vulture 1 | 5.9 | 0.96 | 6.5 | 0.15 | 0.71 |
| Black vulture 2 | 6.07 | 0.98 | 6.36 | 0.16 | 0.71 |
| Black vulture 3 | 5.6 | 1.15 | 6.01 | 0.18 | 0.71 |
| Black vulture 4 | 5.54 | 0.99 | 5.52 | 0.17 | 0.72 |
| Black vulture 5 | 5.9 | 1.08 | 5.63 | 0.16 | 0.71 |
| Mean ± standard deviation | 5.80 ± 0.22 | 1.03 ± 0.08 | 6.0 ± 0.43 | 0.16 ± 0.01 | 0.71 ± 0.01 |
| Bird/Number | 3D-CT Length | 3D-Scanner Length | Macroscopic Length | 3D-CT Height | 3D-Scanner Height | Macroscopic Height | 3D-CT Width | 3D-Scanner Width | Macroscopic Width |
|---|---|---|---|---|---|---|---|---|---|
| Parakeet 1 | 2.09 | 1.65 | 2.23 | 1.36 | 1.51 | 1.31 | 1.43 | 1.35 | 1.4 |
| Parakeet 2 | 2.11 | 1.54 | 2.15 | 1.4 | 1.41 | 1.38 | 1.33 | 1.22 | 1.36 |
| Parakeet 3 | 2.01 | 2 | 2.29 | 1.37 | 1.58 | 1.35 | 1.4 | 1.44 | 1.41 |
| Parakeet 4 | 2.15 | 2.1 | 2.17 | 1.29 | 1.41 | 1.3 | 1.44 | 1.52 | 1.42 |
| Parakeet 5 | 2.05 | 1.76 | 2.13 | 1.3 | 1.42 | 1.3 | 1.45 | 1.55 | 1.43 |
| Mean ± standard deviation | 2.08 ± 0.05 | 1.81 ± 0.23 | 2.19 ± 0.06 | 1.34 ± 0.05 | 1.46 ± 0.07 | 1.33 ± 0.03 | 1.41 ± 0.05 | 1.41 ± 0.13 | 1.4 ± 0.03 |
| Red-legged seriema 1 | 6.44 | 6.7 | 6.58 | 1.28 | 1.27 | 1.35 | 1.66 | 1.68 | 1.62 |
| Red-legged seriema 2 | 6.62 | 7.3 | 6.68 | 1.27 | 1.3 | 1.37 | 1.62 | 1.69 | 1.64 |
| Red-legged seriema 3 | 6.2 | 6.4 | 6.26 | 1.13 | 1 | 1.24 | 1.55 | 1.57 | 1.59 |
| Red-legged seriema 4 | 7.21 | 7 | 7.09 | 1.31 | 1.8 | 1.3 | 1.69 | 1.65 | 1.65 |
| Red-legged seriema 5 | 6.75 | 6.7 | 6.64 | 1.3 | 1.28 | 1.33 | 1.67 | 1.72 | 1.65 |
| Mean ± standard deviation | 6.65 ± 0.29 | 6.82 ± 0.34 | 6.64 ± 0.37 | 1.26 ± 0.07 | 1.33 ± 0.29 | 1.32 ± 0.05 | 1.64 ± 0.05 | 1.66 ± 0.06 | 1.63 ± 0.02 |
| Black vulture 1 | 5.74 | 5.95 | 5.92 | 0.98 | 1.7 | 0.98 | 1.5 | 2 | 1.59 |
| Black vulture 2 | 5.89 | 5.96 | 5.85 | 0.98 | 1.5 | 0.99 | 1.52 | 2 | 1.47 |
| Black vulture 3 | 5.61 | 5.92 | 5.71 | 0.91 | 1 | 0.95 | 1.5 | 1.82 | 1.52 |
| Black vulture 4 | 5.52 | 5.98 | 5.93 | 0.94 | 1.2 | 0.99 | 1.53 | 1.7 | 1.48 |
| Black vulture 5 | 5.9 | 5.97 | 5.96 | 1.04 | 1.3 | 1.01 | 1.59 | 1.7 | 1.55 |
| Mean ± standard deviation | 5.73 ± 0.16 | 5.95 ± 0.02 | 5.87 ± 0.10 | 0.97 ± 0.04 | 1.34 ± 0.27 | 0.98 ± 0.02 | 1.53 ± 0.04 | 1.84 ± 0.15 | 1.52 ± 0.05 |
| Birds | Variables | p Values | Statistical Test |
|---|---|---|---|
| Parakeet | Length: 3D-CT × dental scanner; macroscopy × dental scanner | 0.0070 | Bonferroni |
| Parakeet | Height: 3D-CT × dental scanner; macroscopy × dental scanner | 0.0024 | Bonferroni |
| Black vulture | Length: 3D-CT × dental scanner | 0.0244 | Bonferroni |
| Black vulture | Height: 3D-CT × dental scanner; macroscopy × dental scanner; | 0.0066 | Bonferroni |
| Black vulture | Width: 3D-CT × dental scanner; macroscopy × dental scanner | 0.0012 | Bonferroni |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Camargo, G.C.; Rahal, S.C.; Abdala Junior, R.; da Silva, J.P.; da Silva, D.S.; Castiglioni, M.C.R.; Ichikawa, R.S.; Carvalho, B.C. Computed Tomography and a Dental Intraoral Scanner to Generate Three-Dimensional Models of the Beaks of Three Bird Species. Vet. Sci. 2025, 12, 331. https://doi.org/10.3390/vetsci12040331
de Camargo GC, Rahal SC, Abdala Junior R, da Silva JP, da Silva DS, Castiglioni MCR, Ichikawa RS, Carvalho BC. Computed Tomography and a Dental Intraoral Scanner to Generate Three-Dimensional Models of the Beaks of Three Bird Species. Veterinary Sciences. 2025; 12(4):331. https://doi.org/10.3390/vetsci12040331
Chicago/Turabian Stylede Camargo, Gabriel Corrêa, Sheila Canevese Rahal, Reinaldo Abdala Junior, Jeana Pereira da Silva, Daniel Simões da Silva, Maria Cristina Reis Castiglioni, Ricardo Shoiti Ichikawa, and Bruno Critelli Carvalho. 2025. "Computed Tomography and a Dental Intraoral Scanner to Generate Three-Dimensional Models of the Beaks of Three Bird Species" Veterinary Sciences 12, no. 4: 331. https://doi.org/10.3390/vetsci12040331
APA Stylede Camargo, G. C., Rahal, S. C., Abdala Junior, R., da Silva, J. P., da Silva, D. S., Castiglioni, M. C. R., Ichikawa, R. S., & Carvalho, B. C. (2025). Computed Tomography and a Dental Intraoral Scanner to Generate Three-Dimensional Models of the Beaks of Three Bird Species. Veterinary Sciences, 12(4), 331. https://doi.org/10.3390/vetsci12040331