Pregnancy-Associated Glycoproteins Identification in Skopelos Goat Milk by Means of Mass Spectrometry
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Overview
2.2. Sample Preparation
2.3. LC-MS/MS Analysis
2.4. Bioinformatic Analysis
3. Results
3.1. Pregnancy-Associated Glycoproteins in Caprine Milk on D20 and D45
3.2. Proteins Related to Early Pregnancy in Caprine Milk on D20 and/or D45
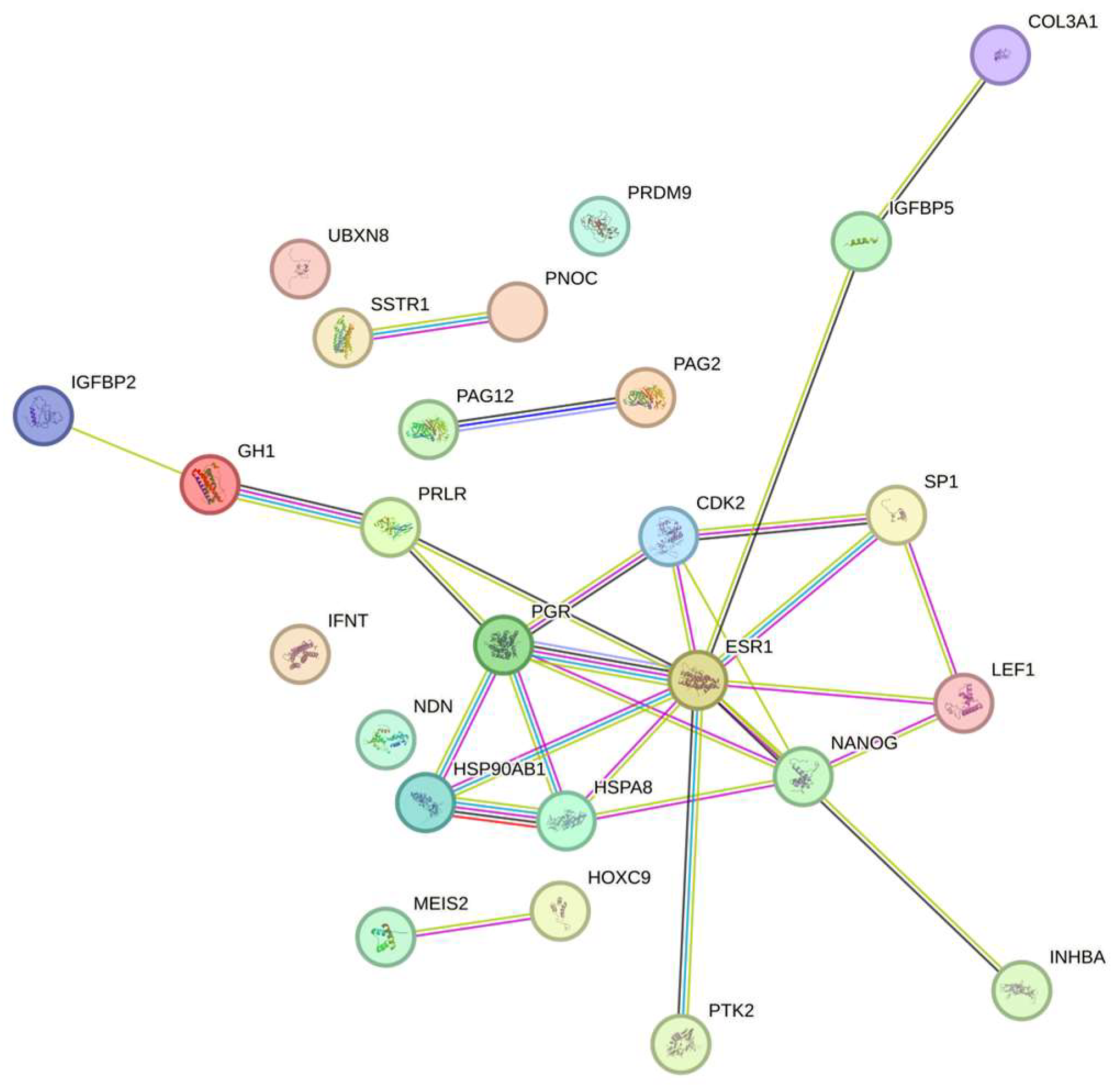
3.3. Bioinformatic Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jirillo, F.; Magrone, T. Anti-inflammatory and anti-allergic properties of donkey’s and goat’s milk. Endocr. Metab. Immune Disord. Drug Targets 2014, 14, 27–37. [Google Scholar] [CrossRef]
- Cunsolo, V.; Fasoli, E.; Saletti, R.; Muccilli, V.; Gallina, S.; Righetti, P.G.; Foti, S. Zeus, Aesculapius, Amalthea and the proteome of goat milk. J. Proteom. 2015, 128, 69–82. [Google Scholar] [CrossRef]
- Bu, H.F.; Zuo, X.L.; Wang, X.; Ensslin, M.A.; Koti, V.; Hsueh, W.; Raymond, A.S.; Shur, B.D.; Tan, X.D. Milk fat globule-EGF factor 8/lactadherin plays a crucial role in maintenance and repair of murine intestinal epithelium. J. Clin. Investig. 2007, 117, 3673–3683. [Google Scholar] [CrossRef]
- González, F.; Sulon, J.; Garbayo, J.M.; Batista, M.; Cabrera, F.; Calero, P.; Gracia, A.; Beckers, J.F. Early pregnancy diagnosis in goats by determination of pregnancy-associated glycoprotein concentrations in plasma samples. Theriogenology 1999, 52, 717–725. [Google Scholar] [CrossRef]
- Goel, A.K.; Agrawal, K.P. A review of pregnancy diagnosis techniques in sheep and goats. Small Rumin. Res. 1992, 9, 255–264. [Google Scholar] [CrossRef]
- Restall, B.J.; Milton, J.T.B.; Klong-yutti, P.; Kochapakdee, S. Pregnancy diagnosis in Thai native goats. Theriogenology 1990, 34, 313–317. [Google Scholar] [CrossRef]
- Szafranska, B.; Xie, S.; Green, J.; Roberts, R.M. Porcine pregnancy-associated glycoproteins: New members of the aspartic proteinase gene family expressed in trophectoderm. Biol. Reprod. 1995, 53, 21–28. [Google Scholar] [CrossRef]
- Xie, S.; Green, J.; Bixby, J.B.; Szafranska, B.; DeMartini, J.C.; Hecht, S.; Roberts, R.M. The diversity and evolutionary relationships of the pregnancy-associated glycoproteins, an aspartic proteinase subfamily consisting of many trophoblast-expressed genes. Proc. Natl. Acad. Sci. USA 1997, 94, 12809–12816. [Google Scholar] [CrossRef]
- Garbayo, J.M.; Green, J.A.; Manikkam, M.; Beckers, J.F.; Kiesling, D.O.; Ealy, A.D.; Roberts, R.M. Caprine pregnancy-associated glycoproteins (PAG): Their cloning, expression, and evolutionary relationship to other PAG. Mol. Reprod. Dev. 2000, 57, 311–322. [Google Scholar] [CrossRef]
- Green, J.A.; Xie, S.; Quan, X.; Bao, B.; Gan, X.; Mathialagan, N.; Beckers, J.F.; Roberts, R.M. Pregnancy-associated bovine and ovine glycoproteins exhibit spatially and temporally distinct expression patterns during pregnancy. Biol. Reprod. 2000, 62, 1624–1631. [Google Scholar] [CrossRef]
- Majewska, M.; Panasiewicz, G.; Majewski, M.; Szafranska, B. Localization of chorionic pregnancy-associated glycoprotein family in the pig. Reprod. Biol. 2006, 6, 205–230. [Google Scholar]
- Wathes, D.C.; Wooding, F.B. An electron microscopic study of implantation in the cow. Am. J. Anat. 1980, 159, 285–306. [Google Scholar] [CrossRef]
- Wooding, F.B. Frequency and localization of binucleate cells in the placentomes of ruminants. Placenta 1983, 4, 527–539. [Google Scholar] [PubMed]
- Wooding, F.B. Role of binucleate cells in fetomaternal cell fusion at implantation in the sheep. Am. J. Anat. 1984, 170, 233–250. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.S.; Gogolin-Ewens, K.; White, T.R.; Brandon, M.R. Studies on the distribution of binucleate cells in the placenta of the sheep with a monoclonal antibody SBU-3. J. Anat. 1985, 140 Pt 4, 565–576. [Google Scholar] [PubMed]
- Wooding, F.B.; Flint, A.P.; Heap, R.B.; Morgan, G.; Buttle, H.L.; Young, I.R. Control of binucleate cell migration in the placenta of sheep and goats. J. Reprod. Fertil. 1986, 76, 499–512. [Google Scholar] [CrossRef]
- Duello, T.M.; Byatt, J.C.; Bremel, R.D. Immunohistochemical localization of placental lactogen in binucleate cells of bovine placentomes. Endocrinology 1986, 119, 1351–1355. [Google Scholar] [CrossRef]
- Faria, T.N.; Deb, S.; Kwok, S.C.; Talamantes, F.; Soares, M.J. Ontogeny of placental lactogen-I and placental lactogen-II expression in the developing rat placenta. Dev. Biol. 1990, 141, 279–291. [Google Scholar] [CrossRef]
- Xie, S.C.; Low, B.G.; Nagel, R.J.; Kramer, K.K.; Anthony, R.V.; Zoli, A.P.; Beckers, J.F.; Roberts, R.M. Identification of the major pregnancy-specific antigens of cattle and sheep as inactive members of the aspartic proteinase family. Proc. Natl. Acad. Sci. USA 1991, 88, 10247–10251. [Google Scholar] [CrossRef]
- Patel, O.V.; Yamada, O.; Kizaki, K.; Todoroki, J.; Takahashi, T.; Imai, K.; Schuler, L.A.; Hashizume, K. Temporospatial expression of placental lactogen and prolactin-related protein-1 genes in the bovine placenta and uterus during pregnancy. Mol. Reprod. Dev. 2004, 69, 146–152. [Google Scholar] [CrossRef]
- Sasser, R.G.; Ruder, C.A.; Ivani, K.A.; Butler, J.E.; Hamilton, W.C. Detection of pregnancy by radioimmunoassay of a novel pregnancy-specific protein in serum of cows and a profile of serum concentrations during gestation. Biol. Reprod. 1986, 35, 936–942. [Google Scholar] [CrossRef]
- Zoli, A.P.; Guilbault, L.A.; Delahaut, P.; Ortiz, W.B.; Beckers, J.F. Radioimmunoassay of a bovine pregnancy-associated glycoprotein in serum: Its application for pregnancy diagnosis. Biol. Reprod. 1992, 46, 83–92. [Google Scholar] [CrossRef]
- Green, J.A.; Parks, T.E.; Avalle, M.P.; Telugu, B.P.; McLain, A.L.; Peterson, A.J.; McMillan, W.; Mathialagan, N.; Hook, R.R.; Xie, S.; et al. The establishment of an ELISA for the detection of pregnancy-associated glycoproteins (PAGs) in the serum of pregnant cows and heifers. Theriogenology 2005, 63, 1481–1503. [Google Scholar] [CrossRef]
- Wallace, R.M.; Pohler, K.G.; Smith, M.F.; Green, J.A. Placental PAGs: Gene origins, expression patterns, and use as markers of pregnancy. Reproduction 2015, 149, R115–R126. [Google Scholar] [CrossRef]
- Green, J.A.; Xie, S.; Michael Roberts, R. Pepsin-related molecules secreted by trophoblast. Rev. Reprod. 1998, 3, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Wooding, F.B.P.; Roberts, R.M.; Green, J.A. Light and electron microscope immunocytochemical studies of the distribution of pregnancy associated glycoproteins (PAGs) throughout pregnancy in the cow: Possible functional implications. Placenta 2005, 26, 807–827. [Google Scholar] [CrossRef]
- Ott, T.L. Immunological detection of pregnancy: Evidence for systemic immune modulation during early pregnancy in ruminants. Theriogenology 2020, 150, 498–503. [Google Scholar] [CrossRef]
- Gonzalez, F.; Sulon, J.; Calero, P.; Batista, M.; Gracia, A.; Beckers, J.F. Pregnancy-associated glycoproteins (PAG) detection in milk samples for pregnancy diagnosis in dairy goats. Theriogenology 2001, 56, 671–676. [Google Scholar] [CrossRef]
- Bouroutzika, E.; Kouretas, D.; Papadopoulos, S.; Veskoukis, A.S.; Theodosiadou, E.; Makri, S.; Paliouras, C.; Michailidis, M.L.; Caroprese, M.; Valasi, I. Effects of Melatonin Administration to Pregnant Ewes under Heat-Stress Conditions, in Redox Status and Reproductive Outcome. Antioxidants 2020, 9, 266. [Google Scholar] [CrossRef] [PubMed]
- Anagnostopoulos, A.K.; Katsafadou, A.I.; Pierros, V.; Kontopodis, E.; Fthenakis, G.C.; Arsenos, G.; Karkabounas, S.C.; Tzora, A.; Skoufos, I.; Tsangaris, G.T. Milk of Greek sheep and goat breeds; characterization by means of proteomics. J. Proteom. 2016, 147, 76–84. [Google Scholar] [CrossRef]
- Sapan, C.V.; Lundblad, R.L.; Price, N.C. Colorimetric protein assay techniques. Biotechnol. Appl. Biochem. 1999, 29, 99–108. [Google Scholar] [CrossRef]
- Velentzas, A.D.; Anagnostopoulos, A.K.; Velentzas, P.D.; Mpakou, V.E.; Sagioglou, N.E.; Tsioka, M.M.; Katarachia, S.; Manta, A.K.; Konstantakou, E.G.; Papassideri, I.S.; et al. Global Proteomic Profiling of Drosophila Ovary: A High-resolution, Unbiased, Accurate and Multifaceted Analysis. Cancer Genom. Proteom. 2015, 12, 369–384. [Google Scholar]
- Buckrell, B.C. Applications of ultrasonography in reproduction in sheep and goats. Theriogenology 1988, 29, 71–84. [Google Scholar] [CrossRef]
- Haibel, G.K. Use of ultrasonography in reproductive management of sheep and goat herds. Vet. Clin. N. Am. Food Anim. Pract. 1990, 6, 597–613. [Google Scholar] [CrossRef]
- Gonzalez-Bulnes, A.; Pallares, P.; Vazquez, M. Ultrasonographic Imaging in Small Ruminant Reproduction. Reprod. Domest. Anim. 2010, 45, 9–20. [Google Scholar] [CrossRef]
- Cruz, C.; Margatho, G.; Simões, M.; Simões, J. The Utilization of a Pregnancy-Associated Glycoprotein Profile and PAG/P4 Ratio Biomarker for the Diagnosis of Pseudopregnancy in Dairy Goats. Vet. Sci. 2024, 11, 574. [Google Scholar]
- Ranilla, M.J.; Sulon, J.; Carro, M.D.; Mantecon, A.R.; Beckers, J.F. Plasmatic profiles of pregnancy-associated glycoprotein and progesterone levels during gestation in Churra and Merino sheep. Theriogenology 1994, 42, 537–545. [Google Scholar] [CrossRef]
- Wallace, J.M.; Aitken, R.P.; Cheyne, M.A.; Humblot, P. Pregnancy-specific protein B and progesterone concentrations in relation to nutritional regimen, placental mass and pregnancy outcome in growing adolescent ewes carrying singleton fetuses. J. Reprod. Fertil. 1997, 109, 53–58. [Google Scholar] [CrossRef][Green Version]
- Karen, A.; Beckers, J.F.; Sulon, J.; de Sousa, N.M.; Szabados, K.; Reczigel, J.; Szenci, O. Early pregnancy diagnosis in sheep by progesterone and pregnancy-associated glycoprotein tests. Theriogenology 2003, 59, 1941–1948. [Google Scholar] [CrossRef]
- Garbayo, J.M.; Serrano, B.; Lopez-Gatius, F. Identification of novel pregnancy-associated glycoproteins (PAG) expressed by the peri-implantation conceptus of domestic ruminants. Anim. Reprod. Sci. 2008, 103, 120–134. [Google Scholar] [CrossRef]
- Spencer, T.E.; Bazer, F.W. Ovine interferon tau suppresses transcription of the estrogen receptor and oxytocin receptor genes in the ovine endometrium. Endocrinology 1996, 137, 1144–1147. [Google Scholar] [CrossRef]
- Martal, J.L.; Chene, N.M.; Huynh, L.P.; L’Haridon, R.M.; Reinaud, P.B.; Guillomot, M.W.; Charlier, M.A.; Charpigny, S.Y. IFN-tau: A novel subtype I IFN1. Structural characteristics, non-ubiquitous expression, structure-function relationships, a pregnancy hormonal embryonic signal and cross-species therapeutic potentialities. Biochimie 1998, 80, 755–777. [Google Scholar] [CrossRef]
- Oosterwegel, M.; van de Wetering, M.; Timmerman, J.; Kruisbeek, A.; Destree, O.; Meijlink, F.; Clevers, H. Differential expression of the HMG box factors TCF-1 and LEF-1 during murine embryogenesis. Development 1993, 118, 439–448. [Google Scholar] [CrossRef]
- Shelton, D.N.; Fornalik, H.; Neff, T.; Park, S.Y.; Bender, D.; DeGeest, K.; Liu, X.; Xie, W.; Meyerholz, D.K.; Engelhardt, J.F.; et al. The role of LEF1 in endometrial gland formation and carcinogenesis. PLoS ONE 2012, 7, e40312. [Google Scholar] [CrossRef]
- Nakaya, Y.; Miyazawa, T. The Roles of Syncytin-Like Proteins in Ruminant Placentation. Viruses 2015, 7, 2928–2942. [Google Scholar] [CrossRef]
- Pannetier, M.; Renault, L.; Jolivet, G.; Cotinot, C.; Pailhoux, E. Ovarian-specific expression of a new gene regulated by the goat PIS region and transcribed by a FOXL2 bidirectional promoter. Genomics 2005, 85, 715–726. [Google Scholar] [CrossRef]
- Shah, M.; Stanek, J.; Handwerger, S. Differential localization of heat shock proteins 90, 70, 60 and 27 in human decidua and placenta during pregnancy. Histochem. J. 1998, 30, 509–518. [Google Scholar] [CrossRef]
- Siu, M.K.; Wong, E.S.; Chan, H.Y.; Ngan, H.Y.; Chan, K.Y.; Cheung, A.N. Overexpression of NANOG in gestational trophoblastic diseases: Effect on apoptosis, cell invasion, and clinical outcome. Am. J. Pathol. 2008, 173, 1165–1172. [Google Scholar] [CrossRef]
- Broxmeyer, H.E.; Lu, L.; Cooper, S.; Schwall, R.H.; Mason, A.J.; Nikolics, K. Selective and indirect modulation of human multipotential and erythroid hematopoietic progenitor cell proliferation by recombinant human activin and inhibin. Proc. Natl. Acad. Sci. USA 1988, 85, 9052–9056. [Google Scholar] [CrossRef]
- Huang, H.M.; Chiou, H.Y.; Chang, J.L. Activin A induces erythroid gene expressions and inhibits mitogenic cytokine-mediated K562 colony formation by activating p38 MAPK. J. Cell. Biochem. 2006, 98, 789–797. [Google Scholar] [CrossRef]
- Zhang, Y.; Fonslow, B.R.; Shan, B.; Baek, M.-C.; Yates, J.R. Protein Analysis by Shotgun/Bottom-up Proteomics. Chem. Rev. 2013, 113, 2343–2394. [Google Scholar] [CrossRef]
- Franzosa, E.A.; Hsu, T.; Sirota-Madi, A.; Shafquat, A.; Abu-Ali, G.; Morgan, X.C.; Huttenhower, C. Sequencing and beyond: Integrating molecular ‘omics’ for microbial community profiling. Nat. Rev. Microbiol. 2015, 13, 360–372. [Google Scholar] [CrossRef]
- Bouroutzika, E.; Proikakis, S.; Anagnostopoulos, A.K.; Katsafadou, A.I.; Fthenakis, G.C.; Tsangaris, G.T. Proteomics Analysis in Dairy Products: Cheese, a Review. Appl. Sci. 2021, 11, 7622. [Google Scholar] [CrossRef]
- Borba, V.V.; Zandman-Goddard, G.; Shoenfeld, Y. Prolactin and autoimmunity: The hormone as an inflammatory cytokine. Best Pract. Res. Clin. Endocrinol. Metab. 2019, 33, 101324. [Google Scholar] [CrossRef]
- Skokowa, J.; Klimiankou, M.; Klimenkova, O.; Lan, D.; Gupta, K.; Hussein, K.; Carrizosa, E.; Kusnetsova, I.; Li, Z.; Sustmann, C.; et al. Interactions among HCLS1, HAX1 and LEF-1 proteins are essential for G-CSF-triggered granulopoiesis. Nat. Med. 2012, 18, 1550–1559. [Google Scholar] [CrossRef]
- Hebenstreit, D.; Giaisi, M.; Treiber, M.K.; Zhang, X.B.; Mi, H.F.; Horejs-Hoeck, J.; Andersen, K.G.; Krammer, P.H.; Duschl, A.; Li-Weber, M. LEF-1 negatively controls interleukin-4 expression through a proximal promoter regulatory element. J. Biol. Chem. 2008, 283, 22490–22497. [Google Scholar] [CrossRef]
- Nguyen, D.X.; Chiang, A.C.; Zhang, X.H.; Kim, J.Y.; Kris, M.G.; Ladanyi, M.; Gerald, W.L.; Massague, J. WNT/TCF signaling through LEF1 and HOXB9 mediates lung adenocarcinoma metastasis. Cell 2009, 138, 51–62. [Google Scholar] [CrossRef][Green Version]
- Bosch, P.J.; Fuller, L.C.; Sleeth, C.M.; Weiner, J.A. Akirin2 is essential for the formation of the cerebral cortex. Neural Dev. 2016, 11, 21. [Google Scholar] [CrossRef]
- Tartey, S.; Matsushita, K.; Imamura, T.; Wakabayashi, A.; Ori, D.; Mino, T.; Takeuchi, O. Essential Function for the Nuclear Protein Akirin2 in B Cell Activation and Humoral Immune Responses. J. Immunol. 2015, 195, 519–527. [Google Scholar] [CrossRef]
- Moutoussamy, S.; Kelly, P.A.; Finidori, J. Growth-hormone-receptor and cytokine-receptor-family signaling. Eur. J. Biochem. 1998, 255, 1–11. [Google Scholar] [CrossRef]
| No. | PAGs | Peptides | Days |
|---|---|---|---|
| 1 | caPAG2 | LNWIPVSQTKSWLITVDR | D20 |
| 2 | caPAG3 | AGDWSVR | D45 |
| DSNVTIVPLRNMR | |||
| 3 | caPAG5 | DKQEGSVVmFGGVDHR | |
| 4 | caPAG6 | IKGKVVHDTVR | |
| 5 | caPAG12 | KTLSGKHMLNNFLK |
| D20 | |
|---|---|
| Protein Name | Peptides |
| Envelope protein syncytin-rum1 | KAVLQNRMALDILTAAQGGTcAIIK |
| Truncated estrogen receptor alpha | LASTSDKGSMAMESAK |
| Progesterone receptor | GEAAEGAAVRPPEK |
| Cyclin-dependent kinase 2 | TLGTPDEVVWPGVTSmPDYKPSFPK |
| Interferon-alpha | AEVmRAFSSSTNLQERFR |
| Collagen type III alpha 1 | DGTSGHPGPIGPPGPR |
| Prepronociceptin | VmARGSWQLSPADPDHVAAAPDQAR |
| Somatostatin receptor 1 | TAANSDGTVAcNMLMPEPAQR |
| Interferon tau BB12 | TEPGLEEVGDMEQK |
| DFAFPQEMVEGGQLQEAQAISVLHEmLQQSFNVFHPER | |
| SP1 transcription factor | IEKGVGGNNGGNGNGSGAFSQAR |
| Lymphocyte enhancer-binding factor-1 isoform 2 | EKLQESASGTGPR |
| Heat shock cognate 71 kDa protein | SINPDEAVAYGAAVQAAILSGDK |
| SQIHDIVLVGGSTR | |
| TTPSYVAFTDTER | |
| NQVAMNPTNTVFDAK | |
| QTQTFTTYSDNQPGVLIQVYEGER | |
| MVNHFIAEFK | |
| IINEPTAAAIAYGLDK | |
| FEELNADLFR | |
| D20 and D45 | |
| Prolactin receptor | VTDSNILVLIPDPQAQKKK |
| NANOG (homeobox protein) | mSATGPISNYYVDSLISHDNEDLLASR |
| Growth hormone receptor | MLILPPVPVPK |
| Heat shock protein HSP 90-beta | YHTSQSGDEMTSLSEYVSR |
| YHTSQSGDEmTSLSEYVSR | |
| NPDDITQEEYGEFYK | |
| HFSVEGQLEFR | |
| GVVDSEDLPLNISR | |
| SLTNDWEDHLAVK | |
| IDIIPNPQER | |
| ELISNASDALDK | |
| D45 | |
| PFOXic protein | QGSPKLTSLNTILHPPLK |
| Insulin-like growth factor binding protein 5 | IAERDSREHEEPTTSEMAEETYSPK |
| Meis homeobox 2 | QRVQNVHGINGSSISSAESR |
| Necdin | EITKmQIMEFLARVFK |
| PRDM9 | THTGEKPYVcREcGRcFSDK |
| INHB (Inhibin beta A chain) | RAEmNELmEQTSEIITFAESGTAR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouroutzika, E.; Theodosiadou, E.K.; Proikakis, S.; Valasi, I.; Tsangaris, G.T. Pregnancy-Associated Glycoproteins Identification in Skopelos Goat Milk by Means of Mass Spectrometry. Vet. Sci. 2025, 12, 1092. https://doi.org/10.3390/vetsci12111092
Bouroutzika E, Theodosiadou EK, Proikakis S, Valasi I, Tsangaris GT. Pregnancy-Associated Glycoproteins Identification in Skopelos Goat Milk by Means of Mass Spectrometry. Veterinary Sciences. 2025; 12(11):1092. https://doi.org/10.3390/vetsci12111092
Chicago/Turabian StyleBouroutzika, Efterpi, Ekaterini K. Theodosiadou, Stavros Proikakis, Irene Valasi, and George Th. Tsangaris. 2025. "Pregnancy-Associated Glycoproteins Identification in Skopelos Goat Milk by Means of Mass Spectrometry" Veterinary Sciences 12, no. 11: 1092. https://doi.org/10.3390/vetsci12111092
APA StyleBouroutzika, E., Theodosiadou, E. K., Proikakis, S., Valasi, I., & Tsangaris, G. T. (2025). Pregnancy-Associated Glycoproteins Identification in Skopelos Goat Milk by Means of Mass Spectrometry. Veterinary Sciences, 12(11), 1092. https://doi.org/10.3390/vetsci12111092