Simple Summary
The emergence of resistance to β-lactam antibiotics and the characteristics of extended-spectrum β-lactamase (ESBL)-producing Escherichia coli (ESBL-E. coli), represent a significant concern in both human and veterinary medicine. Wildlife is recognized as a reservoir of ESBL-E. coli, but the role that wildlife plays in the dissemination of antimicrobial resistance genes (ARGs) is still not fully understood. In the present study, we report the first identification of ESBL-E. coli in captive black bears and reveal a high prevalence of β-lactam resistance. Conjugative transfer assays have demonstrated that these bacteria exhibit high transfer efficiency, and have further demonstrated that multiple ARGs, mobile genetic elements (MGEs), and plasmids were capable of horizontal transmission. These findings indicate that wildlife serves as a reservoir for antibiotic-resistant bacteria with dissemination potential, underscoring the critical importance of antimicrobial resistance (AMR) monitoring and the implementation of a One Health approach.
Abstract
The emergence and global dissemination of extended-spectrum β-lactamase (ESBL)-producing Escherichia coli (ESBL-E. coli) represent a major public health concern. However, the characterization and capacity for horizontal gene transfer (HGT) of ESBL-E. coli in captive black bears remain substantially understudied. In the present study, 19 ESBL-E. coli strains were successfully identified (13.38%, 19/142). A total of 11 sequence types (STs) were identified from 19 ESBL-E. coli strains using MLST. This included eight known types (ST10, ST2690, ST208, ST695, ST4160, ST540, ST3865 and ST2792) and three new STs. Antimicrobial susceptibility testing demonstrated that all 19 ESBL-E. coli exhibited high resistance to KZ (100.00%), CRO (78.95%), and CTX (73.68%). Polymerase chain reaction (PCR) screening for 14 β-lactam antibiotic resistance genes (ARGs) and their variants revealed that blaCTX-M was the most prevalent, followed by blaSHV, blaTEM, and blaDHA. Furthermore, eight β-lactamase variants were detected, including five blaCTX-M variants (blaCTX-M-15, blaCTX-M-3, blaCTX-M-14, blaCTX-M-55, and blaCTX-M-27) and one variant each of blaSHV-1, blaTEM-1, and blaDHA-14. Conjugation assays revealed that eight ESBL-E. coli strains were capable of conjugative transfer. Five plasmid types (IncFII, IncW, IncFrepB, IncY, and IncHI1) and three mobile genetic elements (MGEs) (IS26, ISEcp1, and trbC) were identified as co-transferred with blaCTX-M. ESBL-E. coli poses a potential threat to captive black bears and may lead to further transmission. Consequently, the implementation of continuous surveillance and targeted interventions is imperative to prevent the transmission of ESBL-E. coli.
1. Introduction
Escherichia coli (E. coli) is a Gram-negative bacterium that belongs to the Enterobacteriaceae family. E. coli is a commensal bacterium that is commonly found in the intestines of humans and animals [1]. Among the antibiotics available on the market, β-lactam antibiotics are the most dominant antimicrobial agents globally, accounting for approximately 50% of all prescribed antimicrobial agents [2]. The misuse of clinical antimicrobials exerts selective pressure on bacteria, causing them to evolve or acquire antimicrobial resistance genes (ARGs), thereby developing antimicrobial resistance (AMR). AMR poses a significant challenge in the treatment of bacterial infections in both humans and animals [3]. Furthermore, the presence of extended-spectrum β-lactamase (ESBL)-producing E. coli (ESBL-E. coli) represents a significant global public health threat [4]. ESBLs represent a primary cause of the failure of β-lactam antibiotic treatment [5]. Consequently, the study of resistance to β-lactam antibiotics and the characteristics of ESBL-E. coli has attracted scholarly interest, particularly in the context of veterinary clinical practice [6,7].
Over the past decade, ESBLs, AmpC β-lactamases and carbapenemases have been documented in Enterobacteriaceae originating from both humans and animals [8]. These enzymes have been demonstrated to confer bacterial AMR to a wide range of β-lactam antibiotics, including penicillins, cephalosporins and carbapenems [9]. In addition, among these enzymes, ESBL-E. coli is the most frequently isolated from wild animals and can disseminate rapidly via horizontal gene transfer (HGT) [10,11]. The horizontal transfer of ESBL-resistant genes in ESBL-E. coli is mainly driven by mobile genetic elements (MGEs), including plasmids, transposons, and insertion sequences (ISs) [12,13]. Animals are recognized as potential reservoirs for transmitting AMR bacteria to humans; consequently, bacteria harboring β-lactam AMR pose a serious public health threat [8].
The Asian black bear (Ursus thibetanus) serves as a keystone component of ecosystems, playing critical roles in maintaining ecological balance and stability [14]. In China, there are five subspecies of the Asiatic black bear (U. t. thibetanus, U. t. laniger, U. t. mupinensis, U. t. formosanus, and U. t. ussuricus), and the Sichuan subspecies (U. t. mupinensis) is the most widely distributed black bear in China [15]. In the field of wildlife, the first documented cases of ESBL-E. coli were observed in deer, owls, bird of prey and foxes in Portugal in 2006 [16]. Subsequent to the initial detection, the presence of ESBL-E. coli has been identified in a variety of wildlife species, including cave bats, feral swine, Canis latrans, Orangutan, and giant pandas [17,18,19,20]. Notably, the ESBL-E. coli is one of the most important pathogens at the One Health interface [21]. To date, only two studies have analyzed ESBL-E. coli in bears. A study successfully isolated 17 ESBL-E. coli from Indian sloth bears [22]. Another study isolated only one ESBL-E. coli from Eurasian brown bears in Spain [23]. ESBL-E. coli has not been identified from captive black bears in China, and its HGT potential remains uncharacterized, which underscores the necessity of this investigation.
Our previous study demonstrated a high prevalence of AMR among 142 E. coli isolates from captive black bears (U. t. mupinensis), 65.49% (93/142) of isolates exhibiting resistance to β-lactam antibiotics [24]. In the present study, we performed β-lactam resistance phenotyping, ARG profiling, and an assessment of the HGT potential on ESBL-E. coli isolates from 142 E. coli isolates to better understand the characterization of ESBL-E. coli from captive black bears.
2. Materials and Methods
2.1. Bacterial Strains
The present study utilized 142 E. coli strains isolated from captive black bear fecal samples, with all strains preserved in the laboratory prior to analysis [24]. The 142 fecal samples were collected from a black bear breeding farm in Dujiangyan City, China (103.59° E, 31.02° N), with one sample obtained per individual.
2.2. Screening of ESBL-E. coli Isolates
ESBL-E. coli isolates from captive black bears were screened using the double-disk synergy test, following the CLSI 2023 guidelines. The antimicrobial susceptibility disks employed were ceftazidime (CAZ), ceftazidime-clavulanate (CAL), cefotaxime (CTX), and cefotaxime-clavulanate (CTL). The isolates were cultured on Mueller-Hinton agar plates and incubated at 37 °C for 16–18 h. After incubation, the zones of inhibition were measured. An isolate was confirmed as ESBL-producing if the zone diameter for either combination disk (CAL or CTL) showed a ≥5 mm increase compared to its corresponding β-lactam alone disk (CAZ or CTX, respectively).
2.3. MLST Typing of ESBL-E. coli Isolates
MLST was performed by amplifying of seven housekeeping genes (adk; fumC; gyrB; icd; mdh; purA and recA) according to the standard protocol on PubMLST (https://pubmlst.org/organisms/escherichia-spp (accessed on 10 October 2025)) [25]. The Primer sequences and amplification parameters are detailed in Supplementary Table S1. The positive PCR products were submitted for bidirectional sequencing at Sangon Biotech (China). The bidirectional sequencing reads for each ESBL-E. coli isolate were assembled and aligned against the E. coli MLST database (https://pubmlst.org/ (accessed on 4 November 2025)) to determine their sequence types (STs). Using the goeBURST algorithm in Phyloviz 2.0, clonal clustering analysis assigned STs of successfully typed ESBL-E. coli isolates to distinct clonal complexes (CCs), where each CC comprises closely related STs sharing ≤2 allelic differences.
2.4. Antimicrobial Susceptibility Testing for ESBL-E. coli Isolates
All ESBL-E. coli isolates underwent standardized disk diffusion susceptibility testing against 15 β-lactam antibiotics representing five classes. A total of seven antibacterial agents were tested in our previous study, including ampicillin (AMP), cephalosporins (KZ, CXM, CTX, FEP), monobactams (ATM) and β-lactamase inhibitor combinations (SAM) [24]. The remaining eight antibacterial agents were tested in this study: cephalosporins (cefoxitin, FOX, 30 μg; ceftriaxone, CRO, 30 μg; ceftazidime, CAZ, 30 μg), carbapenems (imipenem, IMP, 10 μg; meropenem, MEM, 10 μg; Ertapenem, ETP, 10 μg), β-lactamase inhibitor combinations (piperacillin/tazobactam, TZP, 100/10 μg; amoxicillin/clavulanic acid, AMC, 20/10 μg). Susceptibility results were interpreted using CLSI 2023 breakpoint criteria. E. coli ATCC25922 was used as the quality control strain. The antibiotics used in this study were based on the information from local farm veterinarians on which antibiotics were used for disease control (CTX, CXM and SAM); AMP, KZ, FEP, FOX, CRO, CAZ, ATM, IMP, MEM, ETP, TZP and AMC have been reported to be found antibiotic resistant in wildlife [26,27,28].
2.5. Screening of β-Lactam ARGs and MGEs from ESBL-E. coli Isolates and Outer Membrane Proteins from ESBLs16
Genomic DNA from ESBL-E. coli isolates was extracted using a Tiangen Biotech kit (Beijing, China) according to the manufacturer’s protocol. The purity and concentration of the DNA were verified by measuring the A260/A280 absorption ratio. Extracted DNA was stored at −20 °C for subsequent PCR amplification. Based on relevant studies, 14 β-lactam ARGs were selected for detection: the ESBL genes blaTEM, blaSHV and blaCTX-M; the AmpC genes blaMOX, blaCIT, blaDHA, blaACC, blaEBC and blaFOX; and the carbapenemase genes blaKPC, blaNDM-1, blaIMP, blaVIM and blaSEM [29,30,31,32]. As blaCTX-M had been characterized in prior experiments [24], blaCTX-M-positive isolates were selected for molecular subtyping to identify genetic subgroups (groups 1, 2, 8, 9, and 25) [29]. We performed PCR amplification of the OmpC and OmpF genes in isolate ESBLs16 to assess potential deficiencies in them [33]. Full primer sequences and amplification parameters are detailed in Supplementary Table S1. The positive PCR products were submitted for unidirectional Sanger sequencing at Sangon Biotech (China). The obtained sequences were aligned against ARG sequences in the GenBank database using the BLASTn algorithm on the NCBI website to assess sequence similarity and identify target fragments (https://blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 4 November 2025)). The data concerning MGEs for the 19 ESBL-E. coli were obtained from a previous study [24].
2.6. Data of AMR, ARGs, and MGEs Analyzed and Association Analysis Between AMR and ARGs or MGEs
The data on AMR, ARGs, and MGEs were analyzed by using SPSS software (version 26.0). Associations among AMR phenotypes, ARGs, and MGEs were analyzed using Spearman’s correlation test, with a p-value < 0.05 considered statistically significant. The results were visualized using the ggplot2 package in RStudio (version 4.2.2) [34].
2.7. Conjugation Assays and PCR-Based Replicon Typing (PBRT)
To investigate the conjugative transfer of β-lactam ARGs and associated MGEs, 19 ESBL-E. coli strains were used as donors, with sodium azide-resistant E. coli J53 as the recipient. The donor and recipient strains were separately cultured in 4.0 mL of Luria–Bertani (LB) broth at 37 °C for 16 h. Then, 0.2 mL of the donor culture was mixed with 0.8 mL of the recipient culture in 4.0 mL of fresh LB broth. A control contained 0.8 mL recipient culture in 4.2 mL LB broth. Both mixtures were incubated at 37 °C for 16 h. Transconjugants were selected and quantified on LB agar plates supplemented with sodium azide (100 μg/mL) and CTX (4 μg/mL), while recipient cell counts were determined on azide-containing plates (100 μg/mL) without antibiotic supplementation. The conjugation frequency was calculated as the number of transconjugants divided by the number of recipient cells, thus representing the HGT frequency. The ESBL phenotype of transconjugants was confirmed by the double-disk synergy testing, and transferred ARGs and MGEs were detected by PCR [35]. Plasmid replicon types were determined by PCR using 18 primer pairs specific for replicon typing (IncHI1, IncHI2, IncI1, IncX, IncL/M, IncN, IncFIA, IncFIB, IncW, IncY, IncP, IncFIC, IncA/C, IncT, IncFII, IncFrepB, IncK/B, IncK and IncB/O) [36]. The subsequent analysis of the PCR products was conducted in accordance with the methodology delineated in Section 2.5. The primer sequences and corresponding amplification conditions are provided in Supplementary Table S1.
3. Results
3.1. ESBL Strains Identified and MLST Analysis
Of the 142 E.coli isolates from captive black bears, 19 (13.38%, 19/142) were successfully confirmed as ESBL-E. coli. Among the 19 ESBL-E. coli strains examined, 18 strains (with the exception of ESBLs15) successfully amplified all seven housekeeping genes (Table 1). MLST analysis revealed that 18 isolates exhibited eight distinct STs (Figure 1). ST10 (38.89%, 7/18) was the most prevalent ST, followed by ST2690 (11.11%, 2/18). The remaining six STs contained only a single strain each. Furthermore, three new STs (designated nST1 to nST3) were identified. Using the goeBURST algorithm, only one clonal complex (CC10) was identified among the 15 ESBL-E. coli isolates that were successfully assigned an ST from the database. This complex comprised eight isolates (53.33%), while the remaining seven isolates were not assigned to any clonal complex.

Table 1.
MLST typing results of 18 ESBL-E. coli.
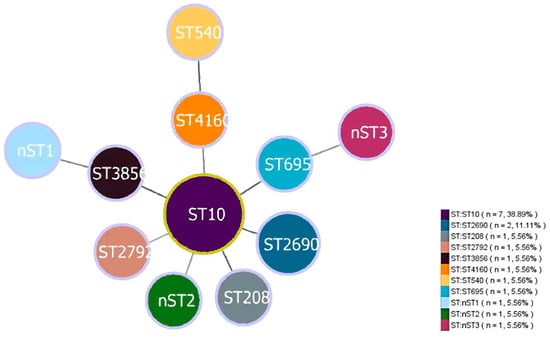
Figure 1.
Minimum spanning tree of MLST types in 18 ESBL-E. coli strains. The size of circle indicates the proportion of isolates belonging to the ST. The yellow outline of the circle represents the adjacent STs belonging to the same clonal complex.
3.2. The Phenotype of Resistance to β-Lactam Antibiotics
As shown in Figure 2, the highest resistance rate was observed for KZ (100%, 19/19), followed by CRO (78.95%, 15/19) and CTX (73.68%, 14/19). The resistance rates for the remaining β-lactam antibiotics ranged from 68.42% (AMP, CXM) to 21.05% (FEP). All 19 ESBL-E. coli isolates were susceptible to CAZ, MEM, ETP, AMC, and SAM. The susceptibility rates for FOX and IMP were all 94.74% (18/19).
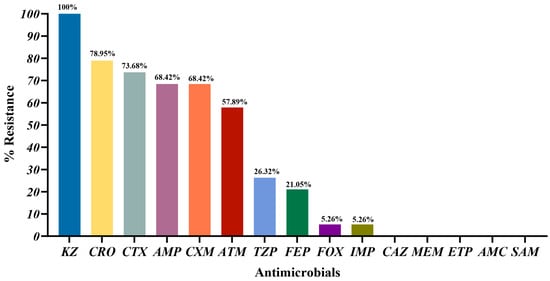
Figure 2.
Percentage of antibiotic resistance in 19 ESBL-E. coli. KZ-cefazolin; CRO-ceftriaxone; CTX-cefotaxime; AMP-Ampicillin; CXM-cefuroxime; ATM-aztreonam; TZP-piperacillin/tazobactam; FEP-cefepime; FOX-cefoxitin; IMP-imipenem; CAZ-ceftazidime; MEM-meropenem; ETP-ertapenem; AMC-amoxicillin/clavulanic acid; SAM-ampicillin/sulbactam.
As illustrated in Figure 3a, the nine resistance patterns are distributed across five classes of β-lactam antibiotics. All 19 ESBL-E. coli isolates exhibited resistance to cephalosporins. It is noteworthy that strains ESBLs03 and ESBLs17 were resistant to all tested β-lactam classes with the exception of carbapenems. Isolate ESBL16 was the only strain exhibiting resistance to carbapenems. As illustrated in Figure 3b, the 14 resistance patterns to 15 β-lactam antibiotics are demonstrated. All 19 ESBL-E. coli isolates exhibited resistance to at least one β-lactam antibiotic. In particular, isolate ESBLs17 exhibited resistance to nine distinct β-lactam antibiotics (AMP/KZ/CXM/FOX/CRO/CTX/FEP/ATM/TZP). The most prevalent pattern (31.58%, 6/19) was resistance to five antibiotics (AMP/KZ/CXM/CRO/CTX, KZ/CXM/CRO/CTX/ATM, AMP/KZ/CRO/CTX/ATM, and AMP/KZ/CRO/CTX/TZP).
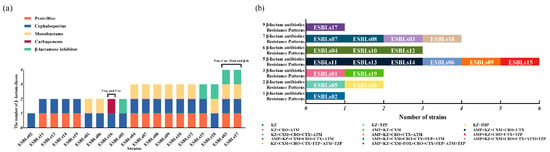
Figure 3.
The resistance patterns of ESBL-E. coli. (a) Color bar chart represents the resistance patterns of ESBL-E. coli to five classes of β-lactam antibiotics. Each color represents a distinct class of β-lactam antibiotics. A total of 9 resistance patterns were observed. Pen-Penicillins; Cep-Cephalosporins; Mon-Monobactams; Car-Carbapenems; β-la-β-lactamase inhibitor. (b) Color bars demonstrate the distribution of phenotypic resistance patterns to 15 β-lactam antibiotics among ESBL-E. coli (n = 19). The specific strain designations are illustrated in the color bars a total of 14 resistance patterns were observed by using disk diffusion assay.
3.3. Prevalence of MGEs and ARGs in 19 ESBL-E. coli Isolates and OMPs in ESBLs16
Table 2 presents the detection rates of β-lactam ARGs among the 19 ESBL-E. coli isolates. A total of four out of the 14 target ARGs were detected. The highest detection rate was for blaCTX-M (78.95%, 15/19), followed by blaSHV (10.53%, 2/19). blaTEM and blaDHA were only detected in one strain (5.26%, 1/19), respectively. In contrast, genes encoding for AmpC β-lactamases (blaMOX, blaCIT, blaACC, blaEBC, blaFOX) and carbapenemases (blaKPC, blaNDM-1, blaIMP, blaVIM, blaSEM) were not detected in the 19 isolates. Subsequent analysis was conducted in order to characterize the subtypes of the β-lactamase genes (blaCTX-M, blaSHV, blaTEM and blaDHA). Five variants of blaCTX-M were identified. Furthermore, the presence of one variant each of blaSHV-1, blaTEM-1, and blaDHA-14 was detected. Among the five blaCTX-M variants, three belonged to the CTX-M-1 group (blaCTX-M-55, blaCTX-M-15 and blaCTX-M-3) and two to the CTX-M-9 group (blaCTX-M-14 and blaCTX-M-27). blaCTX-M-15 was the most prevalent variant (58.82%, 10/17), followed by blaCTX-M-3 (17.65%, 3/17), blaCTX-M-14 (11.76%, 2/17), blaCTX-M-27 (5.88%, 1/17), and blaCTX-M-55 (5.88%, 1/17). It is noteworthy that both ESBLs14 (co-harboring blaCTX-M-15 and blaCTX-M-14) and ESBLs18 (co-harboring blaCTX-M-3 and blaCTX-M-14) contained two coexisting blaCTX-M variants.

Table 2.
Comprehensive data of 19 ESBL-E. coli isolates from captive black bears.
As shown in Figure 4, OmpC and OmpF were successfully amplified from isolate ESBLs16, indicating that the two genes exist in the ESBLs16 strain.
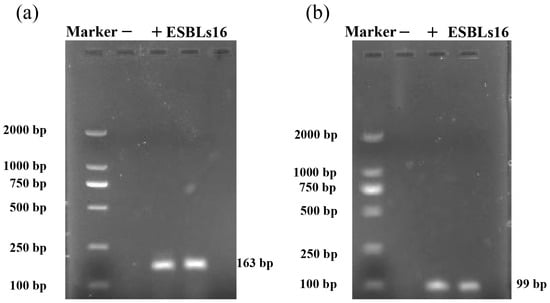
Figure 4.
PCR amplification of OmpC and OmpF genes from isolate ESBLs16. (a) OmpC of isolate ESBLs16. (b) OmpF of isolate ESBLs16. “−” indicates the negative control, and “+” indicates the positive control.
3.4. Associations Between AMR and ARGs or MGEs in 19 ESBL-E. coli Isolates
We analyzed the associations between AMR phenotypes and ARGs in 19 ESBL-E. coli strains. As shown in Figure 5a, two positive association pairs (r > 0, p < 0.05) were observed between AMR and ARGs, specifically between the TZP resistance phenotype and the blaCTX-M-3 gene, and between the FOX resistance phenotype and the blaSHV-1 gene. Furthermore, analysis of the associations between AMR phenotypes and MGEs revealed two additional positive correlations (Figure 5b), involving both the IMP and FOX resistance phenotypes with the tnsA gene.
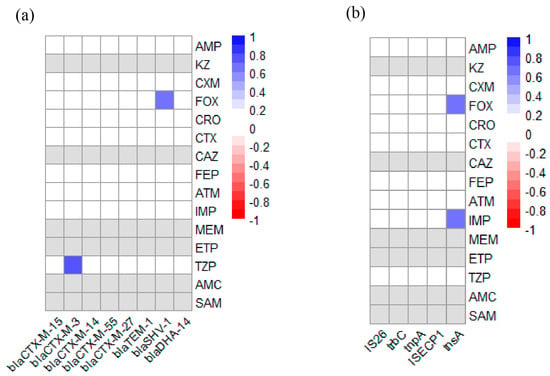
Figure 5.
Heatmap of the correlation-coefficient (r) between AMR and ARGs or MGEs in 19 ESBL-E. coli strains from black bear. Blue indicates positive association (r > 0, p < 0.05) and red indicates negative association (r < 0, p < 0.05). Gray indicates that the resistance rate to the antibiotic was either 0% or 100%; therefore, a valid correlation coefficient could not be computed. The color scale on the right of figure indicates the r-valve: (a) Heatmap of the correlation coefficient between AMR and ARGs. The color scale and corresponding r-valve indicate the association between corresponding abscissa ARGs and ordinate AMR. (b) Heatmap of correlation coefficient between AMR and MGEs. The color scale and corresponding r-valve indicate the association between corresponding MGEs (abscissa) and AMR (ordinate).
3.5. Conjugative Transfer Assays and Replicon Typing
We further investigated the conjugative transferability of ARGs, MGEs and plasmids. In the present study, 19 ESBL-E. coli strains were examined, of which eight (42.11%, 8/19) were found to be capable of conjugative transfer, with frequencies ranging from 8.13 × 10−10 to 1.15 × 10−5. All eight transconjugants were confirmed to have the ESBL-producing phenotype. The CTX-M-9 group variants from isolates ESBLs14 and ESBLs17 exhibited no transferability, whereas other blaCTX-M variants were successfully transferred to the E. coli J53 recipient. Furthermore, blaSHV, blaTEM and blaDHA were not detected in any transconjugants. Figure 6 shows the dissemination details of ARGs, MGEs and plasmids for the eight ESBL-E. coli donor isolates and transconjugants. Among them, trbC was detected in all transconjugants, whereas tnpA was not transferred. The transfer of IS26 was successful in all isolates except for ESBLs12 and ESBLs13. Similarly, ISEcp1 was transferred successfully in all isolates except for ESBLs1.
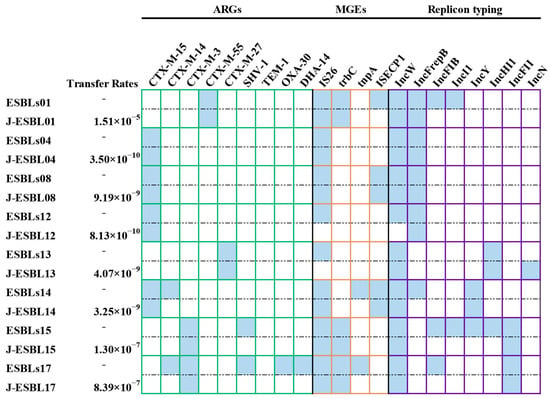
Figure 6.
A heatmap comparing ARGs, MGEs, and plasmid replicon types among 8 ESBL-E. coli donor strains and their corresponding transconjugants. “J” represents that the strain is a transconjugant. Blue squares represent donor bacteria or transconjugants carrying the gene or plasmid.
PBRT identified eight distinct plasmid types in eight ESBL-E. coli isolates, including IncW (100%, 8/8), IncFrepB (62.5%, 5/8), IncFIB (37.5%, 3/8), IncI1 (25%, 2/8), IncY (25%, 2/8), IncHI1 (25%, 2/8), IncFII (25%, 2/8), and IncN (12.5%, 1/8). PBRT analysis of the transconjugants confirmed that five plasmid types (IncFII, IncW, IncFrepB, IncY, and IncHI1) were successfully transferred, while IncFIB, IncI1, and IncN were not observed.
4. Discussion
AMR poses one of the most serious global public health threats to both animals and humans in the 21st century [37]. The global emergence of ESBL-E. coli represents a significant concern because few antibiotics remain active against such bacteria [38,39]. ESBLs confer resistance to a broad spectrum of β-lactam antibiotics, including penicillins, third-generation cephalosporins, and monobactams, thereby complicating the treatment of infections caused by ESBL-E. coli in animals [40]. To date, the presence of ESBL-E. coli has been detected in various wildlife species, including gulls, deer, iguana and coatis [41,42,43,44]. However, reports of ESBL-E. coli in captive black bears remain limited. Only two studies on Indian sloth bears [22] and Spanish Eurasian brown bears [23] have reported ESBL-E. coli. To better understand the characteristics of ESBL-E. coli in captive black bears, 142 fecal isolates preserved in our laboratory were analyzed. Our results showed that 19 ESBL-E. coli strains were successfully identified from 142 E. coli strains (13.38%). The prevalence of ESBL-E. coli from captive black bears was similar to the prevalence from Italian wild boars (15.96%) and wild birds (13.2%) [45,46], but lower than that from captive non-human primates in China (32%) [47]. In contrast, the prevalence of ESBL-E. coli from captive black bears was higher than that reported from wildlife in Portugal (3%) [48].
MLST is a common method employed in the field to differentiate genetic relatedness based on the analysis of allelic profile similarities [49]. In the present study, ST10 was the most prevalent ST, which was consistent with the detection profiles of ESBL-E. coli STs from sheep and primates [50,51]. ST10 is a high-risk clone with a broad host range [52], including livestock (pigs, cattle, sheep and poultry), wildlife (Siberian tigers, North China leopards, and silver gulls), and companion animals (cats and dogs) [53,54,55,56,57,58]. ST10 is also believed to be associated with human-associated infections and has the potential to cause zoonotic transmission [50,59]. The identification of ST10 from captive black bears suggests that continuous monitoring is required to better understand its transmission. In our study, ST2690 was detected as the second prevalent ST (11.11%, 2/18). ST2690 was first identified in ESBL-E. coli from domestic ducks [60]. A recent study has also detected ST2690 in ESBL-E. coli isolates from captive giant panda breeding environments and their animal keepers [30]. This study demonstrated that ST2690 exhibits conjugation capability, thus indicating its potential to spread to giant pandas [30]. The above results imply that ST2690 was co-circulating among the environment, animals, and humans, indicating a significant risk to public health. Furthermore, six additional known STs (ST540, ST4160, ST2792, ST208, ST695, and ST3856) were also detected in ESBL-E. coli from captive black bears. ST540 has been reported in ESBL-E. coli from both wild game meat and wild birds in Switzerland [61,62]. Similarly, ST4160 was identified in equine ESBL-E. coli in the Netherlands [63], while ST2792 was found in ESBL-E. coli from retail chicken meat in Japan [64]. In China, ST208 has been detected in E. coli from retail duck meat [65], and ST3856 was the predominant ST in duck-derived E. coli [66]. Furthermore, ST695 has been identified in E. coli from wild pigeons and penguins in Australia [67]. In summary, ST540, ST4160, ST2792, ST208, ST695, and ST3856 were first detected in ESBL-E. coli from captive black bears, highlighting the necessity for sustained wildlife surveillance.
ESBL-E. coli is characterized by its resistance to β-lactam antibiotics [68]. Our results showed that the highest resistance rate was observed for KZ, CRO and CTX. KZ (a first-generation cephalosporin) has been used clinically for a period exceeding four decades [69]. All 19 ESBL-E. coli isolates were resistant to KZ, which is consistent with the observations reported by Li et al. in ESBL-E. coli from broilers [70]. CRO and CTX are widely used third-generation cephalosporins in clinical practice [71]. In our study, the resistance rates to CRO and CTX were 78.95% and 73.68%, respectively. One study reported that ESBL-E. coli isolated from pigs exhibited high resistance rates to both CRO and CTX [72], which were similar to the resistance rates observed in our study. The high resistance observed to CRO and CTX may be attributable to the treatment of sick captive black bears with these antibiotics (CRO and CTX) during captive management (information provided by the local farm veterinarians for captive black bears). In our study, ESBL-E. coli isolates exhibited strong AMR and a variety of resistance patterns, implying that drug rotation strategies should be considered in the clinical use of antibiotics. These measures aim to reduce the emergence and transmission of AMR bacteria across humans, animals, and environment [73]. Moreover, two ESBL-E. coli isolates (ESBLs03 and ESBLs17) exhibited concomitant resistance to four classes of β-lactam antibiotics, yet remained susceptible to carbapenems. The activity of β-lactamases is suppressed by β-lactamase inhibitors, and the combination of these inhibitors with β-lactam antibiotics helps to preserve and extend the latter’s therapeutic efficacy [74,75]. The concomitant resistance to four classes exhibited by ESBLs03 and ESBLs17 implies that treatment options would be severely limited. It is noteworthy that one ESBL-E. coli (ESBLs16) was resistant to imipenem despite not carrying any carbapenemase genes. Research has demonstrated that imipenem resistance can emerge in non-carbapenemase-producing E. coli strains exhibiting a deficiency in major outer membrane proteins (OMPs) OmpF and OmpC [76]. Consequently, we employed the PCR to amplify the OmpF and OmpC genes in strain ESBLs16. The results of the study demonstrated the presence of both OmpF and OmpC in isolate ESBLs16. The observed imipenem resistance in strains without OmpF or OmpC deficiencies suggests that further investigation is required into potential resistance mechanisms [77]. In summary, ESBL-E. coli from captive black bears demonstrates resistance to various β-lactam antibiotics. This study provides valuable insights that can inform the clinical treatment of infectious diseases in captive black bears.
To date, over 350 natural ESBL variants have been identified and classified into nine structural and evolutionary families (TEM, SHV, CTX-M, PER, VEB, GES, BES, TLA, and OXA) [78]. Among these ESBL variants, CTX-M, TEM, and SHV are frequently detected in wildlife species, including wild boars, chimpanzees, mouflons, and ostriches [45,79]. In our study, CTX-M was the predominant type (78.95%), although its detection rate was lower than that reported in Indian sloth bears (100%) [22] and captive giant pandas (88.9%) [30], yet higher than that in captive wild birds (65.4%) [80]. The detection rates of SHV and TEM in our study were 10.53% and 5.26%, respectively. These rates were found to be significantly lower than those reported in wild bird populations [80,81]. Furthermore, among blaCTX-M variants, blaCTX-M-15 was the most prevalent variant (58.82%, 10/17) in our study. The presence of blaCTX-M-15, which has been primarily identified in human and veterinary clinical samples, has recently been reported in wildlife [82]. In China, blaCTX-M-15 has also been detected in wildlife, including giant pandas (8.82%), swans (21.43%), and primates (55.36%) [30,47,83]. The results obtained demonstrate that the blaCTX-M-15 variant is prevalent among ESBL-E. coli in wildlife in China. In the present study, the blaCTX-M-14 (11.76%, 2/17) variant was also detected in a sample of captive black bears. The blaCTX-M-14 variant is the most common among ESBL-E. coli strains from humans [84]. The presence of blaCTX-M-14 variants in captive black bears in this study suggests that transmission of these variants via animal keepers is a possibility [85].
The uncontrolled dissemination of plasmids carrying ARGs poses a significant public health threat due to rapid HGT [86]. In the present study, four out of the eight ARGs were successfully transferred (including blaCTX-M-15, blaCTX-M-3, blaCTX-M-55, and blaCTX-M-27). The remaining ESBL genes were not transferred, a phenomenon that can be attributed to their predominant chromosomal location, a feature that favors vertical transmission [87]. It is noteworthy that among isolates ESBLs14 and ESBLs18, the blaCTX-M-14 failed to transfer in both conjugation transfer assays. As indicated by previous studies, the blaCTX-M-14 can be located on the chromosome [88], which leads to failure of its horizontal transfer to E. coli J53. Research conducted on blaCTX-M genes located on the chromosome has indicated that these genes are non-transferable to E. coli J53 [89]. In our study, blaCTX-M-14 failed to transfer, possibly due to its chromosomal location. In the present study, six out of eight ESBL-E. coli isolates capable of conjugative transfer were found to co-transfer IS26 with blaCTX-M. blaCTX-M genes are often co-localized with IS26 on plasmids, a configuration that plays a key role in their mobilization and dissemination [90]. Consequently, it can be deduced that transfer of blaCTX-M in isolates ESBLs01, ESBLs04, ESBLs08, ESBLs14, ESBLs15 and ESBLs17 may be mediated by IS26 [91]. PBRT showed that the conjugative plasmids of ESBL-E. coli included IncFII, IncW, IncFrepB, IncY and IncHI1. These incompatibility-group types have been previously detected in ESBL-E. coli plasmids worldwide [92,93,94]. To the best of our knowledge, among ESBL-E. coli isolated from wildlife, the IncW plasmid has only been reported in wild birds prior to the present study [82]. The results of the present study demonstrate the presence of IncW plasmids in ESBL-E. coli from captive black bears, thus indicating a potential expansion of its host range within wildlife species. ESBL-E. coli with HGT capability poses a serious threat to public health by enabling dissemination across the interfaces between humans, animals and the environment, which is a core concern of the One Health framework [95,96]. The Asiatic black bear (U. t. mupinensis), the most widely distributed subspecies in China [15], may be a potential dissemination host due to its extensive contact with all three interfaces.
The limitations of this article are as follows: (1) small sample size and limited geographic scope of sampling; future studies should expand both the sample size and the geographic coverage of sampling; (2) environmental samples and samples from animal keepers were not collected; analyzing multiple interfaces enables a more comprehensive understanding of the transmission mechanisms. Nevertheless, the detection of ESBL-E. coli with HGT capabilities in black bears provides valuable insights into its dissemination potential, despite these limitations.
5. Conclusions
This study reports the first identification of ESBL-E. coli in captive black bears, with the isolates belonging to multiple different STs. The ESBL-E. coli isolates exhibited strong AMR and a diversity of resistance patterns. Furthermore, blaCTX-M genes play a dominant role in mediating ESBL resistance. Conjugative transfer assays have demonstrated high transfer efficiency, and have shown that multiple ARGs, MGEs, and plasmids were capable of horizontal transmission. Based on the One Health framework and the needs of conservation, it is recommended that long-term surveillance be implemented to study the dissemination of ESBL-E. coli from captive black bears.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/vetsci12111085/s1, Table S1: PCR primer and conditions used in this study.
Author Contributions
Conceptualization, Z.Z. (Zhijun Zhong); methodology, Z.Z. (Zhijun Zhong); software, S.L., M.W., R.H., X.L. (Xiaoqi Li), I.L., G.P., H.L. and Z.Z. (Ziyao Zhou); validation, S.L., M.W., R.H., I.L., X.L. (Xiaoqi Li), L.Z., G.P., H.L. and Z.Z. (Ziyao Zhou); investigation, X.L. (Xin Lei), S.P. and Y.Z.; data curation, X.L. (Xin Lei), S.P. and M.C.; writing—original draft preparation, X.L. (Xin Lei), X.Y. and S.P.; writing—review and editing, X.L. (Xin Lei) and Z.Z. (Zhijun Zhong); visualization, X.L. (Xin Lei), X.Y. and K.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Key Research and Development Program of China (2024YFD1800202), and the Study on Key Technologies for Conservation of Wild Giant Panda Populations and Its Habitats within Giant Panda National Park System (CGF2024001).
Institutional Review Board Statement
This work was approved by the Sichuan Agricultural University Animal Ethical and Welfare Committee (ETHICS number: DYY-2020103018).
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bailey, C.; Mansfield, K. Emerging and reemerging infectious diseases of nonhuman primates in the laboratory setting. Vet. Pathol. 2010, 47, 462–481. [Google Scholar] [CrossRef]
- ur Rahman, S.; Ali, T.; Ali, I.; Khan, N.A.; Han, B.; Gao, J. The growing genetic and functional diversity of extended spectrum beta-lactamases. BioMed Res. Int. 2018, 2018, 9519718. [Google Scholar] [CrossRef] [PubMed]
- Kaviani Rad, A.; Balasundram, S.K.; Azizi, S.; Afsharyzad, Y.; Zarei, M.; Etesami, H.; Shamshiri, R.R. An overview of antibiotic resistance and abiotic stresses affecting antimicrobial resistance in agricultural soils. Int. J. Environ. Res. Public Health 2022, 19, 4666. [Google Scholar] [CrossRef]
- Nwafia, I.N.; Ohanu, M.E.; Ebede, S.O.; Ozumba, U.C. Molecular detection and antibiotic resistance pattern of extended-spectrum beta-lactamase producing Escherichia coli in a Tertiary Hospital in Enugu, Nigeria. Ann. Clin. Microbiol. Antimicrob. 2019, 18, 41. [Google Scholar] [CrossRef] [PubMed]
- Kettani Halabi, M.; Lahlou, F.A.; Diawara, I.; El Adouzi, Y.; Marnaoui, R.; Benmessaoud, R.; Smyej, I. Antibiotic Resistance Pattern of Extended Spectrum Beta Lactamase Producing Escherichia coli Isolated from Patients with Urinary Tract Infection in Morocco. Front. Cell. Infect. Microbiol. 2021, 11, 720701. [Google Scholar] [CrossRef]
- Tiwari, A.; Krolicka, A.; Tran, T.T.; Räisänen, K.; Ásmundsdóttir, Á.M.; Wikmark, O.G.; Lood, R.; Pitkänen, T. Antibiotic resistance monitoring in wastewater in the Nordic countries: A systematic review. Environ. Res. 2024, 246, 118052. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, L.M.C.; Consol, P.; Chen, Y. Drug Discovery in the Field of β-Lactams: An Academic Perspective. Antibiotics 2024, 13, 59. [Google Scholar] [CrossRef]
- Madec, J.Y.; Haenni, M.; Nordmann, P.; Poirel, L. Extended-spectrum β-lactamase/AmpC- and carbapenemase-producing Enterobacteriaceae in animals: A threat for humans? Clin. Microbiol. Infect. 2017, 23, 826–833. [Google Scholar] [CrossRef]
- Avershina, E.; Sharma, P.; Taxt, A.M.; Singh, H.; Frye, S.A.; Paul, K.; Kapil, A.; Naseer, U.; Kaur, P.; Ahmad, R. AMR-Diag: Neural network based genotype-to-phenotype prediction of resistance towards β-lactams in Escherichia coli and Klebsiella pneumoniae. Comput. Struct. Biotechnol. J. 2021, 19, 1896–1906. [Google Scholar] [CrossRef]
- Livermore, D.M.; Woodford, N. The beta-lactamase threat in Enterobacteriaceae, Pseudomonas and Acinetobacter. Trends Microbiol. 2006, 14, 413–420. [Google Scholar] [CrossRef]
- Benavides, J.A.; Salgado-Caxito, M.; Opazo-Capurro, A.; González Muñoz, P.; Piñeiro, A.; Otto Medina, M.; Rivas, L.; Munita, J.; Millán, J. ESBL-Producing Escherichia coli Carrying CTX-M Genes Circulating among Livestock, Dogs, and Wild Mammals in Small-Scale Farms of Central Chile. Antibiotics 2021, 10, 510. [Google Scholar] [CrossRef]
- He, Y.Z.; Yan, J.R.; He, B.; Ren, H.; Kuang, X.; Long, T.F.; Chen, C.P.; Liao, X.P.; Liu, Y.H.; Sun, J. A Transposon-Associated CRISPR/Cas9 System Specifically Eliminates both Chromosomal and Plasmid-Borne mcr-1 in Escherichia coli. Antimicrob. Agents Chemother. 2021, 65, e0105421. [Google Scholar] [CrossRef]
- Hounmanou, Y.M.G.; Bortolaia, V.; Dang, S.T.T.; Truong, D.; Olsen, J.E.; Dalsgaard, A. ESBL and AmpC β-Lactamase Encoding Genes in E. coli From Pig and Pig Farm Workers in Vietnam and Their Association with Mobile Genetic Elements. Front. Microbiol. 2021, 12, 629139. [Google Scholar] [CrossRef]
- Dai, Y.; Huang, H.; Qing, Y.; Li, J.; Li, D. Ecological response of an umbrella species to changing climate and land use: Habitat conservation for Asiatic black bear in the Sichuan-Chongqing Region, Southwestern China. Ecol. Evol. 2023, 13, e10222. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Zheng, W.; Guo, X.; Wang, Y.; Zhou, Y.; Zhao, S.; Song, X.; Xu, A. Large Carnivores Persisting in a Human-Dominated Landscape: Suitable Habitat and Connectivity for Asiatic Black Bears in China. Ecol. Evol. 2025, 15, e72181. [Google Scholar] [CrossRef] [PubMed]
- Costa, D.; Poeta, P.; Sáenz, Y.; Vinué, L.; Rojo-Bezares, B.; Jouini, A.; Zarazaga, M.; Rodrigues, J.; Torres, C. Detection of Escherichia coli harbouring extended-spectrum beta-lactamases of the CTX-M, TEM and SHV classes in faecal samples of wild animals in Portugal. J. Antimicrob. Chemother. 2006, 58, 1311–1312. [Google Scholar] [CrossRef]
- Agustin, A.L.D.; Effendi, M.H.; Tyasningsih, W.; Plumeriastuti, H.; Khairullah, A.R.; Ekawasti, F.; Moses, I.B.; Kinasih, K.N.; Kusala, M.K.J.; Mustika, Y.R.; et al. Phylogenetic analysis blaTEM gene of Escherichia coli isolated from cave bats in West Nusa Tenggara Province, Indonesia. Open Vet. J. 2024, 14, 3460–3473. [Google Scholar] [CrossRef]
- Liu, T.; Lee, S.; Kim, M.; Fan, P.; Boughton, R.K.; Boucher, C.; Jeong, K.C. A study at the wildlife-livestock interface unveils the potential of feral swine as a reservoir for extended-spectrum β-lactamase-producing Escherichia coli. J. Hazard. Mater. 2024, 473, 134694. [Google Scholar] [CrossRef]
- Smith, C.M.; Anacker, M.; Bevis, D.L.; Dutton, N.A.M.; Powell, D.; McLaughlin, R.W. Isolation of a CTX-M-55 (ESBL)-Producing Escherichia coli Strain of the Global ST6448 Clone from a Captive Orangutan in the USA. Curr. Microbiol. 2024, 81, 177. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Yan, X.; Li, Y.; Zhang, D.; Li, L.; Geng, Y.; Su, F.; Yue, C.; Hou, R.; Liu, S. Identification of extended-spectrum beta-lactamase (CTX-M)-producing Klebsiella pneumoniae belonging to ST37, ST290, and ST2640 in captive giant pandas. BMC Vet. Res. 2022, 18, 186. [Google Scholar] [CrossRef]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- VinodhKumar, O.R.; Karikalan, M.; Ilayaraja, S.; Sha, A.A.; Singh, B.R.; Sinha, D.K.; Chandra Mohan, S.; Pruthvishree, B.S.; Pawde, A.M.; Sharma, A.K. Multi-drug resistant (MDR), extended spectrum beta-lactamase (ESBL) producing and carbapenem resistant Escherichia coli in rescued Sloth bears (Melursus ursinus), India. Vet. Res. Commun. 2021, 45, 163–170. [Google Scholar] [CrossRef]
- Herrero-García, G.; Barroso, P.; Dashti, A.; González-Barrio, D.; Naves, J.; Fernández-Gil, A.; Ugarte-Ruiz, M.; Pérez-Sancho, M.; Royo, L.J.; Carmena, D.; et al. Non-invasive surveillance of shared pathogens in the Eurasian brown bear (Ursus arctos) human interface. One Health 2024, 18, 100746. [Google Scholar] [CrossRef]
- Liu, H.; Shi, K.; Wang, Y.; Zhong, W.; Pan, S.; Zhou, L.; Cheng, Y.; Yuan, Y.; Zhou, Z.; Liu, H.; et al. Characterization of antibiotic resistance genes and mobile genetic elements in Escherichia coli isolated from captive black bears. Sci. Rep. 2024, 14, 2745. [Google Scholar] [CrossRef]
- PubMLST. Available online: https://pubmlst.org/organisms/escherichia-spp (accessed on 10 October 2025).
- Furlan, J.P.R.; Lopes, R.; Gonzalez, I.H.L.; Ramos, P.L.; Stehling, E.G. Comparative analysis of multidrug resistance plasmids and genetic background of CTX-M-producing Escherichia coli recovered from captive wild animals. Appl. Microbiol. Biotechnol. 2020, 104, 6707–6717. [Google Scholar] [CrossRef]
- Garcias, B.; Aguirre, L.; Seminati, C.; Reyes, N.; Allepuz, A.; Obón, E.; Molina-Lopez, R.A.; Darwich, L. Extended-Spectrum β-Lactam Resistant Klebsiella pneumoniae and Escherichia coli in Wild European Hedgehogs (Erinaceus europeus) Living in Populated Areas. Animals 2021, 11, 2837. [Google Scholar] [CrossRef] [PubMed]
- Garcês, A.; Pires, I. European Wild Carnivores and Antibiotic Resistant Bacteria: A Review. Antibiotics 2023, 12, 1725. [Google Scholar] [CrossRef] [PubMed]
- Eckert, C.; Gautier, V.; Arlet, G. DNA sequence analysis of the genetic environment of various blaCTX-M genes. J. Antimicrob. Chemother. 2006, 57, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Shu, G.; Gan, T.; Lin, Z.; Liu, Y.; Chen, J.; Wang, C.; Deng, L.; Li, C.; Chang, L.J.; Zhang, W.; et al. The resistance patterns and molecular characteristics of ESBL/AmpC-producing Escherichia coli from captive panda ecosystem in China. Ecotoxicol. Environ. Saf. 2024, 278, 116395. [Google Scholar] [CrossRef]
- Dai, W.; Sun, S.; Yang, P.; Huang, S.; Zhang, X.; Zhang, L. Characterization of carbapenemases, extended spectrum β-lactamases and molecular epidemiology of carbapenem-non-susceptible Enterobacter cloacae in a Chinese hospital in Chongqing. Infect. Genet. Evol. 2013, 14, 1–7. [Google Scholar] [CrossRef]
- Karabay, O.; Altindis, M.; Koroglu, M.; Karatuna, O.; Aydemir, Ö.A.; Erdem, A.F. The carbapenem-resistant Enterobacteriaceae threat is growing: NDM-1 epidemic at a training hospital in Turkey. Ann. Clin. Microbiol. Antimicrob. 2016, 15, 6. [Google Scholar] [CrossRef] [PubMed]
- Ganjo, A.R.; Balaky, S.T.J.; Mawlood, A.H.; Smail, S.B.; Shabila, N.P. Characterization of genes related to the efflux pump and porin in multidrug-resistant Escherichia coli strains isolated from patients with COVID-19 after secondary infection. BMC Microbiol. 2024, 24, 122. [Google Scholar] [CrossRef]
- Algammal, A.M.; Hashem, H.R.; Alfifi, K.J.; Hetta, H.F.; Sheraba, N.S.; Ramadan, H.; El-Tarabili, R.M. atpD gene sequencing, multidrug resistance traits, virulence-determinants, and antimicrobial resistance genes of emerging XDR and MDR-Proteus mirabilis. Sci. Rep. 2021, 11, 9476. [Google Scholar] [CrossRef]
- Zhu, Z.; Pan, S.; Wei, B.; Liu, H.; Zhou, Z.; Huang, X.; Luo, Y.; Zhou, L.; Zhang, S.; Ma, X.; et al. High prevalence of multi-drug resistances and diversity of mobile genetic elements in Escherichia coli isolates from captive giant pandas. Ecotoxicol. Environ. Saf. 2020, 198, 110681. [Google Scholar] [CrossRef]
- Carattoli, A.; Bertini, A.; Villa, L.; Falbo, V.; Hopkins, K.L.; Threlfall, E.J. Identification of plasmids by PCR-based replicon typing. J. Microbiol. Methods 2005, 63, 219–228. [Google Scholar] [CrossRef]
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial resistance: A global multifaceted phenomenon. Pathog. Glob. Health 2015, 109, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Das, T.; Nath, C.; Ahmed, T.; Ghosh, K.; Dhar, P.K.; Herrero-Fresno, A.; Barua, H.; Biswas, P.K.; Islam, M.Z.; et al. Whole-genome characterization and global phylogenetic comparison of cefotaxime-resistant Escherichia coli isolated from broiler chickens. J. Microbiol. 2025, 63, e2412009. [Google Scholar] [CrossRef]
- Ruppé, E.; Lixandru, B.; Cojocaru, R.; Büke, C.; Paramythiotou, E.; Angebault, C.; Visseaux, C.; Djuikoue, I.; Erdem, E.; Burduniuc, O.; et al. Relative fecal abundance of extended-spectrum-β-lactamase-producing Escherichia coli strains and their occurrence in urinary tract infections in women. Antimicrob. Agents Chemother. 2013, 57, 4512–4517. [Google Scholar] [CrossRef]
- Duffy, N.; Karlsson, M.; Reses, H.E.; Campbell, D.; Daniels, J.; Stanton, R.A.; Janelle, S.J.; Schutz, K.; Bamberg, W.; Rebolledo, P.A.; et al. Epidemiology of extended-spectrum β-lactamase-producing Enterobacterales in five US sites participating in the Emerging Infections Program, 2017. Infect. Control Hosp. Epidemiol. 2022, 43, 1586–1594. [Google Scholar] [CrossRef]
- Atterby, C.; Börjesson, S.; Ny, S.; Järhult, J.D.; Byfors, S.; Bonnedahl, J. ESBL-producing Escherichia coli in Swedish gulls-A case of environmental pollution from humans? PLoS ONE 2017, 12, e0190380. [Google Scholar] [CrossRef] [PubMed]
- Riwu, K.H.P.; Effendi, M.H.; Rantam, F.A.; Khairullah, A.R.; Kurniawan, S.C.; Kurniawan, A.; Moses, I.B.; Hasib, A.; Widodo, A.; Yanestria, S.M. Molecular detection of blaTEM gene for encoding extended spectrum beta-lactamase (ESBL) on Escherichia coli isolated from deer feces in Indonesia. J. Adv. Vet. Res. 2024, 14, 722–726. [Google Scholar]
- Guyomard-Rabenirina, S.; Reynaud, Y.; Pot, M.; Albina, E.; Couvin, D.; Ducat, C.; Gruel, G.; Ferdinand, S.; Legreneur, P.; Le Hello, S.; et al. Antimicrobial Resistance in Wildlife in Guadeloupe (French West Indies): Distribution of a Single bla (CTX-M-1)/IncI1/ST3 Plasmid Among Humans and Wild Animals. Front. Microbiol. 2020, 11, 1524. [Google Scholar] [CrossRef]
- de Carvalho, M.P.N.; Fernandes, M.R.; Sellera, F.P.; Lopes, R.; Monte, D.F.; Hippólito, A.G.; Milanelo, L.; Raso, T.F.; Lincopan, N. International clones of extended-spectrum β-lactamase (CTX-M)-producing Escherichia coli in peri-urban wild animals, Brazil. Transbound. Emerg. Dis. 2020, 67, 1804–1815. [Google Scholar] [CrossRef]
- Formenti, N.; Calò, S.; Parisio, G.; Guarneri, F.; Birbes, L.; Pitozzi, A.; Scali, F.; Tonni, M.; Guadagno, F.; Giovannini, S.; et al. ESBL/AmpC-Producing Escherichia coli in Wild Boar: Epidemiology and Risk Factors. Animals 2021, 11, 1855. [Google Scholar] [CrossRef] [PubMed]
- Prandi, I.; Bellato, A.; Nebbia, P.; Stella, M.C.; Ala, U.; von Degerfeld, M.M.; Quaranta, G.; Robino, P. Antibiotic resistant Escherichia coli in wild birds hospitalised in a wildlife rescue centre. Comp. Immunol. Microbiol. Infect. Dis. 2023, 93, 101945. [Google Scholar] [CrossRef]
- Wang, Y.; He, T.; Han, J.; Wang, J.; Foley, S.L.; Yang, G.; Wan, S.; Shen, J.; Wu, C. Prevalence of ESBLs and PMQR genes in fecal Escherichia coli isolated from the non-human primates in six zoos in China. Vet. Microbiol. 2012, 159, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Sabença, C.; Romero-Rivera, M.; Barbero-Herranz, R.; Sargo, R.; Sousa, L.; Silva, F.; Lopes, F.; Abrantes, A.C.; Vieira-Pinto, M.; Torres, C.; et al. Molecular Characterization of Multidrug-Resistant Escherichia coli from Fecal Samples of Wild Animals. Vet. Sci. 2024, 11, 469. [Google Scholar] [CrossRef] [PubMed]
- Gashaw, M.; Gudina, E.K.; Froeschl, G.; Matar, R.; Ali, S.; Gabriele, L.; Hohensee, A.; Seeholzer, T.; Kroidl, A.; Wieser, A. Resistome and Phylogenomics of Escherichia coli Strains Obtained from Diverse Sources in Jimma, Ethiopia. Antibiotics 2025, 14, 706. [Google Scholar] [CrossRef]
- Zhao, X.; Zhao, H.; Zhou, Z.; Miao, Y.; Li, R.; Yang, B.; Cao, C.; Xiao, S.; Wang, X.; Liu, H.; et al. Characterization of Extended-Spectrum β-Lactamase-Producing Escherichia coli Isolates That Cause Diarrhea in Sheep in Northwest China. Microbiol. Spectr. 2022, 10, e0159522. [Google Scholar] [CrossRef]
- Bazalar-Gonzales, J.; Silvestre-Espejo, T.; Rodríguez Cueva, C.; Carhuaricra Huamán, D.; Ignación León, Y.; Luna Espinoza, L.; Rosadio Alcántara, R.; Maturrano Hernández, L. Genomic insights into ESBL-producing Escherichia coli isolated from non-human primates in the Peruvian Amazon. Front. Vet. Sci. 2023, 10, 1340428. [Google Scholar] [CrossRef]
- Hu, Z.; Huang, Y.; Cui, X.; Shi, T.; Ji, Q.; Wang, X.; Bao, G.; Liu, Y. Genomic epidemiology of antimicrobial resistance determinants in Chinese swine farm Escherichia coli isolates. Front. Microbiol. 2025, 16, 1575426. [Google Scholar] [CrossRef]
- Kim, J.I.; Moon, B.Y.; Ali, M.S.; Kang, H.S.; Choi, J.H.; Kim, J.M.; Park, S.C.; Lim, S.K. High prevalence of bla(CTX-M-55)-carrying Escherichia coli in both ceftiofur-use and non-use pig farms. Appl. Environ. Microbiol. 2025, 91, e0252524. [Google Scholar] [CrossRef]
- Gu, X.; Wu, Q.; Chai, Y.; Huang, X.; Zhou, X.; Han, M.; Wu, T.; Zhang, X.; Zhong, F. Epidemiological and molecular characteristics of extraintestinal pathogenic Escherichia coli isolated from diseased cattle and sheep in Xinjiang, China from 2015 to 2019. BMC Vet. Res. 2025, 21, 42. [Google Scholar] [CrossRef] [PubMed]
- Stępień-Pyśniak, D.; Hauschild, T.; Łopucki, R.; Kosikowska, U.; Wilczyński, J.; Brzeski, M.; Matusevičius, P.; Christensen, H. Differences in tests of phenotypic colistin resistance in clinical Escherichia coli isolates from poultry and their genetic diversity. Vet. Microbiol. 2025, 307, 110581. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Lan, T.; Zhai, H.; Zhou, M.; Chen, D.; Lu, Y.; Han, L.; Wei, J.; Zhou, S.; Xu, H.; et al. Whole-genome analysis of Escherichia coli isolated from wild Amur tiger (Panthera tigris altaica) and North China leopard (Panthera pardus japonensis). PeerJ 2024, 12, e17381. [Google Scholar] [CrossRef]
- Mukerji, S.; Stegger, M.; Truswell, A.V.; Laird, T.; Jordan, D.; Abraham, R.J.; Harb, A.; Barton, M.; O’Dea, M.; Abraham, S. Resistance to critically important antimicrobials in Australian silver gulls (Chroicocephalus novaehollandiae) and evidence of anthropogenic origins. J. Antimicrob. Chemother. 2019, 74, 2566–2574. [Google Scholar] [CrossRef]
- Jousserand, N.; Auvray, F.; Chagneau, C.; Cavalié, L.; Maurey, C.; Drut, A.; Lavoué, R.; Oswald, E. Zoonotic potential of uropathogenic Escherichia coli lineages from companion animals. Vet. Res. 2025, 56, 69. [Google Scholar] [CrossRef] [PubMed]
- Danzeisen, J.L.; Wannemuehler, Y.; Nolan, L.K.; Johnson, T.J. Comparison of multilocus sequence analysis and virulence genotyping of Escherichia coli from live birds, retail poultry meat, and human extraintestinal infection. Avian Dis. 2013, 57, 104–108. [Google Scholar] [CrossRef]
- Hasan, B.; Sandegren, L.; Melhus, A.; Drobni, M.; Hernandez, J.; Waldenström, J.; Alam, M.; Olsen, B. Antimicrobial drug-resistant Escherichia coli in wild birds and free-range poultry, Bangladesh. Emerg. Infect. Dis. 2012, 18, 2055–2058. [Google Scholar] [CrossRef]
- Nüesch-Inderbinen, M.; Tresch, S.; Zurfluh, K.; Cernela, N.; Biggel, M.; Stephan, R. Finding of extended-spectrum beta-lactamase (ESBL)-producing Enterobacterales in wild game meat originating from several European countries: Predominance of Moellerella wisconsensis producing CTX-M-1, November 2021. Euro Surveill. 2022, 27, 2200343. [Google Scholar] [CrossRef]
- Zurfluh, K.; Albini, S.; Mattmann, P.; Kindle, P.; Nüesch-Inderbinen, M.; Stephan, R.; Vogler, B.R. Antimicrobial resistant and extended-spectrum β-lactamase producing Escherichia coli in common wild bird species in Switzerland. MicrobiologyOpen 2019, 8, e845. [Google Scholar] [CrossRef] [PubMed]
- Apostolakos, I.; Franz, E.; van Hoek, A.; Florijn, A.; Veenman, C.; Sloet-van Oldruitenborgh-Oosterbaan, M.M.; Dierikx, C.; van Duijkeren, E. Occurrence and molecular characteristics of ESBL/AmpC-producing Escherichia coli in faecal samples from horses in an equine clinic. J. Antimicrob. Chemother. 2017, 72, 1915–1921. [Google Scholar] [CrossRef]
- Hayashi, W.; Ohsaki, Y.; Taniguchi, Y.; Koide, S.; Kawamura, K.; Suzuki, M.; Kimura, K.; Wachino, J.I.; Nagano, Y.; Arakawa, Y.; et al. High prevalence of bla(CTX-M-14) among genetically diverse Escherichia coli recovered from retail raw chicken meat portions in Japan. Int. J. Food Microbiol. 2018, 284, 98–104. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, Y.; Chen, M.; Yang, G.; Zhang, J.; Wu, Q.; Wang, J.; Ding, Y.; Ye, Q.; Lei, T.; et al. Characterization of Escherichia coli O157:non-H7 isolated from retail food in China and first report of mcr-1/IncI2-carrying colistin-resistant E. coli O157:H26 and E. coli O157:H4. Int. J. Food Microbiol. 2022, 378, 109805. [Google Scholar] [CrossRef]
- Luo, S.; Liao, C.; Peng, J.; Tao, S.; Zhang, T.; Dai, Y.; Ding, Y.; Ma, Y. Resistance and virulence gene analysis and molecular typing of Escherichia coli from duck farms in Zhanjiang, China. Front. Cell. Infect. Microbiol. 2023, 13, 1202013. [Google Scholar] [CrossRef]
- Mukerji, S.; Gunasekera, S.; Dunlop, J.N.; Stegger, M.; Jordan, D.; Laird, T.; Abraham, R.J.; Barton, M.; O’Dea, M.; Abraham, S. Implications of Foraging and Interspecies Interactions of Birds for Carriage of Escherichia coli Strains Resistant to Critically Important Antimicrobials. Appl. Environ. Microbiol. 2020, 86, e01610-20. [Google Scholar] [CrossRef]
- Ludden, C.; Decano, A.G.; Jamrozy, D.; Pickard, D.; Morris, D.; Parkhill, J.; Peacock, S.J.; Cormican, M.; Downing, T. Genomic surveillance of Escherichia coli ST131 identifies local expansion and serial replacement of subclones. Microb. Genom. 2020, 6, e000352. [Google Scholar] [CrossRef]
- Schmitz, M.L.; Blumer, J.L.; Cetnarowski, W.; Rubino, C.M. Determination of appropriate weight-based cutoffs for empiric cefazolin dosing using data from a phase 1 pharmacokinetics and safety study of cefazolin administered for surgical prophylaxis in pediatric patients aged 10 to 12 years. Antimicrob. Agents Chemother. 2015, 59, 4173–4180. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhao, M.; Liu, J.; Zhou, Y.; Miao, Z. Prevalence and Antibiotic Resistance Profiles of Extended-Spectrum β-Lactamase-Producing Escherichia coli Isolated from Healthy Broilers in Shandong Province, China. J. Food Prot. 2016, 79, 1169–1173. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lu, Q.; Mao, X.; Li, L.; Dou, J.; He, Q.; Shao, H.; Luo, Q. Prevalence of Extended-Spectrum β-Lactamase-Resistant Genes in Escherichia coli Isolates from Central China during 2016–2019. Animals 2022, 12, 3191. [Google Scholar] [CrossRef]
- Tian, G.B.; Wang, H.N.; Zou, L.K.; Tang, J.N.; Zhao, Y.W.; Ye, M.Y.; Tang, J.Y.; Zhang, Y.; Zhang, A.Y.; Yang, X.; et al. Detection of CTX-M-15, CTX-M-22, and SHV-2 extended-spectrum beta-lactamases (ESBLs) in Escherichia coli fecal-sample isolates from pig farms in China. Foodborne Pathog. Dis. 2009, 6, 297–304. [Google Scholar] [CrossRef]
- Kahn, L.H.; Bergeron, G.; Bourassa, M.W.; De Vegt, B.; Gill, J.; Gomes, F.; Malouin, F.; Opengart, K.; Ritter, G.D.; Singer, R.S.; et al. From farm management to bacteriophage therapy: Strategies to reduce antibiotic use in animal agriculture. Ann. N. Y. Acad. Sci. 2019, 1441, 31–39. [Google Scholar] [CrossRef]
- Yahav, D.; Giske, C.G.; Grāmatniece, A.; Abodakpi, H.; Tam, V.H.; Leibovici, L. New β-lactam–β-lactamase inhibitor combinations. Clin. Microbiol. Rev. 2020, 34, 10–1128. [Google Scholar] [CrossRef]
- Schechter, L.M.; Creely, D.P.; Garner, C.D.; Shortridge, D.; Nguyen, H.; Chen, L.; Hanson, B.M.; Sodergren, E.; Weinstock, G.M.; Dunne, W.M., Jr.; et al. Extensive Gene Amplification as a Mechanism for Piperacillin-Tazobactam Resistance in Escherichia coli. mBio 2018, 9, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Oteo, J.; Delgado-Iribarren, A.; Vega, D.; Bautista, V.; Rodríguez, M.C.; Velasco, M.; Saavedra, J.M.; Pérez-Vázquez, M.; García-Cobos, S.; Martínez-Martínez, L.; et al. Emergence of imipenem resistance in clinical Escherichia coli during therapy. Int. J. Antimicrob. Agents 2008, 32, 534–537. [Google Scholar] [CrossRef]
- Roschanski, N.; Fischer, J.; Falgenhauer, L.; Pietsch, M.; Guenther, S.; Kreienbrock, L.; Chakraborty, T.; Pfeifer, Y.; Guerra, B.; Roesler, U.H. Retrospective Analysis of Bacterial Cultures Sampled in German Chicken-Fattening Farms During the Years 2011-2012 Revealed Additional VIM-1 Carbapenemase-Producing Escherichia coli and a Serologically Rough Salmonella enterica Serovar Infantis. Front. Microbiol. 2018, 9, 538. [Google Scholar] [CrossRef]
- Bajpai, T.; Pandey, M.; Varma, M.; Bhatambare, G.S. Prevalence of TEM, SHV, and CTX-M Beta-Lactamase genes in the urinary isolates of a tertiary care hospital. Avicenna J. Med. 2017, 7, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, C.; Araújo, C.; Gonçalves, A.; Vinué, L.; Somalo, S.; Ruiz, E.; Uliyakina, I.; Rodrigues, J.; Igrejas, G.; Poeta, P.; et al. Detection of CTX-M-14 and TEM-52 extended-spectrum beta-lactamases in fecal Escherichia coli isolates of captive ostrich in Portugal. Foodborne Pathog. Dis. 2010, 7, 991–994. [Google Scholar] [CrossRef]
- Adesola, R.O.; Bakre, A.A.; Adekanmbi, A.O.; Ogunro, B.N.; Adeolu Ogundijo, O.; Hamzat, A.; Hossain, D.; Aribana, M.A.; Balogun, L.A. Molecular and Epidemiological Characterization of ESBL-producing Escherichia coli from Captive Wild Birds in Zoological Gardens in Nigeria. Environ. Health Insights 2025, 19, 11786302251329300. [Google Scholar] [CrossRef] [PubMed]
- Furmanek-Blaszk, B.; Sektas, M.; Rybak, B. High Prevalence of Plasmid-Mediated Quinolone Resistance among ESBL/AmpC-Producing Enterobacterales from Free-Living Birds in Poland. Int. J. Mol. Sci. 2023, 24, 12804. [Google Scholar] [CrossRef]
- Ben Yahia, H.; Ben Sallem, R.; Tayh, G.; Klibi, N.; Ben Amor, I.; Gharsa, H.; Boudabbous, A.; Ben Slama, K. Detection of CTX-M-15 harboring Escherichia coli isolated from wild birds in Tunisia. BMC Microbiol. 2018, 18, 26. [Google Scholar] [CrossRef]
- Zeng, Z.; Yang, J.; Gu, J.; Liu, Z.; Hu, J.; Li, X.; Chen, X.; Sun, Z.; Li, J. Prevalence and antimicrobial susceptibility of CTX-M-type-producing Escherichia coli from a wildlife zoo in China. Vet. Med. Sci. 2022, 8, 1294–1299. [Google Scholar] [CrossRef]
- Torres, R.T.; Cunha, M.V.; Araujo, D.; Ferreira, H.; Fonseca, C.; Palmeira, J.D. A walk on the wild side: Wild ungulates as potential reservoirs of multi-drug resistant bacteria and genes, including Escherichia coli harbouring CTX-M beta-lactamases. Environ. Pollut. 2022, 306, 119367. [Google Scholar] [CrossRef]
- Tamang, M.D.; Nam, H.M.; Gurung, M.; Jang, G.C.; Kim, S.R.; Jung, S.C.; Park, Y.H.; Lim, S.K. Molecular characterization of CTX-M β-lactamase and associated addiction systems in Escherichia coli circulating among cattle, farm workers, and the farm environment. Appl. Environ. Microbiol. 2013, 79, 3898–3905. [Google Scholar] [CrossRef]
- Carattoli, A. Plasmids and the spread of resistance. Int. J. Med. Microbiol. 2013, 303, 298–304. [Google Scholar] [CrossRef]
- Naseer, U.; Sundsfjord, A. The CTX-M conundrum: Dissemination of plasmids and Escherichia coli clones. Microb. Drug Resist. 2011, 17, 83–97. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Yue, M.; Zhang, J.; Ruan, Z. Coexistence of two bla(CTX-M-14) genes in a bla(NDM-5)-carrying multidrug-resistant Escherichia coli strain recovered from a bloodstream infection in China. J. Glob. Antimicrob. Resist. 2021, 26, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Liu, D.; Li, X.; Xiao, C.; Mao, Y.; He, J.; Feng, J.; Wang, L. Characterizations of bla(CTX-M-14) and bla(CTX-M-64) in a clinical isolate of Escherichia coli from China. Front. Microbiol. 2023, 14, 1158659. [Google Scholar] [CrossRef]
- Dikaiou, A.; Tzimotoudis, N.; Sergelidis, D.; Papadogiannakis, E.; Giakkoupi, P. Molecular Characterization of Extended-Spectrum ß-Lactamases-Producing Escherichia coli Isolated from a Greek Food Testing Laboratory. Antibiotics 2025, 14, 329. [Google Scholar] [CrossRef]
- Larsen, A.L.; Pedersen, T.; Sundsfjord, A.; Ross, T.A.; Guleng, A.D.; Haug, J.B.; Pöntinen, A.K.; Samuelsen, Ø. Hospital toilets and drainage systems as a reservoir for a long-term polyclonal outbreak of clinical infections with multidrug-resistant Klebsiella oxytoca species complex. Infect. Prev. Pract. 2025, 7, 100430. [Google Scholar] [CrossRef]
- Rozwandowicz, M.; Brouwer, M.S.M.; Fischer, J.; Wagenaar, J.A.; Gonzalez-Zorn, B.; Guerra, B.; Mevius, D.J.; Hordijk, J. Plasmids carrying antimicrobial resistance genes in Enterobacteriaceae. J. Antimicrob. Chemother. 2018, 73, 1121–1137. [Google Scholar] [CrossRef] [PubMed]
- Pungpian, C.; Sinwat, N.; Angkititrakul, S.; Prathan, R.; Chuanchuen, R. Presence and Transfer of Antimicrobial Resistance Determinants in Escherichia coli in Pigs, Pork, and Humans in Thailand and Lao PDR Border Provinces. Microb. Drug Resist. 2021, 27, 571–584. [Google Scholar] [CrossRef] [PubMed]
- Shafiq, M.; Huang, J.; Shah, J.M.; Ali, I.; Rahman, S.U.; Wang, L. Characterization and resistant determinants linked to mobile elements of ESBL-producing and mcr-1-positive Escherichia coli recovered from the chicken origin. Microb. Pathog. 2021, 150, 104722. [Google Scholar] [CrossRef] [PubMed]
- Sharif, N.; Ahmed, S.N.; Khandaker, S.; Monifa, N.H.; Abusharha, A.; Vargas, D.L.R.; Díez, I.T.; Castilla, A.G.K.; Talukder, A.A.; Parvez, A.K.; et al. Multidrug resistance pattern and molecular epidemiology of pathogens among children with diarrhea in Bangladesh, 2019–2021. Sci. Rep. 2023, 13, 13975. [Google Scholar] [CrossRef]
- Amato, H.K.; Wong, N.M.; Pelc, C.; Taylor, K.; Price, L.B.; Altabet, M.; Jordan, T.E.; Graham, J.P. Effects of concentrated poultry operations and cropland manure application on antibiotic resistant Escherichia coli and nutrient pollution in Chesapeake Bay watersheds. Sci. Total Environ. 2020, 735, 139401. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).