Evaluation of Pathogenetic and Immunological Properties of a Vietnamese Isolate of Porcine Reproductive and Respiratory Syndrome Virus of Vietnam in Experimentally Infected Piglets
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Virus and Virus-Neutralization (VN) Test
2.3. Animal Experiment
2.4. Routine Blood Tests
2.5. Detection of Pathogen Load by qRT-PCR
2.6. RNA Extraction, cDNA Synthesis, and qPCR
2.7. Cytokine Assays
2.8. Statistical Analysis
3. Results
3.1. Clinical Evaluation
3.2. Gross Lesion
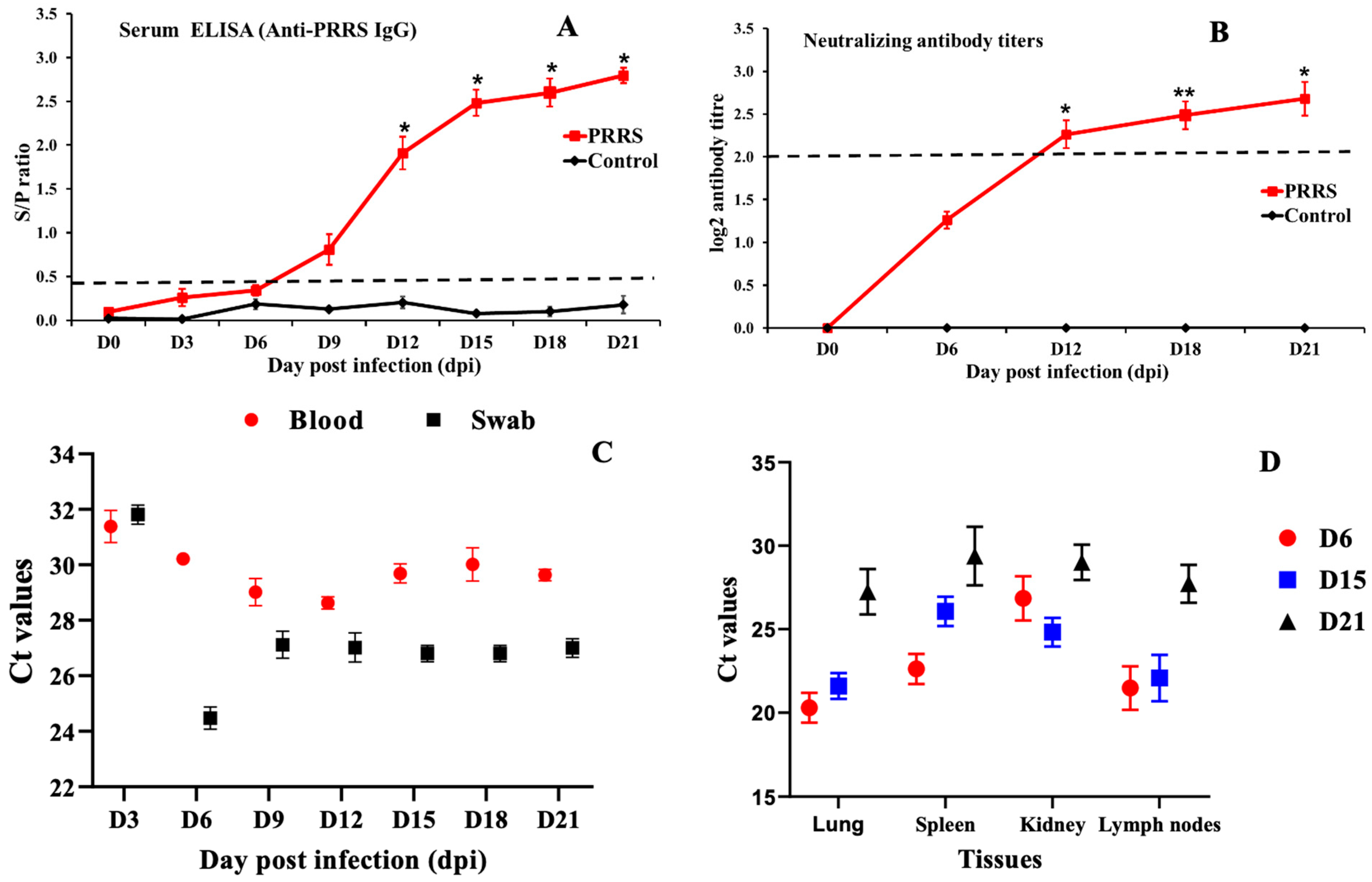
3.3. Viral RNA Load and Antibody
3.4. Blood Count Analysis
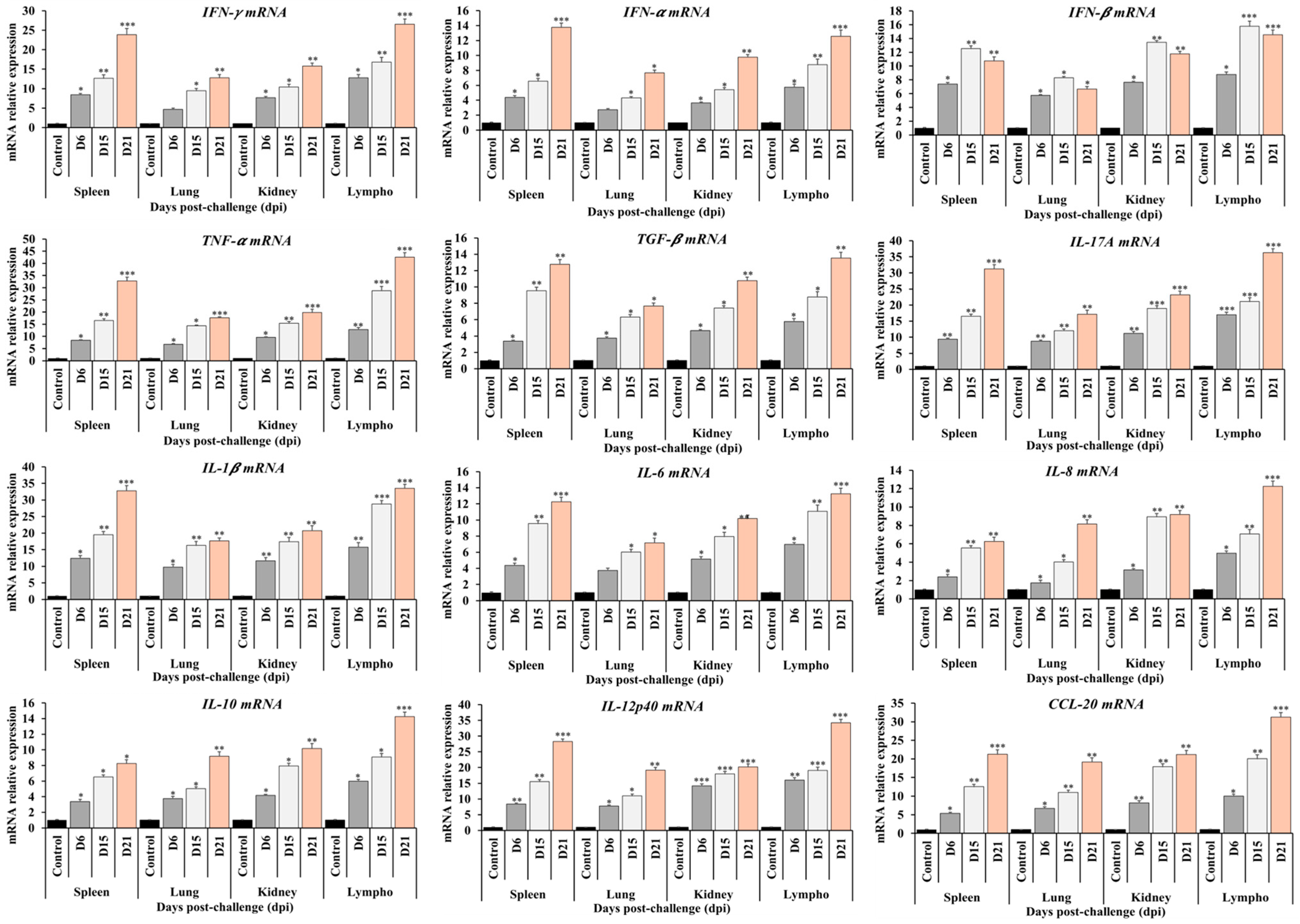
3.5. Cytokine mRNA Expression Profiles in Whole-Blood
3.6. Cytokine mRNA Expression Profiles in Tissues
3.7. Cytokine Assay Analysis
4. Discussion
5. Conclusions
6. Study Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PRRSV | Porcine reproductive and respiratory syndrome virus |
| HP-PRRSV | Highly pathogenic PRRS |
| LP | Low-pathogenic PRRSV |
| PAMs | Porcine alveolar macrophages |
| PBMCs | Peripheral blood mononuclear cells |
| RT-qPCR | Quantitative real-time reverse transcription PCR |
| ELISA | Enzyme-linked immunosorbent assay |
| RPMI | Roswell Park Memorial Institute |
| SVN | Serum virus-neutralizing |
| TNF-α | Tumor necrosis factor-alpha |
| mAbs | Monoclonal antibodies |
| RBCs | Red blood cells |
| TCID | Tissue culture infective dose |
| IFN | Interferon |
| IL | Interleukin |
| MHC | Major histocompatibility complex |
| CPE | Cytopathic effect |
| DMEM | Dulbecco’s modified Eagle’s medium |
| Dpc | Days post-challenge |
| FBS | Fetal bovine serum |
| ANOVA | Analysis of variance |
| SEM | Standard error of the mean |
| CCL | Chemokine (C-C motif) ligand |
| CXCR | C-X-C chemokine receptor |
References
- Done, S.H.; Paton, D.J.; White, M.E. Porcine reproductive and respiratory syndrome (PRRS): A review, with emphasis on pathological, virological and diagnostic aspects. Br. Vet. J. 1996, 152, 153–174. [Google Scholar] [CrossRef]
- Dea, S.; Gagnon, C.A.; Mardassi, H.; Pirzadeh, B.; Rogan, D. Current knowledge on the structural proteins of porcine reproductive and respiratory syndrome (PRRS) virus: Comparison of the North American and European isolates. Arch. Virol. 2000, 145, 659–688. [Google Scholar] [CrossRef]
- Amonsin, A.; Kedkovid, R.; Puranaveja, S.; Wongyanin, P.; Suradhat, S.; Thanawongnuwech, R. Comparative analysis of complete nucleotide sequence of porcine reproductive and respiratory syndrome virus (PRRSV) isolates in Thailand (US and EU genotypes). Virol. J. 2009, 6, 143. [Google Scholar] [CrossRef]
- Nguyen Tung, N.H.D.; Vui, D.T.; Tho, N.D.; Inui, K. Molecular Epidemiology of Highly Pathogenic PRRS in Vietnam in 2010. In Proceedings of the 5th Asian Pig Veterinary Society Congress, Pattaya, Thailand, 7–9 March 2011. [Google Scholar]
- WOAH. Chapter 3.9.6. Porcine reproductive and respiratory syndrome (infection with procine reproductive and respiratory syndrome virus). In Manual of Diagnostic Tests and Vaccines for Terrestrial Animals 2022; WOAH: Paris, France, 2022. [Google Scholar]
- Han, M.; Yoo, D. Engineering the PRRS virus genome: Updates and perspectives. Vet. Microbiol. 2014, 174, 279–295. [Google Scholar] [CrossRef]
- Feng, Y.; Zhao, T.; Nguyen, T.; Inui, K.; Ma, Y.; Nguyen, T.H.; Nguyen, V.C.; Liu, D.; Bui, Q.A.; To, L.T.; et al. Porcine respiratory and reproductive syndrome virus variants, Vietnam and China, 2007. Emerg. Infect. Dis. 2008, 14, 1774–1776. [Google Scholar] [CrossRef] [PubMed]
- Long, T.L. Kết quả chẩn đoán và nghiên cứu gây hội chứng rối loạn sinh sản và hô hấp trên lợn Việt Nam từ tháng 3/2007 đến 5/2008. Tạp Chí KHKT Thú Y 2008, 15, 5–13. [Google Scholar]
- Lee, H.S.; Pham, T.L.; Nguyen, T.N.; Lee, M.; Wieland, B. Seasonal patterns and space-time clustering of porcine reproductive and respiratory syndrome (PRRS) cases from 2008 to 2016 in Vietnam. Transbound. Emerg. Dis. 2019, 66, 986–994. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, L.; Xu, Z.; Deng, H.; Li, F.; Sun, X.; Zhou, Y.; Zhu, L. Emergence and spread of NADC34-like PRRSV in Southwest China. Transbound. Emerg. Dis. 2022, 69, e3416–e3424. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Ji, X.; Fu, C.; Hu, D.; Pang, H.; Wang, T.; Li, C.; Wang, G.; Peng, J. Evolution Characterization and Pathogenicity of a Porcine Reproductive and Respiratory Syndrome Virus Isolate from a Pig Farm in Shandong Province, China. Viruses 2022, 14, 1194. [Google Scholar] [CrossRef]
- Wu, Y.; Peng, O.; Xu, Q.; Li, Q.; Li, W.; Lin, L.; Zhou, Q.; Cai, X.; Hu, G.; He, Z.; et al. Characterization and Pathogenicity of Two Novel PRRSVs Recombined by NADC30-like and NADC34-like Strains in China. Viruses 2022, 14, 2174. [Google Scholar] [CrossRef]
- Do, D.T.; Park, C.; Choi, K.; Jeong, J.; Nguyen, T.T.; Nguyen, K.D.; Vo, D.T.; Chae, C. Comparison of two genetically distant type 2 porcine reproductive and respiratory syndrome virus (PRRSV) modified live vaccines against Vietnamese highly pathogenic PRRSV. Vet. Microbiol. 2015, 179, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.H.; Tran, H.A.T.; Nguyen, T.Q.; Nguyen, P.B.T.; Le, T.H.T.; Lai, D.C.; Nguyen, M.N. Phylogenetic analysis of porcine reproductive and respiratory syndrome virus in Vietnam, 2021. Virus Genes 2022, 58, 361–366. [Google Scholar] [CrossRef]
- TCVN 8400-21:2014; Animal Disease—Diagnostic Procedure—Part 21: Porcine Reproductive and Respiratory Syndrome (PRRS). Ministry of Agriculture and Rural Development (MARD) Vietnam: Hanoi, Vietnam.
- Kim, H.; Kim, H.; Jung, J.H.; Choi, Y.J.; Kim, J.; Um, C.G.; Hyun, S.B.; Shin, S.; Lee, B.; Jang, G.; et al. The assessment of efficacy of porcine reproductive respiratory syndrome virus inactivated vaccine based on the viral quantity and inactivation methods. Virol. J. 2011, 8, 323–327. [Google Scholar] [CrossRef]
- Zuckermann, F.A.; Garcia, E.A.; Luque, I.D.; Christopher-Hennings, J.; Doster, A.; Brito, M.; Osorio, F. Assessment of the efficacy of commercial porcine reproductive and respiratory syndrome virus (PRRSV) vaccines based on measurement of serologic response, frequency of gamma-IFN-producing cells and virological parameters of protection upon challenge. Vet. Microbiol. 2007, 123, 69–85. [Google Scholar] [CrossRef]
- Li, Z.; He, Y.; Xu, X.; Leng, X.; Li, S.; Wen, Y.; Wang, F.; Xia, M.; Cheng, S.; Wu, H. Pathological and immunological characteristics of piglets infected experimentally with a HP-PRRSV TJ strain. BMC Vet. Res. 2016, 12, 230. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Guan, Z.; Pang, L.; Ouyang, Y.; Jiang, Y.; Zhang, J.; Qiu, Y.; Li, Z.; Li, B.; Liu, K.; Shao, D.; et al. Secondary Highly Pathogenic Porcine Reproductive and Respiratory Syndrome Virus (HP-PRRSV2) Infection Augments Inflammatory Responses, Clinical Outcomes, and Pathogen Load in Glaesserella-parasuis-Infected Piglets. Vet. Sci. 2023, 10, 365. [Google Scholar] [CrossRef]
- Bloemraad, M.; de Kluijver, E.P.; Petersen, A.; Burkhardt, G.E.; Wensvoort, G. Porcine reproductive and respiratory syndrome: Temperature and pH stability of Lelystad virus and its survival in tissue specimens from viraemic pigs. Vet. Microbiol. 1994, 42, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Yang, H. Porcine reproductive and respiratory syndrome in China. Virus Res. 2010, 154, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ma, W.; Pan, Y.; Wang, X.; Wang, M.; Zhang, H.; Gao, J.; Zhang, H.; Tian, Z.; Li, C.; et al. Characterization of Rongchang piglets after infection with type 2 porcine reproductive and respiratory syndrome virus strains differing in pathogenicity. Front. Microbiol. 2023, 14, 1283039. [Google Scholar] [CrossRef]
- Brockmeier, S.L.; Loving, C.L.; Nelson, E.A.; Miller, L.C.; Nicholson, T.L.; Register, K.B.; Grubman, M.J.; Brough, D.E.; Kehrli, M.E., Jr. The presence of alpha interferon at the time of infection alters the innate and adaptive immune responses to porcine reproductive and respiratory syndrome virus. Clin. Vaccine Immunol. 2012, 19, 508–514. [Google Scholar] [CrossRef]
- Tian, K.; Yu, X.; Zhao, T.; Feng, Y.; Cao, Z.; Wang, C.; Hu, Y.; Chen, X.; Hu, D.; Tian, X.; et al. Emergence of fatal PRRSV variants: Unparalleled outbreaks of atypical PRRS in China and molecular dissection of the unique hallmark. PLoS ONE 2007, 2, e526. [Google Scholar] [CrossRef] [PubMed]
- Diaz, I.; Darwich, L.; Pappaterra, G.; Pujols, J.; Mateu, E. Different European-type vaccines against porcine reproductive and respiratory syndrome virus have different immunological properties and confer different protection to pigs. Virology 2006, 351, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Rossow, K.D.; Morrison, R.B.; Goyal, S.M.; Singh, G.S.; Collins, J.E. Lymph node lesions in neonatal pigs congenitally exposed to porcine reproductive and respiratory syndrome virus. J. Vet. Diagn. Investig. 1994, 6, 368–371. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Fuertes, L.; Campos, E.; Domenech, N.; Ezquerra, A.; Castro, J.M.; Dominguez, J.; Alonso, F. Porcine reproductive and respiratory syndrome (PRRS) virus down-modulates TNF-alpha production in infected macrophages. Virus Res. 2000, 69, 41–46. [Google Scholar] [CrossRef]
- Lopez-Lorenzo, G.; Prieto, A.; Diaz-Cao, J.M.; Lopez-Novo, C.; Garcia-Dios, D.; Lopez, C.; Panadero, R.; Iglesias, A.; Díez-Baños, P.; Fernández, G. Evaluation of the efficacy of two postweaning colibacillosis vaccines in a field herd with PRRS circulation during postweaning stage. Vet. Microbiol. 2023, 285, 109870. [Google Scholar] [CrossRef]
- Galliher-Beckley, A.; Li, X.; Bates, J.T.; Madera, R.; Waters, A.; Nietfeld, J.; Henningson, J.; He, D.; Feng, W.; Chen, R.; et al. Pigs immunized with Chinese highly pathogenic PRRS virus modified live vaccine are protected from challenge with North American PRRSV strain NADC-20. Vaccine 2015, 33, 3518–3525. [Google Scholar] [CrossRef]
- Zhang, X.; Paget, M.; Wang, C.; Zhu, Z.; Zheng, H. Innate immune evasion by picornaviruses. Eur. J. Immunol. 2020, 50, 1268–1282. [Google Scholar] [CrossRef]
- Rajkhowa, T.K.; Jagan Mohanarao, G.; Gogoi, A.; Hauhnar, L.; Isaac, L. Porcine reproductive and respiratory syndrome virus (PRRSV) from the first outbreak of India shows close relationship with the highly pathogenic variant of China. Vet. Q. 2015, 35, 186–193. [Google Scholar] [CrossRef]
- Wu, J.; Li, J.; Tian, F.; Ren, S.; Yu, M.; Chen, J.; Lan, Z.; Zhang, X.; Yoo, D.; Wang, J. Genetic variation and pathogenicity of highly virulent porcine reproductive and respiratory syndrome virus emerging in China. Arch. Virol. 2009, 154, 1589–1597. [Google Scholar] [CrossRef]
- Kiang, J.G.; Woods, A.K.; Cannon, G. Effects of Hemorrhage on Hematopoietic Cell Depletion after a Combined Injury with Radiation: Role of White Blood Cells and Red Blood Cells as Biomarkers. Int. J. Mol. Sci. 2024, 25, 2988. [Google Scholar] [CrossRef]
- Thomas, T.A.; Francis, R.O.; Zimring, J.C.; Kao, J.P.; Nemkov, T.; Spitalnik, S.L. The Role of Ergothioneine in Red Blood Cell Biology: A review and perspective. Antioxidants 2024, 13, 717. [Google Scholar] [CrossRef]
- Mulas, O.; Mola, B.; Madeddu, C.; Caocci, G.; Maccio, A.; Nasa, G. Prognostic Role of Cell Blood Count in Chronic Myeloid Neoplasm and Acute Myeloid Leukemia and Its Possible Implications in Hematopoietic Stem Cell Transplantation. Diagnostics 2022, 12, 2493. [Google Scholar] [CrossRef]
- Lyu, X.; Xiong, Y.; Jiang, L.; Qin, F. The independent predictive role of platelet to white blood cell ratio on all-cause mortality: A 7-year nationwide follow-up study in China. Int. J. Surg. 2024, 110, 5923–5925. [Google Scholar] [CrossRef]
- Morgan, S.B.; Frossard, J.P.; Pallares, F.J.; Gough, J.; Stadejek, T.; Graham, S.P.; Steinbach, F.; Drew, T.W.; Salguero, F.J. Pathology and Virus Distribution in the Lung and Lymphoid Tissues of Pigs Experimentally Inoculated with Three Distinct Type 1 PRRS Virus Isolates of Varying Pathogenicity. Transbound. Emerg. Dis. 2016, 63, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Tornimbene, B.; Frossard, J.P.; Chhim, V.; Sorn, S.; Guitian, J.; Drew, T.W. Emergence of highly pathogenic porcine reproductive and respiratory syndrome (HP-PRRS) in medium-scale swine farms in southeastern Cambodia. Prev. Vet. Med. 2015, 118, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Karakhanova, S.; Mosl, B.; Harig, S.; von Ahn, K.; Fritz, J.; Schmidt, J.; Jäger, D.; Werner, J.; Bazhin, A.V. Influence of interferon-alpha combined with chemo (radio) therapy on immunological parameters in pancreatic adenocarcinoma. Int. J. Mol. Sci. 2014, 15, 4104–4125. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.W.; de Waal Malefyt, R.; Coffman, R.L.; O’Garra, A. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 2001, 19, 683–765. [Google Scholar] [CrossRef]
- Marcal, P.H.F.; Gama, R.S.; Pereira de Oliveira, L.B.; Martins-Filho, O.A.; Pinheiro, R.O.; Sarno, E.N.; Moraes, M.O.; Fraga, L.A.d.O. Functional biomarker signatures of circulating T-cells and its association with distinct clinical status of leprosy patients and their respective household contacts. Infect. Dis. Poverty 2020, 9, 167. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, Y.; Luo, J.; Ding, H.; Liu, S.; Amer, S.; Xie, L.; Lyv, W.; Su, W.; Li, M.; et al. Identification of miRNomes reveals ssc-miR-30d-R_1 as a potential therapeutic target for PRRS viral infection. Sci. Rep. 2016, 6, 24854. [Google Scholar] [CrossRef]
- Deng, Z.; Fan, T.; Xiao, C.; Tian, H.; Zheng, Y.; Li, C.; He, J. TGF-beta signaling in health, disease, and therapeutics. Signal Transduct. Target. Ther. 2024, 9, 61. [Google Scholar] [CrossRef] [PubMed]
- Tie, Y.; Tang, F.; Peng, D.; Zhang, Y.; Shi, H. TGF-beta signal transduction: Biology, function and therapy for diseases. Mol. Biomed. 2022, 3, 45. [Google Scholar] [CrossRef]
- Zhao, L.; Xia, J.; Wang, X.; Xu, F. Transcriptional regulation of CCL20 expression. Microbes Infect. 2014, 16, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Kwantwi, L.B.; Wang, S.; Sheng, Y.; Wu, Q. Multifaceted roles of CCL20 (C-C motif chemokine ligand 20): Mechanisms and communication networks in breast cancer progression. Bioengineered 2021, 12, 6923–6934. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Li, Y.; He, C.; Liu, X.; Lv, C.; Liu, K.; Yu, X.; Zhao, M. Sequence analysis of the GP5 protein of porcine reproductive and respiratory syndrome virus in Vietnam from 2007 to 2023. Front. Microbiol. 2024, 15, 1475208. [Google Scholar] [CrossRef]
- Cui, X.; Xia, D.; Huang, X.; Sun, Y.; Shi, M.; Zhang, J.; Li, G.; Yang, Y.; Wang, H.; Cai, X.; et al. Analysis of Recombinant Characteristics Based on 949 PRRSV-2 Genomic Sequences Obtained from 1991 to 2021 Shows That Viral Multiplication Ability Contributes to Dominant Recombination. Microbiol. Spectr. 2022, 10, e02934-22. [Google Scholar] [CrossRef]



| Category of Clinical Sign | Symptom | Score |
|---|---|---|
| Systemic: | Normal | 0 |
| Apathy | 1 | |
| Anorexia | 2 | |
| Severe depression | 5 | |
| Death | 10 | |
| Respiratory: | Normal | 0 |
| Sneezing, Coughing | 1 | |
| Tachypnea | 2 | |
| Labored breathing | 3 | |
| Abdominal breathing | 5 | |
| Other symptoms: | Normal | 0 |
| Diarrhea | 1 | |
| Conjunctivitis | 2 | |
| Reddening of the ears | 3 | |
| Skin cyanopathy | 5 |
| Genes | Oligonucleotide Sequences (5′-3′) | GenBank Acc. No |
|---|---|---|
| IL-1β | F: TGAAGAGAGAAGTGGTGTTCTGC | AJ747049 |
| R: GGTACAGATTCTTTCCCTTGATCC | ||
| IL-6 | F: GCATCCACTTCCAGGCCA | AF518322.1 |
| R: CTTCCTCATCTTCATCGTCA | ||
| IL-8 | F: TGCACTTACTCTTGCCAGAACTG | NM_213867.1 |
| R: CAAACTGGCTGTTGCCTTCTT | ||
| IL-10 | F: GCCTTCGGCCCAGTGAA | NM_214041.1 |
| R: AGAGACCCGGTCAGCAACAA | ||
| IL-12p40 | F: GATGCTGGCCAGTACACC | U08317 |
| R: TCCAGCACGACCTCAATG | ||
| IL-17A | F: CAAGCGGTGGCGTTTTGCCT | NM_001005729.1 |
| R: GTCTCCGTCGGGGATGGGCT | ||
| IFN-α | F: CCCCTGTGCCTGGGAGAT | XM_003480507.1 |
| R: AGGTTTCTGGAGGAAGAGAAGGA | ||
| IFN-β | F: AGTTGCCTGGGACTCCTCAA | GQ415073.1 |
| R: CCTCAGGGACCTCAAAGTTCAT | ||
| IFN-γ | F: CAGCTTTGCGTGACTTTGTG | X53085 |
| R: GATGAGTTCACTGATGGCTTT | ||
| TNF-α | F: CGTTGTAGCCAATGTCAAAGCC | X54859 |
| R: TGCCCAGATTCAGCAAAGTCCA | ||
| TGF-β | F: GAACAAACTCTTGGGCAATG | NM_214015.2 |
| R: ACTTCCAGCTTGTCACCTTG | ||
| CCL-20 | F: TGCTCCTGGCTGCTTTGATGTC | NM_001024589 |
| R: TCATTGGCGAGCTGCTGTGTG | ||
| β-Actin | F: CTGGCATTGTCATGGACTCT | U07786 |
| R: GCGATGATCTTGATCTTCAT | ||
| PRRS Realtime RT-PCR | F: ATG ATG RGC TGG CAT TCT | WOAH/TCVN |
| R: ACA CGG TCG CCC TAA TTG | ||
| FAM-TGT GGT GAA TGG CAC TGA TTG ACA-BHQ1 |
| Items | Reference | Day Post-Infection (dpi) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rank | Unit | D0 | D3 | D6 | D9 | D12 | D15 | D18 | D21 | |||
| White Blood cells | WBC | 10.20–30.00 | 109/L | Control | 18.17 ± 3.73 | 17.45 ± 1.61 | 20.97 ± 3.39 | 21.38 ± 1.95 | 18.16 ± 2.43 | 18.75 ± 1.80 | 18.35 ± 1.75 | 19.10 ± 2.68 |
| PRRS | 17.63 ± 2.57 | 14.58 ± 2.18 | 10.99 ± 2.79 | 15.84 ± 2.55 | 22.94 ± 2.84 | 28.71 ± 3.24 | 29.71 ± 0.27 | 30.71 ± 2.75 | ||||
| Red blood cells | RBC | 5.50–9.00 | 1012/L | Control | 7.04 ± 0.74 | 7.19 ± 0.38 | 6.93 ± 0.48 | 7.11 ± 0.53 | 7.02 ± 0.53 | 7.21 ± 0.36 | 7.20 ± 0.53 | 7.22 ± 0.65 |
| PRRS | 7.01 ± 0.69 | 6.51 ± 0.47 | 6.54 ± 0.58 | 5.56 ± 0.38 | 6.60 ± 0.39 | 7.04 ± 0.44 | 7.28 ± 0.33 | 7.04 ± 0.65 | ||||
| Hemoglobin | HGB | 100–160 | g/L | Control | 120.20 ±8.23 | 122.00 ± 5.31 | 124.25 ± 6.63 | 121.50 ± 7.63 | 120.00 ± 6.29 | 120.60 ± 6.50 | 121.20 ± 8.25 | 121.00 ± 7.67 |
| PRRS | 123.86 ± 9.74 | 121.49 ± 7.65 | 105.20 ± 6.02 | 96 ± 1.85 | 101.00 ± 2.06 | 110 ± 1.70 | 118 ± 2.00 | 115.00 ± 7.65 | ||||
| Hematocrit | HCT | 0.330–0.520 | Control | 0.36 ± 0.03 | 0.36 ± 0.02 | 0.38 ± 0.02 | 0.38 ± 0.02 | 0.40 ± 0.01 | 0.39 ± 0.00 | 0.39 ± 0.00 | 0.38 ± 0.00 | |
| PRRS | 0.38 ± 0.00 | 0.32 ± 0.02 | 0.30 ± 0.03 | 0.283 ± 0.02 | 0.31 ± 0.02 | 0.31 ± 0.02 | 0.319 ± 0.02 | 0.31 ± 0.02 | ||||
| Mean Corpuscular Volume | MCV | 51.0–73.0 | fL | Control | 52.60 ± 3.13 | 53.88 ± 2.27 | 53.98 ± 3.42 | 52.83 ± 2.10 | 54.98 ± 1.81 | 54.74 ± 2.44 | 54.10 ± 2.28 | 52.96 ± 2.45 |
| PRRS | 53.23 ± 2.25 | 52.34 ± 3.59 | 51.11 ± 3.48 | 51.22 ± 2.95 | 52.43 ± 3.27 | 53.25 ± 3.76 | 53.56 ± 2.90 | 52.70 ± 3.13 | ||||
| Mean Corpuscular Hemoglobin | MCH | 14.0–22.0 | pg | Control | 16.32 ± 0.77 | 16.62 ± 0.86 | 17.13 ± 0.78 | 16.75 ± 0.35 | 16.20 ± 0.33 | 16.18 ± 0.52 | 16.08 ± 0.45 | 16.76 ± 0.71 |
| PRRS | 16.19 ± 0.65 | 16.06 ± 0.81 | 16.06 ± 0.53 | 16.4 ± 0.59 | 15.60 ± 0.48 | 16.4 ± 0.26 | 15.5 ± 0.25 | 16.40 ± 0.66 | ||||
| Mean Corpuscular Hemoglobin concentration | MCHC | 300–360 | g/L | Control | 322.12 ±12.36 | 323.02 ± 15.48 | 331.28 ± 19.03 | 332.48 ± 10.32 | 323.84 ± 9.23 | 318.72 ± 8.90 | 321.88 ± 4.71 | 316.80 ± 2.86 |
| PRRS | 317.77 ± 10.13 | 318.54 ± 12.56 | 326.66 ± 12.37 | 312 ± 16.01 | 282.00 ± 17.57 | 312 ± 16.69 | 285 ± 19.50 | 312.00 ± 13.37 | ||||
| Platelet | PLT | 200–1000 | 109/L | Control | 245.00 ±36.09 | 236.40 ± 32.67 | 288.75 ± 22.21 | 306.25 ± 78.60 | 271.00 ± 76.15 | 259.40 ± 69.28 | 269.40 ± 56.60 | 253.80 ± 42.44 |
| PRRS | 253.81 ± 44.39 | 218.60 ± 33.88 | 203.40 ± 61.01 | 198 ± 36.41 | 213.00 ± 40.55 | 229 ± 26.40 | 234 ± 12.00 | 229.00 ± 47.38 | ||||
| Neutrophil | Neu | 2.80–16.10 | 109/L | Control | 5.18 ± 0.67 | 5.15 ± 1.89 | 4.96 ± 1.47 | 4.93 ± 2.00 | 4.86 ± 1.10 | 4.80 ± 0.67 | 4.28 ± 0.80 | 5.34 ± 0.77 |
| PRRS | 6.04 ± 0.69 | 9.23 ± 1.87 | 9.56 ± 0.72 | 8.34 ± 1.79 | 6.35 ± 1.31 | 5.87 ± 1.27 | 5.28 ± 1.34 | 5.23 ± 1.64 | ||||
| Lymphocyte | Lym | 4.80–16.20 | 109/L | Control | 11.92 ± 2.09 | 12.10 ± 2.22 | 12.09 ± 1.66 | 12.15 ± 1.69 | 10.86 ± 1.67 | 11.29 ± 1.92 | 11.94 ± 2.37 | 11.84 ± 1.84 |
| PRRS | 12.26 ± 1.67 | 10.17 ± 1.78 | 9.78 ± 1.92 | 8.65 ± 1.71 | 12.04 ± 1.83 | 13.46 ± 2.11 | 14.1 ± 2.28 | 14.46 ± 1.86 | ||||
| Monocytes | Mon | 0.20–2.25 | 109/L | Control | 1.44 ± 0.42 | 1.30 ± 0.25 | 1.46 ± 0.42 | 1.33 ± 0.36 | 1.36 ± 0.19 | 1.50 ± 0.19 | 1.34 ± 0.07 | 1.48 ± 0.07 |
| PRRS | 1.62 ± 0.42 | 1.92 ± 0.44 | 2.01 ± 0.41 | 1.74 ± 0.20 | 1.79 ± 0.22 | 1.7 ± 0.09 | 1.86 ± 0.11 | 1.70 ± 0.43 | ||||
| Eosinophil | Eos | 0.00–1.80 | 109/L | Control | 0.19 ± 0.10 | 0.20 ± 0.11 | 0.22 ± 0.17 | 0.22 ± 0.10 | 0.24 ± 0.12 | 0.22 ± 0.12 | 0.21 ± 0.12 | 0.20 ± 0.15 |
| PRRS | 0.26 ± 0.13 | 0.78 ± 0.10 | 0.84 ± 0.16 | 1.13 ± 0.10 | 1.22 ± 0.08 | 1.12 ± 0.09 | 1.19 ± 0.00 | 1.06 ± 0.14 | ||||
| Basophil | Bas | 0.00–0.46 | 109/L | Control | 0.29 ± 0.07 | 0.31 ± 0.04 | 0.29 ± 0.06 | 0.27 ± 0.07 | 0.29 ± 0.06 | 0.26 ± 0.05 | 0.26 ± 0.04 | 0.26 ± 0.04 |
| PRRS | 0.31 ± 0.04 | 0.35 ± 0.05 | 0.39 ± 0.06 | 0.33 ± 0.04 | 0.30 ± 0.05 | 0.298 ± 0.05 | 0.29 ± 0.01 | 0.31 ± 0.05 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dang, H.V.; Truong, A.D.; Chu, N.T.; Phan, H.T.; Nguyen, H.M.; Dam, Q.K.; Nguyen, L.P.; Le, K.V.; Vu, H.T.; Vo, L.T.H.; et al. Evaluation of Pathogenetic and Immunological Properties of a Vietnamese Isolate of Porcine Reproductive and Respiratory Syndrome Virus of Vietnam in Experimentally Infected Piglets. Vet. Sci. 2025, 12, 1084. https://doi.org/10.3390/vetsci12111084
Dang HV, Truong AD, Chu NT, Phan HT, Nguyen HM, Dam QK, Nguyen LP, Le KV, Vu HT, Vo LTH, et al. Evaluation of Pathogenetic and Immunological Properties of a Vietnamese Isolate of Porcine Reproductive and Respiratory Syndrome Virus of Vietnam in Experimentally Infected Piglets. Veterinary Sciences. 2025; 12(11):1084. https://doi.org/10.3390/vetsci12111084
Chicago/Turabian StyleDang, Hiep Van, Anh Duc Truong, Nhu Thi Chu, Hoai Thi Phan, Hieu Minh Nguyen, Quoc Khanh Dam, Linh Phuong Nguyen, Kien Van Le, Hao Thi Vu, Le Thi Hai Vo, and et al. 2025. "Evaluation of Pathogenetic and Immunological Properties of a Vietnamese Isolate of Porcine Reproductive and Respiratory Syndrome Virus of Vietnam in Experimentally Infected Piglets" Veterinary Sciences 12, no. 11: 1084. https://doi.org/10.3390/vetsci12111084
APA StyleDang, H. V., Truong, A. D., Chu, N. T., Phan, H. T., Nguyen, H. M., Dam, Q. K., Nguyen, L. P., Le, K. V., Vu, H. T., Vo, L. T. H., Nguyen, T. D., Tran, H. T. T., & Dang, H. V. (2025). Evaluation of Pathogenetic and Immunological Properties of a Vietnamese Isolate of Porcine Reproductive and Respiratory Syndrome Virus of Vietnam in Experimentally Infected Piglets. Veterinary Sciences, 12(11), 1084. https://doi.org/10.3390/vetsci12111084






