Simple Summary
This review explores how the Chlorella vulgaris microalga affects broiler chickens, specifically their growth performance and blood health markers. Our analysis shows that a cumulative intake of approximately 20 g per bird improved growth and feed efficiency, with benefits peaking at this level before levelling off. This amount also enhanced plasma health markers, suggesting that C. vulgaris can support overall broiler health. However, exceeding 20 g diminished these benefits, emphasising the need to determine the optimal intake levels of C. vulgaris.
Abstract
This systematic review examines the effects of cumulative Chlorella vulgaris intake levels on broiler chickens, focusing on growth performance and systemic health markers. The review establishes a clear relationship between cumulative C. vulgaris intake and significant outcomes in poultry nutrition and health through a detailed analysis of various studies. The correlation analysis revealed that cumulative C. vulgaris intake levels ranging from 0.8 to 718 g/bird influenced growth rates and feed efficiency, following sigmoid models. Specifically, intakes of approximately 20 g/bird maximized final body weight (R2 = 0.616, p < 0.001), cumulative body weight gain (R2 = 0.627, p < 0.001) and daily weight gain (R2 = 0.639, p < 0.001). The feed conversion ratio also improved with increasing C. vulgaris intakes up to this level, although this was non-significant (R2 = 0.289, p = 0.117). In addition, similar cumulative C. vulgaris intake levels impacted plasma health markers in broilers, leading to reductions in triacylglycerols and cholesterol and improvements in immunoglobulin levels. These findings underscore the importance of carefully calibrated C. vulgaris supplementation strategies to optimise poultry growth and health without adverse effects. Future research should focus on refining C. vulgaris dosing guidelines and further exploring its long-term effects and mechanisms of action to enhance poultry health and production sustainability.
1. Introduction
The world’s population is increasing and is estimated to reach 9.7 billion by 2050 [1]. As such, the search for sustainable and health-enhancing livestock feed has become increasingly urgent, with a growing emphasis on natural additives and feedstocks that improve the well-being and productivity of farm animals [2,3]. In this context, Chlorella vulgaris, a protein-rich microalga, has garnered significant attention as a potent feed supplement in poultry diets. Its potential to substantially influence the growth performance and health status of broiler chickens, in addition to its nutritional benefits, is an area of extensive research and interest [4,5].
C. vulgaris is valued for its high protein content and presence of vitamins, minerals and essential fatty acids. These nutritional components improve growth rates and enhance immune responses, leading to better overall health in broilers. Studies have shown that incorporating C. vulgaris into poultry diets can significantly improve feed conversion ratios (FCR), carcass quality and various health markers [4,6,7]. As such, C. vulgaris represents a promising alternative to conventional feed additives, aligning with the growing demand for more sustainable and health-promoting livestock feed options.
The unicellular nature of C. vulgaris makes it highly valued for its rich nutritional composition, which includes essential nutrients like proteins, vitamins, minerals and bioactive compounds such as chlorophylls, carotenoids and omega-3 (n−3) fatty acids [8,9,10,11,12]. Additionally, C. vulgaris has a favourable essential amino acid composition [13]. These components not only serve as nutritional supplements but also significantly enhance the growth and health of broilers. Chlorophylls, for instance, play crucial roles in detoxification processes, while carotenoids, including beta-carotene, contribute to immune function and visual health. The presence of omega-3 fatty acids in C. vulgaris also supports cardiovascular health and reduces inflammation, further promoting the overall well-being of broilers [4].
Studies have demonstrated that C. vulgaris supplementation leads to marked improvements in broiler growth performance and feed efficiency. C. vulgaris’s high protein content and balanced amino acid profile contribute to muscle development and weight gain. For example, the dietary inclusion of C. vulgaris improved broilers’ final body weights and FCRs, indicating a more efficient conversion of feed into body mass [14]. Additionally, C. vulgaris supplementation has been shown to enhance the nutritional quality of meat by increasing the levels of beneficial fatty acids and reducing harmful lipid oxidation, thereby improving meat quality [3,15,16] and consumer acceptability.
In addition to their substantial protein contents, microalgae are rich in a variety of bioactive compounds, including polysaccharides, polyphenols and pigments [17]. C. vulgaris, in particular, demonstrates potent immune-enhancing properties. The bioactive compounds in C. vulgaris, such as beta-glucans and other polysaccharides, modulate the immune system, enhancing both innate and adaptive immunity in broilers, leading to increased antibody production, improved disease resistance and overall health [8,11,18,19]. For instance, supplementation with C. vulgaris has been associated with increased levels of immunoglobulin IgA, IgM and IgG, which are critical for immune defence. Moreover, the antioxidant properties of C. vulgaris’s bioactive compounds [20] are effective in reducing oxidative stress, thereby protecting broiler cells from damage and supporting healthy physiological functions [6,7]. This positions C. vulgaris as a valuable addition to broiler diets, contributing to productivity and animal welfare.
However, integrating C. vulgaris into broiler diets presents several challenges, particularly concerning the appropriate levels of inclusion, the duration of feeding and cell wall indigestibility for monogastric animals like broilers. These factors are critical in determining the overall impact on growth performance and health. While certain levels of C. vulgaris supplementation have shown promise in enhancing growth performance, variations in dosage and feeding duration can produce different outcomes in terms of health benefits and physiological responses. For instance, studies have indicated that low inclusion levels (up to 2% of the diet) can improve FCR without negatively affecting growth, while higher levels might not provide additional benefits or could potentially lead to adverse effects [6,16].
One significant challenge is the cell wall of C. vulgaris, which is highly resistant to digestion in monogastrics due to its rigid structure composed of sporopollenin-like biopolymers. This indigestibility can limit the bioavailability of nutrients contained within this microalga, reducing its effectiveness as a feed supplement. Techniques such as mechanical disruption, enzymatic treatment, fermentation or a pulse-electric field are often required to break down its cell walls and enhance its nutrient availability, adding to the complexity and cost of using C. vulgaris in broiler diets [4,21,22,23,24].
The main aim of this review was to systematically assess and synthesise existing scientific literature from databases such as Google Scholar (Google LLC, Mountain View, CA, USA), PubMed (NCBI, Bethesda, MD, USA), Scopus (Elsevier B.V., Amsterdam, The Netherlands) and Web of Science (Clarivate Analytics, Philadelphia, PA, USA). The goal was to determine the dose–response relationship between various cumulative levels of C. vulgaris intake and its impact on key performance parameters and health markers in broiler chickens. Cumulative microalga intake was calculated by multiplying the total feed consumed by a bird by the proportion of C. vulgaris in its diet. We hypothesised that the observed effects resulted from the unique transfer kinetics of C. vulgaris’s bioactive compounds to the birds. This review aimed to identify the optimal C. vulgaris dosage ranges that maximised growth performance and health benefits in broiler chickens. It also aimed to highlight any potential thresholds or limits beyond which C. vulgaris incorporation could lead to diminishing returns or adverse effects on these parameters.
2. Impact of Cumulative Chlorella vulgaris Intake Levels on the Growth Performance of Broilers
Table 1 summarizes the data from various studies that examined the effects of different cumulative intake levels of C. vulgaris on broiler growth performance. An analysis of the nutritional profile of C. vulgaris was previously performed [25]. C. vulgaris, known for its rich nutritional profile, has been the subject of numerous studies aiming to quantify its benefits on broiler performance [25]. These studies have systematically investigated C. vulgaris’s effects, considering variables such as the ages and initial weights of the broilers, the percentage of microalgae in their diets, the durations of the supplementation periods and the cumulative intakes alongside critical growth performance indicators. The cumulative intake levels and their impacts varied across the studies, providing a broad perspective on the effects of this microalga. The data show varying cumulative C. vulgaris intake levels, ranging from 0.8 g/bird [26] to 718 g/bird [6], with associated changes in the birds’ growth outcomes.
In one study, the effects of a 1.40 g cumulative C. vulgaris intake per bird over 34 days were investigated, observing a final body weight of 1533 g, a cumulative body weight gain of 1488 g, and a daily body weight gain of 43.8 g, with an FCR of 1.88 [27], and all of these effects were not significantly different from the control treatment. In another part of this study, with a cumulative intake of 4.27 g over the same period, the final body weight was 1619 g, with similar body weight gain patterns and an FCR of 1.81 [27]. Another study using a cumulative intake of 14.13 g showed a final body weight of 1643 g and an FCR of 1.78, indicating a slight improvement in feed efficiency with increased C. vulgaris levels [27]. In these two studies, the authors presented final body weights and feed conversion ratios that were better than the controls. Another study reported on the effects of a 3.52 g cumulative C. vulgaris intake over 31 days, noting a final body weight of 1990 g and a body weight gain of 1916 g, with an FCR of 1.84 [28]. In other studies, higher intake levels of C. vulgaris, such as 20.0 g over 41 days, resulted in a final body weight of 2166 g and an FCR of 1.571, with a dressing percentage of 71.69% and a breast-meat water-holding capacity of 88.33% [29]. In contrast, the control treatment achieved a final body weight of 1791 g, with an FCR of 1.784, indicating significant improvements in growth performance at these higher intake levels of C. vulgaris. Additionally, studies have examined even higher cumulative C. vulgaris intake levels, such as 401 g, 561 g and 718 g over 34 days [6]. They reported final body weights of 2819 g, 2587 g and 2342 g, respectively. The FCRs were 1.5, 1.53 and 1.61, respectively, indicating that while higher C. vulgaris intake levels could promote growth, the efficiency gains might diminish at very high inclusion rates.

Table 1.
Impact of cumulative Chlorella vulgaris intake levels on the growth performance of broilers.
Table 1.
Impact of cumulative Chlorella vulgaris intake levels on the growth performance of broilers.
| Initial Age and Weight | Alga Level (% Feed) and Duration of Trial (Days) 1 | Cumulative Alga Intake (g/bird) 2 | Growth Performance | Reference | |||
|---|---|---|---|---|---|---|---|
| Final Body Weight (g) | Cumulative Body Weight Gain (g) | Body Weight Gain (g/d) | Feed Conversion Ratio | ||||
| 39.43 g, 1 d-old 3 | 0.50%, 9 d | 0.800 | 190.3 | 150.9 | 16.8 | 1.12 | [26] |
| 45.1 g, 1 d-old 3 | 0.05%, 34 d | 1.40 | 1533 | 1488 | 43.8 | 1.88 | [27] |
| 1 d-old 3 | 0.07%, 41 d | 2.95 | - | 2723.1 | 66.4 | 1.55 | [30] |
| 72.56 g, 4 d-old 3,4 | 0.10%, 31 d | 3.52 | 1990 | 1916 | 61.8 | 1.84 | [28] |
| 45.1 g, 1 d-old 3 | 0.15%, 34 d | 4.27 | 1619 | 1574 | 46.3 | 1.81 | [27] |
| 40.03 g, 1 d-old | 0.10%, 41 d | 4.35 | 2501.3 | 2461.3 | 60 | 1.77 | [31] |
| 1 d-old 3 | 0.14%, 41 d | 5.94 | - | 2755 | 67.2 | 1.54 | [30] |
| 41.8 g, 1 d-old | 0.20%, 41 d | 6.71 | 2001 | 1959 | 47.8 | 1.713 | [29] |
| 40.03 g, 1 d-old | 0.20%, 41 d | 8.73 | 2520.8 | 2480.8 | 60.5 | 1.76 | [31] |
| 1 d-old 3 | 0.21%, 41 d | 9.22 | - | 2850.8 | 69.5 | 1.54 | [30] |
| 41.8 g, 1 d-old | 0.40%, 41 d | 13.0 | 2077 | 2035 | 49.6 | 1.602 | [29] |
| 45.1 g, 1 d-old 3 | 0.50%, 34 d | 14.1 | 1643 | 1598 | 47 | 1.78 | [27] |
| 41.8 g, 1 d-old | 0.60%, 41 d | 20.0 | 2166 | 2124 | 51.8 | 1.571 | [29] |
| 1 d-old 3 | 1.0%, 34 d | 24.4 | - | 1603 | 47.1 | 1.52 | [32] |
| 1 d-old 3,5 | 1.0%, 34 d | 25.2 | - | 1647 | 48.4 | 1.53 | [32] |
| 47.1 g, 1 d-old 3 | 0.80%, 34 d | 28.9 | 2606.3 | 2559 | 73.1 | 1.45 | [33] |
| 788 g, 21 d-old 3 | 10%, 14 d | 176 | 1928 | 1140 | 81.4 | 1.54 | [15] |
| 107 g, 5 d-old 3 | 10%, 34 d | 401 | 2819 | 2712 | 77.49 | 1.5 | [6] |
| 109 g, 5 d-old 3 | 15%, 34 d | 561 | 2587 | 2478 | 70.8 | 1.53 | [6] |
| 106 g, 5 d-old 4 | 20%, 34 d | 718 | 2342 | 2236 | 63.87 | 1.61 | [6] |
1 The final day of the trial, which involved slaughtering, was not included in the trial’s duration. 2 Calculated by multiplying the total feed consumed per animal during the experimental period by the dietary percentage of microalga. For some of the studies, no information about the cumulative feed intake was available, and therefore, an estimation of this was completed as follows: cumulative feed intake (g/bird) (An et al. [27] and Roques et al. [33]) = CFI (g/d/bird) × number of trial days; cumulative feed intake (g/bird) (Alfaia et al. [15]) = cumulative feed intake (g/d/pen) × number of trial days/number of birds; cumulative feed intake (g/bird) (Cabrol et al. [6]) = cumulative feed intake (g/pen)/number of birds; and cumulative feed intake (g/bird) (Rezvani et al. [30]) = cumulative body weight gain (g) × feed conversion ratio. 3 Male broilers. 4 Female broilers. 5 In this trial, fresh liquid Chlorella vulgaris was used.
Table 2 summarises the correlation analysis for predicting the dependent performance variables based on cumulative C. vulgaris intake. Only variables with three or more degrees of freedom (dof) were analysed for correlation, ensuring the reliability of the statistical analysis. Data analysis for the correlations was conducted using SPSS software (version 29.0, 2024), employing various regression and curve estimation techniques. These included linear, logarithmic, inverse, quadratic, cubic, compound, power, sigmoid, growth, exponential and logistic models. The analysis focused on cumulative C. vulgaris intake as the independent variable that influenced the growth performance metrics.

Table 2.
Summary of the correlation analysis for predicting the dependent performance variables based on cumulative Chlorella vulgaris intake.
For growth performance, the final body weights of the broilers showed a strong correlation with the cumulative C. vulgaris intake. The sigmoid model (R2 = 0.616, dof = 18, p < 0.001) suggested a threshold effect where the body weights increased sharply up to a certain intake level before plateauing. This indicated that the optimal cumulative C. vulgaris intake for maximising final body weight was approximately 20 g/bird. Similarly, cumulative body weight gain followed a sigmoid pattern (R2 = 0.627, dof = 18, p < 0.001), indicating rapid weight gain up to a certain point of C. vulgaris intake, beyond which the gains plateaued. This suggested that the optimal cumulative intake for maximum body weight gain was also approximately 20 g/bird. Daily weight gain also showed a strong correlation with C. vulgaris intake, following a sigmoid model (R2 = 0.639, dof = 18, p < 0.001). This supported the threshold effect observed in other growth performance metrics, indicating that the daily weight gain optimised at a specific cumulative intake level of approximately 20 g/bird before levelling off. The FCR, a key indicator of broiler production efficiency, exhibited a lower sigmoid correlation (R2 = 0.289, dof = 18, p = 0.014), indicating that feed efficiency improved with increasing C. vulgaris intake up to a specific level. Beyond this level, the improvements in FCR may diminish, suggesting an optimal intake level of approximately 20 g/bird, where feed efficiency is maximised.
The intake of C. vulgaris in the broiler diets significantly affected various growth performances (Figure 1). Key performance indicators such as final body weight, cumulative body weight gain, and daily weight gain exhibited strong sigmoid correlations with cumulative C. vulgaris intake, indicating optimal intake levels of approximately 20 g/bird where these metrics were maximised. The FCR presented moderate to high correlations, suggesting specific intake levels of approximately 20 g/bird for optimal performance. These findings underscore the potential benefits and limitations of C. vulgaris supplementation in poultry diets, particularly concerning growth performance.
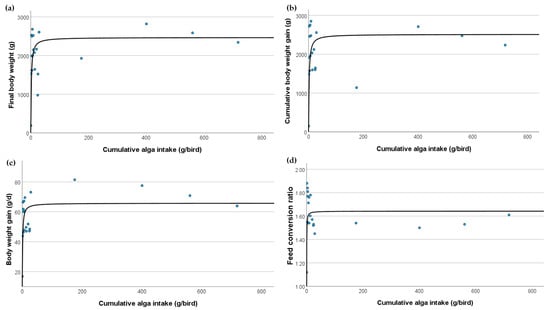
Figure 1.
The relationship between Chlorella vulgaris dosage and key broiler growth indicators characterized by sigmoid models: (a) final body weight, (b) cumulative body weight gain, (c) body weight gain, and (d) feed conversion ratio.
3. Impact of Cumulative Chlorella vulgaris Intake Levels on Plasma Metabolites and Immunoglobulin Levels in Broilers
Table 3 presents data from various studies examining the effects of different cumulative intake levels of C. vulgaris on plasma metabolites and immunoglobulin levels in broilers. The cumulative intake levels varied, providing insights into how C. vulgaris affects these health markers. The data showed cumulative C. vulgaris intake levels ranging from 1.40 g/bird [27] to 175 g/bird [16], with corresponding changes in plasma metabolites and immunoglobulin levels.

Table 3.
Impact of cumulative Chlorella vulgaris intake levels on plasma metabolites and immunoglobulin levels in broilers.
An et al. [27] investigated the effects of a 1.40 g cumulative C. vulgaris intake per bird over 34 days, observing total protein levels of 2.77 g/dL and triacylglycerols at 30.7 mg/dL, cholesterol at 120.3 mg/dL, high-density lipoproteins (HDL) at 97.1 mg/dL and albumin at 1.16 g/dL. The aspartate aminotransferase (AST) levels were 236.6 U/L, with plasma IgA at 721 µg/mL, IgM at 480 µg/mL and IgG at 3814 µg/mL. With a cumulative intake of 4.27 g, the final body weight was 1619 g, and the plasma metabolites showed the following slight changes: total protein at 2.78 g/dL, triacylglycerols at 34.4 mg/dL, cholesterol at 120.1 mg/dL, HDL at 93.2 mg/dL and albumin at 1.11 g/dL. The AST levels were 237 U/L, with plasma IgA at 710 µg/mL, IgM at 501 µg/mL and IgG at 3563 µg/mL. Another study [29] explored higher intake levels, such as 6.71 g and 13.04 g over 41 days. For the 6.71 g intake, the total protein was 5.050 g/dL, with triacylglycerols at 79.66 mg/dL, cholesterol at 161.00 mg/dL, HDL at 31.66 mg/dL and albumin at 2.9 g/dL. AST levels were 132.6 U/L, with plasma IgA at 290.30 µg/mL, IgM at 431.00 µg/mL and IgG at 4065.0 µg/mL. For the 13.04 g intake, the total protein was 5.600 g/dL, with triacylglycerols at 85.00 mg/dL, cholesterol at 143.30 mg/dL, HDL at 36.33 mg/dL and albumin at 3.3 g/dL. The AST levels were 97.0 U/L, with plasma IgA at 280.60 µg/mL, IgM at 328.30 µg/mL and IgG at 4600.0 µg/mL. The effects of cumulative C. vulgaris levels of 401 g, 561 g, and 718 g over 34 days were reported in [6]. They noted varied plasma metabolites and immunoglobulin levels across these intake levels, emphasizing the nuanced impact of high cumulative C. vulgaris intake on broiler health markers.
Table 4 summarises the correlation analysis for predicting the plasma metabolites and immunoglobulin levels based on cumulative C. vulgaris intake. Only variables with three or more degrees of freedom were analysed for correlation, ensuring the reliability of the statistical analysis. Additional data on broiler blood profiles and immune responses are provided in Appendix A. Table A1 summarises the impact of cumulative C. vulgaris intake levels on plasma metabolites and phitohemoglotenine-P response, while Table A2 presents the effects on the haematological profiles of the broilers.

Table 4.
Summary of correlation analysis for predicting the dependent variables, plasma metabolites and immunoglobulins, based on the cumulative Chlorella vulgaris intake.
For the systemic health indicators, the total protein levels showed a quadratic relationship with the cumulative C. vulgaris intake (R2 = 0.222, dof = 4, p = 0.605). The triacylglycerol levels also followed a quadratic pattern (R2 = 0.296, dof = 4, p = 0.495). The cholesterol levels showed an exponential relationship (R2 = 0.559, dof = 5, p = 0.053), suggesting that optimal cholesterol levels are achieved at lower cumulative intakes. The high-density lipoprotein levels followed a cubic pattern (R2 = 0.369, dof = 4, p = 0.399), and the albumin levels are best described by a power model (R2 = 0.253, dof = 4, p = 0.310). The AST levels showed a cubic relationship (R2 = 0.592, dof = 4, p = 0.166), suggesting that there were varying effects of C. vulgaris intake on liver function.
For plasma immunoglobulin levels, IgA showed a logarithmic relationship with the cumulative C. vulgaris intake (R2 = 0.569, dof = 6, p = 0.031), indicating that the IgA levels increased with C. vulgaris intake up to a point. The IgM levels followed a quadratic pattern (R2 = 0.746, dof = 5, p = 0.033), suggesting that there were optimal IgM levels at certain cumulative intakes. The IgG levels showed a cubic relationship (R2 = 0.842, dof = 4, p = 0.045), indicating that IgG levels are optimised at specific cumulative C. vulgaris intake levels.
The intake of C. vulgaris in broiler diets significantly affects plasma metabolite and plasma immunoglobulin levels. For instance, cholesterol levels are optimised at lower cumulative intakes, while IgA, IgM and IgG levels are optimised at specific intake levels, highlighting the potential health benefits of C. vulgaris supplementation. These findings underscore the potential benefits and limitations of C. vulgaris supplementation in poultry diets, particularly concerning growth performance and health markers.
4. Safety Precautions and Regulatory Aspects
Several critical considerations have emerged in assessing the safety precautions and regulatory aspects related to the use of C. vulgaris as a feed additive or ingredient in broiler diets. The safety of dietary C. vulgaris is generally acknowledged [34], particularly when it is free from contaminants. C. vulgaris is widely considered safe by regulatory authorities such as the European Food Safety Authority (EFSA) and the U.S. Food and Drug Administration (FDA), provided it is produced and processed under stringent quality control measures. Studies have shown that contaminants from freshwater sources are typically present in C. vulgaris at levels below detectable thresholds, reinforcing its safety profile [4]. Ensuring that C. vulgaris is free from contaminants like heavy metals or harmful microorganisms is essential, as these can pose significant health risks to poultry and consumers. The potential for bioaccumulation of these contaminants in broiler tissues, especially with higher levels of C. vulgaris intake, necessitates rigorous quality controls and regular safety assessments. This aspect underscores the need for well-established safety protocols in the production and processing of C. vulgaris intended for animal feed.
Proper cultivation and production conditions can ensure that the levels of contaminants such as heavy metals and harmful microorganisms remain within acceptable limits. Contamination with bacteria like Leucobacter sp., Aeromicrobium sp., Staphylococcus spp. and Halomonas spp., which can originate from various sources during the cultivation and sub-culturing processes, must be monitored and controlled. Adhering to stringent quality control measures, including regular toxicity analyses and monitoring microcystin levels, is crucial to maintaining the safety of C. vulgaris as a feed additive [5].
Additionally, the regulatory landscape surrounding the use of C. vulgaris in animal feed is complex and varies across different regions. Compliance with local and international regulations concerning feed safety, permissible additive levels, and labelling requirements is paramount. These regulations have been designed to ensure the safety of animal feed additives and, by extension, the safety of animal-derived food products for human consumption. Regulatory standards often evolve in response to new scientific findings and public health considerations. For instance, studies contribute to a growing body of evidence that regulators may use to review and update the guidelines on the use of C. vulgaris in poultry diets [4].
The long-term safety of C. vulgaris, particularly at high inclusion levels and over extended feeding durations, remains an area requiring further research. While short-term studies have indicated beneficial effects, the long-term implications for animal and human health are not fully understood. This knowledge gap calls for ongoing research and monitoring to detect any potential adverse effects, including the cumulative impacts of bioactive compounds in C. vulgaris on animal health and food safety.
In summary, while C. vulgaris offers potential health benefits as a poultry feed additive, its safe inclusion in broiler diets demands a comprehensive approach encompassing rigorous quality control, adherence to evolving regulatory standards and continuous research into its long-term safety and efficacy. Such an approach is essential to ensure that C. vulgaris-enhanced broiler meat is not only beneficial but also safe and compliant with regulatory requirements, thereby maintaining consumer trust and market viability.
5. Conclusions and Future Research
The intake of C. vulgaris in broiler diets significantly influences broiler growth performance and health-related compounds. Key performance indicators such as final body weight, cumulative body weight gain and daily weight gain exhibit significant sigmoid correlations with cumulative C. vulgaris intake, with optimal intake levels at approximately 20 g/bird, where these metrics are maximised. However, the feed conversion ratio presents lower significant sigmoid correlations, which also suggests specific intake levels of approximately 20 g/bird for optimal performance.
For health-related compounds, plasma metabolites and immunoglobulin levels demonstrate significant correlations with cumulative C. vulgaris intake. For instance, the levels of total protein, triacylglycerols and cholesterol have shown varying relationships with C. vulgaris intake, indicating that optimal intake levels can help maintain balanced plasma metabolites. Plasma immunoglobulin levels, particularly IgA, IgM and IgG, have exhibited significant correlations with C. vulgaris intake, suggesting enhanced immune responses at specific intake levels. Higher levels of immunoglobulins inside the normal range, particularly IgM and IgG, are generally indicative of an enhanced immune response. Increased IgM levels typically suggest a primary immune response, indicating that the bird’s immune system is effectively recognizing and responding to antigens. Elevated IgG levels are associated with long-term immunity and memory response, suggesting that the birds are better prepared to fight off infections.
These findings underscore the potential benefits of C. vulgaris supplementation in broiler diets. Optimal cumulative intake levels of approximately 20 g/bird maximise growth performance and positively influence health markers, including plasma metabolites and immunoglobulin levels. However, it is crucial to balance these benefits with potential diminishing returns or adverse effects at higher intake levels, ensuring that C. vulgaris is used effectively within the dietary framework of broilers.
Future research on C. vulgaris in poultry nutrition should concentrate on identifying the optimal dosage and duration of supplementation to maximise growth performance and health benefits while avoiding adverse effects. Longitudinal studies are essential to evaluate the long-term implications of C. vulgaris use on broiler health, particularly concerning the potential accumulation of bioactive compounds. Additionally, understanding the mechanisms by which C. vulgaris influences broiler physiology will help refine supplementation strategies for targeted outcomes, ensuring that broilers receive the maximum benefit from C. vulgaris’s nutritional properties.
Moreover, ensuring the safety and regulatory compliance of C. vulgaris supplementation is crucial. Rigorous quality control measures must be implemented to prevent contamination risks, and adherence to regulatory guidelines is necessary to maintain consumer confidence in C. vulgaris-supplemented poultry products. Comparative studies with other feed additives could also provide valuable insights into C. vulgaris’s relative effectiveness and economic viability, helping producers make informed decisions about its use in poultry diets. By addressing these key areas, future research can enhance our understanding of how to optimise C. vulgaris supplementation, ultimately improving both the productivity and health of broilers.
Author Contributions
Conceptualization, J.A.M.P.; data curation, A.R.M. and M.P.S.; writing—original draft preparation, J.A.M.P.; writing—review and editing, A.R.M., M.P.S., M.L. and J.A.M.P.; project administration, J.A.M.P.; funding acquisition, J.A.M.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the following Fundação para a Ciência e a Tecnologia grants (Lisbon, Portugal): 2022.11690.BD to A.R.M. and UI/BD/153071/2022 to M.P.S., as well as grants UIDB/04129/2020 to LEAF, UIDB/00276/2020 to CIISA and LA/P/0059/2020 to AL4AnimalS.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A

Table A1.
Impact of cumulative Chlorella vulgaris intake levels on plasma metabolites and phitohemoglotenine-P response of broilers.
Table A1.
Impact of cumulative Chlorella vulgaris intake levels on plasma metabolites and phitohemoglotenine-P response of broilers.
| Initial Age and Weight | Alga Level (% Feed) and Duration of Trial (days) 1 | Cumulative Alga Intake (g/bird) 2 | Plasma Metabolites | Phitohemoglotenine-P Response | Reference | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total Lipid (mg/dL) | LDL (mg/dL) | Glucose (mg/dL) | ALT (U/L) | SOD (U/mL) | MDA (nmol/mL) | |||||
| 45.1 g, 1 d-old 3 | 0.05%, 34 d | 1.40 | 314.6 | - | - | - | - | - | - | [27] |
| 1 d-old 3 | 0.07%, 41 d | 2.95 | - | - | - | - | - | - | 1.39 | [30] |
| 45.1 g, 1 d-old 3 | 0.15%, 34 d | 4.27 | 308.4 | - | - | - | - | - | - | [27] |
| 1 d-old 3 | 0.14%, 41 d | 5.94 | - | - | - | - | - | - | 1.51 | [30] |
| 41.8 g, 1 d-old | 0.20%, 41 d | 6.71 | - | 113.4 | 220.0 | 8.333 | 381.7 | 59.26 | - | [29] |
| 1 d-old 3 | 0.21%, 41 d | 9.22 | - | - | - | - | - | 1.54 | [30] | |
| 41.8 g, 1 d-old | 0.40%, 41 d | 13.0 | - | 90.00 | 208.3 | 7.000 | 434.5 | 43.66 | - | [29] |
| 45.1 g, 1 d-old 3 | 0.50%, 34 d | 14.1 | 252.0 | - | - | - | - | - | - | [27] |
| 41.8 g, 1 d-old | 0.60%, 41 d | 20.0 | - | 125.5 | 231.6 | 8.333 | 475.6 | 42.66 | - | [29] |
| 788.3 g, 21 d-old 3 | 10%, 14 d | 175 | 350 | 15 | 246.3 | 4.60 | - | - | - | [16] |
1 The final day of the trial, which involved slaughtering, was not included in the trial’s duration. 2 Calculated by multiplying the total feed consumed per animal during the experimental period by the dietary percentage of microalga. For some of the studies, no information about the cumulative feed intake was available, and therefore, an estimation of this was completed as follows: cumulative feed intake (g/bird) (An et al. [27]) = cumulative feed intake (g/d/bird) × number of trial days; cumulative feed intake (g/bird) (Rezvani et al. [30]) = cumulative body weight gain (g) × feed conversion ratio; and cumulative feed intake (g/bird) (Coelho et al. [16]) = cumulative feed intake (g/pen)/number of birds. 3 Male broilers. ALT, alanine aminotransferase; LDL, low-density lipoproteins; MDA, malondialdehyde; SOD, superoxide dismutase.

Table A2.
Impact of cumulative Chlorella vulgaris intake levels on the haematological profiles of broilers.
Table A2.
Impact of cumulative Chlorella vulgaris intake levels on the haematological profiles of broilers.
| Initial Age and Weight | Alga Level (% feed) and Duration of Trial (days) 1 | Cumulative Alga Intake (g/bird) 2 | Blood Leucocytes | Hb (×103/µL) | RBC (×106/µL) | PCV (%) | MCV (fL) | MCH (pg) | MCHC (%) | PL (×103/µL) | Reference | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WBC (×103/µL) | HE (×103/µL) | LY (×103/µL) | MO (×103/µL) | EO (×103/µL) | BA (×103/µL) | |||||||||||
| 41.8 g, 1 d-old | 0.20%, 41 d | 6.71 | 19.33 | 19.33 | 74.66 | 5.666 | 0.333 | - | 13.53 | 4.066 | 38.36 | 86.53 | 28.20 | 34.20 | 429.3 | [29] |
| 41.8 g, 1 d-old | 0.40%, 41 d | 13.0 | 22.66 | 14.66 | 83.33 | 2.000 | 0.000 | - | 15.40 | 5.400 | 44.53 | 85.76 | 28.93 | 35.06 | 403.3 | [29] |
| 41.8 g, 1 d-old | 0.60%, 41 d | 20.0 | 20.66 | 16.66 | 79.00 | 2.666 | 1.666 | - | 13.93 | 4.933 | 40.46 | 87.50 | 28.56 | 32.73 | 413.0 | [29] |
| 1 d-old 3 | 1.0%, 34 d | 24.4 | 23.81 | 6.6 | 13.5 | 2.69 | 0.82 | 0.19 | - | - | - | - | - | - | - | [32] |
| 1 d-old 3,4 | 1.0%, 34 d | 25.2 | 31.65 | 8.92 | 17.93 | 3.39 | 1.10 | 0.30 | - | - | - | - | - | - | - | [32] |
1 The final day of the trial, which involved slaughtering, was not included in the trial’s duration. 2 Calculated by multiplying the total feed consumed per animal during the experimental period by the dietary percentage of microalga. 3 Male broilers. 4 In this trial, fresh liquid Chlorella vulgaris was used. BA, basophils; EO, eosinophils; Hb, haemoglobin; HE, heterophils; LY, lymphocytes; MCH, mean corpuscular haemoglobin; MCHC, mean concentration of haemoglobin in red blood cells; MCV, mean corpuscular volume; MO, monocytes; PCV, packed cell volume; PL, platelets; RBC, red blood cells; WBC, white blood cells.
References
- United Nations. Department of Economic and Social Affairs, Population Division. World Population Prospects 2022: Summary of Results. 2022. UN DESA/POP/2022/TR/NO. 3. Available online: https://www.un.org/development/desa/pd/sites/www.un.org.development.desa.pd/files/wpp2022_summary_of_results.pdf (accessed on 28 May 2024).
- Abdel-Wareth, A.A.A.; Williams, A.N.; Salahuddin, M.; Gadekar, S.; Lohakare, J. Algae as an alternative source of protein in poultry diets for sustainable production and disease resistance: Present status and future considerations. Front. Vet. Sci. 2024, 11, 1382163. [Google Scholar] [CrossRef] [PubMed]
- Chaves, A.A.M.; Martins, C.F.; Carvalho, D.F.P.; Ribeiro, D.M.; Lordelo, M.; Freire, J.P.B.; Almeida, A.M. A viewpoint on the use of microalgae as an alternative feedstuff in the context of pig and poultry feeding-a special emphasis on tropical regions. Trop. Anim. Health Prod. 2021, 53, 396. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA). Chlorella vulgaris safety assessment. EFSA J. 2021. Available online: https://www.efsa.europa.eu/en/efsajournal/pub/ (accessed on 26 May 2024).
- Allmicroalgae. Quality control in the production of Chlorella vulgaris. 2021. Available online: https://allmicroalgae.com/quality-control (accessed on 26 May 2024).
- Cabrol, M.B.; Martins, J.C.; Malhão, L.P.; Alves, S.P.; Bessa, R.J.; Almeida, A.M.; Raymundo, A.; Lordelo, M. Partial replacement of soybean meal with Chlorella vulgaris in broiler diets influences performance and improves breast meat quality and fatty acid composition. Poult. Sci. 2022, 101, 101955. [Google Scholar] [CrossRef] [PubMed]
- Cabrol, M.B.; Martins, J.C.; Malhão, L.P.; Alfaia, C.M.; Prates, J.A.M.; Almeida, A.M.; Lordelo, M.; Raymundo, A. Digestibility of Meat Mineral and Proteins from Broilers Fed with Graded Levels of Chlorella vulgaris. Foods 2022, 11, 1345. [Google Scholar] [CrossRef] [PubMed]
- Andrade, L.M.; Andrade, C.J.; Dias, M.; Nascimento, C.A.O.; Mendes, M.A. Chlorella and spirulina microalgae as sources of functional foods, nutraceuticals, and food supplements; an overview. MOJ Food Process. Technol. 2018, 6, 45–58. [Google Scholar] [CrossRef]
- Maurício, T.; Couto, D.; Lopes, D.; Conde, T.; Pais, R.; Batista, J.; Melo, T.; Pinho, M.; Moreira, A.S.P.; Trovão, M.; et al. Differences and Similarities in Lipid Composition, Nutritional Value, and Bioactive Potential of Four Edible Chlorella vulgaris Strains. Foods 2023, 12, 1625. [Google Scholar] [CrossRef] [PubMed]
- Lum, K.K.; Kim, J.; Lei, X.G. Dual potential of microalgae as a sustainable biofuel feedstock and animal feed. J. Anim. Sci. Biotechnol. 2013, 4, 53. [Google Scholar] [CrossRef]
- Ru, I.T.K.; Sung, Y.Y.; Jusoh, M.; Wahid, M.E.A.; Nagappan, T. Chlorella vulgaris: A perspective on its potential for combining high biomass with high-value bioproducts. Appl. Phycol. 2020, 1, 2–11. [Google Scholar] [CrossRef]
- Safi, C.; Zebib, B.; Merah, O.; Pontalier, P.-Y.; Vaca-Garcia, C. Morphology, composition, production, processing and applications of Chlorella vulgaris: A review. Renew. Sustain. Energy Rev. 2014, 35, 265–278. [Google Scholar] [CrossRef]
- Becker, E.W. Micro-algae as a source of protein. Biotechnol. Adv. 2007, 25, 207–210. [Google Scholar] [CrossRef]
- Coudert, E.; Baéza, E.; Berri, C. Use of algae in poultry production: A review. J. World’s Poult. Sci. 2020, 76, 767–786. [Google Scholar] [CrossRef]
- Alfaia, C.M.; Pestana, J.M.; Rodrigues, M.; Coelho, D.; Aires, M.J.; Ribeiro, D.M.; Major, V.T.; Martins, C.F.; Santos, H.; Lopes, P.A.; et al. Influence of dietary Chlorella vulgaris and carbohydrate-active enzymes on growth performance, meat quality and lipid composition of broiler chickens. Poult. Sci. 2021, 100, 926–937. [Google Scholar] [CrossRef]
- Coelho, D.F.M.; Alfaia, C.M.R.P.M.; Assunção, J.M.P.; Costa, M.; Pinto, R.M.A.; Fontes, C.M.G.A.; Lordelo, M.M.; Prates, J.A.M. Impact of dietary Chlorella vulgaris and carbohydrate-active enzymes incorporation on plasma metabolites and liver lipid composition of broilers. BMC Vet. Res. 2021, 17, 229. [Google Scholar] [CrossRef] [PubMed]
- Korczyński, M.; Witkowska, Z.; Opaliński, S.; Świniarska, M.; Dobrzański, Z. Algae extract as a potential feed additive. In Marine Algae Extracts: Processes, Products, Applications; Kim, S.K., Chojnacka, K., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2015; pp. 603–626. [Google Scholar] [CrossRef]
- Coronado-Reyes, J.A.; Salazar-Torres, J.A.; Juárez-Campos, B.; González-Hernández, J.C. Chlorella Vulgaris, a Microalgae Important to Be Used in Biotechnology: A Review. Food Sci. Technol. 2022, 42, e37320. [Google Scholar] [CrossRef]
- Pantami, H.A.; Ahamad Bustamam, M.S.; Lee, S.Y.; Ismail, I.S.; Mohd Faudzi, S.M.; Nakakuni, M.; Shaari, K. Comprehensive GCMS and LC-MS/MS Metabolite Profiling of Chlorella vulgaris. Mar. Drugs 2020, 18, 367. [Google Scholar] [CrossRef] [PubMed]
- Coulombier, N.; Jauffrais, T.; Lebouvier, N. Antioxidant Compounds from Microalgae: A Review. Mar. Drugs 2021, 19, 549. [Google Scholar] [CrossRef] [PubMed]
- Agarwalla, A.; Komandur, J.; Mohanty, K. Current trends in the pretreatment of microalgal biomass for efficient and enhanced bioenergy production. Bioresour. Technol. 2023, 369, 128330. [Google Scholar] [CrossRef] [PubMed]
- Alhattab, M.; Kermanshahi-Pour, A.; Brooks, M.S.L. Microalgae disruption techniques for product recovery: Influence of cell wall composition. J. Appl. Phycol. 2019, 31, 61–88. [Google Scholar] [CrossRef]
- Costa, M.M.; Spínola, M.P.; Alves, V.D.; Prates, J.A.M. Improving protein extraction and peptide production from Chlorella vulgaris using combined mechanical/physical and enzymatic pre-treatments. Heliyon 2024, 10, i32704. [Google Scholar] [CrossRef]
- Van Nerom, S.; Buyse, K.; Van Immerseel, F.; Robbens, J.; Delezie, E. Pulsed electric field (PEF) processing of microalga Chlorella vulgaris and its digestibility in broiler feed. Poult. Sci. 2024, 103, 103721. [Google Scholar] [CrossRef]
- Spínola, M.P.; Costa, M.M.; Prates, J.A.M. Enhancing Digestibility of Chlorella vulgaris Biomass in Monogastric Diets: Strategies and Insights. Animals 2023, 13, 1017. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Yoon, J.H.; An, S.H.; Cho, I.H.; Lee, C.W.; Jeon, Y.J.; Joo, S.S.; Ban, B.C.; Lee, J.Y.; Jung, H.J.; et al. Intestinal Immune Cell Populations, Barrier Function, and Microbiomes in Broilers Fed a Diet Supplemented with Chlorella vulgaris. Animals 2023, 13, 2380. [Google Scholar] [CrossRef] [PubMed]
- An, B.-K.; Kim, K.-E.; Jeon, J.-Y.; Lee, K.W. Effect of dried Chlorella vulgaris and Chlorella growth factor on growth performance, meat qualities and humoral immune responses in broiler chickens. SpringerPlus 2016, 5, 718. [Google Scholar] [CrossRef] [PubMed]
- El-Bahr, S.; Shousha, S.; Shehab, A.; Khattab, W.; Ahmed-Farid, O.; Sabike, I.; El-Garhy, O.; Albokhadaim, I.; Albosadah, K. Effect of dietary microalgae on growth performance, profiles of amino and fatty acids, antioxidant status, and meat quality of broiler chickens. Animals 2020, 10, 761. [Google Scholar] [CrossRef] [PubMed]
- El-Gogary, M.; Dorra, T.; Megahed, A. Evaluation of the Role of Spirulina platensis and Chlorella vulgaris on Growth Performance, Meat Quality and Blood Parameters of Broiler Chickens. J. Anim. Poult. Prod. 2023, 14, 149–156. [Google Scholar] [CrossRef]
- Rezvani, M.; Com, M.R.; Shivazad, M.; Zaghari, M. A survey on Chlorella vulgaris effects on performance and cellular immunity in broilers. Int. J. Agric. Sci. 2012, 3, 9–15. [Google Scholar]
- Abou-Zeid, A.E.; El-Damarawy, S.Z.; Mariey, Y.A.; El-Mansy, M.M. Effect of using Spirulina platensis and/or Chlorella vulgaris algae as feed additives on productive performance of broiler chicks. J. Anim. Poult. Prod. 2015, 6, 623–634. [Google Scholar] [CrossRef]
- Kang, H.K.; Salim, H.M.; Akter, N.; Kim, D.W.; Kim, J.H.; Bang, H.T.; Kim, M.J.; Na, J.C.; Hwangbo, J.; Choi, H.C.; et al. Effect of various forms of dietary Chlorella supplementation on growth performance, immune characteristics, and intestinal microflora population of broiler chickens. J. Appl. Poult. Res. 2013, 22, 100–108. [Google Scholar] [CrossRef]
- Roques, S.; Koopmans, S.J.; Mens, A.; van Harn, J.; van Krimpen, M.; Kar, S.K. Effect of Feeding 0.8% Dried Powdered Chlorella vulgaris Biomass on Growth Performance, Immune Response, and Intestinal Morphology during Grower Phase in Broiler Chickens. Animals 2022, 12, 1114. [Google Scholar] [CrossRef]
- Takyar, M.B.T.; Khajavi, S.H.; Safari, R. Evaluation of antioxidant properties of Chlorella vulgaris and Spirulina platensis and their application to extend the shelf life of rainbow trout (Oncorhynchus mykiss) fillets during refrigerated storage. LWT—Food Sci. Technol. 2019, 100, 244–249. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).